
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 07 February 2018
Sec. Microbial Immunology
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.00178
Hospital-acquired infections caused by Acinetobacter baumannii have become problematic because of high rates of drug resistance. A. baumannii is usually harmless, but it may cause infectious diseases in an immunocompromised host. Although neutrophils are the key players of the initial immune response against bacterial infection, their interactions with A. baumannii remain largely unknown. A new biological defense mechanism, termed neutrophil extracellular traps (NETs), has been attracting attention. NETs play a critical role in bacterial killing by bacterial trapping and inactivation. Many pathogenic bacteria have been reported to induce NET formation, while an inhibitory effect on NET formation is rarely reported. In the present study, to assess the inhibition of NET formation by A. baumannii, bacteria and human neutrophils were cocultured in the presence of phorbol 12-myristate 13-acetate (PMA), and NET formation was evaluated. NETs were rarely observed during the coculture despite neutrophil PMA stimulation. Furthermore, A. baumannii prolonged the lifespan of neutrophils by inhibiting NET formation. The inhibition of NET formation by other bacteria was also investigated. The inhibitory effect was only apparent with live A. baumannii cells. Finally, to elucidate the mechanism of this inhibition, neutrophil adhesion was examined. A. baumannii suppressed the adhesion ability of neutrophils, thereby inhibiting PMA-induced NET formation. This suppression of cell adhesion was partly due to suppression of the surface expression of CD11a in neutrophils. The current study constitutes the first report on the inhibition of NET formation by a pathogenic bacterium, A. baumannii, and prolonging the neutrophil lifespan. This novel pathogenicity to inhibit NET formation, thereby escaping host immune responses might contribute to a development of new treatment strategies for A. baumannii infections.
Acinetobacter baumannii is an aerobic gram-negative bacillus that is widely distributed in nature. Recently, hospital-acquired infections caused by A. baumannii have become a severe problem because of high rates of drug resistance. The incidence of multidrug-resistant A. baumannii (MDRA) has increased rapidly worldwide since the late 1990s. Although, to control and treat A. baumannii infections, many studies have evaluated drug-resistance mechanisms and drug usage, MDRA is difficult to manage and remains a critical issue globally. The number of global fatalities associated with A. baumannii infections continues to rise (1–5).
Bacterial capsule, biofilm formation, secretion systems, high adhesion capacity, and high-affinity iron acquisition have been reported as the virulence factors of A. baumannii (6–11). Although A. baumannii possesses multiple potential pathogenicity factors, specific factors contributing to its virulence remain unclear, and, hence, it is currently not possible to satisfactorily manage infections caused by this bacterium. A. baumannii is usually harmless but it may cause various infectious diseases in an immunocompromised host (1, 3, 5). Therefore, bacterial interaction with host cells should be investigated to understand diseases linked to A. baumannii infection. However, details of the interaction between A. baumannii and host cells remain to be elucidated.
Bacteremia and severe sepsis are caused by A. baumannii in an immunocompromised host with high frequency, and are associated with high mortality rates (12–14). In a previous study, we reported that A. baumannii adheres to neutrophils and enhances their invasive ability, with the bacteria transported together with the invading neutrophils. A. baumannii appears to spread throughout the body by attracting and hijacking neutrophils, similar to a commuter calling a taxi cab, and causing bacteremia. This novel mechanism of bacterial mobility is referred to as the “bacterial immunity taxi” (15).
Neutrophils are immune cells that play a pivotal role during the initial immune response to various bacterial infections (16). Recently, neutrophils were shown to release their nuclear content, including unfolded chromatin and lysosomal enzymes. This phenomenon plays a critical role in bacterial killing by trapping and inactivating the bacteria. This biological defense mechanism is termed neutrophil extracellular traps (NETs) (17–20). We have previously investigated neutrophil responsiveness to A. baumannii and Pseudomonas aeruginosa (a bacterium closely related to A. baumannii), focusing on NETs (21). We found that A. baumannii does not induce NET formation and cannot sterilize bacteria, in contrast to P. aeruginosa. Moreover, phagocytosis and the production of reactive oxygen species (ROS) were less pronounced in neutrophils stimulated by A. baumannii than by P. aeruginosa. Our previous studies suggest that A. baumannii is able to escape neutrophil defense mechanisms (15, 21).
Recently, it was paper reported that the probiotic bacterium Lactobacillus rhamnosus inhibits NET formation via its antioxidative activity (22). The notion that bacteria control NET formation, a defense mechanism of neutrophils against infection, is worth pursuing. Moreover, NETs are considered to be a type of cell death mechanism, termed NETosis (19, 23), and it would be very interesting if bacteria could suppress the death of immune cells. Many pathogenic bacteria have been reported to induce NET formation, while an inhibitory effect on NET formation is rarely reported (20, 24, 25). A. baumannii may possess a number of escape mechanisms enabling it to evade several immune responses, as demonstrated above. In the current study, we investigated the inhibitory effect of A. baumannii on NET formation. We found that A. baumannii inhibits NET formation via the action of phorbol 12-myristate 13-acetate (PMA)-induced neutrophils. Furthermore, we provide evidence that suppression of neutrophil adhesion underpins this phenomenon.
Acinetobacter baumannii (ATCC 19606), A. calcoaceticus (ATCC 14987), A. haemolyticus (ATCC 17906), and Escherichia coli (ATCC 25922) were used as reference strains. Bacteria were grown in LB broth (Sigma-Aldrich, St. Louis, MO, USA) for 16 h at 37°C. The cells were then washed with phosphate-buffered saline (PBS), and suspended in fresh RPMI 1640 medium (Sigma-Aldrich).
Neutrophils were isolated from the human peripheral blood of healthy volunteers (n = 10), as described previously (26). Heparinized human blood was mixed with dextran 200,000/saline (final concentration of 1%) to sediment most of the erythrocytes. The supernatant was then processed by density gradient centrifugation using lymphosepar I (Wako Pure Chemical Industries, Osaka, Japan). Neutrophils were purified from the pelleted cells after hypotonic conditions induced lysis of the remaining erythrocytes. Purity of the neutrophil preparation was greater than 95%, as assessed by Diff Quick staining.
Written informed consent was obtained from all study participants, in accordance with the Declaration of Helsinki. The study was approved by the Ethical Review Committee of the School of Medicine of Teikyo University.
Neutrophils (1 × 106 cells/mL) and A. baumannii cells [5 × 107 colony-forming units, i.e., multiplicity of infection (MOI) of 50] were cocultured on non-coated glass slides (As One, Osaka, Japan) for 3 h in RPMI 1640 containing 2% human serum, at 37°C and under an atmosphere of 5% CO2. In some experiments, neutrophils were stimulated with 200-nM PMA simultaneously with the culture. The cells were fixed with 5% formaldehyde at room temperature for 10 min, and washed three times with PBS. DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI; Southern Biotech, Birmingham, AL, USA) and visualized under a fluorescence microscope (BX53; Olympus, Tokyo, Japan).
Neutrophils (1 × 106 cells/mL) and A. baumannii cells (MOI 50) were cocultured in 96-well culture plates (AGC Techno Glass, Shizuoka, Japan) for 0–5 h in RPMI 1640 containing 2% human serum, at 37°C and under an atmosphere of 5% CO2, and stimulated with 200-nM PMA. Extracellular DNA was stained with 5-µM SYTOX green (Life Technologies, Gaithersburg, MD, USA), a fluorescent membrane-impermeable DNA dye. Fluorescence was quantified using a microplate reader equipped with filters to detect the excitation and emission maxima at 485 and 535 nm, respectively (ARVO MX; PerkinElmer, Waltham, MA, USA). In some experiments, the assay was performed in the presence of 0.1-µg/mL CD11a-blocking antibody or control IgG (BioLegend, San Diego, CA, USA).
The release of lactate dehydrogenase (LDH) from neutrophils was detected by using an MTX-LDH assay kit (Kyokuto Pharmaceutical Industrial Co., Ltd., Tokyo, Japan). Briefly, LDH reduces NAD to NADH, leading to the production of formazan in proportion to the LDH levels in cell supernatants; the formazan produced was determined at 560 nm in a microplate reader. Cytotoxicity was calculated as a percentage compared with LDH levels of lysed neutrophils.
Neutrophils (1 × 106 cells/mL) were cultured for 3 h in RPMI 1640 medium supplemented with 2% human serum, and viable neutrophils, which were not stained with trypan blue, were counted. In some experiments, neutrophils were stimulated with 200-nM PMA and cocultured with A. baumannii (MOI 50).
Neutrophils (2.5 × 105 cells/0.25 mL), fluorescently labeled using PKH 26 (Sigma-Aldrich), were cultured in RPMI 1640 supplemented with 2% human serum, in the presence or absence of A. baumannii (MOI 50), and stimulated with 200-nM PMA. The cells were cultured in a glass-bottom dish (CELLview, Greiner Bio-One, Frickenhausen, Germany) at 37°C under an atmosphere of 5% CO2. Extracellular DNA was visualized by staining with 5-µM SYTOX green. The cells were evaluated every minute by confocal microscopy, for 5 h (FV10i, Olympus).
Neutrophils (1 × 106 cells/mL, 0.1 mL) were placed in a 96-well culture plate, and incubated at 37°C for 30 min in RPMI 1640 supplemented with 2% human serum. In some experiments, neutrophils were stimulated with 200-nM PMA when cocultured with A. baumannii (MOI 50), before adding them to the wells. The non-adhering neutrophils were removed by washing the wells three times with PBS. The adherent cells remaining in the wells were lysed with 1% Nonidet P-40, and LDH from adherent neutrophils was detected using an MTX-LDH assay kit, as described above. The adhesion rate of neutrophils was calculated as a percentage of the lysed neutrophils. In some experiments, the assay was performed in the presence of 0.1-µg/mL CD11a-blocking antibody or control IgG.
The surface expression of CD11a and CD11b on neutrophils was determined by flow cytometry (FACSCanto II, BD Biosciences, San Jose, CA, USA), using specific antibodies [FITC-labeled anti-CD11a antibody and PE-labeled anti-CD11b antibody (BD Biosciences)].
Data are presented as the mean ± SD. The values were compared using paired Student’s t-test, and differences at p < 0.01 were considered statistically significant.
Previously, we reported that A. baumannii does not induce NET formation after a 1-h coculture with neutrophils and that neutrophils are unable to kill this bacterium (21). Thus, A. baumannii appeared to possess escape mechanisms to evade immune responses. In the present study, we investigated the effect of A. baumannii on PMA-induced NET formation. Neutrophils and A. baumannii were cocultured for 3 h in the presence of PMA, DNA was stained with DAPI, and observed under a fluorescence microscope. NET formation was observed upon PMA stimulation. However, NETs were rarely observed when neutrophils were cocultured with A. baumannii, regardless of PMA stimulation (Figure 1A). To quantify the amount of the released extracellular DNA, the signal of SYTOX green-stained DNA in the medium was measured. The amount of extracellular neutrophil DNA increased after PMA stimulation, but it was significantly reduced upon coculture with A. baumannii (Figure 1B). Furthermore, neutrophil cell death was evaluated by determining the amount of released LDH. Extracellular LDH levels were elevated upon PMA stimulation, but, in comparison, they were significantly reduced upon coculture with A. baumannii (Figure 1C). Next, the survival of PMA-stimulated neutrophils was evaluated in coculture with A. baumannii. Upon PMA stimulation, most neutrophils died; in contrast, many remained alive during coculture with A. baumannii (Figure 1D). Furthermore, A. baumannii alone did not induce neutrophil cell death. Consequently, A. baumannii prolonged the lifespan of neutrophils by inhibiting NET formation, i.e., a cell death pathway, in PMA stimulation.
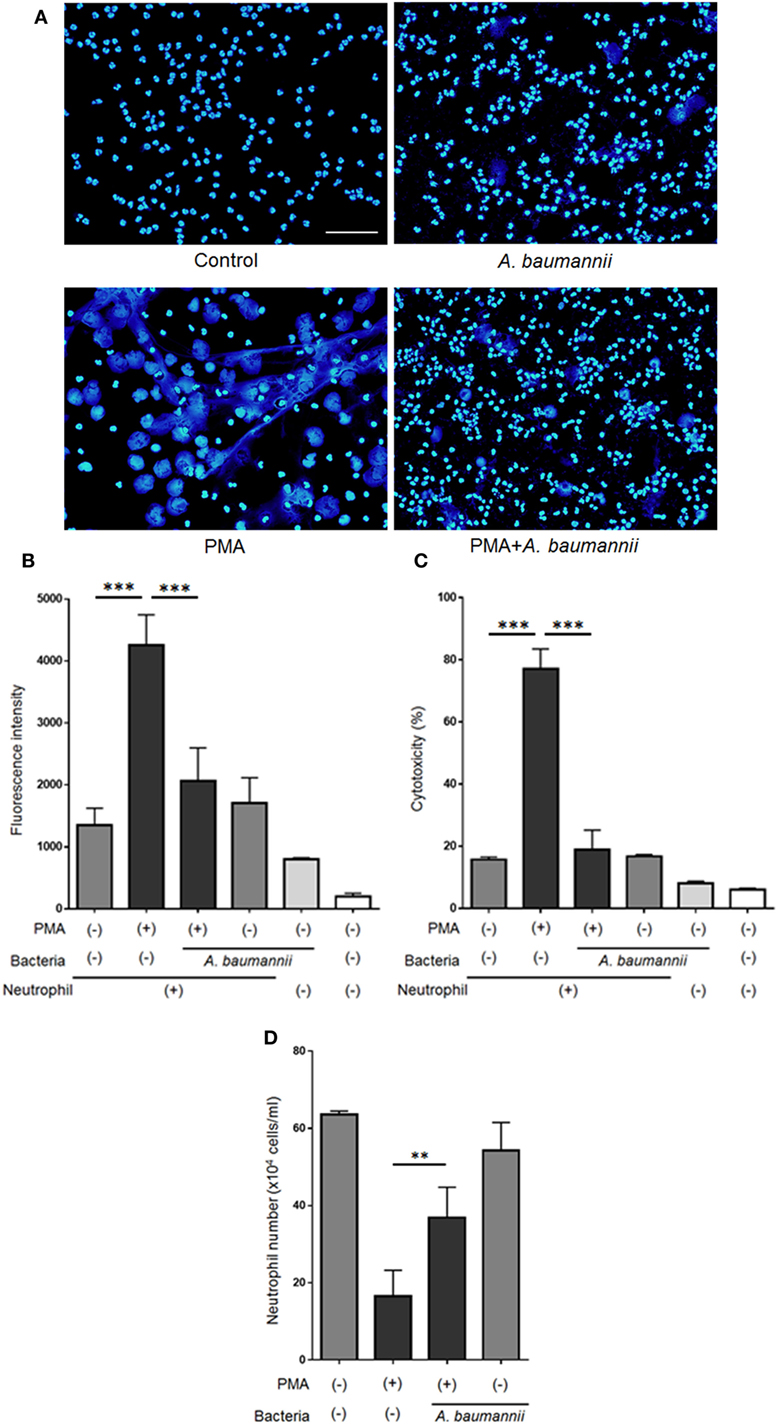
Figure 1. Acinetobacter baumannii inhibiting the formation of NETs. (A) Neutrophils and A. baumannii (MOI 50) were cocultured for 3 h, in the presence or absence of 200-nM phorbol 12-myristate 13-acetate (PMA). After incubation, the cells were fixed and DNA were stained with DAPI. Scale bar, 50 µm. (B) Extracellular DNA signal, after staining with SYTOX green and quantification. (C) Levels of extracellular lactate dehydrogenase (LDH) released by neutrophils, determined by the MTX-LDH assay kit. Cytotoxicity was calculated as a percentage based on LDH levels of lysed neutrophils. (D) Cell survival, based on staining with trypan blue. The data are shown as the mean ± SD; n ≥ 3 per group. ***p < 0.001 and **p < 0.01. The results are representative of at least three experiments.
Furthermore, to investigate the kinetics of the inhibitory effect of A. baumannii on NET formation, NET formation was monitored during a 5-h neutrophil culture with PMA, in the presence or absence of A. baumannii by time-lapse microscopy (Figure 2A; Videos S1 and S2 in Supplementary Material), and detection of extracellular DNA (Figure 2B). As observed, PMA-induced NET formation was inhibited by coculture with A. baumannii for up to 5 h. Compared with PMA stimulation alone, the amount of extracellular neutrophil DNA was also inhibited by coculture with A. baumannii. Hence, A. baumannii inhibited PMA-induced NET formation, thereby inhibiting PMA-stimulated cell death.
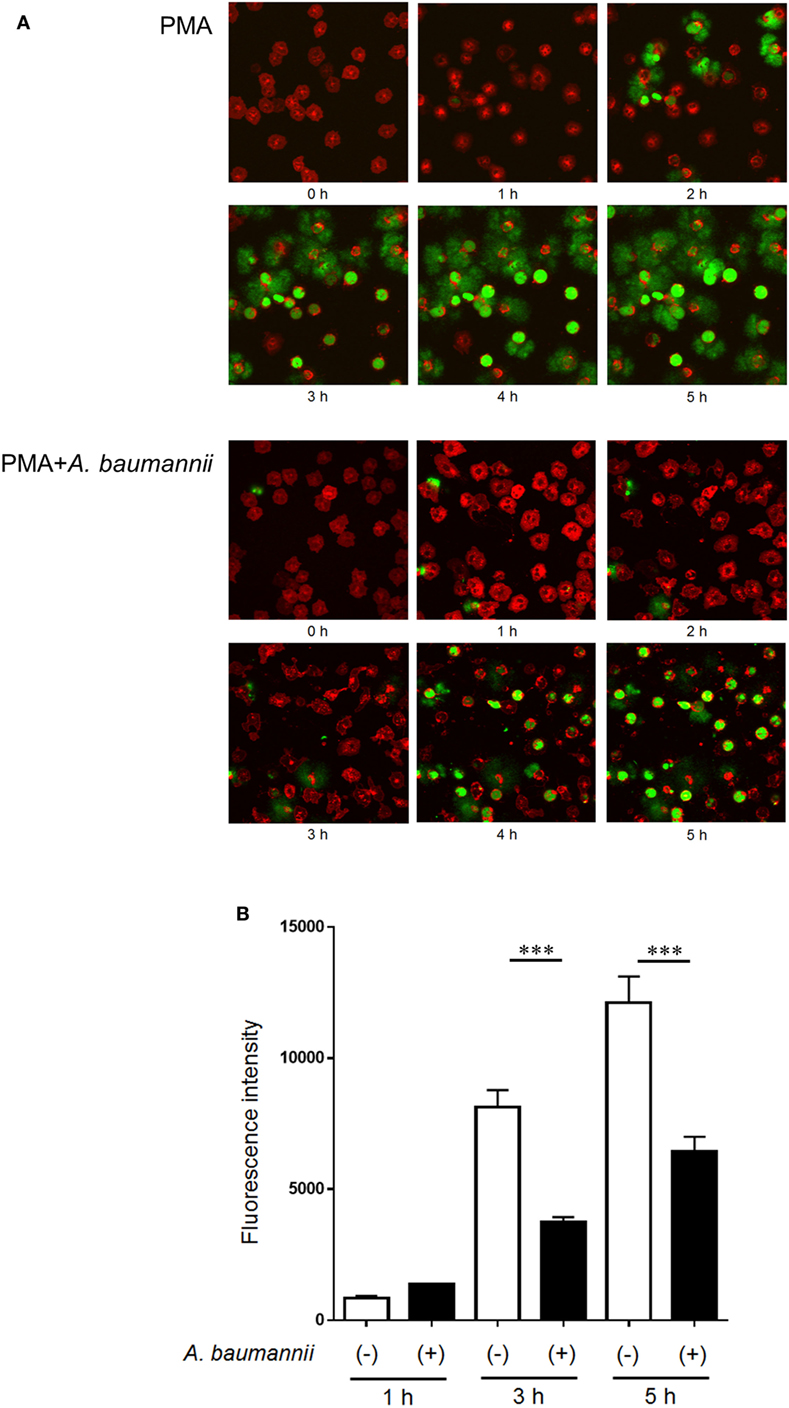
Figure 2. Kinetics of the inhibition of NET formation by Acinetobacter baumannii. (A) Fluorescently labeled neutrophils (red) and A. baumannii (MOI 50) were cocultured for 0–5 h in the presence of 200-nM phorbol 12-myristate 13-acetate (PMA); SYTOX green (green) was used to visualize the extracellular DNA. Cells were observed every minute by confocal microscopy. Hourly images are shown. (B) Quantification of DNA released by neutrophils during the experiment described in (A). The data are shown as the mean ± SD; n ≥ 3 per group. ***p < 0.001. The results are representative of at least three experiments.
To investigate the inhibitory effect of A. baumannii on NET formation in more detail, the formation of NETs was quantified in the presence of various bacteria. A. baumannii reference strain (ATCC 19606) suppressed PMA-induced NET formation in an MOI-dependent manner. MDRA clinical isolates (T14 and T2) exerted the same inhibitory effect (Figure 3A). In another experiment, A. baumannii cells were heat-killed or formalin-killed, and the NET quantification assay was performed. Upon killing, the bacteria lost the ability to inhibit NET formation (Figure 3B).
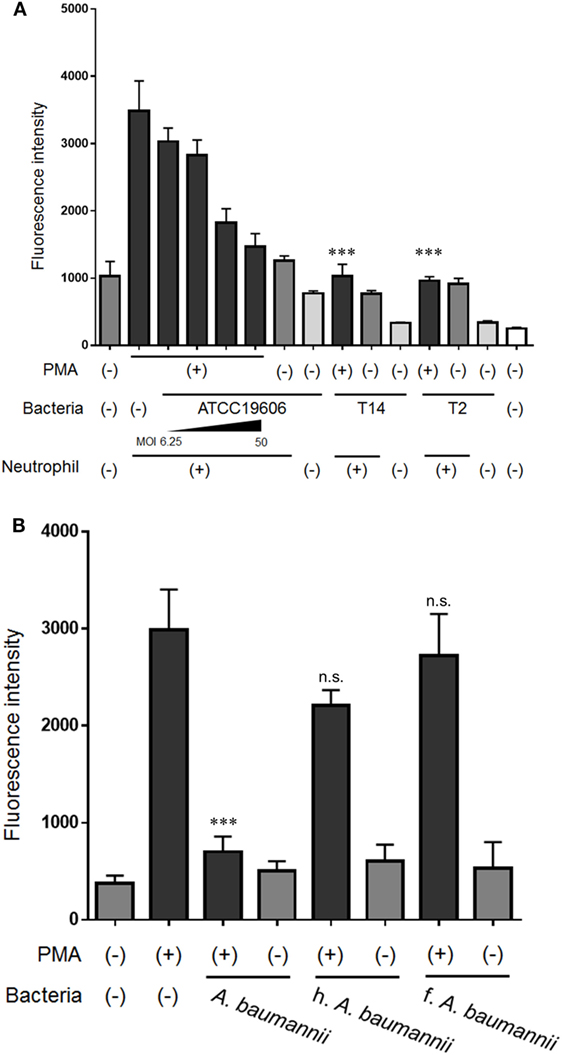
Figure 3. Inhibition of NET formation by Acinetobacter baumannii under different experimental conditions. (A) Neutrophils and ATCC 19606 (A. baumannii reference strain; MOI 6.25, 12.5, 25, and 50), or T14 or T2 (MDRA clinical isolates; MOI 50) were cocultured for 3 h, in the presence or absence of 200-nM phorbol 12-myristate 13-acetate (PMA). Extracellular DNA was stained with SYTOX green and the signal quantified. (B) NET formation in the presence of dead A. baumannii cells (MOI 50). The bacteria were either heat-killed (h.) or formalin-killed (f.). The data are shown as the mean ± SD; n ≥ 3 per group; n.s., not significant; ***p < 0.001 relative to PMA stimulation in the absence of bacteria. The results are representative of at least three experiments.
Next, the ability of bacteria other than A. baumannii to inhibit NET formation was examined. Other bacteria from the same genus, A. calcoaceticus and A. hemolyticus, and bacteria from a different genus, E. coli, did not inhibit NET formation (Figure 4). These observations suggested that only live A. baumannii cells are able to inhibit PMA-induced NET formation.
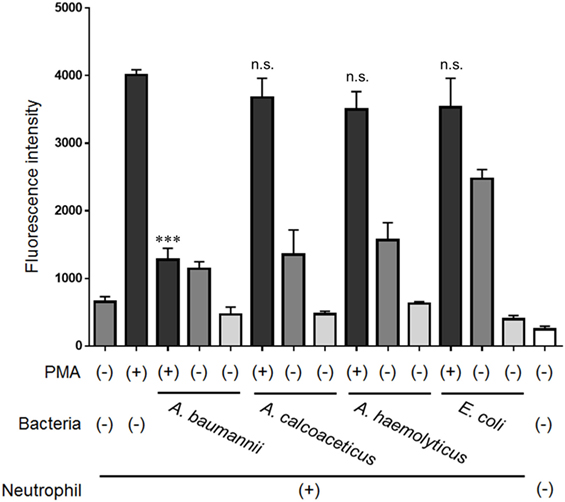
Figure 4. Inhibition of NET formation by bacteria other than Acinetobacter baumannii. Neutrophils and A. baumannii, A. calcoaceticus, A. hemolyticus, or Escherichia coli (MOI 50) were cocultured for 3 h, in the presence or absence of 200-nM phorbol 12-myristate 13-acetate (PMA). Extracellular DNA was stained with SYTOX green and the signal quantified. The data are shown as the mean ± SD; n ≥ 3 per group; n.s., not significant; ***p < 0.001 relative to PMA stimulation in the absence of bacteria. The results are representative of at least three experiments.
Neutrophil adhesion to the scaffold is thought to be required for NET formation. However, we previously demonstrated that neutrophil mobility is enhanced upon stimulation with A. baumannii (15). Furthermore, we evaluated neutrophil morphology at 1 h post PMA stimulation. The cytoplasm in PMA-stimulated neutrophils was thin and roundly spread, and these neutrophils appeared to adhere strongly. In contrast, PMA and A. baumannii-stimulated neutrophils appeared to adhere weakly compared with only PMA-stimulated neutrophils (Figure S1 in Supplementary Material). Therefore, we speculated that the adhesion ability of neutrophils was reduced upon A. baumannii stimulation. Cell-adhesion assay was performed to evaluate the adhesion ability of neutrophils upon PMA stimulation and coculture with A. baumannii. The adhesion ability was enhanced by PMA stimulation, but this enhancement was suppressed during coculture with A. baumannii (Figure 5A). Next, NET quantification assay was performed using plates coated with a cell-adhesion inhibitor, poly-2-hydroxyethyl methacrylate (poly-HEMA), to examine whether inhibition of adhesion would affect NET formation. Indeed, PMA-induced NET formation was suppressed to the A. baumannii coculture levels when the neutrophil adhesion was inhibited (Figure 5B). These observations suggested that the inhibition of neutrophil adhesion suppresses NET formation. Furthermore, we found that A. baumannii could suppress the adhesion of PMA-stimulated neutrophils.
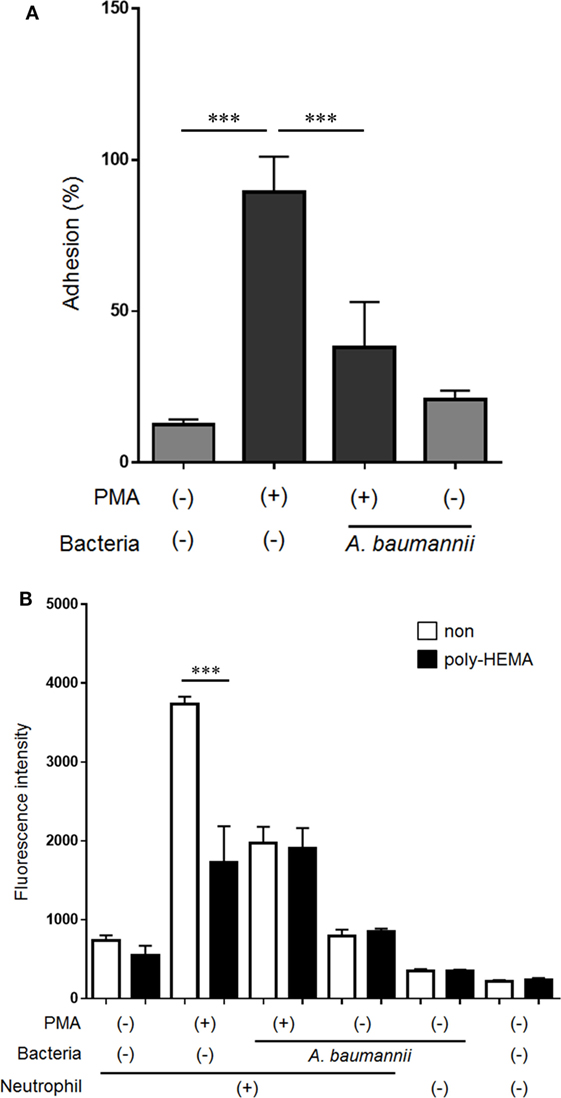
Figure 5. Acinetobacter baumannii inhibiting NET formation by suppressing neutrophil adhesion. (A) Neutrophils were stimulated with 200-nM phorbol 12-myristate 13-acetate (PMA) and cocultured with A. baumannii (MOI 50) for 30 min. The non-adhering neutrophils were removed, the adhering cells were lysed, and LDH levels determined. The adhesion rate was calculated as a percentage compared with lysed neutrophils. (B) Neutrophils were cultured for 3 h in poly-HEMA-coated plates (poly-HEMA inhibits cell adhesion), in the absence or presence of 200-nM PMA, and A. baumannii (MOI 50). Extracellular DNA was stained with SYTOX green and the signal quantified. The data are shown as the mean ± SD; n ≥ 3 per group. ***p < 0.001. The results are representative of at least three experiments.
Finally, to investigate the mechanism underlying the suppression of neutrophil adhesion upon stimulation with A. baumannii, flow cytometry was used to analyze the expression of neutrophil cell surface adhesion molecules. The levels of CD11a (integrin αL) and CD11b (integrin αM) were increased upon PMA stimulation. The enhancement of CD11a expression by PMA was suppressed upon coculture with A. baumannii (Figures 6A,C). In contrast, the enhancement of CD11b expression was further increased in the presence of A. baumannii (Figures 6B,D).
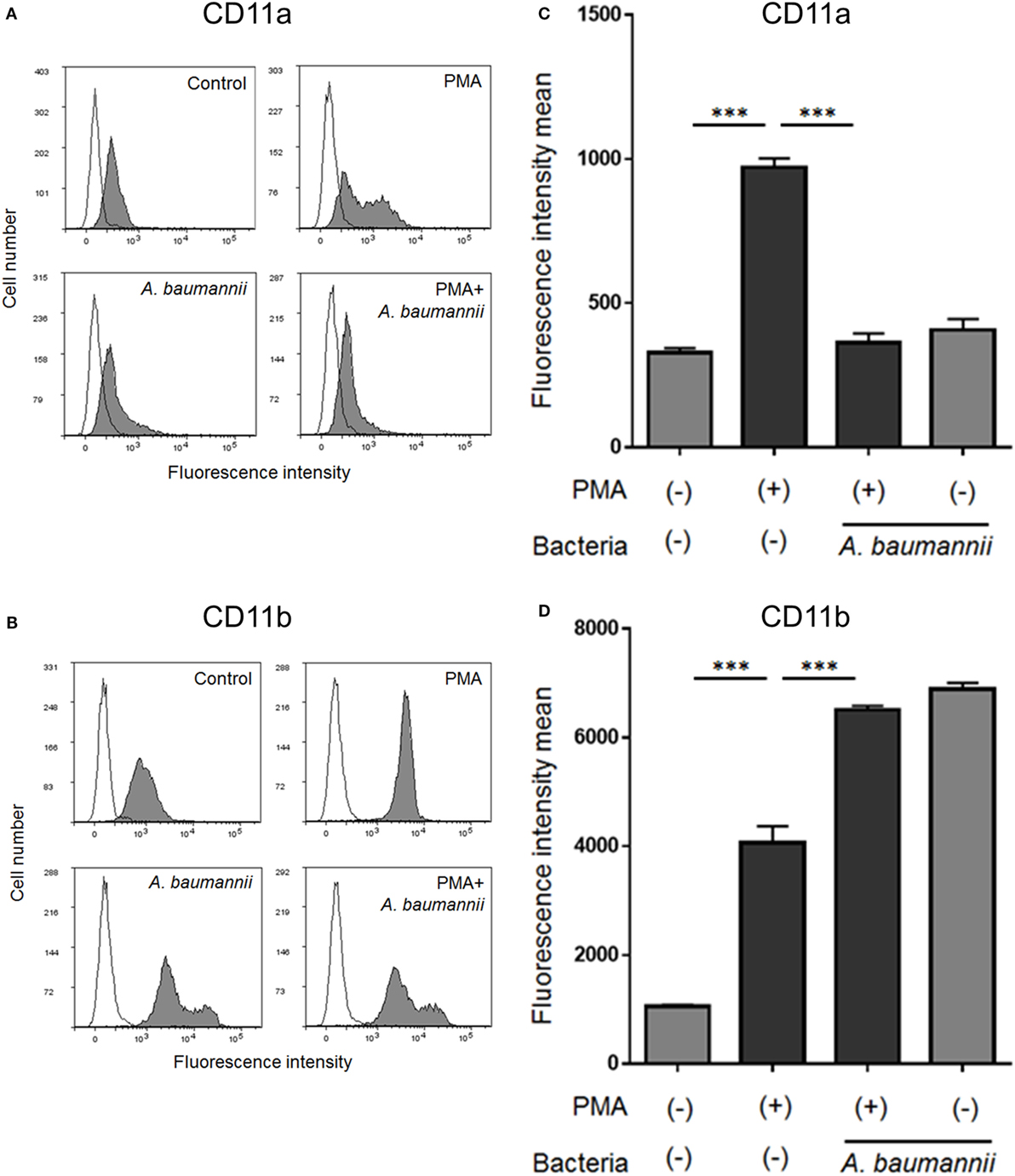
Figure 6. Changes in the cell surface expression of neutrophil molecules CD11a and CD11b. Neutrophils were stimulated with 200-nM phorbol 12-myristate 13-acetate (PMA) and cocultured with Acinetobacter baumannii (MOI 50) for 1 h. The expression of CD11a and CD11b was determined by flow cytometry using specific antibodies. Representative histograms are shown. Fluorescence profiles in the absence of antibody staining are indicated by unfilled shapes (A,B). The determined mean fluorescence intensity of samples is shown in (C,D). The data are shown as the mean ± SD; n ≥ 3 per group. ***p < 0.001. The results are representative of at least three experiments.
Because the expression of CD11a in neutrophils was suppressed by A. baumannii, the effect of CD11a on the neutrophil adhesion ability and NET formation was next assessed. The enhancement of neutrophil adhesion by PMA was reduced in the presence of a CD11a-blocking antibody (Figure 7A). Furthermore, PMA-induced NET formation was also reduced when a CD11a-blocking antibody was present (Figure 7B). Collectively, these observations suggested that A. baumannii suppressed the neutrophil expression of CD11a, thereby inhibiting PMA-induced NET formation.
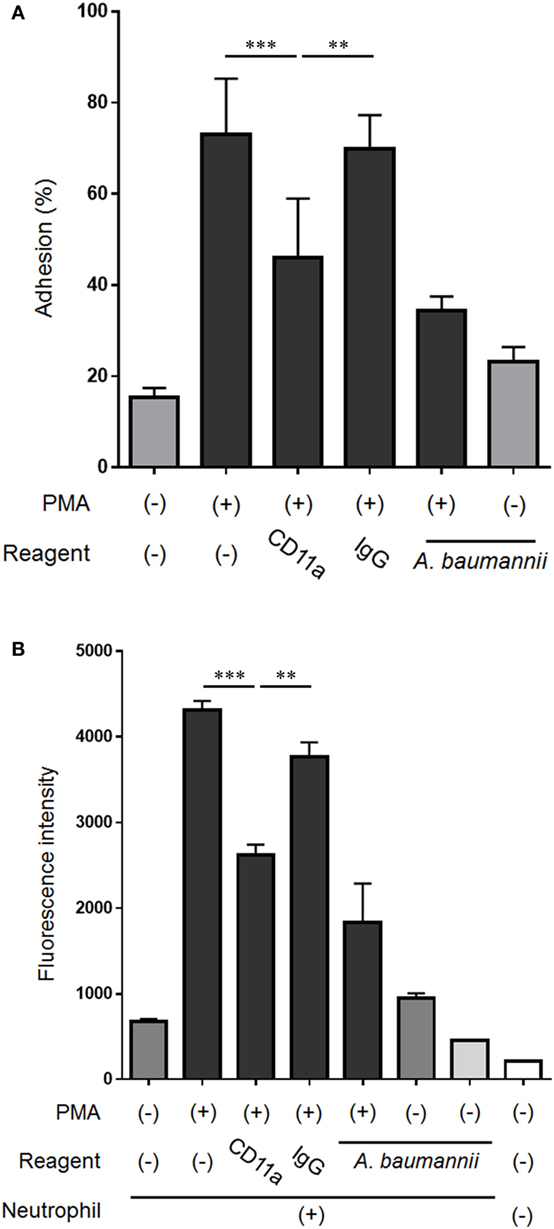
Figure 7. Effect of the neutrophil cell surface molecule CD11a on NET formation. (A) Neutrophils were stimulated with 200-nM phorbol 12-myristate 13-acetate (PMA) for 30 min, the non-adherent neutrophils were removed, the adhering cells were lysed, and LDH levels were determined. In some experiments, the assay was performed in the presence of 0.1-µg/mL CD11a-blocking antibody or control IgG, and Acinetobacter baumannii (MOI 50). The adhesion rate was calculated as a percentage compared with lysed neutrophils. (B) Neutrophils were cultured for 3 h, and the extracellular DNA was stained with SYTOX green and the signal quantified. Some experiments were performed in the presence of 0.1-µg/mL CD11a-blocking antibody or control IgG, and A. baumannii (MOI 50). The data are shown as the mean ± SD; n ≥ 3 per group. ***p < 0.001 and **p < 0.01. The results are representative of at least three experiments.
A previous study reported that the probiotic bacterium L. rhamnosus inhibits NET formation (22). The immunomodulatory effect of probiotic bacteria, including lactic acid bacteria such as L. rhamnosus, on host immune cells is well known (27). Furthermore, it was reported that sugar constituents of the Cryptococcus neoformans capsule and of the Candida albicans or Streptococcus suis biofilms modulate NET formation (28–30). However, many common pathogenic bacteria were reported to induce NET formation, while the inhibition of NET formation is rarely reported (20, 24, 25). The current study constitutes the first-ever report that A. baumannii, a common pathogenic gram-negative bacterium, exerts such an inhibitory effect. Furthermore, it is surprising that the pathogenic bacterium A. baumannii prolongs the lifespan of neutrophils by inhibiting NET formation. Previous studies reported that L. rhamnosus and microbial sugars suppress ROS production in neutrophils, thereby inhibiting NET formation. Therefore, ROS production by A. baumannii-stimulated neutrophils was also investigated. The enhancement of ROS production by PMA was not suppressed by A. baumannii stimulation (Figure S2 in Supplementary Material). Thus, A. baumannii did not appear to possess an antioxidative activity. Moreover, no capsule or biofilm formation by A. baumannii was observed under the culture conditions employed in the current study. Furthermore, L. rhamnosus was shown to inhibit bacterium-induced NET formation (22). The effect of A. baumannii on E. coli-induced NET formation was hence investigated. However, A. baumannii did not inhibit this process (Figure S3 in Supplementary Material). Various pathways of NET formation are known (25, 31, 32). Based on data presented in the current study, we suggest that A. baumannii inhibits the formation of NETs that require long (3 h or more) and strong neutrophil activation, e.g., PMA stimulation, independently of antioxidative activity.
Cell adhesion is important for neutrophil activation (16, 33). We thought that NETs are also similar, with the neutrophils unable to release their DNA into the extracellular space without adhesion, but this was clearly not the case. In the current study, we showed that PMA-induced neutrophil NET formation was suppressed when the assay was performed using plates treated with a cell-adhesion inhibitor. Furthermore, we showed that A. baumannii inhibits neutrophil adhesion by suppressing the PMA-induced CD11a expression, thereby inhibiting NET formation. However, CD11a inhibition did not completely suppress the enhancement in adhesion stimulated by PMA. CD11b inhibition also suppressed adhesion and NET formation of neutrophils induced by PMA (Figure S4 in Supplementary Material). Thus, cell adhesion is important for NET formation, and other cell-adhesion molecules may also be partially involved in this effect. In the current study, we demonstrated that CD11a is an important player in neutrophil adhesion and NET formation after PMA stimulation, and its levels are modulated by A. baumannii stimulation.
Many neutrophil surface molecules also change upon stimulation and regulate neutrophil activity (34, 35). Recently, Zawrotniak et al. reported neutrophil surface molecules and signals that are important for promoting NET formation stimulated by C. albicans components such as mannan (36). Also, glucuronoxylomannan from the C. neoformans capsule (28) and matrix mannan from the C. albicans biofilm (29) inhibit NET formation. Thus, NET formation appears to be regulated via a complex mechanism involving the microorganism component and its neutrophil surface receptors. Indeed, in this study, CD11b, CD14, CD16, TLR4, and TLR2 expression was also affected by PMA stimulation and A. baumannii cocultivation (Figures 6B,D and Figure S5 in Supplementary Material). Investigating the bacterial factor(s), associated receptor(s), and signals is thus a challenge for future studies.
Acinetobacter baumannii appears to inhibit the activation of neutrophils by suppressing their adhesion, and extends their survival after PMA stimulation. Furthermore, when the neutrophils were cocultured with A. baumannii, the extracellular LDH levels were low and the majority of neutrophils remained alive. In contrast, many neutrophils died upon coculture with E. coli or P. aeruginosa (Figure S6 and Video S3 in Supplementary Material). Although it is generally known that many common bacteria kill neutrophils by inducing apoptosis or exerting cytotoxicity (37, 38), A. baumannii did not exert such a killing effect. Furthermore, our previous studies suggested that A. baumannii avoids neutrophil defense mechanisms, such as phagocytosis, at an initial point (at 1 h) (21). It appears that A. baumannii is not recognized as a foreign body that stimulates a strong inflammatory response by neutrophils. However, this is clearly in contrast to the findings of other (39). Lazaro-Diez et al. showed that A. baumannii is indeed phagocytosed by neutrophils and induces NET formation. These discrepancies may be associated with the difference in the experimental conditions, such the duration of coculture, MOI, and culture conditions. In another report, macrophages rather than neutrophils were shown to contribute to phagocytosis during the initial stages of A. baumannii infection (40).
Neutrophils are key immune cells indispensable for the control of A. baumannii infection. Several studies reported the increase of mortality associated with A. baumannii infection upon neutrophil depletion in mouse models (41–43). The recruitment of neutrophils to the A. baumannii infection site via cytokines and chemokines has been evidenced in many studies, including our own (15, 41, 44). Immune strategies to combat A. baumannii may involve the triggering of oxidative burst and cytokine/chemokine production to amplify the immune response against the pathogen by the accumulated neutrophils (45). However, a part of A. baumannii can escape the immune response, including NETs, as demonstrated in the current study. In this case, A. baumannii would contribute to the dissemination of bacteria by utilizing the migratory capacity of neutrophils, as reported previously (15). Indeed, bacteremia and severe sepsis are caused by A. baumannii with high frequency in a compromised host (12–14). Furthermore, the bacteria are also detected in other organs, including the blood, in several mouse models of respiratory A. baumannii infection (42). The data reported in the current study suggest that A. baumannii inhibits NET formation, thereby escaping host immune responses and causing infection. This novel mechanism to inhibit NET formation might contribute to a development of new treatment strategies for A. baumannii infections.
In summary, we discovered that neutrophil adhesion is important for PMA-induced NET formation and that A. baumannii inhibits NET formation by suppressing neutrophil cell adhesion. Future studies should investigate the involved molecule(s) in detail and the mechanisms that control the expression of neutrophil CD11a, including bacterial factor(s) and intracellular signals.
Written informed consent was obtained from all study participants, in accordance with the Declaration of Helsinki. The study was approved by the Ethical Review Committee of the School of Medicine of Teikyo University.
GK designed and performed the experiments and analyzed the data. TK-U, SN, ST-N, and TU performed some of the experiments and participated in data interpretation. YO supervised the study. GK and YO cowrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to Shouichi Higashi (Yokohama City University), Dr. Teruo Akuta, Mr. Keitaro Imaizumi (Kyokuto Pharmaceutical Industrial Co.), Dr. Hirotoshi Kikuchi, Dr. Kenji Hikosaka, Dr. Ryuichi Nakano, Ms. Akiyo Nakano, Dr. Yuka Unno, and Dr. Yoshinori Sato (Teikyo University School of Medicine) for helpful discussions about this study. We would also like to thank Ms. Chizuru Miyazaki (Teikyo University School of Medicine) for technical assistance.
This work was supported in part by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (JSPS KAKENHI, 17K16230, 25890019) and The Science Research Promotion Fund of the Promotion and Mutual Aid Corporation for Private Schools of Japan.
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.00178/full#supplementary-material.
Video S1. Time-lapse observation of PMA-induced NET formation. Fluorescently-labeled neutrophils (red) were cultured for 0–5 h in the presence of 200 nM PMA; SYTOX green (green) was used to visualize the extracellular DNA. The images were captured every minute by a confocal microscope.
Video S2. Time-lapse observation of the inhibition of PMA-induced NET formation by A. baumannii. Fluorescently-labeled neutrophils (red) and A. baumannii (MOI 50) were co-cultured for 0–5 h in the presence of 200 nM PMA; SYTOX green (green) was used to visualize the extracellular DNA. The images were captured every minute by a confocal microscope.
Video S3. Time-lapse observation of neutrophils co-cultured with A. baumannii. Fluorescently-labeled neutrophils (red) and A. baumannii (MOI 50) were co-cultured for 0–5 h; SYTOX green (green) was used to visualize the extracellular DNA. The images were captured every minute by a confocal microscope.
HEMA, 2-hydroxyethyl methacrylate; LDH, lactate dehydrogenase; MDRA, multidrug-resistant Acinetobacter baumannii; MOI, multiplicity of infection; NET, neutrophil extracellular trap; PMA, phorbol 12-myristate 13-acetate; ROS, reactive oxygen species.
1. Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol (2007) 5(12):939–51. doi:10.1038/nrmicro1789
2. Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis (2008) 46(8):1254–63. doi:10.1086/529198
3. Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med (2008) 358(12):1271–81. doi:10.1056/NEJMra070741
4. Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev (2008) 21(3):538–82. doi:10.1128/CMR.00058-07
5. Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev (2017) 30(1):409–47. doi:10.1128/CMR.00058-16
6. Gaddy JA, Arivett BA, McConnell MJ, Lopez-Rojas R, Pachon J, Actis LA. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect Immun (2012) 80(3):1015–24. doi:10.1128/IAI.06279-11
7. Mortensen BL, Skaar EP. Host-microbe interactions that shape the pathogenesis of Acinetobacter baumannii infection. Cell Microbiol (2012) 14(9):1336–44. doi:10.1111/j.1462-5822.2012.01817.x
8. McConnell MJ, Actis L, Pachon J. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev (2013) 37(2):130–55. doi:10.1111/j.1574-6976.2012.00344.x
9. Mortensen BL, Skaar EP. The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host. Front Cell Infect Microbiol (2013) 3:95. doi:10.3389/fcimb.2013.00095
10. Geisinger E, Isberg RR. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog (2015) 11(2):e1004691. doi:10.1371/journal.ppat.1004691
11. Weber BS, Hennon SW, Wright MS, Scott NE, de Berardinis V, Foster LJ, et al. Genetic dissection of the type VI secretion system in Acinetobacter and identification of a novel peptidoglycan hydrolase, TagX, required for its biogenesis. MBio (2016) 7(5):e1253–1216. doi:10.1128/mBio.01253-16
12. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis (2004) 39(3):309–17. doi:10.1086/421946
13. Wisplinghoff H, Paulus T, Lugenheim M, Stefanik D, Higgins PG, Edmond MB, et al. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J Infect (2012) 64(3):282–90. doi:10.1016/j.jinf.2011.12.008
14. Leao AC, Menezes PR, Oliveira MS, Levin AS. Acinetobacter spp. are associated with a higher mortality in intensive care patients with bacteremia: a survival analysis. BMC Infect Dis (2016) 16:386. doi:10.1186/s12879-016-1695-8
15. Kamoshida G, Tansho-Nagakawa S, Kikuchi-Ueda T, Nakano R, Hikosaka K, Nishida S, et al. A novel bacterial transport mechanism of Acinetobacter baumannii via activated human neutrophils through interleukin-8. J Leukoc Biol (2016) 100(6):1405–12. doi:10.1189/jlb.4AB0116-023RR
16. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol (2006) 6(3):173–82. doi:10.1038/nri1785
17. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science (2004) 303(5663):1532–5. doi:10.1126/science.1092385
18. Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol (2007) 5(8):577–82. doi:10.1038/nrmicro1710
19. Wartha F, Henriques-Normark B. ETosis: a novel cell death pathway. Sci Signal (2008) 1(21):e25. doi:10.1126/stke.121pe25
20. Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol (2012) 198(5):773–83. doi:10.1083/jcb.201203170
21. Kamoshida G, Kikuchi-Ueda T, Tansho-Nagakawa S, Nakano R, Nakano A, Kikuchi H, et al. Acinetobacter baumannii escape from neutrophil extracellular traps (NETs). J Infect Chemother (2015) 21(1):43–9. doi:10.1016/j.jiac.2014.08.032
22. Vong L, Lorentz RJ, Assa A, Glogauer M, Sherman PM. Probiotic Lactobacillus rhamnosus inhibits the formation of neutrophil extracellular traps. J Immunol (2014) 192(4):1870–7. doi:10.4049/jimmunol.1302286
23. Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol (2007) 176(2):231–41. doi:10.1083/jcb.200606027
24. White PC, Chicca IJ, Cooper PR, Milward MR, Chapple IL. Neutrophil extracellular traps in periodontitis: a web of intrigue. J Dent Res (2016) 95(1):26–34. doi:10.1177/0022034515609097
25. Delgado-Rizo V, Martinez-Guzman MA, Iniguez-Gutierrez L, Garcia-Orozco A, Alvarado-Navarro A, Fafutis-Morris M. Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol (2017) 8:81. doi:10.3389/fimmu.2017.00081
26. Kamoshida G, Kikuchi-Ueda T, Nishida S, Tansho-Nagakawa S, Kikuchi H, Ubagai T, et al. Spontaneous formation of neutrophil extracellular traps in serum-free culture conditions. FEBS Open Bio (2017) 7(6):877–86. doi:10.1002/2211-5463.12222
27. Erickson KL, Hubbard NE. Probiotic immunomodulation in health and disease. J Nutr (2000) 130(2S Suppl):403S–9S. doi:10.1093/jn/130.2.403S
28. Rocha JD, Nascimento MT, Decote-Ricardo D, Corte-Real S, Morrot A, Heise N, et al. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci Rep (2015) 5:8008. doi:10.1038/srep08008
29. Johnson CJ, Cabezas-Olcoz J, Kernien JF, Wang SX, Beebe DJ, Huttenlocher A, et al. The extracellular matrix of Candida albicans biofilms impairs formation of neutrophil extracellular traps. PLoS Pathog (2016) 12(9):e1005884. doi:10.1371/journal.ppat.1005884
30. Ma F, Yi L, Yu N, Wang G, Ma Z, Lin H, et al. Streptococcus suis serotype 2 biofilms inhibit the formation of neutrophil extracellular traps. Front Cell Infect Microbiol (2017) 7:86. doi:10.3389/fcimb.2017.00086
31. Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol (2010) 185(12):7413–25. doi:10.4049/jimmunol.1000675
32. Rochael NC, Guimaraes-Costa AB, Nascimento MT, DeSouza-Vieira TS, Oliveira MP, Garcia e Souza LF, et al. Classical ROS-dependent and early/rapid ROS-independent release of neutrophil extracellular traps triggered by Leishmania parasites. Sci Rep (2015) 5:18302. doi:10.1038/srep18302
33. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol (2011) 11(8):519–31. doi:10.1038/nri3024
34. Kuijpers TW, Tool AT, van der Schoot CE, Ginsel LA, Onderwater JJ, Roos D, et al. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood (1991) 78(4):1105–11.
35. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol (2013) 13(3):159–75. doi:10.1038/nri3399
36. Zawrotniak M, Bochenska O, Karkowska-Kuleta J, Seweryn-Ozog K, Aoki W, Ueda M, et al. Aspartic proteases and major cell wall components in Candida albicans trigger the release of neutrophil extracellular traps. Front Cell Infect Microbiol (2017) 7:414. doi:10.3389/fcimb.2017.00414
37. Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res (2009) 43(1–3):25–61. doi:10.1007/s12026-008-8049-6
38. Los FC, Randis TM, Aroian RV, Ratner AJ. Role of pore-forming toxins in bacterial infectious diseases. Microbiol Mol Biol Rev (2013) 77(2):173–207. doi:10.1128/MMBR.00052-12
39. Lazaro-Diez M, Chapartegui-Gonzalez I, Redondo-Salvo S, Leigh C, Merino D, Segundo DS, et al. Human neutrophils phagocytose and kill Acinetobacter baumannii and A. pittii. Sci Rep (2017) 7(1):4571. doi:10.1038/s41598-017-04870-8
40. Qiu H, KuoLee R, Harris G, Van Rooijen N, Patel GB, Chen W. Role of macrophages in early host resistance to respiratory Acinetobacter baumannii infection. PLoS One (2012) 7(6):e40019. doi:10.1371/journal.pone.0040019
41. van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun (2007) 75(12):5597–608. doi:10.1128/IAI.00762-07
42. Breslow JM, Meissler JJ Jr, Hartzell RR, Spence PB, Truant A, Gaughan J, et al. Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance. Infect Immun (2011) 79(8):3317–27. doi:10.1128/IAI.00069-11
43. Tsuchiya T, Nakao N, Yamamoto S, Hirai Y, Miyamoto K, Tsujibo H. NK1.1(+) cells regulate neutrophil migration in mice with Acinetobacter baumannii pneumonia. Microbiol Immunol (2012) 56(2):107–16. doi:10.1111/j.1348-0421.2011.00402.x
44. Bhuiyan MS, Ellett F, Murray GL, Kostoulias X, Cerqueira GM, Schulze KE, et al. Acinetobacter baumannii phenylacetic acid metabolism influences infection outcome through a direct effect on neutrophil chemotaxis. Proc Natl Acad Sci U S A (2016) 113(34):9599–604. doi:10.1073/pnas.1523116113
Keywords: Acinetobacter baumannii, neutrophil, neutrophil extracellular trap, adhesion, CD11a
Citation: Kamoshida G, Kikuchi-Ueda T, Nishida S, Tansho-Nagakawa S, Ubagai T and Ono Y (2018) Pathogenic Bacterium Acinetobacter baumannii Inhibits the Formation of Neutrophil Extracellular Traps by Suppressing Neutrophil Adhesion. Front. Immunol. 9:178. doi: 10.3389/fimmu.2018.00178
Received: 07 November 2017; Accepted: 19 January 2018;
Published: 07 February 2018
Edited by:
Tamás Laskay, University of Lübeck, GermanyReviewed by:
Maria Rapala-Kozik, Jagiellonian University, PolandCopyright: © 2018 Kamoshida, Kikuchi-Ueda, Nishida, Tansho-Nagakawa, Ubagai and Ono. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Go Kamoshida, a2Ftb3NoaWRhQG1lZC50ZWlreW8tdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.