
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Immunol., 27 July 2017
Sec. Nutritional Immunology
Volume 8 - 2017 | https://doi.org/10.3389/fimmu.2017.00850
This article is part of the Research TopicXenobiotics and the gut microbiome in health and diseaseView all 15 articles
A commentary on
Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome
by Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, et al. Diabetes (2015) 64:2847–58. doi: 10.2337/db14-1916
Dietary polyphenols exert a range of beneficial outcomes on the intestinal microbiota, and in metabolic syndrome, such as anti-inflammatory, antioxidant, anticarcinogenic, and antidiabetic effects. Research by Roopchand and colleagues demonstrated that concord grape polyphenols (GP) led to changes in the gut microbiota and reduction in conditions associated with metabolic syndrome arising from high-fat diet (HFD) in mice (1). Mice were divided into three groups and fed with HFD only or HFD supplemented with 10% soy-protein isolate (SPI) or HFD supplemented with 10% GP-SPI, respectively, for 13 weeks. When compared to the other diet groups, mice on the GP-SPI diet had lower body weight and adiposity though the food intake was similar across the groups (1).
Also, mice on the GP-SPI diet reduced markers of systemic inflammation as IL-6 was undetectable, and low levels of TNF-α and bacterial lipopolysaccharide was detected in the serum in comparison to the SPI group. However, levels of cholesterol, triglycerides, and IL-1β in the serum were not significantly different from mice fed with the SPI diet (1).
So, how did the GP-based diet impact the gastrointestinal tract? Just as observed in the serum, lower expression of TNF-α and IL-6 was detected in the colon. Fasting-induced adipose factor, a circulating lipoprotein lipase inhibitor, was significantly increased in the ileum compared to the SPI diet (1). This suggests that the GPs may aid in the suppression of fatty acid storage thereby attenuating the effects of diet-based metabolic syndrome. Further evidence in the ileum was provided by increased gene expression of proglucagon, a precursor of proteins associated with production of insulin and maintenance of gut barrier integrity (1). However, it would have been nice to see how these evidences compared with the controls used in this study as the data were not shown. Glut2, a gene for glucose transport, was also significantly lower in the jejunum tissue when compared to the mice on the SPI-diet (1).
Grape polyphenol-based diet led to decreased ratio of Firmicutes to Bacteroidetes and significant increase in the relative abundance of Akkermansia muciniphila in the cecal and fecal microbiota (1). This decrease in the proportion of Firmicutes to Bacteroidetes has been reported in other diet-induced obesity studies (2, 3). However, another study did not find a causal effect of Firmicutes to Bacteroidetes ratio in relation to obesity (4).
Similarly, the effects of dietary polyphenol from cranberry extract were evaluated in mice fed with high-fat/high-sucrose diet for 8 weeks (5). Cranberry extract prevented weight gain, enhanced insulin sensitivity, and reduced triglycerides in the jejunum. Cranberry extract-supplemented diet also led to a dramatic increase in Akkermansia (5). Furthermore, a reduction in body weight gain and insulin was observed in rats fed with a standard-chow diet supplemented with pterostilbene (6). Changes in the gut microbiota with increase in Akkermansia were also observed (6).
Of what importance is Akkermansia in the intestinal microbiota and how does it influence diet-induced obesity and metabolic disorders? A. muciniphila is a mucin degrading bacterium present in the mucus layer of the intestinal epithelium and may represent 3–5% of the gut microbiota in healthy adults (7). Several studies have shown an increase in Akkermansia in diet-induced obesity studies and correlates with the reduction of weight gain, adiposity, and improved glucose tolerance (1, 5, 8). Administration of live A. muciniphila reversed the symptoms of obesity and metabolic syndrome in HFD mice by reducing adiposity, inflammatory markers, insulin resistance, and improved gut barrier (7). Recently, it was shown that the introduction of capsaicin, a dietary polyphenol, led to an abundance of the genera Akkermansia, Bacteroides, and Coprococcus in mice fed with HFD and a decrease in weight gain (9). Potential mechanisms by which Akkermansia influences the host microbiota leading to these beneficial outcomes are depicted below (Figure 1).
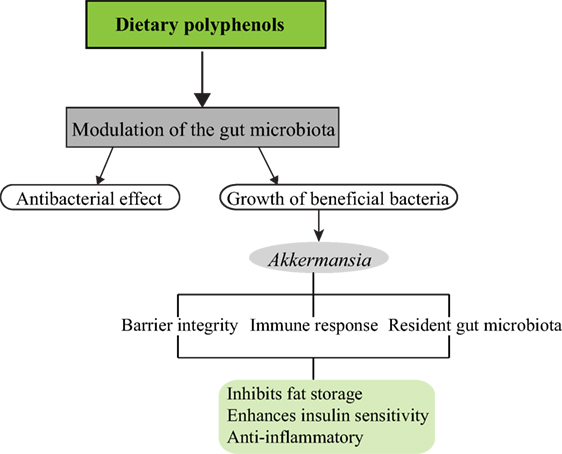
Figure 1. Dietary polyphenols and impact on the gut microbiota. Different classes of dietary polyphenols modulate the gut microbiota by various means. This could be by exerting antibacterial effect as observed in flavonoids on six pure cultures of intestinal bacteria (10) and stimulating the growth of beneficial bacteria in the gut microbiota such as Akkermansia. Akkermansia, in turn, generates short-chain fatty acids from the breakdown of mucins, which stimulates the goblet cells to produce more mucus thereby preserving/replenishing the intestinal barrier integrity. Mucus secretion is associated with the activation of the immune system, by preventing increased interaction of microbe-associated molecular patterns with intestinal epithelial cells, and stimulating other immune responses. This helps to reduce intestinal inflammation. Akkermansia may also influence resident gut bacteria by acting as an oxygen scavenger thereby, creating a favorable environment for the growth of strict anaerobes, which could have a synergistic effect on the host.
These and other studies suggest that dietary polyphenols play a role in the modulation of the gut microbiota that may favor positive outcomes. Understanding the mechanism of action of dietary polyphenols is likely to be key in the development of new diet-based therapies. This is because two different polyphenols can give complementary and dissimilar effects on the gut microbiota as observed in black tea and red wine grape extracts (11). As such, more studies are needed to unravel the bioactivity of this class of xenobiotics to fully understand their effects on the host.
The author confirms being the sole contributor of this work and approved it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes (2015) 64(8):2847. doi:10.2337/db14-1916
2. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature (2006) 444(7122):1027–31. doi:10.1038/nature05414
3. Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe (2008) 3(4):213–23. doi:10.1016/j.chom.2008.02.015
4. Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (2008) 32(11):1720–4. doi:10.1038/ijo.2008.155
5. Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut (2015) 64(6):872. doi:10.1136/gutjnl-2014-307142
6. Etxeberria U, Hijona E, Aguirre L, Milagro FI, Bujanda L, Rimando AM, et al. Pterostilbene-induced changes in gut microbiota composition in relation to obesity. Mol Nutr Food Res (2017) 61(1):1500906. doi:10.1002/mnfr.201500906
7. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A (2013) 110(22):9066–71. doi:10.1073/pnas.1219451110
8. Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut (2014) 63(5):727. doi:10.1136/gutjnl-2012-303839
9. Shen W, Shen M, Zhao X, Zhu H, Yang Y, Lu S, et al. Anti-obesity effect of capsaicin in mice fed with high-fat diet is associated with an increase in population of the gut bacterium Akkermansia muciniphila. Front Microbiol (2017) 8:272. doi:10.3389/fmicb.2017.00272
10. Duda-Chodak A. The inhibitory effect of polyphenols on human gut microbiota. J Physiol Pharmacol (2012) 63(5):497–503.
Keywords: gut microbiota, polyphenols, Akkermansia, diet, obesity
Citation: Anonye BO (2017) Commentary: Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Front. Immunol. 8:850. doi: 10.3389/fimmu.2017.00850
Received: 08 April 2017; Accepted: 05 July 2017;
Published: 27 July 2017
Edited by:
Stephen J. Pandol, Cedars-Sinai Medical Center, United StatesReviewed by:
Francisco José Pérez-Cano, University of Barcelona, SpainCopyright: © 2017 Anonye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Blessing O. Anonye, Yi5hbm9ueWVAd2Fyd2ljay5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.