
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 02 March 2018
Sec. Viral Immunology
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.00423
This article is part of the Research TopicLymph Node T Cell Dynamics and Novel Strategies for HIV CureView all 17 articles
Immunological inductive tissues, such as secondary lymphoid organs, are composed of distinct anatomical microenvironments for the generation of immune responses to pathogens and immunogens. These microenvironments are characterized by the compartmentalization of highly specialized immune and stromal cell populations, as well as the presence of a complex network of soluble factors and chemokines that direct the intra-tissue trafficking of naïve and effector cell populations. Imaging platforms have provided critical contextual information regarding the molecular and cellular interactions that orchestrate the spatial microanatomy of relevant cells and the development of immune responses against pathogens. Particularly in HIV/SIV disease, imaging technologies are of great importance in the investigation of the local interplay between the virus and host cells, with respect to understanding viral dynamics and persistence, immune responses (i.e., adaptive and innate inflammatory responses), tissue structure and pathologies, and changes to the surrounding milieu and function of immune cells. Merging imaging platforms with other cutting-edge technologies could lead to novel findings regarding the phenotype, function, and molecular signatures of particular immune cell targets, further promoting the development of new antiviral treatments and vaccination strategies.
Investigation of the human immune system in the context of infectious diseases has been accomplished primarily based on studies utilizing circulating cells. However, use of such biological material may not capture the in vivo timing or mechanisms governing the initiation and development of immune responses to pathogens at important anatomical sites, such as secondary lymphoid organs, mucosal-associated lymphoid tissues (MALTs), and mucosae. Therefore, the need for comprehensive analysis of tissues central to disease pathogenesis, and interactions between theses tissues, is of great importance. The application of multidimensional methodologies, like polyparametric flow cytometry, has provided critical information regarding the phenotype and functionality of tissue-resident immune cells, especially T and B cells (1–5). Despite their analytical power, these methodologies cannot address the tissue distribution/localization of lymphoid populations in vivo, as well as the anatomical context in which their highly dynamic interactions occur. On the other hand, tissue investigation using histopathological assays, like immunohistochemistry, has provided critical information regarding the impact of HIV/SIV on the organization of the human immune system at a tissue level (6–14).
Imaging technologies are continuingly advancing, with new hardware (i.e., new types of cameras, laser lines, hybrid detectors, etc.) and software, improving the quality of images obtained at the level of acquisition, segmentation, and deconvolution of cells. Furthermore, the availability of steadily increasing antibody specificities and appropriate labels/probes further facilitates the application of imaging technologies to biological material. The introduction of advanced imaging technologies, such as multispectral confocal (15) and multiphoton microscopy, as well as imaging mass cytometry, positron emission tomography (PET), and magnetic resonance imaging (MRI) (16–18), opens new opportunities for the investigation of molecular and cellular events at dimensions that range from the nanoscale to the entire body and for visualizing the dynamic changes occurring in living tissues and individuals. Furthermore, the availability of technologies like stimulated emission depletion microscopy (STED) can provide unprecedented resolution (~20–50 nm) using light microscopy (19) for the detailed analysis and quantification of molecular dynamics at a subcellular level (20). Therefore, the application of cutting-edge imaging technologies can provide substantial novel insights into host–pathogen interactions that are simply not feasible with other approaches (Table 1), which may be critical for the development of vaccines, especially those aiming to elicit broadly neutralizing antibodies, as well as for the discovery of novel immunotherapy targets to eliminate HIV.
Secondary lymphoid organs (i.e., lymph nodes and spleen) and MALT create an extended tissue network that provides a unique microenvironment for pathogen capture, antigen presentation, and induction of adaptive immune responses (21–23). The ex vivo and in vitro analysis of cells derived from such tissues using powerful methodologies like polyparametric flow cytometry and sequencing of sorted cell subsets has provided important information about the character and molecular profile of cells involved in the development of these responses (4, 15, 24, 25). The application of imaging technologies, however, can provide relevant information about cell populations in their “natural environment” and with respect to their spatial positioning, displacement, surrounding cells, and milieu microenvironment. Furthermore, estimating the possible role of parameters, like cell shape and polarization (26), in the biological process under investigation is impossible for cells removed from their natural tissue microenvironment. To this end, the combination of ex vivo organ culture models (27) with imaging analysis and whole-body in vivo studies would significantly increase our knowledge about the role of particular cells and soluble factors in HIV/SIV pathogenesis.
The compromised immune response against pathogens in subjects with genetic defects that affect the architecture and development of follicles demonstrates the importance of tissue integrity for an effective response against pathogens (28–30). It is well established that HIV/SIV infections are associated with extensive changes/damage of tissue architecture, especially in LNs and gut mucosa (31). Stromal cells, like fibroblastic reticular cells (FRCs) and follicular dendritic cells (FDCs), represent critical elements of the lymphoid tissue architecture, which are significantly affected by HIV/SIV (32–35), and because of their biology and function forming extended interdigitating networks within the follicular (FDC) (36) and extra-follicular (FRC) (37) areas, their isolation and in vitro analysis is challenging. Thus, imaging these stromal elements in their native intact tissue environments, with 3D volumetric analysis, will likely be essential to fully understand the importance of these networks in HIV/SIV infections. A comprehensive understanding of tissue perturbations in terms of cellularity and architecture will further elucidate defects in adaptive cellular responses and in the generation of antibody responses with functionalities that effectively control the virus, including broadly neutralizing antibodies.
The development of effective adaptive immune responses against pathogens is a multistep process that requires the orchestrated function of several cell types and soluble factors within the LN environment. A critical aspect of this process is the compartmentalization of immune cell subsets with different origins or maturation status, as well as the presence of chemokine gradients that direct this compartmentalization and trafficking of cells between and within areas of LN. For example, the development of high-affinity, antigen-specific B-cell responses requires interactions between CD4 + T cells and B cells in the follicle. The identification of human follicular helper CD4 + T cells (Tfh) revealed a highly specialized CD4 + T-cell subset with a unique phenotypic, functional, and molecular signature (38–40). Still, Tfh cells represent a heterogeneous cellular population with different combinations of expressed surface receptors, such as PD-1, CD150, and CD57 (4, 41, 42). Likewise, follicular B cells represent a diverse population with different phenotypic profiles depending on their localization in germinal cell areas [light and dark zone (43, 44)]. Analysis of these populations based on their phenotype using flow-cytometry assays has been particularly informative with respect to their relative frequencies and dynamics in human and animal disease models. However, their phenotype does not always indicate their localization within tissue microenvironments. For example, although the dark zone is the site where B-cell division takes place, many proliferating (Ki67 +) B cells can be found in the light zone, and under physiological conditions, Tfh subsets have a distinct localization pattern (Figure 1). This type of imaging analysis can provide additional unique information regarding the juxtaposition/clustering of Tfh cells and B cells, the “polarization” pattern of germinal centers, as well as the distribution of Tfh cells, B cells, and FDCs within these LN follicular areas. Investigation of the impact that HIV/SIV infection has on the microanatomy of tissue environments could provide information about the cellular and molecular mechanisms mediating the development of humoral responses, as well as the local interplay between the host and virus during HIV/SIV disease progress.
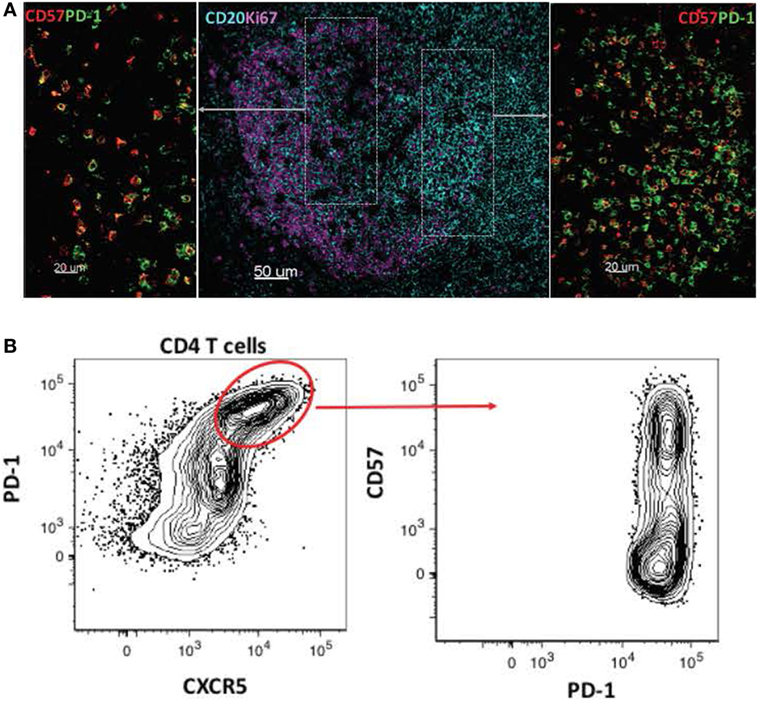
Figure 1. Heterogeneity of follicular cell populations. (A) Confocal images showing the relative distribution of proliferating B cells (CD20hi/dimKi67hi, CD20/cyan, and Ki67/magenta) and CD4 Tfh subsets (PD-lhiCD57hi, PD-lhiCD57lo, PD-1/green, and CD57/red) in a tonsillar follicular area. (B) Flow-cytometry plots showing the phenotype of tonsillar Tfh subsets based on the combined expression of PD-1, CXCR5, and CD57 surface receptors.
The introduction of novel imaging technologies could generate new perspectives regarding the role of particular immune cell subsets. Traditionally, the quality of CD8 + T cells in HIV/SIV infection has been evaluated based on (i) their capacity to produce multiple cytokines (poly-functionality) (45), (ii) the expression of an “exhausted” phenotype related to their function and survival/proliferative capacity (46–48), and (iii) their potential for killing infected targets, mainly through perforin/granzyme protein expression (49, 50). However, an effective CTL response requires trafficking of activated and differentiated CD8 + T cells in areas with HIV/SIV-infected cells, followed by their ability to sense, efficiently engage the infected cells and perform their CTL effector function. Development of imaging-based methods allowing for the evaluation of such biological processes/steps (10) could provide a comprehensive analysis of CTL responses in HIV/SIV infection and contribute to a holistic view of the efficiency of adaptive responses needed for virus elimination.
Classic immunohistochemistry studies have provided valuable information regarding the impact of HIV/SIV infection on the structure of tissues, such as LNs and gut mucosa. Early histological studies revealed lymphoid tissue pathologies (i.e., follicular hyperplasia, follicular lysis, and depletion and fibrosis) that are hallmarks of HIV infection (31, 51). Further work demonstrated a process of progressive deposition of fibrotic collagen, beginning early after HIV infection, driven by TGFβ regulatory CD4 + T cells (34, 52, 53), leading to the loss of stromal cells, like FRCs, and CD4 + T-cell populations (34). Additional significant changes take place in the follicular areas, manifested as enlarged/less-defined follicles and germinal centers, with presumably an important effect on Tfh cell dynamics.
Follicular helper CD4 + T cells represent a highly differentiated CD4 + T-cell subset with a unique phenotype (4, 40, 41, 54, 55) and molecular signature (4, 39), which provides critical help to follicular B cells during the development of B-cell responses against pathogens and immunogens (44). The chronic phase of HIV/SIV infection is characterized by accumulation of Tfh cells, at least in a group of individuals (4, 56, 57). Furthermore, SIV infection has a significant impact on the gene signature of Tfh cells, characterized by increased expression of IFNγ- and TGFβ-related genes (4). Imaging studies have facilitated the characterization/localization of Tfh cells within the follicular areas during HIV/SIV infection, based on the expression of surface receptors like PD-1 and CXCR5 (4, 8, 13, 58, 59). Furthermore, imaging analysis has revealed that Tfh cells populate different areas of the follicle (marginal zone, surrounding GC, or mainly within the light zone) (Figure 1) (4, 8, 58), presumably exposed to different local signals. Given the dramatic effect of HIV/SIV infection on the LN structure and follicular organization, imaging provides critical information about the distribution of Tfh cells within the follicular areas, as well as their proximity and engagement with B cells. The cellular and molecular mechanisms regulating the dynamics of Tfh cells during HIV/SIV infection are not well understood (4, 60). To this end, tissue imaging can contribute valuable information regarding:
1. The heterogeneity of follicular cells (Tfh subsets like Th1-like Tfh cells (61), dark zone vs. light zone B cells, etc.) and how these populations are impacted during different stages of infection.
2. The possible role of local immune activation/inflammation on T- and B-cell dynamics during HIV/SIV disease progression.
3. The impact of follicular damage/alteration (i.e., loss of FDC) on Tfh and B-cell dynamics.
4. The possible role of locally expressed cytokines (i.e., IL-21, TGFβ, IL-10) or chemokines (i.e., CXCL-13) on Tfh, B-cell dynamics.
5. The distribution of Tfh-infected cells and the dynamics of local HIV/SIV replication.
6. The impact of LN pathologies (i.e., fibrosis, etc.) on LN function (i.e., antigen capture, vaccine responses, etc.).
7. The impact of cART and HIV cure strategies on the FDC reservoir.
Chronic HIV/SIV infections are characterized by accumulation of CD8 + T cells in the LN and particularly in the follicle, a process referred to as “follicular lysis” (15, 24, 62). Imaging studies have shown that trafficking of virus-specific CD8 + T cells into the follicular area is relatively compromised (63–65). Flow-cytometry-based assays have shown that similar to Tfh cells, follicular CD8 + T cells are characterized by low expression of CCR7 and upregulated CXCR5, and have a unique transcriptional profile (15, 24). What regulates the trafficking of CD8 + T cells, particularly the cytotoxic effector cells, into the LN and distinct microenvironments, like follicles, is not well understood. It was recently shown that expression of viral proteins per se may not represent the main force behind this trafficking (15). Imaging analysis can provide critical information regarding the role of local inflammatory cells/signals as mediators of CD8 + T-cell trafficking in the follicular areas during HIV/SIV infection, potentially leading to novel targets for the in vivo manipulation of LN CD8 + T-cell dynamics. FRCs provide the cellular network for trafficking of T cells in the T-cell zones (37). Chronic HIV/SIV infection is associated with significant damage to both FRC (34) and follicular structures (31, 33). Whether this tissue damage creates an environment where T-cell trafficking becomes highly stochastic and/or dysfunctional is not known. Thus, imaging studies could be highly informative in addressing these unresolved questions, for example, by assessing the relationship between the magnitude of FDC changes, follicular lysis, and altered chemokine gradients on one hand with follicular CD8 + T-cell enrichment on the other hand.
Innate immune cells play an important role in HIV/SIV infections and disease at multiple levels, including (i) virus capture and dissemination (66), (ii) expression of pro- and anti-inflammatory mediators (i.e., IFNα/β, TNFα, IL6, IL10, etc.) (67–71), and (iii) expression of pro-inflammatory chemokines (i.e., IP-10, MCP, MIP-1α/β, etc.) (72, 73). Flow-cytometry studies have shown an increased recruitment of hyporesponsive monocyte/macrophages and plasmacytoid dendritic cells early after SIV infection that could affect the ability of IFNα production in the LN (74–76). Complementary to flow-cytometry data, imaging studies have revealed an accumulation of monocytic-lineage cells in areas surrounding the follicle and in close proximity to CD8 + T cells in chronic HIV infection (15) as well as in pathogenic SIV infection of rhesus macaques but not in non-pathogenic SIV infection in nature hosts (i.e., sooty mangabeys) (77). Furthermore, pharmacological manipulation of monocyte activation results in reduced recruitment of activated monocytes to the LN and reduced viral replication (78). More recently, tissue imaging has shown that infected macrophages could contribute to the rapid disease progression in SIV-infected non-human primate (NHP) infants (79). While monocytes/macrophages can clearly become infected with HIV/SIV, the relative contribution of infected monocytes/macrophages as long-lived viral reservoirs in vivo is still an open question. Novel, high-resolution imaging approaches allowing for the simultaneous detection of viral RNA and DNA could shed light upon this issue. Furthermore, it is not known if the viral dynamics of infected monocytes/macrophages occurs in a similar fashion in LNs from different anatomical sites, for example, comparing axillary and mesenteric LNs or MALT (80), and warrants further investigation.
Natural killer (NK) cells play an essential role in antiviral immunity, but knowledge of their function in secondary lymphoid organs is incomplete. Contrary to SIV-infected macaques, in situ approaches demonstrated that NK cells in secondary lymphoid organs from chronically SIVagm-infected African green monkeys (AGMs) were frequently CXCR5 + and entered and persisted in lymph node follicles where they seem to play a major role in viral reservoir control (81). The relative positioning/compartmentalization of innate cells and associated soluble factors could inform on the role of these cells in the generation and maintenance of effective adaptive immune responses during HIV/SIV.
Mucosal barriers are the body’s first defense against external pathogenic threats. Although they represent the boundary between the external environment and the host, mucosal surfaces are often the sites of pathogen transmission (82). In the context of HIV infection, mucosal surfaces represent the major routes of transmission, with the most relevant mucosal tissues being the genital mucosa and gastrointestinal tract (82). Imaging studies utilizing SIV NHP models have been absolutely instrumental in dissecting key aspects of HIV-1 transmission across mucosal surfaces and the early events surrounding mucosal infection, including (i) understanding the unique cellular composition and characteristics of different mucosal tissues and their susceptibility to viral transmission, (ii) defining the early host–viral dynamics within mucosal tissues, including characterizing the principal target cells in vivo, and (iii) demonstrating the process and principal pattern of viral dissemination and establishment (83–86).
Disruption of the intestinal barrier and subsequent microbial translocation and inflammation is one of the hallmarks of HIV/SIV pathogenesis and disease progression (87–89). Damaged epithelial integrity (90, 91), as well as the loss of relevant cells from the gut mucosa (92–94), has been associated with HIV/SIV pathogenesis. Imaging studies have been instrumental in investigating the impact of these tissue perturbations in the context of HIV/SIV infections. Besides the documentation of the magnitude of barrier damage, imaging studies have shown: (i) the possible role of gut macrophages with respect to their phagocytic activity (80, 91) or capacity to produce pro-inflammatory cytokines (95) in chronic immune activation and progression to AIDS, (ii) that blocking microbial translocation can reduce viral replication and dissemination in LNs (96), and (iii) that barrier damage and microbial translocation differentiate pathogenic and non-pathogenic SIV infections (91). Furthermore, tissue imaging has been very informative concerning the verification of animal models for SIV pathogenesis––such as the use of pigtail macaques (97) or experimental colitis as an alternative model to investigate the impact of barrier integrity in SIV pathogenesis (16). Similar to LNs, imaging studies will be instrumental in our understanding of the local interplay between the virus and innate/adaptive immunity.
Besides lymphoid organs, other tissues have also been shown to play a role in the pathogenesis of HIV/SIV infections. Imaging studies have been instrumental for our understanding of the CD3 + (98) and CD8 + T cell (99), NK (100) as well as myeloid (Kuppfer) cell (101) dynamics in liver during SIV infection. In situ hybridization imaging assays have also shown insufficient viral replication in liver (99, 101). Besides the liver, RNA in situ hybridization imaging has been widely used for the detection of cells harboring transcribed virus in several tissues including the following:
(1) adipose tissue and specifically in the stromal vascular fraction (6);
(2) lungs, where macrophages represent a main source of virus production in infant NHP (79, 102) with lung tissue damage associated with infection of interstitial rather than alveolar macrophages (103); and
(3) brain (104) in line with other assays showing that CNS macrophages represents a latent reservoir in cART-treated animals. Confocal imaging of protein markers has revealed the heterogeneity and possible role of monocytes/macrophages, especially recently infiltrating cells, in HIV/SIV encephalitis (105, 106). Increased frequency of perivascular proliferating macrophages (107) could account for the accumulation of macrophages in SIV-infected animals. CNS lesions, found in monkeys receiving cART, were associated with inflammation dominated by lymphocyte and low levels of SIV RNA in the brain (108). Complementary to these imaging studies, use of laser capture microdissection revealed a compartmentalization of viral sequences in brain from animals infected with a neurotropic virus (109). In addition to tissue imaging assays, MRI-based methodologies have been widely used for the study of HIV/SIV neuropahtogenesis (110–113) as well as the in vivo viral dynamics in the brain of experimental models (17).
A major obstacle for HIV eradication is the establishment of long-lived viral reservoirs, particularly in “immunologically privileged” areas, like B-cell follicles (114, 115). Therefore, the molecular characterization of cells contributing to these reservoirs, as well as their tissue topology, is of great importance in the development of novel strategies for virus reactivation and elimination. Sensitive PCR-based assays have contributed significantly to our knowledge regarding the dynamics/kinetics of virus replication, the efficacy of cART, and the characterization of cell subsets harboring actively transcribed or latent virus (116–119). Early studies have shown sequestration of viral RNA in follicles using in situ hybridization techniques (120). More recently, novel next-generation in situ hybridization platforms have been developed with great potential for the comprehensive analysis of viral reservoirs at a tissue level. These platforms allow for the detection of viral RNA (RNAscope) and/or viral DNA (DNAscope) (114, 121, 122). Merging this technology with multispectral confocal microscopy will allow for a comprehensive analysis of (i) the viral reservoir with respect to relevant molecular markers of cells harboring the virus, (ii) the local microenvironment (surrounding immune cells, inflammatory cells, cytokines/chemokines), and (iii) virus dynamics (based on the simultaneous detection of viral RNA, DNA, and viral particles).
In addition to identification of individual cells harboring virus at a tissue level, imaging assays have contributed significantly to our understanding of viral dynamics in vivo. Confocal imaging has provided important information regarding viral transmission across and infection in the female reproductive tract (86), as well as revealed that Th17-lineage CD4 + T cells as a preferential target for the virus early after vaginal inoculation (12). Application of technologies like whole-body immune-PET has provided additional insight into the distribution of virus among different organs in chronic SIV infection (123), as well as the impact of antiretroviral or immune-based treatments on viral dynamics (123, 124). Non-invasive whole-body imaging, although of relatively low resolution, provides a “real time” and non-invasive monitoring of viral or relevant immune cell dynamics and could guide the performance of tissue imaging assays for a high-resolution analysis of related cells. Additional information can be obtained from whole-body PET-TDM for drug distribution dynamics (125) allowing the identification of pharmacological sanctuaries, drug interactions and helping the optimization of drug delivery use and drug design.
Today, several imaging technologies and platforms are available for tissue analysis. Several factors should be taken into consideration regarding the choice of the most relevant platform, including the following:
(i) The scientific question under investigation: tissue cell composition and viral reservoirs [light microscopy, confocal microscopy, ion beam imaging (126), Laser Capture Microdissection (127)], subcellular structure and virus–host protein interactions (confocal microscopy, electron microscopy, high-resolution optical imaging technologies), or assessment of cellular and viral dynamics at organ or whole-body level [MRI (17), PET scan (123), confocal endoscopy].
(ii) The requirement for high-resolution, “volumetric” analysis or live imaging (two-photon microscopy) to address the biological process under investigation. The introduction of the two-photon intravital microscopy in the NHP SIV model could revolutionize the field of HIV/SIV pathogenesis and vaccine development by providing real time, in vivo measurements of immune cell trafficking, tissue cell dynamics, and interactions between host cells and virus.
(iii) The ability to simultaneously use multiple probes (dimensionality), allowing for the comprehensive analysis of several cells, proteins, and RNA/DNA sequence within the same imaged plane (confocal or imaging mass cytometry). Although high-resolution technologies like electron microscopy-based platforms or super resolution confocal microscopy can provide unprecedented information for the tissue, cell structure, and molecular dynamics, they are lacking their capacity to simultaneously use multiple probes, at least in their current form.
(iv) The potential for “fusion” with other high-throughput platforms. Although current confocal microscopy assays can visualize several probes simultaneously (15), the selection of probes/antibodies is hypothesis-driven. Merging multiplexed confocal microscopy assays with technologies like tissue imaging mass cytometry (128) could provide unique information at multiple levels, including unbiased pathway analysis, discovery of novel therapeutic molecular targets, and pharmacokinetics of antiretroviral regimens.
Modern imaging technologies allow for the acquisition of high-dimensional data. To foster new discoveries derived from such data sets, the development and application of sophisticated algorithms is required. Accurate tissue reconstruction (using advanced 3D tomography algorithms) (129, 130), quantitative analysis of imaging objects using platforms like histocytometry (15, 131), algorithms allowing for the fusion of imaging with other high-throughput platforms (128), as well as modeling tissue cell and virus dynamics based on imaging data could significantly improve our understanding of the highly complex tissue immunobiology, especially during HIV/SIV infection.
Although tissue imaging is a powerful tool, we should keep in mind that there are also limitations that could lead to misinterpretation of tissue immune dynamics. Limited access to tissue material, especially from human subjects, represents a major limitation for tissue analysis. Collecting images from one or two random tissue sections could potentially lead to inaccurate measurements (“sampling error”). Ideally, application of novel, large-volume imaging techniques, like optically cleared tissue imaging (132), could overcome the “sampling error” limitation. However, the need for multiple assays and measurements from usually limited tissue material precludes these types of imaging applications, especially when human tissues are under investigation. Therefore, one should be very cautious with the interpretation of imaging data generated from limited tissue sections. One common practice for the validation of imaging data could be their comparison to data derived from other types of assays, such as flow cytometry.
Imaging studies have significantly improved our understanding of cellular and molecular mechanisms for HIV/SIV pathogenesis both in humans and NHP SIV models (133). Several imaging studies have validated SIV infection of NHPs as models for HIV pathogenesis, including the illustration of early events resulting in HIV/SIV transmission (86, 134, 135), the documentation and role of gut mucosal barrier damage in HIV/SIV pathogenesis (89, 91), as well as the impact of infection in secondary lymphoid tissues (31). Particularly for lymph node dynamics, a similar profile for follicular CD4 + (4, 56, 57) and CD8 + T cells (15, 24) has been shown in infected humans and NHPs, while in situ hybridization assays have established the importance of these sites for virus persistence (64, 122). Given the difficulty in obtaining human tissues from different anatomical sites, the performance of imaging studies in SIV models will continue to provide unpresented information regarding the anatomical compartmentalization of these immune dynamics.
We are witnessing a boom of imaging technologies that expand our capacity for comprehensive spatial analysis of tissue cells and molecules with high definition. Given the complexity of tissue immunobiology, the performance of different imaging-based assays, as well as their merging with other high-throughput assays, is of great importance for the generation of high-dimensional data. Besides the characterization of virus and cells at the tissue level, imaging technologies could prove useful in the analysis of other biological parameters, such as metabolic status, monitoring of therapeutics (pharmacokinetic studies), or novel immunotherapies (i.e., administration of multi-specific antibodies). Although HIV/SIV infections lead to major changes in tissue architecture, imaging the immune system in infected humans and NHPs can potentially provide insight into the overall anatomy and organization of the immune system in disease and contribute to the generation of a human cellular atlas.
All authors have contributed equally to this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank the personnel of Tissue Analysis Core at VRC, NIAID for useful suggestions. This research was supported by the Intramural Research Program of the Vaccine Research Center, NIAID, National Institutes of Health, a CAVD (OP1032325) from the Bill and Melinda Gates Foundation and by the Oregon National Primate Research Center NIH (P51OD011092).
1. Bolton DL, McGinnis K, Finak G, Chattopadhyay P, Gottardo R, Roederer M. Combined single-cell quantitation of host and SIV genes and proteins ex vivo reveals host-pathogen interactions in individual cells. PLoS Pathog (2017) 13(6):e1006445. doi:10.1371/journal.ppat.1006445
2. De Rosa SC, Roederer M. Eleven-color flow cytometry. A powerful tool for elucidation of the complex immune system. Clin Lab Med (2001) 21(4):697–712, vii.
3. Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, et al. Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity (2017) 46(6): 1073–88.e6. doi:10.1016/j.immuni.2017.05.007
4. Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest (2012) 122(9):3281–94. doi:10.1172/JCI63039
5. Yamamoto T, Lynch RM, Gautam R, Matus-Nicodemos R, Schmidt SD, Boswell KL, et al. Quality and quantity of TFH cells are critical for broad antibody development in SHIVAD8 infection. Sci Transl Med (2015) 7(298):298ra120. doi:10.1126/scitranslmed.aab3964
6. Damouche A, Lazure T, Avettand-Fenoel V, Huot N, Dejucq-Rainsford N, Satie AP, et al. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog (2015) 11(9):e1005153. doi:10.1371/journal.ppat.1005153
7. Estes JD, Reilly C, Trubey CM, Fletcher CV, Cory TJ, Piatak M Jr, et al. Antifibrotic therapy in simian immunodeficiency virus infection preserves CD4+ T-cell populations and improves immune reconstitution with antiretroviral therapy. J Infect Dis (2015) 211(5):744–54. doi:10.1093/infdis/jiu519
8. Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol (2012) 188(7):3247–56. doi:10.4049/jimmunol.1103138
9. Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature (2005) 434(7037):1148–52. doi:10.1038/nature03513
10. Li Q, Skinner PJ, Ha SJ, Duan L, Mattila TL, Hage A, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science (2009) 323(5922):1726–9. doi:10.1126/science.1168676
11. Malleret B, Maneglier B, Karlsson I, Lebon P, Nascimbeni M, Perie L, et al. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood (2008) 112(12):4598–608. doi:10.1182/blood-2008-06-162651
12. Stieh DJ, Matias E, Xu H, Fought AJ, Blanchard JL, Marx PA, et al. Th17 cells are preferentially infected very early after vaginal transmission of SIV in macaques. Cell Host Microbe (2016) 19(4):529–40. doi:10.1016/j.chom.2016.03.005
13. Xu H, Wang X, Malam N, Lackner AA, Veazey RS. Persistent simian immunodeficiency virus infection causes ultimate depletion of follicular Th cells in AIDS. J Immunol (2015) 195(9):4351–7. doi:10.4049/jimmunol.1501273
14. Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science (1999) 286(5443):1353–7. doi:10.1126/science.286.5443.1353
15. Petrovas C, Ferrando-Martinez S, Gerner MY, Casazza JP, Pegu A, Deleage C, et al. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci Transl Med (2017) 9:373. doi:10.1126/scitranslmed.aag2285
16. Hao XP, Lucero CM, Turkbey B, Bernardo ML, Morcock DR, Deleage C, et al. Experimental colitis in SIV-uninfected rhesus macaques recapitulates important features of pathogenic SIV infection. Nat Commun (2015) 6:8020. doi:10.1038/ncomms9020
17. Song J, Cai Z, White AG, Jin T, Wang X, Kadayakkara D, et al. Visualization and quantification of simian immunodeficiency virus-infected cells using non-invasive molecular imaging. J Gen Virol (2015) 96(10):3131–42. doi:10.1099/jgv.0.000245
18. Wu WE, Tal A, Zhang K, Babb JS, Ratai EM, Gonzalez RG, et al. Structure-specific glial response in a macaque model of neuroAIDS: multivoxel proton magnetic resonance spectroscopic imaging at 3 Tesla. AIDS (2013) 27(16):2519–28. doi:10.1097/01.aids.0000433244.32105.96
19. Klar TA, Jakobs S, Dyba M, Egner A, Hell SW. Fluorescence microscopy with diffraction resolution barrier broken by stimulated emission. Proc Natl Acad Sci U S A (2000) 97(15):8206–10. doi:10.1073/pnas.97.15.8206
20. Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature (2009) 457(7233):1159–62. doi:10.1038/nature07596
21. Barone F, Patel P, Sanderson JD, Spencer J. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol (2009) 2(6):495–503. doi:10.1038/mi.2009.106
22. Costiniuk CT, Angel JB. Human immunodeficiency virus and the gastrointestinal immune system: does highly active antiretroviral therapy restore gut immunity? Mucosal Immunol (2012) 5(6):596–604. doi:10.1038/mi.2012.82
23. Gasteiger G, Ataide M, Kastenmuller W. Lymph node – an organ for T-cell activation and pathogen defense. Immunol Rev (2016) 271(1):200–20. doi:10.1111/imr.12399
24. Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavinger M, et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci U S A (2017) 114(8):1976–81. doi:10.1073/pnas.1621418114
25. Sankaran S, Guadalupe M, Reay E, George MD, Flamm J, Prindiville T, et al. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc Natl Acad Sci U S A (2005) 102(28):9860–5. doi:10.1073/pnas.0503463102
26. Russell S. How polarity shapes the destiny of T cells. J Cell Sci (2008) 121(Pt 2):131–6. doi:10.1242/jcs.021253
27. Grivel JC, Margolis L. Use of human tissue explants to study human infectious agents. Nat Protoc (2009) 4(2):256–69. doi:10.1038/nprot.2008.245
28. Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol (2006) 177(7):4927–32. doi:10.4049/jimmunol.177.7.4927
29. Tangye SG, Deenick EK, Palendira U, Ma CS. T cell-B cell interactions in primary immunodeficiencies. Ann N Y Acad Sci (2012) 1250:1–13. doi:10.1111/j.1749-6632.2011.06361.x
30. Warnatz K, Bossaller L, Salzer U, Skrabl-Baumgartner A, Schwinger W, van der Burg M, et al. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood (2006) 107(8):3045–52. doi:10.1182/blood-2005-07-2955
31. Estes JD. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol Rev (2013) 254(1):65–77. doi:10.1111/imr.12070
32. Orenstein JM, Feinberg M, Yoder C, Schrager L, Mican JM, Schwartzentruber DJ, et al. Lymph node architecture preceding and following 6 months of potent antiviral therapy: follicular hyperplasia persists in parallel with p24 antigen restoration after involution and CD4 cell depletion in an AIDS patient. AIDS (1999) 13(16):2219–29. doi:10.1097/00002030-199911120-00004
33. Pantaleo G, Graziosi C, Demarest JF, Cohen OJ, Vaccarezza M, Gantt K, et al. Role of lymphoid organs in the pathogenesis of human immunodeficiency virus (HIV) infection. Immunol Rev (1994) 140:105–30. doi:10.1111/j.1600-065X.1994.tb00867.x
34. Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest (2011) 121(3):998–1008. doi:10.1172/JCI45157
35. Zhang ZQ, Schuler T, Cavert W, Notermans DW, Gebhard K, Henry K, et al. Reversibility of the pathological changes in the follicular dendritic cell network with treatment of HIV-1 infection. Proc Natl Acad Sci U S A (1999) 96(9):5169–72. doi:10.1073/pnas.96.9.5169
36. Kranich J, Krautler NJ. How follicular dendritic cells shape the B-cell antigenome. Front Immunol (2016) 7:225. doi:10.3389/fimmu.2016.00225
37. Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol (2008) 181(6):3947–54. doi:10.4049/jimmunol.181.6.3947
38. Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol (2004) 173(1):68–78. doi:10.4049/jimmunol.173.1.68
39. Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood (2004) 104(7):1952–60. doi:10.1182/blood-2004-03-1206
40. Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med (2001) 193(12):1373–81. doi:10.1084/jem.193.12.1373
41. Sattarzadeh A, Diepstra A, Rutgers B, van den Berg A, Visser L. CD57+ T-cells are a subpopulation of T-follicular helper cells in nodular lymphocyte predominant Hodgkin lymphoma. Exp Hematol Oncol (2015) 4:27. doi:10.1186/s40164-015-0022-1
42. Wallin EF, Jolly EC, Suchanek O, Bradley JA, Espeli M, Jayne DR, et al. Human T-follicular helper and T-follicular regulatory cell maintenance is independent of germinal centers. Blood (2014) 124(17):2666–74. doi:10.1182/blood-2014-07-585976
43. Klein U, Tu Y, Stolovitzky GA, Keller JL, Haddad J Jr, Miljkovic V, et al. Gene expression dynamics during germinal center transit in B cells. Ann N Y Acad Sci (2003) 987:166–72. doi:10.1111/j.1749-6632.2003.tb06045.x
44. Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol (2012) 30:429–57. doi:10.1146/annurev-immunol-020711-075032
45. Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood (2006) 107(12):4781–9. doi:10.1182/blood-2005-12-4818
46. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature (2006) 443(7109):350–4. doi:10.1038/nature05115
47. Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med (2006) 203(10):2281–92. doi:10.1084/jem.20061496
48. Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med (2006) 12(10):1198–202. doi:10.1038/nm1482
49. Makedonas G, Hutnick N, Haney D, Amick AC, Gardner J, Cosma G, et al. Perforin and IL-2 upregulation define qualitative differences among highly functional virus-specific human CD8 T cells. PLoS Pathog (2010) 6(3):e1000798. doi:10.1371/journal.ppat.1000798
50. Roberts ER, Carnathan DG, Li H, Shaw GM, Silvestri G, Betts MR. Collapse of cytolytic potential in SIV-specific CD8+ T cells following acute SIV infection in rhesus macaques. PLoS Pathog (2016) 12(12):e1006135. doi:10.1371/journal.ppat.1006135
51. Vago L, Antonacci MC, Cristina S, Parravicini C, Lazzarin A, Moroni M, et al. Morphogenesis, evolution and prognostic significance of lymphatic tissue lesions in HIV infection. Appl Pathol (1989) 7(5):298–309.
52. Cockerham LR, Yukl SA, Harvill K, Somsouk M, Joshi SK, Sinclair E, et al. A randomized controlled trial of lisinopril to decrease lymphoid fibrosis in antiretroviral-treated, HIV-infected individuals. Pathog Immun (2017) 2(3):310–34. doi:10.20411/pai.v2i3.207
53. Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis (2007) 195(4):551–61. doi:10.1086/510852
54. Wang C, Hillsamer P, Kim CH. Phenotype, effector function, and tissue localization of PD-1-expressing human follicular helper T cell subsets. BMC Immunol (2011) 12:53. doi:10.1186/1471-2172-12-53
55. Xu H, Wang X, Lackner AA, Veazey RS. PD-1(HIGH) follicular CD4 T helper cell subsets residing in lymph node germinal centers correlate with B cell maturation and IgG production in rhesus macaques. Front Immunol (2014) 5:85. doi:10.3389/fimmu.2014.00085
56. Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest (2012) 122(9):3271–80. doi:10.1172/JCI64314
57. Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med (2013) 210(1):143–56. doi:10.1084/jem.20121932
58. Johansson-Lindbom B, Ingvarsson S, Borrebaeck CA. Germinal centers regulate human Th2 development. J Immunol (2003) 171(4):1657–66. doi:10.4049/jimmunol.171.4.1657
59. Schmitt N, Bustamante J, Bourdery L, Bentebibel SE, Boisson-Dupuis S, Hamlin F, et al. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood (2013) 121(17):3375–85. doi:10.1182/blood-2012-08-448902
60. Chowdhury A, Del Rio Estrada PM, Tharp GK, Trible RP, Amara RR, Chahroudi A, et al. Decreased T follicular regulatory cell/T follicular helper cell (TFH) in simian immunodeficiency virus-infected rhesus macaques may contribute to accumulation of TFH in chronic infection. J Immunol (2015) 195(7):3237–47. doi:10.4049/jimmunol.1402701
61. Velu V, Mylvaganam GH, Gangadhara S, Hong JJ, Iyer SS, Gumber S, et al. Induction of Th1-biased T follicular helper (Tfh) cells in lymphoid tissues during chronic simian immunodeficiency virus infection defines functionally distinct germinal center Tfh cells. J Immunol (2016) 197(5):1832–42. doi:10.4049/jimmunol.1600143
62. Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol (2016) 17(10):1187–96. doi:10.1038/ni.3543
63. Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, Ray MG, et al. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol (2007) 178(11):6975–83. doi:10.4049/jimmunol.178.11.6975
64. Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med (2015) 21(2):132–9. doi:10.1038/nm.3781
65. Li S, Folkvord JM, Rakasz EG, Abdelaal HM, Wagstaff RK, Kovacs KJ, et al. Simian immunodeficiency virus-producing cells in follicles are partially suppressed by CD8+ cells in vivo. J Virol (2016) 90(24):11168–80. doi:10.1128/JVI.01332-16
66. Ploquin MJ, Diop OM, Sol-Foulon N, Mortara L, Faye A, Soares MA, et al. DC-SIGN from African green monkeys is expressed in lymph nodes and mediates infection in trans of simian immunodeficiency virus SIVagm. J Virol (2004) 78(2):798–810. doi:10.1128/JVI.78.2.798-810.2004
67. Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol (2013) 190(7):3049–53. doi:10.4049/jimmunol.1203032
68. Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One (2011) 6(3):e17739. doi:10.1371/journal.pone.0017739
69. Lal G, Kulkarni N, Nakayama Y, Singh AK, Sethi A, Burrell BE, et al. IL-10 from marginal zone precursor B cells controls the differentiation of Th17, Tfh and Tfr cells in transplantation tolerance. Immunol Lett (2016) 170:52–63. doi:10.1016/j.imlet.2016.01.002
70. Tian Y, Mollo SB, Harrington LE, Zajac AJ. IL-10 regulates memory T cell development and the balance between Th1 and follicular Th cell responses during an acute viral infection. J Immunol (2016) 197(4):1308–21. doi:10.4049/jimmunol.1502481
71. Wu H, Chen Y, Liu H, Xu LL, Teuscher P, Wang S, et al. Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice. Eur J Immunol (2016) 46(5):1152–61. doi:10.1002/eji.201546094
72. Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol (2011) 89(2):207–15. doi:10.1038/icb.2010.158
73. Rabin RL, Alston MA, Sircus JC, Knollmann-Ritschel B, Moratz C, Ngo D, et al. CXCR3 is induced early on the pathway of CD4+ T cell differentiation and bridges central and peripheral functions. J Immunol (2003) 171(6):2812–24. doi:10.4049/jimmunol.171.6.2812
74. Bruel T, Dupuy S, Demoulins T, Rogez-Kreuz C, Dutrieux J, Corneau A, et al. Plasmacytoid dendritic cell dynamics tune interferon-alfa production in SIV-infected cynomolgus macaques. PLoS Pathog (2014) 10(1):e1003915. doi:10.1371/journal.ppat.1003915
75. Durudas A, Milush JM, Chen HL, Engram JC, Silvestri G, Sodora DL. Elevated levels of innate immune modulators in lymph nodes and blood are associated with more-rapid disease progression in simian immunodeficiency virus-infected monkeys. J Virol (2009) 83(23):12229–40. doi:10.1128/JVI.01311-09
76. Wonderlich ER, Wijewardana V, Liu X, Barratt-Boyes SM. Virus-encoded TLR ligands reveal divergent functional responses of mononuclear phagocytes in pathogenic simian immunodeficiency virus infection. J Immunol (2013) 190(5):2188–98. doi:10.4049/jimmunol.1201645
77. Tabb B, Morcock DR, Trubey CM, Quinones OA, Hao XP, Smedley J, et al. Reduced inflammation and lymphoid tissue immunopathology in rhesus macaques receiving anti-tumor necrosis factor treatment during primary simian immunodeficiency virus infection. J Infect Dis (2013) 207(6):880–92. doi:10.1093/infdis/jis643
78. Campbell JH, Burdo TH, Autissier P, Bombardier JP, Westmoreland SV, Soulas C, et al. Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. PLoS One (2011) 6(4):e18688. doi:10.1371/journal.pone.0018688
79. Sugimoto C, Merino KM, Hasegawa A, Wang X, Alvarez XA, Wakao H, et al. Critical role for monocytes/macrophages in rapid progression to AIDS in pediatric simian immunodeficiency virus-infected rhesus macaques. J Virol (2017) 91(17):e00379–17. doi:10.1128/JVI.00379-17
80. Swan ZD, Bouwer AL, Wonderlich ER, Barratt-Boyes SM. Persistent accumulation of gut macrophages with impaired phagocytic function correlates with SIV disease progression in macaques. Eur J Immunol (2017) 47(11):1925–35. doi:10.1002/eji.201646904
81. Huot N, Jacquelin B, Garcia-Tellez T, Rascle P, Ploquin MJ, Madec Y, et al. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat Med (2017) 23(11):1277–86. doi:10.1038/nm.4421
82. Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Curr Opin Immunol (2015) 36:22–30. doi:10.1016/j.coi.2015.06.004
83. Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med (2011) 62:127–39. doi:10.1146/annurev-med-080709-124959
84. Keele BF, Estes JD. Barriers to mucosal transmission of immunodeficiency viruses. Blood (2011) 118(4):839–46. doi:10.1182/blood-2010-12-325860
85. Smedley J, Turkbey B, Bernardo ML, Del Prete GQ, Estes JD, Griffiths GL, et al. Tracking the luminal exposure and lymphatic drainage pathways of intravaginal and intrarectal inocula used in nonhuman primate models of HIV transmission. PLoS One (2014) 9(3):e92830. doi:10.1371/journal.pone.0092830
86. Stieh DJ, Maric D, Kelley ZL, Anderson MR, Hattaway HZ, Beilfuss BA, et al. Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS Pathog (2014) 10(10):e1004440. doi:10.1371/journal.ppat.1004440
87. Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol (2012) 30:149–73. doi:10.1146/annurev-immunol-020711-075001
88. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med (2006) 12(12):1365–71. doi:10.1038/nm1511
89. Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol (2005) 5(10):783–92. doi:10.1038/nri1705
90. Canary LA, Vinton CL, Morcock DR, Pierce JB, Estes JD, Brenchley JM, et al. Rate of AIDS progression is associated with gastrointestinal dysfunction in simian immunodeficiency virus-infected pigtail macaques. J Immunol (2013) 190(6):2959–65. doi:10.4049/jimmunol.1202319
91. Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog (2010) 6(8):e1001052. doi:10.1371/journal.ppat.1001052
92. Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med (2004) 200(6):749–59. doi:10.1084/jem.20040874
93. Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol (2012) 5(6):646–57. doi:10.1038/mi.2012.38
94. Pallikkuth S, Micci L, Ende ZS, Iriele RI, Cervasi B, Lawson B, et al. Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. PLoS Pathog (2013) 9(7):e1003471. doi:10.1371/journal.ppat.1003471
95. Swan ZD, Wonderlich ER, Barratt-Boyes SM. Macrophage accumulation in gut mucosa differentiates AIDS from chronic SIV infection in rhesus macaques. Eur J Immunol (2016) 46(2):446–54. doi:10.1002/eji.201545738
96. Kristoff J, Haret-Richter G, Ma D, Ribeiro RM, Xu C, Cornell E, et al. Early microbial translocation blockade reduces SIV-mediated inflammation and viral replication. J Clin Invest (2014) 124(6):2802–6. doi:10.1172/JCI75090
97. Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol (2010) 3(4):387–98. doi:10.1038/mi.2010.14
98. Ahsan MH, Gill AF, Lackner AA, Veazey RS. Acute and chronic T cell dynamics in the livers of simian immunodeficiency virus-infected macaques. J Virol (2012) 86(9):5244–52. doi:10.1128/JVI.07080-11
99. Schmitz JE, Kuroda MJ, Veazey RS, Seth A, Taylor WM, Nickerson CE, et al. Simian immunodeficiency virus (SIV)-specific CTL are present in large numbers in livers of SIV-infected rhesus monkeys. J Immunol (2000) 164(11):6015–9. doi:10.4049/jimmunol.164.11.6015
100. Evans TI, Li H, Schafer JL, Klatt NR, Hao XP, Traslavina RP, et al. SIV-induced translocation of bacterial products in the liver mobilizes myeloid dendritic and natural killer cells associated with liver damage. J Infect Dis (2016) 213(3):361–9. doi:10.1093/infdis/jiv404
101. Ahsan MH, Gill AF, Alvarez X, Lackner AA, Veazey RS. Kinetics of liver macrophages (Kupffer cells) in SIV-infected macaques. Virology (2013) 446(1–2):77–85. doi:10.1016/j.virol.2013.07.026
102. Li Y, Kang G, Duan L, Lu W, Katze MG, Lewis MG, et al. SIV infection of lung macrophages. PLoS One (2015) 10(5):e0125500. doi:10.1371/journal.pone.0125500
103. Cai Y, Sugimoto C, Arainga M, Midkiff CC, Liu DX, Alvarez X, et al. Preferential destruction of interstitial macrophages over alveolar macrophages as a cause of pulmonary disease in simian immunodeficiency virus-infected rhesus macaques. J Immunol (2015) 195(10):4884–91. doi:10.4049/jimmunol.1501194
104. Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, et al. Brain macrophages in simian immunodeficiency virus-infected, antiretroviral-suppressed macaques: a functional latent reservoir. MBio (2017) 8(4):e1186–1117. doi:10.1128/mBio.01186-17
105. Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog (2010) 6(4):e1000842. doi:10.1371/journal.ppat.1000842
106. Soulas C, Conerly C, Kim WK, Burdo TH, Alvarez X, Lackner AA, et al. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol (2011) 178(5):2121–35. doi:10.1016/j.ajpath.2011.01.023
107. Filipowicz AR, McGary CM, Holder GE, Lindgren AA, Johnson EM, Sugimoto C, et al. Proliferation of perivascular macrophages contributes to the development of encephalitic lesions in HIV-infected humans and in SIV-infected macaques. Sci Rep (2016) 6:32900. doi:10.1038/srep32900
108. Mangus LM, Beck SE, Queen SE, Brill SA, Shirk EN, Metcalf Pate KA, et al. Lymphocyte-dominant encephalitis and meningitis in simian immunodeficiency virus-infected macaques receiving antiretroviral therapy. Am J Pathol (2018) 188(1):125–34. doi:10.1016/j.ajpath.2017.08.035
109. Matsuda K, Brown CR, Foley B, Goeken R, Whitted S, Dang Q, et al. Laser capture microdissection assessment of virus compartmentalization in the central nervous systems of macaques infected with neurovirulent simian immunodeficiency virus. J Virol (2013) 87(16):8896–908. doi:10.1128/JVI.00874-13
110. Hakkers CS, Arends JE, Barth RE, Du Plessis S, Hoepelman AI, Vink M. Review of functional MRI in HIV: effects of aging and medication. J Neurovirol (2017) 23(1):20–32. doi:10.1007/s13365-016-0483-y
111. Li CX, Herndon JG, Novembre FJ, Zhang X. A longitudinal magnetization transfer imaging evaluation of brain injury in a macaque model of neuroAIDS. AIDS Res Hum Retroviruses (2015) 31(3):335–41. doi:10.1089/aid.2014.0166
112. Li CX, Zhang X, Komery A, Li Y, Mao H, Herndon JG, et al. Longitudinal cerebral metabolic changes in pig-tailed macaques infected with the neurovirulent virus SIVsmmFGb. J Neurovirol (2014) 20(6):612–9. doi:10.1007/s13365-014-0286-y
113. Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, et al. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest (2005) 115(9):2534–45. doi:10.1172/JCI22953
114. Deleage C, Wietgrefe SW, Del Prete G, Morcock DR, Hao XP, Piatak M Jr, et al. Defining HIV and SIV reservoirs in lymphoid tissues. Pathog Immun (2016) 1(1):68–106. doi:10.20411/pai.v1i1.100
115. Miles B, Connick E. TFH in HIV latency and as sources of replication-competent virus. Trends Microbiol (2016) 24(5):338–44. doi:10.1016/j.tim.2016.02.006
116. Cohn LB, Silva IT, Oliveira TY, Rosales RA, Parrish EH, Learn GH, et al. HIV-1 integration landscape during latent and active infection. Cell (2015) 160(3):420–32. doi:10.1016/j.cell.2015.01.020
117. Hosmane NN, Kwon KJ, Bruner KM, Capoferri AA, Beg S, Rosenbloom DI, et al. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J Exp Med (2017) 214(4):959–72. doi:10.1084/jem.20170193
118. Reece JC, Martyushev A, Petravic J, Grimm A, Gooneratne S, Amaresena T, et al. Measuring turnover of SIV DNA in resting CD4+ T cells using pyrosequencing: implications for the timing of HIV eradication therapies. PLoS One (2014) 9(4):e93330. doi:10.1371/journal.pone.0093330
119. Saune K, Delaugerre C, Raymond S, Nicot F, Boineau J, Pasquier C, et al. Analytical sensitivity of three real-time PCR assays for measuring subtype B HIV-1 RNA. J Clin Virol (2013) 57(1):80–3. doi:10.1016/j.jcv.2012.12.017
120. Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature (1993) 362(6418):355–8. doi:10.1038/362355a0
121. Deleage C, Turkbey B, Estes JD. Imaging lymphoid tissues in nonhuman primates to understand SIV pathogenesis and persistence. Curr Opin Virol (2016) 19:77–84. doi:10.1016/j.coviro.2016.07.002
122. Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med (2017) 23(11):1271–6. doi:10.1038/nm.4411
123. Santangelo PJ, Rogers KA, Zurla C, Blanchard EL, Gumber S, Strait K, et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods (2015) 12(5):427–32. doi:10.1038/nmeth.3320
124. Byrareddy SN, Arthos J, Cicala C, Villinger F, Ortiz KT, Little D, et al. Sustained virologic control in SIV+ macaques after antiretroviral and alpha4beta7 antibody therapy. Science (2016) 354(6309):197–202. doi:10.1126/science.aag1276
125. Di Mascio M, Srinivasula S, Bhattacharjee A, Cheng L, Martiniova L, Herscovitch P, et al. Antiretroviral tissue kinetics: in vivo imaging using positron emission tomography. Antimicrob Agents Chemother (2009) 53(10):4086–95. doi:10.1128/AAC.00419-09
126. Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD, et al. Multiplexed ion beam imaging of human breast tumors. Nat Med (2014) 20(4):436–42. doi:10.1038/nm.3488
127. Tjernlund A, Burgener A, Lindvall JM, Peng T, Zhu J, Ohrmalm L, et al. In situ staining and laser capture microdissection of lymph node residing SIV gag-specific CD8+ T cells – a tool to interrogate a functional immune response ex vivo. PLoS One (2016) 11(3):e0149907. doi:10.1371/journal.pone.0149907
128. Van de Plas R, Yang J, Spraggins J, Caprioli RM. Image fusion of mass spectrometry and microscopy: a multimodality paradigm for molecular tissue mapping. Nat Methods (2015) 12(4):366–72. doi:10.1038/nmeth.3296
129. Arganda-Carreras I, Fernandez-Gonzalez R, Munoz-Barrutia A, Ortiz-De-Solorzano C. 3D reconstruction of histological sections: application to mammary gland tissue. Microsc Res Tech (2010) 73(11):1019–29. doi:10.1002/jemt.20829
130. Villinger C, Gregorius H, Kranz C, Hohn K, Munzberg C, von Wichert G, et al. FIB/SEM tomography with TEM-like resolution for 3D imaging of high-pressure frozen cells. Histochem Cell Biol (2012) 138(4):549–56. doi:10.1007/s00418-012-1020-6
131. Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity (2012) 37(2):364–76. doi:10.1016/j.immuni.2012.07.011
132. Li W, Germain RN, Gerner MY. Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D). Proc Natl Acad Sci U S A (2017) 114(35):E7321–30. doi:10.1073/pnas.1708981114
133. Sanders-Beer BE, Voronin Y, McDonald D, Singh A. Harnessing novel imaging approaches to guide HIV prevention and cure discoveries—a national institutes of health and global HIV vaccine enterprise 2017 meeting report. AIDS Res Hum Retroviruses (2018) 34(1):12–26. doi:10.1089/AID.2017.0216
134. Dinh MH, Anderson MR, McRaven MD, Cianci GC, McCoombe SG, Kelley ZL, et al. Visualization of HIV-1 interactions with penile and foreskin epithelia: clues for female-to-male HIV transmission. PLoS Pathog (2015) 11(3):e1004729. doi:10.1371/journal.ppat.1004729
Keywords: HIV, lymph nodes, mucosa, immune cells, T cells, imaging
Citation: Estes JD, LeGrand R and Petrovas C (2018) Visualizing the Immune System: Providing Key Insights into HIV/SIV Infections. Front. Immunol. 9:423. doi: 10.3389/fimmu.2018.00423
Received: 14 December 2017; Accepted: 16 February 2018;
Published: 02 March 2018
Edited by:
Leonidas Stamatatos, Fred Hutchinson Cancer Research Center, United StatesReviewed by:
Donald Sodora, Center for Infectious Disease Research, United StatesCopyright: © 2018 Estes, LeGrand and Petrovas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Constantinos Petrovas, cGV0cm92YXNjQG1haWwubmloLmdvdg==
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.