- 1Department of Gastroenterology and Hematology, Hirosaki University Graduate School of Medicine, Hirosaki, Japan
- 2Department of Gastroenterology and Hematology, Hirosaki National Hospital, Hirosaki, Japan
- 3Department of Social Medicine, Hirosaki University Graduate School of Medicine, Hirosaki, Japan
Background: Suppression of gastric acid by proton pump inhibitors is associated with the increase of Lactobacillus in human gut microbiota. Gastric acid secretion is also suppressed by Helicobacter pylori infection and following atrophic gastritis. However, few studies have examined the association between H. pylori infection and Lactobacillus species in gut microbiota particularly in Japan.
Methods: A total of 1,123 adult subjects who participated in a health survey in Hirosaki City were studied. Infection of H. pylori was defined by both serum antibody and stool antigen test. The presence and the severity of atrophic gastritis were defined by the serum level of serum pepsinogens. Using 16S ribosomal RNA amplification from fecal samples, the relative abundance of Lactobacillus was calculated, and the composition ratio of each Lactobacillus species was surveyed.
Results: The relative abundance of the Lactobacillus in H. pylori-infected subjects with severe atrophic gastritis was higher comparing with those in subjects with mild atrophic gastritis and without atrophic gastritis (0.591 vs 0.068% and 0.033%, respectively; p < 0.001) and also that of non-infected subjects (0.033%; p < 0.001). In H. pylori non-infected subjects, both gender and age were not associated with the relative abundance of Lactobacillus in fecal samples. The proportion of Lactobacillus salivarius was high in H. pylori-infected subjects while that of Lactobacillus acidophilus was high in non-infected subjects.
Conclusion: Lactobacillus in human gut microbiota could be influenced by H. pylori infection and severity of atrophic gastritis in Japanese subjects.
Introduction
Lactobacilli are a well-known probiotic and have been introduced into many fermented dairy products. A recent meta-analysis of randomized controlled trials showed products containing Lactobacillus species increased stool frequency in constipated adults (1). Several randomized controlled studies also reported that some Lactobacillus species such as Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus casei, and Lactobacillus rhamnosus GG prevented antibiotic-associated diarrhea and Clostridium difficile-associated colitis (2–5).
Recent studies suggested that the use of proton pump inhibitors (PPIs) was associated with the increase in the Lactobacillus population in the human gut microbiota (6, 7). This phenomenon is thought to be due to long-term acid suppression by PPIs. Gastric acid secretion is also suppressed by Helicobacter pylori infection and following atrophic gastritis (8–12). However, it is unclear whether the decrease of gastric acid caused by H. pylori infection and atrophic gastritis would cause an increase in Lactobacillus in the human gut microbiota. Most patients infected with H. pylori develop atrophic gastritis in Japan (13). Gastric acid secretion is also lower in Japanese people comparing with Western populations in healthy subjects (14). The lower gastric acid secretion might result in different gut microbiota in Japanese from those in Western people. Indeed, a recent study demonstrated that gut microbiome of the Japanese is considerably different from those of other populations (15). In Japan, however, few studies have examined the association between H. pylori infection and gut microbiota, particularly Lactobacillus species. Although a previous German study showed a modulation of Lactobacillus in the gut microbiota after successful eradication of H. pylori, next-generation sequence analysis was not used (16). Furthermore, previous studies have been performed regardless of the extent of atrophic gastritis.
The aim of this study was to evaluate the influence of H. pylori infection and the progress of atrophic gastritis on the amount and diversity of Lactobacillus species in the human gut microbiota in Japan using next-generation sequence analysis.
Materials and Methods
Study Subjects
A total of 1,123 adults participated in the Iwaki Health Promotion Projects held in June 2014, in Hirosaki City, north Japan (Figure 1). Of these, we excluded 207 subjects who had previously received H. pylori eradication therapy, 12 subjects who had a previous history of gastric surgery, and 20 subjects who were taking PPI. After the exclusion, there was no subject whose serum level of creatinine was larger than 2.0 mg/dL. Finally, 884 subjects were analyzed.
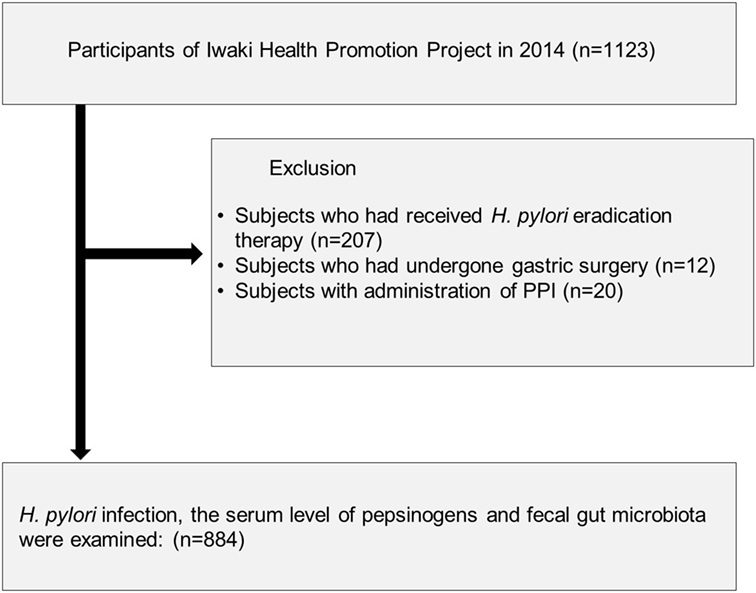
Figure 1. Study flow of subjects. A total of 884 subjects were enrolled from 1,123 adults who participated in the Iwaki Health Promotion Projects in 2014.
Diagnosis of H. pylori Infection
Serum samples were collected after one night of fasting and stored at −20°C. The titer of serum IgG antibody to H. pylori was measured by E-plate (Eiken, Tokyo, Japan) (17). Stool samples were collected and stored at −80°C. Stool samples were tested for H. pylori antigen by using Testmate EIA (Wakamoto and Kyowa Medex, Tokyo, Japan) (18). H. pylori status was defined as positive when the stool antigen test was positive and serum antibody titer ≥10 U/mL, and as negative when the stool antigen test was negative and serum antibody titer <3 U/mL.
Evaluation of Atrophic Gastritis
The serum level of pepsinogen (PG) I and II was measured and used as markers of atrophic gastritis (12, 19, 20). The result was considered indicative of atrophic gastritis when both a PG I level of <70 μg/L and a PG I/II ratio of <3.0 were observed, and severe atrophic gastritis when both a PG I level of <30 μg/L and a PG I/II ratio <2.0 were observed.
DNA Extraction From Fecal Samples
Two to three grams of fecal samples were collected by each participant in commercial containers (TechnoSuruga Laboratory Co., Ltd., Shizuoka, Japan) and suspended in guanidine thiocyanate solution [100 mM Tris–HCl (pH 9.0), 40 mM Tris–EDTA (pH 8.0), 4 M guanidine thiocyanate]. These samples were kept at −80°C until DNA extraction. Frozen fecal solids were beaten with zirconia beads at 5 m/s for 2 min by using a FastPrep 24 Instrument (MP Biomedicals, Santana Ana, CA, USA). DNA was extracted from 200 µL of the suspension by using a Magtration System 12 GC (Precision System Science, Japan), with MagdDEA DNA 200 (Precision System Science, Japan) as the reagent for automatic nucleic acid extraction.
Next-Generation Sequence Analysis and 16S rDNA-Based Taxonomic Analysis
According to previous studies, a series of representative bacteria in the human gut microbiota was analyzed using the primers for the V3–V4 region of 16S rDNA of prokaryotes (21, 22). Sequencing was conducted using an Illumina MiSeq system (Illumina, San Diego, CA, USA). The method for quality filtering the sequences was as follows: only reads that had quality value scores of scores of ≥20 for more than 99% of the sequence were extracted for the analysis. Detection and identification of bacteria from sequences were performed using Metagenome@KIN software (World Fusion Co., Tokyo, Japan) and the TechnoSuruga Lab Microbial Identification database DB-BA 10.0 (TechnoSuruga Laboratory) at 97% sequence similarity.
We compared the relative abundance of Lactobacillus in the gut microbiota between H. pylori-infected and non-infected subjects and among subjects with different degrees of atrophic gastritis. Relative abundance is the percent composition of reads of Lactobacillus relative to the total reads of gut microbiota. The composition ratio of each Lactobacillus species was surveyed in each subject. Moreover, to evaluate the influence of gender and age, in H. pylori non-infected subjects, we surveyed the relative abundance of Lactobacillus in gut microbiota between male and female, and among different age groups by decades.
Statistical Analysis
Statistical analyses of the clinical data were performed using the Statistical Package for the Social Sciences (SPSS) version 20.0 (SPSS Inc., Chicago, IL, USA). Categorical variables are shown as frequencies and percentages and, continuous variables are shown as the mean with standard deviation or the median with interquartile range. Categorical variables were compared using the chi-square test and continuous variables were compared using the Student’s t-test. One-way ANOVA was used for comparing more than two groups. Mann–Whitney U-test and Steel–Dwass test were used to compare the abundance of Lactobacillus. A p value of less than 0.05 was considered significant for all tests.
Ethics Statement
This study was performed in accordance with the ethical standards of the Declaration of Helsinki and approved by the ethics committee at Hirosaki University Medical Ethics Committee (2014-377). All patients provided written informed consent for this study.
Results
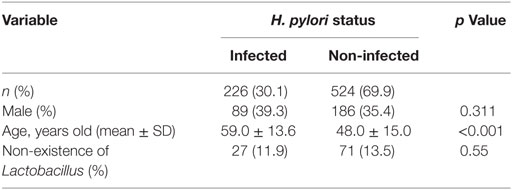
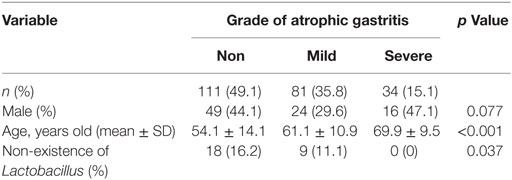
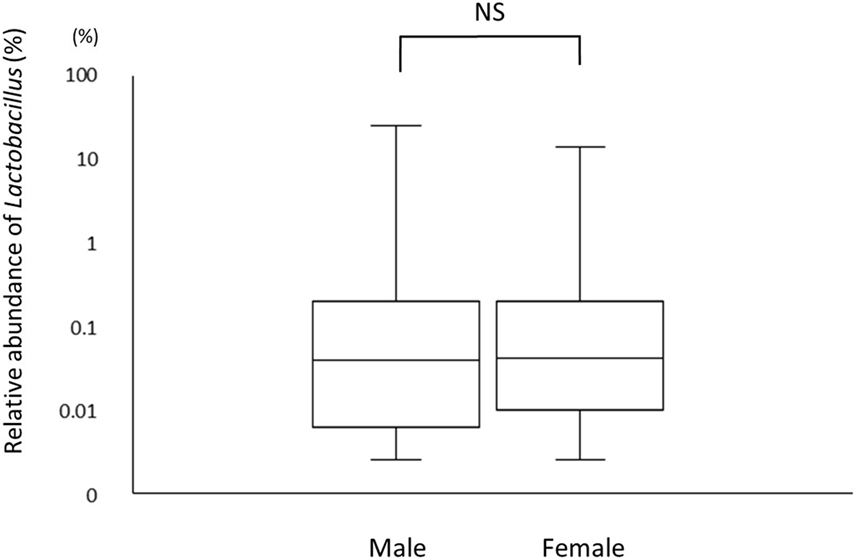
A total of 226 subjects were defined as infected with H. pylori, and 524 subjects who were defined as non-infected (Table 1). The median relative abundance of Lactobacillus in subjects with H. pylori infection and non-infected subjects was 0.071 (interquartile range: IQR; 0.350)% and 0.033 (0.143)%. In H. pylori-infected subjects, there were 111 subjects without atrophic gastritis, 81 subjects with mild atrophic gastritis and 34 subjects with severe atrophic gastritis (Table 2). The median relative abundance of Lactobacillus in subjects with severe atrophic gastritis was significantly higher than those in subjects with mild atrophic gastritis, without atrophic gastritis and non-infected [median (IQR) 0.591 (1.837) vs 0.068 (0.258)%, 0.033 (0.205)% and 0.033 (0.143)%; p < 0.001] (Figure 2). The relative abundance of Lactobacillus was not significantly different between subjects with mild atrophic gastritis, without atrophic gastritis and non-infected subjects. In H. pylori non-infected subjects, there were no significant differences in the relative abundance of Lactobacillus between male and female subjects (p = 0.332) (Figure 3), or among the six age groups by decades (p = 0.532) (Figure 4).
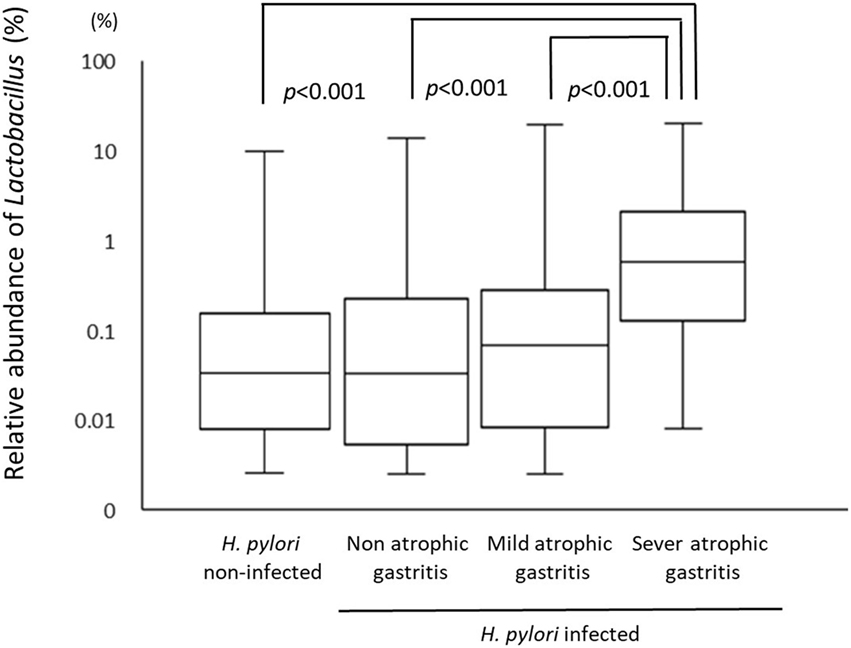
Figure 2. The relative abundance of the Lactobacillus in gut microbiota among Helicobacter pylori non-infected subjects and infected subjects with different degree of atrophic gastritis.
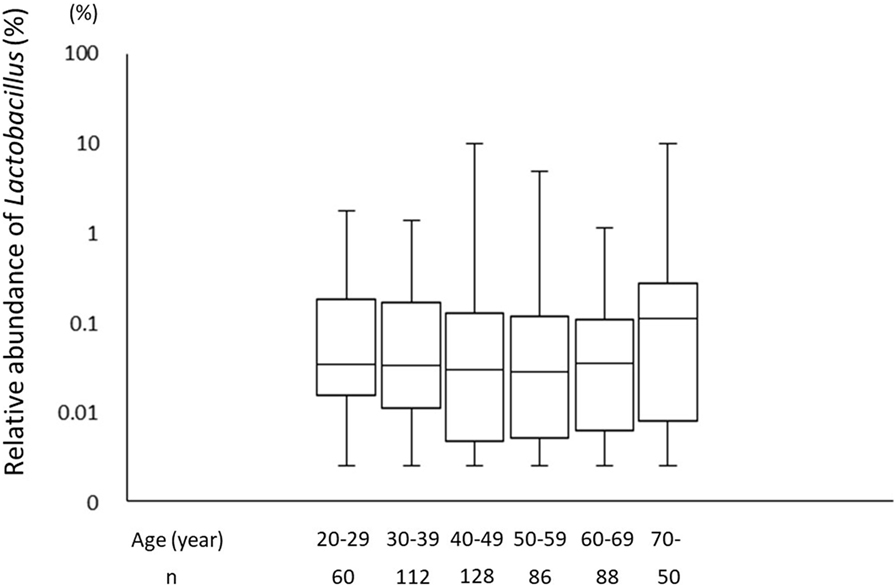
Figure 4. The relative abundance of the Lactobacillus in gut microbiota among the six age groups by decades.
Figure 5 shows the proportion of Lactobacillus species in H. pylori-infected subjects divided by degree of atrophic gastritis and non-infected subjects. The proportion of Lactobacillus salivarius was higher in H. pylori-infected subjects than non-infected subjects. By contrast, the proportion of L. acidophilus in H. pylori non-infected subjects was higher than that in H. pylori-infected subjects regardless the degree of atrophic gastritis.
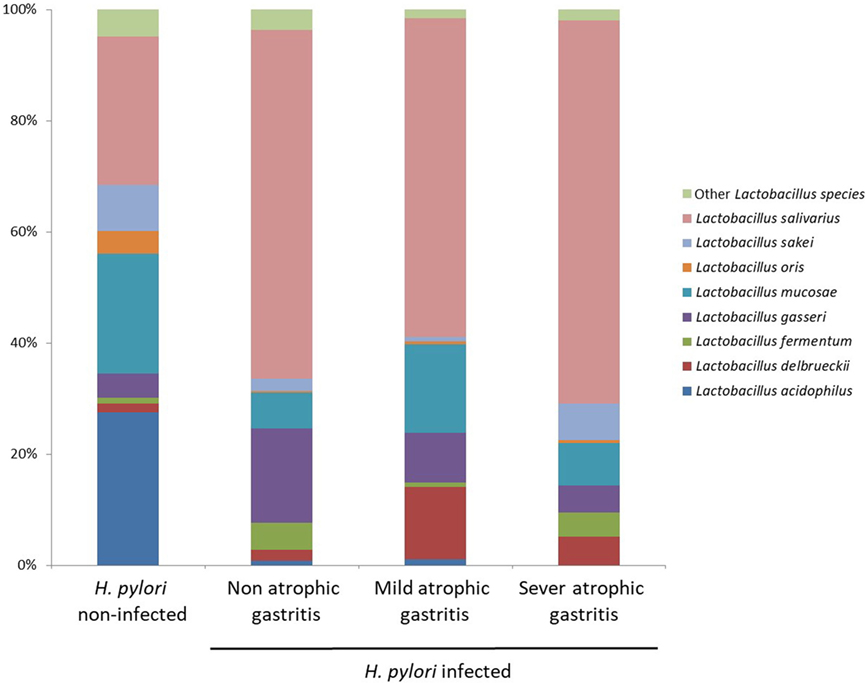
Figure 5. Proportion of Lactobacillus species in Helicobacter pylori-infected subjects divided by degree of atrophic gastritis and non-infected subjects.
Discussion
This study demonstrated that infection with H. pylori modulates the proportion of species of Lactobacillus in gut microbiota. The relative abundance of Lactobacillus in human gut microbiota may increase after the development of severe atrophic gastritis. By contrast, in H. pylori non-infected subjects, relative abundance of Lactobacillus in gut microbiota was not affected by gender or age.
A previous German study reported that the presence of H. pylori led to an increased growth of lactobacilli (16). In this study, however, H. pylori-infected subjects showed higher rates of Lactobacillus only when the subjects had severe atrophic gastritis. Therefore, the rates of Lactobacillus in the gut microbiota could be associated with the progress of atrophic gastritis rather than with H. pylori infection in Japan. Severe gastric atrophy indicated by serum level of PGs has been significantly correlated with gastric acid secretion (12, 23). The use of PPIs has been associated with an increase of Lactobacillus in the gut microbiota (6, 7). In Japan, the reduction of gastric acid by severe atrophic gastritis could influence the rates of Lactobacillus in gut microbiota even though the effects were smaller than with the use of PPI or potassium-competitive acid blockers. By contrast, in Western people, H. pylori-infected in only gastric antrum to pylorus, so the gastric acid would be increased by H. pylori infection. Therefore, species of Lactobacillus would be different between Japanese and Western people even though H. pylori infection is associated with the increase of Lactobacillus in both populations.
Generally, the development of atrophic gastritis in males is observed at a younger age than in females. Furthermore, the degree of atrophic gastritis increases with age, alongside the duration of H. pylori infection. Therefore, to eliminate the potential influence of gender or age on our results, we compared the relative abundance of Lactobacillus between males and females, and among the different age groups (grouped by decades) in H. pylori non-infected subjects. The results showed the rate of Lactobacillus was not affected by gender and age suggesting that the proportion of Lactobacillus in the gut microbiota could be associated with the severity of atrophic gastritis rather than both gender and age.
The mechanisms of the increase of Lactobacillus in microbiota due to a reduction of gastric acid have not been clearly elucidated. In this study, in H. pylori-infected subjects, the intestinal flora at species level was characterized by an increase of L. salivarius and a decrease of L. acidophilus. In H. pylori-infected subjects, the composition ratio of each Lactobacillus species was similar regardless of the severity of atrophic gastritis. Therefore, H. pylori infection initially influences the composition ratio of each Lactobacillus species in the gut microbiota before the progression of atrophic gastritis. Subsequently, the relative abundance of Lactobacillus increases following the decrease of gastric acid in Japanese subjects. However, in a previous German study, the intestinal flora was characterized by an increase in growth of L. acidophilus in H. pylori-infected patients (16). In that study, the microbiota was examined via bacterial culture, and some of H. pylori-infected patients had duodenal ulcers, indicating a higher gastric acid secretion. The uniqueness of the gut microbiome of Japanese might also cause the difference (15). It is necessary to examine the influence of H. pylori infection on the gut microbiota in different populations using next-generation analysis.
Several limitations associated with this study should be mentioned. First, we did not exclude the subjects with chronic inflammation of the gastrointestinal tract, including bacterial infection of the gut microbiota. However, prevalence rates of such diseases would be low, and the number of study subjects was sufficiently to counteract this. Second, instead of measuring gastric pH, PG I and PG I/II were used as markers of atrophic gastritis. As this study was based on a mass survey, it was not possible to measure individual gastric acid production and gastric pH. Indeed, a good correlation between serum PG levels and gastric acid secretion level has previously been shown (20). Third, we did not exclude patients with autoimmune gastritis from patients with severe atrophic gastritis. However, autoimmune gastritis is a rare disease in Japan, and the results of serum level of PGs indicated that none of the non-infected subjects had atrophic gastritis in this study. Therefore, influence of autoimmune gastritis on our results could be small.
In conclusion, the species of Lactobacillus in the human gut microbiota would be associated with H. pylori infection, and a significant increase of the rate of Lactobacillus appeared in subjects with severe atrophic gastritis. Efficacy of Lactobacilli as a probiotic might be influenced by H. pylori infection and subsequent atrophic gastritis, which associate with reduced gastric acid.
Ethics Statement
This study was performed in accordance with the ethical standards of the Declaration of Helsinki and approved by the ethics committee at Hirosaki University Medical Ethics Committee (2014-377).
Author Contributions
TS designed study, interpreted study results, and participated in drafting and editing of manuscript. CI performed statistical analysis and participated in drafting and editing of manuscript. DC assisted in study design and participated in the collection of materials. DC, TA, SN, and SF participated in the collection of materials and performed experiments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This study was based on the Iwaki Health Promotion Project as a project by Hirosaki University Graduate School of Medicine, in collaboration with Aomori Heath Evaluation and Promotion Center and Hirosaki City Office, Department of Health Promotion.
Funding
Supported in part by JSPS KAKENHI Grant Numbers 23659342, 17K09098, and 26460761.
References
1. Miller LE, Ouwehand AC, Ibarra A. Effects of probiotic-containing products on stool frequency and intestinal transit in constipated adults: systematic review and meta-analysis of randomized controlled trials. Ann Gastroenterol (2017) 30:629–39. doi:10.20524/aog.2017.0192
2. Beniwal RS, Arena VC, Thomas L, Narla S, Imperiale TF, Chaudhry RA, et al. A randomized trial of yogurt for prevention of antibiotic-associated diarrhea. Dig Dis Sci (2003) 48:2077–82. doi:10.1023/A:1021711204498
3. Plummer S, Weaver MA, Harris JC, Dee P, Hunter J. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol (2004) 7:59–62.
4. Hickson M, D’Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ (2007) 14:80. doi:10.1136/bmj.39231.599815.55
5. Wenus C, Goll R, Loken EB, Biong AS, Halvorsen DS, Florholmen J. Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur J Clin Nutr (2008) 62:299–301. doi:10.1038/sj.ejcn.1602718
6. Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, et al. Proton pump inhibitors affect the gut microbiome. Gut (2016) 65:740–8. doi:10.1136/gutjnl-2015-310376
7. Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut (2016) 65:749–56. doi:10.1136/gutjnl-2015-310861
8. Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut (2001) 48:765–73. doi:10.1136/gut.48.6.765
9. Hammond CE, Beeson C, Suarez G, Peek RM Jr, Backert S, Smolka AJ. Helicobacter pylori virulence factors affecting gastric proton pump expression and acid secretion. Am J Physiol Gastrointest Liver Physiol (2015) 309:G193–201. doi:10.1152/ajpgi.00099.2015
10. Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet (1995) 17:1525–8. doi:10.1016/S0140-6736(95)91084-0
11. Weck MN, Gao L, Brenner H. Helicobacter pylori infection and chronic atrophic gastritis: associations according to severity of disease. Epidemiology (2009) 20:569–74. doi:10.1097/EDE.0b013e3181a3d5f4
12. Kishikawa H, Nishida J, Ichikawa H, Kaida S, Takarabe S, Matsumkubo T, et al. Fasting gastric pH of Japanese subjects stratified by IgG concentration against Helicobacter pylori and pepsinogen status. Helicobacter (2011) 16:427–33. doi:10.1111/j.1523-5378.2011.00868.x
13. Shimoyama T, Aoki M, Sasaki Y, Matsuzaka M, Nakaji S, Fukuda S. ABC screening for gastric cancer is not applicable in a Japanese population with high prevalence of atrophic gastritis. Gastric Cancer (2012) 15:331–4. doi:10.1007/s10120-012-0141-x
14. Ishimura N, Owada Y, Aimi M, Oshima T, Kamada T, Inoue K, et al. No increase in gastric acid secretion in healthy Japanese over the past two decades. J Gastroenterol (2015) 50:844–52. doi:10.1007/s00535-014-1027-y
15. Nishijima S, Suda W, Oshima K, Kim SW, Hirose Y, Morita H, et al. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res (2016) 23(2):125–33. doi:10.1093/dnares/dsw002
16. Bühling A, Radun D, Müller WA, Malfertheiner P. Influence of anti-Helicobacter triple-therapy with metronidazole, omeprazole and clarithromycin on intestinal microflora. Aliment Pharmacol Ther (2001) 15:1445–52. doi:10.1046/j.1365-2036.2001.01033.x
17. Kawai T, Kawakami K, Kudo T, Ogiahara S, Handa Y, Moriyasu F. A new serum antibody test kit (E plate) for evaluation of Helicobacter pylori eradication. Intern Med (2002) 41:780–3. doi:10.2169/internalmedicine.41.780
18. Sato M, Shimoyama T, Takahashi R, Kajiyama H, Sano Y, Sakaedani N, et al. Characterization and usefulness of stool antigen tests using a monoclonal antibody to Helicobacter pylori catalase. J Gastroenterol Hepatol (2012) 27:23–8. doi:10.1111/j.1440-1746.2012.07066.x
19. Miki K, Ichinose M, Kawamura N, Matsushima M, Ahmad HB, Kimura M, et al. The significance of low serum pepsinogen levels to detect stomach cancer associated with extensive chronic gastritis in Japanese subjects. Jpn J Cancer Res (1989) 80:111–4. doi:10.1111/j.1349-7006.1989.tb02276.x
20. Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer (2006) 9:245–53. doi:10.1007/s10120-006-0397-0
21. Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and Archaea using next-generation sequencing. PLoS One (2014) 9:e105592. doi:10.1371/journal.pone.0105592
22. Hisada T, Endoh K, Kuriki K. Inter- and intra-individual variations in seasonal and daily stabilities of the human gut microbiota in Japanese. Arch Microbiol (2015) 197:919–34. doi:10.1007/s00203-015-1125-0
Keywords: Lactobacillus, Helicobacter pylori, atrophic gastritis, gut microbiota, pepsinogen
Citation: Iino C, Shimoyama T, Chinda D, Arai T, Chiba D, Nakaji S and Fukuda S (2018) Infection of Helicobacter pylori and Atrophic Gastritis Influence Lactobacillus in Gut Microbiota in a Japanese Population. Front. Immunol. 9:712. doi: 10.3389/fimmu.2018.00712
Received: 30 October 2017; Accepted: 22 March 2018;
Published: 06 April 2018
Edited by:
Shigeru Kamiya, Kyorin University, JapanReviewed by:
Xu Zhang, The Scripps Research Institute, United StatesKatsuhiro Mabe, Hokkaido University, Japan
Copyright: © 2018 Iino, Shimoyama, Chinda, Arai, Chiba, Nakaji and Fukuda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadashi Shimoyama, dHNpbW9AaGlyb3Nha2ktdS5hYy5qcA==
 Chikara Iino
Chikara Iino Tadashi Shimoyama
Tadashi Shimoyama Daisuke Chinda
Daisuke Chinda Tetsu Arai
Tetsu Arai Daisuke Chiba1
Daisuke Chiba1