- 1Department of Virology, Nagoya University Graduate School of Medicine, Nagoya, Japan
- 2Medical Virology Section, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, United States
Chronic active Epstein–Barr virus (CAEBV) disease is a rare disorder in which persons are unable to control infection with the virus. The disease is progressive with markedly elevated levels of EBV DNA in the blood and infiltration of organs by EBV-positive lymphocytes. Patients often present with fever, lymphadenopathy, splenomegaly, EBV hepatitis, or pancytopenia. Over time, these patients develop progressive immunodeficiency and if not treated, succumb to opportunistic infections, hemophagocytosis, multiorgan failure, or EBV-positive lymphomas. Patients with CAEBV in the United States most often present with disease involving B or T cells, while in Asia, the disease usually involves T or NK cells. The only proven effective treatment for the disease is hematopoietic stem cell transplantation. Current studies to find a cause of this disease focus on immune defects and genetic abnormalities associated with the disease.
Introduction
Primary infection of adolescents and young adults often results in infectious mononucleosis with fever, lymphadenopathy, and sore throat (1). Additional signs and symptoms include splenomegaly, hepatomegaly, lymphocytosis, and liver dysfunction. Fever and lymphadenopathy usually resolve within 2 weeks after onset but can persist for a month, or in rare cases even longer. EBV is present in circulating B cells, and the level of EBV DNA is elevated in the blood for the first month of the illness. Both the innate immune response (especially NK cells) and the acquired immune response (virus-specific CD4 and CD8 cells) have a critical role in clearing the infection (2).
Initial control of EBV in healthy persons involves NK cells that can kill virus-infected cells (3, 4) and secrete IFN-γ, which inhibits B cell proliferation, and monocytes, which release chemokines in response to virus infection (5). A large clonal or oligoclonal expansion of CD8 cells is observed during infectious mononucleosis (6). Most CD8 cells are directed to lytic antigens initially, and these cells rapidly undergo apoptosis (7). These patients have modestly elevated antibodies to EBV lytic antigens as well as antibodies to the EBV nuclear antigens (EBNAs), including EBNA1.
Rare patients who become infected with EBV, or reactivate EBV, develop disease that does not resolve. Some of these patients develop fulminant infectious mononucleosis and die within days or weeks of primary infection. Others develop a more chronic course with persistent or intermittent infectious mononucleosis-like symptoms including fever, persistent lymphadenopathy, splenomegaly, and EBV hepatitis. These patients are unable to control EBV infection and have infiltration of tissues by EBV positive T, NK, or less often B cells. They have markedly elevated levels of EBV that persist in the blood. This entity is referred to as chronic active EBV (CAEBV) disease.
Some patients with CAEBV have been reported to have impaired NK cell (8) or T cell activity (9–13) against EBV-infected cells. In addition, reduced numbers of EBV-specific T cells have been described in patients with CAEBV disease (10). Unlike healthy persons with infectious mononucleosis, patients with CAEBV disease often have low numbers of EBV-specific CD8 cells (10). A recent study showed that patients with CAEBV or infectious mononucleosis have a decrease in the TCR-beta repertoire and expanded T cell clones in their peripheral blood compared with healthy carriers of EBV (14). Many have extremely high levels of antibodies to EBV lytic proteins and lack antibody to EBNA1 (13).
CAEBV Definition and Features
Chronic active Epstein–Barr virus disease is usually defined as a chronic illness lasting at least 6 months, an increased EBV level in either the tissue or the blood, and lack of evidence of a known underlying immunodeficiency (15). Other authors, particularly when defining severe CAEBV disease, require both an elevated level of EBV in the blood as well as infiltration of tissues by EBV-positive lymphocytes (16). Recently, the duration of illness required for defining the disease has been shortened to 3 months (17). Former definitions required elevated levels of antibody to EBV viral capsid or early antigen in the blood (18); however, we have found that elevated levels of EBV DNA in the blood are more specific for CAEBV than elevated levels of EBV antibodies. Most laboratories now perform ELISA tests for EBV antibodies, and these are often less helpful than the previously used quantitative immunofluorescent assay using endpoint dilution of serum. It is important that DNA PCR is done using either whole blood or peripheral blood mononuclear cells, rather than plasma or serum which is much less sensitive for diagnosis of CAEBV disease.
Chronic active Epstein–Barr virus disease was originally reported in children during primary infection, but in recent years, perhaps with increasing recognition of the disease, CAEBV disease has been reported in adults as well (19). CAEBV disease may be indolent with episodic fever, lymphadenopathy, and viral hepatitis followed by periods that are nearly asymptomatic; however, during these asymptomatic periods, the Epstein–Barr viral load remains very elevated. Alternatively, the disease can have a persistent or even fulminant presentation with death occurring in a few weeks. CAEBV disease is more frequent in Asians and in persons from South and Central America and Mexico. In these patients, EBV is predominantly present in T cells (Figure 1) or NK cells (20). In contrast, patients from the United States with CAEBV more often have EBV in B or T cells (16). In most healthy persons, EBV is latent in B cells; however, EBV can sometimes be detected in T and NK cells in the tonsils (21), and virus has been detected in T cells in persons with HIV (22) and other lymphoproliferative diseases (23, 24). At present, it is unclear how the virus enters T and NK cells; these cells do not express CD21, the EBV receptor.
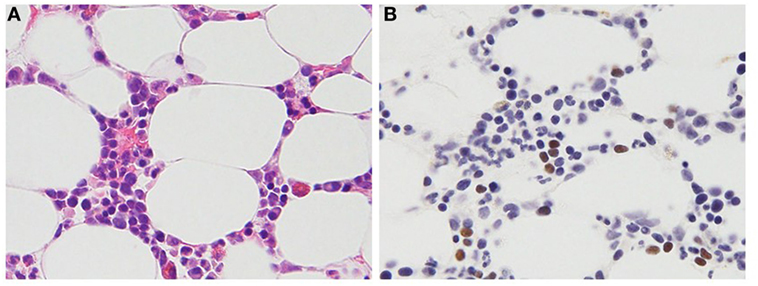
Figure 1. Histopathologic features of a 47-year-old female patient with T cell chronic active Epstein–Barr virus (EBV) disease. (A) Hematoxylin and eosin stain. Small- to medium-sized lymphocytes without significant atypia infiltrate the bone marrow clot. (B) EBV-encoded RNA in situ hybridization. The brown staining lymphocytes are positive for EBV RNA.
Epstein–Barr virus gene expression in patients with CAEBV disease varies. There are four patterns of EBV gene expression, ranging from type 0 with no viral proteins expressed, although EBV EBV-encoded RNA and BART RNAs are expressed, to type 3 with all the latent viral proteins expressed including the EBV nuclear antigens (EBNAs) 1, 2, 3A–C, and LP, and latent membrane proteins (LMP) 1 and 2. Type 1 latency involves expression of EBNA1 and no other proteins; with type 2 latency, EBNA1, LMP1, and LMP2 are expressed. Patients with infectious mononucleosis have type 3 latency, whereas healthy EBV carriers have type 0 latency. Type 1 latency is seen in Burkitt lymphoma and type 2 in nasopharyngeal carcinoma, Hodgkin lymphoma, peripheral T cell lymphoma, angioimmunoblastic T cell lymphoma, and extranodal NK/T cell lymphoma (25). Most patients with CAEBV disease express a limited number of EBV latency genes. Although many patients have been reported with a type 2 latency pattern (26, 27), other patterns of EBV gene expression have also been reported, including type 3 (28). Thus, patients with T and NK cell CAEBV have a latency pattern that resembles that seen in EBV-positive T cell and NK cell lymphomas. These findings are consistent with a recent study showing that the cellular gene expression profile in patients with NK cell CAEBV is similar to that in NK cell lymphoma (29).
Epstein–Barr virus can be clonal, oligoclonal, or polyclonal in peripheral blood mononuclear cells of patients with CAEBV disease. Clonality for CAEBV has been based on PCR of the T cell receptor genes (for T cell CAEBV) or IgH genes (for EBV B cell disease) (16) or on the terminal repeat structure of the EBV genome (20). In one study of 17 patients, most patients had clonal EBV (27). Clonality does not necessarily indicate a worse prognosis (20).
Cells from patients with CAEBV can express both T-helper (TH1) (e.g., interferon-γ, IL-1β, IL-2) and TH2 (IL-4, IL-10, IL-13) cytokines (30). This failure to express a predominantly antiviral TH1 pattern has been referred to as an “unbalanced cytokine profile.” Patients with NK cell CAEBV disease were reported to have higher levels of IL-13 than those with T cell disease (27). Plasma levels of certain EBV microRNAs expressed from the BamH1 A fragment rightward transcript (BART) are higher in persons with CAEBV disease than in those with infectious mononucleosis or healthy controls (31). These findings suggest that these may be biomarkers useful for following these patients.
Etiology
Initial reports suggested that CAEBV disease may be due to an unusual strain of EBV that results in lytic replication, but is impaired for transformation (32, 33), or a strain with a deletion in the viral genome (34). However, a subsequent study by one of these groups (35) showed that the unaffected father of the patient with CAEBV disease and some healthy controls had the same lytic strain of the virus as the patient with CAEBV, indicating that the unusual strain of EBV was not the cause of the disease.
Several features of CAEBV suggest that there is likely a genetic etiology. First, the impaired cytotoxic activity of T or NK cells (cited above) suggests that the disease could be due to an immunodeficiency. Second, the increased rate of the disease in Asians or natives of Central or South America suggests that the genetic background may play a role in the disease.
One study reported CAEBV in family members (36); however most recent cases do not describe multiple family members with the disease (16, 37). Studies have not found a consistent cause for CAEBV disease. Patients with meeting the definition of CAEBV B cell disease were subsequently found to have compound heterozygous mutations in perforin (38), compound heterozygous mutations in Munc13-4 (39), homozygous or compound heterozygous mutations in Munc 18-2 (39, 40), a heterozygous gain-of-function mutation in phosphoinositide 3-kinase p110δ (41), a mutation in MAGT1 (42), a mutation in GATA2 (43), and homozygous mutations in CTPS1 (44). In each of the patients tested, EBV was predominantly in B cells. At present, no single genetic defect has been associated with a large proportion of patients with CAEBV disease.
Recent comprehensive genetic analysis by whole-exome sequencing showed that germline mutations are rare in CAEBV, but somatic driver mutations are frequently found in EBV-infected cells (45). Driver mutations including DDX3X and other genes associated with hematologic malignancies have been shown to accumulate in EBV-infected T/NK cells. In a case in which serial samples were obtained, clonal evolution of EBV-infected cells was confirmed with branching mutations in DDX3X. Mutations in DDX3X are frequently seen in Burkitt lymphoma and extranodal NK/T cell lymphoma (46, 47). These results indicate that serial acquisition of mutations in EBV-infected NK or T cells have the potential to result in transformation of the cells and may contribute to lymphomagenesis in this disease.
Although no single genetic defect has been identified in CAEBV disease, a positive association with human leukocyte antigen (HLA) A26 and a negative association with B52 were observed (48). Interestingly, both the A26 and B52 alleles are frequently seen in East Asia and Mexico, where the prevalence of the disease is high. Associations with HLA loci have been reported in other EBV-associated malignancies that show geographically distinct distributions (49, 50).
CAEBV in the United States
In the largest series of CAEBV reported in the United States, EBV was often detected in B cells in tissues from patients, with cases of T and NK cell disease less common (16). The age of onset ranged from 4 to 51 years (mean 19 years). Patients with T cell disease were younger (mean age 7 years) than those with B cell disease (mean age 23 years). Lymphadenopathy and splenomegaly were the most frequent signs and symptoms, followed by fever, hepatitis, hypogammaglobulinemia, pancytopenia, hemophagocytosis, and hepatomegaly. Less common symptoms included pneumonitis, central nervous system disease, and periphery neuropathy. Some patients had B cell lymphopenia, others had reduced numbers of NK cells, and some had low numbers of both cells. Deaths were most often due to progressive EBV lymphoproliferative disease or opportunistic infections.
CAEBV in Asia
T or NK cell CAEBV has a geographical predisposition, with most cases occurring in East Asians and some cases in Native American populations in the Western hemisphere (16). This distribution is analogous to that of extranodal NK/T cell lymphoma, also referred to as nasal NK/T-cell lymphoma. In Japan, nearly 60% of cases of CAEBV are T cell type, while 40% are NK cell type (37). EBV-infected T cells are variable: CD4+ T cells, CD8+ T cells, CD4+ and CD8+ T cells, CD4− and CD8− T cells, and γδ T cells have all been reported as the predominant cell type in individual patients with CAEBV. EBV-infected T or NK cells usually express cytotoxic molecules, such as perforin, granzyme, and T-cell intracytoplasmic antigen (TIA)-1 (51, 52), indicating that they have a cytotoxic cell phenotype.
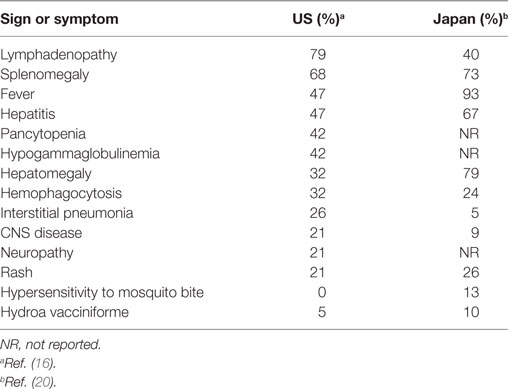
The age at the onset of CAEBV in Asia ranged from 9 months to 53 years (mean, 11.3 years) (20). The signs and symptoms of CAEBV differ in frequency in the US and in Asia (Table 1). Typically in Asia, patients develop fever, hepatosplenomegaly, and lymphadenopathy; other common symptoms are thrombocytopenia, anemia, skin rash, diarrhea, and uveitis (20). The disease is sometimes complicated by hemophagocytic syndrome, coagulopathy, digestive tract ulcer/perforation, central nervous system involvement, myocarditis, interstitial pneumonia, multi-organ failure and sepsis (20). Interstitial pneumonia, calcifications in basal ganglia, and coronary aneurysms are occasionally seen without any symptoms. Some patients may have skin symptoms, such as hypersensitivity to mosquito bites and hydroa vacciniforme. Patients with severe mosquito bite allergy generally have EBV-infected NK cells, whereas those with hydroa vacciniforme often have EBV-infected γδ T cells (37). Patients with CAEBV sometimes develop T or NK cell neoplasms such as extranodal NK/T cell lymphoma, aggressive NK cell leukemia, and peripheral T cell lymphoma (37).
Treatment and Prognosis
In the absence of treatment, patients with CAEBV develop progressive cellular and humoral immunodeficiencies and develop opportunistic infections, hemophagocytosis, multi-organ failure, or EBV-positive B, T, or NK cell lymphomas (53). CAEBV is refractory to antiviral therapy, interferon, intravenous immunoglobulin, and conventional chemotherapy and thus has a poor prognosis. Many other treatments have been tried including immunosuppressive agents such as cyclosporine or corticosteroids, autologous EBV-specific cytotoxic T cells, rituximab in the case of B cell CAEV, and the combination of bortezomib and ganciclovir. In some cases, these other treatments have resulted in transient reductions in systemic symptoms with improvement in laboratory abnormalities; however, the disease eventually returns and patients succumb to their disease if they do not undergo hematopoietic stem cell transplantation.
The survival of patients with T cell-type CAEBV is significantly lower, compared with that of patients with NK cell-type CAEBV (20). Hematopoietic stem cell transplantation alone is a curative treatment for the disease, although the incidence of transplantation-related complications is high (54, 55).
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases. We thank Dr. Seiichi Kato, Aichi Cancer Institute, Japan for the photomicrograph of the pathology slides.
References
1. Balfour HH Jr, Holman CJ, Hokanson KM, Lelonek MM, Giesbrecht JE, White DR, et al. A prospective clinical study of Epstein-Barr virus and host interactions during acute infectious mononucleosis. J Infect Dis (2005) 192:1505–12. doi:10.1086/491740
2. Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol (2015) 33:787–821. doi:10.1146/annurev-immunol-032414-112326
3. Djaoud Z, Guethlein LA, Horowitz A, Azzi T, Nemat-Gorgani N, Olive D, et al. Two alternate strategies for innate immunity to Epstein-Barr virus: one using NK cells and the other NK cells and γδ T cells. J Exp Med (2017) 214:1827–41. doi:10.1084/jem.20161017
4. Chijioke O, Landtwing V, Münz C. NK cell influence on the outcome of primary Epstein-Barr virus infection. Front Immunol (2016) 7:323. doi:10.3389/fimmu.2016.00323
5. Gaudreault E, Fiola S, Olivier M, Gosselin J. Epstein-Barr virus induces MCP-1 secretion by human monocytes via TLR2. J Virol (2007) 81:8016–24. doi:10.1128/JVI.00403-07
6. Callan MF, Steven N, Krausa P, Wilson JD, Moss PA, Gillespie GM, et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med (1996) 2:906–11. doi:10.1038/nm0896-906
7. Callan MF, Fazou C, Yang H, Rostron T, Poon K, Hatton C, et al. CD8(+) T-cell selection, function, and death in the primary immune response in vivo. J Clin Invest (2000) 106:1251–61. doi:10.1172/JCI10590
8. Joncas J, Monczak Y, Ghibu F, Alfieri C, Bonin A, Ahronheim G, et al. Brief report: killer cell defect and persistent immunological abnormalities in two patients with chronic active Epstein-Barr virus infection. J Med Virol (1989) 28:110–7. doi:10.1002/jmv.1890280211
9. Fujieda M, Wakiguchi H, Hisakawa H, Kubota H, Kurashige T. Defective activity of Epstein-Barr virus (EBV) specific cytotoxic T lymphocytes in children with chronic active EBV infection and in their parents. Acta Paediatr Jpn (1993) 35:394–9. doi:10.1111/j.1442-200X.1993.tb03079.x
10. Sugaya N, Kimura H, Hara S, Hoshino Y, Kojima S, Morishima T, et al. Quantitative analysis of Epstein-Barr virus (EBV)-specific CD8+ T cells in patients with chronic active EBV infection. J Infect Dis (2004) 190:985–8. doi:10.1086/423285
11. Tsuge I, Morishima T, Kimura H, Kuzushima K, Matsuoka H. Impaired cytotoxic T lymphocyte response to Epstein-Barr virus-infected NK cells in patients with severe chronic active EBV infection. J Med Virol (2001) 64:141–8. doi:10.1002/jmv.1029
12. Kimura H, Tsuge I, Imai S, Yamamoto M, Kuzushima K, Osato T, et al. Intact antigen presentation for Epstein-Barr virus (EBV)-specific CTL by a lymphoblastoid cell line established from a patient with severe chronic active EBV infection. Med Microbiol Immunol (1995) 184:63–8. doi:10.1007/BF00221388
13. Xing Y, Song HM, Wei M, Liu Y, Zhang YH, Gao L. Clinical significance of variations in levels of Epstein-Barr virus (EBV) antigen and adaptive immune response during chronic active EBV infection in children. J Immunotoxicol (2013) 10:387–92. doi:10.3109/1547691X.2012.758199
14. Liu S, Zhang Q, Huang D, Zhang W, Zhong F, Feng J, et al. Comprehensive assessment of peripheral blood TCRβ repertoire in infectious mononucleosis and chronic active EBV infection patients. Ann Hematol (2017) 96:665–80. doi:10.1007/s00277-016-2911-8
15. Kimura H. Pathogenesis of chronic active Epstein-Barr virus infection: is this an infectious disease, lymphoproliferative disorder, or immunodeficiency? Rev Med Virol (2006) 16:251–61. doi:10.1002/rmv.505
16. Cohen JI, Jaffe ES, Dale JK, Pittaluga S, Heslop HE, Rooney CM, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood (2011) 117:5835–49. doi:10.1182/blood-2010-11-316745
17. Quintanilla-Martinez L, Ko YH, Kimura H, Jaffe ES. EBV-positive T-cell and NK-cell lymphoproliferative diseases of childhood. 4th ed. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press (2017). p. 355–62.
18. Okano M, Kawa K, Kimura H, Yachie A, Wakiguchi H, Maeda A, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol (2005) 80:64–9. doi:10.1002/ajh.20398
19. Arai A, Imadome K, Watanabe Y, Yoshimori M, Koyama T, Kawaguchi T, et al. Clinical features of adult-onset chronic active Epstein-Barr virus infection: a retrospective analysis. Int J Hematol (2011) 93:602–9. doi:10.1007/s12185-011-0831-x
20. Kimura H, Morishima T, Kanegane H, Ohga S, Hoshino Y, Maeda A, et al. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis (2003) 187:527–33. doi:10.1086/367988
21. Hudnall SD, Ge Y, Wei L, Yang NP, Wang HQ, Chen T. Distribution and phenotype of Epstein-Barr virus-infected cells in human pharyngeal tonsils. Mod Pathol (2005) 18:519–27. doi:10.1038/modpathol.3800369
22. Bekker V, Scherpbier H, Beld M, Piriou E, van Breda A, Lange J, et al. Epstein-Barr virus infects B and non-B lymphocytes in HIV-1-infected children and adolescents. J Infect Dis (2006) 194:1323–30. doi:10.1086/508197
23. Pallesen G, Hamilton-Dutoit SJ, Zhou X. The association of Epstein-Barr virus (EBV) with T-cell lymphoproliferations and Hodgkin’s disease: two new developments in the EBV field. Adv Cancer Res (1993) 62:179–239. doi:10.1016/S0065-230X(08)60319-X
24. Calattini S, Sereti I, Scheinberg P, Kimura H, Childs RW, Cohen JI. Detection of EBV genomes in plasmablasts/plasma cells and non-B cells in the blood of most patients with EBV lymphoproliferative disorders by using immuno-FISH. Blood (2010) 116:4546–59. doi:10.1182/blood-2010-05-285452
25. Longnecker L, Kieff E, Cohen JI. Epstein-Barr virus. 6th ed. In: Knipe DM, Howley PM, Cohen JI, Griffith DE, Lamb RA, Martin MA, et al., editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins (2013). p. 1898–959.
26. Iwata S, Wada K, Tobita S, Gotoh K, Ito Y, Demachi-Okamura A, et al. Quantitative analysis of Epstein-Barr virus (EBV)-related gene expression in patients with chronic active EBV infection. J Gen Virol (2010) 91:42–50. doi:10.1099/vir.0.013482-0
27. Kimura H, Hoshino Y, Hara S, Sugaya N, Kawada J, Shibata Y, et al. Differences between T cell-type and natural killer cell-type chronic active Epstein-Barr virus infection. J Infect Dis (2005) 191:531–9. doi:10.1086/427239
28. Yoshioka M, Ishiguro N, Ishiko H, Ma X, Kikuta H, Kobayashi K. Heterogeneous, restricted patterns of Epstein-Barr virus (EBV) latent gene expression in patients with chronic active EBV infection. J Gen Virol (2001) 82:2385–92. doi:10.1099/0022-1317-82-10-2385
29. Washio K, Oka T, Abdalkader L, Muraoka M, Shimada A, Oda M, et al. Gene expression analysis of hypersensitivity to mosquito bite, chronic active EBV infection and NK/T-lymphoma/leukemia. Leuk Lymphoma (2017) 58:2683–94. doi:10.1080/10428194.2017.1304762
30. Ohga S, Nomura A, Takada H, Ihara K, Kawakami K, Yanai F, et al. Epstein-Barr virus (EBV) load and cytokine gene expression in activated T cells of chronic active EBV infection. J Infect Dis (2001) 183:1–7. doi:10.1086/317653
31. Kawano Y, Iwata S, Kawada J, Gotoh K, Suzuki M, Torii Y, et al. Plasma viral microRNA profiles reveal potential biomarkers for chronic active Epstein-Barr virus infection. J Infect Dis (2013) 208:771–9. doi:10.1093/infdis/jit222
32. Alfieri C, Ghibu F, Joncas JH. Lytic, nontransforming Epstein-Barr virus (EBV) from a patient with chronic active EBV infection. Can Med Assoc J (1984) 131:1249–52.
33. Schwarzmann F, von Baehr R, Jager M, Prang N, Böhm S, Reischl U, et al. A case of severe chronic active infection with Epstein-Barr virus: immunologic deficiencies associated with a lytic virus strain. Clin Infect Dis (1999) 29:626–31. doi:10.1086/598645
34. Schooley RT, Carey RW, Miller G, Henle W, Eastman R, Mark EJ, et al. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis. Clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med (1986) 104:636–43. doi:10.7326/0003-4819-104-5-636
35. Alfieri C, Joncas JH. Biomolecular analysis of a defective nontransforming Epstein-Barr virus (EBV) from a patient with chronic active EBV infection. J Virol (1987) 61:3306–9.
36. Joncas JH, Ghibu F, Blagdon M, Montplaisir S, Stefanescu I, Menezes J. A familial syndrome of susceptibility to chronic active Epstein-Barr virus infection. Can Med Assoc J (1984) 130:280–4.
37. Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood (2012) 119:673–86. doi:10.1182/blood-2011-10-381921
38. Katano H, Ali MA, Patera AC, Catalfamo M, Jaffe ES, Kimura H, et al. Chronic active Epstein-Barr virus infection associated with mutations in perforin that impair its maturation. Blood (2004) 103:1244–52. doi:10.1182/blood-2003-06-2171
39. Rohr J, Beutel K, Maul-Pavicic A, Vraetz T, Thiel J, Warnatz K, et al. Atypical familial hemophagocytic lymphohistiocytosis due to mutations in UNC13D and STXBP2 overlaps with primary immunodeficiency diseases. Haematologica (2010) 95:2080–7. doi:10.3324/haematol.2010.029389
40. Cohen JI, Niemela JE, Stoddard JL, Pittaluga S, Heslop H, Jaffe ES, et al. Late-onset severe chronic active EBV in a patient for five years with mutations in STXBP2 (MUNC18-2) and PRF1 (perforin 1). J Clin Immunol (2015) 35:445–8. doi:10.1007/s10875-015-0168-y
41. Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat Immunol (2014) 15:88–97. doi:10.1038/ni.2771
42. Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature (2011) 475:471–6. doi:10.1038/nature10246
43. Cohen JI, Dropulic L, Hsu AP, Zerbe CS, Krogmann T, Dowdell K, et al. Association of GATA2 deficiency with severe primary Epstein-Barr virus (EBV) infection and EBV-associated cancers. Clin Infect Dis (2016) 63:41–7. doi:10.1093/cid/ciw160
44. Kucuk ZY, Zhang K, Filipovich L, Bleesing JJ. CTP synthase 1 deficiency in successfully transplanted siblings with combined immune deficiency and chronic active EBV infection. J Clin Immunol (2016) 36:750–3. doi:10.1007/s10875-016-0332-z
45. Okuno Y, Murata T, Ito Y, Sato Y, Kojima S, Ogawa S, et al. Comprehensive genetic study of chronic active EBV infection. 17th International Symposium on Epstein Barr Virus and Associated Diseases. Zurich (2016). 114 p. Abstract number EBV2016-1149.
46. Jiang L, Gu ZH, Yan ZX, Zhao X, Xie YY, Zhang ZG, et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet (2015) 47:1061–6. doi:10.1038/ng.3358
47. Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature (2012) 490:116–20. doi:10.1038/nature11378
48. Ito Y, Suzuki R, Torii Y, Kawa K, Kikuta A, Kojima S. HLA-A*26 and HLA-B*52 are associated with a risk of developing EBV-associated T/NK lymphoproliferative disease. Blood (2013). Available from: http://www.bloodjournal.org/content/early/2011/11/16/blood-2011-10-381921/tab-e-letters
49. Hildesheim A, Apple RJ, Chen CJ, Wang SS, Cheng YJ, Klitz W, et al. Association of HLA class I and II alleles and extended haplotypes with nasopharyngeal carcinoma in Taiwan. J Natl Cancer Inst (2002) 94:1780–9. doi:10.1093/jnci/94.23.1780
50. Niens M, Jarrett RF, Hepkema B, Nolte IM, Diepstra A, Platteel M, et al. HLA-A*02 is associated with a reduced risk and HLA-A*01 with an increased risk of developing EBV+ Hodgkin lymphoma. Blood (2007) 110:3310–5. doi:10.1182/blood-2007-05-086934
51. Ohshima K, Suzumiya J, Shimazaki K, Kato A, Tanaka T, Kanda M, et al. Nasal T/NK cell lymphomas commonly express perforin and Fas ligand: important mediators of tissue damage. Histopathology (1997) 31:444–50. doi:10.1046/j.1365-2559.1997.2880887.x
52. Quintanilla-Martinez L, Kumar S, Fend F, Reyes E, Teruya-Feldstein J, Kingma DW, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood (2000) 96:443–51.
53. Okano M. Overview and problematic standpoints of severe chronic active Epstein-Barr virus infection syndrome. Crit Rev Oncol Hematol (2002) 44:273–82. doi:10.1016/S1040-8428(02)00118-X
54. Gotoh K, Ito Y, Shibata-Watanabe Y, Kawada J, Takahashi Y, Yagasaki H, et al. Clinical and virological characteristics of 15 patients with chronic active Epstein-Barr virus infection treated with hematopoietic stem cell transplantation. Clin Infect Dis (2008) 46:1525–34. doi:10.1086/587671
Keywords: chronic active Epstein–Barr virus, Epstein–Barr virus lymphoma, infectious mononucleosis, hemophagocytosis, DDX3X
Citation: Kimura H and Cohen JI (2017) Chronic Active Epstein–Barr Virus Disease. Front. Immunol. 8:1867. doi: 10.3389/fimmu.2017.01867
Received: 09 September 2017; Accepted: 08 December 2017;
Published: 22 December 2017
Edited by:
Stuart G. Tangye, Garvan Institute of Medical Research, AustraliaReviewed by:
Umaimainthan Palendira, Centenary Institute Australia, AustraliaClaire Shannon-Lowe, University of Birmingham, United Kingdom
At least a portion of this work is authored by Jeffrey I. Cohen on behalf of the U.S. Government and, as regards Dr. Cohen and the US government, is not subject to copyright protection in the United States. Foreign and other copyrights may apply. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeffrey I. Cohen, jcohen@niaid.nih.gov
 Hiroshi Kimura
Hiroshi Kimura Jeffrey I. Cohen
Jeffrey I. Cohen