
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 26 April 2018
Sec. Molecular Innate Immunity
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.00804
This article is part of the Research Topic Lectins and Their Ligands in Shaping Immune Responses View all 19 articles
Myeloid C-type lectin receptors (CLRs) are important sensors of self and non-self that work in concert with other pattern recognition receptors (PRRs). CLRs have been previously classified based on their signaling motifs as activating or inhibitory receptors. However, specific features of the ligand binding process may result in distinct signaling through a single motif, resulting in the triggering of non-canonical pathways. In addition, CLR ligands are frequently exposed in complex structures that simultaneously bind different CLRs and other PRRs, which lead to integration of heterologous signaling among diverse receptors. Herein, we will review how sensing by myeloid CLRs and crosstalk with heterologous receptors is modulated by many factors affecting their signaling and resulting in differential outcomes for immunity and inflammation. Finding common features among those flexible responses initiated by diverse CLR-ligand partners will help to harness CLR function in immunity and inflammation.
The expression of diverse pattern recognition receptors (PRRs), including differential expression of CLRs, provides different subsets of immune cells with a repertoire to interpret and respond distinctly to the information coming from the environment. Myeloid cells are central for initiation and regulation of innate and adaptive immunity or tolerance and the CLR repertoire essentially contributes to myeloid cell function. We previously proposed a classification of myeloid CLRs based on their intracellular signaling motifs (1). While signaling motifs allow to predict effector responses following sensing by CLRs, this canonical response is subjected to modulation by the physical nature, affinity, and avidity of the ligand (2). Based on their intracellular signaling motifs, myeloid CLRs can be classified into the following broad categories (Figure 1): immunoreceptor tyrosine-based activating motif (ITAM)-coupled CLRs, hemi-ITAM-(hemITAM)-bearing CLRs, immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing CLRs, and a group of CLRs lacking typical signaling motifs (1, 3, 4).
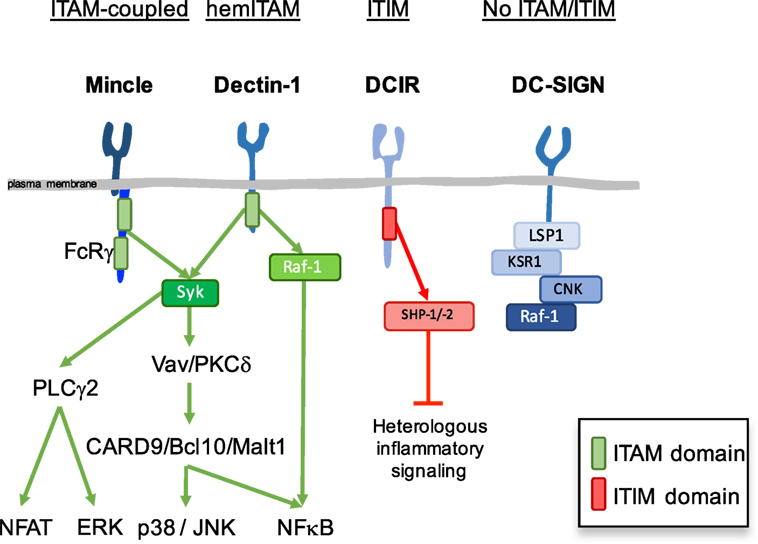
Figure 1. Canonical signaling modules in myeloid C-type lectin receptors (CLRs). Based on canonical intracellular signaling motifs, myeloid CLRs can be classified into immunoreceptor tyrosine-based activating motif (ITAM)-coupled CLRs, hemi-ITAM-(hemITAM)-bearing CLRs, immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing CLRs, and a group of CLRs lacking typical signaling motifs. Mincle, Dectin-1, DCIR, DC-SIGN, and their corresponding canonical signaling pathways and adaptors are depicted as prototypical examples of each category.
Immunoreceptor tyrosine-based activating motif-coupled CLRs have a classical ITAM motifs in their intracellular tail, consisting of YXXL tandem repeats, or can interact with ITAM-containing adaptor proteins, as Fc receptor γ (FcRγ) chain or DNAX-activation protein 12 (DAP12) (5). The majority of them, including Dectin-2 (CLEC6A in human, Clec4n in the mouse), Mincle (CLEC4E), MCL (CLEC4D), BDCA-2 (human CLEC4C), DCAR (mouse Clec4b1), DCAR1 (mouse Clec4b2), and mannose receptor (MR) (MRC1, CD206) utilize the FcRγ chain adaptor, while MDL-1 (CLEC5A) interacts with DAP12 (6–12). Hemi-ITAM-bearing CLRs contain a single tyrosine within an YXXL motif in their cytoplasmic domain (13, 14). Dectin-1 (CLEC7A), CLEC-2 (CLEC1B), DNGR-1 (CLEC9A), and SIGN-R3 (mouse Cd209d) belong to the hemITAM-based CLRs category (15–20).
These ITAM or hemITAM CLRs are considered activating receptors that couple to the spleen tyrosine kinase (Syk) (Figure 1) (15, 21, 22). Phosphorylation of the tyrosine(s) in the ITAM or hemITAM motifs generates docking sites for the SH2 domains of Syk, which undergoes a conformational change that permits autophosphorylation and activation (23). Mincle acts as a prototypical activating CLR after recognition of glycolipids in the cell wall of some fungal and bacterial pathogens (24–26). Through the full ITAM of the FcRγ chain adaptor, Mincle couples to Syk and activates Vav proteins and PKCδ, which lead to downstream activation of CARD9/Bcl10/Malt1 and MAPK pathways, thus resulting in the induction of several cytokines and chemokines, including TNF-α, macrophage inflammatory protein 2 (MIP-2; CXCL2), keratinocyte-derived chemokine (KC; CXCL1), and IL-6 (7, 27, 28). Production of inflammatory cytokines by myeloid cells, together with the generation of Th1 and Th17 responses, contribute to protective immunity upon recognition of some Mincle ligands (29–38).
Spleen tyrosine kinase activation downstream of the hemITAM-bearing CLR Dectin-1 leads to similar signaling pathways to those described for Mincle (Figure 1), with activation of the CARD9/Bcl10/Malt-1 module that promotes canonical NF-κB signaling (27, 28, 39). Dectin-1 can also activate MAPK (40, 41), NFAT through phospholipase C-γ2 (42, 43), and a Syk-independent non-canonical NF-κB activation relying on the activation of the Raf-1 kinase (44). These integrated pathways mediate production of reactive oxygen species (ROS) and cytokines, such as IL-1β, IL-6, IL-10, IL-12, TNF-α, and IL-23 to drive Th1 and Th17 differentiation, being essential for the development of antifungal immune responses (45–48). This axis is also activated in response to intestinal fungi, where Dectin-1 contributes to gut homeostasis (49).
Immunoreceptor tyrosine-based inhibitory motif-containing CLRs negatively regulate signaling initiated by kinase-associated heterologous receptors through the recruitment of tyrosine phosphatases, such as Src homology region 2 domain-containing phosphatase (SHP)-1 or -2 (Figure 1). Myeloid CLRs included in this group are human DCIR (CLEC4A), mDcir1 (Clec4a2), mDcir2 (Clec4a4), Clec12a (MICL, DCAL-2, KLRL1, CLL1), MAgH (CLEC12B), and Ly49Q (1, 50, 51). The ITIMs of both hDCIR and mDCIR1 have been shown to mediate inhibitory signaling through activation of the phosphatases, SHP-1 and SHP-2 (52–54). Activation of hDCIR on dendritic cells (DCs) leads to inhibition of TLR8-mediated IL-12 and TNF-α production and TLR9-induced IFN-α production (55, 56). Sensing endogenous ligands by DCIR modulates innate immunity to pathogens, such as Plasmodium or Mycobacterium (57, 58).
Myeloid CLRs that do not bear evident ITAM or ITIM domains include MMR (MRC1), DEC-205 (LY75), human DC-SIGN (CD209), mouse SIGN-R1 (Cd209b), Langerin (CD207), human MGL (CLEC10A), mouse Mgl1 (Clec10a), mouse Mgl2 (Mgl2), CLEC-1 (CLEC1A), human DCAL-1 (CLECL1), LOX-1 (OLR1), and LSECtin (CLEC4G). As an example, DC-SIGN intracellular tail is associated with a signalosome composed of the scaffold proteins LSP1, KSR1, and CNK and the kinase Raf-1 in unstimulated DCs (59) (Figure 1). Similar to other CLRs in this group, DC-SIGN cannot promote DCs activation or cytokine secretion per se, but it rather modulates signaling by heterologous receptors (see below) or engages the endocytic machinery contributing to antigen processing and presentation to T cells (3).
Along this review, we will provide illustrative examples of how signaling pathways triggered by a CLR coupled to a particular canonical motif can vary depending on many factors. We will focus on Mincle, Dectin-1, DNGR-1, DCIR, and DC-SIGN as myeloid CLRs representative of each category of signaling motif. Table 1 includes the signaling module coupled to each CLR surveyed in this review, common and gene names, category of flexible signaling source, signaling pathway involved, and the inflammatory outcome provided by such flexibility. In this Table 1, CLRs are grouped based on the signaling module they bear (left column) and graphically illustrates how the signaling pathways triggered by these receptors are more complex and versatile (right columns) than expected by their signaling modules.
Classifications of receptors based on intracellular structural motifs stand on the fact that those domains determine the molecular signaling pathways initiated after ligand recognition (1). However, in addition to the basic ITAM and ITIM motifs, subtle variations in the context of the canonical motifs profoundly affect the signal delivered. For example, DNGR-1 is a DC-specific hemITAM-bearing receptor that detects dead cells and promotes cross-presentation in sterile or infectious settings, without contributing to inflammation (Figure 2A), in contrast to the close-related Dectin-1 (19, 60–63). This deficiency to promote cytokine production through DNGR-1 hemITAM was linked to an isoleucine that precedes the tyrosine in DNGR-1 hemITAM and rescued by mutation to the glycine present in Dectin-1 hemITAM (60). Signaling flexibility can thus be intrinsically provided by the amino acid sequence of those motifs present in a CLR. In this regard, residues in the neck region of DNGR-1 allow the receptor to adopt different conformations that depend on pH and ionic strength, modulating its function as the receptor progresses through the endocytic pathway (64). Even the inflammatory response of mouse and human Dectin-1 to the same ligand varies because of minor interspecies variations in the signaling motif, with low valency ligands inducing proinflammatory genes through human but not mouse Dectin-1 (65).
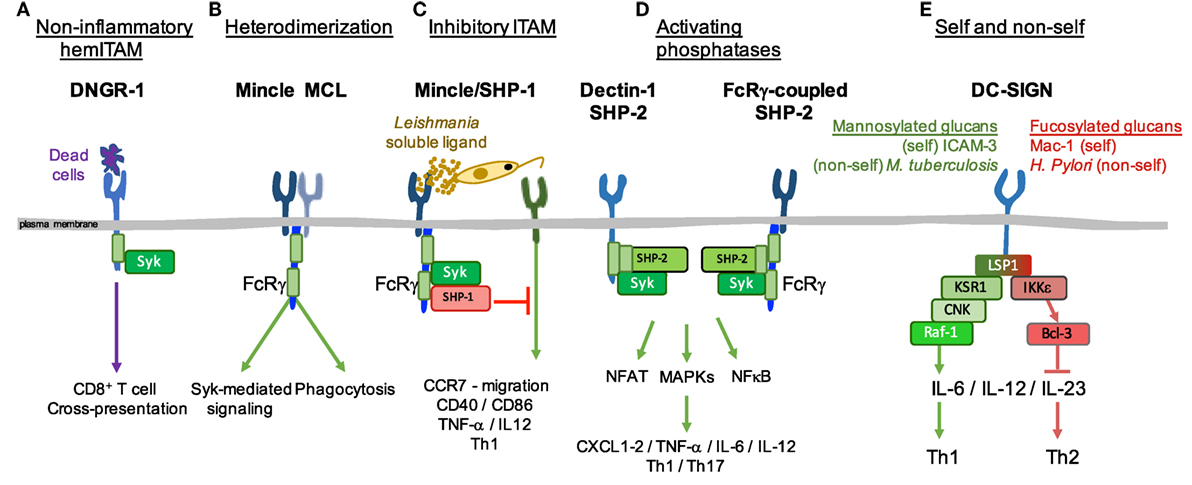
Figure 2. Signaling flexibility downstream of C-type lectin receptors (CLRs). Signaling triggered downstream of CLRs goes beyond the canonical modules present in their intracellular domains and can be modulated by different processes. Some examples of such plasticity are represented. (A) DNGR-1 promotes cross-presentation of antigens to CD8+ T cells, yet not directly contributing to inflammation. (B) Mincle and MCL dimerize, boosting phagocytosis, and spleen tyrosine kinase (Syk)-mediated inflammatory responses. (C) Sensing of a soluble ligand from Leishmania by Mincle triggers an inhibitory immunoreceptor tyrosine-based activating motif conformation downstream of Fc receptor γ (FcRγ), where SHP-1 dampens inflammatory responses triggered by heterologous receptors. (D) The phosphatase SHP-2 acts as a scaffold downstream of Dectin-1 and FcRγ-coupled CLRs, facilitating the recruitment of Syk and its inflammatory signaling. (E) Both self and non-self ligands share signaling pathways downstream of DC-SIGN depending on whether they are mannosylated or fucosylated glucans.
Receptor location also affects CLR signaling and functions. A single CLR may be expressed in different cell types (66) as diverse isoforms that may differ in subcellular location. For example, two isoforms of Dectin-1 have been described to bind β-glucans (67); isoform A is characterized by the presence of a stalk region including an N-linked glycosylation site, which is missing in isoform B (68). This glycosylation determines the cell surface expression of isoform A, while non-glycosylated isoform B is retained intracellularly, thus conditioning the response to ligands (69) and the sensitivity to proteolytic cleavage (70).
The subcellular location of a CLR may not only depend on intrinsic features in its sequence, but also on the size of the particle where the ligand is recognized. For example, “frustrated” phagocytosis mediated by Dectin-1 in response to ligands exposed in large particles leads to enhanced cytokine response and ROS production compared with soluble ligands (71–73). Blockade of Dectin-1 internalization following ligand exposure leads to sustained MAPK activation (72), suggesting that endocytosis dampens Dectin-1 production of cytokines. Thus, formation of a phagocytic synapse by particulate β-glucan redistributes Dectin-1 and phosphatases along the cellular membrane, favoring proinflammatory signals including ROS production (73). In addition, the size of the ligand-containing particle and the consequent location of the receptor, can lead to qualitatively different responses. Dectin-1-mediated phagocytosis dampens the nuclear translocation of neutrophil elastase, controlling the extent of neutrophil extracellular traps (NET) formation in response to small pathogens (bacteria or yeast). Consequently, Dectin-1 blockade or deficiency leads to enhanced NETosis, as observed in response to non-phagocytic large pathogens (hyphae) (74).
Thus, the expected canonical response based on signaling modules can be altered both by slight modifications in motif context and the subcellular location of CLRs, taking into account that the latter may be affected by the size of the ligand recognized.
The signal transduction through several myeloid CLRs may also depend on their capacity to form dimers or multimers with other CLRs. CLRs bearing hemITAMs may require two phosphorylated tyrosines in a homodimer to bind Syk. It has been shown that CLEC-2 preexists as a dimer that aggregates following ligand binding (75, 76). The hemITAM motif of CLEC-2 is crucial for blood-lymph separation during development (77, 78). Of note, thrombus stability is dependent on CLEC-2 but not on the hemITAM, revealing a hemITAM-independent signaling for CLEC-2 (79).
DC-SIGN provides another example of homomultimerization, despite lacking ITAM or ITIM domains. This CLR appears assembled as a tetramer, allowing multiple interactions with diverse pathogens that differ in size, but also increasing ligand avidity (80). In addition, some CLRs form heterodimers, such as MCL and Mincle (11, 81). These two CLRs are interrelated as they both sense the mycobacterial glycolipid trehalose-6,6-dimycolate (TDM), triggering an FcRγ-dependent pathway (11). Indeed, MCL and Mincle are co-regulated and depend on each other for their mutual surface expression (82, 83). However, the association of MCL with FcRγ in this complex is species-specific, being direct in mouse cells (11) but requiring Mincle in rat (81). Thus, the interaction between these CLRs would facilitate MCL signaling capacity via association with Mincle and translocation to the plasma membrane. On the other hand, Mincle would benefit the endocytic capacity of MCL (Figure 2B) and both receptors could increase affinity or specificity for their ligands (84). MCL also forms a heterodimeric pattern-recognition receptor with Dectin-2 (85), which has a high affinity for α-mannans on the surface of Candida albicans (C. albicans) hyphae.
Cooperative interaction is also found in the case of dengue virus binding with high affinity to MR and DC-SIGN, receptors that subsequently handle the virus to the lower affinity receptor CLEC5A, which mediates signal transduction (86).
All these examples illustrate how multimerization of CLRs, forming either homo- or hetero-complexes, facilitates a cooperative response to the ligand.
Another layer of complexity in CLR signaling stems from the ability of a single CLR to bind different ligands through its plastic C-type lectin domain. For instance, depending on their relative affinity or avidity, ligands may fine-tune signaling pathways downstream of ITAM motifs. Whereas the binding of high-avidity ligands to these receptors induces activating signals, the binding of low-avidity ligands leads to hypophosphorylation of the ITAM domain and preferential association of SH2-containing phosphatases like SHP-1, a configuration known as “inhibitory ITAM” (87). Although FcαRI receptor, which associates for signaling with the FcRγ chain, is the paradigmatic example of this inhibitory pathway (88–90), we have shown that CLRs associated with FcRγ chain may behave in the same fashion.
As an example, Mincle senses a soluble ligand derived from Leishmania that induces phosphorylation of SHP-1 coupled to FcRγ chain, inhibiting DC activation through heterologous receptors (Figure 2C) (91). In addition, SHP-1 contributes to deceleration of phagosome maturation upon TDM binding, suggesting an inhibitory signal downstream of Mincle during phagocytic processes (92). MR binds the FcRγ chain and, upon sensing Mycobacterium tuberculosis, recruits SHP-1 to the phagosome, thus limiting PI(3)P generation and delaying fusion with the lysosome, which promotes M. tuberculosis growth (12). Following treatment of DCs with curdlan or depleted zymosan (lacking TLR-stimulating properties), Dectin-1 signaling is modulated by the association of SHP-1 and PTEN to the FcRγ chain, hindering cytokine expression, DC maturation, and T-cell proliferation (93). ROS production downstream of Dectin-1 sensing of C. albicans is also tightly regulated by the SH2-domain containing inositol 5′ phosphatase (SHIP)-1 in response to Dectin-1 ligands (94). Thus, association of phosphatases to “activating” CLRs depending on the ligand nature, binding affinity, or avidity may contribute to maintenance of immune homeostasis.
Conversely, tyrosine phosphatases can contribute to activation. Contrary to SHP-1, the related tyrosine phosphatase SHP-2 acts as a scaffold, facilitating the recruitment of Syk to Dectin-1 or the adaptor FcRγ chain (95) (Figure 2D). In this way, DC-derived SHP-2 was crucial in vivo for the induction of TNF-α, IL-6, IL-12, and Th1 and Th17 anti-fungal responses upon C. albicans infection (95).
Immunoreceptor tyrosine-based inhibitory motif-coupled receptors can also deliver an activating signal. In a model of tuberculosis infection in non-human primates, DCIR deficiency impairs STAT1-mediated type I IFN signaling in DCs, leading to increased production of IL-12 and differentiation of T lymphocytes toward Th1. Thus, DCIR-deficient mice with increased Th1 immunity control M. tuberculosis better than WT animals, but also shown increased inflammation in the lungs mediated by TNF-α and inducible nitric oxide synthase (iNOS) (58). This study suggests that DCIR acts as an activating receptor for the STAT1-type I IFN signaling, and speculates that DCIR may function as a molecular sink binding unphosphorylated inactive SHP-2, therefore, limiting SHP-2′s capacity to deactivate STAT1.
The examples explained above illustrate a lack of correspondence between the canonical motif coupled to a CLR and the resulting signaling pathway. Association to kinases would lead to activating routes, while association to phosphatases would result in regulatory pathways, with some exceptions like the SHP-2-mediated CLR-induced activation (95). Association of kinases or phosphatases could be related to the strength of the initiating signal, with suboptimal phosphorylation leading to phosphatase binding to the hypo-phosporylated ITAM (inhibitory ITAM) (87). Due to the signaling flexibility offered by CLRs, a detailed empiric analysis for each CLR-ligand interaction in terms of type of ligand, concentration, and kinetics of exposition would be required to predict the signaling outcome.
C-type lectin receptors act as plastic receptors, some of them detecting self-ligands, other detecting non-self ligands, and many of them acting as dual receptors sensing self and non-self. It is possible that CLRs will behave as activating receptors when they sense non-self ligands, while CLRs bearing an ITIM motif will preferably bind self to dampen inflammation. However, in opposition to non-dangerous self, also known as “self-associated molecular patterns” (96, 97), Polly Matzinger proposed the existence of dangerous-self (damage-associated molecular patterns or DAMPs) exposed and/or released upon necrotic cell death (98, 99). In addition, tissue damage signals concomitant to infection can contribute to effector responses. Thus, DNGR-1 senses tissue damage concomitant with viral infections and facilitates antigen processing of viral antigens for cross-presentation to CD8+ T cells, decoding the antigenicity rather than the adjuvanticity of the cargo (60–63). Some examples of CLRs dealing with self and non-self ligands are explained below.
Mincle is a plastic CLR promoting proinflammatory signals after sensing glycolipids in the cell wall of bacteria and fungi (24–26), but also sensing damaged self in the form of soluble SAP-130 following necrosis (7). Mincle sensing of β-glucosylceramide (100) or cholesterol sulfate (101) promotes immunopathology (102, 103). Conversely, there are reports suggesting that Mincle sensing of SAP-130 can also drive immunosuppression (104). Moreover, human albumin abolishes innate immunity by directly binding Mincle receptor in the microglia after subarachnoid hemorrhage (105). Thus, Mincle is an example of CLR that deals with self and non-self ligands that may result in activating or inhibitory signals. However, the correlation of sensing self with an inhibitory response and sensing non-self with an activating response is not established. In this regard, non-self signals from pathogens may mimic self-inhibitory signals to escape immune surveillance, which could be the case for Mincle sensing of Leishmania (91).
DCIR is a myeloid CLR endowed with an ITIM motif that behaves as a self PRR. DCIR maintains the homeostasis of the immune system (106), since aged mice deficient for this CLR spontaneously develop several autoimmune disorders (107). Intravenous immunoglobulins bearing sialic acid induce a DCIR-mediated negative signal in DCs via SHP-2 and SHIP-1 that promotes Treg differentiation and dampens allergy (108). DCIR self-sensing can also occur in the context of infection, thus modulating the inflammatory response. DCIR-deficient mice exhibited severe inflammatory disease following Chikungunya virus infection (109). However, reduced adaptive T-cell responses in DCIR-deficient mice following cerebral malaria caused by Plasmodium berghei renders them more resistant (57). Since no evidence for direct interactions between DCIR and Chikungunya virus and P. berghei exists, we could hypothesize that DCIR may be recognizing DAMPs released during infection.
DC-SIGN illustrates how a single CLR deals differently with a variety of self and non-self ligands. DC-SIGN binds high mannose and fucose (LeX, LeY, LeA, LeB) that can be exposed in a variety of self receptors, such as ICAM-2, ICAM-3, CEACAM-1, Mac1 and CEA, or non-self proteins (structures in pathogens, including viruses, bacteria, fungi, and eukaryote parasites) (3, 110–115). Upon binding of mannosylated glucans, either self as those present on ICAM-3 (110) or non-self from M. tuberculosis (59), DC-SIGN couples to a LSP1–KSR1–CNK signalosome, leading to activation of Raf-1 and acetylation of the NF-κB p65 subunit, which results in enhancement of proinflammatory responses, including IL-12p70 and IL-6, although also promotes IL-10 transcription (59) (Figure 2E). In contrast, DC-SIGN recognition of fucosylated glucans as presented in self proteins, such as Mac1 (113) or non-self pathogens (Helicobacter pylori) (114), leads to dissociation of the LSP1-based signalosome and leaves just LSP1 associated with DC-SIGN. Phosphorylated LSP1 subsequently recruits IKKε and CYLD. IKKε activation inhibits CYLD deubiquitinase activity, facilitating nuclear translocation of ubiquitinated Bcl3 that represses TLR-induced proinflammatory cytokine expression, enhancing expression of IL-10 and Th2-attracting chemokines, and thus promoting Th2 polarization (114) (Figure 2E). In addition, IKKε collaborates with type I IFNR signaling to induce and activate the transcription factor ISGF3 that induces IL-27p28, a key cytokine for induction of T follicular helper cells (115). These results point to DC-SIGN as a dual receptor that, depending on the nature of the ligand, contributes to maintain homeostasis or initiates the immune response against some pathogens.
All these examples illustrate how a single CLR can trigger different signaling pathways depending on the recognition of self or non-self ligands. Current understanding of these processes is based on the study of individual CLRs. Deciphering common signaling patterns for self versus non-self sensing would allow harnessing immunity and inflammation by CLRs.
In addition to the diverse response of a single CLR depending on the stimulus, it is fascinating how these signaling pathways interact with signals from heterologous receptors and lead to complex responses to stimuli that are simultaneously detected by several myeloid PRRs expressed in myeloid cells [see also Ref. (116, 117) for reviews focused on this topic]. In this section, we illustrate some examples of how myeloid CLRs cross-talk with surrounding heterologous receptors.
Dectin-1 triggers a response after sensing infectious agents, such as diverse fungi and mycobacteria (118), Salmonella typhimurium (119) or Leishmania infantum (120). Dectin-1 may also promote proinflammatory signals following the detection of endogenous factors, such as vimentin from atherosclerotic plaques (121), galectin-9 from pancreatic carcinoma (122), or N-glucans on tumor cells (123). In addition to a prototypical activating CLR, Dectin-1 modulates signals simultaneously triggered through other PRRs. Dectin-1 cooperates with signals from TLR2/MyD88 to increase proinflammatory cytokine production (124–126). This synergy is exerted at the level of effector responses resulting in increased production of TNF-α, IL-12, and ROS (124) (Figure 3A, left). Dectin-1 also positively cooperates in the full activation of the NLRP3 inflammasome, participating in the priming and generation of pro-IL-1β and the induction of ROS required for NLRP3 activation (127). Conversely, Dectin-1 stimulation with depleted zymosan in bone marrow macrophages leads to Syk and Pyk2-ERK-dependent activation of SOCS-1 that downregulates IL-10 and IL-12p40 production induced by TLR9 stimulation (128) (Figure 3A, right). This effect would contribute to the Dectin-1 signature in priming Th17 responses (40, 128). In addition, Dectin-1 protects against chronic liver disease by suppressing TLR4 signaling. This effect is mediated by reducing TLR4 and CD14 expression, which are regulated by Dectin-1-dependent macrophage colony stimulating factor expression (129).
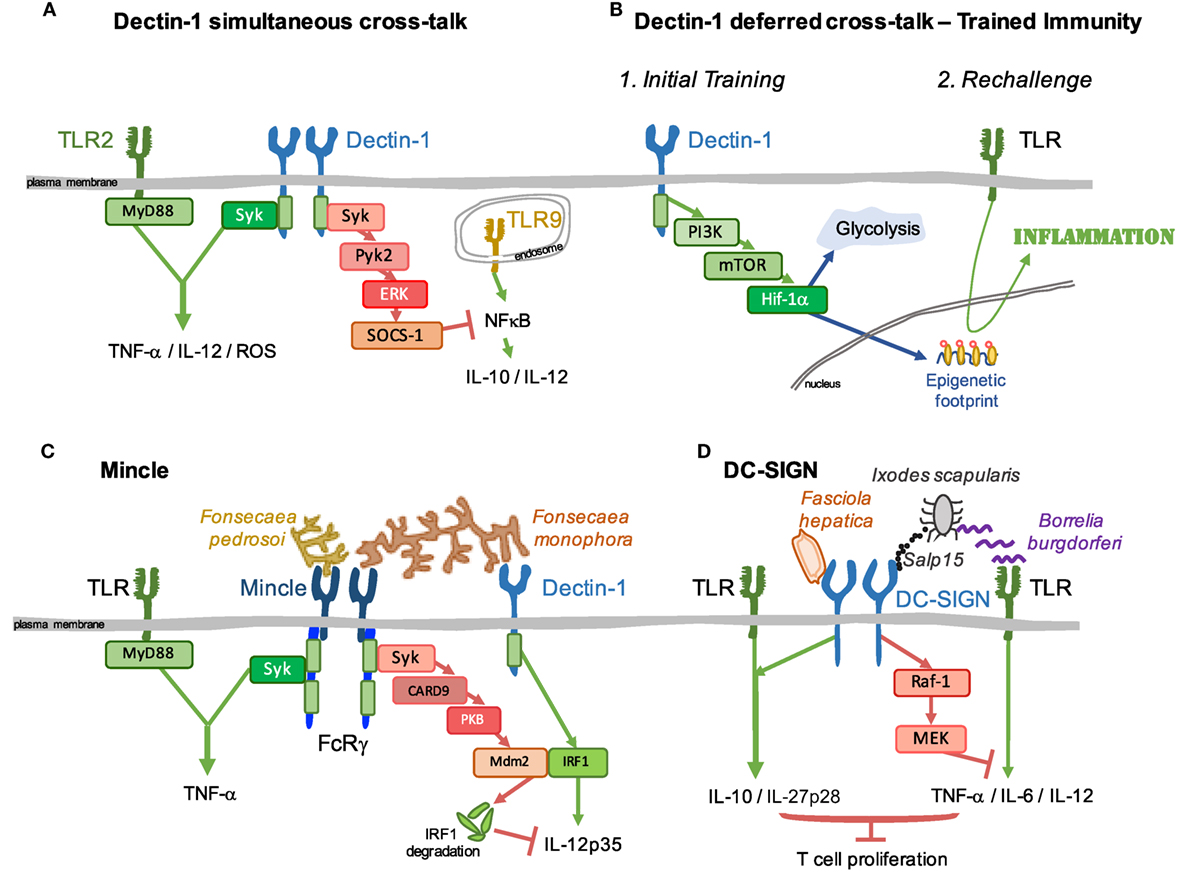
Figure 3. Signaling crosstalk between myeloid C-type lectins and heterologous receptors. Signaling pathways initiated downstream of C-type lectin receptors (CLRs) interact with surrounding cascades triggered by heterologous receptors. Examples of such crosstalk are illustrated. (A) Dectin-1 coordinates with simultaneous signals from diverse TLRs to modulate the inflammatory response; this interaction can be either positive, as for TLR2/MyD88 (left), or negative, as for TLR9 through a Pyk2/ERK/SOCS-1-dependent pathway (right). (B) In addition, the axis Dectin-1/PI3K/mTOR/Hif-1α generates a switch toward glycolytic metabolism together with an epigenetic footprint, allowing for a “deferred” improved response to TLRs, boosting the inflammatory response. This process is known as trained immunity. (C) A full inflammatory response against Fonsecaea pedrosoi is achieved by synergistic stimulation between Mincle and ligands for TLRs coupled to the MyD88 adaptor (left). However, simultaneous recognition of Fonsecaea monophora by Mincle and Dectin-1 triggers a Mincle-PKB-Mdm2-dependent degradation of Dectin-1-activated IRF1, dampening the expression of protective IL-12p35 (right). (D) DC-SIGN recognition of Fasciola hepatica enhances TLR-induced IL-10 and IL-27p28 (left). Moreover, DC-SIGN sensing of the salivary protein Salp15 from the tick vector Ixodes scapularis dampens inflammatory responses triggered by Borrelia burgdorferi through TLRs (right). Both examples illustrate strategies to escape immune surveillance based on inhibition of T cell proliferation.
Apart from direct modulation of signaling pathways triggered simultaneously, Dectin-1 can leave a footprint that affects deferred signaling by heterologous receptors, a process named as trained immunity (130). Trained immunity after sensing of C. albicans or purified β-glucan via Dectin-1 results in enhanced protection to a lethal challenge with Candida and cross-protection to Staphylococcus aureus infection (130, 131). This increased protection upon a later infection is linked to increased proinflammatory responses to delayed rechallenge with different TLR ligands, such as LPS or Pam3Cys4 (130) (Figure 3B), or bacteria, i.e., Bacteroides fragilis, Escherichia coli, Staphylococcus aureus, Borrelia burgdorferi, or M. tuberculosis (130, 132, 133). In monocytes, Dectin-1 signaling triggers the PI3K-Akt pathway, leading to activation of mTOR and HIF-1α (131). This leads to a shift from oxidative phosphorylation to aerobic glycolysis. Accumulation of fumarate, associated with glutamine replenishment of the TCA cycle, inhibits KDM5 histone demethylases, a key step for induction of monocyte epigenetic reprogramming that underlies the long-lasting effects of trained immunity (130, 134) (Figure 3B).
Apart from β-glucan or Candida, several other self and non-self ligands, such as chitin (135), BCG vaccine (136), and uric acid (137) induce trained immunity (137, 138). It would thus not be surprising that more CLRs could contribute to trained immunity. In this regard, although C. albicans mannans, potentially sensed by MR, Dectin-2, or Mincle (46), have shown not to prime human monocytes directly (130), they are essential for C. albicans-induced training (133). Furthermore, both Dectin-1 and MR are needed to trigger glycolysis upon C. albicans stimulation (139); this glycolytic switch constitutes a critical metabolic step in trained immunity induction (131, 139). Trained immunity triggered by Dectin-1 and potentially other CLRs is thus a consequence of metabolic switch and epigenetic programming that affects deferred heterologous signaling.
As described before, Mincle triggers an FcRγ-mediated activating signal in response to different stimuli. In addition, Mincle engagement can deliver regulatory responses affecting signaling pathways triggered by heterologous PRRs, such as TLRs or other CLRs, for example, Dectin-1. This section will explore modulation of heterologous receptors by Mincle.
Mincle is induced following TLR activation (7). Following sensing of Fonsecaea pedrosoi, Mincle triggers an incomplete inflammatory response that requires synergistic TLR stimulation to induce a potent proinflammatory response (Figure 3C, left), needed to clear the infection in a mouse model of chromoblastomycosis (140). This cooperative activation through Mincle and TLRs is particularly effective in human newborn DCs. Co-stimulation using the Mincle agonist trehalose-6,6-dibehenate and the TLR7/8 agonist R848 led to enhanced caspase-1 and NF-κB activation, Th1 polarizing cytokine production and autologous Th1 polarization (141).
However, Mincle exhibits a dual role in promotion and subsequent resolution of inflammation. Mycobacteria express ligands for TLRs which induce expression of Mincle that can then detect TDM and contribute to inflammation. Mincle via the Syk/p38 axis can also lead to eIF5A hypusination that increases translation efficiency of iNOS, which is transcriptionally induced by TLR2 ligation (142). In this way, Mincle favors NO production that inhibits late-stage activation of NLRP3 inflammasome in TDM-induced inflammation, contributing to termination (142). Similarly, TLR2 sensing of Corynebacterium induces robust Mincle expression, which cooperatively detects corynebacterial glycolipids favoring production of granulocyte colony stimulating factor and NO (143).
Dectin-1 and Mincle are involved in the recognition of Fonsecaea monophora, a pleomorphic fungus also responsible for chromoblastomycosis (144, 145). Signaling triggered by Dectin-1 initiates protective immunity against the fungus by activating IRF1 and IL-12p35 transcription. However, these responses are dampened by the Mincle/Syk axis, in a process involving PI3K/PKB-mediated activation of the E3 ubiquitin ligase Mdm2, leading to degradation of IRF1 and repression of IL-12p35 production (Figure 3C, right). In this way, Mincle sensing of F. monophora dampens induction of protective Th1 immunity triggered by Dectin-1 (146). Mincle is also targeted by Leishmania parasites to evade the priming of Th1 immunity initiated by DCs. As explained above, Mincle recruits SHP-1 to an inhibitory ITAM configuration in the coupled FcRγ chain, and this results in inhibition of DC activation by heterologous receptors sensing Leishmania or LPS (91) (Figure 2C). Mincle ligation can also reduce TLR4-mediated inflammation, whereas Mincle deletion or knockdown results in exaggerated inflammation in response to LPS. This effect is mediated through the control of TLR4 correceptor CD14 expression (147).
DC-SIGN engagement does not generally induce the expression of cytokines by itself, but rather modulates responses initiated by TLRs. Thus, glycans from the helminth Fasciola hepatica are recognized by DC-SIGN leading to enhanced TLR-induced IL-10 and IL-27p28, triggering a tolerogenic program that differentiates naive CD4+ T cells into regulatory T cells (148) (Figure 3D, left). However, the interaction of DC-SIGN with the salivary protein Salp15 from the tick Ixodes scapularis dampens inflammatory responses triggered by Borrelia burgdorferi. Raf-1 activation downstream of DC-SIGN sensing Salp15 results in MEK-dependent decrease of IL-6 and TNF mRNA stability and impaired nucleosome remodeling at the IL-12p35 promoter, modulating TLR-induced DC activation and T cell proliferation (112) (Figure 3D, right).
All these examples clearly illustrate how signaling pathways triggered by CLRs can have an impact on responses mediated by surrounding heterologous receptors, adding an extra layer of complexity to our understanding of CLR-mediated responses.
Classical sorting of myeloid CLRs based on the structure of the C-type lectin domain does not have functional significance. A more recent classification based on the presence of ITAM, hemITAM, or ITIM intracellular signaling motifs associated with the receptors has been useful as a starting point to predict the functional outcome of signaling CLRs (1). However, many factors may alter the expected canonical response. Minor variations in the context of the canonical motifs result in different signaling and effector outcomes (60, 65). Subcellular location depending on the isoform (69) or conformation of the receptor based on specific residues (64) also affects the function of the receptor. CLR signaling also depends on the size of the particle, where the ligand is recognized, affecting quantitatively the strength of the reaction (71–73) and also leading to qualitatively different responses (74, 149). Cooperative binding and signal transduction may be a consequence of multimerization. There are examples of homodimerization (75, 76) and formation of hetero-complexes (11, 81, 84–86). Hetero-complexes result in a mutual benefit for involved receptors, combining avidity for the ligand, capacity for endocytosis and/or signal transduction capabilities.
The plasticity of the C-type lectin domain allows binding to different ligands that, depending on their relative affinity or avidity, may trigger activating or inhibitory signaling pathways downstream of the same motifs. For example, low-avidity ligands drive a Syk-dependent association with SHP-1 to the ITAM domain (87, 88, 90), with a growing list of examples illustrating CLRs coupled to the FcRγ chain (12, 91–93). Conversely, tyrosine phosphatases may contribute to activation (95) and ITIM-containing CLRs may trigger activating signals (58). These results evidence the fine regulation of signaling though a single receptor based on differential interaction with diverse ligands, leading to the hypothesis that sensing self-ligands through CLRs could drive tolerance while non-self ligands could provoke immunity. However, dangerous-self could rather contribute to immunity and some non-self ligands could inhibit immune response for evasion, making the final outcome of a single response rather unpredictable. In addition, the concerted sensing of complex ligands by a variety of PRRs leads to complex integrated responses. CLRs may affect signals of heterologous receptors that are simultaneously triggered, either enhancing or modulating the response (59, 91, 115, 124–126, 128, 142, 146). Of note, Dectin-1 induces a metabolic switch and epigenetic programming that affects deferred heterologous signaling (130, 131). In conclusion, understanding how different signaling pathways triggered by CLRs and heterologous receptors act in concert during sensing self and non-self remain a fascinating endeavor.
Research in the field of CLRs has gained much attention considering the diversity of members, ligands, expression pattern on clinically relevant cellular populations and their relevant function on the initiation, and regulation of immunity and inflammation. Some of these features have been illustrated here and offer multiple possibilities to harness CLR-triggered responses. However, CLR manipulation may lead to unexpected outcomes and needs to be tested empirically. In addition, deciphering molecular signatures common to signaling pathways triggered by CLRs in response to different ligands will help to understand their precise role in immunity and inflammation.
CF, SI, PS-L, MM-L, and DS conceived and wrote the manuscript. CF did the figures that were edited by all the authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the members of the immunobiology lab for useful discussions.
CF is supported by AECC Foundation as recipient of an “Ayuda Fundación Científica AECC a personal investigador en cancer.” SI is funded by grant SAF2015-74561-JIN from the Spanish Ministry of Economy, Industry and Competitiveness (MINECO) and European Fund for Regional Development (FEDER). PS-L is funded by grant BES-2015-072699 (“Ayudas para contratos predoctorales para la formación de doctores 2015”) from MINECO. MM-L received a FPU fellowship (AP2010-5935) from the Spanish Ministry of Education. Work in the DS laboratory is funded by the CNIC and grant SAF2016-79040-R from MINECO and FEDER; B2017/BMD-3733 Immunothercan-CM from Comunidad de Madrid; RD16/0015/0018-REEM from FIS-Instituto de Salud Carlos III, MINECO, and FEDER; Foundation Acteria; Constantes y Vitales prize (Atresmedia); Foundation La Marató de TV3 (201723); the European Commission (635122-PROCROP H2020); and the European Research Council (ERC-Consolidator Grant 725091). The CNIC is supported by the MINECO and the Pro-CNIC Foundation, and is a Severo Ochoa Center of Excellence (MINECO award SEV-2015-0505). The authors have no conflicting financial interests.
1. Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol (2012) 30:491–529. doi:10.1146/annurev-immunol-031210-101352
2. Iborra S, Sancho D. Signalling versatility following self and non-self sensing by myeloid C-type lectin receptors. Immunobiology (2015) 220(2):175–84. doi:10.1016/j.imbio.2014.09.013
3. Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol (2009) 9(7):465–79. doi:10.1038/nri2569
4. Monteiro JT, Lepenies B. Myeloid C-type lectin receptors in viral recognition and antiviral immunity. Viruses (2017) 9(3):1–22. doi:10.3390/v9030059
5. Underhill DM, Goodridge HS. The many faces of ITAMs. Trends Immunol (2007) 28(2):66–73. doi:10.1016/j.it.2006.12.004
6. Sato K, Yang X-l, Yudate T, Chung J-S, Wu J, Luby-Phelps K, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem (2006) 281(50):38854–66. doi:10.1074/jbc.M606542200
7. Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol (2008) 9(10):1179–88. doi:10.1038/ni.1651
8. Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med (2001) 194(12):1823–34. doi:10.1084/jem.194.12.1823
9. Kaden SA, Kurig S, Vasters K, Hofmann K, Zaenker KS, Schmitz J, et al. Enhanced dendritic cell-induced immune responses mediated by the novel C-type lectin receptor mDCAR1. J Immunol (2009) 183(8):5069–78. doi:10.4049/jimmunol.0900908
10. Bakker AB, Baker E, Sutherland GR, Phillips JH, Lanier LL. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc Natl Acad Sci U S A (1999) 96(17):9792–6. doi:10.1073/pnas.96.17.9792
11. Miyake Y, Toyonaga K, Mori D, Kakuta S, Hoshino Y, Oyamada A, et al. C-type lectin MCL is an FcRgamma-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity (2013) 38(5):1050–62. doi:10.1016/j.immuni.2013.03.010
12. Rajaram MVS, Arnett E, Azad AK, Guirado E, Ni B, Gerberick AD, et al. M. tuberculosis-initiated human mannose receptor signaling regulates macrophage recognition and vesicle trafficking by FcRgamma-chain, grb2, and SHP-1. Cell Rep (2017) 21(1):126–40. doi:10.1016/j.celrep.2017.09.034
13. Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol (2006) 7(12):1258–65. doi:10.1038/ni1417
14. Osorio F, Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity (2011) 34(5):651–64. doi:10.1016/j.immuni.2011.05.001
15. Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity (2005) 22(4):507–17. doi:10.1016/j.immuni.2005.03.004
16. Fuller GLJ, Williams JAE, Tomlinson MG, Eble JA, Hanna SL, Pöhlmann S, et al. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem (2007) 282(17):12397–409. doi:10.1074/jbc.M609558200
17. Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem (2008) 283(24):16693–701. doi:10.1074/jbc.M709923200
18. Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest (2008) 118(6):2098–110. doi:10.1172/JCI34584
19. Sancho D, Joffre OP, Keller AM, Rogers NC, Martínez D, Hernanz-Falcón P, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature (2009) 458(7240):899–903. doi:10.1038/nature07750
20. Tanne A, Ma B, Boudou F, Tailleux L, Botella H, Badell E, et al. A murine DC-SIGN homologue contributes to early host defense against Mycobacterium tuberculosis. J Exp Med (2009) 206(10):2205–20. doi:10.1084/jem.20090188
21. Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood (2005) 106(7):2543–50. doi:10.1182/blood-2005-03-1239
22. Kerrigan AM, Brown GD. Syk-coupled C-type lectins in immunity. Trends Immunol (2011) 32(4):151–6. doi:10.1016/j.it.2011.01.002
23. Mócsai A, Ruland J, Tybulewicz VLJ. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol (2010) 10(6):387–402. doi:10.1038/nri2765
24. Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med (2009) 206(13):2879–88. doi:10.1084/jem.20091750
25. Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol (2010) 184(6):2756–60. doi:10.4049/jimmunol.0904013
26. Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, et al. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe (2013) 13(4):477–88. doi:10.1016/j.chom.2013.03.008
27. Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, et al. Syk kinase-coupled C-type lectin receptors engage protein kinase C-sigma to elicit Card9 adaptor-mediated innate immunity. Immunity (2012) 36(1):32–42. doi:10.1016/j.immuni.2011.11.015
28. Roth S, Bergmann H, Jaeger M, Yeroslaviz A, Neumann K, Koenig PA, et al. Vav proteins are key regulators of Card9 signaling for innate antifungal immunity. Cell Rep (2016) 17(10):2572–83. doi:10.1016/j.celrep.2016.11.018
29. Vijayan D, Radford KJ, Beckhouse AG, Ashman RB, Wells CA. Mincle polarizes human monocyte and neutrophil responses to Candida albicans. Immunol Cell Biol (2012) 90(9):889–95. doi:10.1038/icb.2012.24
30. Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, et al. The macrophage-inducible C-type lectin, Mincle, is an essential component of the innate immune response to Candida albicans. J Immunol (2008) 180(11):7404–13. doi:10.4049/jimmunol.180.11.7404
31. Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci U S A (2009) 106(6):1897–902. doi:10.1073/pnas.0805177106
32. Lee W-B, Kang J-S, Yan J-J, Lee MS, Jeon B-Y, Cho S-N, et al. Neutrophils promote mycobacterial trehalose dimycolate-induced lung inflammation via the Mincle pathway. PLoS Pathog (2012) 8(4):e1002614. doi:10.1371/journal.ppat.1002614
33. Schweneker K, Gorka O, Schweneker M, Poeck H, Tschopp J, Peschel C, et al. The mycobacterial cord factor adjuvant analogue trehalose-6,6’-dibehenate (TDB) activates the Nlrp3 inflammasome. Immunobiology (2013) 218(4):664–73. doi:10.1016/j.imbio.2012.07.029
34. Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG, et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through Mincle/CARD9 signaling and the inflammasome. J Immunol (2013) 190:5722–30. doi:10.4049/jimmunol.1203343
35. Ostrop J, Jozefowski K, Zimmermann S, Hofmann K, Strasser E, Lepenies B, et al. Contribution of Mincle-SYK signaling to activation of primary human APCs by mycobacterial cord factor and the novel adjuvant TDB. J Immunol (2015) 195(5):2417–28. doi:10.4049/jimmunol.1500102
36. Behler F, Maus R, Bohling J, Knippenberg S, Kirchhof G, Nagata M, et al. Macrophage-inducible C-type lectin Mincle-expressing dendritic cells contribute to control of splenic Mycobacterium bovis BCG infection in mice. Infect Immun (2015) 83(1):184–96. doi:10.1128/IAI.02500-14
37. Rabes A, Zimmermann S, Reppe K, Lang R, Seeberger PH, Suttorp N, et al. The C-Type lectin receptor Mincle binds to Streptococcus pneumoniae but plays a limited role in the anti-pneumococcal innate immune response. PLoS One (2015) 10(2):e0117022. doi:10.1371/journal.pone.0117022
38. Kottom TJ, Hebrink DM, Jenson PE, Nandakumar V, Wuthrich M, Wang H, et al. The interaction of pneumocystis with the C-type lectin receptor Mincle exerts a significant role in host defense against infection. J Immunol (2017) 198(9):3515–25. doi:10.4049/jimmunol.1600744
39. Gross O, Gewies A, Finger K, Schäfer M, Sparwasser T, Peschel C, et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature (2006) 442(7103):651–6. doi:10.1038/nature04926
40. LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol (2007) 8(6):630–8. doi:10.1038/ni1460
41. Slack EC, Robinson MJ, Hernanz-Falcón P, Brown GD, Williams DL, Schweighoffer E, et al. Syk-dependent ERK activation regulates IL-2 and IL-10 production by DC stimulated with zymosan. Eur J Immunol (2007) 37(6):1600–12. doi:10.1002/eji.200636830
42. Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol (2007) 178(5):3107–15. doi:10.4049/jimmunol.178.5.3107
43. Xu S, Huo J, Lee K, Kurosaki T, Lam K. Phospholipase Cgamma 2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J Biol Chem (2009) 284:7038–46. doi:10.1074/jbc.M806650200
44. Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol (2009) 10(2):203–13. doi:10.1038/ni.1692
45. Rothfuchs AG, Bafica A, Feng CG, Egen JG, Williams DL, Brown GD, et al. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J Immunol (2007) 179(6):3463–71. doi:10.4049/jimmunol.179.6.3463
46. Drummond RA, Brown GD. Signalling C-type lectins in antimicrobial immunity. PLoS Pathog (2013) 9(7):e1003417. doi:10.1371/journal.ppat.1003417
47. Saijo S, Fujikado N, Furuta T, Chung S-H, Kotaki H, Seki K, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol (2007) 8(1):39–46. doi:10.1038/ni1425
48. Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol (2007) 8(1):31–8. doi:10.1038/ni1408
49. Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science (2012) 336(6086):1314–7. doi:10.1126/science.1221789
50. Kanazawa N. Dendritic cell immunoreceptors: C-type lectin receptors for pattern-recognition and signaling on antigen-presenting cells. J Dermatol Sci (2007) 45(2):77–86. doi:10.1016/j.jdermsci.2006.09.001
51. Marshall ASJ, Willment JA, Lin H-H, Williams DL, Gordon S, Brown GD. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J Biol Chem (2004) 279(15):14792–802. doi:10.1074/jbc.M313127200
52. Kanazawa N, Okazaki T, Nishimura H, Tashiro K, Inaba K, Miyachi Y. DCIR acts as an inhibitory receptor depending on its immunoreceptor tyrosine-based inhibitory motif. J Invest Dermatol (2002) 118(2):261–6. doi:10.1046/j.0022-202x.2001.01633.x
53. Richard M, Thibault N, Veilleux P, Gareau-Pagé G, Beaulieu AD. Granulocyte macrophage-colony stimulating factor reduces the affinity of SHP-2 for the ITIM of CLECSF6 in neutrophils: a new mechanism of action for SHP-2. Mol Immunol (2006) 43(10):1716–21. doi:10.1016/j.molimm.2005.10.006
54. Lambert AA, Barabe F, Gilbert C, Tremblay MJ. DCIR-mediated enhancement of HIV-1 infection requires the ITIM-associated signal transduction pathway. Blood (2011) 117(24):6589–99. doi:10.1182/blood-2011-01-331363
55. Meyer-Wentrup F, Cambi A, Joosten B, Looman MW, de Vries IJM, Figdor CG, et al. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J Leukoc Biol (2009) 85(3):518–25. doi:10.1189/jlb.0608352
56. Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJA, Figdor CG, de Vries IJM, et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood (2008) 111(8):4245–53. doi:10.1182/blood-2007-03-081398
57. Maglinao M, Klopfleisch R, Seeberger PH, Lepenies B. The C-type lectin receptor DCIR is crucial for the development of experimental cerebral malaria. J Immunol (2013) 191(5):2551–9. doi:10.4049/jimmunol.1203451
58. Troegeler A, Mercier I, Cougoule C, Pietretti D, Colom A, Duval C, et al. C-type lectin receptor DCIR modulates immunity to tuberculosis by sustaining type I interferon signaling in dendritic cells. Proc Natl Acad Sci U S A (2017) 114(4):E540–9. doi:10.1073/pnas.1613254114
59. Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol (2009) 10(10):1081–8. doi:10.1038/ni.1778
60. Zelenay S, Keller AM, Whitney PG, Schraml BU, Deddouche S, Rogers NC, et al. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. J Clin Invest (2012) 122(5):1615–27. doi:10.1172/JCI60644DS1
61. Iborra S, Izquierdo HM, Martinez-Lopez M, Blanco-Menendez N, Reis e Sousa C, Sancho D. The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice. J Clin Invest (2012) 122(5):1628–43. doi:10.1172/JCI60660
62. Sancho D, Reis e Sousa C. Sensing of cell death by myeloid C-type lectin receptors. Curr Opin Immunol (2013) 25:46–52. doi:10.1016/j.coi.2012.12.007
63. Iborra S, Martinez-Lopez M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, et al. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1+ dendritic cells. Immunity (2016) 45(4):847–60. doi:10.1016/j.immuni.2016.08.019
64. Hanc P, Schulz O, Fischbach H, Martin SR, Kjaer S, Reis ESC. A pH- and ionic strength-dependent conformational change in the neck region regulates DNGR-1 function in dendritic cells. EMBO J (2016) 35(22):2484–97. doi:10.15252/embj.201694695
65. Takano T, Motozono C, Imai T, Sonoda KH, Nakanishi Y, Yamasaki S. Dectin-1 intracellular domain determines species-specific ligand spectrum by modulating receptor sensitivity. J Biol Chem (2017) 292(41):16933–41. doi:10.1074/jbc.M117.800847
66. Goodridge HS, Shimada T, Wolf AJ, Hsu Y-MS, Becker CA, Lin X, et al. Differential use of CARD9 by Dectin-1 in macrophages and dendritic cells. J Immunol (2009) 182(2):1146–54. doi:10.4049/jimmunol.182.2.1146
67. Willment JA, Gordon S, Brown GD. Characterization of the human beta-glucan receptor and its alternatively spliced isoforms. J Biol Chem (2001) 276(47):43818–23. doi:10.1074/jbc.M107715200
68. Yokota K, Takashima A, Bergstresser PR, Ariizumi K. Identification of a human homologue of the dendritic cell-associated C-type lectin-1, Dectin-1. Gene (2001) 272(1–2):51–60. doi:10.1016/S0378-1119(01)00528-5
69. Fischer M, Muller JP, Spies-Weisshart B, Grafe C, Kurzai O, Hunniger K, et al. Isoform localization of Dectin-1 regulates the signaling quality of anti-fungal immunity. Eur J Immunol (2017) 47(5):848–59. doi:10.1002/eji.201646849
70. Griffiths JS, Thompson A, Stott M, Benny A, Lewis NA, Taylor PR, et al. Differential susceptibility of Dectin-1 isoforms to functional inactivation by neutrophil and fungal proteases. FASEB J (2018). doi:10.1096/fj.201701145R
71. Rosas M, Liddiard K, Kimberg M, Faro-Trindade I, McDonald JU, Williams DL, et al. The induction of inflammation by Dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J Immunol (2008) 181(5):3549–57. doi:10.4049/jimmunol.181.5.3549
72. Hernanz-Falcón P, Joffre O, Williams DL, Reis e Sousa C. Internalization of Dectin-1 terminates induction of inflammatory responses. Eur J Immunol (2009) 39(2):507–13. doi:10.1002/eji.200838687
73. Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature (2011) 472(7344):471–5. doi:10.1038/nature10071
74. Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol (2014) 15(11):1017–25. doi:10.1038/ni.2987
75. Hughes CE, Pollitt AY, Mori J, Eble JA, Tomlinson MG, Hartwig JH, et al. CLEC-2 activates Syk through dimerization. Blood (2010) 115(14):2947–55. doi:10.1182/blood-2009-08-237834
76. Watson AA, Christou CM, James JR, Fenton-May AE, Moncayo GE, Mistry AR, et al. The platelet receptor CLEC-2 is active as a dimer. Biochemistry (2009) 48(46):10988–96. doi:10.1021/bi901427d
77. Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem (2010) 285(32):24494–507. doi:10.1074/jbc.M110.130575
78. Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood (2010) 116(4):661–70. doi:10.1182/blood-2010-02-270876
79. Haining EJ, Cherpokova D, Wolf K, Becker IC, Beck S, Eble JA, et al. CLEC-2 contributes to hemostasis independently of classical hemITAM signaling in mice. Blood (2017) 130(20):2224–8. doi:10.1182/blood-2017-03-771907
80. Garcia-Vallejo JJ, van Kooyk Y. The physiological role of DC-SIGN: a tale of mice and men. Trends Immunol (2013) 34(10):482–6. doi:10.1016/j.it.2013.03.001
81. Lobato-Pascual A, Saether PC, Fossum S, Dissen E, Daws MR. Mincle, the receptor for mycobacterial cord factor, forms a functional receptor complex with MCL and FcepsilonRI-gamma. Eur J Immunol (2013) 43(12):3167–74. doi:10.1002/eji.201343752
82. Miyake Y, Masatsugu OH, Yamasaki S. C-type lectin receptor MCL facilitates Mincle expression and signaling through complex formation. J Immunol (2015) 194(11):5366–74. doi:10.4049/jimmunol.1402429
83. Kerscher B, Dambuza IM, Christofi M, Reid DM, Yamasaki S, Willment JA, et al. Signalling through MyD88 drives surface expression of the mycobacterial receptors MCL (Clecsf8, Clec4d) and Mincle (Clec4e) following microbial stimulation. Microbes Infect (2016) 18(7–8):505–9. doi:10.1016/j.micinf.2016.03.007
84. Yamasaki S. Signaling while eating: MCL is coupled with Mincle. Eur J Immunol (2013) 43(12):3156–8. doi:10.1002/eji.201344131
85. Zhu L-L, Zhao X-Q, Jiang C, You Y, Chen X-P, Jiang Y-Y, et al. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity (2013) 39(2):324–34. doi:10.1016/j.immuni.2013.05.017
86. Lo YL, Liou GG, Lyu JH, Hsiao M, Hsu TL, Wong CH. Dengue virus infection is through a cooperative interaction between a mannose receptor and CLEC5A on macrophage as a multivalent hetero-complex. PLoS One (2016) 11(11):e0166474. doi:10.1371/journal.pone.0166474
87. Blank U, Launay P, Benhamou M, Monteiro RC. Inhibitory ITAMs as novel regulators of immunity. Immunol Rev (2009) 232(1):59–71. doi:10.1111/j.1600-065X.2009.00832.x
88. Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffié C, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity (2005) 22(1):31–42. doi:10.1016/j.immuni.2004.11.017
89. Aloulou M, Ben Mkaddem S, Biarnes-Pelicot M, Boussetta T, Souchet H, Rossato E, et al. IgG1 and IVIg induce inhibitory ITAM signaling through FcγRIII controlling inflammatory responses. Blood (2012) 119(13):3084–96. doi:10.1182/blood-2011-08-376046
90. Ben Mkaddem S, Hayem G, Jonsson F, Rossato E, Boedec E, Boussetta T, et al. Shifting FcgammaRIIA-ITAM from activation to inhibitory configuration ameliorates arthritis. J Clin Invest (2014) 124(9):3945–59. doi:10.1172/JCI74572
91. Iborra S, Martinez-Lopez M, Cueto FJ, Conde-Garrosa R, Del Fresno C, Izquierdo HM, et al. Leishmania uses Mincle to target an inhibitory ITAM signaling pathway in dendritic cells that dampens adaptive immunity to infection. Immunity (2016) 45(4):788–801. doi:10.1016/j.immuni.2016.09.012
92. Patin EC, Geffken AC, Willcocks S, Leschczyk C, Haas A, Nimmerjahn F, et al. Trehalose dimycolate interferes with FcgammaR-mediated phagosome maturation through Mincle, SHP-1 and FcgammaRIIB signalling. PLoS One (2017) 12(4):e0174973. doi:10.1371/journal.pone.0174973
93. Pan YG, Yu YL, Lin CC, Lanier LL, Chu CL. FcepsilonRI gamma-chain negatively modulates Dectin-1 responses in dendritic cells. Front Immunol (2017) 8:1424. doi:10.3389/fimmu.2017.01424
94. Blanco-Menendez N, Del Fresno C, Fernandes S, Calvo E, Conde-Garrosa R, Kerr WG, et al. SHIP-1 couples to the Dectin-1 hemITAM and selectively modulates reactive oxygen species production in dendritic cells in response to Candida albicans. J Immunol (2015) 195(9):4466–78. doi:10.4049/jimmunol.1402874
95. Deng Z, Ma S, Zhou H, Zang A, Fang Y, Li T, et al. Tyrosine phosphatase SHP-2 mediates C-type lectin receptor-induced activation of the kinase Syk and anti-fungal TH17 responses. Nat Immunol (2015) 16(6):642–52. doi:10.1038/ni.3155
96. Elward K, Gasque P. “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol Immunol (2003) 40(2–4):85–94. doi:10.1016/S0161-5890(03)00109-3
97. Varki A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology (2011) 21(9):1121–4. doi:10.1093/glycob/cwr087
98. Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol (1994) 12:991–1045. doi:10.1146/annurev.iy.12.040194.005015
99. Matzinger P. The danger model: a renewed sense of self. Science (2002) 296(5566):301–5. doi:10.1126/science.1071059
100. Nagata M, Izumi Y, Ishikawa E, Kiyotake R, Doi R, Iwai S, et al. Intracellular metabolite beta-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc Natl Acad Sci U S A (2017) 114(16):E3285–94. doi:10.1073/pnas.1618133114
101. Kostarnoy AV, Gancheva PG, Lepenies B, Tukhvatulin AI, Dzharullaeva AS, Polyakov NB, et al. Receptor Mincle promotes skin allergies and is capable of recognizing cholesterol sulfate. Proc Natl Acad Sci U S A (2017) 114(13):E2758–65. doi:10.1073/pnas.1611665114
102. Suzuki Y, Nakano Y, Mishiro K, Takagi T, Tsuruma K, Nakamura M, et al. Involvement of Mincle and Syk in the changes to innate immunity after ischemic stroke. Sci Rep (2013) 3:3177. doi:10.1038/srep03177
103. Arumugam TV, Manzanero S, Furtado M, Biggins PJ, Hsieh YH, Gelderblom M, et al. An atypical role for the myeloid receptor Mincle in central nervous system injury. J Cereb Blood Flow Metab (2017) 37(6):2098–111. doi:10.1177/0271678X16661201
104. Seifert L, Werba G, Tiwari S, Ly NNG, Alothman S, Alqunaibit D, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature (2016) 532(7598):245–9. doi:10.1038/nature17403
105. Xie Y, Guo H, Wang L, Xu L, Zhang X, Yu L, et al. Human albumin attenuates excessive innate immunity via inhibition of microglial Mincle/Syk signaling in subarachnoid hemorrhage. Brain Behav Immun (2017) 60:346–60. doi:10.1016/j.bbi.2016.11.004
106. Fujikado N, Saijo S, Yonezawa T, Shimamori K, Ishii A, Sugai S, et al. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med (2008) 14:176–80. doi:10.1038/nm1697
107. Maruhashi T, Kaifu T, Yabe R, Seno A, Chung SH, Fujikado N, et al. DCIR maintains bone homeostasis by regulating IFN-gamma production in T cells. J Immunol (2015) 194(12):5681–91. doi:10.4049/jimmunol.1500273
108. Massoud AH, Yona M, Xue D, Chouiali F, Alturaihi H, Ablona A, et al. Dendritic cell immunoreceptor: a novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells. J Allergy Clin Immunol (2014) 133(3):853–63.e5. doi:10.1016/j.jaci.2013.09.029
109. Long KM, Whitmore AC, Ferris MT, Sempowski GD, McGee C, Trollinger B, et al. Dendritic cell immunoreceptor regulates Chikungunya virus pathogenesis in mice. J Virol (2013) 87(10):5697–706. doi:10.1128/JVI.01611-12
110. Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell (2000) 100(5):575–85. doi:10.1016/S0092-8674(00)80693-5
111. Geijtenbeek TBH, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CMJE, Appelmelk B, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med (2003) 197(1):7–17. doi:10.1084/jem.20021229
112. Hovius JWR, de Jong MAWP, den Dunnen J, Litjens M, Fikrig E, van der Poll T, et al. Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog (2008) 4(2):e31. doi:10.1371/journal.ppat.0040031
113. van Gisbergen KPJM, Sanchez-Hernandez M, Geijtenbeek TBH, van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J Exp Med (2005) 201(8):1281–92. doi:10.1084/jem.20041276
114. Gringhuis SI, Kaptein TM, Wevers BA, Mesman AW, Geijtenbeek TB. Fucose-specific DC-SIGN signalling directs T helper cell type-2 responses via IKKepsilon- and CYLD-dependent Bcl3 activation. Nat Commun (2014) 5:3898. doi:10.1038/ncomms4898
115. Gringhuis SI, Kaptein TM, Wevers BA, van der Vlist M, Klaver EJ, van Die I, et al. Fucose-based PAMPs prime dendritic cells for follicular T helper cell polarization via DC-SIGN-dependent IL-27 production. Nat Commun (2014) 5:5074. doi:10.1038/ncomms6074
116. Thaiss CA, Levy M, Itav S, Elinav E. Integration of innate immune signaling. Trends Immunol (2016) 37(2):84–101. doi:10.1016/j.it.2015.12.003
117. Ostrop J, Lang R. Contact, collaboration, and conflict: signal integration of Syk-coupled C-type lectin receptors. J Immunol (2017) 198(4):1403–14. doi:10.4049/jimmunol.1601665
118. Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol (2006) 6(1):33–43. doi:10.1038/nri1745
119. Jackson N, Compton E, Trowsdale J, Kelly AP. Recognition of Salmonella by Dectin-1 induces presentation of peptide antigen to type B T cells. Eur J Immunol (2014) 44(4):962–9. doi:10.1002/eji.201344065
120. Lefevre L, Lugo-Villarino G, Meunier E, Valentin A, Olagnier D, Authier H, et al. The C-type lectin receptors Dectin-1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum. Immunity (2013) 38(5):1038–49. doi:10.1016/j.immuni.2013.04.010
121. Thiagarajan PS, Yakubenko VP, Elsori DH, Yadav SP, Willard B, Tan CD, et al. Vimentin is an endogenous ligand for the pattern recognition receptor Dectin-1. Cardiovasc Res (2013) 99(3):494–504. doi:10.1093/cvr/cvt117
122. Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med (2017) 23(5):556–67. doi:10.1038/nm.4314
123. Chiba S, Ikushima H, Ueki H, Yanai H, Kimura Y, Hangai S, et al. Recognition of tumor cells by Dectin-1 orchestrates innate immune cells for anti-tumor responses. Elife (2014) 3:e04177. doi:10.7554/eLife.04177
124. Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by Dectin-1 and toll-like receptor 2. J Exp Med (2003) 197(9):1107–17. doi:10.1084/jem.20021787
125. Viriyakosol S, Fierer J, Brown GD, Kirkland TN. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on toll-like receptor 2 and Dectin-1. Infect Immun (2005) 73(3):1553–60. doi:10.1128/iai.73.3.1553-1560.2005
126. Yadav M, Schorey JS. The beta-glucan receptor Dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood (2006) 108(9):3168–75. doi:10.1182/blood-2006-05-024406
127. Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature (2009) 459:433–6. doi:10.1038/nature07965
128. Eberle ME, Dalpke AH. Dectin-1 stimulation induces suppressor of cytokine signaling 1, thereby modulating TLR signaling and T cell responses. J Immunol (2012) 188(11):5644–54. doi:10.4049/jimmunol.1103068
129. Seifert L, Deutsch M, Alothman S, Alqunaibit D, Werba G, Pansari M, et al. Dectin-1 regulates hepatic fibrosis and hepatocarcinogenesis by suppressing TLR4 signaling pathways. Cell Rep (2015) 13(9):1909–21. doi:10.1016/j.celrep.2015.10.058
130. Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe (2012) 12(2):223–32. doi:10.1016/j.chom.2012.06.006
131. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science (2014) 345(6204):1250684. doi:10.1126/science.1250684
132. Buffen K, Oosting M, Quintin J, Ng A, Kleinnijenhuis J, Kumar V, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog (2014) 10(10):e1004485. doi:10.1371/journal.ppat.1004485
133. Ifrim DC, Joosten LA, Kullberg BJ, Jacobs L, Jansen T, Williams DL, et al. Candida albicans primes TLR cytokine responses through a Dectin-1/Raf-1-mediated pathway. J Immunol (2013) 190(8):4129–35. doi:10.4049/jimmunol.1202611
134. Arts RJ, Novakovic B, Ter Horst R, Carvalho A, Bekkering S, Lachmandas E, et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab (2016) 24(6):807–19. doi:10.1016/j.cmet.2016.10.008
135. Rizzetto L, Ifrim DC, Moretti S, Tocci N, Cheng SC, Quintin J, et al. Fungal chitin induces trained immunity in human monocytes during cross-talk of the host with Saccharomyces cerevisiae. J Biol Chem (2016) 291(15):7961–72. doi:10.1074/jbc.M115.699645
136. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A (2012) 109(43):17537–42. doi:10.1073/pnas.1202870109
137. Crisan TO, Netea MG, Joosten LA. Innate immune memory: implications for host responses to damage-associated molecular patterns. Eur J Immunol (2016) 46(4):817–28. doi:10.1002/eji.201545497
138. Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science (2016) 352(6284):aaf1098. doi:10.1126/science.aaf1098
139. Domínguez-Andrés J, Arts RJW, ter Horst R, Gresnigt MS, Smeekens SP, Ratter JM, et al. Rewiring monocyte glucose metabolism via C-type lectin signaling protects against disseminated candidiasis. PLoS Pathog (2017) 13:e1006632. doi:10.1371/journal.ppat.1006632
140. Sousa, MdG, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, et al. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe (2011) 9(5):436–43. doi:10.1016/j.chom.2011.04.005
141. van Haren SD, Dowling DJ, Foppen W, Christensen D, Andersen P, Reed SG, et al. Age-specific adjuvant synergy: dual TLR7/8 and Mincle activation of human newborn dendritic cells enables Th1 polarization. J Immunol (2016) 197(11):4413–24. doi:10.4049/jimmunol.1600282
142. Lee WB, Kang JS, Choi WY, Zhang Q, Kim CH, Choi UY, et al. Mincle-mediated translational regulation is required for strong nitric oxide production and inflammation resolution. Nat Commun (2016) 7:11322. doi:10.1038/ncomms11322
143. Schick J, Etschel P, Bailo R, Ott L, Bhatt A, Lepenies B, et al. Toll-like receptor 2 and Mincle cooperatively sense corynebacterial cell wall glycolipids. Infect Immun (2017) 85(7):1–14. doi:10.1128/IAI.00075-17
144. Wuthrich M, Wang H, Li M, Lerksuthirat T, Hardison SE, Brown GD, et al. Fonsecaea pedrosoi-induced Th17-cell differentiation in mice is fostered by Dectin-2 and suppressed by Mincle recognition. Eur J Immunol (2015) 45(9):2542–52. doi:10.1002/eji.201545591
145. Siqueira IM, de Castro RJA, Leonhardt LCM, Jeronimo MS, Soares AC, Raiol T, et al. Modulation of the immune response by Fonsecaea pedrosoi morphotypes in the course of experimental chromoblastomycosis and their role on inflammatory response chronicity. PLoS Negl Trop Dis (2017) 11(3):e0005461. doi:10.1371/journal.pntd.0005461
146. Wevers BA, Kaptein TM, Zijlstra-Willems EM, Theelen B, Boekhout T, Geijtenbeek TB, et al. Fungal engagement of the C-type lectin Mincle suppresses Dectin-1-induced antifungal immunity. Cell Host Microbe (2014) 15(4):494–505. doi:10.1016/j.chom.2014.03.008
147. Greco SH, Mahmood SK, Vahle AK, Ochi A, Batel J, Deutsch M, et al. Mincle suppresses toll-like receptor 4 activation. J Leukoc Biol (2016) 100(1):185–94. doi:10.1189/jlb.3A0515-185R
148. Rodriguez E, Kalay H, Noya V, Brossard N, Giacomini C, van Kooyk Y, et al. Fasciola hepatica glycoconjugates immuneregulate dendritic cells through the dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin inducing T cell anergy. Sci Rep (2017) 7:46748. doi:10.1038/srep46748
Keywords: lectin receptors, signaling, monocytes, macrophages, dendritic cells, innate immunity, inflammation
Citation: del Fresno C, Iborra S, Saz-Leal P, Martínez-López M and Sancho D (2018) Flexible Signaling of Myeloid C-Type Lectin Receptors in Immunity and Inflammation. Front. Immunol. 9:804. doi: 10.3389/fimmu.2018.00804
Received: 19 December 2017; Accepted: 03 April 2018;
Published: 26 April 2018
Edited by:
Roland Lang, Universitätsklinikum Erlangen, GermanyReviewed by:
Michael Rory Daws, University of Oslo, NorwayCopyright: © 2018 del Fresno, Iborra, Saz-Leal, Martínez-López and Sancho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos del Fresno, Y2FybG9zLmRlbGZyZXNub0BjbmljLmVz;
David Sancho, ZHNhbmNob0BjbmljLmVz
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.