
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Immunol. , 12 June 2017
Sec. Alloimmunity and Transplantation
Volume 8 - 2017 | https://doi.org/10.3389/fimmu.2017.00683
This article is part of the Research Topic Tailoring NK Cell Receptor-Ligand Interactions: an Art in Evolution View all 34 articles
 Meriem Messaoudene1,2
Meriem Messaoudene1,2 Alexandra Frazao3
Alexandra Frazao3 Pierre Jean Gavlovsky3
Pierre Jean Gavlovsky3 Antoine Toubert3
Antoine Toubert3 Nicolas Dulphy3
Nicolas Dulphy3 Anne Caignard3*
Anne Caignard3*
Natural killer (NK) cells are potent antitumor effectors, involved in hematological malignancies and solid tumor immunosurveillance. They infiltrate various solid tumors, and their numbers are correlated with good outcome. The function of NK cells extends their lytic capacities toward tumor cells expressing stress-induced ligands, through secretion of immunoregulatory cytokines, and interactions with other immune cells. Altered NK cell function due to tumor immune escape is frequent in advanced tumors; however, strategies to release the function of NK infiltrating tumors are emerging. Recent therapies targeting specific oncogenic mutations improved the treatment of cancer patients, but patients often relapse. The actual development consists in combined therapeutic strategies including agents targeting the proliferation of tumor cells and others restorating functional antitumor immune effectors for efficient and durable efficacy of anticancer treatment. In that context, we discuss the recent results of the literature to propose hypotheses concerning the potential use of NK cells, potent antitumor cytotoxic effectors, to design novel antitumor strategies.
Natural killer cells have been known and actively studied for more than four decades. They were first described as large granular lymphocytes cytotoxic for various tumor cells without prior stimulation (1, 2). In addition to their cytolytic activity against neoplastic and virus-infected cells, NK cells also display immunomodulatory functions by their ability to release cytokines, like interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα), and chemokines. NK cells represent 5–15% of blood lymphocytes. They are present in the bone marrow, liver, uterus, spleen, lungs, in mucosa-associated lymphoid tissues, thymus, and secondary lymphoid tissues (SLT) and are recruited in inflamed sites. In SLT, NK cells provide an early source of IFNγ and interact with dendritic cells to promote T helper cell type 1 responses (3).
Natural killer cells are now grouped in the system of innate lymphoid cells (4). These populations, mostly tissue resident and characterized by their capacity to produce high amounts of cytokines, constitute innate homologs of T helper cell (CD4) and cytotoxic T cell (CD8) subsets. ILCs are implicated in tissue homeostasis and autoimmune diseases. Their distribution and capacity to produce cytokines suggest that they may also be involved in the development or evolution of cancer. NK cells are considered as cytotoxic counterparts of ILC1, both depending on the T-bet transcription factor for their development.
Human NK cells, defined as CD45+/CD3−/CD56+ cells (5), are classically subdivided in two subsets based on the relative membrane expression of CD56 and CD16, the low-affinity receptor for the Fc portion of IgG (FcγRIIIA): CD56dim NK cells that express high levels of CD16 mediate antibody dependent cell cytotoxicity (ADCC), whereas CD56bright NK cells express no or low levels of CD16. These two subsets are present in different proportions in the different tissues. CD56dim NK cells represent 90% of blood and splenic NK cells, while CD56bright NK cells predominate over CD56dim in the SLT [lymph nodes (LN) and tonsils] representing up to 90% of NK cells and also constitute the major NK subset in tissues. It is accepted that CD56bright NK cells are less mature than CD56dim NK cells and display an immunoregulatory function, secreting high amounts of IFNγ and TNFα. CD56dim NK cells represent mature NK cells with a high cytotoxic activity (6).
The activation of NK cells is tightly regulated by a balance between activating and inhibitory signals delivered through engagement of numerous activating and inhibitory receptors with ligands on the target cell. Natural cytotoxicity receptors (NCRs), such as NKp46 and NKp30, are expressed by resting NK cells while NKp44 is induced after activation by cytokines, such as IL-2 and IL-15 (7, 8). The NCRs are implicated in the lysis of various tumor cells (9). The activating NK group 2 member D (NKG2D) receptor is expressed by most circulating NK cells and binds the stress-induced MHC-class I polypeptide-related sequence (MIC)-A/B molecules and UL16-binding proteins 1–6 (ULBP1–6) (10). DNAX accessory molecule-1 (DNAM-1) binds Nectin family molecules CD155 and CD112.
Natural killer cell activation is efficiently controlled by specific inhibitory NK receptors binding human leukocyte antigen of class I (HLA-class I) molecules. The C-type lectin CD94/NKG2A receptor binds HLA-E molecules (11) sensing the global HLA-class I molecules on the target while killer Ig-like receptors (KIRs) bind classical HLA-class I molecules, including HLA-C, HLA-Bw4, and some HLA-A alleles.
A link between NK cell function and cancer development was reported in a Japanese 11-year follow-up study including 3,625 patients in which cancer incidence was negatively correlated with blood NK-mediated cytotoxicity (12). Authors further showed that individuals with particular NKG2D haplotypes, HNK1/HNK1 haplotype (correlated with high NK activity) had a decreased risk of cancer compared to those with an LNK1/LNK1 haplotype (correlated with low NK activity) (13).
Additional results including ours showed the impact of NCR transcripts in the evolution of melanoma, lung cancers, and gastrointestinal stromal tumors (GIST) patients (14–16). High NKp46 correlated with better survival in metastatic melanoma patients and particular profiles of NKp30 isoforms was associated with better outcome and response to treatment in GIST patients.
The cancer immunoediting process (17) resumes cancer progression in three phases. In the elimination phase, immune cells and among them NK cells eradicate developing tumor cells. During the equilibrium phase, the immune system may select tumor variants with less immunogenicity gradually leading to the tumor escape phase and tumor progression. It is considered that most tumors at diagnosis are in the phase of immune escape associated with functionally altered tumor infiltrating NK cells (18). Tumor immunoediting selecting variants with decreased expression of stress-induced ligands provide tumor escape to NK cell-mediated lysis through activating receptors NKG2D or NKp46 (19, 20).
The challenge is thus to overcome tumor immunosuppression and restore NK cell activities. To this aim, understanding the mechanisms that lead to NK cell defects in tumor is required.
Numerous studies showed that severe quantitative and qualitative alterations of NK cells are associated with different hematological malignancies, particularly in myeloid disorders. In chronic myelogenous leukemia patients, low numbers of NK cells are associated with defects in their proliferation, and weak NK cell cytolytic functions in comparison with healthy donor blood NK cells (21). Furthermore, profound alterations in the activating receptors profile have also been reported including downregulation of NKp30 and NKp46 as well as DNAM-1, 2B4, and NKG2C on NK cells from acute myeloid leukemia (AML) patients. Decreased NKp30 and NKp46 expression was correlated with reduced NK cell killing and poor leukemia prognosis (22–25). Recently, Khaznadar et al. analyzed by cell imaging the lytic NK immunological synapse following interaction with AML cells and showed defective lytic granule polarization in NK cell-AML conjugates leading to impaired NK cell cytotoxic function (26).
Importantly, the intimate relationship between immune pressure and leukemogenesis has been suggested in two recent studies. Stringaris et al. described an immunoediting process induced by AML blasts that limits NK cell control of leukemia. They showed that abnormal NKG2A expression and TNFα production predict a poor response to chemotherapy in AML patients (27). Conversely, Khaznadar et al. showed that NK cell defects in AML patients at diagnosis could be associated with a specific transcriptional program in AML blasts and with patient’s outcome including relapse occurrence (28).
Furthermore, the beneficial role in the graft-versus-leukemia (GvL) of allogeneic NK cells for leukemic patients receiving allogeneic hematopoietic stem cell transplantation (HSCT) is well documented (29). Several studies showed that NK cells have a potent GvL effect in both KIR/HLA-class I-mismatched and -matched donor–recipient combinations after allogenic HSCT in AML patients (30–32). Moreover, rapid NK recovery after HSCT is also associated with a greater GvL effect and improved outcome in AML patients (33).
In situ detection of NK cells infiltrating various human tumors/tissues was carried out, leading sometimes to divergent results due to the disparity of NK cell markers used (CD57, CD56, NKp46, double CD3/CD56 staining). However, several reports showed that NK cells can infiltrate clear-cell renal cell carcinoma (34), melanoma (35), non-small cell lung cancer (NSCLC) (36), breast cancer (BC) (37), GIST (38), and colorectal carcinoma (CRC) (39) although NK cells were mainly localized at the tumor’s periphery. In several tumors, infiltrations by NK cells were reported to have a prognostic value. Increased overall survival was associated with a high NK cell infiltrate within the tumor or tumor stroma in lung adenocarcinoma (40), metastatic renal carcinoma (41), and lung metastasis of renal cancer (42). Elevated number of NK cells was associated with reduced risk of cancer progression in prostate cancer (43), with a reduced risk of death in squamous cell lung cancer (44), and a better prognosis in gastric carcinoma (45) and CRC (46). In addition, the number of NKp46+ NK cells was found inversely correlated with metastasis occurrence in patients with GIST (47). Furthermore, a positive association between a high numbers of tumor infiltrating CD56+ NK cells with a regression of melanocytic lesions was observed (48).
In most tumor types studied, ex vivo tumor-infiltrating NK cells displayed severe phenotypic and functional alterations compared to blood NK cells and more interestingly compared to NK cells present in adjacent normal tissues. Those alterations affected the expression of activating receptors including NKp30, CD16, DNAM-1, and ILT2 on NK cells from patients with non-invasive and invasive BC (49) or NSCLC (36). A concomitant-increased expression of the inhibitory molecule NKG2A was also observed in BC (49). This deficient phenotype was associated with impaired functions including decreased cytotoxicity against tumor cells (36, 49) and reduced IFNγ production (36). Recently, Carrega et al. reported that lung and BC tissues were highly enriched in CD56brightperforinlow NK cell subset compared to matched normal tissues (37). It is of note that comparison between NK cells from tumor and normal adjacent tissue is required for better understanding of the effect of the tumor environment on their activation.
Interestingly, our team recently identified in tumor draining LN from melanoma and BC patients, the presence of a CD56brightCD16+ NK-cell subset that displays higher expression of activating receptors, perforin molecules, and performs ADCC (50). We found that different NK receptors regulate the two LN-NK cell subsets in melanoma and BC (personal communication) and that NK-infiltrating LN recapitulate the alterations reported in the primary tumors. The presence of CD16+ NK cells in certain tumors (51) and metastatic LN emphasizes the interest for ADCC function of such NK cells.
Alterations in blood NK cells from patients with solid tumors were also reported, but in a lesser extent than in tumor infiltrating NK cells. Compared to healthy donors, a downregulation of NKG2D and an increase of the inhibitory receptor CD158b expression were correlated with impaired NK cell function (52–54) in metastatic melanoma patients. Our group showed a progressive decrease of NKp46 expression on blood NK cells with the disease progression in melanoma patients (55). In BC patients with invasive tumor, blood NK cells display altered expression of activating receptors NKp30, NKG2D, DNAM-1, 2B4, and CD16 and an upregulation of the inhibitory receptors NKG2A and CD85j. This phenotypic change was correlated with decreased NK cell cytotoxicity function and cytokine production (IFNγ and TNFα) (49). Blood NK cells from soft-tissue sarcoma patients displayed reduced proportions of CD56dim NK cells. Low percentages of blood NK cells associated with a reduced NKp30, NKp46, and NKG2D expression were reported in patients with invasive squamous cervical cancer (56).
The advent of targeted therapies that counteract a vital cellular process within the tumor cell greatly improved cancer treatment strategies. Thus, mitogen-activated protein kinase (MAPK) inhibitors that control the mutation-driven oncogenic pathway present in most cancers are new efficient players in the arsenal of therapies for cancer patients. In addition, monoclonal antibodies (mAbs) that recognize tumor-associated antigens have been established as one of the most successful therapeutic strategies for both hematologic malignancies and solid tumors. These mAbs may activate antibody-dependent cell-mediated cytotoxicity involving NK cells.
Combining targeted therapies and methods to stimulate patient’s immune players is actively evaluated and represents a promising and natural evolution in cancer treatment as this could ally immediate efficiency, specificity, and long-term antitumor efficacy.
It is of note that targeted therapies also display off-target effects, connecting oncogenesis to immunosurveillance. We discuss below the interest of NK cell-based therapies in the context of such tumor-targeted therapies (Figure 1).
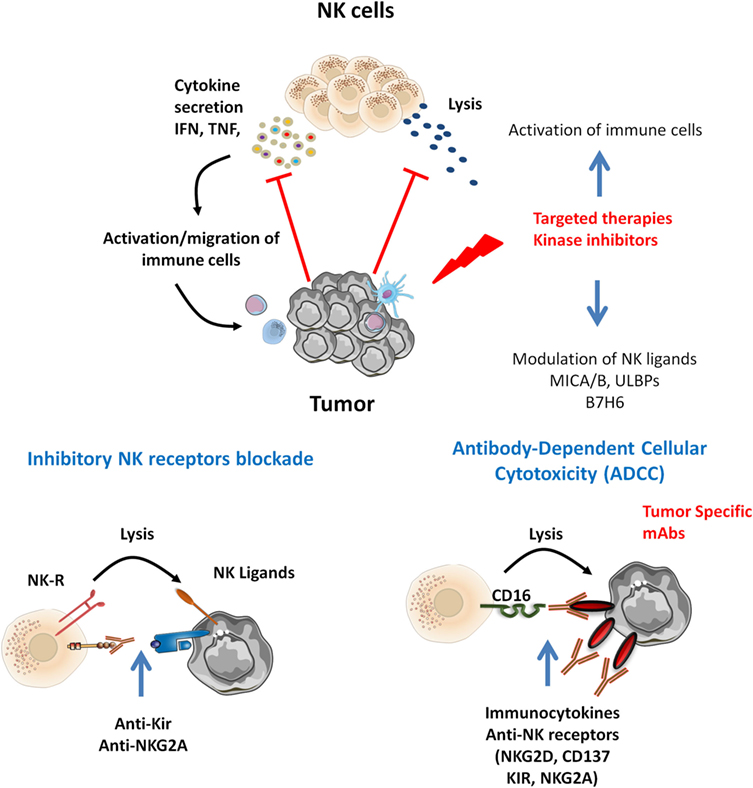
Figure 1. Natural killer (NK) cells infiltrating various tumor types display altered functions. Tumor-specific therapies may potentiate NK cell activation. Inhibitors targeting MAP kinase reduce tumor growth and display off-target effect modulating NK ligands expression and immune cell activation. Tumor-specific monoclonal antibodies (mAbs) may trigger ADCC by CD16+ NK cells. In that context, target NK-based immunotherapies may be proposed. Combined mitogen-activated protein kinase inhibitors with cytokines release NK function by inhibitory NK receptors [killer Ig-like receptor (KIR), NKG2A] blockade and promote NK-mediated ADCC of tumor antigen-specific mAbs and combined immunocytokines, anti-NK receptors (NKG2D, CD137, KIR, NKG2A).
Most melanoma patients (65%) bear a BRAF-mutated tumor and receive specific inhibitors targeting mutated BRAFV600E alone or in combination with MEK inhibitors, upstream of ERK (57). These inhibitors may exert bystander effects on certain immune cells that depend on MAPK for their activation and/or proliferation. BRAF inhibitors do not affect NK cell phenotype in vivo and in vitro, but blood NK cell numbers were increased in vemurafenib-treated patients (58, 59). MEK inhibition alters the expression of the main NK receptors and the function of cytokine-activated NK cells, but the combined BRAF and MEK inhibitors did not (60).
In addition, targeted therapies may interfere with the NK/target interactions through modulation of NK ligands on cancer cells. We have shown that a BRAF inhibitor modulates the expression of MICA and ULBP2 (ligands of NKG2D), changing the ratio between membrane expression and soluble form, and increases B7H6 (ligand of NKp30) expression and HLA-A,B,C and HLA-E molecules expression that engage inhibitory receptors (KIRs, NKG2A), thus interfering with NK cell-mediated lysis (in revision). Resistance to a BRAF inhibitor is accompanied by higher NK ligands expression (personal communications).
Our findings and recent results from the literature emphasize that therapeutics designed to limit cancer cell growth by acting through kinase inhibitors should also be considered in terms of their impact on immunosurveillance (61). In a murine model of BRAF-mutated melanoma, host NK cells and perforin were required for the effect of a BRAF inhibitor (62) and correlated with the reduction of tumor growth, and an increased NK and T cell infiltration of the tumors (63).
Combining specific MAPK inhibitors with immunotherapies to increase response rates is evaluated leading to yet discordant results. BRAF inhibition augments melanoma antigen expression and maintains T cell function (64). However, inhibition of BRAF in a murine model of human melanoma was associated with decreased tumor-resident lymphocytes and resistance to CTLA-4 mAb (65). MEK inhibitors increased antigen-specific T cell within the tumor sparing their cytotoxicity and combined with anti-PD-L1 mAb they exerted a synergic effect of tumor growth inhibition (66). Other kinase inhibitors such as those targeting Jak involved in the signaling cascade of cytokine receptors may influence NK (67).
A better understanding of off-target efficacy of MAPK inhibition affecting tumor–host interactions is required to develop strategies aimed at facilitating antitumor immune responses. The emerging findings indicate a potential synergy between targeted therapies, which change the balance between ligands of activating and inhibitory NK receptors, and NK-based immunotherapies, opening new interesting opportunities for the design of clinical trials.
One promising approach is to release NK cell function with anti-KIR or anti-NKG2A mAbs as NK cells are strictly controlled by receptors specific for HLA-class I molecules. Fully human anti-KIR mAbs, 1-7F9 mAb, and then lirilumab (recombinant version with a stabilized hinge) were generated (68). They prevent the binding of KIR2DL1, KIR2DL2, and KIR2DL3 receptors to their HLA-C ligands and blocking their inhibitory signaling. In vitro and in vivo studies showed that anti-KIR mAbs augmented NK cell-mediated lysis of HLA-C+ tumor cells, including autologous AML blasts and autologous CD138+ multiple myeloma cells (68–71). In addition, transient increases of TNFα and MIP-1β serum concentrations and CD69 expression on NK cells were observed from treated patients (72). In a clinical trial, Benson et al. showed that 1-7F9 mAb is safe in patients with multiple myeloma and enhances ex vivo patient-derived NK cell cytotoxicity against tumor cells (73).
Other immune receptors highly expressed by NK cells are in development, such as anti-NKG2A (monalizumab).
Targeting inhibitory pathways in NK cell/tumor interactions may be complementary to small-molecule inhibitors for the treatment of advanced tumors such as melanoma. The prospect of combining NK cell-based immunotherapy with approaches to target the immunosuppressive tumor microenvironment or immune checkpoints, such as KIR blockade, is especially relevant to the treatment of solid tumors (74, 75) and particularly for tumors refractory to targeted therapies.
Natural killer cells express activating low-affinity FcgRIIIa (CD16) and are key mediators of antibody-dependent cellular cytotoxicity. The relevance of ADCC in tumor control using therapeutic mAbs was evaluated in several cancers. The contribution of ADCC to the clinical efficacy of a therapeutic mAb has been observed in non-Hodgkin’s lymphoma patients treated by anti-CD20 (rituximab) (76). Other therapeutic mAbs likely inducing NK cell-mediated ADCC are anti-CD19 in patients with B malignancies, anti-GD2 in neuroblastoma patients, and anti-HER2 mAbs (trastuzumab) in metastatic breast and gastric cancer patients (76–78). Anti-EGFR mAb (cetuximab) was shown to increase ADCC-mediated lysis of colon tumor cells by blood NK cells from colorectal cancer patients that display altered natural cytotoxic activity (51).
Several modifications of the antibody structure, such as class switching, humanization, and point mutations to reduce complement interaction/activation, are developed to engineer mAbs with increased NK cell ADCC function and limit their toxicity. Thus, humanized anti-GD2 mAb (hu3F8-IgG1) exerts reduced toxicity compared to other anti-GD2 mAbs, by leveraging ADCC over complement-mediated cytotoxicity (79). Higher FcγRIIIA-binding affinity of anti-CD19 antibody significantly increased NK cell-mediated ADCC, leading to malignant B-cell clearing in non-human primates (78, 80). Other strategies to enhance the effect of ADCC include the coadministration of cytokines, IL-12 with anti-HER2/neu (trastuzumab) (81) to stimulate IFNγ production by NK cells and T cells and promote the CD56dimCD16+ NK cell differentiation to mediate ADCC (82). Co-infusion of anti-CD20 (rituximab) and TLR9 agonist (CpG) that is known to raise the membrane expression of CD20 on malignant B cells enhances ADCC (83). The infusion of immunocytokines, cytokines linked to the Fc terminus of humanized Abs, is also evaluated to potentiate ADCC. In preclinical study, Buhtoiarov et al. demonstrated that the humanized anti-GD2 immunocytokine hu14.18-IL-2 exerts higher antitumor effect than the reagents given separately (84).
Combining tumor-specific mAbs and mAbs targeting NK receptors (NKG2D, costimulatory molecule CD137) is another option. Anti-CD137 coadministred with rituximab led to a subsequent stimulation of these NK cells and enhanced rituximab-dependent cytotoxicity against the lymphoma cells (85). Furthermore, combination of rituximab with antibodies that block KIR2DL1 significantly improved NK cell-mediated lysis of tumor targets (86).
Restoring NK cell functions in addition to administration of tumor-specific therapies with kinase inhibitors or tumor-specific mAbs may benefit patients. It would increase the control of residual tumor cells, enhance mAbs efficiency, and promote the adaptive immune response necessary for long-lasting protective immunity. In that context, cytokines, blockade of inhibitory NK receptors (KIRs, NKG2A), or transfer of alloreactive NK cells are promising NK-based therapies.
The study protocol was approved by an ethic committee “Ile de France” (CPP: 2834), and the Declaration of Helsinki protocols were followed.
MM, ND, and AC wrote the manuscript; MM did the figure; AF, PG, and AT read and corrected the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was funded by INSERM, Association Laurette Fugain. AF has a PhD grant from Canceropole Ile de France. The work was funded by Institut du Cancer (INCa, PAIR Melanoma Program 2013-066).
1. Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer (1975) 16:230–9. doi:10.1002/ijc.2910160204
2. Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol (1975) 5:112–7. doi:10.1002/eji.1830050209
3. Sun H, Sun C, Tian Z, Xiao W. NK cells in immunotolerant organs. Cell Mol Immunol (2013) 10:202–12. doi:10.1038/cmi.2013.9
4. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells – a proposal for uniform nomenclature. Nat Rev Immunol (2013) 13:145–9. doi:10.1038/nri3365
5. Lanier LL, Testi R, Bindl J, Phillips JH. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med (1989) 169:2233–8. doi:10.1084/jem.169.6.2233
6. Caligiuri MA. Human natural killer cells. Blood (2008) 112:461–9. doi:10.1182/blood-2007-09-077438
7. Lanier L. Turning on natural killer cells. J Exp Med (2000) 191:1259–62. doi:10.1084/jem.191.8.1259
8. Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J (2004) 23:255–9. doi:10.1038/sj.emboj.7600019
9. Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol (2001) 19:197–223. doi:10.1146/annurev.immunol.19.1.197
10. Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol (2003) 3:781–90. doi:10.1038/nri1199
11. Braud V, Allan D, O’Callaghan C, Soderstrom K, D’Andrea A, Ogg G, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature (1998) 391:795–9. doi:10.1038/35869
12. Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet (2000) 356:1795–9. doi:10.1016/S0140-6736(00)03231-1
13. Hayashi T, Imai K, Morishita Y, Hayashi I, Kusunoki Y, Nakachi K. Identification of the NKG2D haplotypes associated with natural cytotoxic activity of peripheral blood lymphocytes and cancer immunosurveillance. Cancer Res (2006) 66:563–70. doi:10.1158/0008-5472.CAN-05-2776
14. Fend L, Rusakiewicz S, Adam J, Bastien B, Caignard A, Messaoudene M, et al. Prognostic impact of the expression of NCR1 and NCR3 NK cell receptors and PD-L1 on advanced non-small cell lung cancer. Oncoimmunology (2017) 6:e1163456. doi:10.1080/2162402X.2016.1163456
15. Messaoudene M, Fregni G, Enot D, Jacquelot N, Neves E, Germaud N, et al. NKp30 isoforms and NKp46 transcripts in metastatic melanoma patients: unique NKp30 pattern in rare melanoma patients with favorable evolution. Oncoimmunology (2016) 5:e1154251. doi:10.1080/2162402X.2016.1154251
16. Rusakiewicz S, Perier A, Semeraro M, Pitt JM, Pogge von Strandmann E, Reiners KS, et al. NKp30 isoforms and NKp30 ligands are predictive biomarkers of response to imatinib mesylate in metastatic GIST patients. Oncoimmunology (2017) 6:e1137418. doi:10.1080/2162402X.2015.1137418
17. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol (2004) 22:329–60. doi:10.1146/annurev.immunol.22.012703.104803
18. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol (2011) 29:235–71. doi:10.1146/annurev-immunol-031210-101324
19. Elboim M, Gazit R, Gur C, Ghadially H, Betser-Cohen G, Mandelboim O. Tumor immunoediting by NKp46. J Immunol (2010) 184:5637–44. doi:10.4049/jimmunol.0901644
20. Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity (2008) 28:571–80. doi:10.1016/j.immuni.2008.02.016
21. Pierson BA, Miller JS. CD56+bright and CD56+dim natural killer cells in patients with chronic myelogenous leukemia progressively decrease in number, respond less to stimuli that recruit clonogenic natural killer cells, and exhibit decreased proliferation on a per cell basis. Blood (1996) 88:2279–87.
22. Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood (2002) 99:3661–7. doi:10.1182/blood.V99.10.3661
23. Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood (2007) 109:323–30. doi:10.1182/blood-2005-08-027979
24. Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, et al. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol (2012) 90:109–15. doi:10.1038/icb.2011.15
25. Lion E, Willemen Y, Berneman ZN, Van Tendeloo VF, Smits EL. Natural killer cell immune escape in acute myeloid leukemia. Leukemia (2012) 26:2019–26. doi:10.1038/leu.2012.87
26. Khaznadar Z, Henry G, Setterblad N, Agaugue S, Raffoux E, Boissel N, et al. Acute myeloid leukemia impairs natural killer cells through the formation of a deficient cytotoxic immunological synapse. Eur J Immunol (2014) 44:3068–80. doi:10.1002/eji.201444500
27. Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R, et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica (2014) 99:836–47. doi:10.3324/haematol.2013.087536
28. Khaznadar Z, Boissel N, Agaugue S, Henry G, Cheok M, Vignon M, et al. Defective NK cells in acute myeloid leukemia patients at diagnosis are associated with blast transcriptional signatures of immune evasion. J Immunol (2015) 195:2580–90. doi:10.4049/jimmunol.1500262
29. Ruggeri L, Capanni M, Urbani E, Perrucio K, Shlomchik W, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (2002) 295:2375–82. doi:10.1126/science.1068440
30. Beelen DW, Ottinger HD, Ferencik S, Elmaagacli AH, Peceny R, Trenschel R, et al. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood (2005) 105:2594–600. doi:10.1182/blood-2004-04-1441
31. Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood (2010) 116:2411–9. doi:10.1182/blood-2010-05-283051
32. Stringaris K, Adams S, Uribe M, Eniafe R, Wu CO, Savani BN, et al. Donor KIR genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant (2010) 16:1257–64. doi:10.1016/j.bbmt.2010.03.004
33. Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong AS, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia (2007) 21:2145–52. doi:10.1038/sj.leu.2404892
34. Wittnebel S, Da Rocha S, Giron-Michel J, Jalil A, Opolon P, Escudier B, et al. Membrane-bound interleukin (IL)-15 on renal tumor cells rescues natural killer cells from IL-2 starvation-induced apoptosis. Cancer Res (2007) 67:5594–9. doi:10.1158/0008-5472.CAN-06-4406
35. Kornstein MJ, Stewart R, Elder DE. Natural killer cells in the host response to melanoma. Cancer Res (1987) 47:1411–2.
36. Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res (2011) 71:5412–22. doi:10.1158/0008-5472.CAN-10-4179
37. Carrega P, Bonaccorsi I, Di Carlo E, Morandi B, Paul P, Rizzello V, et al. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol (2014) 192:3805–15. doi:10.4049/jimmunol.1301889
38. Rusakiewicz S, Semeraro M, Sarabi M, Desbois M, Locher C, Mendez R, et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res (2013) 73:3499–510. doi:10.1158/0008-5472.CAN-13-0371
39. Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res (2011) 17:678–89. doi:10.1158/1078-0432.CCR-10-2173
40. Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg (2001) 121:1058–63. doi:10.1067/mtc.2001.113026
41. Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol (2006) 24:1997–2005. doi:10.1200/JCO.2005.03.9594
42. Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res (2013) 19:4079–91. doi:10.1158/1078-0432.CCR-12-3847
43. Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods (2009) 348:9–17. doi:10.1016/j.jim.2009.06.004
44. Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer (2002) 35:23–8. doi:10.1016/S0169-5002(01)00292-6
45. Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer (2000) 88:577–83. doi:10.1002/(SICI)1097-0142(20000201)88:3<577::AID-CNCR13>3.0.CO;2-V
46. Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer (1997) 79:2320–8. doi:10.1002/(SICI)1097-0142(19970615)79:12<2320::AID-CNCR5>3.0.CO;2-P
47. Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med (2011) 17:700–7. doi:10.1038/nm.2366
48. McKay K, Moore PC, Smoller BR, Hiatt KM. Association between natural killer cells and regression in melanocytic lesions. Hum Pathol (2011) 42:1960–4. doi:10.1016/j.humpath.2011.02.019
49. Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest (2011) 121:3609–22. doi:10.1172/JCI45816
50. Messaoudene M, Fregni G, Fourmentraux-Neves E, Chanal J, Maubec E, Mazouz-Dorval S, et al. Mature cytotoxic CD56bright/CD16+ natural killer cells can infiltrate lymph nodes adjacent to metastatic melanoma. Cancer Res (2014) 74:81–92. doi:10.1158/0008-5472.CAN-13-1303
51. Rocca YS, Roberti MP, Julia EP, Pampena MB, Bruno L, Rivero S, et al. Phenotypic and functional dysregulated blood NK cells in colorectal cancer patients can be activated by cetuximab plus IL-2 or IL-15. Front Immunol (2016) 7:413. doi:10.3389/fimmu.2016.00413
52. Mirjacic Martinovic K, Konjevic G, Babovic N, Inic M. The stage dependent changes in NK cell activity and the expression of activating and inhibitory NK cell receptors in melanoma patients. J Surg Res (2011) 171:637–49. doi:10.1016/j.jss.2010.05.012
53. Konjevic G, Mirjacic Martinovic K, Vuletic A, Jovic V, Jurisic V, Babovic N, et al. Low expression of CD161 and NKG2D activating NK receptor is associated with impaired NK cell cytotoxicity in metastatic melanoma patients. Clin Exp Metastasis (2007) 24:1–11. doi:10.1007/s10585-006-9043-9
54. Konjevic G, Mirjacic Martinovic K, Vuletic A, Babovic N. In-vitro IL-2 or IFN-alpha-induced NKG2D and CD161 NK cell receptor expression indicates novel aspects of NK cell activation in metastatic melanoma patients. Melanoma Res (2010) 20:459–67. doi:10.1097/CMR.0b013e32833e3286
55. Fregni G, Messaoudene M, Fourmentraux-Neves E, Mazouz-Dorval S, Chanal J, Maubec E, et al. Phenotypic and functional characteristics of blood natural killer cells from melanoma patients at different clinical stages. PLoS One (2013) 8:e76928. doi:10.1371/journal.pone.0076928
56. Garcia-Iglesias T, Del Toro-Arreola A, Albarran-Somoza B, Del Toro-Arreola S, Sanchez-Hernandez PE, Ramirez-Duenas MG, et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer (2009) 9:186. doi:10.1186/1471-2407-9-186
57. Eroglu Z, Ribas A. Combination therapy with BRAF and MEK inhibitors for melanoma: latest evidence and place in therapy. Ther Adv Med Oncol (2016) 8:48–56. doi:10.1177/1758834015616934
58. Comin-Anduix B, Chodon T, Sazegar H, Matsunaga D, Mock S, Jalil J, et al. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res (2010) 16:6040–8. doi:10.1158/1078-0432.CCR-10-1911
59. Schilling B, Sondermann W, Zhao F, Griewank KG, Livingstone E, Sucker A, et al. Differential influence of vemurafenib and dabrafenib on patients’ lymphocytes despite similar clinical efficacy in melanoma. Ann Oncol (2014) 25:747–53. doi:10.1093/annonc/mdt587
60. Manzini C, Vene R, Cossu I, Gualco M, Zupo S, Dono M, et al. Cytokines can counteract the inhibitory effect of MEK-i on NK-cell function. Oncotarget (2016) 7:60858–71. doi:10.18632/oncotarget.11504
61. Chretien AS, Le Roy A, Vey N, Prebet T, Blaise D, Fauriat C, et al. Cancer-induced alterations of NK-mediated target recognition: current and investigational pharmacological strategies aiming at restoring NK-mediated anti-tumor activity. Front Immunol (2014) 5:122. doi:10.3389/fimmu.2014.00122
62. Ferrari de Andrade L, Ngiow SF, Stannard K, Rusakiewicz S, Kalimutho M, Khanna KK, et al. Natural killer cells are essential for the ability of BRAF inhibitors to control BRAFV600E-mutant metastatic melanoma. Cancer Res (2014) 74:7298–308. doi:10.1158/0008-5472.CAN-14-1339
63. Knight DA, Ngiow SF, Li M, Parmenter T, Mok S, Cass A, et al. Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest (2013) 123:1371–81. doi:10.1172/JCI66236
64. Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res (2010) 70:5213–9. doi:10.1158/0008-5472.CAN-10-0118
65. Hooijkaas A, Gadiot J, Morrow M, Stewart R, Schumacher T, Blank CU. Selective BRAF inhibition decreases tumor-resident lymphocyte frequencies in a mouse model of human melanoma. Oncoimmunology (2012) 1:609–17. doi:10.4161/onci.20226
66. Ebert PJ, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, et al. Promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity (2016) 44:609–21. doi:10.1016/j.immuni.2016.01.024
67. Bottos A, Gotthardt D, Gill JW, Gattelli A, Frei A, Tzankov A, et al. Decreased NK-cell tumour immunosurveillance consequent to JAK inhibition enhances metastasis in breast cancer models. Nat Commun (2016) 7:12258. doi:10.1038/ncomms12258
68. Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood (2009) 114:2667–77. doi:10.1182/blood-2009-02-206532
69. Benson DM Jr, Bakan CE, Zhang S, Collins SM, Liang J, Srivastava S, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood (2011) 118:6387–91. doi:10.1182/blood-2011-06-360255
70. Sola C, Andre P, Lemmers C, Fuseri N, Bonnafous C, Blery M, et al. Genetic and antibody-mediated reprogramming of natural killer cell missing-self recognition in vivo. Proc Natl Acad Sci U S A (2009) 106:12879–84. doi:10.1073/pnas.0901653106
71. Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood (2014) 123:678–86. doi:10.1182/blood-2013-08-519199
72. Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood (2012) 120:4317–23. doi:10.1182/blood-2012-06-437558
73. Benson DM Jr, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood (2012) 120:4324–33. doi:10.1182/blood-2012-06-438028
74. Murray S, Lundqvist A. Targeting the tumor microenvironment to improve natural killer cell-based immunotherapies: on being in the right place at the right time, with resilience. Hum Vaccin Immunother (2016) 12:607–11. doi:10.1080/21645515.2015.1096458
75. Navarro A, Bjorklund A, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol (2015) 29:202–7. doi:10.3389/fimmu.2015.00202
76. Alderson KL, Sondel PM. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol (2011) 2011:379123. doi:10.1155/2011/379123
77. Breton CS, Nahimana A, Aubry D, Macoin J, Moretti P, Bertschinger M, et al. A novel anti-CD19 monoclonal antibody (GBR 401) with high killing activity against B cell malignancies. J Hematol Oncol (2014) 7:33. doi:10.1186/1756-8722-7-33
78. Ahmed M, Cheung NK. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett (2014) 588:288–97. doi:10.1016/j.febslet.2013.11.030
79. Cheung NK, Guo H, Hu J, Tassev DV, Cheung IY. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. Oncoimmunology (2012) 1:477–86. doi:10.4161/onci.19864
80. Zalevsky J, Leung IW, Karki S, Chu SY, Zhukovsky EA, Desjarlais JR, et al. The impact of Fc engineering on an anti-CD19 antibody: increased Fcgamma receptor affinity enhances B-cell clearing in nonhuman primates. Blood (2009) 113:3735–43. doi:10.1182/blood-2008-10-182048
81. Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest (2002) 110:983–92. doi:10.1172/JCI0215950
82. Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol (2004) 172:2048–58. doi:10.4049/jimmunol.172.4.2048
83. Betting DJ, Yamada RE, Kafi K, Said J, van Rooijen N, Timmerman JM. Intratumoral but not systemic delivery of CpG oligodeoxynucleotide augments the efficacy of anti-CD20 monoclonal antibody therapy against B cell lymphoma. J Immunother (2009) 32:622–31. doi:10.1097/CJI.0b013e3181ab23f1
84. Buhtoiarov IN, Neal ZC, Gan J, Buhtoiarova TN, Patankar MS, Gubbels JA, et al. Differential internalization of hu14.18-IL2 immunocytokine by NK and tumor cell: impact on conjugation, cytotoxicity, and targeting. J Leukoc Biol (2011) 89:625–38. doi:10.1189/jlb.0710422
85. Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood (2011) 117:2423–32. doi:10.1182/blood-2010-08-301945
Keywords: tumour immunosurveillance, natural killer ligands, immune checkpoint inhibitors, BRAF inhibitor, AMLMDS, melanoma
Citation: Messaoudene M, Frazao A, Gavlovsky PJ, Toubert A, Dulphy N and Caignard A (2017) Patient’s Natural Killer Cells in the Era of Targeted Therapies: Role for Tumor Killers. Front. Immunol. 8:683. doi: 10.3389/fimmu.2017.00683
Received: 07 March 2017; Accepted: 26 May 2017;
Published: 12 June 2017
Edited by:
Ulrike Koehl, Hannover Medical School, GermanyReviewed by:
Christian Kalberer, University of Basel, SwitzerlandCopyright: © 2017 Messaoudene, Frazao, Gavlovsky, Toubert, Dulphy and Caignard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne Caignard, YW5uZS5jYWlnbmFyZEBpbnNlcm0uZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.