
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 16 November 2017
Sec. Inflammation
Volume 8 - 2017 | https://doi.org/10.3389/fimmu.2017.01534
This article is part of the Research TopicRegulation of Inflammation in Chronic DiseaseView all 48 articles
Observed in many central nervous system diseases, neuroinflammation (NI) proceeds from peripheral immune cell infiltration into the parenchyma, from cytokine secretion and from oxidative stress. Astrocytes and microglia also get activated and proliferate. NI manifestations and consequences depend on its context and on the acute or chronic aspect of the disease. The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)/Fn14 pathway has been involved in chronic human inflammatory pathologies such as neurodegenerative, autoimmune, or malignant diseases. New data now describe its regulatory effects in tissues or fluids from patients with neurological diseases. In this mini-review, we aim to highlight the role of TWEAK/Fn14 in modulating NI in multiple sclerosis, neuropsychiatric systemic lupus erythematosus, stroke, or glioma. TWEAK/Fn14 can modulate NI by activating canonical and non-canonical nuclear factor-κB pathways but also by stimulating mitogen-activated protein kinase signaling. These downstream activations are associated with (i) inflammatory cytokine, chemokine and adhesion molecule expression or release, involved in NI propagation, (ii) matrix-metalloproteinase 9 secretion, implicated in blood–brain barrier disruption and tissue remodeling, (iii) astrogliosis and microgliosis, and (iv) migration of tumor cells in glioma. In addition, we report several animal and human studies pointing to TWEAK as an attractive therapeutic target.
Initially described as an accumulation of leukocytes in multiple sclerosis (MS) brain, neuroinflammation (NI) now also applies to other central nervous system (CNS) diseases (1). Among others factors, it results from peripheral immune cell infiltration through the brain barriers, from cytokine secretion and from oxidative stress (2). NI is based upon and is regulated by bidirectional communication pathways involving especially cytokines that connect the CNS and immune system (3). However, using the generic term “NI” for such a multifaceted process could be inaccurate (1). NI features depend on disease-specific conditions and differ in CNS infection, ischemia, and malignant or autoimmune diseases. Whether NI is acute or chronic is a major point to consider. In fact, transient NI is usually beneficial and must be preserved, while chronic NI is preferentially associated with diseases resulting in neurodegeneration (4, 5).
Neuroinflammation highly involves cellular components of the neurovascular unit (6, 7), which consists in microvascular endothelial cells surrounded by basal lamina, astrocytic end-feet, pericytes, and neurons. It underlies the concept of a blood–brain barrier (BBB) actively separating the parenchyma from the circulation. Resident CNS cells such as astrocytes and microglia are also involved in regulating NI and respond to CNS insults by a proliferation and an abnormal activation, respectively called astrogliosis and microgliosis. This response is not always a harmful process, it can also be a beneficial and crucial process involved in CNS repair (8–10). During NI, immune cells, microglia, and astrocytes release soluble proteins, called cytokines, which mediate cell–cell communication (11). Among them, the tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) plays a dual role in the physiological versus inflammatory pathological responses of tissues, including the CNS (12–15).
Tumor necrosis factor-like weak inducer of apoptosis is a member of TNF superfamily initially shown to induce apoptosis of malignant cells (16). It is synthesized as a transmembrane protein form (mTWEAK) and proteolytically processed as a soluble cytokine (sTWEAK). Monocytes/macrophages are the main source of sTWEAK in inflammatory tissues. Until now, mTWEAK has only been described on freshly isolated monocytes (17, 18). Unlike TNF-α, TWEAK curtails the innate immune response and attenuates the transition to adaptive Th1 immunity (19). Moreover, TWEAK inhibits TNF receptor-1 signaling that promotes inflammation (20). TWEAK signaling mainly requires binding to fibroblast growth factor inducible 14 (Fn14), a member of the TNF receptor superfamily. Although Fn14 is poorly expressed in healthy endothelial cells, neurons, astrocytes, microglia, and progenitor cells, it is highly inducible in these cells. TWEAK interaction with its Fn14 receptor induces multiple molecular events and biological responses depending on cell type and microenvironment. These downstream signaling pathways have been compiled from the literature in 2012 by Bhattacharjee et al. (21), who cataloged 46 proteins and 28 induced genes. Thus, Fn14 engagement primarily activates nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) via interaction with intracellular TNF-receptor-associated-factors (21, 22). mTWEAK seems to activate more efficiently the canonical NF-κB pathway while both membrane and soluble TWEAK can induce the non-canonical NF-κB pathway (23). Secondary TWEAK signaling pathways have also been described, such as the phosphatidylinositol 3-kinase/Akt pathway (14).
In the CNS, TWEAK targets endothelial cells, astrocytes, and neurons. Interestingly, in murine and human astrocytes, the TWEAK/Fn14 pathway can stimulate reactivity, i.e., when cells proliferate, are activated and produce inflammation factors (24, 25). This associated mitogenic potency is mediated by the TWEAK-induced MAPK signaling pathway (26). Besides, in an in vitro model of human BBB, TWEAK induced microvascular cerebral endothelial cells to display an inflammatory profile: they increased (i) their secretion of proinflammatory cytokines, (ii) their production and activation of matrix-metalloproteinase 9 (MMP-9) involved in BBB disruption, and (iii) their expression of intercellular adhesion molecule-1 implicated in leukocyte adhesion to endothelium (27).
Here, we aimed to review TWEAK role in NI modulation in neurodegenerative, autoimmune, ischemic, and malignant CNS inflammatory diseases. Considering available data about TWEAK involvement in their pathogenesis, we will successively focus on MS, neuropsychiatric systemic lupus erythematosus (NPSLE), cerebral ischemia, glioma, amyotrophic lateral sclerosis (ALS), Parkinson’s disease, and schizophrenia (Table 1).
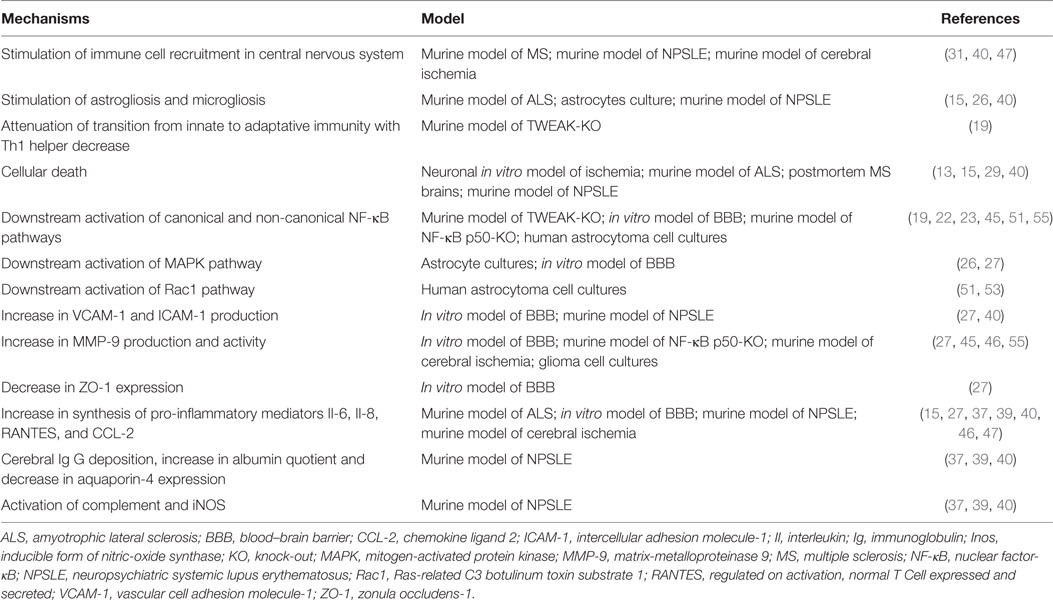
Table 1. Mechanisms underlying the role of tumor necrosis factor-like weak inducer of apoptosis (TWEAK) in neuroinflammation regulation.
Multiple sclerosis is a multifactorial disease involving auto-immunity against myelin components, NI, BBB disruption, tissue remodeling, and demyelination/remyelination (28).
Serafini et al. showed that both TWEAK and Fn14 were upregulated in postmortem MS brain sections (29). Furthermore, this increase was related to the degree of inflammation and demyelination. Perivascular and intrameningeal macrophages, microglia, and astrocytes were the main sources of TWEAK, with a different contribution according to lesion location and degree of inflammation. Fn14 was expressed by neurons and astrocytes in the cortex of highly infiltrated MS brains (29). The absence of TWEAK/Fn14 expression in healthy brain reinforces the idea that TWEAK/Fn 14 pathway could play a role in MS pathogeny. In blood, Desplat-Jégo et al. demonstrated that mTWEAK was expressed at the cell surface of monocytes derived from MS patients but not from non-MS patients (18). In MS, such mTWEAK-expressing monocytes could represent immune cells that infiltrate CNS by interacting with Fn14 molecules at the membrane of BBB endothelial cells. In the same study, sTWEAK serum and cerebrospinal fluid levels were similar in MS and non-MS patients (18), suggesting that sTWEAK is not a reliable diagnosis MS biomarker.
Myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (EAE) is the best-characterized animal model of MS. In chronic EAE induced in C57Bl/6 mice, TWEAK transcript levels increased in the spinal cord (24). Moreover, transgenic mice overexpressing sTWEAK developed a more severe EAE than wild-type mice (24). Besides, in cuprizone-treated mice, a model of demyelination/remyelination, TWEAK and Fn14 transcription were upregulated during both demyelination and remyelination phases (30).
Treated with cuprizone TWEAK-knock-out mice displayed a significant delay in microglia accumulation and in demyelination phases (30). Reinforcing the role of TWEAK/Fn14 in MS, our team showed that EAE severity and CNS leukocyte infiltration were reduced in mice treated with blocking monoclonal anti-TWEAK antibody after the priming phase (31). Moreover, an Fn14-TNF-related apoptosis-inducing ligand (TRAIL) fusion protein was designed to simultaneously block endogenous TWEAK and mediate TRAIL inhibitory signals on activated T cells (32). Injected in EAE-mice, this protein reduced EAE severity (32, 33). Additionally, vaccinating mice or rats with TWEAK extracellular domain or with Fn14 induced the production of specific inhibitory antibodies. Such treatment was associated with reduced inflammatory spinal cord infiltration and with clinical amelioration during EAE (34). However, in humans, a raising question is the relevance of targeting TWEAK in MS when anti-TNF-α has failed to improve disease status (35). One could argue that the TWEAK/Fn14 pathway could also play a role in neuroprotection. In this way, Iocca et al. observed a modest but significant delay in remyelination of TWEAK-knock-out mice treated with cuprizone. However, this marginal delay did not result in prolonged defect in remyelination (30). Later, Echeverry et al. described a TNF-α-dependent neuroprotective effect of TWEAK in another NI model (13), but data in MS are lacking. Further studies are then needed to definitively establish that TWEAK is a relevant therapeutic target in MS.
Neuropsychiatric systemic lupus erythematosus is an autoimmune disease underlied by hyperactivation of B and T lymphocytes, leading to overproduction of autoantibodies, tissue deposition of immune complexes, and increase in proinflammatory cytokines. This inflammatory state potentially targets all the organs, with marked joint, renal, hematologic and skin damages. In a significant number of patients, neuropsychiatric manifestations can also occur and thus define NPSLE. A pathogenic role of TWEAK was shown in lupus nephritis, a renal manifestation of the disease (36). Besides, evidences of TWEAK involvement in NPSLE are growing.
Putterman et al. observed an increased expression of TWEAK and Fn14 in the cerebral cortex of the MRL/lpr mouse strain with NPSLE (37, 38). Furthermore, knocking out Fn14 in this strain (i) significantly improved cognitive function and (ii) decreased depression and anhedonia in comparison with MRL/lpr wild-type mice. These clinical parameters were associated with decreased levels of proinflammatory cytokines and preserved BBB integrity (37). Further, CNS anatomopathological studies in these mice showed (i) a reduction of fibronectin deposition and inducible form of nitric-oxyde synthase (iNOS) production, (ii) a diminution of immune infiltrates, and (iii) a decrease of IgG deposition and complement activation (39). Finally, reduced cortex neuronal degeneration, apoptosis, and gliosis were associated with improved cognitive functions in such mice (40).
These results were consolidated by intracerebroventricular injection of Fc-TWEAK in non-autoimmune mice. Just like MRL/lpr strain, these mice developed NPSLE symptoms, associated with high levels of TWEAK pathway downstream components, complement and iNOS activation, IgG brain deposition, neurodegeneration, and apoptosis (39). Trysberg et al. suggested that NPSLE could be associated with high levels of MMP-9 and sTWEAK in cerebrospinal fluid (41, 42). They also found a significant correlation between intrathecal MMP-9 and levels of tau (a neuronal degeneration marker) and glial fibrillary acidic protein (an astrocytic degeneration marker) (41). These results suggest that TWEAK/Fn14 interaction might be involved in brain damages during NPSLE since it promotes synthesis and activation of MMP-9 in CNS (27). Nevertheless, a recent study concluded that TWEAK levels in serum and cerebro-spinal fluid did not seem relevant biomarkers for NPSLE (43).
Finally, these findings highly support the involvement of TWEAK/Fn14 interaction in NPSLE pathogenesis and symptom occurrence.
Cerebral ischemia or stroke is the second cause of mortality in the world. The onset of cerebral ischemia is followed by inflammatory events that affect neurological patient’s outcome. Several studies implicate TWEAK in stroke.
Experimental focal cerebral ischemia was associated with an increase in TWEAK and Fn14 mRNA levels (44) and with BBB disruption (45, 46). In this context, TWEAK-induced NF-κB activation resulted in both astrocytic chemokine (C-C motif) ligand-2 (CCL-2) expression, leading to polynuclear neutrophil recruitment into the damaged zone, and in upregulation of MMP-9 activity (45–47). The effective inhibition of this neuroinflammatory process by Fn14-Fc decoy injections (46, 48) was associated with a reduced ischemic zone and hastened motor function recovery (46). Stroke patients displayed significantly elevated TWEAK serum levels and, in postmortem stroke human brains, elevated Fn14 mRNA levels were associated with upregulated Fn14 immunostaining in the ischemic zone (49).
Hypoxic-preconditioning of mice or cultured neurons promotes neuron survival and reduces ischemic lesion volume. In this context, TWEAK and Fn14 levels increase. Moreover, the protective effect of hypoxic preconditioning was abrogated in TWEAK or Fn14 KO mice and restored in cultured neurons after addition of TWEAK (13). This suggests that the TWEAK/Fn14 pathway contributes to the protective effect of hypoxic preconditioning. This is likely mediated by neuronal TNF-α and the activation of the MAPK pathway, resulting in the inactivation of the Bcl-2-associated death promoter protein (13).
These results suggest that a relevant therapeutic strategy would be administration of TWEAK in order to protect the brain in patients at high risk of stroke.
Glioblastoma, the most common primary brain tumor, is associated with a high mortality related to its high local invasiveness, angiogenesis, and immunosuppressive potency. The initiation and development of tumors are associated with repetitive tissue damage and are tightly linked to chronic inflammatory processes. Tumor cell migration is essential in glioblastoma malignancy progression and depends on its inflammatory microenvironment.
Tumor necrosis factor-like weak inducer of apoptosis upregulates Fn14 in migrating glioblastoma cells. This overexpression correlates with tumor aggressiveness and poor outcome (50, 51). In this way, Fn14 expression may help classifying glioma histological subtype (52). Moreover, canonical NF-κB pathway, stimulated by TWEAK, promotes Rac1 and Cdc42 expression, key mediators in the regulation of glioblastoma cell migration and invasion (51, 53, 54). Interestingly, Cherry et al. showed that MMP-9 inhibition abolished TWEAK-induced glioma invasion (55).
Therapeutic strategies targeting Fn14 in malignant cells have been evaluated. In these preclinical studies, manipulating Fn14 expression levels or suppressing TWEAK-Fn14-NFκB-dependent signaling decreased glioblastoma cell invasion capacity (56, 57). However, further experiments are needed to evaluate whether these strategies can significantly impact patients’ survival.
Amyotrophic lateral sclerosis is a fatal neurodegenerative disease affecting motor neurons in the brain and spinal cord, and associated with NI hallmark (astrogliosis and microgliosis) and skeletal muscle atrophy. Mutations within the superoxide dismutase 1 (SOD1) are sometimes found in familial or sporadic cases. Mutant SOD1 expression was associated with inflammatory astrocytes and microglia and with skeletal muscle atrophy (58).
Bowerman et al. used a model of transgenic mice overexpressing human mutant SOD1 and recapitulating the main traits of ALS to study TWEAK/Fn14 involvement in ALS (15). In this model, mTWEAK was upregulated in the spinal cord and it induced astrocyte proliferation and IL-6 release. TWEAK and Fn14 were also found in skeletal muscle with different patterns of expression linked to disease development. In this study, the authors demonstrated a TWEAK induced-motor neuron death (15). Surprisingly, it was mediated by TWEAK interaction with CD163, a putative alternative TWEAK receptor. This neuronal death involved caspase-3 activation and was totally independent of Fn14. This work is the first implicating CD163 in mediating TWEAK effects in an inflammatory neurological disease. CD163 is a member of the scavenger receptors and is exclusively expressed by monocytes and macrophages. Increasing in vivo and in vitro evidences suggest that the interaction between TWEAK and CD163 may affect the development of atherosclerosis and related diseases (59).
Tumor necrosis factor-like weak inducer of apoptosis deletion reduced astrogliosis and microgliosis in the spinal cord, which substantiated the implication of the TWEAK pathway in ALS. However, muscle pathology was only partially reduced and TWEAK deletion did not prevent motor deterioration nor increase life span. In the same way, anti-TWEAK antibodies injected in ALS mice decreased microgliosis without improving motor functions. Bowerman et al. thus proposed a model combining the action of peripheral and central TWEAK, which could both contribute to the sustained activation of microglia in ALS (15).
This work suggests that blocking TWEAK in ALS is interesting but not sufficient and requires being included in a combinatory therapeutic approach.
Parkinson’s disease is a neurodegenerative pathology characterized by movement impairment due to dopaminergic loss in the nigrostriatal pathway. It may also have a neuroinflammatory component, where NF-κB may play a dual role, both promoting and protecting against neurodegeneration (60). The role of TWEAK itself has been studied in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) murine model and in Parkinson’s disease human brain samples (61). There was no difference in substantia nigra TWEAK concentration between MPTP mice, Parkinson’s disease patients, and controls. While TWEAK or Fn14-KO failed to prevent MPTP toxicity, anti-TWEAK antibodies treatment attenuated the MPTP-induced death of dopaminergic neurons (61). These apparently conflicting results could be explained in part by existing compensatory systems, including the TNF-α pathway, since TNF is expressed in microglia of Parkinson’s disease patients.
Neuroinflammation is highly suspected to be involved in psychiatric pathologies like schizophrenia or bipolar disorder. Recently, TWEAK plasma levels of patients suffering from schizophrenia were compared with controls. Although no difference was shown globally, TWEAK levels in male patients were significantly lower than in male controls, suggesting TWEAK involvement in subgroups of schizophrenia patients (62). In bipolar disorder, conflicting results were observed, perhaps due to the heterogeneity in patients’ groups: Cingi et al. measured lower TWEAK plasma levels compared to healthy controls (63), while Barbosa et al. described increased concentrations, regardless of the mood (64). Nevertheless, as the interest for studying TWEAK/Fn14 pathway in psychiatric diseases is very recent, further studies will certainly be available soon.
This mini-review reveals that the TWEAK/Fn14 pathway modulates NI in neurodegenerative, immune, ischemic, and malignant CNS inflammatory diseases. In fact, as supported by both animal and human in vitro and in vivo studies, TWEAK/Fn14 can modulate NI by activating canonical and non-canonical NF-κB pathways but also MAPK signaling. Then, expression or release of inflammatory cytokines, chemokines, and adhesion molecules are upregulated and astrogliosis and microgliosis occur. Additionally, MMP-9 secretion is stimulated and reinforces BBB disruption and tissue remodeling. Note that monoclonal anti-TWEAK antibodies have been injected in patients in phase I clinical studies. In healthy volunteers or patients with solid tumors or rheumatoid arthritis, they (i) displayed a classical therapeutic antibody pharmacokinetic pattern, (ii) were associated with a favorable safety profile, and (iii) induced a decrease in circulating TWEAK serum level (65–68). Additionally, in these studies, TWEAK/Fn14 pathway inhibition yielded encouraging results, such as reduced clinical and biological inflammatory markers, including sTWEAK. All these data support the concept that the TWEAK/Fn14 axis represents a promising therapeutic target for modulating inflammation, including NI.
AB and SD-J both collected the publications and wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
We thank Nicolas Simon for reading and critical review of this paper, and Isabelle Virard for proofreading it.
This work was supported by Aix-Marseille University, CNRS, and AP-HM.
1. Masgrau R, Guaza C, Ransohoff RM, Galea E. Should we stop saying ‘glia’ and ‘neuroinflammation’? Trends Mol Med (2017) 23(6):486–500. doi:10.1016/j.molmed.2017.04.005
2. Schwartz M, Deczkowska A. Neurological disease as a failure of brain-immune crosstalk: the multiple faces of neuroinflammation. Trends Immunol (2016) 37(10):668–79. doi:10.1016/j.it.2016.08.001
3. Kempuraj D, Thangavel R, Selvakumar G, Zaheer S, Ahmed M, Raikwar P, et al. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front Cell Neurosci (2017) 11:216. doi:10.3389/fncel.2017.00216
4. Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res (2014) 79:1–12. doi:10.1016/j.neures.2013.10.004
5. Kielian T. Multifaceted roles of neuroinflammation: the need to consider both sides of the coin. J Neurochem (2016) 136(Suppl 1):5–9. doi:10.1111/jnc.13530
6. Wohleb ES, Godbout JP. Basic aspects of the immunology of neuroinflammation. Mod Trends Pharmacopsychiatry (2013) 28:1–19. doi:10.1159/000343964
7. Yepes M. TWEAK and Fn14 in the neurovascular unit. Front Immunol (2013) 4:367. doi:10.3389/fimmu.2013.00367
8. Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett (2014) 565:30–8. doi:10.1016/j.neulet.2013.12.071
9. Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, Lyck R, et al. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta (2016) 1862(3):461–71. doi:10.1016/j.bbadis.2015.10.018
10. Rustenhoven J, Jansson D, Smyth LC, Dragunow M. Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci (2017) 38(3):291–304. doi:10.1016/j.tips.2016.12.001
11. Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol (2017) 17(1):49–59. doi:10.1038/nri.2016.123
12. Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine (2007) 40(1):1–16. doi:10.1016/j.cyto.2007.09.007
13. Echeverry R, Wu F, Haile WB, Wu J, Yepes M. The cytokine tumor necrosis factor-like weak inducer of apoptosis and its receptor fibroblast growth factor-inducible 14 have a neuroprotective effect in the central nervous system. J Neuroinflammation (2012) 9:45. doi:10.1186/1742-2094-9-45
14. Burkly LC. TWEAK/Fn14 axis: the current paradigm of tissue injury-inducible function in the midst of complexities. Semin Immunol (2014) 26(3):229–36. doi:10.1016/j.smim.2014.02.006
15. Bowerman M, Salsac C, Coque E, Eiselt É, Deschaumes RG, Brodovitch A, et al. Tweak regulates astrogliosis, microgliosis and skeletal muscle atrophy in a mouse model of amyotrophic lateral sclerosis. Hum Mol Genet (2015) 24(12):3440–56. doi:10.1093/hmg/ddv094
16. Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem (1997) 272(51):32401–10. doi:10.1074/jbc.272.51.32401
17. Nakayama M, Kayagaki N, Yamaguchi N, Okumura K, Yagita H. Involvement of TWEAK in interferon gamma-stimulated monocyte cytotoxicity. J Exp Med (2000) 192(9):1373–80. doi:10.1084/jem.192.9.1373
18. Desplat-Jégo S, Feuillet L, Creidy R, Malikova I, Rance R, Khrestchatisky M, et al. TWEAK is expressed at the cell surface of monocytes during multiple sclerosis. J Leukoc Biol (2009) 85(1):132–5. doi:10.1189/jlb.0608347
19. Maecker H, Varfolomeev E, Kischkel F, Lawrence D, LeBlanc H, Lee W, et al. TWEAK attenuates the transition from innate to adaptive immunity. Cell (2005) 123(5):931–44. doi:10.1016/j.cell.2005.09.022
20. Wicovsky A, Salzmann S, Roos C, Ehrenschwender M, Rosenthal T, Siegmund D, et al. TNF-like weak inducer of apoptosis inhibits proinflammatory TNF receptor-1 signaling. Cell Death Differ (2009) 16(11):1445–59. doi:10.1038/cdd.2009.80
21. Bhattacharjee M, Raju R, Radhakrishnan A, Nanjappa V, Muthusamy B, Singh K, et al. A bioinformatics resource for TWEAK-Fn14 signaling pathway. J Signal Transduct (2012) 2012:376470. doi:10.1155/2012/376470
22. Burkly LC. Regulation of tissue responses: the TWEAK/Fn14 pathway and other TNF/TNFR superfamily members that activate non-canonical NFκB signaling. Front Immunol (2015) 6:92. doi:10.3389/fimmu.2015.00092
23. Roos C, Wicovsky A, Müller N, Salzmann S, Rosenthal T, Kalthoff H, et al. Soluble and transmembrane TNF-like weak inducer of apoptosis differentially activate the classical and noncanonical NF-kappa B pathway. J Immunol (2010) 185(3):1593–605. doi:10.4049/jimmunol.0903555
24. Desplat-Jégo S, Varriale S, Creidy R, Terra R, Bernard D, Khrestchatisky M, et al. TWEAK is expressed by glial cells, induces astrocyte proliferation and increases EAE severity. J Neuroimmunol (2002) 133(1–2):116–23. doi:10.1016/S0165-5728(02)00368-5
25. Saas P, Boucraut J, Walker PR, Quiquerez AL, Billot M, Desplat-Jego S, et al. TWEAK stimulation of astrocytes and the proinflammatory consequences. Glia (2000) 32(1):102–7. doi:10.1002/1098-1136(200010)32:1<102::AID-GLIA100>3.0.CO;2-U
26. Rousselet E, Traver S, Monnet Y, Perrin A, Mandjee N, Hild A, et al. Tumor necrosis factor-like weak inducer of apoptosis induces astrocyte proliferation through the activation of transforming-growth factor-α/epidermal growth factor receptor signaling pathway. Mol Pharmacol (2012) 82(5):948–57. doi:10.1124/mol.112.079608
27. Stephan D, Sbai O, Wen J, Couraud PO, Putterman C, Khrestchatisky M, et al. TWEAK/Fn14 pathway modulates properties of a human microvascular endothelial cell model of blood brain barrier. J Neuroinflammation (2013) 10:9. doi:10.1186/1742-2094-10-9
28. Nazeri A, Heydarpour P, Sadaghiani S, Sahraian MA, Burkly LC, Bar-Or A. A further TWEAK to multiple sclerosis pathophysiology. Mol Neurobiol (2014) 49(1):78–87. doi:10.1007/s12035-013-8490-y
29. Serafini B, Magliozzi R, Rosicarelli B, Reynolds R, Zheng TS, Aloisi F. Expression of TWEAK and its receptor Fn14 in the multiple sclerosis brain: implications for inflammatory tissue injury. J Neuropathol Exp Neurol (2008) 67(12):1137–48. doi:10.1097/NEN.0b013e31818dab90
30. Iocca HA, Plant SR, Wang Y, Runkel L, O’Connor BP, Lundsmith ET, et al. TNF superfamily member TWEAK exacerbates inflammation and demyelination in the cuprizone-induced model. J Neuroimmunol (2008) 194(1–2):97–106. doi:10.1016/j.jneuroim.2007.12.003
31. Desplat-Jégo S, Creidy R, Varriale S, Allaire N, Luo Y, Bernard D, et al. Anti-TWEAK monoclonal antibodies reduce immune cell infiltration in the central nervous system and severity of experimental autoimmune encephalomyelitis. Clin Immunol (2005) 117(1):15–23. doi:10.1016/j.clim.2005.06.005
32. Razmara M, Hilliard B, Ziarani AK, Murali R, Yellayi S, Ghazanfar M, et al. Fn14-TRAIL, a chimeric intercellular signal exchanger, attenuates experimental autoimmune encephalomyelitis. Am J Pathol (2009) 174(2):460–74. doi:10.2353/ajpath.2009.080462
33. Prinz-Hadad H, Mizrachi T, Irony-Tur-Sinai M, Prigozhina TB, Aronin A, Brenner T, et al. Amelioration of autoimmune neuroinflammation by the fusion molecule Fn14·TRAIL. J Neuroinflammation (2013) 10:36. doi:10.1186/1742-2094-10-36
34. Mueller AM, Pedré X, Kleiter I, Hornberg M, Steinbrecher A, Giegerich G. Targeting fibroblast growth factor-inducible-14 signaling protects from chronic relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol (2005) 159(1–2):55–65. doi:10.1016/j.jneuroim.2004.10.001
35. Van Oosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, von Blomberg BM, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology (1996) 47(6):1531–4. doi:10.1212/WNL.47.6.1531
36. Chen J, Wei L, Xia Y. Roles of tumour necrosis factor-related weak inducer of apoptosis/fibroblast growth factor-inducible 14 pathway in lupus nephritis. Nephrology (Carlton) (2017) 22(2):101–6. doi:10.1111/nep.12957
37. Wen J, Xia Y, Stock A, Michaelson JS, Burkly LC, Gulinello M, et al. Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway. J Autoimmun (2013) 43:44–54. doi:10.1016/j.jaut.2013.03.002
38. Stock AD, Wen J, Putterman C. Neuropsychiatric lupus, the blood brain barrier, and the TWEAK/Fn14 pathway. Front Immunol (2013) 4:484. doi:10.3389/fimmu.2013.00484
39. Wen J, Chen CH, Stock A, Doerner J, Gulinello M, Putterman C. Intracerebroventricular administration of TNF-like weak inducer of apoptosis induces depression-like behavior and cognitive dysfunction in non-autoimmune mice. Brain Behav Immun (2016) 54:27–37. doi:10.1016/j.bbi.2015.12.017
40. Wen J, Doerner J, Weidenheim K, Xia Y, Stock A, Michaelson JS, et al. TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice. J Autoimmun (2015) 60:40–50. doi:10.1016/j.jaut.2015.03.005
41. Trysberg E, Blennow K, Zachrisson O, Tarkowski A. Intrathecal levels of matrix metalloproteinases in systemic lupus erythematosus with central nervous system engagement. Arthritis Res Ther (2004) 6(6):R551–6. doi:10.1186/ar1228
42. Trysberg EBL, Su L, Michaelson J, Putterman C. Cerebrospinal fluid (CSF) TWEAK: a novel biomarker for neuropsychiatric SLE? Arthritis Rheum (2007) 56:S750.
43. Fragoso-Loyo H, Atisha-Fregoso Y, Nuñez-Alvarez CA, Llorente L. Utility of TWEAK to assess neuropsychiatric disease activity in systemic lupus erythematosus. Lupus (2016) 25(4):364–9. doi:10.1177/0961203315610206
44. Potrovita I, Zhang W, Burkly L, Hahm K, Lincecum J, Wang MZ, et al. Tumor necrosis factor-like weak inducer of apoptosis-induced neurodegeneration. J Neurosci (2004) 24(38):8237–44. doi:10.1523/JNeurosc.1089-04.2004
45. Polavarapu R, Gongora MC, Winkles JA, Yepes M. Tumor necrosis factor-like weak inducer of apoptosis increases the permeability of the neurovascular unit through nuclear factor-kappa B pathway activation. J Neurosci (2005) 25(44):10094–100. doi:10.1523/JNEUROSCI.3382-05.2005
46. Zhang X, Winkles JA, Gongora MC, Polavarapu R, Michaelson JS, Hahm K, et al. TWEAK-Fn14 pathway inhibition protects the integrity of the neurovascular unit during cerebral ischemia. J Cereb Blood Flow Metab (2007) 27(3):534–44. doi:10.1038/sj.jcbfm.9600368
47. Haile WB, Echeverry R, Wu J, Yepes M. The interaction between tumor necrosis factor-like weak inducer of apoptosis and its receptor fibroblast growth factor-inducible 14 promotes the recruitment of neutrophils into the ischemic brain. J Cereb Blood Flow Metab (2010) 30(6):1147–56. doi:10.1038/jcbfm.2009.280
48. Yepes M, Brown S, Moore E, Smith E, Lawrence D, Winkles J. A soluble Fn14-Fc decoy receptor reduces infarct volume in a murine model of cerebral ischemia. Am J Pathol (2005) 166(2):511–20. doi:10.1016/S0002-9440(10)62273-0
49. Inta I, Frauenknecht K, Dörr H, Kohlhof P, Rabsilber T, Auffarth GU, et al. Induction of the cytokine TWEAK and its receptor Fn14 in ischemic stroke. J Neurol Sci (2008) 275(1–2):117–20. doi:10.1016/j.jns.2008.08.005
50. Tran NL, McDonough WS, Donohue PJ, Winkles JA, Berens TJ, Ross KR, et al. The human Fn14 receptor gene is up-regulated in migrating glioma cells in vitro and overexpressed in advanced glial tumors. Am J Pathol (2003) 162(4):1313–21. doi:10.1016/S0002-9440(10)63927-2
51. Tran NL, McDonough WS, Savitch BA, Fortin SP, Winkles JA, Symons M, et al. Increased fibroblast growth factor-inducible 14 expression levels promote glioma cell invasion via Rac1 and nuclear factor-kappaB and correlate with poor patient outcome. Cancer Res (2006) 66(19):9535–42. doi:10.1158/0008-5472.CAN-06-0418
52. Pelekanou V, Notas G, Kampa M, Tsentelierou E, Stathopoulos EN, Tsapis A, et al. BAFF, APRIL, TWEAK, BCMA, TACI and Fn14 proteins are related to human glioma tumor grade: immunohistochemistry and public microarray data meta-analysis. PLoS One (2013) 8(12):e83250. doi:10.1371/journal.pone.0083250
53. Fortin SP, Ennis MJ, Schumacher CA, Zylstra-Diegel CR, Williams BO, Ross JT, et al. Cdc42 and the guanine nucleotide exchange factors Ect2 and trio mediate Fn14-induced migration and invasion of glioblastoma cells. Mol Cancer Res (2012) 10(7):958–68. doi:10.1158/1541-7786.MCR-11-0616
54. Dhruv HD, Whitsett TG, Jameson NM, Patel F, Winkles JA, Berens ME, et al. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) promotes glioblastoma cell chemotaxis via Lyn activation. Carcinogenesis (2014) 35(1):218–26. doi:10.1093/carcin/bgt289
55. Cherry EM, Lee DW, Jung JU, Sitcheran R. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) promotes glioma cell invasion through induction of NF-κB-inducing kinase (NIK) and noncanonical NF-κB signaling. Mol Cancer (2015) 14:9. doi:10.1186/s12943-014-0273-1
56. Perez JG, Tran NL, Rosenblum MG, Schneider CS, Connolly NP, Kim AJ, et al. The TWEAK receptor Fn14 is a potential cell surface portal for targeted delivery of glioblastoma therapeutics. Oncogene (2016) 35(17):2145–55. doi:10.1038/onc.2015.310
57. Roos A, Dhruv HD, Mathews IT, Inge LJ, Tuncali S, Hartman LK, et al. Identification of aurintricarboxylic acid as a selective inhibitor of the TWEAK-Fn14 signaling pathway in glioblastoma cells. Oncotarget (2017) 8(7):12234–46. doi:10.18632/oncotarget.14685
58. Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell (2010) 140(6):918–34. doi:10.1016/j.cell.2010.02.016
59. Liu H, Lin D, Xiang H, Chen W, Zhao S, Peng H, et al. The role of tumor necrosis factor-like weak inducer of apoptosis in atherosclerosis via its two different receptors. Exp Ther Med (2017) 14(2):891–7. doi:10.3892/etm.2017.4600
60. Baiguera C, Alghisi M, Pinna A, Bellucci A, De Luca MA, Frau L, et al. Late-onset parkinsonism in NFκB/c-Rel-deficient mice. Brain (2012) 135(Pt 9):2750–65. doi:10.1093/brain/aws193
61. Mustafa S, Martin HL, Burkly L, Costa A, Martins ML, Schwaninger M, et al. The role of TWEAK/Fn14 signaling in the MPTP-model of Parkinson’s disease. Neuroscience (2016) 319:116–22. doi:10.1016/j.neuroscience.2016.01.034
62. Tatlıdil Yaylacı E, Yüksel RN, Ünal K, Altunsoy N, Cingi M, Yalçın Şahiner Ş, et al. TNF-related weak inducer of apoptosis (TWEAK) levels in schizophrenia. Psychiatry Res (2015) 229(3):755–9. doi:10.1016/j.psychres.2015.08.006
63. Cingi Yirün M, Yirün O, Ünal K, Yüksel RN, Altunsoy N, Tatlidil Yaylaci E, et al. Serum TNF-related weak inducer of apoptosis (TWEAK) and TNF-related apoptosis-inducing ligand (TRAIL) levels of patients with bipolar disorder in manic episode, in remission and healthy controls. Psychiatry Res (2017) 257:338–45. doi:10.1016/j.psychres.2017.07.067
64. Barbosa IG, Vaz GN, Rocha NP, Machado-Vieira R, Ventura MRD, Huguet RB, et al. Plasma levels of tumor necrosis factor superfamily molecules are increased in bipolar disorder. Clin Psychopharmacol Neurosci (2017) 15(3):269–75. doi:10.9758/cpn.2017.15.3.269
65. Cheng E, Armstrong CL, Galisteo R, Winkles JA. TWEAK/Fn14 axis-targeted therapeutics: moving basic science discoveries to the clinic. Front Immunol (2013) 4:473. doi:10.3389/fimmu.2013.00473
66. Wisniacki N, Amaravadi L, Galluppi GR, Zheng TS, Zhang R, Kong J, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of anti-TWEAK monoclonal antibody in patients with rheumatoid arthritis. Clin Ther (2013) 35(8):1137–49. doi:10.1016/j.clinthera.2013.06.008
67. Galluppi GR, Wisniacki N, Stebbins C. Population pharmacokinetic and pharmacodynamic analysis of BIIB023, an anti-TNF-like weak inducer of apoptosis (anti-TWEAK) monoclonal antibody. Br J Clin Pharmacol (2016) 82(1):118–28. doi:10.1111/bcp.12914
Keywords: tumor necrosis factor-like weak inducer of apoptosis, Fn14, central nervous system, neuroinflammation, multiple sclerosis, neuropsychiatric systemic lupus erythematosus, stroke, glioma
Citation: Boulamery A and Desplat-Jégo S (2017) Regulation of Neuroinflammation: What Role for the Tumor Necrosis Factor-Like Weak Inducer of Apoptosis/Fn14 Pathway? Front. Immunol. 8:1534. doi: 10.3389/fimmu.2017.01534
Received: 21 August 2017; Accepted: 27 October 2017;
Published: 16 November 2017
Edited by:
Jixin Zhong, Case Western Reserve University, United StatesReviewed by:
Yumin Xia, Second Affiliated Hospital of Xi’an Jiaotong University, ChinaCopyright: © 2017 Boulamery and Desplat-Jégo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sophie Desplat-Jégo, c29waGllLmRlc3BsYXRAYXAtaG0uZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.