
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 16 February 2018
Sec. Primary Immunodeficiencies
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.00289
This article is part of the Research Topic Primary Immunodeficiencies Worldwide View all 23 articles
 Gesmar R. S. Segundo1,2*
Gesmar R. S. Segundo1,2* Anh T. V. Nguyen3
Anh T. V. Nguyen3 Huyen T. Thuc3
Huyen T. Thuc3 Le N. Q. Nguyen3
Le N. Q. Nguyen3 Roger H. Kobayashi4
Roger H. Kobayashi4 Hai T. Le3
Hai T. Le3 Huong T. M. Le3
Huong T. M. Le3 Troy R. Torgerson1
Troy R. Torgerson1 Hans D. Ochs1*
Hans D. Ochs1*
Background: New sequencing techniques have revolutionized the identification of the molecular basis of primary immunodeficiency disorders (PID) not only by establishing a gene-based diagnosis but also by facilitating defect-specific treatment strategies, improving quality of life and survival, and allowing factual genetic counseling. Because these techniques are generally not available for physicians and their patients residing in developing countries, collaboration with overseas laboratories has been explored as a possible, albeit cumbersome, strategy. To reduce the cost of time and temperature-sensitive shipping, we selected Guthrie cards, developed for newborn screening, to collect dried blood spots (DBS), as a source of DNA that can be shipped by regular mail at minimal cost.
Method: Blood was collected and blotted onto the filter paper of Guthrie cards by completely filling three circles. We enrolled 20 male patients with presumptive X-linked agammaglobulinemia (XLA) cared for at the Vietnam National Children’s Hospital, their mothers, and several sisters for carrier analysis. DBS were stored at room temperature until ready to be shipped together, using an appropriately sized envelope, to a CLIA-certified laboratory in the US for sequencing. The protocol for Sanger sequencing was modified to account for the reduced quantity of gDNA extracted from DBS.
Result: High-quality gDNA could be extracted from every specimen. Bruton tyrosine kinase (BTK) mutations were identified in 17 of 20 patients studied, confirming the diagnosis of XLA in 85% of the study cohort. Type and location of the mutations were similar to those reported in previous reviews. The mean age when XLA was suspected clinically was 4.6 years, similar to that reported by Western countries. Two of 15 mothers, each with an affected boy, had a normal BTK sequence, suggesting gonadal mosaicism.
Conclusion: DBS collected on Guthrie cards can be shipped inexpensively by airmail across continents, providing sufficient high-quality gDNA for Sanger sequencing overseas. By using this method of collecting gDNA, we were able to confirm the diagnosis of XLA in 17 of 20 Vietnamese patients with the clinical diagnosis of agammaglobulinemia.
Recent technical advances in gene analysis have facilitated diagnosis and treatment of primary immunodeficiency diseases (PID), with more than 320 genes identified that cause PID when mutated (1). While those technologies are readily available in developed countries, most developing countries do not have the medical facilities to perform gene sequencing and protein analysis (2, 3). Many physicians caring for PID patients in these parts of the world enter into partnerships with researchers in the United States, Europe, and selected Asian countries to initiate genetic evaluation of their patients (2, 3). However, shipping blood or cryopreserved peripheral blood mononuclear cells between continents is hampered by bureaucratic road blocks, is expensive, and is sometimes unsuccessful.
X-linked agammaglobulinemia (XLA), a genetic disorder first described by Bruton in 1952 (4), is caused by mutations in the Bruton tyrosine kinase (BTK) gene (5). Patients with XLA have low or absence of circulating B cells and markedly reduced serum immunoglobulin and specific antibody levels, resulting in increased susceptibility to bacterial and selective viral infections. Affected boys present typically with respiratory tract infections, but may also develop colitis, arthritis, and neurologic findings (6–8). The BTK gene consists of 18 coding exons and directs the production of a 659 amino acid protein with 5 distinct structural domains (Figure 1) that plays a crucial role in B cell development and function (6, 9).
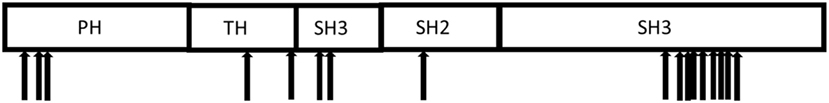
Figure 1. Distribution of the mutations in the Bruton tyrosine kinase gene identified in the 17 Vietnamese X-linked agammaglobulinemia patients.
The clinical criteria sufficient for a probable diagnosis of XLA include a male with less than 2% CD19+ B cells in whom other causes of hypogammaglobulinemia have been excluded, who has at least one of the following: onset of recurrent bacterial infections in the first 5 years of life, serum IgG, IgM and IgA that are more than 2 SD below normal for age, and poor responses to vaccines. The “definitive” diagnosis of XLA requires gene sequencing confirming a mutation in BTK and/or absence of BTK protein expression in monocytes or platelets (10). Because analysis of protein expression and sequencing of the BTK gene are not commonly available in developing countries, a definitive diagnosis of XLA cannot be established.
To provide an inexpensive and effective method of performing genetic analyses in patients from developing countries with clinical and laboratorial findings compatible with a diagnosis of XLA, we tested the procedure established more than 50 years ago to collect and ship blood samples from hospitals to State laboratories for newborn screening. Originally designed for early detection of metabolic disorders such as phenylketonuria (11), dried blood spots (DBS) collected on filter paper (Guthrie cards) have been used in recent years as a source of gDNA to screen for severe combined immune deficiency, based on the presence or absence of T cell receptor excision circles, representing recent thymic emigrants (12, 13). We used Standard Guthrie cards to collect DBS, which can be shipped in a regular envelop by air mail (20 cards/envelop) from Vietnam to a CLIA-approved laboratory in Seattle, WA, USA, where the DNA was extracted and sequenced for mutations in the BTK gene. The approach has been validated by the demonstration that gDNA, stored for years in DBS, is stable and suitable for sequence analysis (14).
The DBS analysis for BTK mutations was approved by the institutional review boards at Seattle Children’s Hospital and Vietnam National Children’s Hospital. Written informed consent for genetic testing was obtained from the parents of Vietnamese patients by local physicians.
Twenty patients with a clinical diagnosis of XLA, cared for by physicians at the Vietnam National Children’s Hospital, Hanoi, were enrolled in the study from May to July 2017, and DBS were collected on Guthrie cards. A possible clinical diagnosis of XLA was based on the increased susceptibility to bacterial infections at an early age, low serum immunoglobulin levels, and marked decrease or absence of peripheral blood B lymphocytes (10). Mothers and sisters of these patients were invited to provide DBS for investigation of carrier status.
Vietnamese physicians caring for the patients collected venous blood or blood from a finger stick and completely filled three circles on the paper filter cards (ID Biological, Greenville, SC, USA) used by the Washington State Department of Health for newborn screening. The samples were stored at room temperature until DBS from all 20 patients were collected and were shipped in a single standard envelope by regular air mail to the Immunology Diagnostic Laboratory at Seattle Children’s Research Institute. Upon arrival, gDNA was extracted from one of the three blood spots collected from each patient using the Quiagen DNA blood Mini Kit (Quiagen, Germantown, USA). The average yield was 3–20 ng/µl (total 150 µl). The unused DBS were stored at room temperature for future use.
The targeted Sanger sequencing protocol for regular DNA analysis was modified, as the final concentration of gDNA extracted from DBS (5–25 ng/μl) was lower than that obtained from peripheral blood. We optimized PCR amplification using only 2 μl of gDNA per exon or 5 µl gDNA for the longest exons or multiple exons based on the (modified) primers reported previously (15). Briefly, samples were amplified in 10 µl reactions containing 1 µl each of forward and reverse primers (5 mM each), 1 µl of dNTP mix, 1 µl of 10× buffer, 0.5 µl of MgSO4 (50 mM), and 0.1 µl of undiluted Taq DNA Polymerase. All exons were amplified under the same PCR conditions: denaturation at 94°C during 2 min and then 38 cycles at 94°C for 30 s, 55°C for 30 s, and 68°C for 1.5 min, with final extension at 68°C for 5 min. Amplicons were sequenced according to the standard Big Dye protocol, analyzed, and aligned using the Mutation Surveyor and BioEdit software to detect mutations in the coding sequence and exon/intron junctions of BTK. The nomenclature for mutations in BTK is according to guidelines by the Human Genome Variation Society as determined by Mutalyzer 2.0.26-Name checker (NM_000061.2).
Table 1 presents clinical findings and laboratorial data of the 20 patients and lists BTK mutations identified in 17 participants. The mean age at the onset of symptoms was 9.52 months (range, 1–27 months), and the mean age at diagnosis was 54.70 months (range, 6–153 months). There was no difference between those patients with BTK mutations and those patients without. The mean age when the diagnosis was confirmed by BTK genetic analysis was 84.50 months (range, 21–197 months). The diagnosis of XLA based on the clinical characteristics was suspected at the age of 6 months in an asymptomatic boy (p06) who had an older diseased brother with an XLA phenotype. All patients started to receive IVIG as soon as the diagnosis of XLA was suspected clinically.
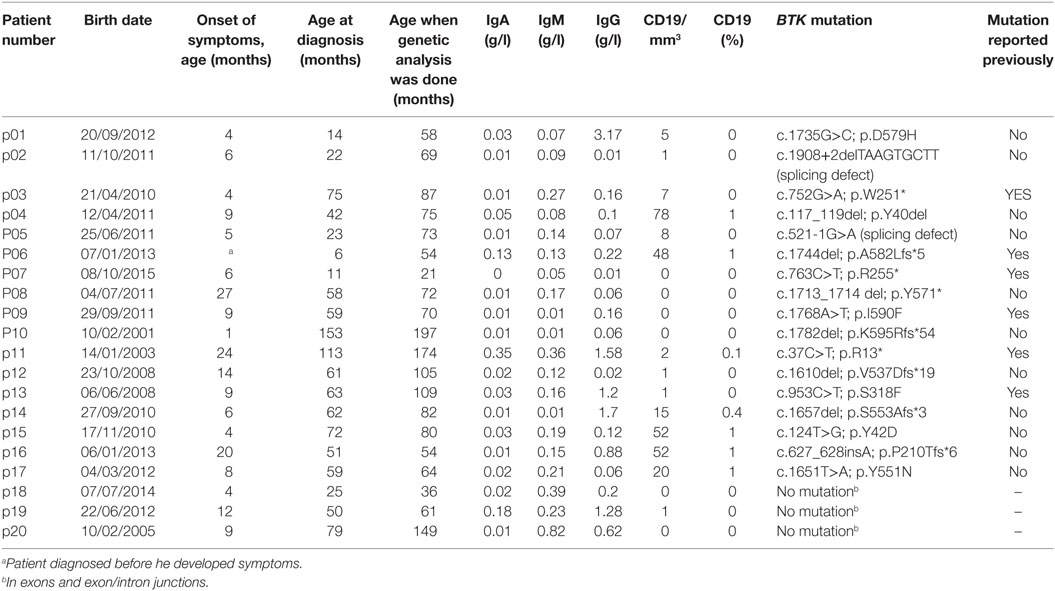
Table 1. Clinical findings, laboratory results, and BTK mutations in 20 Vietnamese patients with agammaglobulinemia.
The most prevalent infections observed in this cohort of 20 patients were otitis media (121 episodes) and pneumonia (61 episodes), followed by conjunctivitis, arthritis, sepsis, sinusitis, and meningitis (Figure 2). The mean IgG level before IVIG therapy was 0.58 g/l (range, 0.01–3.17 g/l).
Pathogenic mutations in the BTK gene were identified in 17 of our cohort of 20 unrelated symptomatic patients tested by sequencing the 18 coding exons and exon–intron junctions. The mutations included missense and nonsense mutations, insertions, deletions, and splicing defects and were located throughout the BTK gene (Table 1; Figure 1). Additional samples were provided from 15 mothers of the 17 unrelated patients with BTK mutations (Table 2). Thirteen of the mothers had the same mutation as their sons and are considered XLA carriers. Of three girls, each having a full brother with a BTK mutation, one was found to be a carrier. The daughter of one of the two mothers with normal BTK sequence was found to have the same BTK mutation as her brother (p01), suggesting gonadal mosaicism.
The diagnosis of agammaglobulinemia can be suspected based on the clinical and laboratory characteristics. While the majority of patients with agammaglobulinemia are male, suggesting X-linked inheritance and mutations in BTK, there are a handful of genes that, if mutated, result in autosomal recessive agammaglobulinemia, including IGHM (μ heavy chain deficiency), IGLL1 (λ5 deficiency), CD79A (Igα deficiency), CD79B (Igβ deficiency), BLNK, PIK3R1 (1), IKAROS (16), and one gene (TCF3, E47 deficiency) causing an autosomal-dominant agammaglobulinemia (17). Because of this heterogeneity, molecular analysis is imperative for optimal therapy, prognostic considerations, and genetic counseling of patients with agammaglobulinemia. While sequence analysis, either by Sanger sequencing of individual genes or by next-generation sequencing is broadly available in the developed world, most developing countries do not have access to these advanced techniques.
This pilot study demonstrates the feasibility of collecting blood on Guthrie cards, shipping the cards at minimal cost from one continent to another, and identifying causative mutations in BTK by Sanger sequencing in 17 of 20 (85%) unrelated male patients with clinical and laboratory findings compatible with XLA. We have used this technique to collect and ship DBS from a number of South American countries to identify disease-causing mutations in >25 PID-associated genes including WAS, CD40L, PIK3CD, FOXP3, CTLA4, STAT1, STAT3, RAG1, and RAG2. Because of the limited quantity of patient material, we had to slightly modify the PCR amplification technique that we routinely use for gDNA extraction from peripheral blood or saliva.
The type and distribution of the BTK mutations were similar to those reported previously. Since only the coding region and the intron/exon junctions of BTK were sequenced, mutations in the promoter or Poly A regions, or deep intronic variants, would have been missed. Identifying those mutations will require analysis of mRNA or protein expression, we hope to develop in the future. The clinical characteristics and laboratory findings of the 17 Vietnamese XLA patients with BTK mutations were similar to those observed in Western countries, with episodes of pneumonia reported by 80% of the Vietnamese patients, 62% of US patients (7), 100% of patients from the Netherlands (18), and 77% of patients from China (8).
The mean age at diagnosis, based on clinical and immunological criteria, was similar to Western countries: 4.6 years in Vietnamese patients, compared with 5.37 in the United States (7) and 6.5 years in patients from the Netherlands (18), but slightly lower than in those from China (7.09 years) (8).
In conclusion, DBS are easy to collect, can be stored and shipped at room temperature, and transported inexpensively across continents. DBS are an excellent source of high-quality gDNA in sufficient quantity for Sanger sequencing, making this collection technique an affordable option for developing countries to interact and collaborate with sophisticated laboratories anywhere in the world.
Institutional review boards (IRBs) at Seattle Children’s Hospital and Vietnam National Children’s Hospital.
HO and TT conceived and designed the study; GS developed the technique and sequenced BTK from DBS; AN, HTML, HT, LN, and HTL identified the patients and collected DBS; RK established contact with the Hanoi group and facilitated the shipment of samples.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the C.H.I.L.D.R.E.N grant from the Jeffrey Modell Foundation (GRS Segundo).
1. Picard C, Bobby Gaspar H, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol (2017) 38:96–128. doi:10.1007/s10875-017-0464-9
2. Costa-Carvalho B, González-Serrano M, Espinosa-Padilla S, Segundo G. Latin American challenges with the diagnosis and treatment of primary immunodeficiency diseases. Expert Rev Clin Immunol (2017) 13:483–9. doi:10.1080/1744666X.2017.1255143
3. Espinosa-Rosales FJ, Condino-Neto A, Franco JL, Sorensen RU. Into action: improving access to optimum care for all primary immunodeficiency patients. J Clin Immunol (2016) 36:415–7. doi:10.1007/s10875-016-0277-2
5. Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell (1993) 72:279–90. doi:10.1016/0092-8674(93)90667-F
6. Conley ME, Rohrer J, Minegishi Y. X-linked agammaglobulinemia. Clin Rev Allergy Immunol (2000) 19:183–204. doi:10.1385/CRIAI:19:2:183
7. Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) (2006) 85:193–202. doi:10.1097/01.md.0000229482.27398.ad
8. Chen XF, Wang WF, Zhang YD, Zhao W, Wu J, Chen TX. Clinical characteristics and genetic profiles of 174 patients with X-linked agammaglobulinemia: report from Shanghai, China (2000–2015). Medicine (Baltimore) (2016) 95:e4544. doi:10.1097/MD.0000000000004544
9. Desiderio S. Role of Btk in B cell development and signaling. Curr Opin Immunol (1997) 9:534–40. doi:10.1016/S0952-7915(97)80107-0
10. Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol (1999) 93:190–7. doi:10.1006/clim.1999.4799
11. Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics (1963) 32:338–43.
12. Hazenberg MD, Verschuren MC, Hamann D, Miedema F, van Dongen JJ. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J Mol Med (Berl) (2001) 79:631–40. doi:10.1007/s001090100271
13. Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol (2005) 115:391–8. doi:10.1016/j.jaci.2004.10.012
14. Hollegaard MV, Grove J, Grauholm J, Kreiner-Møller E, Bønnelykke K, Nørgaard M, et al. Robustness of genome-wide scanning using archived dried blood spot samples as a DNA source. BMC Genet (2011) 12:58. doi:10.1186/1471-2156-12-58
15. Chan K-W, Chen T, Jiang L, Fok F-S, Lee T-L, Lee B-W, et al. Identification of Bruton tyrosine kinase mutations in 12 Chinese patients with X-linked agammaglobulinaemia by long PCR-direct sequencing. Intl J Immunogenet (2006) 33:205–9. doi:10.1111/j.1744-313X.2006.00598.x
16. Kuehn HS, Boisson B, Cunningham-Rundles C, Reichenbach J, Stray-Pedersen A, Gelfand EW, et al. Loss of B cells in patients with heterozygous mutations in IKAROS. N Engl J Med (2016) 374:1032–43. doi:10.1056/NEJMoa1512234
17. Boisson B, Wang YD, Bosompem A, Ma CS, Lim A, Kochetkov T, et al. A recurrent dominant negative E47 mutation causes agammaglobulinemia and BCR(-) B cells. J Clin Invest (2013) 123:4781–5. doi:10.1172/JCI71927
Keywords: X-linked agammaglobulinemia, genetic sequencing, primary immunodeficiencies, Btk, dried blood spots
Citation: Segundo GRS, Nguyen ATV, Thuc HT, Nguyen LNQ, Kobayashi RH, Le HT, Le HTM, Torgerson TR and Ochs HD (2018) Dried Blood Spots, an Affordable Tool to Collect, Ship, and Sequence gDNA from Patients with an X-Linked Agammaglobulinemia Phenotype Residing in a Developing Country. Front. Immunol. 9:289. doi: 10.3389/fimmu.2018.00289
Received: 10 December 2017; Accepted: 01 February 2018;
Published: 16 February 2018
Edited by:
Antonio Condino-Neto, University of São Paulo, BrazilReviewed by:
Anders Fasth, University of Gothenburg, SwedenCopyright: © 2018 Segundo, Nguyen, Thuc, Nguyen, Kobayashi, Le, Le, Torgerson and Ochs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gesmar R. S. Segundo, Z2VzbWFyMkBnbWFpbC5jb20=;
Hans D. Ochs, aGFucy5vY2hzQHNlYXR0bGVjaGlsZHJlbnMub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.