
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 24 April 2018
Sec. Microbial Immunology
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.00823
This article is part of the Research TopicThe Mononuclear Phagocyte System in Infectious DiseaseView all 61 articles
Sepsis, in essence, is a serious clinical condition that can subsequently result in death as a consequence of a systemic inflammatory response syndrome including febrile leukopenia, hypotension, and multiple organ failures. To date, such life-threatening organ dysfunction remains one of the leading causes of death in intensive care units, with an increasing incidence rate worldwide and particularly within the rapidly growing senior population. While most of the clinical trials are aimed at dampening the overwhelming immune response to infection that spreads through the bloodstream, based on several human immunological investigations, it is now widely accepted that susceptibility to nosocomial infections and long-term sepsis mortality involves an immunosuppressive phase that is characterized by a decrease in some subsets of dendritic cells (DCs). Only recently substantial advances have been made in terms of the origin of the mononuclear phagocyte system that is now likely to allow for a better understanding of how the paralysis of DCs leads to sepsis-related death. Indeed, the unifying view of each subset of DCs has already improved our understanding of the pivotal pathways that contribute to the shift in commitment of their progenitors that originate from the bone marrow. It is quite plausible that this anomaly in sepsis may occur at the single level of DC-committed precursors, and elucidating the immunological basis for such a derangement during the ontogeny of each subset of DCs is now of particular importance for restoring an adequate cell fate decision to their vulnerable progenitors. Last but not least, it provides a direct perspective on the development of sophisticated myelopoiesis-based strategies that are currently being considered for the treatment of immunosenescence within different tissue microenvironments, such as the kidney and the spleen.
The mononuclear phagocyte system has been initially formulated by the late 1960. It consists of a network of cells, comprising monocytes, macrophages, and dendritic cells (DCs) that are dis-seminated throughout the organism. These cells are characterized by their morphology, their phenotypic characteristics (including phagocytic activity), and their roles in orchestrating the immune system. The majority of their committed progenitors are quiescent at homeostasis, although their very high proliferative potential provides them with the capacity to continuously maintain their numbers. Significant progresses in system biology have been made only recently in regard to understanding of the ontogeny and the function of mononucleated cells (referred to as myelopoiesis). This led to the discovery of committed precursors for adult-derived monocytes, conventional, plasmacytoid, or monocyte-derived dendritic cells (Mo-DCs), which are primarily described in the present perspective article. For more details on the embryonically derived phagocytes, we direct the reader to the following outstanding review (1).
Macrophage and DC precursor cells (referred to as MDP) does not constitute a homogeneous population but rather consists in a mixture of progenitors committed either to the DC lineage or the monocyte/macrophage lineage when they are transferred into the bone marrow (BM) of hosts that have previously been irradiated (2, 3). While less is known about the ontogeny of monocytes, macrophages, and DCs in humans than in mice, recent studies have allowed a link to be made with what has been observed in animal models. Notably, a homolog of murine MDP has been identified based on the in vitro differentiation of human CD34+ hematopoietic progenitors into type 1 conventional DC (cDC1) (4). There has since been a concerted effort to identify precursors restricted to either cDCs or those derived from the monocytic lineage. MDP express M-CSF-R (or CD115) and the Flt3 receptor (CD135), which are receptors for cytokines that play important roles in the development of monocytes or DCs, respectively. It is likely that the commitment shift of MDP depends on the balance between signals linked to the activation of these receptors (5). This hypothesis is bolstered by the fact that the expression of M-CSF-R decreases in the precursors of cDCs and plasmacytoid DCs (pDCs), although it is not detectable in mature cells. Conversely, Flt3 is not found in the precursors restricted to the monocytic lineage (6, 7). Signaling by the aforementioned growth factors could induce changes at the level of the expression of certain transcription factors. For example, the hematopoietic transcription factors PU.1 and MAFB (for MAF BZIP Transcription Factor B) are crucial for the development of DCs or monocytes, respectively, and they could be implicated in engagement in one of these lineages (8).
Apart from the MDP, the precursor CDP stands for common DC progenitor (Figure 1). Like the MDP, it expresses M-CSF-R and Flt3 (9–11). The CDP on the one hand generates pDCs, and on the other hand generates pre-cDCs, which are the direct circulating precursors of the cDCs in tissues. In parallel, other teams have elegantly shown that, as is the case with mice, the generation of cDC1 and cDC2 by common DC progenitor (hCDP) occurs by production of a circulating progenitor, namely the hPre-cDC, which is incapable of generating pDCs (12). Like their murine homologs, hPre-cDCs are heterogeneous and they comprise various fractions already committed to become cDC1 or cDC2 (13–15). Pre-cDCs leave the BM via blood circulation and then penetrate into lymphoid and non-lymphoid tissues in order to differentiate into cDCs (9–11). The factors that influence the differentiation of pre-cDCs into cDC1 or DC2 are still unknown. However, it appears that this decision is taken at the CDP stage, which can already exhibit a transcriptional signature similar to cDC1 or cDC2. Moreover, the pre-cDC population appears to be heterogeneous, comprising a mixture of pre-cDC1 and pre-cDC2 in mice (16) and in humans (15).
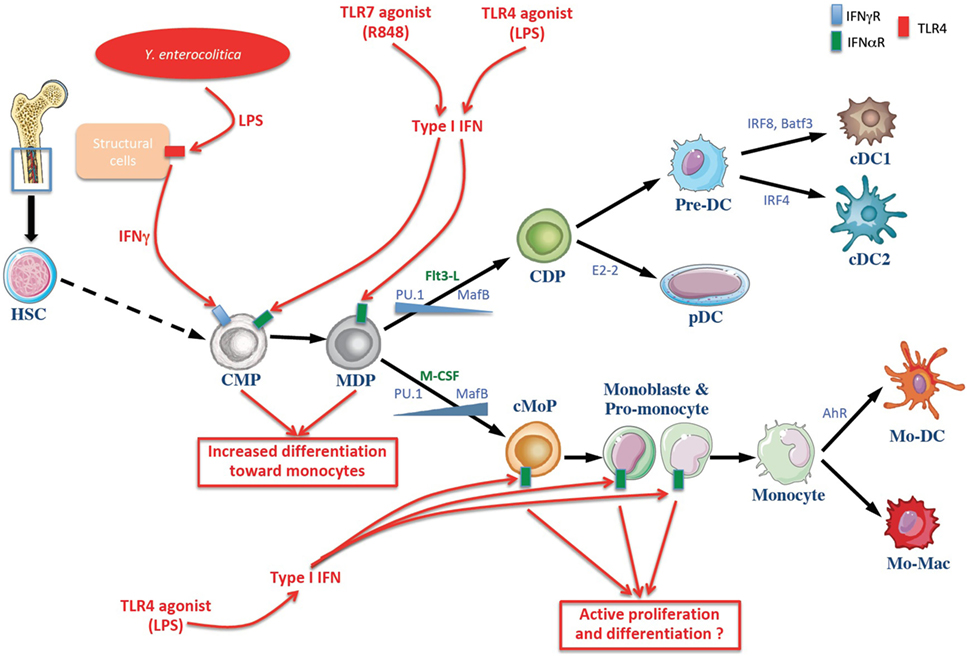
Figure 1. Schematic overview of dendritic cell (DC) and monocytes generation at homeostasis and in systemic infection or endotexemia murine models. The common myeloid progenitor (CMP) derived from hematopoietic stem cells (HSCs) in the bone marrow and can give rise to the monocyte and DC progenitor (MDP) which in turn differentiates into the DC or monocytic lineages. The differentiation toward DC and monocytes is influenced by cytokines and growth factors (noted in green), notably Flt3-L and M-CSF. Transcription factors involved in cell’s fate choice are noted in blue. Infectious stimuli (in red) can affect this process. Lipopolysaccharides (LPS) of the Gram negative bacilli Yersinia enterocolitica are sensed by radio-resistant cells that produce IFNγ, inducing a selective differentiation of myeloid progenitors toward the monocytic lineage (monocytopoiesis) at the expense of conventional DC (cDC) (17). Moreover, R848 and LPS induce the production of type I IFN involved in the differentiation of myeloid progenitors toward the monocytic lineage (18, 19). cDC, conventional dendritic cell; CDP, common dendritic cell progenitor; Pre-DC, precursor of cDCs; pDC, plasmacytoide DC; cMoP, common monocyte progenitor; Mo-DC, monocyte-derived dendritic cells, Mo-Mac, monocyte-derived macrophages; IFNγ, interferon γ; TLR toll-like receptor.
More recently, a progenitor restricted to monocytes and derived directly from MDP was identified and designated as cMoP, for common monocyte progenitor (Figure 1). It differs phenotypically from MDP by the loss of Flt3 expression. Consequently, cMoPs differentiate into monocytes and their descendants, but they do not generate cDCs (7). The development of cMoPs into monocytes also takes place as monoblast and then as pro-monocyte stages. They are characterized by the expression of stem cell antigen 1 (Sca-1) and they undergo very fast turn-over in the BM (20, 21). The monocytes generated in this manner then migrate from the BM to the tissues where they differentiate depending on the microenvironment (22). Furthermore, a recent study has shown that the generation of human monocytes by hMDP occurs by production of restricted precursors referred to as cMoPs (23), as in mice (7).
These novel concepts are not yet set in stone, however, as the single cell genomic era is already leading to refinements in ontogeny of each subsets of DCs and macrophages. For instance, Helft and colleagues recently demonstrated that human cDC1 are derived more efficiently from the multipotent lymphoid progenitor than from the common myeloid progenitor (CMP) (24). In parallel, it is proposed that the MDP does not constitute a homogeneous population but rather consists in a mixture of progenitors committed either to the DC lineage or the monocyte/macrophage lineage, with no or only very few individual cells able to yield both cell lineages in their progeny (25, 26).
Monocytopoiesis is a dynamic process that occurs in the BM as well as in other organs as an adaption to several physiological stresses that varies over time, while emergency myelopoiesis refers to the rapid generation of myeloid effector cells in response to purified lipopolysaccharide (LPS) (27). A hallmark of septicemia is a profound decrease of circulating DCs, which is also an indicator of a poor prognosis for septic patients (28–30). Two main types of murine models of sepsis or acute inflammation are generally used. On the one hand, the model of peritoneal or intravenous injection of purified endotoxins constitutes a simple model of acute inflammation. On the other hand, the other widely used murine sepsis model is based on cecal ligature and puncture (CLP). This chirurgical model induces intestinal bacterial translocation into the peritoneal cavity, generating a systemic infection and massive inflammation. Meanwhile, the extent to which murine models adequately reflect the complexity of human sepsis or acute inflammation is a matter of debate (31, 32). Although differences in TLR distribution among the various mononuclear phagocyte subsets exist between humans and mice (33), the latter are widely used to understand part of these complex disorders.
In order for this emergency myelopoiesis to be induced, TLR4 needs to be expressed by the radiation-resistant cells of the host. These cells then produce the growth factor G-CSF, which is sufficient to induce this phenomenon (34). G-CSF can also be produced following activation of inflammasomes, which depends on the cytokines IL-1beta and IL-1alpha, thereby inducing emergency myelopoiesis (35, 36). A recent study has also provided evidence for the production of IL-3 by B lymphocytes in a murine model of septicemia. This IL-3 allows for a significant increase in the production of monocytes and neutrophils, which are involved in systemic inflammatory respiratory syndrome (SIRS) and the “cytokine storm.” Furthermore, an elevated level of IL-3 in serum is predictive of a poor prognosis in septic patients (37). The hematopoietic progenitors can hence be indirectly activated in case of severe infection, so as to reorient the production of cells toward the myeloid lineage. However, the mechanisms causing this decrease in DCs during sepsis remain unclear, possibly encompassing both enhanced cell death of at least some subsets of DCs (and their defective reconstitution from their progenitor cells, e.g., originated from cMop and/or pre-cDC). Using the recently accepted nomenclature (38), we herein discuss the potential mechanisms causing this decrease in some DCs during sepsis that is linked to long-term sepsis-related mortality, especially in elderly and diabetic populations. In addition to studies of DCs in sepsis and endotoxemia models, we also review the contribution of macrophages, as these phagocytes have sometimes been incorrectly classified as Mo-DCs with the use of non-discriminating markers (38), such as CD64.
Monocytes are circulating hematopoietic cells generated in the BM, with a very short half-life that does not exceed a few days. In mice, they are generally divided into two subpopulations that are distinguished based on the expression of Ly6C surface molecules (39). Ly6Chi monocytes, or inflammatory monocytes, are rapidly recruited at sites of infection and inflammation in a CCR2 chemokine-dependent manner. Once inside tissues, diverse signals from the microenvironment can induce an increase in phagocytosis, the production of cytokines, antimicrobial activity, and antigen presentation by the cells, thereby inducing a phenotype that is sometimes very similar to that of macrophages or DCs (22, 40). Ly6Clow monocytes, also called patrolling monocytes, are less common than inflammatory monocytes, and they express the CX3C chemokine receptor 1 (CX3CR1) also known as the G-protein coupled receptor 13 (GPR13) or fractalkine receptor. Indeed it appears that their main function is to ensure endothelial integrity, by patrolling in the lumen of the blood vessels along the endothelium (41). These cells are the product of the differentiation of Ly6Chi blood monocytes at homeostasis (42). Ly6Clow monocytes could hence be considered as macrophages of the vascular system (22, 40). Two monocyte populations are also present in humans, and they correlate with those found in mice (43, 44). However, they are not distinguished based on the same surface markers as in mice. Rather, they are distinguished by the expression of the LPS CD14 coreceptor and of the CD16 receptor for crystalizable fragments of antibody. Human CD14+CD16− monocytes appear to be the homologs of the murine Ly6Chi population, while the CD14+CD16+ population appears to be analogous to the murine Ly6Clow population (22, 40, 41). During polymicrobial sepsis, inflammatory monocytes prevent renal damage in a CX3CR1-dependent adhesion mechanism (45) and a decrease in circulating patrolling monocytes is associated with unfavorable outcome (46). While reactivity to the subsequent endotoxin challenge is enhanced by muramyldipedtide, it remains to be determined whether the anaphylactic reactions are influenced by the muramyldipeptide-induced conversion of Ly6Chi toward Ly6Clow monocytes (47) or by other mechanisms involved in leukocyte binding and adhesion.
Adult-derived macrophages have been assumed to be the progeny of monocytes in tissues (48). However, although monocytes can indeed generate macrophages under certain conditions, circulating monocytes do not appear to be the main source of these cells. With the aim of simplifying nomenclatures, Martin Guilliams and his collaborators recently proposed that the use of the term “macrophage” should be restricted to mononucleated phagocytes of embryonic origin (49). Indeed, recent studies have shown that the majority of macrophages residing in the brain, the liver, the lungs, and even the spleen are derived from embryonic precursors in the vitellin vesicle and in the fetal liver. These macrophages disseminate to the various tissues of the body once the blood circulation becomes established, and they are maintained there by proliferating locally throughout the individual’s lifetime (42, 50–57). These embryonic macrophages can be progressively displaced by blood-derived monocytes. For instance, the intestinal macrophages that are of embryonic origin are replaced by the differentiation of blood monocytes that are recruited into tissues several weeks after birth (58). Moreover, monocytes constitute a major source of tissue macrophages already at steady state, including the skin (59) and the oral mucosa (60), and this phenomenon is amplified by inflammatory and/or aging processes in a range of organs such as the intestine (61), the heart (62, 63), the peritoneal cavity (64), and the liver (65, 66). Hotchkiss et al. reported that the number of splenic macrophages is not reduced in septic and trauma patients (67). These observations still need to be investigated with up-to-date markers to decipher the exact changes in the mononuclear phagocytes at the subset level. Indeed, the identification of splenic macrophages with CD14 is not sufficient.
Upon homeostasis in certain tissues such as the kidney, or in case of either infection or inflammation, numerous studies have shown that some DCs and macrophages are two sides of the same coin, as they both are derived from monocytes. These cells will hence be referred to here as monocyte-derived antigen-presenting cells (Mo-APCs). The cells derived from monocytes can express high levels of major histocompatibility complex class II (MHC-II) and CD11c, and they can migrate and efficiently present Ag to T lymphocytes (59). Certain studies have also shown their efficacy at cross-presentation of Ag, although these cells appear to use different intracellular components than cDC1 to achieve this (68–71). As suggested by their cross-presenting activity, like cDC1, APCs derived from monocytes have been implicated in cytotoxic Th1 responses (72, 73). However, like cDC2, Mo-APCs have also been reported to induce Th2 and Th17 types of responses (74–76). Depending on the context, Mo-APCs could develop functions similar to those of the various populations of cDCs. However, the lack of markers to discriminate these cells from cDCs, macrophages, or active monocytes greatly complicates the study of Mo-APCs. A study has shown the presence of MHC-II+CD11c+ cells derived from monocytes in skeletal muscles under conditions of homeostasis. The intramuscular administration of alum adjuvant induced a very pronounced increase in the representation of these cells and the simultaneous administration of LPS greatly increased their capacity to migrate to lymph nodes and the spleen. These cells are capable of presenting Ag to naive T lymphocyte by normal as well as cross-presentation. They are characterized by the expression of inducible nitric oxide synthase (iNOS) and of the Fc receptor CD64 (FcγRI), which are not expressed by cDCs and pDCs (69). The CD64 marker can also be used to distinguish CDP-derived cells from monocyte-derived cells at the level of the intestine and the skin under homeostatic or inflammatory conditions in mice (59, 61), but not at the level of the kidney (77). The immunoglobulin FcεRI receptor has also recently been reported to be expressed by Mo-APCs in mice and in humans (70, 74), and on human cDC2 (15, 78, 79), it appears that Fc receptors are mainly restricted to phagocytes of monocytic origin (80), thereby they may facilitate their identification in conjunction with other discriminative traits (80). Improvements in the characterization of these cells within distinct tissue microenvironments will undoubtedly increase our knowledge of their biology and presumably provide an explanation for the poor clinical impact of past investigations regarding each DC subsets in sepsis. Despite these limitations, Kassianos et al. demonstrated that human Mo-APCs are the major subsets responsive to Escherichia coli in terms of inflammatory cytokine secretion, antigen presentation to CD8+ T cells, and phagocytosis (33).
Dendritic cells are the main antigen-presenting cells (APCs) of the organism. They are characterized by the expression of MHC-II, integrin CD11c, and the transcription factor Zbtb46 (sometimes referred to as zDC) (81–83). However, these markers are also expressed by certain macrophages or other cells that are derived from monocytes. Generally, a distinction is made between cDCs, plasmacytoid (pDCs), which are present in the basal state, and DCs derived from monocytes (Mo-DCs), which are recruited extensively in case of inflammation (6). cDCs are found in the vast majority of lymphoid and non-lymphoid tissues. The term “conventional” refers to DCs that are non-plasmacytoid and that are not derived from monocytes but from a precursor restricted to these cells. cDCs induce either immunity or tolerance toward the Ag that they present to lymphocytes (6). There is a general consensus that there are two populations of cDCs, namely cDC1 and cDC2, which are endowed with distinct functional specialization and thus play complementary roles in the shaping of immune responses (6, 49, 83, 84). Numerous studies have shown a dramatic decrease in DCs during septicemia. Integrin CD11c is often used as a marker of DCs, although it is also expressed to a varying degree by other cell populations such as certain macrophages, neutrophils, and lymphocytes (Table 1). A dramatic decrease in CD11c+ cells in the periphery has been observed over the first days of a murine model of polymicrobial sepsis (85–90), and in the BM (91) (Table 2). Wen and colleagues found that there was a significant reduction in the percentage of CD11c+CD11b+MHCIIhi cells in lung and spleen from 3 to 14 days post-CLP procedure in comparison to sham mice (86). They also noted a decrease in the percentage of lung CD11c+CD11b+ and CD11c+B220+ cells at days 2 and 8 in post-CLP mice infected by S. mansoni eggs, which was associated with a diminished ability of lung CD11c+ cells to produce IL12p70 after TLR agonist stimulation (88).
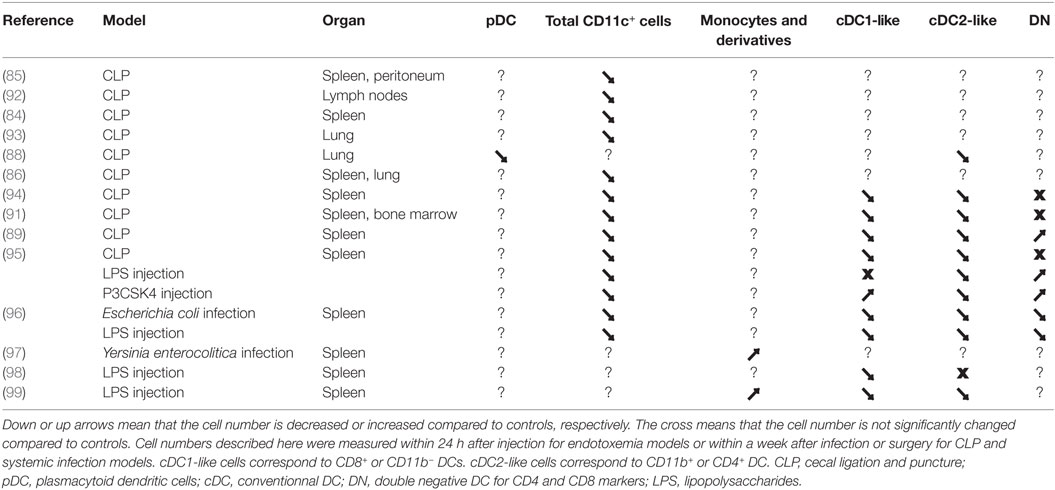
Table 2. Comparison of cell numbers for mononuclear phagocytes populations in murine models of polymicrobial sepsis, systemic inflammation, or endotoxemia.
In order to evaluate the impact of septicemia on different populations of DCs, Flohé and his collaborators used a mouse CLP model, and they distinguished CD11c+ cells on the basis of expression of CD8 (expressed by cDC1) and CD4 (expressed by cDC2), from double-negative cells that might be cDC precursors, for example (108). In light of this, the observed loss of DCs at 36 h postoperative to the procedure was due to the CD8+ and CD4+ populations, while the total number of double-negative cells itself was increased in this model (94). Another CLP study has also provided evidence for a depletion of DCs from the local mesenteric and systemic inguinal lymph nodes, with a preferential loss of cDC1 expressing CD8, which was associated with increased apoptosis (92). This splenic cDC1 loss was maintained up to 5 days post-CLP procedure, and the cells repopulated the spleen at day-7 post-CLP procedure in an NF-kB signaling-dependent pathway (90).
It hence appears that, in mice, polymicrobial septicemia induces a specific depletion of certain DC populations of the lymphoid organs. Some populations may be depleted more so than others, as has been observed in the spleen with an increase in the CD4– CD8– population, for which the exact link with cDC1 and cDC2 is not known (94, 108). In keeping with a less-mature phenotype of this double-negative population, when cell proliferation was measured, during a CLP procedure, by BrdU incorporation after 4 days, these cells had a significantly higher BrdU content compared to sham control mice (91). The double-negative splenic cells were differently affected according to the model selected. Thus, they were not affected after CLP, although they were significantly increased after LPS or Pam3CSK4 injection (95).
Type 1 conventional DCs are characterized by the expression of TLR3 that is required for sensing of viral RNA, and by a greater capacity for secretion of the cytokine IL-12p70 following their activation. This cytokine allows for differentiation of type 1 T helper cells (Th1) implicated in cytotoxic anti-viral and anti-tumor immunity (100, 109, 110), and promotes CD4+ T helper cells for CD8+ responses (101). This subpopulation of cDCs has also been reported to be efficient in terms of a particular mechanism of antigen presentation that is called “cross-presentation”, which allows these cells that are constitutively resistant to viral infection to acquire exogenous antigens from the infectious agent (111). This cross-presentation process consists of the processing of exogenous antigens into peptides and their loading onto MHC-I molecules so as to be presented to CD8+ T cells. This process is called cross-priming if it results in their activation (112, 113). In humans, a very similar population has been reported to be present in the blood and in the spleen, expressing CLEC9A, as do their murine homologs (4, 114–117) (Table 1). Overall, the data in humans suggest that cDC1 excels at cross-presentation of cell-associated antigens (115, 118–122) or of antigens that are delivered to late endosomes/lysosomes (123, 124). However, the data in mice show that other DC subsets are also capable of cross-presentation, provided that they have been properly stimulated (125, 126). Meanwhile, this function could depend on a number of variables such as the type of antigen, its intracellular route of delivery, and the accompanying adjuvant signal sensed by the DCs. cDC1 cells are more effective in regard to this function in specific pathophysiological contexts including viral infections or tumor development/treatment (127–129). During LPS-induced endotoxemia in mice, a reduced cross-priming activity of splenic cDC1 (130) correlates with a prominent loss of splenic cDC1, defined as CD8+ DCs, which is glucocorticoid dependent (98). Indeed, endogenous glucocorticoids blunt LPS-induced inflammation and they promote tolerance by suppressing cDC1 IL-12 production. In the absence of glucocorticoid signaling in CD11c-expressing cells, LPS treatment induces higher serum levels of IL-12, type I IFN, TNF-α, and IFN-γ (98). In terms of epigenomic reprogramming, the inflammatory function of TNF is potentiated by type I IFN by prevention of the silencing of genes encoding inflammatory molecules in human macrophages (131). A similar decrease in mouse splenic cDC1 has also been observed after CLP procedures (89, 95). However, injection of different PAMPs induced various effects on cDC numbers. Indeed, LPS does significantly affect splenic cDC1 numbers within 2 days after LPS injection (96), which is followed by a cDC1 number recovery (95, 96) (Table 2). On the other hand, Pam3CSK4 does induce an increase in cDC1 cells after 4 days (95) (Table 2). Like their mouse counterpart (114, 132), human blood cDC1 cells were found to not express or very low level of TLR4 and they failed to ingest E. coli (33). It remains to be investigated whether these differences are still maintained in human lymphoid and non-lymphoid tissues during sepsis and endotoxemia.
By contrast, cDC2 are often characterized by the expression of integrin CD11b and SIRPα (also referred to as CD172a) (80), and in the spleen as CD4+CD8– cells (Table 1). They are found in lymphoid and non-lymphoid tissues, and they predominate over the cDC1 population in nearly all tissues. The development and maintenance of cDC2 appear to be dependent, for example, on the IRF4 transcription factor (102, 103, 133) and the activation of Notch-2 receptors (134). This population of cDCs appears to be more efficient than the cDC1 in terms of the interaction with CD4+ T lymphocyte and the polarization of helper T lymphocytes, particularly for Th2 and Th17, which are implicated in immune responses toward extracellular pathogens and the regulation of immunity (74, 133, 135–137). However, the heterogeneity of CD11b+ cells has greatly complicated the study of this population. Indeed, distinguishing cDC2 from macrophages and cells derived from monocytes is difficult as they can express numerous markers that they have in common. To date, it has hence been difficult to assign them non-overlapping immunological properties as well as to establish their dependence on specific transcription factors. New markers have recently been described to assist with the discrimination between cDC2 and macrophages and the cells derived from monocytes in various tissues, such as the lungs, muscles, and the intestines. A decrease in splenic cDC2 has been observed in CLP models (91, 94, 95), associated with a decrease in proliferation of the residual cells (91) (Table 2). Proper definition of cDC2 cells in various species and tissues, and in various acute inflammatory models and human samples to define their decline in numbers and alteration of their function, still needs to be investigated with the improved definition of the markers (Table 1). Indeed it has been proposed a set of markers to define properly cDC2, and cDC1, across species and tissues (38), and the new refinements by single cell approaches would need to be taken into account to further study cDC2 cell modulations during sepsis and endotoxemia (15, 79, 138).
Already in the first papers describing their discovery more than 15 years ago, human and mouse pDCs have been shown to lack any antigen presenting functions at steady state but to acquire it upon proper stimulation. Relative to human pDCs that do not express CD11c, mouse pDCs may express intermediate, not low, levels of this marker. While mouse and human pDCs have been found to express Siglec-H or Blood Dendritic Cell Antigen 2 (BDCA-2) markers, respectively, they are not sufficient to characterize them, since they are also expressed on subsets of macrophages in mouse (16, 139) and of pre-cDCs in human (15, 79). pDCs are found in blood, as well as peripheral lymphoid and non-lymphoid tissues. The main function of pDCs is the rapid and pronounced release of type 1 IFN in case of viral infection, due to activation of TLR7 and TLR9 by viral nucleic acids (140). Despite their role in secreting type I IFNs during endotoxemia, pDCs may also be critically involved in regulating endotoxemia through their function in cross-priming and cross-presentation of antigen to T cells (141–143). While the inability to present antigens of steady state human pDC is widely accepted since their discoveries, measuring this function necessitates both to properly purify pDC ensuring lack of contamination by other DCs (15, 79) and also to segregate pDC according to their different activation states that may be linked to distinct functional specialization (138, 144). Indeed, a proper preparation of pDC from human blood can be reached by studying Lin− (by using these markers: CD14, CD16, CD19, CD20 and CD56), CD123+HLA-DR+AXL−CD11c− cells (Table 1) (15, 79, 138, 144). As few studies have investigated pDC during endotoxemia and sepsis (Table 2) (88), there is a need to revisit the role of pDCs during endotoxemia and sepsis. In the future, these refinements to ensure proper purification of pDCs should allow for a better delineation of the roles of these various populations in immunological processes, such as sepsis (61, 69, 74, 102, 132).
The different types of mononuclear phagocytes might be affected during sepsis by a reduction in their number, by cell death or precursor fate mechanisms, or by their resolutive functions. Numerous studies have shown that immune cell death contributes to immunosuppression and damage to organs during the development of septicemia (145). Apoptosis appears to at least partially explain the loss of DCs observed in a murine model of septicemia (146). For instance, sera from sepsis patients has been shown to induce death of circulating CD11c+ CD123− DCs, CD14+ monocytes and of in vitro generated monocyte-derived DCs (147). However, the markers used do not allow the subset specificity to be determined. Moreover, the potentially lowered survival of pDC needs to be evaluated with more specific markers that preclude pre-DC contamination (15, 79). This programmed cell death is in part due to the engagement of some TLRs (89, 96, 148). For instance, cDC1 apoptosis in the spleen within 48 h following live E. coli injection is TLR4- and TRIF-dependent (96). Furthermore, phagocytosis of apoptotic cells by DCs renders them tolerogenic. Immunosuppression induced by an endotoxic shock is restrained by the expression of an anti-apoptotic protein by DCs, or by an increase in their number and their activation state by treatment with Flt3L (106, 107, 149). In conclusion, the increase in apoptotic cells with septicemia could contribute to immunosuppression by, on the one hand, the loss of effector cells, and, on the other hand, the induction of tolerance (150).
In addition to death-mediated modulation of the mononuclear phagocyte system, these cells can also be affected by their functions. For instance, during sepsis, monocytes are reprogrammed to enhance protective functions such as anti-microbial functions, which are dependent on hypoxia inducible factor-1α (151). In contrast to this beneficial modulation, the mononuclear phagocyte system can also be modulated during the course of sepsis to promote immunosuppression. For instance, the mononuclear phagocyte system might lose the ability to drive a suitable adaptive immune response. DCs of septic patients, for example, exhibit a decrease in the expression of HLA-DR, thereby reducing their capacity to interact with T lymphocytes (152). Similarly, DCs of septic mice also exhibit a decrease in the expression of MHC-II (91). Moreover, numerous studies in humans as well as in mice have provided evidence for a pronounced decrease in the production of pro-inflammatory cytokines such as IL-12 or TNF by septic DCs stimulated by several PAMPs, while DC dysfunction during sepsis is partly mimicked by the TLR2 agonist Pam3CSK4, rather than the TLR4 agonist LPS (95). Conversely, their capacity to produce the anti-inflammatory cytokines IL-10 or TGF-β is significantly increased (86, 94, 152, 153). Like DCs, monocytes isolated from the blood of septic patients exhibit decreased expression of HLA-DR molecules and lower production of the pro-inflammatory cytokine IL-12 following stimulation after an increase in the production of the anti-inflammatory cytokine IL-10. A high concentration of IL-10 is particularly associated with a poor prognosis for septic patients (93). In the same way, human blood cDC2 produced immunoregulatory molecules, such as IDO, upregulated PD-L1 (a ligand of the inhibitory co-receptor PD1 on T cells), produced high levels of IL-10, and were immunosuppressive in response to E. coli (33). Similarly, PD-1 or PD-L1 was expressed at higher levels in septic shock patients (154), and their functional blockade by antibodies restored monocyte functions (155). In terms of helper T cell (Th) polarization, in mice it appears that the interaction between septic DCs and CD4+ T lymphocytes induces preferential polarization of the latter toward a Th2 type or T regulatory profile (86, 91, 153, 156). In addition, GM-CSF-derived BM DCs from CLP- and Pam3CSK4-treated mice were less effective in vivo at Th1 priming compared to GM-CSF-derived DCs from LPS-treated mice (95). It is possible that the loss of the capacity to induce Th1 responses is due, at least in part, to a specific loss of cDC1 which appear to be crucial for the development of such responses (92, 94, 107, 112, 113). Although progress has been made in this regard, the exact molecular mechanism of such functional difference remains unclear. Moreover, it has been reported that the failure of DCs generated by post-septic mice to produce IL-12 with the CLP model was observed at least 6 weeks after this process. This failure to produce IL-12 appears to be due to epigenetic changes induced at the level of promoters for genes coding for this cytokine (86). In summary, these long lasting events might occur in myeloid progenitors as DCs are short lived. Future molecular investigations should consider their epigenetic regulation.
In contrast to modulation of the mononuclear phagocyte system at a functional level, sepsis may affect the developmental fate of myeloid progenitor cells. Monocytes can also acquire phenotypic and functional characteristics of DCs, although the factors influencing this differentiation are still unknown. In keeping with the high level of plasticity of monocytes, the differentiation of these cells depends on local mediators such as cytokines, PAMPs, or DAMPs (76, 157). Some of these cytokines are induced by these danger signals, such as type I IFN. Indeed, type I IFN gives rise to Mo-APCs by acting through the IFNAR receptor on direct monocyte progenitors (Figure 1) (18). Similar to DAMPs, microbiota-dependent metabolites affect the balance between Mo-DCs and macrophages. For instance, aryl hydrocarbon receptor (AHR) ligands, derived either from dietary food intake or from tryptophan catabolism at the mucosal barrier, shift the monocyte cell fate toward monocyte-derived DCs in a PRDM1- (also known as BLIMP1) and IRF4-dependent manner (99, 158). It appears that activation of IRF4 allows monocytes to differentiate into Mo-DC while it remains controversial that only monocytes re-expressing Flt3 can generate Mo-APCs (71, 99, 159). It might be of interest to understand the molecular mechanism of how reprogramming of each monocytes impacts their subsequent ability to differentiate into either DCs or macrophages within different microenvironment. In summary, the generation of monocytes at the expense of cDCs could limit the availability of innate immune effector cells that can counter the infection, and this process might be involved in the immunosuppression in septic animals and patients.
Recent studies have shown that hematopoietic progenitors themselves express PRR, such as TLR (160, 161). They can hence theoretically directly detect PAMP and react as a consequence. In vitro culture experiments of murine and human HSC stimulated by agonists of TLR have shown their preferential differentiation into phagocytes at the expense of cells of the lymphoid lineage (161–164). Furthermore, experiments with parabiotics have ele-gantly demonstrated that a low number of HSC continuously enter the blood circulation before returning to the BM (165). This phenomenon could allow HSC to locally generate effector cells, directly after encountering a circulating microorganism and in a way that is tailored to the molecular signature of the invading pathogens (166).
Type I IFNs can have an effect on hematopoiesis in vivo, particularly by induction of the proliferation of quiescent HSC following injection of the TLR3 agonist polyinosinic: poly cytidylic acid (poly I:C) into mice (167). However, excessive signaling by type 1 IFNs, induced for example by a deficiency in the negative regulator IRF-2, leads to attenuation of HSC proliferation over time, as evidenced by the low capacity of these hematopoietic cells to repopulate following transplantation (168, 169). Additionally, chronic administration of poly I:C induces a selective depletion of WT hematopoietic stem cells (HSCs) in chimeric mice with WT: Ifnar1−/− BM cells. Excessive proliferation could, as a matter of fact, induce a state of attenuation of the function of stem cells by differentiation, senescence, or also apoptosis, thereby decreasing the risk of malignant transformation and of perturbation of the tolerogenic tissue architecture (167, 170). This attenuation of stem cells could, over time, lead to leukopenias and hematopoietic anomalies. There is still scant documentation regarding the influence of the infectious context on the potential for differentiation of myeloid precursors. In addition to their roles in regard to HSC, type 1 IFNs are involved in the differentiation of common myeloid precursors into macrophages, following the direct activation of TLR7 of these cells by R848 (19). Also, a study has shown that the precursor cells of common DC progenitors express several TLR in mice, including TLR4. In vitro activation of these TLR induces a reduction in the expression of the chemokine receptor CXCR4, which is involved in the retention of CDPs in the BM. In vitro activation of the TLR of CDP also induces an increase in the expression of the chemokine receptor CCR7, which is involved in migration of DCs toward the lymphoid organs. When these active CDP were transferred to mice, they were preferentially found at the level of lymph nodes rich in agonists of TLR, subsequent to local TLR agonist injection, where they underwent differentiation. As the various populations of DCs were not studied in detail in this study, it is hence not possible to draw conclusions regarding the potential selective differentiation of stimulated CDP (160). However, a study has shown that the in vitro differentiation by the cytokine GM-CSF of hematopoietic cells derived from CLP septic mice induced the generation of DCs with an immunosuppressive phenotype, aggravating the susceptibility to secondary infections with Pseudomonas aeruginosa when they were injected into post-CLP septic mice (91, 171). Conversely, the injection of DCs derived in vitro from the BM of healthy mice considerably increased the resistance of septic mice to secondary infections (171). Similarly, when injected intratracheally at day-5 after CLP surgery, cultures of DCs isolated from mouse BM with GM-CSF and IL-4 protected recipient mice from Aspergilus fumigatus-induced death (153). The exact contribution of each DC subset in cultures of mouse BM with GM-CSF remains to be investigated, as in vitro generated CD11c+MHC II+ cells are a heterogeneous population of cells, with some resembling macrophages more than DCs (172).
Similarly, IL-4 may favor monocyte development toward monocyte-derived DCs to the detriment of monocyte-derived macrophages (99, 172). Aside from a role for IL-4 in the ontogenic shift between DCs and macrophages, IL-4 plays an important role at the functional level as it is required for optimal cross-priming by GM-CSF-induced Mo-DCs (71). A side-by-side comparison of these in vitro generated DC subsets in the protection of septic mice needs to be undertaken. Moreover, the contribution of in vitro generated cDC1 and cDC2 needs to be investigated by using cultures of mouse BM with Flt3L (173). Finally, supplementation of mouse BM cultures with GM-CSF and IL-4 should be studied so as to determine the contribution of each DCsubset in the resolution of sepsis. As done recently in a cancer model, and because in vitro generated DCs might lack environmental cues, the benefit of directly ex vivo extracted DC in sepsis models might be of interest (174). Despite this comparison between in vitro and ex vivo generated DC subsets, their ability to reach the organs of interest, such as lung-draining lymph nodes in the case of intratracheally injected cells, remains to be verified in each sepsis model (153). Meanwhile, limitations of diphtheria toxin-mediated models of cell type depletion have been described such as for DC targeting in CD11c-hDTR mice where many other cell types are affected (175). This implies performing complementation studies with each DC subset obtained in vitro or ex vivo, for proper interpretation of the phenotype of diphtheria toxin-treated mice. For instance, adoptive transfer of GM-CSF-derived DCs into DC depleted mice prevents CLP-induced mortality (176).
It hence appears that DCs generated during septicemia have different effects compared to those produced under homeostatic conditions, and that they are involved in the immunosuppression observed in septic patients. In various models of bacterial infection, the chemokine CCR2 receptor-dependent mobilization of monocytes is crucial for the control of the pathology. Pasquevich and his collaborators have recently shown that the infection of mice with Gram-negative Y. enterocolitica bacilli induces a selective differentiation of myeloid progenitors toward the monocytic lineage (monocytopoiesis) at the expense of cDCs. This process depends on the activation of TLR4 and on the production and the detection of IFN-γ by non-hematopoietic cells (17).
The word “Septicemia” is of Greek origin and it means blood putrefaction. Aside from septicemia, sepsis is a medical term defined as “life-threatening organ dysfunction due to a dysregulated host response to infection” (97, 104, 177). Sepsis is a common and lethal syndrome for which no specific treatments exist (105). In the United States, severe sepsis has been shown to occur in about 2% of patients admitted to hospital. The number of cases in the United States exceeds 750,000 per year and was recently reported to be increasing. Whereas the estimated annual economic burden of this condition is about € 2 billion, the lifetime therapeutic management of sepsis is still far from optimal. Sepsis develops when an initial immune response to an endotoxin derived from an infectious agent becomes amplified and deregulated, leading to persistent inflammation and, in the most severe cases, multiorgan failure and death. Sepsis is often thought to result from systemic invasion of the bloodstream by pathogenic organisms. However, several lines of evidence have converged in support of the notion that it also develops in the absence of any invading pathogens as a consequence of tissue injury and/or unrestrained translocation of commensals. Roquilly et al. showed that resolution of the primary infection changed the local lung environment, which led to the development of tolerogenic DCs and macrophages that contributed to immune suppression (178).
As a conceptual framework, we herein propose that sepsis-mediated mononuclear phagocyte system deregulation might occur at the mononuclear phagocyte precursor level. Therefore, strategies aiming to restore the differentiation of DCs, and maintenance of their physiological functions (178) could be beneficial in the treatment of sepsis. However, it remains to be clearly established whether these tolerogenic cells have been biased in their development at the precursor level in a specific environment, such as the kidney. Such a precursor effect is supported by the fact that the impaired capacity of antigen presentation through MHCII molecules lasts for 21 days or more after recovery from the primary infection, which exceeds the short lifetime of DCs. Moreover, if BM precursors are affected by the primary infection, their degree of resilience needs to be measured in the framework of the new paradigm of infection memory (179).
It is worth noting that similar inflammatory pathways that are required for host protection against infectious agents can also be induced in response to sterile tissue damage (180). For instance, type I interferons and TNF cooperatively induce signals to epigenetically reprogram macrophages, thereby rendering them more sensitive to weak signals, such as responses to LPS, while also making them resistant to suppression by IL-10 (131). In other words, the hematopoietic cells integrate infection marks with deleterious consequences and they are then reinitialized differently in a subsequent challenge. It remains to be investigated whether the epigenetic reprogramming of the mononuclear phagocyte system and their precursors may influence long-term disease outcomes. Indeed, new technologies such as single cell RNA sequencing, epigenomic approaches such as ATAC-seq, mass cytometry, and mass histology may improve our knowledge regarding the developmental and functional changes that affect mononuclear phagocytes during various inflammatory conditions such as sepsis and endotoxemia. The availability of different conditional mice to deplete specific genes or subsets would shed light on their specific requirements during sepsis and endotoxemia situations.
Overall, these data indicate functional changes in various populations of myeloid cells over the course of septicemia. However, these results also suggest that sepsis could induce modulations of myeloid cells in terms of the overall populations; promoting the production, survival, differentiation, or proliferation of certain cells at the expense of others. These results therefore suggest that therapeutic strategies aimed at maintaining the number and the functions of the mononuclear phagocyte system, in particular DCs, are likely to limit the immunosuppressive state that is commonly found during septicemia and infectious situations (178).
LFP, CL, and MC wrote the manuscript, gave feedback and revised the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by grants from the Fondation pour la Recherche Médicale (DEQ20130326475) for M.C. LFP also received a fellowship from the ATIP-Avenir program. CL received of a PhD fellowship funded by the INSERM, the Nord-Pas de Calais Regional Council, and the “Association pour la Recherche sur le Cancer” cancer charity.
DC(s), dendritic cell(s); cDC, conventional DC; pre-cDC, precursor of cDCs; pDC, plasmacytoid dendritic cell; IFN, interferon; LPS, lipopolysaccharide; MDP, macrophage and DC progenitor; CDP, cDC precursor; cMoP, common monocyte progenitor; MHC-II, major histocompatibility complex class II; BM, bone marrow; Mo-APC, monocyte-derived antigen presenting cells; Mo-DC, monocyte-derived dendritic cell.
1. Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity (2016) 44:439–49. doi:10.1016/j.immuni.2016.02.024
2. Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science (2006) 311:83–7. doi:10.1126/science.1117729
3. Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med (2009) 206:595–606. doi:10.1084/jem.20081385
4. Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med (2010) 207:1261–71. doi:10.1084/jem.20092618
5. Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol Rev (2010) 234:32–44. doi:10.1111/j.0105-2896.2009.00877.x
6. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol (2013) 31:563–604. doi:10.1146/annurev-immunol-020711-074950
7. Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol (2013) 14:821–30. doi:10.1038/ni.2638
8. Bakri Y, Sarrazin S, Mayer UP, Tillmanns S, Nerlov C, Boned A, et al. Balance of MafB and PU.1 specifies alternative macrophage or dendritic cell fate. Blood (2005) 105:2707–16. doi:10.1182/blood-2004-04-1448
9. Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol (2006) 7:663–71. doi:10.1038/ni1340
10. Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol (2007) 8:1217–26. doi:10.1038/ni1522
11. Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol (2007) 8:1207–16. doi:10.1038/ni1518
12. Lee J, Breton G, Oliveira TY, Zhou YJ, Aljoufi A, Puhr S, et al. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med (2015) 212:385–99. doi:10.1084/jem.20141442
13. Breton G, Lee J, Zhou YJ, Schreiber JJ, Keler T, Puhr S, et al. Circulating precursors of human CD1c+ and CD141+ dendritic cells. J Exp Med (2015) 212:401–13. doi:10.1084/jem.20141441
14. Breton G, Zheng S, Valieris R, Tojal da Silva I, Satija R, Nussenzweig MC. Human dendritic cells (DCs) are derived from distinct circulating precursors that are precommitted to become CD1c+ or CD141+ DCs. J Exp Med (2016) 213:2861–70. doi:10.1084/jem.20161135
15. See P, Dutertre CA, Chen J, Günther P, McGovern N, Irac SE, et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science (2017) 356. doi:10.1126/science.aag3009
16. Schlitzer A, Sivakamasundari V, Chen J, Sumatoh HR, Schreuder J, Lum J, et al. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol (2015) 16:718–28. doi:10.1038/ni.3200
17. Pasquevich KA, Bieber K, Günter M, Grauer M, Pötz O, Schleicher U, et al. Innate immune system favors emergency monopoiesis at the expense of DC-differentiation to control systemic bacterial infection in mice. Eur J Immunol (2015) 45:2821–33. doi:10.1002/eji.201545530
18. Lasseaux C, Fourmaux MP, Chamaillard M, Poulin LF. Type I interferons drive inflammasome-independent emergency monocytopoiesis during endotoxemia. Sci Rep (2017) 7:16935. doi:10.1038/s41598-017-16869-2
19. Buechler MB, Akilesh HM, Hamerman JA. Cutting edge: direct sensing of TLR7 ligands and type I IFN by the common myeloid progenitor promotes mTOR/PI3K-dependent emergency myelopoiesis. J Immunol (2016) 197:2577–82. doi:10.4049/jimmunol.1600813
20. Serbina NV, Hohl TM, Cherny M, Pamer EG. Selective expansion of the monocytic lineage directed by bacterial infection. J Immunol (2009) 183:1900–10. doi:10.4049/jimmunol.0900612
21. Takahashi K, Naito M, Takeya M. Development and heterogeneity of macrophages and their related cells through their differentiation pathways. Pathol Int (1996) 46:473–85. doi:10.1111/j.1440-1827.1996.tb03641.x
22. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol (2014) 14:392–404. doi:10.1038/nri3671
23. Kawamura S, Onai N, Miya F, Sato T, Tsunoda T, Kurabayashi K, et al. Identification of a human clonogenic progenitor with strict monocyte differentiation potential: a counterpart of mouse cMoPs. Immunity (2017) 46:835–48.e4. doi:10.1016/j.immuni.2017.04.019
24. Helft J, Anjos-Afonso F, van der Veen AG, Chakravarty P, Bonnet D, Reis E Sousa C. Dendritic cell lineage potential in human early hematopoietic progenitors. Cell Rep (2017) 20:529–37. doi:10.1016/j.celrep.2017.06.075
25. Onai N, Ohteki T. Bipotent or oligopotent? A macrophage and DC progenitor revisited. Immunity (2014) 41:5–7. doi:10.1016/j.immuni.2014.07.004
26. Sathe P, Metcalf D, Vremec D, Naik SH, Langdon WY, Huntington ND, et al. Lymphoid tissue and plasmacytoid dendritic cells and macrophages do not share a common macrophage-dendritic cell-restricted progenitor. Immunity (2014) 41:104–15. doi:10.1016/j.immuni.2014.05.020
27. Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol (2014) 14:302–14. doi:10.1038/nri3660
28. Grimaldi D, Louis S, Pène F, Sirgo G, Rousseau C, Claessens YE, et al. Profound and persistent decrease of circulating dendritic cells is associated with ICU-acquired infection in patients with septic shock. Intensive Care Med (2011) 37:1438–46. doi:10.1007/s00134-011-2306-1
29. Guisset O, Dilhuydy MS, Thiébaut R, Lefèvre J, Camou F, Sarrat A, et al. Decrease in circulating dendritic cells predicts fatal outcome in septic shock. Intensive Care Med (2007) 33:148–52. doi:10.1007/s00134-006-0436-7
30. Elsayh KI, Zahran AM, Lotfy Mohamad I, Aly SS. Dendritic cells in childhood sepsis. J Crit Care (2013) 28(881):e887–813. doi:10.1016/j.jcrc.2013.05.007
31. Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci U S A (2015) 112:1167–72. doi:10.1073/pnas.1401965111
32. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A (2013) 110:3507–12. doi:10.1073/pnas.1222878110
33. Kassianos AJ, Hardy MY, Ju X, Vijayan D, Ding Y, Vulink AJ, et al. Human CD1c (BDCA-1)+ myeloid dendritic cells secrete IL-10 and display an immuno-regulatory phenotype and function in response to Escherichia coli. Eur J Immunol (2012) 42:1512–22. doi:10.1002/eji.201142098
34. Boettcher S, Ziegler P, Schmid MA, Takizawa H, van Rooijen N, Kopf M, et al. Cutting edge: LPS-induced emergency myelopoiesis depends on TLR4-expressing nonhematopoietic cells. J Immunol (2012) 188:5824–8. doi:10.4049/jimmunol.1103253
35. Suzuki A, Takahashi T, Okuno Y, Tsuyuoka R, Fukumoto M, Nakamura K, et al. IL-1 production as a regulator of G-CSF and IL-6 production in CSF-producing cell lines. Br J Cancer (1992) 65:515–8. doi:10.1038/bjc.1992.106
36. Dubois CM, Neta R, Keller JR, Jacobsen SE, Oppenheim JJ, Ruscetti F. Hematopoietic growth factors and glucocorticoids synergize to mimic the effects of IL-1 on granulocyte differentiation and IL-1 receptor induction on bone marrow cells in vivo. Exp Hematol (1993) 21:303–10.
37. Weber GF, Chousterman BG, He S, Fenn AM, Nairz M, Anzai A, et al. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science (2015) 347:1260–5. doi:10.1126/science.aaa4268
38. Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, et al. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity (2016) 45:669–84. doi:10.1016/j.immuni.2016.08.015
39. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity (2003) 19:71–82. doi:10.1016/S1074-7613(03)00174-2
40. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol (2011) 11:762–74. doi:10.1038/nri3070
41. Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity (2010) 33:375–86. doi:10.1016/j.immuni.2010.08.012
42. Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity (2013) 38:79–91. doi:10.1016/j.immuni.2012.12.001
43. Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood (2010) 116:e74–80. doi:10.1182/blood-2010-02-258558
44. Vu Manh TP, Elhmouzi-Younes J, Urien C, Ruscanu S, Jouneau L, Bourge M, et al. Defining mononuclear phagocyte subset homology across several distant warm-blooded vertebrates through comparative transcriptomics. Front Immunol (2015) 6:299. doi:10.3389/fimmu.2015.00299
45. Chousterman BG, Boissonnas A, Poupel L, Baudesson de Chanville C, Adam J, Tabibzadeh N, et al. Ly6Chigh monocytes protect against kidney damage during sepsis via a CX3CR1-dependent adhesion mechanism. J Am Soc Nephrol (2016) 27:792–803. doi:10.1681/ASN.2015010009
46. Gainaru G, Papadopoulos A, Tsangaris I, Lada M, Giamarellos-Bourboulis EJ, Pistiki A, et al. Increases in inflammatory and CD14(dim)/CD16(pos)/CD45(pos) patrolling monocytes in sepsis: correlation with final outcome. Crit Care (2018) 22:56. doi:10.1186/s13054-018-1977-1
47. Lessard AJ, LeBel M, Egarnes B, Préfontaine P, Thériault P, Droit A, et al. Triggering of NOD2 receptor converts inflammatory Ly6C(high) into Ly6C(low) monocytes with patrolling properties. Cell Rep (2017) 20:1830–43. doi:10.1016/j.celrep.2017.08.009
48. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science (2010) 327:656–61. doi:10.1126/science.1178331
49. Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol (2014) 14:571–8. doi:10.1038/nri3712
50. Aziz A, Soucie E, Sarrazin S, Sieweke MH. MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science (2009) 326:867–71. doi:10.1126/science.1176056
51. Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science (2012) 336:86–90. doi:10.1126/science.1219179
52. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (2010) 330:841–5. doi:10.1126/science.1194637
53. Naito M, Hasegawa G, Takahashi K. Development, differentiation, and maturation of Kupffer cells. Microsc Res Tech (1997) 39:350–64. doi:10.1002/(SICI)1097-0029(19971115)39:4<350::AID-JEMT5>3.0.CO;2-L
54. Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res (1999) 117:145–52. doi:10.1016/S0165-3806(99)00113-3
55. Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med (2013) 210:1977–92. doi:10.1084/jem.20131199
56. Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, Brazowski E, Shibolet O, Halpern Z, et al. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol (2014) 193:344–53. doi:10.4049/jimmunol.1400574
57. Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity (2015) 42:665–78. doi:10.1016/j.immuni.2015.03.011
58. Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol (2014) 15:929–37. doi:10.1038/ni.2967
59. Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity (2013) 39:925–38. doi:10.1016/j.immuni.2013.10.004
60. Capucha T, Mizraji G, Segev H, Blecher-Gonen R, Winter D, Khalaileh A, et al. Distinct murine mucosal langerhans cell subsets develop from pre-dendritic cells and monocytes. Immunity (2015) 43:369–81. doi:10.1016/j.immuni.2015.06.017
61. Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol (2012) 42:3150–66. doi:10.1002/eji.201242847
62. Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med (2014) 211:2151–8. doi:10.1084/jem.20140639
63. Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity (2014) 40:91–104. doi:10.1016/j.immuni.2013.11.019
64. Bain CC, Hawley CA, Garner H, Scott CL, Schridde A, Steers NJ, et al. Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat Commun (2016) 7:ncomms11852. doi:10.1038/ncomms11852
65. Blériot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity (2015) 42:145–58. doi:10.1016/j.immuni.2014.12.020
66. Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun (2016) 7:10321. doi:10.1038/ncomms10321
67. Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, et al. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol (2002) 168:2493–500. doi:10.4049/jimmunol.168.5.2493
68. Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell (2010) 143:416–29. doi:10.1016/j.cell.2010.09.039
69. Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Grégoire C, et al. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol (2012) 188:1751–60. doi:10.4049/jimmunol.1102744
70. Segura E, Albiston AL, Wicks IP, Chai SY, Villadangos JA. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc Natl Acad Sci U S A (2009) 106:20377–81. doi:10.1073/pnas.0910295106
71. Briseño CG, Haldar M, Kretzer NM, Wu X, Theisen DJ, Kc W, et al. Distinct transcriptional programs control cross-priming in classical and monocyte-derived dendritic cells. Cell Rep (2016) 15:2462–74. doi:10.1016/j.celrep.2016.05.025
72. Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity (2007) 26:519–31. doi:10.1016/j.immuni.2007.01.017
73. Ji Q, Castelli L, Goverman JM. MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8(+) T cells. Nat Immunol (2013) 14:254–61. doi:10.1038/ni.2513
74. Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity (2013) 38:322–35. doi:10.1016/j.immuni.2012.10.016
75. Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity (2010) 32:557–67. doi:10.1016/j.immuni.2010.03.017
76. Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol (2013) 34:440–5. doi:10.1016/j.it.2013.06.001
77. Schraml BU, van Blijswijk J, Zelenay S, Whitney PG, Filby A, Acton SE, et al. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell (2013) 154:843–58. doi:10.1016/j.cell.2013.07.014
78. Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity (2013) 38:336–48. doi:10.1016/j.immuni.2012.10.018
79. Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science (2017) 356. doi:10.1126/science.aah4573
80. Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol (2014) 14:94–108. doi:10.1038/nri3666
81. Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med (2012) 209:1153–65. doi:10.1084/jem.20112675
82. Satpathy AT, KC W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med (2012) 209:1135–52. doi:10.1084/jem.20120030
83. Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol (2012) 13:1145–54. doi:10.1038/ni.2467
84. Sichien D, Lambrecht BN, Guilliams M, Scott CL. Development of conventional dendritic cells: from common bone marrow progenitors to multiple subsets in peripheral tissues. Mucosal Immunol (2017) 10:831–44. doi:10.1038/mi.2017.8
85. Ding Y, Chung CS, Newton S, Chen Y, Carlton S, Albina JE, et al. Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock (2004) 22:137–44. doi:10.1097/01.shk.0000131194.80038.3f
86. Wen H, Dou Y, Hogaboam CM, Kunkel SL. Epigenetic regulation of dendritic cell-derived interleukin-12 facilitates immunosuppression after a severe innate immune response. Blood (2008) 111:1797–804. doi:10.1182/blood-2007-08-106443
87. Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, et al. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol (2003) 171:909–14. doi:10.4049/jimmunol.171.2.909
88. Wen H, Hogaboam CM, Gauldie J, Kunkel SL. Severe sepsis exacerbates cell-mediated immunity in the lung due to an altered dendritic cell cytokine profile. Am J Pathol (2006) 168:1940–50. doi:10.2353/ajpath.2006.051155
89. Pène F, Courtine E, Ouaaz F, Zuber B, Sauneuf B, Sirgo G, et al. Toll-like receptors 2 and 4 contribute to sepsis-induced depletion of spleen dendritic cells. Infect Immun (2009) 77:5651–8. doi:10.1128/IAI.00238-09
90. Courtine E, Pène F, Cagnard N, Toubiana J, Fitting C, Brocheton J, et al. Critical role of cRel subunit of NF-kappaB in sepsis survival. Infect Immun (2011) 79:1848–54. doi:10.1128/IAI.00021-11
91. Pastille E, Didovic S, Brauckmann D, Rani M, Agrawal H, Schade FU, et al. Modulation of dendritic cell differentiation in the bone marrow mediates sustained immunosuppression after polymicrobial sepsis. J Immunol (2011) 186:977–86. doi:10.4049/jimmunol.1001147
92. Efron PA, Martins A, Minnich D, Tinsley K, Ungaro R, Bahjat FR, et al. Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis. J Immunol (2004) 173:3035–43. doi:10.4049/jimmunol.173.5.3035
93. Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis (2013) 13:260–8. doi:10.1016/S1473-3099(13)70001-X
94. Flohé SB, Agrawal H, Schmitz D, Gertz M, Flohé S, Schade FU. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J Leukoc Biol (2006) 79:473–81. doi:10.1189/jlb.0705413
95. Bruns S, Pastille E, Wirsdorfer F, Frisch M, Flohe SB. Lipopeptides rather than lipopolysaccharide favor the development of dendritic cell dysfunction similar to polymicrobial sepsis in mice. Inflamm Res (2013) 62:627–36. doi:10.1007/s00011-013-0616-1
96. De Trez C, Pajak B, Brait M, Glaichenhaus N, Urbain J, Moser M, et al. TLR4 and toll-IL-1 receptor domain-containing adapter-inducing IFN-beta, but not MyD88, regulate Escherichia coli-induced dendritic cell maturation and apoptosis in vivo. J Immunol (2005) 175:839–46. doi:10.4049/jimmunol.175.2.839
97. Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA (2016) 315:762–74. doi:10.1001/jama.2016.0288
98. Li CC, Munitic I, Mittelstadt PR, Castro E, Ashwell JD. Suppression of dendritic cell-derived IL-12 by endogenous glucocorticoids is protective in LPS-induced sepsis. PLoS Biol (2015) 13:e1002269. doi:10.1371/journal.pbio.1002269
99. Goudot C, Coillard A, Villani AC, Gueguen P, Cros A, Sarkizova S, et al. Aryl hydrocarbon receptor controls monocyte differentiation into dendritic cells versus macrophages. Immunity (2017) 47:582–96.e6. doi:10.1016/j.immuni.2017.08.016
100. Edelson BT, KC W, Juang R, Kohyama M, Benoit LA, Klekotka PA, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med (2010) 207:823–36. doi:10.1084/jem.20091627
101. Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H, et al. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell (2015) 162:1322–37. doi:10.1016/j.cell.2015.08.004
102. Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity (2013) 38:970–83. doi:10.1016/j.immuni.2013.04.011
103. Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun (2013) 4:2990. doi:10.1038/ncomms3990
104. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA (2016) 315:801–10. doi:10.1001/jama.2016.0287
105. Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, et al. Sepsis: a roadmap for future research. Lancet Infect Dis (2015) 15:581–614. doi:10.1016/S1473-3099(15)70112-X
106. Wysocka M, Montaner LJ, Karp CL. Flt3 ligand treatment reverses endotoxin tolerance-related immunoparalysis. J Immunol (2005) 174:7398–402. doi:10.4049/jimmunol.174.11.7398
107. Strother RK, Danahy DB, Kotov DI, Kucaba TA, Zacharias ZR, Griffith TS, et al. Polymicrobial sepsis diminishes dendritic cell numbers and function directly contributing to impaired primary CD8 T cell responses in vivo. J Immunol (2016) 197:4301–11. doi:10.4049/jimmunol.1601463
108. Torres D, Köhler A, Delbauve S, Caminschi I, Lahoud MH, Shortman K, et al. IL-12p40/IL-10 producing preCD8alpha/Clec9A+ dendritic cells are induced in neonates upon Listeria monocytogenes infection. PLoS Pathog (2016) 12:e1005561. doi:10.1371/journal.ppat.1005561
109. del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunol Rev (2010) 234:268–81. doi:10.1111/j.0105-2896.2009.00874.x
110. Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol (2001) 166:5448–55. doi:10.4049/jimmunol.166.9.5448
111. Silvin A, Yu CI, Lahaye X, Imperatore F, Brault JB, Cardinaud S, et al. Constitutive resistance to viral infection in human CD141+ dendritic cells. Sci Immunol (2017) 2. doi:10.1126/sciimmunol.aai8071
112. den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo. J Exp Med (2002) 196:817–27. doi:10.1084/jem.20020295
113. Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science (2008) 322:1097–100. doi:10.1126/science.1164206
114. Robbins SH, Walzer T, Dembélé D, Thibault C, Defays A, Bessou G, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol (2008) 9:R17. doi:10.1186/gb-2008-9-1-r17
115. Bachem A, Güttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med (2010) 207:1273–81. doi:10.1084/jem.20100348
116. Poulin LF, Reyal Y, Uronen-Hansson H, Schraml BU, Sancho D, Murphy KM, et al. DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues. Blood (2012) 119:6052–62. doi:10.1182/blood-2012-01-406967
117. Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity (2012) 37:60–73. doi:10.1016/j.immuni.2012.04.012
118. Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med (2010) 207:1283–92. doi:10.1084/jem.20100223
119. Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J Exp Med (2013) 210:1035–47. doi:10.1084/jem.20121103
120. Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med (2010) 207:1247–60. doi:10.1084/jem.20092140
121. Balan S, Ollion V, Colletti N, Chelbi R, Montanana-Sanchis F, Liu H, et al. Human XCR1+ dendritic cells derived in vitro from CD34+ progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells. J Immunol (2014) 193:1622–35. doi:10.4049/jimmunol.1401243
122. Deauvieau F, Ollion V, Doffin AC, Achard C, Fonteneau JF, Verronese E, et al. Human natural killer cells promote cross-presentation of tumor cell-derived antigens by dendritic cells. Int J Cancer (2015) 136:1085–94. doi:10.1002/ijc.29087
123. Cohn L, Chatterjee B, Esselborn F, Smed-Sörensen A, Nakamura N, Chalouni C, et al. Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation. J Exp Med (2013) 210:1049–63. doi:10.1084/jem.20121251
124. Flinsenberg TW, Compeer EB, Koning D, Klein M, Amelung FJ, van Baarle D, et al. Fcgamma receptor antigen targeting potentiates cross-presentation by human blood and lymphoid tissue BDCA-3+ dendritic cells. Blood (2012) 120:5163–72. doi:10.1182/blood-2012-06-434498
125. Desch AN, Gibbings SL, Clambey ET, Janssen WJ, Slansky JE, Kedl RM, et al. Dendritic cell subsets require cis-activation for cytotoxic CD8 T-cell induction. Nat Commun (2014) 5:4674. doi:10.1038/ncomms5674
126. Neubert K, Lehmann CH, Heger L, Baranska A, Staedtler AM, Buchholz VR, et al. Antigen delivery to CD11c+CD8- dendritic cells induces protective immune responses against experimental melanoma in mice in vivo. J Immunol (2014) 192:5830–8. doi:10.4049/jimmunol.1300975
127. Nierkens S, Tel J, Janssen E, Adema GJ. Antigen cross-presentation by dendritic cell subsets: one general or all sergeants? Trends Immunol (2013) 34:361–70. doi:10.1016/j.it.2013.02.007
128. Alloatti A, Rookhuizen DC, Joannas L, Carpier JM, Iborra S, Magalhaes JG, et al. Critical role for Sec22b-dependent antigen cross-presentation in antitumor immunity. J Exp Med (2017) 214:2231–41. doi:10.1084/jem.20170229
129. Sánchez-Paulete AR, Cueto FJ, Martínez-López M, Labiano S, Morales-Kastresana A, Rodríguez-Ruiz ME, et al. Cancer immunotherapy with immunomodulatory anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov (2016) 6:71–9. doi:10.1158/2159-8290.CD-15-0510
130. Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, et al. Systemic activation of dendritic cells by toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol (2006) 7:165–72. doi:10.1038/ni1300
131. Park SH, Kang K, Giannopoulou E, Qiao Y, Kang K, Kim G, et al. Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat Immunol (2017) 18:1104–16. doi:10.1038/ni.3818
132. Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol (2012) 13:1118–28. doi:10.1038/ni.2419
133. Gao Y, Nish SA, Jiang R, Hou L, Licona-Limón P, Weinstein JS, et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity (2013) 39:722–32. doi:10.1016/j.immuni.2013.08.028
134. Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity (2011) 35:780–91. doi:10.1016/j.immuni.2011.08.013
135. Tussiwand R, Everts B, Grajales-Reyes GE, Kretzer NM, Iwata A, Bagaitkar J, et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity (2015) 42:916–28. doi:10.1016/j.immuni.2015.04.017
136. Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hägerbrand K, Marsal J, et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity (2013) 38:958–69. doi:10.1016/j.immuni.2013.03.009
137. Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med (2007) 204:1775–85. doi:10.1084/jem.20070602
138. Alcántara-Hernández M, Leylek R, Wagar LE, Engleman EG, Keler T, Marinkovich MP, et al. High-dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity (2017) 47:1037–50.e6. doi:10.1016/j.immuni.2017.11.001
139. Swiecki M, Wang Y, Riboldi E, Kim AH, Dzutsev A, Gilfillan S, et al. Cell depletion in mice that express diphtheria toxin receptor under the control of SiglecH encompasses more than plasmacytoid dendritic cells. J Immunol (2014) 192:4409–16. doi:10.4049/jimmunol.1303135
140. Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol (2011) 29:163–83. doi:10.1146/annurev-immunol-031210-101345
141. Mouriès J, Moron G, Schlecht G, Escriou N, Dadaglio G, Leclerc C. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood (2008) 112:3713–22. doi:10.1182/blood-2008-03-146290
142. Zhang H, Gregorio JD, Iwahori T, Zhang X, Choi O, Tolentino LL, et al. A distinct subset of plasmacytoid dendritic cells induces activation and differentiation of B and T lymphocytes. Proc Natl Acad Sci U S A (2017) 114:1988–93. doi:10.1073/pnas.1610630114
143. Hoeffel G, Ripoche AC, Matheoud D, Nascimbeni M, Escriou N, Lebon P, et al. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity (2007) 27:481–92. doi:10.1016/j.immuni.2007.07.021
144. Alculumbre SG, Saint-André V, Di Domizio J, Vargas P, Sirven P, Bost P, et al. Diversification of human plasmacytoid predendritic cells in response to a single stimulus. Nat Immunol (2018) 19:63–75. doi:10.1038/s41590-017-0012-z
145. Pinheiro da Silva F, Nizet V. Cell death during sepsis: integration of disintegration in the inflammatory response to overwhelming infection. Apoptosis (2009) 14:509–21. doi:10.1007/s10495-009-0320-3
146. Peck-Palmer OM, Unsinger J, Chang KC, McDonough JS, Perlman H, McDunn JE, et al. Modulation of the Bcl-2 family blocks sepsis-induced depletion of dendritic cells and macrophages. Shock (2009) 31:359–66. doi:10.1097/SHK.0b013e31818ba2a2
147. Raffray L, Douchet I, Augusto JF, Youssef J, Contin-Bordes C, Richez C, et al. Septic shock sera containing circulating histones induce dendritic cell-regulated necrosis in fatal septic shock patients. Crit Care Med (2015) 43:e107–16. doi:10.1097/CCM.0000000000000879
148. Zhang L, Cardinal JS, Pan P, Rosborough BR, Chang Y, Yan W, et al. Splenocyte apoptosis and autophagy is mediated by interferon regulatory factor 1 during murine endotoxemia. Shock (2012) 37:511–7. doi:10.1097/SHK.0b013e318249cfa2
149. Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Chapman MJ, Lesnik P. Enhanced dendritic cell survival attenuates lipopolysaccharide-induced immunosuppression and increases resistance to lethal endotoxic shock. J Immunol (2008) 180:6941–6. doi:10.4049/jimmunol.180.10.6941
150. Kushwah R, Wu J, Oliver JR, Jiang G, Zhang J, Siminovitch KA, et al. Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3+ Treg. Eur J Immunol (2010) 40:1022–35. doi:10.1002/eji.200939782
151. Shalova IN, Lim JY, Chittezhath M, Zinkernagel AS, Beasley F, Hernández-Jiménez E, et al. Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1alpha. Immunity (2015) 42:484–98. doi:10.1016/j.immuni.2015.02.001
152. Poehlmann H, Schefold JC, Zuckermann-Becker H, Volk HD, Meisel C. Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Crit Care (2009) 13:R119. doi:10.1186/cc7969
153. Benjamim CF, Lundy SK, Lukacs NW, Hogaboam CM, Kunkel SL. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood (2005) 105:3588–95. doi:10.1182/blood-2004-08-3251
154. Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline science: defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol (2016) 100:1239–54. doi:10.1189/jlb.4HI0616-255R
155. Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care (2011) 15:R99. doi:10.1186/cc10112
156. Faivre V, Lukaszewicz AC, Alves A, Charron D, Payen D, Haziot A. Human monocytes differentiate into dendritic cells subsets that induce anergic and regulatory T cells in sepsis. PLoS One (2012) 7:e47209. doi:10.1371/journal.pone.0047209
157. Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev (2010) 234:90–104. doi:10.1111/j.0105-2896.2009.00876.x
158. Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med (2016) 22:598–605. doi:10.1038/nm.4102
159. Menezes S, Melandri D, Anselmi G, Perchet T, Loschko J, Dubrot J, et al. The heterogeneity of Ly6Chi monocytes controls their differentiation into iNOS+ macrophages or monocyte-derived dendritic cells. Immunity (2016) 45:1205–18. doi:10.1016/j.immuni.2016.12.001
160. Schmid MA, Takizawa H, Baumjohann DR, Saito Y, Manz MG. Bone marrow dendritic cell progenitors sense pathogens via toll-like receptors and subsequently migrate to inflamed lymph nodes. Blood (2011) 118:4829–40. doi:10.1182/blood-2011-03-344960
161. Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity (2006) 24:801–12. doi:10.1016/j.immuni.2006.04.008
162. De Luca K, Frances-Duvert V, Asensio MJ, Ihsani R, Debien E, Taillardet M, et al. The TLR1/2 agonist PAM(3)CSK(4) instructs commitment of human hematopoietic stem cells to a myeloid cell fate. Leukemia (2009) 23:2063–74. doi:10.1038/leu.2009.155
163. Sioud M, Floisand Y, Forfang L, Lund-Johansen F. Signaling through toll-like receptor 7/8 induces the differentiation of human bone marrow CD34+ progenitor cells along the myeloid lineage. J Mol Biol (2006) 364:945–54. doi:10.1016/j.jmb.2006.09.054
164. Sioud M, Floisand Y. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th1-type response. Eur J Immunol (2007) 37:2834–46. doi:10.1002/eji.200790042
165. Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science (2001) 294:1933–6. doi:10.1126/science.1064081
166. Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell (2007) 131:994–1008. doi:10.1016/j.cell.2007.09.047
167. Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature (2009) 458:904–8. doi:10.1038/nature07815
168. Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the toll-like receptor signaling. J Immunol (2003) 171:4304–10. doi:10.4049/jimmunol.171.8.4304
169. Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med (2005) 202:1599–611. doi:10.1084/jem.20050967
170. Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet (2008) 9:115–28. doi:10.1038/nrg2269
171. Pène F, Zuber B, Courtine E, Rousseau C, Ouaaz F, Toubiana J, et al. Dendritic cells modulate lung response to Pseudomonas aeruginosa in a murine model of sepsis-induced immune dysfunction. J Immunol (2008) 181:8513–20. doi:10.4049/jimmunol.181.12.8513
172. Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity (2015) 42:1197–211. doi:10.1016/j.immuni.2015.05.018
173. Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, et al. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol (2005) 174:6592–7. doi:10.4049/jimmunol.174.11.6592
174. Laoui D, Keirsse J, Morias Y, Van Overmeire E, Geeraerts X, Elkrim Y, et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat Commun (2016) 7:13720. doi:10.1038/ncomms13720
175. van Blijswijk J, Schraml BU, Reis e Sousa C. Advantages and limitations of mouse models to deplete dendritic cells. Eur J Immunol (2013) 43:22–6. doi:10.1002/eji.201243022
176. Scumpia PO, McAuliffe PF, O’Malley KA, Ungaro R, Uchida T, Matsumoto T, et al. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J Immunol (2005) 175:3282–6. doi:10.4049/jimmunol.175.5.3282
177. Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA (2016) 315:775–87. doi:10.1001/jama.2016.0289
178. Roquilly A, McWilliam HEG, Jacqueline C, Tian Z, Cinotti R, Rimbert M, et al. Local modulation of antigen-presenting cell development after resolution of pneumonia induces long-term susceptibility to secondary infections. Immunity (2017) 47:135–47.e5. doi:10.1016/j.immuni.2017.06.021
179. Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, et al. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature (2017) 550:475–80. doi:10.1038/nature24271
Keywords: dendritic cell, monocytes, ontogeny, sepsis, endotoxemia
Citation: Poulin LF, Lasseaux C and Chamaillard M (2018) Understanding the Cellular Origin of the Mononuclear Phagocyte System Sheds Light on the Myeloid Postulate of Immune Paralysis in Sepsis. Front. Immunol. 9:823. doi: 10.3389/fimmu.2018.00823
Received: 22 September 2017; Accepted: 04 April 2018;
Published: 24 April 2018
Edited by:
Etienne Meunier, UMR5089 Institut de Pharmacologie et de Biologie Structurale (IPBS), FranceReviewed by:
Marc DALOD, Centre national de la recherche scientifique (CNRS), FranceCopyright: © 2018 Poulin, Lasseaux and Chamaillard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lionel Franz Poulin, bGlvbmVsLnBvdWxpbkBjbnJzLmZy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.