
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 13 March 2018
Sec. Inflammation
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.00302
Background: The mechanisms underlying the non-antimicrobial immunomodulatory properties of macrolides are not well understood.
Objectives: To systematically review the evidence for the immunomodulatory properties of macrolides in humans and to describe the underlying mechanism and extent of their influence on the innate and adaptive immune system.
Methods: A systematic literature search was done in MEDLINE using the OVID interface from 1946 to December 2016 according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA). Original articles investigating the influence of four macrolides (azithromycin, clarithromycin, erythromycin, and roxithromycin) on immunological markers in humans were included.
Results: We identified 22 randomized, controlled trials, 16 prospective cohort studies, and 8 case–control studies investigating 47 different immunological markers (186 measurements) in 1,834 participants. The most frequently reported outcomes were a decrease in the number of neutrophils, and the concentrations of neutrophil elastase, interleukin (IL)-8, IL-6, IL-1beta, tumor necrosis factor (TNF)-alpha, eosinophilic cationic protein, and matrix metalloproteinase 9. Inhibition of neutrophil function was reported more frequently than eosinophil function. A decrease in T helper (Th) 2 cells cytokines (IL-4, IL-5, IL-6) was reported more frequently than a decrease in Th1 cytokines (IL-2, INF-gamma).
Conclusion: Macrolides influence a broad range of immunological mechanisms resulting in immunomodulatory effects. To optimize the treatment of chronic inflammatory diseases by macrolides, further studies are necessary, particularly comparing different macrolides and dose effect relationships.
Macrolides are mainly used as antibiotics to treat respiratory, skin and soft tissue, and urogenital infections (1, 2). They derive from Streptomyces species and are characterized by a macrocyclic lactone ring, which is either 14- [erythromycin (ERM), clarithromycin (CAM) and roxithromycin (RXM)], 15- [azithromycin (AZM)], or 16-membered (spiramycin, josamycin, midecamycin) (3). The antimicrobial activity of macrolides results from inhibition of bacterial protein synthesis through reversible binding to the peptide exit tunnel of ribosomes (4).
In addition to their antibiotic activity, macrolides have immunomodulatory properties, which were first described soon after their introduction in the 1950s (3, 5–7). The concept of using macrolides primarily for their immunomodulatory activities was introduced in the 1970s (8). The seminal study that distinguished between macrolides’ antimicrobial and their immunomodulatory effects was in adults with diffuse panbronchiolitis (DPB) in whom treatment with ERM dramatically improved survival independent of bacterial colonization (9). These results encouraged further research on the use of macrolides for the treatment of other chronic inflammatory conditions (10–14).
The mechanisms underlying the non-antimicrobial effects of macrolides are less well understood. Aside from ribosomal-mediated inhibition of pathogen virulence factor production, a number of other mechanisms have been proposed, including action on host immunity.
The objective of this review was to systematically summarize studies which investigated immunomodulatory properties of macrolides in humans and to describe the underlying mechanism and extent of their influence on the innate and adaptive immune system.
This review was done according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) (15). A literature review was done in December 2016 searching MEDLINE using the OVID interface from 1946 to 2016 using the search terms: (macrolide OR azithromycin OR clarithromycin OR erythromycin OR roxithromycin) AND (anti-inflammatory OR immunomodulatory OR immunolides) without any language limitations or limitation of study design (Figure 1). Only studies in humans, in which the participants received one of the four mentioned macrolides and which investigated immunological markers involved in inflammation were included. Studies reporting clinical endpoints only or studies in which macrolides were investigated for their antimicrobial activity were excluded. References were hand-searched for additional publications. Search results were independently screened by one reviewer, and checked by a second reviewer. Potentially eligible full-text articles were assessed according to our inclusion and exclusion criteria. The following variables were extracted from the included studies: year of study, country, study design, number of participants, age of participants, underlying disease, type, dose and duration of macrolide use, type of samples collected, and measured immune markers. Changes were classified as being significant when the p-value was ≤0.05.
We identified 2,107 studies, of which 45 were included in the final analysis; 22 randomized, controlled trials, 16 prospective cohort studies, and 7 case–control studies (Figure 1). Studies originated from 17 countries (Japan n = 12, United States of America n = 6, China n = 4, Australia n = 4, United Kingdom n = 4, Turkey n = 2, Serbia n = 2, Croatia n = 2, and one each from Belgium, Canada, Greece, the Netherlands, Italy, South Korea, Russia, Sweden, and Switzerland) and included a total of 1,834 participants. Six studies, including 423 participants, were done in children and adolescents (<18 years of age). Details of all studies including a risk of bias analysis are summarise in Table 1 and Table 2.
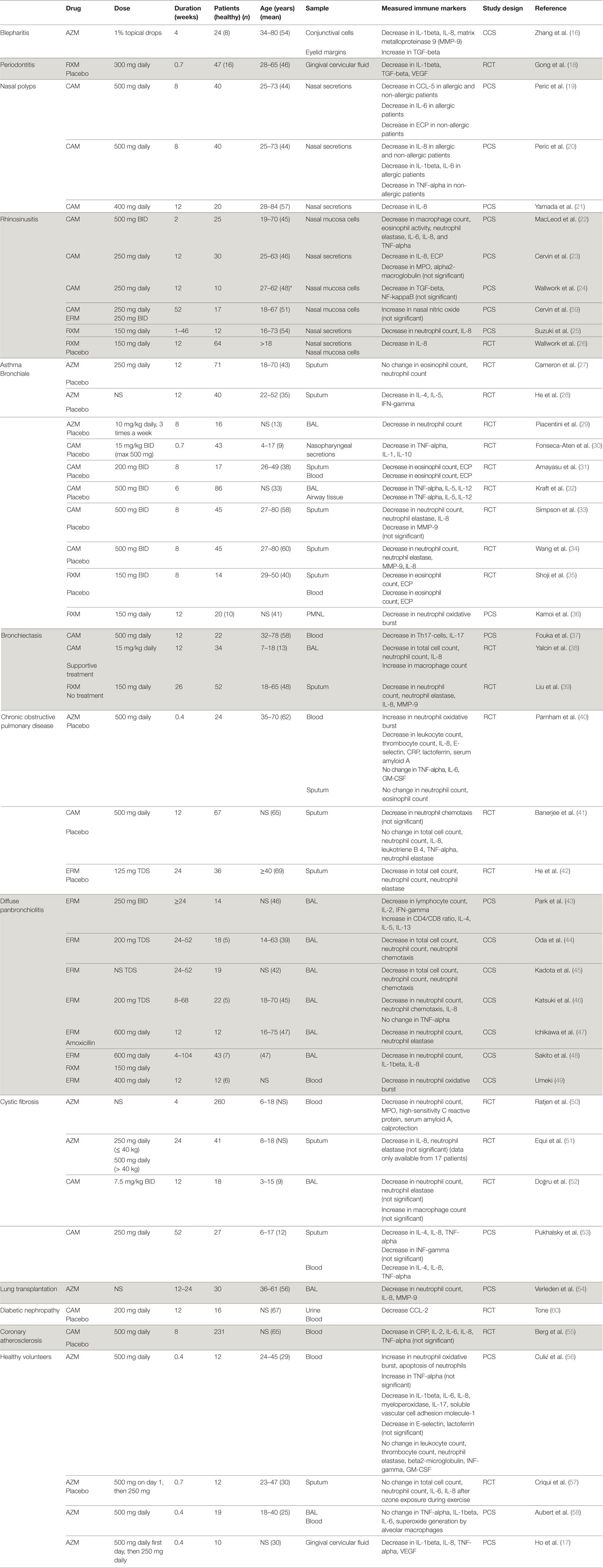
Table 1. Macrolide-induced changes in immunological markers in 45 studies in humans categorized by disease (NS = not stated).
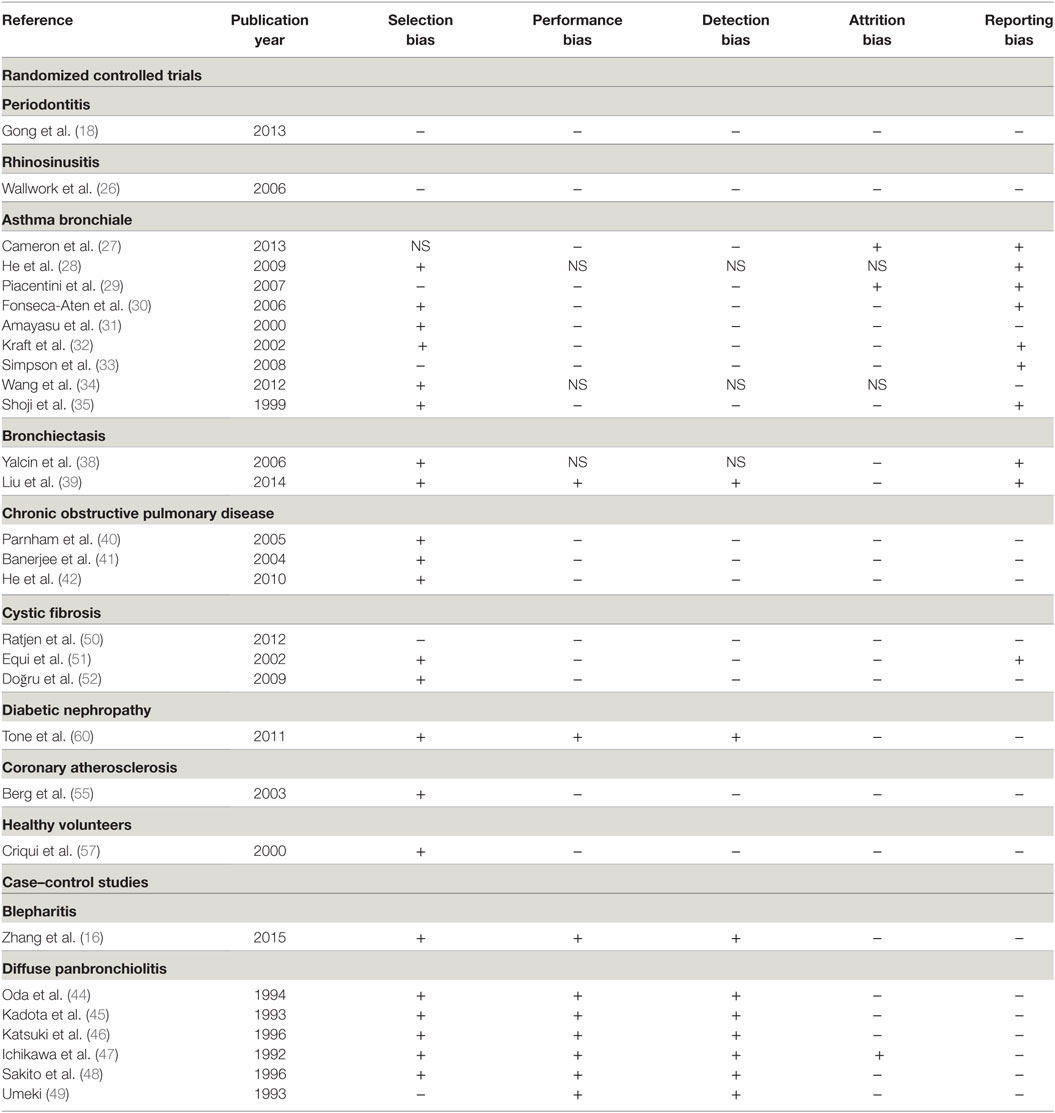
Table 2. Risk of bias summary of the randomized controlled trials and case–control studies included in the review (NS = not stated).
A total of 47 different immunological markers were investigated. On average, four markers were investigated per study resulting in a total of 186 measurements (Table 3; Figure 2). The immunological markers were classified into groups: cell counts (n = 9 markers/41 total measurements), neutrophil function (n = 6/25), eosinophil function (n = 2/7), macrophage function (n = 1/1), cytokine concentrations (n = 16/81), inflammatory proteins (n = 6/8), cell adhesion molecules (n = 2/3), molecules involved in inflammatory signaling pathway (n = 1/1), and other markers (n = 5/5, alpha-2-macroglobulin, beta-2-microglobulin, high-sensitivity C reactive protein, calprotectin, nasal nitric oxide).
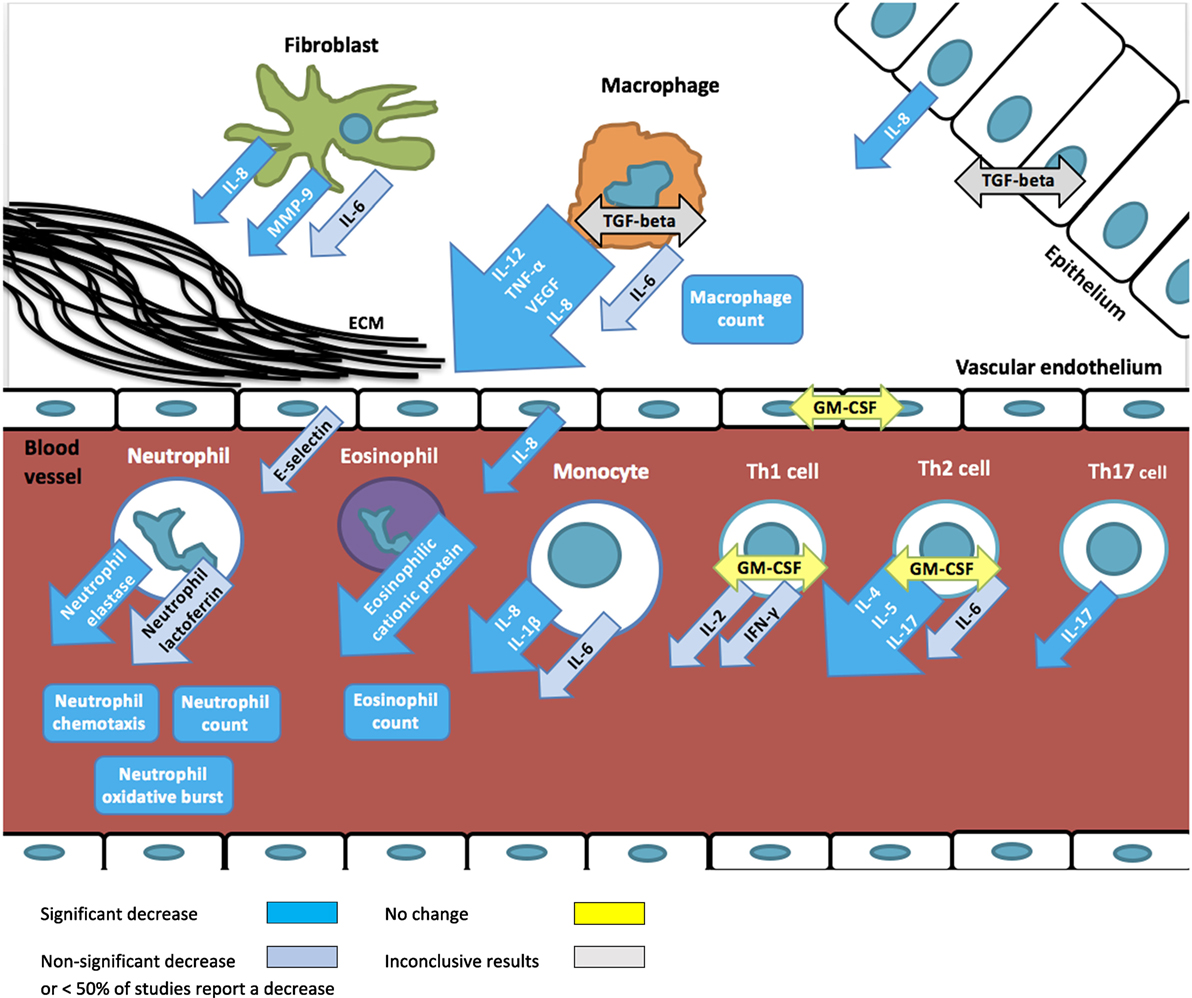
Figure 2. Overview of immunomodulatory effects of macrolides based on studies summarized in Table 1. Arrows depict excreted proteins, boxes depict cell counts or functions.
Overall, a decrease in immunological markers, number, or function was more frequently observed than an increase (139 measurements vs 19). No change of immunological markers reported in 11 immunological markers (28 measurements) in 7 studies. The most frequently reported macrolide-induced changes were a decrease in interleukin (IL)-8 concentration (n = 21), neutrophil count (n = 15), tumor necrosis factor-alpha (TNF-alpha) (n = 9), neutrophil elastase (n = 8), IL-1beta (n = 7), eosinophilic cationic protein (ECP, n = 6), IL-6 (n = 5), matrix metalloproteinase 9 (MMP-9) (n = 5), and oxidative burst activity (n = 5).
Immunomodulatory effects were investigated for four types of macrolides, including CAM (n = 73), AZM (n = 69), ERM (n = 27), and RXM (n = 17). AZM was more frequently associated with no influence on the immunological markers investigated (21/69) compared to any of the other macrolides (Table 4).
In the following, the immunomodulatory properties of macrolides are summarized and categorized by the disease in which they were investigated (Table 1).
Blepharitis is a common chronic inflammation of the eye lid leading to dry, itchy, and erythematous eyes. Anterior blepharitis is often associated with bacterial infections, while posterior blepharitis is linked to dysfunction of Meibomian glands. Many studies report clinical improvement in patients with blepharitis treated with topical AZM, due to a decrease in secretions and plugging of the Meibomian glands but did not investigate the underlying immunological mechanisms. The one study which did investigate immunological markes shows that concentrations of IL-1beta, IL-8, and MMP-9 in conjunctival cells of patients with blepharitis are higher than in healthy controls (16). Concentrations of these cytokines decrease with local AZM treatment, but return to pre-treatment levels after discontinuation (16).
Periodontitis is an inflammatory process of the gums with a complex pathogenesis including microorganisms as well as neutrophils, macrophages and fibroblasts. One key immunological mechanism underlying the pathogensis of periodontitis has been described as a TNF-alpha-induced increase in vascular endothelial growth factor (VEGF) leading to an aberrant angiogenesis (61). Both AZM and RXM decreased TNF-alpha and VEGF concentrations as well as other cytokines including IL-1beta, IL-8, and transforming growth factor beta (TGF-beta) in gingival crevicular fluid (17, 18). Since oral bacteria play an important role in periodontitis, however, some of the some of the benefits of macrolides may be attributable to antimicrobial rather than to immunomodulatory effects.
Chronic rhinosinusitis (CRS) with nasal polyps is characterized by a T helper (Th) 2 cells-dominated inflammation with upregulation of IL-4, IL-5, and IL-13 and an increase in eosinophil count, ECP, and immunoglobulin E. CRS without nasal polyps is characterized by Th1-dominated inflammation with upregulation of IL-2, TGF-beta, and IFN-gamma. Studies in patients with CRS treated with CAM and RXM show a significant reduction in macrophage, neutrophil, and eosinophil counts and concentrations of neutrophil elastase, ECP, CC-chemokine ligand-5 (CCL-5), IL-1beta, IL-6, IL-8, interferon (IFN)-gamma, TNF-alpha, myeloperoxidase (MPO), and alpha-macroglobulin in nasal secretions (19–23, 25, 26, 62). One of the postulated mechanisms by which macrolides inhibit the development of nasal polyps is through their anti-oxidative effects inhibiting the TGF-beta-induced production of reactive oxygen species (24). However, the immunomodulatory mechanisms differ in allergic and non-allergic nasal polyposis patients. While CAM reduces IL-6 and CCL-5 in all patients, it reduces IL-1beta and IL-6 only in patients with allergic CRS and TNF-alpha and ECP only in patients with non-allergic CRS (19, 20).
Asthma is characterized by chronic airway inflammation, reversible airway obstruction, and airway hyper-responsiveness. In eosinophilic asthma, eosinophils, mast cells, and Th2-mediated inflammation play an important role. Concentrations of IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, vascular cell adhesion molecule-1, CC chemokines, and granulocyte macrophage colony-stimulating factor (GM-CSF) are elevated. In severe asthma, in addition to eosinophils, increased neutrophils and IL-8 concentrations are found in airways. In patients with asthma, AZM, CAM, and RXM decrease eosinophil and neutrophil counts, inhibit neutrophil migration and oxidative burst activity in phagocytes, decrease concentrations of neutrophil elastase, ECP, IL-1, IL-4, IL-5, IL-8, IL-10, IL-12, MMP-9, TNF-alpha, and INF-gamma in nasopharyngeal secretions, sputum, or bronchoalveolar lavage (BAL) samples (27–30, 32–36). In addition, CAM and RXM also decrease the eosinophil counts and concentrations of ECP in blood and inhibit oxidative burst activity in phagocytes (31, 35, 36).
Bronchiectasis is characterized by permanent enlargement of bronchi and cytokines play an important role in the pathogenesis. In BAL samples of patients with bronchiectasis, elevated concentrations of IL-1beta and IL-8, as well as Th17-cytokines (IL-17A and IL-23), are found. In this setting, CAM and RXM lead to a decrease in total cell and neutrophil counts, concentrations of neutrophil elastase, IL-8, and MMP-9 in sputum or BAL of patients with bronchiectasis (38, 39). Interestingly, in BAL samples, these drugs significantly increase macrophage counts (38). Furthermore, macrolides lead to a decrease in peripheral blood Th17 cells and IL-17 concentrations (37).
Chronic obstructive pulmonary disease (COPD) is characterized by chronic inflammation of lung parenchyma and peripheral airways with an increase in alveolar macrophages, neutrophils, T cells (predominantly Th1-, and Th17- cells), and innate lymphoid cells. These cells, as well as structural cells, such as epithelial cells, endothelial cells, and fibroblasts, secrete a variety of pro-inflammatory cytokines. Although most patients with COPD have a predominantly neutrophilic inflammation, some also have elevated eosinophil counts in sputum. Oxidative stress plays a key role in COPD, and can result in activation of the pro-inflammatory transcription factor nuclear factor (NF)-kappaB. Moreover, COPD is associated with increased apoptosis and defective phagocytosis in the airways. In patients with COPD, IL-1beta, IL-4, IL-8, and TNF-alpha concentrations in blood are elevated, while IL-10 concentrations are lower compared to healthy adults. In patients with COPD, AZM leads to a decrease in white blood cell and platelet counts and concentrations of CRP, IL-8, E-selectin, and lactoferrin in blood (40). By contrast, macrolides increase neutrophil oxidative burst and neutrophil glutathione peroxidase activity in blood (40). In the sputum of COPD patients, CAM and ERM lead to a significant decrease in total cell and neutrophil count and inhibit neutrophil chemotaxis and decrease concentrations of neutrophil elastase (41, 42).
Diffuse panbronchiolitis (DPB) is a chronic distal airway inflammation characterized by diffuse micronodular pulmonary lesions mostly consisting of neutrophils. Neutrophils and epithelial cells produce IL-8, which is an important chemotactic factor to attract more neutrophils. The neutrophil count in BAL samples of patients with DPB correlates to the concentrations of IL-1beta and IL-8 (48). ERM reduces IL-1beta concentrations in BAL samples of patients with DPB which leads to a subsequent reduction of IL-8 concentrations and a decrease in neutrophil count and neutrophil chemotactic activity (44–48, 63). Furthermore, ERM treatment also results in a decrease in lymphocyte count, IL-2, interferon-gamma, and to increase in CD4/CD8 ratio, IL-4, IL-5, IL-13 in BAL samples of patients with DPB (43).
In patients with cystic fibrosis (CF), chronic airway inflammation results from cytokines secreted by epithelial and immune cells, which leads to neutrophil influx into airways. The release of neutrophil proteases, including neutrophil elastase, contributes to the development of bronchiectasis. Sustained inflammation is mainly due to an increase in the transcription of NF-kappaB activity, which leads to an increase in IL-8 production. These immunological mechanisms are influenced by AZM and CAM, which in CF-patients lead to a decrease in neutrophil count, concentrations of neutrophil elastase, IL-4, IL-8, TNF-alpha, and INF-gamma, and to an increase in numbers of macrophages in BAL samples or in sputum (51–53). In CF-patients macrolides also lead to a decrease in neutrophil count, concentrations of IL-4, IL-8, TNF-alpha, MPO, high-sensitivity C reactive protein, serum amyloid A, and calprotectin in blood (50, 53).
Macrolides are important therapeutic options in the treatment of many chronic inflammatory diseases because of their immunomodulatory effects. To understand the mechanisms underlying these effects, we reviewed all human studies that analyzed the influence of macrolides on immunological markers. The non-antimicrobial effects of macrolides are extensive and range from changes in cell counts and function, up- and downregulation of cytokine production to expression of adhesion molecules.
The most frequently and consistently reported immunomodulatory effect of macrolides is a reduced neutrophilic inflammation. Reduced numbers of neutrophils and inhibition of neutrophilic function lead to lower concentrations of neutrophil elastase and IL-8, and ultimately to a decrease in tissue injury. Furthermore, macrolides also reduce IL-1beta concentrations, another key mediator of the inflammatory response that is most abundantly produced by monocytes and macrophages. Evidence from animal and in vitro studies show that the inhibition of the key pro-inflammatory cytokines IL-8 and IL-1beta results from macrolides’ ability to alter intracellular signaling, particularly through the inhibition of NF-kappaB activation and expression of activator protein-1 (64–66). Notably, this effect has been observed in the absence of an infectious agent.
On the basis of these observed in vitro immunological effects of macrolides, patients with diseases mediated by neutrophilic inflammation such as periodontitis, severe asthma, DPB, bronchiectasis, COPD, and CF should benefit from treatment with this class of antibiotics. Indeed, clinically beneficial effects have been shown in randomized controlled studies in patients with COPD and CF with improved symptom scores, respiratory function and decreased frequency of exacerbations (67–69). For DPB, bronchiectasis and asthma, however, there is an absence of randomized controlled studies showing clinical beneficial effects of macrolides (70–72).
Macrolides are more commonly and consistently reported to inhibit neutrophilic than eosinophilic function. This is consistent with clinical studies that show patients with eosinophil-driven chronic inflammatory diseases associated with increased IgE (such as CRS or atopic asthma) have significantly lower improvement rates with macrolide treatment than those with normal serum IgE (26, 62, 73). Although the effect of macrolides on eosinophils has been less commonly investigated, a few studies report decreased eosinophil counts, and concentration of ECP (a ribonuclease secreted by eosinophils responsible for local cytotoxic effect). This suggests that there may be a role for the use of macrolides in allergic chronic inflammatory diseases (43, 74, 75). The possible influence of macrolides on eosinophilic inflammation is further supported by the finding that Th2 cytokines, such as IL-4 and IL-5, are more frequently reduced than Th1 cytokines, such as IL-2 and INF-gamma (19, 20, 22, 32, 42, 43, 53, 55, 56). The stronger effect of macrolides on Th2 compared with Th1 responses is further supported by evidence from animal and in vitro studies (74, 75). However, some of the anti-inflammatory effects might also be explained through their antibiotic effect on (undiagnosed) pathogens which trigger and sustain inflammation.
It is likely that immunomodulatory effects vary between different macrolides. Although some studies included more than one macrolide, none of the human studies directly compared different macrolides. Interestingly, AZM was less frequently associated with changes in measured immunological markers compared to the other macrolides. However, most of these studies were either in healthy volunteers or AZM was administered for only a few days (56–58, 76). By contrast, clinical studies in patients with CF suggest that AZM, but not CAM, leads to an improvement in respiratory function and reduction in pulmonary exacerbations (69, 77). In vitro studies comparing the immunomodulatory effects of different macrolides suggest that CAM has less immunomodulatory activity compared to other macrolides. For example, RXM, but not CAM or ERM, was shown to decreased chemotaxis of Th1 and Th2 cells (78). Similarly, CAM had a significantly weaker effect on reducing IL-6 production by human macrophages than ERM (79). Furthermore, another study showed that AZM, but not CAM or RXM, inhibits IL-1alpha and IL-1beta production (80).
Immunomodulatory effects of macrolides have been described with the recommended dose for antimicrobial treatment. Macrolides have excellent tissue penetration compared to other classes of antibiotics resulting in tissue concentrations generally exceeding serum concentrations (except for RXM). For the immunomodulatory effects macrolides’ ability to accumulate in neutrophils and macrophages is particularly important. Concentrations in macrophages have been shown to be 400- to 800-fold higher compared to serum for CAM and AZM and 5- to 100-fold higher in tissue compared to serum for ERM, CAM and AZM (81–85). This drug accumulation in immune cells may result in immunomodulatory effects occurring at lower doses and lasting longer compared to the antimicrobial effects. The relationship between macrolide dose and immunomodulatory effect is, therefore, an interesting avenue for future research.
The main limitation of this review is the heterogeneity of study populations, underlying diseases, type of macrolide and methods used to assess the immunomodulatory effect. A further limitation is selection and reporting bias and based on study types other biases including carry-over effect in cross-over trials and recall bias in case–control studies.
In summary, there is substantial evidence that macrolides exhibit immunomodulatory effects through inhibition of neutrophilic inflammation and macrophage activation. However, there is considerable heterogeneity between studies and in the immunological markers measured. Further studies will help delineate the exact mechanisms underlying the immunomodulatory properties of macrolides and the relative activity of different macrolides. This will enable the optimal use of this class of antibiotics in the treatment of chronic inflammatory diseases.
PZ and NC designed the study. PZ drafted the initial manuscript and approved the final manuscript as submitted. PZ, VZ, and NR did the risk of bias analysis. VZ, NC, and NR critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
PZ was supported by a Melbourne International Research Scholarship and a scholarship from the Ettore-Rossi-Foundation.
1. Piscitelli SC, Danziger LH, Rodvold KA. Clarithromycin and azithromycin: new macrolide antibiotics. Clin Pharm (1992) 11(2):137–52.
2. Retsema J, Girard A, Schelkly W, Manousos M, Anderson M, Bright G, et al. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother (1987) 31(12):1939–47. doi:10.1128/AAC.31.12.1939
3. Zuckerman JM. Macrolides and ketolides: azithromycin, clarithromycin, telithromycin. Infect Dis Clin North Am (2004) 18(3):621–49. doi:10.1016/j.idc.2004.04.010
4. Tenson T, Lovmar M, Ehrenberg M. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J Mol Biol (2003) 330(5):1005–14. doi:10.1016/S0022-2836(03)00662-4
5. Poliak MS. [Content of monomycin in bile and its effectiveness in inflammatory diseases and surgery of the biliary tract]. Antibiotiki (1963) 8:83–7.
6. Parfenova EN, Ryviakova EV. [Use of erythromycin in non-specific inflammatory diseases of the Urogenital System]. Urol Mosc (1963) 28:29–31.
7. Gluzman IS. [Erythromycin ointment therapy of inflammatory diseases of eyelid, conjunctiva and cornea. (Clinico-Experimental Studies)]. Oftalmol Zh (1964) 19:450–4.
8. Plewig G, Schopf E. Anti-inflammatory effects of antimicrobial agents: an in vivo study. J Invest Dermatol (1975) 65(6):532–6. doi:10.1111/1523-1747.ep12610281
9. Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med (1998) 157(6 Pt 1):1829–32. doi:10.1164/ajrccm.157.6.9710075
10. Hahn DL, Grasmick M, Hetzel S, Yale S, AZMATICS (AZithroMycin-Asthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med (2012) 25(4):442–59. doi:10.3122/jabfm.2012.04.110309
11. Koutsoubari I, Papaevangelou V, Konstantinou GN, Makrinioti H, Xepapadaki P, Kafetzis D, et al. Effect of clarithromycin on acute asthma exacerbations in children: an open randomized study. Pediatr Allergy Immunol (2012) 23(4):385–90. doi:10.1111/j.1399-3038.2012.01280.x
12. Clement A, Tamalet A, Leroux E, Ravilly S, Fauroux B, Jais JP. Long term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled trial. Thorax (2006) 61(10):895–902. doi:10.1136/thx.2005.057950
13. Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet (2012) 380(9842):660–7. doi:10.1016/S0140-6736(12)60953-2
14. Sadreddini S, Noshad H, Molaeefard M, Moloudi R, Ardalan MR, Ghojazadeh M. A double blind, randomized, placebo controlled study to evaluate the efficacy of erythromycin in patients with knee effusion due to osteoarthritis. Int J Rheum Dis (2009) 12(1):44–51. doi:10.1111/j.1756-185X.2009.01379.x
15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (2009) 339:b2700. doi:10.1136/bmj.b2700
16. Zhang L, Su Z, Zhang Z, Lin J, Li DQ, Pflugfelder SC. Effects of azithromycin on gene expression profiles of proinflammatory and anti-inflammatory mediators in the eyelid margin and conjunctiva of patients with meibomian gland disease. JAMA Ophthalmol (2015) 133(10):1117–23. doi:10.1001/jamaophthalmol.2015.2326
17. Ho W, Eubank T, Leblebicioglu B, Marsh C, Walters J. Azithromycin decreases crevicular fluid volume and mediator content. J Dent Res (2010) 89(8):831–5. doi:10.1177/0022034510368650
18. Gong Y, Lu J, Ding X, Yu Y. Effect of adjunctive roxithromycin therapy on interleukin-1beta, transforming growth factor-beta1 and vascular endothelial growth factor in gingival crevicular fluid of cyclosporine A-treated patients with gingival overgrowth. J Periodontal Res (2014) 49(4):448–57. doi:10.1111/jre.12123
19. Peric A, Vojvodic D, Matkovic-Jozin S. Effect of long-term, low-dose clarithromycin on T helper 2 cytokines, eosinophilic cationic protein and the ‘regulated on activation, normal T cell expressed and secreted’ chemokine in the nasal secretions of patients with nasal polyposis. J Laryngol Otol (2012) 126(5):495–502. doi:10.1017/S0022215112000485
20. Peric A, Vojvodic D, Baletic N, Peric A, Miljanovic O. Influence of allergy on the immunomodulatory and clinical effects of long-term low-dose macrolide treatment of nasal polyposis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub (2010) 154(4):327–33. doi:10.5507/bp.2010.049
21. Yamada T, Fujieda S, Mori S, Yamamoto H, Saito H. Macrolide treatment decreased the size of nasal polyps and IL-8 levels in nasal lavage. Am J Rhinol (2000) 14(3):143–8. doi:10.2500/105065800782102717
22. MacLeod CM, Hamid QA, Cameron L, Tremblay C, Brisco W. Anti-inflammatory activity of clarithromycin in adults with chronically inflamed sinus mucosa. Adv Ther (2001) 18(2):75–82. doi:10.1007/BF02852391
23. Cervin A, Wallwork B, Mackay-Sim A, Coman WB, Greiff L. Effects of long-term clarithromycin treatment on lavage-fluid markers of inflammation in chronic rhinosinusitis. Clin Physiol Funct Imaging (2009) 29(2):136–42. doi:10.1111/j.1475-097X.2008.00848.x
24. Wallwork B, Coman W, Mackay-Sim A, Cervin A. Effect of clarithromycin on nuclear factor-kappa B and transforming growth factor-beta in chronic rhinosinusitis. Laryngoscope (2004) 114(2):286–90. doi:10.1097/00005537-200402000-00019
25. Suzuki H, Shimomura A, Ikeda K, Oshima T, Takasaka T. Effects of long-term low-dose macrolide administration on neutrophil recruitment and IL-8 in the nasal discharge of chronic sinusitis patients. Tohoku J Exp Med (1997) 182(2):115–24. doi:10.1620/tjem.182.115
26. Wallwork B, Coman W, Mackay-Sim A, Greiff L, Cervin A. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope (2006) 116(2):189–93. doi:10.1097/01.mlg.0000191560.53555.08
27. Cameron EJ, Chaudhuri R, Mair F, McSharry C, Greenlaw N, Weir CJ, et al. Randomised controlled trial of azithromycin in smokers with asthma. Eur Respir J (2013) 42(5):1412–5. doi:10.1183/09031936.00093913
28. He J, Zhu N, Chen X. Clinical impacts of azithromycin on lung function and cytokines for athmatic patients. JMS (2009) 36(6):719–22.
29. Piacentini GL, Peroni DG, Bodini A, Pigozzi R, Costella S, Loiacono A, et al. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: a preliminary report. Allergy Asthma Proc (2007) 28(2):194–8. doi:10.2500/aap.2007.28.2958
30. Fonseca-Aten M, Okada PJ, Bowlware KL, Chavez-Bueno S, Mejias A, Rios AM, et al. Effect of clarithromycin on cytokines and chemokines in children with an acute exacerbation of recurrent wheezing: a double-blind, randomized, placebo-controlled trial. Ann Allergy Asthma Immunol (2006) 97(4):457–63. doi:10.1016/S1081-1206(10)60935-0
31. Amayasu H, Yoshida S, Ebana S, Yamamoto Y, Nishikawa T, Shoji T, et al. Clarithromycin suppresses bronchial hyperresponsiveness associated with eosinophilic inflammation in patients with asthma. Ann Allergy Asthma Immunol (2000) 84(6):594–8. doi:10.1016/S1081-1206(10)62409-X
32. Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest (2002) 121(6):1782–8. doi:10.1378/chest.121.6.1782
33. Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med (2008) 177(2):148–55. doi:10.1164/rccm.200707-1134OC
34. Wang Y, Zhang S, Qu Y. Effect of clarithromycin on non-eosinophilic refractory asthma. J Clin Pulm Med (2012) 17(11):1948–51.
35. Shoji T, Yoshida S, Sakamoto H, Hasegawa H, Nakagawa H, Amayasu H. Anti-inflammatory effect of roxithromycin in patients with aspirin-intolerant asthma. Clin Exp Allergy (1999) 29(7):950–6. doi:10.1046/j.1365-2222.1999.00551.x
36. Kamoi H, Kurihara N, Fujiwara H, Hirata K, Takeda T. The macrolide antibacterial roxithromycin reduces bronchial hyperresponsiveness and superoxide anion production by polymorphonuclear leukocytes in patients with asthma. J Asthma (1995) 32(3):191–7. doi:10.3109/02770909509089507
37. Fouka E, Lamprianidou E, Arvanitidis K, Filidou E, Kolios G, Miltiades P, et al. Low-dose clarithromycin therapy modulates Th17 response in non-cystic fibrosis bronchiectasis patients. Lung (2014) 192(6):849–55. doi:10.1007/s00408-014-9619-0
38. Yalçin E, Kiper N, Ozçelik U, Doğru D, Firat P, Sahin A, et al. Effects of claritromycin on inflammatory parameters and clinical conditions in children with bronchiectasis. J Clin Pharm Ther (2006) 31(1):49–55. doi:10.1111/j.1365-2710.2006.00708.x
39. Liu J, Zhong X, He Z, Wei L, Zheng X, Zhang J, et al. Effect of low-dose, long-term roxithromycin on airway inflammation and remodeling of stable noncystic fibrosis bronchiectasis. Mediators Inflamm (2014) 2014:708608. doi:10.1155/2014/708608
40. Parnham MJ, Culić O, Eraković V, Munić V, Popović-Grle S, Barisić K, et al. Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short-term azithromycin treatment. Eur J Pharmacol (2005) 517(1–2):132–43. doi:10.1016/j.ejphar.2005.05.023
41. Banerjee D, Honeybourne D, Khair OA. The effect of oral clarithromycin on bronchial airway inflammation in moderate-to-severe stable COPD: a randomized controlled trial. Treat Respir Med (2004) 3(1):59–65. doi:10.2165/00151829-200403010-00007
42. He ZY, Ou LM, Zhang JQ, Bai J, Liu GN, Li MH, et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration (2010) 80(6):445–52. doi:10.1159/000321374
43. Park SJ, Lee YC, Rhee YK, Lee HB. The effect of long-term treatment with erythromycin on Th1 and Th2 cytokines in diffuse panbronchiolitis. Biochem Biophys Res Commun (2004) 324(1):114–7. doi:10.1016/j.bbrc.2004.09.018
44. Oda H, Kadota J, Kohno S, Hara K. Erythromycin inhibits neutrophil chemotaxis in bronchoalveoli of diffuse panbronchiolitis. Chest (1994) 106(4):1116–23. doi:10.1378/chest.106.4.1116
45. Kadota J, Sakito O, Kohno S, Sawa H, Mukae H, Oda H, et al. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am Rev Respir Dis (1993) 147(1):153–9. doi:10.1164/ajrccm/147.1.153
46. Katsuki M. Neutrophil chemotactic activity in bronchoalveolar lavage fluid recovered from patients with diffuse panbronchiolitis. Kurume Med J (1996) 43(4):279–87. doi:10.2739/kurumemedj.43.279
47. Ichikawa Y, Ninomiya H, Koga H, Tanaka M, Kinoshita M, Tokunaga N, et al. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am Rev Respir Dis (1992) 146(1):196–203. doi:10.1164/ajrccm/146.1.196
48. Sakito O, Kadota J, Kohno S, Abe K, Shirai R, Hara K. Interleukin 1 beta, tumor necrosis factor alpha, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: a potential mechanism of macrolide therapy. Respiration (1996) 63(1):42–8. doi:10.1159/000196514
49. Umeki S. Anti-inflammatory action of erythromycin. Its inhibitory effect on neutrophil NADPH oxidase activity. Chest (1993) 104(4):1191–3.
50. Ratjen F, Saiman L, Mayer-Hamblett N, Lands LC, Kloster M, Thompson V, et al. Effect of azithromycin on systemic markers of inflammation in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa. Chest (2012) 142(5):1259–66. doi:10.1378/chest.12-0628
51. Equi A, Balfour-Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet (2002) 360(9338):978–84. doi:10.1016/S0140-6736(02)11081-6
52. Doğru D, Dalgiç F, Kiper N, Ozçelik U, Yalçin E, Aslan AT, et al. Long-term clarithromycin in cystic fibrosis: effects on inflammatory markers in BAL and clinical status. Turk J Pediatr (2009) 51(5):416–23.
53. Pukhalsky AL, Shmarina GV, Kapranov NI, Kokarovtseva SN, Pukhalskaya D, Kashirskaja NJ. Anti-inflammatory and immunomodulating effects of clarithromycin in patients with cystic fibrosis lung disease. Mediators Inflamm (2004) 13(2):111–7. doi:10.1080/09629350410001688495
54. Verleden SE, Vandooren J, Vos R, Willems S, Dupont LJ, Verleden GM, et al. Azithromycin decreases MMP-9 expression in the airways of lung transplant recipients. Transpl Immunol (2011) 25(2–3):159–62. doi:10.1016/j.trim.2011.06.006
55. Berg HF, Maraha B, Scheffer GJ, Peeters MF, Kluytmans JA. Effect of clarithromycin on inflammatory markers in patients with atherosclerosis. Clin Diagn Lab Immunol (2003) 10(4):525–8.
56. Culić O, Eraković V, Cepelak I, Barisić K, Brajsa K, Ferencić Z, et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol (2002) 450(3):277–89. doi:10.1016/S0014-2999(02)02042-3
57. Criqui GI, Solomon C, Welch BS, Ferrando RE, Boushey HA, Balmes JR. Effects of azithromycin on ozone-induced airway neutrophilia and cytokine release. Eur Respir J (2000) 15(5):856–62. doi:10.1034/j.1399-3003.2000.15e08.x
58. Aubert JD, Juillerat-Jeanneret L, Fioroni P, Dayer P, Plan PA, Leuenberger P. Function of human alveolar macrophages after a 3-day course of azithromycin in healthy volunteers. Pulm Pharmacol Ther (1998) 11(4):263–9. doi:10.1006/pupt.1998.0123
59. Cervin A, Kalm O, Sandkull P, Lindberg S. One-year low-dose erythromycin treatment of persistent chronic sinusitis after sinus surgery: clinical outcome and effects on mucociliary parameters and nasal nitric oxide. Otolaryngol Head Neck Surg (2002) 126(5):481–9. doi:10.1067/mhn.2002.124849
60. Tone A, Shikata K, Nakagawa K, Hashimoto M, Makino H. Renoprotective effects of clarithromycin via reduction of urinary MCP-1 levels in type 2 diabetic patients. Clin Exp Nephrol (2011) 15(1):79–85. doi:10.1007/s10157-010-0357-1
61. Booth V, Young S, Cruchley A, Taichman NS, Paleolog E. Vascular endothelial growth factor in human periodontal disease. J Periodontal Res (1998) 33(8):491–9. doi:10.1111/j.1600-0765.1998.tb02349.x
62. Suzuki H, Ikeda K, Honma R, Gotoh S, Oshima T, Furukawa M, et al. Prognostic factors of chronic rhinosinusitis under long-term low-dose macrolide therapy. ORL J Otorhinolaryngol Relat Spec (2000) 62(3):121–7. doi:10.1159/000027731
63. Akiyoshi H, Honda J, Nakahara S, Tokisawa S, Tokunaga N, Ichikawa Y, et al. [Mechanism of efficacy of erythromycin on diffuse panbronchiolitis – effect of erythromycin on cytokine mRNA expression in human whole blood model]. Kansenshogaku Zasshi (1994) 68(2):209–16. doi:10.11150/kansenshogakuzasshi1970.68.209
64. Bosnar M, Čužić S, Bošnjak B, Nujić K, Ergović G, Marjanović N, et al. Azithromycin inhibits macrophage interleukin-1beta production through inhibition of activator protein-1 in lipopolysaccharide-induced murine pulmonary neutrophilia. Int Immunopharmacol (2011) 11(4):424–34. doi:10.1016/j.intimp.2010.12.010
65. Cheung PS, Si EC, Hosseini K. Anti-inflammatory activity of azithromycin as measured by its NF-kappaB, inhibitory activity. Ocul Immunol Inflamm (2010) 18(1):32–7. doi:10.3109/09273940903359725
66. Cigana C, Nicolis E, Pasetto M, Assael BM, Melotti P. Anti-inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun (2006) 350(4):977–82. doi:10.1016/j.bbrc.2006.09.132
67. Yao GY, Ma YL, Zhang MQ, Gao ZC. Macrolide therapy decreases chronic obstructive pulmonary disease exacerbation: a meta-analysis. Respiration (2013) 86(3):254–60. doi:10.1159/000350828
68. Donath E, Chaudhry A, Hernandez-Aya LF, Lit L. A meta-analysis on the prophylactic use of macrolide antibiotics for the prevention of disease exacerbations in patients with Chronic Obstructive Pulmonary Disease. Respir Med (2013) 107(9):1385–92. doi:10.1016/j.rmed.2013.05.004
69. Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev (2012) 11:Cd002203. doi:10.1002/14651858.CD002203.pub4
70. Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev (2015) 7:Cd010337. doi:10.1002/14651858.CD010337.pub2
71. Kew KM, Undela K, Kotortsi I, Ferrara G. Macrolides for chronic asthma. Cochrane Database Syst Rev (2015) 9:Cd002997. doi:10.1002/14651858.CD002997.pub4
72. Yang M, Dong BR, Lu J, Lin X, Wu HM. Macrolides for diffuse panbronchiolitis. Cochrane Database Syst Rev (2013) 2:Cd007716. doi:10.1002/14651858.CD007716.pub3
73. Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax (2013) 68(4):322–9. doi:10.1136/thoraxjnl-2012-202698
74. Kobayashi M, Shimauchi T, Hino R, Tokura Y. Roxithromycin downmodulates Th2 chemokine production by keratinocytes and chemokine receptor expression on Th2 cells: its dual inhibitory effects on the ligands and the receptors. Cell Immunol (2004) 228(1):27–33. doi:10.1016/j.cellimm.2004.03.011
75. Ivetić Tkalčević V, Cužić S, Kramarić MD, Parnham MJ, Eraković Haber V. Topical azithromycin and clarithromycin inhibit acute and chronic skin inflammation in sensitized mice, with apparent selectivity for Th2-mediated processes in delayed-type hypersensitivity. Inflammation (2012) 35(1):192–205. doi:10.1007/s10753-011-9305-9
76. Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther (2014) 143(2):225–45. doi:10.1016/j.pharmthera.2014.03.003
77. Robinson P, Schechter MS, Sly PD, Winfield K, Smith J, Brennan S, et al. Clarithromycin therapy for patients with cystic fibrosis: a randomized controlled trial. Pediatr Pulmonol (2012) 47(6):551–7. doi:10.1002/ppul.21613
78. Ito T, Ito N, Hashizume H, Takigawa M. Roxithromycin inhibits chemokine-induced chemotaxis of Th1 and Th2 cells but regulatory T cells. J Dermatol Sci (2009) 54(3):185–91. doi:10.1016/j.jdermsci.2009.01.007
79. Sato Y, Kaneko K, Inoue M. Macrolide antibiotics promote the LPS-induced upregulation of prostaglandin E receptor EP2 and thus attenuate macrolide suppression of IL-6 production. Prostaglandins Leukot Essent Fatty Acids (2007) 76(3):181–8. doi:10.1016/j.plefa.2006.12.005
80. Gualdoni GA, Lingscheid T, Schmetterer KG, Hennig A, Steinberger P, Zlabinger GJ. Azithromycin inhibits IL-1 secretion and non-canonical inflammasome activation. Sci Rep (2015) 5:12016. doi:10.1038/srep12016
81. Rodvold KA. Clinical pharmacokinetics of clarithromycin. Clin Pharmacokinet (1999) 37(5):385–98. doi:10.2165/00003088-199937050-00003
82. Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother (1990) 25(Suppl A):73–82. doi:10.1093/jac/25.suppl_A.73
83. Fraschini F, Scaglione F, Pintucci G, Maccarinelli G, Dugnani S, Demartini G. The diffusion of clarithromycin and roxithromycin into nasal mucosa, tonsil and lung in humans. J Antimicrob Chemother (1991) 27(Suppl A):61–5. doi:10.1093/jac/27.suppl_A.61
84. Dette GA. [Tissue penetration of erythromycin (author’s transl)]. Infection (1979) 7(3):129–45. doi:10.1007/BF01641314
Keywords: azalides, azithromycin, clarithromycin, erythromycin, immunolides, roxithromycin
Citation: Zimmermann P, Ziesenitz VC, Curtis N and Ritz N (2018) The Immunomodulatory Effects of Macrolides—A Systematic Review of the Underlying Mechanisms. Front. Immunol. 9:302. doi: 10.3389/fimmu.2018.00302
Received: 10 November 2017; Accepted: 02 February 2018;
Published: 13 March 2018
Edited by:
Heiko Mühl, Goethe University Frankfurt, GermanyReviewed by:
Klaus G. Schmetterer, Medizinische Universität Wien, AustriaCopyright: © 2018 Zimmermann, Ziesenitz, Curtis and Ritz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petra Zimmermann, cGV0cmEuemltbWVybWFubkByY2gub3JnLmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.