- 1Centre for Trophoblast Research, University of Cambridge, Cambridge, UK
- 2Department of Obstetrics and Gynaecology, National Institute for Health Research Cambridge Biomedical Research Centre, University of Cambridge School of Clinical Medicine, Cambridge, UK
Our understanding of development and function of natural killer (NK) cells has progressed significantly in recent years. However, exactly how uterine NK (uNK) cells develop and function is still unclear. To help investigators that are beginning to study tissue NK cells, we summarize in this review our current knowledge of the development and function of uNK cells, and what is yet to be elucidated. We compare and contrast the biology of human and mouse uNK cells in the broader context of the biology of innate lymphoid cells and with reference to peripheral NK cells. We also review how uNK cells may regulate trophoblast invasion and uterine spiral arterial remodeling in human and murine pregnancy.
Introduction
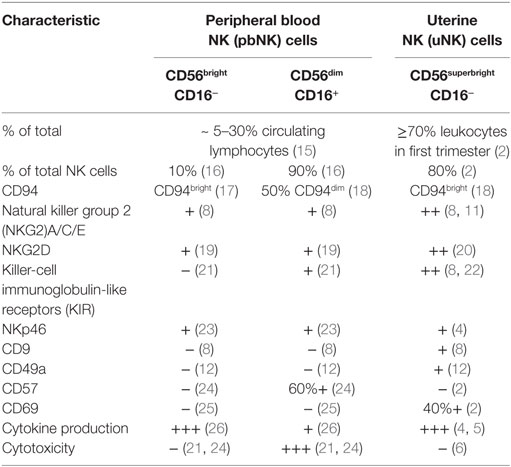
CD56superbright uterine natural killer (uNK) cells are present in human endometrium prior to the initiation of pregnancy, and markedly expand and become progressively more granulated during the progesterone-dominated secretory phase after ovulation and throughout the first trimester (1–3). uNK cells within the decidua have a distinct phenotype compared to peripheral blood NK (pbNK) cells and share features of both CD56bright and CD56dim pbNK subsets (Table 1). Similarly to CD56bright pbNK cells, uNK preferentially produce cytokines and are poorly cytotoxic, despite their abundant intracellular granules containing granzymes, granulysin, and perforin (4–9). Killer-cell immunoglobulin-like receptors (KIR) and natural killer group 2 (NKG2)A/C/E receptors, which recognize trophoblast MHC class I human leukocyte antigen (HLA)-C and HLA-E, respectively, are expressed at higher levels among uNK than their pbNK cell counterparts, and are skewed toward recognition of their respective ligands (8, 10, 11). All human decidual uNK cells are CD49a+, also known as very late antigen-1 (VLA-1) or integrin α1β1, and express CD69 (2, 12). uNK cells peak in frequency during the first trimester, before becoming progressively less granular and beginning to diminish in numbers midway through gestation, so that only small numbers are present at term (13, 14).
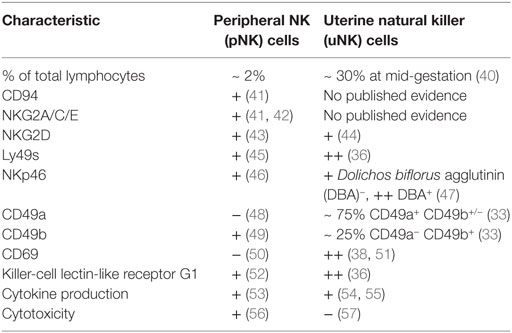
In comparison to humans, two functionally disparate populations of uNK cells have been identified in mice, which are distinguished in most studies to date by their reactivity to Dolichos biflorus agglutinin (DBA). Gene expression studies show that DBA+ uNK cells predominantly express transcripts for angiogenic factors, whereas interferon (IFN)-γ transcripts dominate in the DBA− subset (27). Murine uNK do not begin to mature into large, granulated lymphocytes until blastocyst implantation, and they acquire reactivity to DBA after g.d. 5 alongside their increase in granularity (28, 29). As in humans, murine uNK cells are poorly cytotoxic despite containing granules encasing perforin and granzymes (30–32). At the mesometrial pole of the implantation site and adjacent to the decidua basalis, a lymphocyte-rich accretion of leukocytes composed largely of uNK cells, macrophages, and dendritic cells develops (29, 33, 34). This mesometrial lymphoid aggregate of pregnancy (MLAp) is a feature of pregnancy unique to rodents, which is established by g.d. 8. Mature uNK cells are most abundant throughout the decidua basalis and MLAp approximately halfway through gestation (Figure 1) (28, 29, 35). uNK undergoing apoptosis begin to appear from mid-gestation onwards and are highly prevalent by g.d. 12 (28, 35). Expression of lectin-like Ly49 receptors, which recognize MHC class I, is higher among uNK than peripheral (pNK) cells and, as in humans, some receptors are mildly skewed toward recognition of trophoblast MHC ligands (36, 37). uNK in mice also express killer-cell lectin-like receptor G1 (KLRG1) more highly than their pNK cell counterparts, indicating a more mature phenotype (36, 38). The features of murine uNK cells are summarized in Table 2.
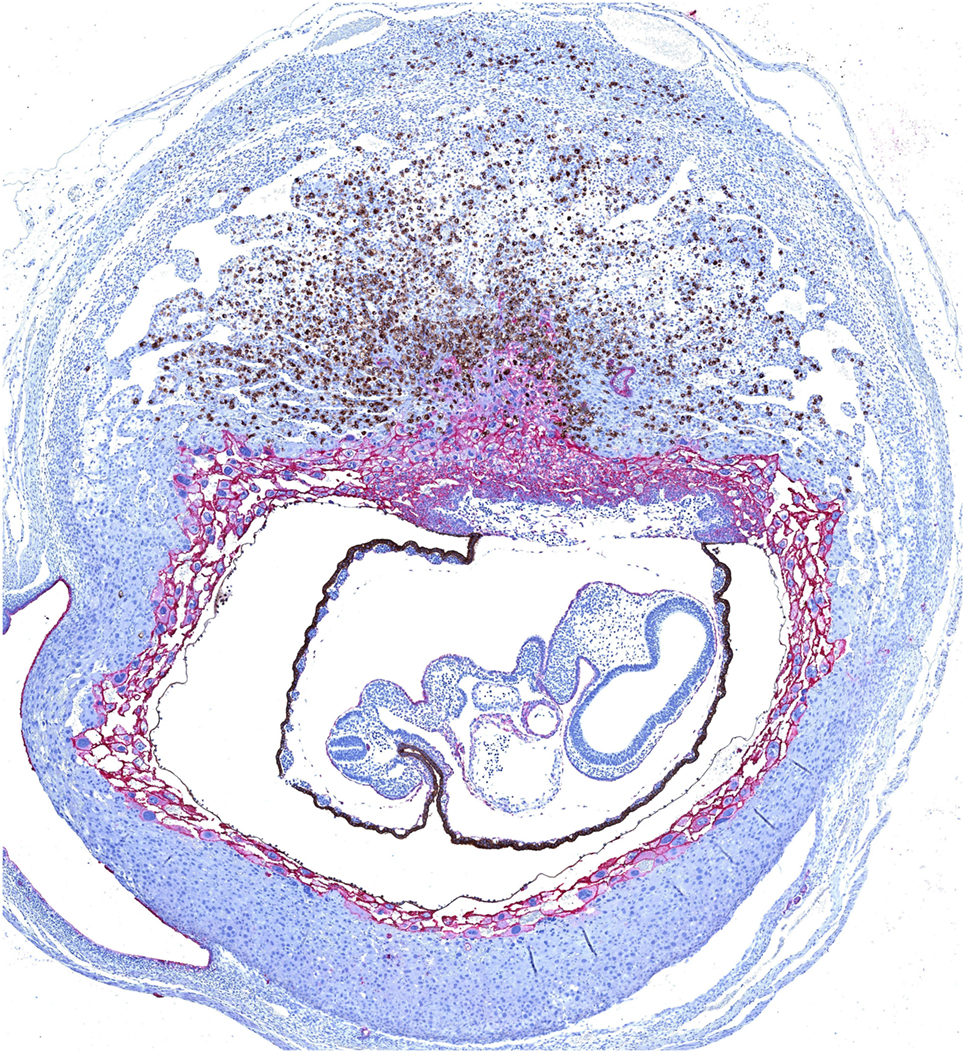
Figure 1. Dual immunohistochemical staining of Dolichos biflorus agglutinin (DBA)+ uterine natural killer (uNK) cells and trophoblast in a mouse implantation site at mid-gestation. Trophoblast (shown in pink) migrate centrally into the decidua to form an ectoplacental cone, which is surrounded by a layer of moderately invasive trophoblast giant cells expressing MHC class I. Mature uNK are abundant throughout the decidua basalis, and in a lymphocyte-rich accretion at the uppermost pole of the implantation site, known as the mesometrial lymphoid aggregate of pregnancy. Interactions between uNK Ly49 receptors and trophoblast MHC class I can modulate the activity of DBA+ uNK (shown in brown) and DBA− uNK cells, and impact on their production of angiogenic factors and interferon (IFN)-γ respectively. Reproduced from Moffett and Colucci (39) with permission.
The relatively recent designation of CD49a as a marker of tissue residency and its inclusion in the cytometric analysis of uterine lymphocytes alongside common NK cell markers such as CD49b (DX5) has enabled the redefinition of murine uNK subsets (33, 48). uNK cells in mice can now be classified as CD49a+ DX5+/− uterine tissue-resident NK (trNK) cells and CD49a− DX5+ uterine conventional NK (cNK) cell populations (33, 48, 58, 59), which will be described in greater depth later in this review. DBA reactivity is strongest on uterine CD49a+ trNK, and is weak on DX5+ uterine cNK (40, 58). As in DBA+ uNK, decidual CD49a+ DX5+/− trNK cells produce less total IFN-γ at mid-gestation than CD49a− DX5+ cNK cells, which further supports the correlation between CD49a and DBA reactivity (27, 58, 59). Although the correlation between CD49a and DBA co-expression is not sufficiently clear-cut to consider DBA as a specific marker of uterine trNK cells, it does enable some reconsideration of historical histological studies.
Despite numerous anatomical and physiological differences between murine and human pregnancies, the functions and regulation of uNK cells are reasonably comparable between these species. In both species, uNK contribute to fundamental physiological processes of pregnancy within the decidua, but there are key differences in how these effects are mediated (Figure 2). Human uNK assist in the initial stages of decidua-associated vascular remodeling and control the depth of invasion of extravillous trophoblast (EVT), which are responsible for the majority of arterial transformation in human pregnancy. Comparatively, murine uNK are composed of two subsets, with largely differing roles. uNK-derived IFN-γ is essential for remodeling of the decidual vasculature in mice, whereas the contribution of trophoblast is relatively insignificant and, indeed, rodent uNK predominantly suppress trophoblast invasion. In both species, uNK produce angiogenic factors, but in mice this is predominantly mediated by the DBA+ subset. As such, considering the broader themes of the decidual adaptations to pregnancy, mice provide a useful animal model in which to study reproductive immunology.
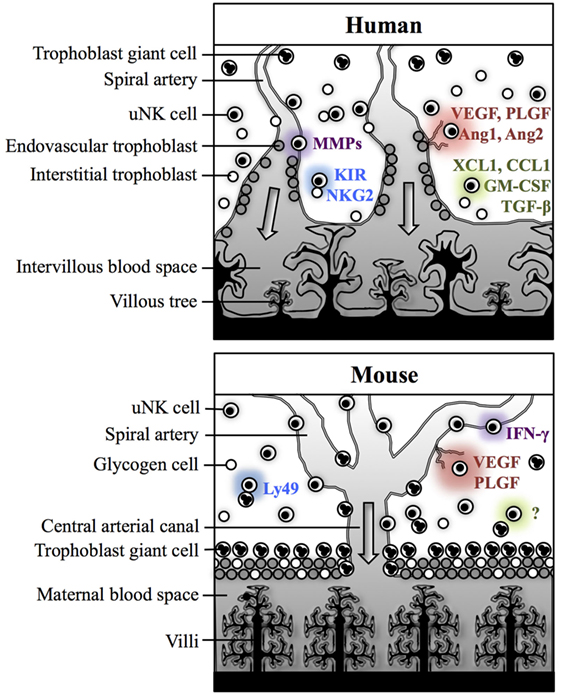
Figure 2. Physiological processes of pregnancy within the decidua in human and mice. In both humans and mice, uterine natural killer (uNK) cells are thought to contribute to spiral arterial remodeling (purple), angiogenesis (red), and control of trophoblast invasion (green). Interactions between uNK receptors and trophoblast MHC class I antigens may modulate uNK cell activity (blue). In humans, uNK cells may contribute directly to decidua-associated vascular remodeling through secretion of matrix metalloproteinases (MMPs). Human uNK may also influence trophoblast-mediated vascular remodeling through secreting factors which enhance extravillous trophoblast (EVT) invasion [XCL1, CCL1, granulocyte-macrophage colony-stimulating factor (GM-CSF)] or suppress EVT migration [transforming growth factor (TGF)-β]. uNK cells in humans also secrete several angiogenic factors including vascular endothelial growth factor (VEGF), placental growth factor (PLGF), Ang1, and Ang2. Their activity may be modulated by killer-cell immunoglobulin-like receptors (KIR) and natural killer group 2 (NKG2) receptors, which recognize human leukocyte antigen class I ligands expressed by EVT. In mice, IFN-γ secreted by Dolichos biflorus agglutinin (DBA)− uNK cells is essential for decidual vascular remodeling. DBA+ uNK cells are predominantly responsible for producing angiogenic factors, including VEGF and PLGF. Evidence from studies in rats and mice suggests that uNK cells primarily suppress trophoblast motility, but the mechanisms for this are not currently understood. Murine uNK cell function can be modulated by Ly49 receptors, which recognize MHC class I expressed by trophoblast giant cells.
Innate Lymphoid Cells
Natural killer cells are the most abundant and well-characterized subset of innate lymphoid cells (ILCs), which comprise lymphocytes belonging to the innate arm of the immune system exhibiting features of both innate and adaptive immunity (60, 61). ILCs are an important component in the immune response to a wide range of pathogens, particularly at epithelial barrier surfaces. They also contribute to tissue and metabolic homeostasis and have been implicated in the pathogenesis of cancer and inflammatory diseases. Features common to all ILCs are the absence of recombination activating gene (RAG)-dependent antigen-specific receptors, absence of myeloid lineage markers, and a lymphoid cellular morphology. Based upon this classification, ILCs can be broadly categorized into three major groups according to their development, cell surface markers, and functions (62, 63).
Our understanding of the origins and functions of ILCs is rapidly evolving, and the identities of disparate ILC populations are becoming increasingly apparent (64–67). While the pathways of ILC development in mice have been well defined, ILC differentiation in humans has yet to be determined. There is also a degree of phenotypic plasticity among ILCs, indicating that ILCs are capable of adapting their identities and functions in vivo in response to other immune cells and secreted factors in the local environment.
Functional comparisons have been made between ILCs and T cells, as the stimuli and cytokine profiles of ILC1s, ILC2s, and ILC3s are analogous to those of the TH cell subsets TH1, TH2, and TH17, respectively. Group 1 ILCs comprise bona fide helper-like ILC1s and NK cells. NK cells can be further subdivided into cNK and trNK subsets that differ in their phenotype, function, and development. cNK cells are the only ILC population to exhibit cytotoxicity mediated by exocytosis of cytotoxic granules containing perforin and granzymes, similarly to CD8+ cytotoxic T-lymphocytes (60).
Uterine ILCs
Since the amalgamation of diverse innate lymphocyte populations into the ILC family, there has been considerable focus on determining the distribution and biological significance of these cells in vivo (68). Other than uNK cells, first defined in 1991, ILC1s and ILC3s have also been identified in human decidua (2, 33, 69, 70). Uterine ILC3s (uILC3s) were initially classified as stage 3 uNK cell progenitors based upon their CD34− CD117+ CD94− CD56+ KIR− phenotype. These cells produced interleukin (IL)-22 and expressed RORC and LTA, encoding the transcription factor RORγ and lymphotoxin (LT)-α, respectively, which makes them indistinguishable from uILC3s (62, 69). The presence of human uILC3s and lymphoid tissue inducer (LTi)-like uILC3s has since been confirmed in accordance with currently accepted ILC definitions (33, 70). However, it has since been proposed that a population of CD34+ CD122+ CD309− lymphoid-like cells in human decidua represent NK-committed decidual hematopoietic progenitor cells (HPCs) (71). If these cells can be more definitively characterized as such, through detection of multiple co-expressed cell surface markers and transcription factors, it is likely that the population described by Male et al. were a heterogeneous mix of stage 3 uNK cell precursors and uILC3s. A proportion of uILC3s have been shown to differentiate to stage 3-like CD117+ CD56+ CD94+ uNK cells upon in vitro culture with IL-15 (70). A similar report indicates that uILCs can differentiate into stage 4 CD117− CD56+ CD94+ uNK cells in vitro, further suggesting that uNK cell precursors were present (69). In view of the recent finding that tonsillar ILC3s can differentiate to stage 4 CD94+ CD56bright NK cells upon aryl hydrocarbon receptor (AhR) silencing in vitro, it would also be interesting to ascertain whether AhR is expressed by uILC3s and whether its manipulation is similarly able to induce differentiation to an NK cell phenotype (72).
All groups of ILCs are present in the uteri of virgin and pregnant mice (33). Uterine trNK cells were initially considered to develop independently of the transcription factors nuclear factor, interleukin-3 regulated (Nfil3) and T-box transcription factor Tbx21 (T-bet), but their dependency on Eomesodermin (Eomes) was not ascertained. As such, it was not possible to deduce their identity as belonging to an NK cell or bona fide ILC1 lineage (48). Similarly to those in the salivary gland, trNK cells in the uterus do express Eomes and, together with uterine cNK cells and Eomes− uILC1s, they are found throughout the decidua and myometrium during pregnancy. uILC1s can produce tumor necrosis factor (TNF)-α and IFN-γ but, owing to the fact that uILC1-sufficient Nfil3−/− females exhibit poor decidual vascular remodeling, the contribution of uILC1-derived IFN-γ to vascular modification is seemingly negligible (33, 58, 59). uILC2s, uILC3s, and LTi-like uILC3s are found only in the myometrium and in the MLAp (33). The MLAp is of unknown function but, since it is traversed by branches of the uterine artery, it is possible that it exerts some effect on the perfusion of individual implantation sites through leukocyte-mediated modification of vessels proximal to the spiral arteries (73, 74). Unlike lymph nodes, MLAp formation does not depend on LTα and LTβ-receptor signaling, making a role for LTi-like uILC3s in MLAp development unlikely (35, 75). Whether uILC2s and uILC3s and their derived cytokines, IL-5, IL-13, IL-17, and IL-22 participate in local immune regulation or tissue remodeling is currently unknown (33).
NK Cell Development
Murine cNK cells arise from NK progenitors (NKP), which represent the first of six defined stages of murine NK cell development (76–78). In contrast to helper-like ILCs, cNK cells develop independently of IL-7 and become CD127− CD122+ (IL-2Rβ; IL-15Rβ) at the NKP stage. IL-15 signaling is essential for the differentiation of NKPs to immature NK (iNK) cells (79–81). Subsequently, iNK cells acquire functionally modulatory receptors such as NK1.1, NKp46, Ly49, and NKG2 receptors. Expression of the integrin CD49b (DX5) denotes the transition of iNK cells to a mature phenotype, which correlates with the development of functional competence such as IFN-γ production and cytolytic potential (82, 83). Three further stages of maturation can be defined by the differential expression of CD27 and CD11b, which culminate in the development of terminally mature NK cells expressing KLRG1 and CD43 (52, 84).
However, NK cell development can occur via alternative pathways. NK cells of thymic origin have been identified, which depend upon the GATA-binding protein-3 (GATA-3) transcription factor and IL-7 signaling. These cells appear phenotypically immature compared to cNK cells, but are more effective cytokine producers (85). More recently, Nfil3-independent NK cells have been described in skin, uterus, and salivary glands, which all express the integrin CD49a as a marker of tissue residency but which differ in their dependency on T-bet (33, 48, 86–88). However, since the population originally classified as CD49a+ DX5− hepatic trNK cells does not express the transcription factor Eomes, it is more appropriate to consider these as hepatic ILC1s. These exhibit a broader cytokine profile than cNK and highly express TNF-related apoptosis-inducing ligands (TRAIL), which confer potential to induce apoptosis (48, 89, 90).
Ontogenesis of human NK cells is broadly analogous to that in mice, but there are notable differences in the sequence and anatomical sites of each developmental stage. Human NK cells arise from bone marrow (BM)-derived CD34+ HPCs. Although elusive until recently, NK lineage-restricted progenitors have been identified in adult and fetal bone marrow, fetal liver, and adult tonsils (91). Evidence suggests that CD34dim pro-NK cells are exported from BM comparatively early and home to secondary lymphoid tissues where they continue to differentiate (92). Five continuous stages of human NK cell development have been characterized in lymph node and tonsil. IL-15 acts on stage two pre-NK cells to support their transition to stage three. Human NK cells do not begin to express receptors for class I HLA antigens, including KIR and CD94/NKG2 dimers, until they reach a mature CD56bright phenotype (17). At this stage, human NK cells are competent cytokine producers, which either remain in situ or terminally differentiate in peripheral blood to acquire cytotoxic potential as CD56dim CD16+ NK cells (21, 26, 93).
The transcriptional control of human NK cell development has not been delineated clearly, but GATA-3 transcripts are abundant in stage 3 NK cells, and T-bet and Eomes are highly expressed in stage four and stage five NK cells (17, 94). However, as in mouse, subpopulations of CD49a+ trNK cells have been identified in uterine endometrium and liver (59, 95). A subpopulation of CD127− CD56+ Eomes+ tonsillar and intestinal intraepithelial ILC1s are phenotypically and functionally resemblant of NK cells, but their murine counterparts develop independently of IL-15 (96). As such, it is possible that these, and perhaps other ILC1s, arise from pre-NK cells, and are more closely developmentally linked to NK cells than we currently appreciate.
Origin of uNK Cells
Uterine natural killer cells account for over 70% of decidual leukocytes in the first trimester of human pregnancy and for approximately 30% of lymphocytes in murine decidua at mid-gestation (2, 40). The origin of these distinct and specialized NK cells has been a subject of investigation for over 30 years, but it is becoming increasingly accepted that uNK cells are likely to be a heterogeneous population arising from in situ progenitors and from homing of NKPs and/or pNK cells (97).
When mice were lethally irradiated in the presence of a protective lead shield covering one uterine horn, and subsequently rat BM cells were adoptively transferred, only uNK cells of rat origin could be identified in the irradiated uterine tissue, indicating that peripherally derived NKPs contribute to the generation of uNK cells (98). This is supported by observations that uteri from NK-sufficient mice are devoid of uNK cells when engrafted into NK-deficient hosts (99). Leukocytes of donor origin can be found in both murine and human decidua, following experimental transgenic labeling of BM cells and hematopoietic stem cell transplantation (HSCT) respectively, which suggests that decidual leukocytes are derived, at least in part, from BM HPCs in vivo (100, 101). A very small population of stage 3 NK precursors in peripheral blood, which are capable of maturing to stage 4 cells in the presence of IL-15, also raises the possibility that NK precursors home to the uterus and differentiate to mature uNK cells in situ (69). As pbNK cells can be induced to acquire phenotypic and functional attributes of uNK cells under the influence of hypoxia, transforming growth factor (TGF)-β, and demethylating agents, it is also possible that some uNK cells develop as a result of pbNK cell recruitment (102).
However, in the study by Peel and Stewart, no uNK cells could be detected in the irradiated uterine horn in half of the mice which had retained functional BM as a result of lead shielding of their legs during irradiation. This suggests that uNK cell precursors present in uterine tissue prior to irradiation were either destroyed or rendered incapable of proliferation, and that recruitment of circulating NKPs was insufficient to restore the uNK cell population (98). The proposed NK-committed decidual HPCs identified by Vacca et al. can differentiate to mature uNK cells in the presence of IL-15, which is expressed abundantly in first trimester decidua and placenta (71, 103). The presence of in situ HPCs would also account for the CD56+ NK cells detected in human endometrial tissue which had been xenografted into hormone-treated immunodeficient mice (104). NK cell development from resident hematopoietic progenitors has also been documented in mice (105). Taken together, it seems probable that uNK cells arise from proliferation of peripherally derived HPCs and/or NK precursors which have homed to the pregravid uterus, but a potential contribution by pbNK cells which undergo phenotypic adaptation in situ cannot be discounted.
Effector Functions of NK Cells
Soluble factors secreted by other leukocytes can stimulate cytokine production by NK cells, which provides a means by which these immune cells can indirectly interact with each other and reciprocally induce effector functions. NK cells are responsive to a number of cytokines released by monocytes, including IL-1, IL-10, IL-12, IL-15, and IL-18, and TH lymphocytes, including IL-2 and IL-21. These induce production of key NK cell-mediated cytokines such as IFN-γ, granulocyte-macrophage colony stimulating factor (GM-CSF), TNF-α, and macrophage inflammatory protein (MIP)-1 (106). Of these, IFN-γ has the most diverse immunomodulatory roles and promotes TH1 cell differentiation, activation of macrophages and enhancement of antigen presentation via upregulation of class I and class II MHC molecules; all of which cumulatively contribute to antimicrobial, antiviral, and anti-tumor immunity (107).
All mature murine cNK cells have the capacity to produce cytokines and mediate perforin-dependent cytotoxicity. Distinct tissue-specific NK cell subpopulations display variation in functionality, such that salivary gland trNK cells only induce TRAIL-dependent cytolysis and uNK cells are weakly cytotoxic under physiological conditions (57, 87). In humans, CD56dim CD16+ NK cells contain lytic granules, and are less effective cytokine producers and express KIR at far higher frequencies than their CD56bright CD16− counterparts (21, 26). That the CD56dim CD16+ subset accounts for 90% of circulating NK cells emphasizes the importance of HLA class I recognition as a means of immunosurveillance by pNK cells. Indeed, the absence of NK cells in vivo enhances susceptibility to viral infections and metastatic progression of malignant tumors (108–110).
Natural killer cells express a broad repertoire of modulatory receptors, of which many are common to both human and mouse. Among these are the activating receptors NKp46, which recognizes viral hemagglutinins, NKG2D which binds cellular stress-induced ligands, and CD16, which mediates antibody-dependent cellular cytotoxicity (ADCC) in response to immunoglobulin G (IgG) (111, 112). The induction of cytotoxic effector responses is tightly regulated and, with the exception of CD16, requires the synergistic input of signaling via two activating receptors, reduced inhibition and/or the presence of stimulatory cytokines (113). As many inhibitory NK cell receptors recognize MHC class I ligands, reduced inhibition predominantly occurs in the context of downregulation of MHC class I molecules by virally infected and malignant cells.
The probable roles of uNK in both human and mouse are the production of cytokines, chemokines and angiogenic factors, which may mediate the key physiological processes required for successful pregnancy, discussed in greater depth later in this review (Figure 2). Comprehensive gene expression analyzes have demonstrated the extent to which human NK cells in the uterus functionally and phenotypically differ from those in peripheral blood (4, 8). Although uNK cells are phenotypically and functionally distinct from pNK cells, their activity can be similarly modulated through interactions with soluble factors and cell-bound ligands, including MHC class I.
NK Cell Recognition of MHC Molecules
Recognition of class I MHC is mediated by KIR in humans, Ly49 receptors in mice, and by CD94/NKG2 heterodimers in both species (114). KIRs are highly polymorphic receptors encoded within the leukocyte receptor complex (LRC) on chromosome 19, which bind to HLA class I molecules (115). Sixteen KIR genes have been identified and, for each, between 18 and 112 alleles are currently known (116, 117). Fourteen of these genes encode functional receptors for classical HLA, of which six are inhibitory and eight are activating.
KIR genes can be grouped into two main haplotypes, termed A and B. With the exception of KIR2DS4, which is most commonly truncated, haplotype A encodes only inhibitory receptors whereas haplotype B contains genes for both inhibitory and activating KIR. The majority of KIR2D receptors exhibit binding specificity for one of two epitopes of all HLA-C allotypes, C1 and C2, which differ due to diallelic polymorphism at positions 77 and 80 of the α1 chain (116, 118). Binding affinities between KIR2DL and HLA-C molecules also influence functional responses, such that weak interactions induce less inhibition. KIR A haplotypes are typified by KIR2DL1, which binds C2 epitopes with high avidity, and KIR2DL3, which weakly binds C1 epitopes. Comparatively, KIR B haplotypes are characterized by an allotype of KIR2DL1 which binds C2 epitopes with low affinity, and KIR2DL2, which binds more strongly than KIR2DL3 to C1 epitopes (119).
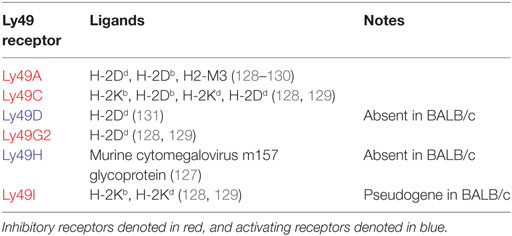
The functionally analogous receptors for classical MHC class I molecules in the mouse are polymorphic lectin-like Ly49 receptors. These are encoded within the natural killer complex (NKC) on chromosome 6 and bind classical H-2 antigens. Ly49 gene content varies considerably between strains, ranging from eight in BALB/c mice to 22 in non-obese diabetic (NOD) mice (120, 121). Ly49 receptors use the same signaling pathways as KIR, including intracytoplasmic immunoreceptor tyrosine-based inhibition motifs (ITIM) for inhibitory receptors and signaling through DAP12 for activating receptors (122–124). Nomenclature of Ly49 genes, which are synonymous with killer cell lectin-like receptor subfamily A (Klra) genes, is non-descriptive and each receptor is designated a letter between A and X (125). The major Ly49 receptors in C57BL/6 (H-2b) and BALB/c (H-2d) strains and their respective ligands are summarized in Table 3. Of particular functional significance is the activating Ly49H receptor, which recognizes the murine cytomegalovirus (MCMV) m157 glycoprotein. BALB/c mice notably lack this receptor and, as such, are highly susceptible to MCMV, with high viral titers and increased mortality following infection (126, 127).
In both humans and mice, recognition of non-classical MHC class I molecules is predominantly mediated by CD94/NKG2 heterodimers. Inhibitory CD94/NKG2A dimers signal via an ITIM-dependent pathway, whereas activating CD94/NKG2C and CD94/NKG2E associate with DAP12 (132–136). CD94/NKG2 dimers recognize HLA-E in humans and Qa-1b in mice, which are expressed in complex with peptides derived from leader sequences of other MHC class I molecules (41, 42, 137, 138). As such, HLA-E and Qa-1b provide an additional means by which aberrant MHC class I expression in diseased cells can be detected by NK cells. There are potentially some species-related differences in the expression profiles of these receptors, as human CD94/NKG2E exists only in an intracellular form, and cell surface expression of neither CD94/NKG2C nor CD94/NKG2E has been definitively detected in mice (41, 42, 134).
It has long been considered that acquisition of individual KIR and Ly49 receptors occurs stochastically, such that the co-expression frequencies of individual receptors do not deviate markedly from the product rule (139). This generates subsets of NK cells expressing anywhere between zero and the full complement of NK cell receptors for MHC class I herein referred to as NKRs. However, deviations in the NKR repertoire in accordance with the MHC environment indicate that there are some selective influences (140–143). Specific NKRs are downregulated in the presence of their cognate MHC ligands in a manner that is both MHC dose-dependent and reflective of receptor–ligand binding avidity (142, 144, 145). Refinement of the NKR repertoire is an important aspect of the adaptation of NK cells to their host environment, and is complementary to a process referred to as NK cell education, during which interactions with self-MHC calibrate NK cell responsiveness. Taken together, these processes may allow for selection of the most biologically useful and least self-reactive NK cell subsets in vivo.
NK Cell Education
The concept of NK cell education, or “licensing,” was first proposed in 2005 when it was observed that cells expressing inhibitory NKRs for self-MHC are functionally more responsive than those that do not, both in terms of cytotoxicity and IFN-γ production. This was proposed as a mechanism for NK cell self-tolerance, so that uneducated cells lacking NKRs for self-MHC respond poorly to activating stimuli, such as cross-linking of activating receptors and MHC class I deficient cells. This negates the requirement for NKR-mediated counter-inhibition and reduces the potential for autoreactivity (140, 146). However, NK cell education is by no means an essential requirement for functionality, since responsiveness can be at least partially restored among uneducated NK cells in the presence of pro-inflammatory cytokines (140, 146–148).
The outcome of the educative process is that NK cells attain the capacity to respond to aberrant MHC class I expression. This occurs through “missing-self” recognition, which may result from the absence of self-MHC class I or in the presence of allogeneic MHC class I ligands. The latter effect can be harnessed for therapeutic benefit in HSCT, used in the treatment of hematological malignancies. NK cells from HLA haplotype-mismatched donors enhance graft tolerance in patients with acute myeloid leukemia and induce disease remission with protection against relapse (149). The only physiological situation in which allogeneic class I MHC is presented to a host is during pregnancy. uNK cells are a sufficiently distinct subset that their behavior cannot be effectively modeled on pNK cells, owing to significant phenotypic and functional differences. However, a wealth of evidence from human genetic association studies and mouse models suggests that uNK cell activity can be modulated through interactions with class I MHC from both parents, and that this has the potential to significantly impact on reproductive outcome (150).
Regulation of Spiral Arterial Remodeling by uNK Cells
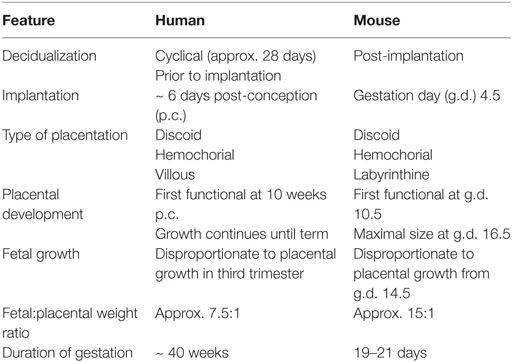
The placenta was originally thought to provide the means of “anatomical separation of fetus from mother,” enabling development of the semi-allogeneic fetus without maternal immune rejection (151). A modern view of immunogenetics of pregnancy proposes that in fact maternal NK cells regulate placentation and vascular remodeling through direct interactions with fetal trophoblast cells (150). Despite numerous anatomical and physiological differences between human and murine pregnancy, mice can provide a useful model in which to study trophoblast differentiation and immune regulation of placental development because both species exhibit hemochorial placentation, where placental trophoblast cells invade the maternal decidua and come into direct contact with maternal blood (152–154). Key features of human and murine pregnancy are summarized in Table 4.
Remodeling of the maternal spiral arteries is an essential local vascular adaptation to pregnancy, which transforms the arteries supplying the feto-placental unit to large bore, high conductance vessels with non-turbulent flow (158). The initial stages of vascular transformation in humans occur during the secretory phase of the menstrual cycle and become more pronounced in early pregnancy independently of trophoblast invasion (159). The maternal vessels during these stages are closely apposed by leukocytes, particularly macrophages and uNK cells, which may contribute to this decidua-associated remodeling through secretion of proteolytic matrix metalloproteinases (MMPs). CD56+ cells have been shown histologically to express MMP-7, MMP-9, MMP-19, and MMP-23 (160–162). However, since CD56 is also expressed by endovascular EVT, it cannot be asserted from dual immunohistochemical staining alone that intramural and endovascular CD56+ MMP+ cells are uNK cells (159). Subsequent stages of remodeling in humans are dependent upon the deep invasion of EVT by interstitial and endovascular routes. Interstitial EVT migrate through the decidua and are thought to intravasate into the walls of the maternal spiral arteries to contribute to disorganization of the vascular smooth muscle (159, 163). Perivascular trophoblast may intravasate further into the vascular lumen to account for some of the endovascular trophoblast which migrates retrogradely along the lumen of the arteries (164). It is considered that EVT from both interstitial and endovascular routes become incorporated into the vascular wall and replace vascular smooth muscle cells (VSMCs) with fibrinoid material, which maintains the vessel in a dilated state and renders it incapable of vasoconstriction (165).
The contribution of trophoblast to decidual vascular transformation in mice is less well defined. Moderately invasive trophoblast giant cells (TGCs) associate with decidual vessels in their more distal segments, and line the arterial canals which supply the feto-placental unit (166). It seems likely that vascular modification in mice is predominantly mediated by other decidual cells, including uNK cells which become integrated into the vascular media (167). Indeed, it is well established that IFN-γ of uNK cell origin is essential for spiral arterial remodeling in murine pregnancy (54). NK cell-deficient mice consistently have defective decidual vascular remodeling, characterized by narrow vascular lumens, thick vascular walls, and retention of vascular smooth muscle actin (58, 168–170). Through utilizing alymphoid mice which were engrafted with BM from IFN-γ−/− mice or severe combined immunodeficient (SCID) mice, which lack T- and B-lymphocytes, it has been elegantly and conclusively demonstrated that IFN-γ of uNK cell origin is essential for murine spiral arterial remodeling (54). NK cell-deficient mice also exhibit IFN-γ-dependent morphological abnormalities such as decidual hypocellularity and failure of MLAp formation, which can be restored through adoptive transfer of BM from C57BL/6 or SCID mice (54, 169, 171). The mechanisms by which IFN-γ mediates vascular remodeling have not been elucidated. Although murine uNK cells have not been reported to produce MMPs, decidual macrophages do produce MMP-9, and MMP-2 and MMP-9 expression can be observed throughout the decidua basalis and in close proximity of decidual arteries (172, 173). Since uNK cells produce IFN-γ and MIP-1α, which are key cytokines involved in macrophage activation, it is possible that uNK cells mediate vascular remodeling through stimulation of MMP production by macrophages (36, 107). Indeed, MMP-2 and macrophage-derived MMP-9 are essential in the pathogenesis of murine abdominal aortic aneurysms, in which pathological destruction of the aortic vascular media leads to extensive dilatation and risk of rupture (174).
More similarly to humans, modification of the spiral arteries in rats involves initial medial disorganization by uNK cells and subsequent destruction of the smooth muscle layer by interstitial and endovascular trophoblast, which invade deep into the decidua and myometrium (175, 176). This demonstrates that, even among species that exhibit hemochorial placentation, there is significant variability in the dependence upon trophoblast and uNK cells for transformation of the spiral arteries supplying the feto-placental unit.
Regulation of Trophoblast Invasion by uNK Cells
Uterine natural killer cells may also contribute to modification of spiral arteries indirectly, through their influence on EVT. A recent study shows that human uNK produce the chemokines XCL1 and CCL1. The receptor for XCL1, XCR1, is expressed by several cell types in the placenta, including fetal endothelial cells and EVT. XCR1 is also expressed by decidual cells, including a small population of CD14+ macrophages. The CCL1 receptor, CCR8, has been identified on all decidual macrophages and on a small proportion of uNK (177). It has been determined by intracellular cytometry that uNK secrete GM-CSF, and the chemokines IL-8 and interferon-inducible protein (IP)-10 have been detected in supernatants of uNK cells in vitro. All of these factors enhance motility of primary trophoblast in cell migration and invasion assays (4, 177–180). Intracellular cytometry has since shown that macrophages are probably the predominant source of IL-8 among decidual leukocytes, although activated uNK cells were not assessed in this study (181). IL-8 stimulates production of MMP-2 and MMP-9 in a first trimester EVT cell line, which is suggestive of a mechanism by which leukocyte-derived factors may promote EVT-induced vascular remodeling (180). However, uNK also secrete TGF-β, which impairs the invasive properties of primary trophoblast in vitro (5, 182). As such, human uNK seemingly mediate a balance between enhancing and inhibiting EVT invasion, and alterations in their function may lead to placental pathology and associated disorders of pregnancy. Supernatants from IL-15-activated uNK cell isolates from women with high uterine artery resistance, which denotes incomplete arterial remodeling, do not effectively induce motility of a first trimester EVT cell line and apoptosis of VSMC and endothelial cell lines in vitro (183). While this likely indicates that uNK cell-derived factors contribute to vascular remodeling and modulating trophoblast migration in vivo, uNK cells were harvested in this study at 9–14 weeks gestation, when transformation of the decidual sections of the spiral arteries is advanced and uNK cell function is declining (184). Assessment of uNK function at an earlier gestational time-point would be more informative for understanding the relative contribution of uNK cells to these physiological processes.
Whereas human uNK cells have been demonstrated to both enhance and inhibit EVT invasion, evidence from studies in rats and mice suggests that uNK cells primarily suppress trophoblast motility. The onset of trophoblast invasion in both rats and mice was observed to correlate with the demise of uNK cells, at around g.d. 14 and was accelerated in NK cell-deficient and IFN-γ−/− mice (176). This is seemingly dependent upon a profound deficit in uNK cell number and/or function, as no effect on depth of trophoblast invasion could be determined in a model of more subtle, MHC-dependent uNK inhibition (36). It has also been suggested that, through contributing to decidual angiogenesis, uNK cells contribute to increased oxygen tensions at the maternal–fetal interface, which prevents trophoblast adopting an invasive phenotype (175). This would most likely be mediated by murine DBA+ uNK cells, which are known to produce angiogenic factors including vascular endothelial growth factor (VEGF) and placental growth factor (PLGF) (27, 55, 185).
Human uNK also secrete several angiogenic factors including VEGF, PLGF, angiopoietin (Ang)1, and Ang2 (4, 5, 179). Production of all factors mentioned can be modulated through KIR/HLA interactions and by the activating receptors NKG2D, NKp30, NKp44, and NKp46 (4, 178, 179). Human trophoblast express ligands for NKp44, but not for NKG2D (4, 20, 179). However, since decidual stromal cells express ligands for NKp30 and NKG2D, it is likely that uNK cell function is also modulated through interactions with maternal tissues (4, 186). There is some evidence to suggest that ligation of NKp30 also induces production of IFN-γ, TNF-α, MIP-1α, and MIP-1β, but since uNK cells were stimulated in the presence of IL-2, the physiological significance of these results is questionable (187). Moreover, recent work suggests that the decidual microenvironment influences the expression of NKp30 and NKp44 splicing variants that may be responsible for decreased cytotoxicity and altered cytokine secretion of uNK cells compared to pbNK cells (188).
Sequelae of Defective Placentation
Defective vascular remodeling, characterized by the absence of intramural EVT and retention of VSMCs, particularly within the myometrial segments of the spiral arteries, is a common pathologic feature in cases of pre-eclampsia, early miscarriage, unexplained stillbirth, and fetal growth restriction (FGR) (189–191). As such, these conditions may reasonably be considered as a spectrum of disorders that can arise from a common primary pathology and, collectively, they are often referred to as the Great Obstetric Syndromes. Some cases of recurrent miscarriage (RM) may also be caused by insufficient trophoblast invasion (192).
To date, many of the studies investigating impaired decidual vascular transformation in mice have focused on the causative mechanisms and histological features. Defective remodeling of spiral arteries does not spontaneously induce systemic hypertension in mice, but is linked to poor fetal growth, indicating that pre-eclampsia only occurs as a response to placental stress from underperfusion in humans (36, 193).
Human EVT invasion may also occur excessively when a blastocyst implants in poorly or non-decidualized tissue. Placenta accreta occurs due to pathological trophoblastic invasion of the myometrium, which most commonly occurs as a result of implantation at the site of uterine scar tissue from previous intrauterine surgery (194). Similar pathological features are observed in ectopic pregnancies, in which the thin wall of the Fallopian tube is commonly entirely infiltrated by EVT in the absence of decidual tissue (195). Given that trophoblast migration is enhanced in mice and rats depleted of NK cells, excessive invasion of EVT in non-decidualized tissue in humans is highly suggestive of a fundamental role for human uNK cells in regulating trophoblast invasion (175, 176).
Immunogenetics of Trophoblast and uNK Cell Interactions
Uterine natural killer cell activity can be directly modulated through interactions with decidual stromal cells, uterine leukocytes, and invasive trophoblast. Of these, the regulation of uNK cell function by trophoblast has been particularly well explored, owing to the association between certain KIR/HLA interactions and disorders of pregnancy. Trophoblast cells express a distinct repertoire of HLA ligands in comparison to somatic cells. Syncytiotrophoblast, which directly contacts maternal blood, and villous cytotrophoblast are HLA negative (196). Invasive EVT express a unique combination of polymorphic HLA-C and oligomorphic HLA-E and HLA-G, but not HLA-A, HLA-B, or MHC class II (197). Each of the EVT HLA class I ligands is able to interact with uNK cell receptors (uNKRs), as outlined in Table 5. Soluble HLA-G is reportedly produced by trophoblast, and is suggested to modulate uNK cell activity (198, 199). However, assessment of the crystal structure of KIR2DL4 and its potential interaction with HLA-G has revealed no evidence of direct receptor–ligand binding (200). In view of this, and in the absence of functional data using non-preactivated uNK cells, hypotheses regarding the role of uNK-expressed KIR2DL4 remain unsubstantiated.
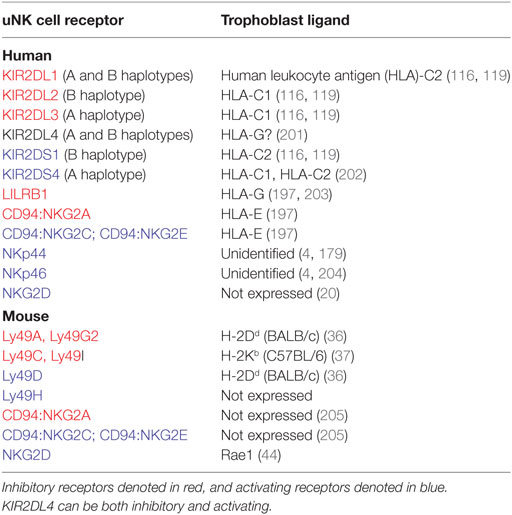
Table 5. Uterine natural killer (uNK) cell receptors and respective trophoblast ligands in human and mouse.
As in humans, murine trophoblast cells at the site of physiological exchange are MHC negative, whereas invasive trophoblast does express MHC class I antigens (36, 37, 206). Invasive TGCs from C57BL/6 mice have been shown to express H-2Kb ligands at far greater intensity than H-2Db, which is only detectable at very low levels (37). Trophoblast expression of transgenic H-2Dd epitopes in C57BL/6 mice has also been demonstrated by immunofluorescence staining (36). uNK cells interact with trophoblast class I MHC through Ly49 receptors (Table 5), but since trophoblast do not express Qa-1b, any functional modulation of uNK cells through NKG2A/C/E is most likely mediated by decidual stromal cells (205, 207).
Trophoblast and uNK Cell Interactions in Disorders of Pregnancy
Immunogenetic associations between maternal KIR/fetal HLA variants and disorders of pregnancy show that combinations of a maternal KIR AA genotype and fetal C2 epitopes are present at significantly higher frequencies in pregnancies complicated by pre-eclampsia (208). Extension of this work later showed that this increased risk of developing pre-eclampsia is highest when trophoblast C2 epitopes are paternally inherited (209). An increased frequency of maternal KIR AA and paternally derived C2 epitopes has also been observed in cases of RM (209, 210). There is an additional weaker correlation, between maternal KIR AA and FGR (209). Within the KIR A haplotype are two genes for inhibitory KIR, KIR2DL1 and KIR2DL3, which encode receptors for C2 and C1 epitopes, respectively (Table 5). It may be reasonably considered that KIR2DL1/C2 interactions are particularly detrimental to uNK cell function, as binding between KIR2DL1 and C2 epitopes is stronger and more specific than that between KIR2DL3 and C1 epitopes (211). Furthermore, interaction between KIR2DL1 and its cognate HLA-C ligand significantly reduces production of chemokines and angiogenic factors by IL-2-activated uNK cells in vitro (4).
Conversely, the presence of the telomeric region of the KIR B haplotype (Tel-B) was shown to be protective against RM and pre-eclampsia, particularly when trophoblast expressed C2 epitopes (208–210). The Tel-B region contains KIR2DS1, which encodes an activating KIR that binds C2 epitopes (Table 5). Maternal KIR2DS1 predisposes to high birth weights, above the 90th centile, when the fetus expresses paternally derived C2 epitopes (212). Interaction between uNK cell KIR2DS1 and C2-expressing target cells in vitro induces GM-CSF production, which enhances trophoblast migration in a Transwell assay (178). Taken together, these data strongly indicate that uNK cell activity is modulated through KIR/HLA interactions and further support the hypothesis that imbalance in uNK cell function, potentially leading to dysregulation of physiological processes essential to pregnancy, can lead to undesirable reproductive outcomes in humans.
Most studies investigating murine uNK cell function to date have assessed the contribution of uNK cell deficiency or uNK cell-derived factors to reproductive success. Only more recently have mouse models been used to examine the impact of more subtle variations in uNK cell activity, such as that mediated by parental MHC disparity. Allogeneic, paternally inherited MHC class I is sufficient in isolation to modulate uNK cell function, and to directly impact on spiral arterial remodeling and fetal growth (36, 37). BALB/c females mated with BALB.B males, which express the C57BL/6 H-2b MHC allotype, exhibit enhanced decidual vascular remodeling and increased fetal growth (37). However, the underlying mechanisms for this remain unclear. Paternally derived trophoblast H-2Dd has been convincingly demonstrated to inhibit uNK cell function in C57BL/6 females. Reduced production of IFN-γ by uNK cells was mediated by inhibitory interactions between H-2Dd with Ly49A and Ly49G2, which resulted in incomplete spiral arterial remodeling and reduced fetal growth. Maternally expressed H-2Dd was also disadvantageous for vascular transformation and fetal growth, which suggests that murine uNK cell education does not confer protective benefits during pregnancy (36).
The relative impact of maternal NKR variability as a determinant of pregnancy outcome in mice has been less well investigated. Ncr1−/− mice, which lack NKp46, exhibit impaired decidual vascular remodeling and disrupted angiogenesis, which suggests that NKp46-mediated activation of uNK is important for optimal reproductive outcome in mice (47). Expression of Ly49 receptors can also influence pregnancy, as Ly49 knockdown (Ly49KD) mice, in which Ly49s are expressed by only 50% of DX5+ and 6% of DBA+ uNK, exhibit subfertility, impaired angiogenesis, reduced vascular remodeling and, unexpectedly, enhanced fetal growth (213). However, given the incongruence of the fetal phenotype in this model, it is feasible that extraneous factors are contributing to the outcomes observed. As the transcriptionally silenced region in Ly49KD mice spans approximately 10.2 megabase pairs (Mbp), encompassing the ~2 Mbp NKC region, the potential impact of genes irrelevant to NK and/or leukocyte function cannot be discounted (214, 215). In keeping with defective angiogenesis, VEGF expression within the decidua and MLAp was reduced in Ly49KD females. The total concentration of IFN-γ within these tissues was unaffected but, as uNK function was not specifically assessed in this study, it is not possible to determine whether uNK dysregulation is responsible for the vascular phenotype observed (213). As such, the strongest data relating to the consequences of NKR-MHC interactions in murine pregnancy are from studies investigating parental MHC disparity.
Concluding Remarks
Results from many studies using mouse models to date have substantiated data from human genetic association studies, which strongly suggest that reduced uNK cell activation is disadvantageous for reproductive outcome. However, it is apparent that the success of pregnancy depends upon a highly complex network of interactions between trophoblast, uNK cells, decidual stromal cells, and other decidual leukocytes. New technology, including improved mouse models, high-throughput genotyping, mass cytometry, and single-cell RNA sequencing should help to define the role of immune cells in pregnancy, including tissue NK cells and other ILCs in human and mouse uterus.
Author Contributions
The manuscript was written by LMG and edited by FC.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Ashley Moffett and Jens Kieckbusch for critically reading the manuscript and all members of the laboratory which is funded by the Wellcome Trust and the Centre for Trophoblast Research. LMG was funded by fellowships from the British Heart Foundation (FS/12/4/29254) and the Centre for Trophoblast Research.
Abbreviations
cNK, conventional natural killer cells; NKR, natural killer receptor; pbNK, peripheral blood natural killer cells; pNK, peripheral natural killer cells; trNK, tissue-resident natural killer cells; uNK, uterine natural killer cells.
References
1. King A, Wellings V, Gardner L, Loke YW. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol (1989) 24:195–205. doi: 10.1016/0198-8859(89)90060-8
2. King A, Balendran N, Wooding P, Carter NP, Loke YW. CD3- leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol (1991) 1:169–90. doi:10.1155/1991/83493
3. Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol (2002) 2:656–63. doi:10.1038/nri967
4. Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med (2006) 12:1065–74. doi:10.1038/nm1452
5. Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, et al. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol (2006) 80:572–80. doi:10.1189/jlb.0406250
6. Kopcow HD, Allan DS, Chen X, Rybalov B, Andzelm MM, Ge B, et al. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci U S A (2005) 102:15563–8. doi:10.1073/pnas.0507835102
7. King A, Wooding P, Gardner L, Loke YW. Immunology: expression of perforin, granzyme A and Tia-1 by human uterine CD56+ NK cells implies they are activated and capable of effector functions. Hum Reprod (1993) 8:2061–7. doi:10.1093/oxfordjournals.humrep.a137982
8. Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med (2003) 198:1201–12. doi:10.1084/jem.20030305
9. Vujaklija DV, Gulic T, Sucic S, Nagata K, Ogawa K, Laskarin G, et al. First trimester pregnancy decidual natural killer cells contain and spontaneously release high quantities of granulysin. Am J Reprod Immunol (2011) 66:363–72. doi:10.1111/j.1600-0897.2011.01015.x
10. Sharkey AM, Gardner L, Hiby S, Farrell L, Apps R, Masters L, et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J Immunol (2008) 181:39–46. doi:10.4049/jimmunol.181.1.39
11. King A, Allan DS, Bowen M, Powis SJ, Joseph S, Verma S, et al. Hla-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol (2000) 30:1623–31. doi:10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M
12. Dietl J, Ruck P, Marzusch K, Horny HP, Kaiserling E, Handgretinger R. Uterine granular lymphocytes are activated natural killer cells expressing VLA-1. Immunol Today (1992) 13:236. doi:10.1016/0167-5699(92)90161-Y
13. Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol (2010) 54:281–94. doi:10.1387/ijdb.082763jb
14. Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol (2009) 82:24–31. doi:10.1016/j.jri.2009.08.001
15. Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol (2004) 72:203–12. doi:10.1046/j.0902-4441.2003.00199.x
16. Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol (1986) 136:4480–6.
17. Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med (2006) 203:1033–43. doi:10.1084/jem.20052507
18. King A, Hiby SE, Verma S, Burrows T, Gardner L, Loke YW. Uterine NK cells and trophoblast HLA class I molecules. Am J Reprod Immunol (1997) 37:459–62. doi:10.1111/j.1600-0897.1997.tb00260.x
19. Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science (1999) 285:727–9. doi:10.1126/science.285.5428.727
20. Apps R, Gardner L, Traherne J, Male V, Moffett A. Natural-killer cell ligands at the maternal-fetal interface: UL-16 binding proteins, MHC class-I chain related molecules, HLA-F and CD48. Hum Reprod (2008) 23:2535–48. doi:10.1093/humrep/den223
21. Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, et al. CD56bright cells differ in their Kir repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol (2001) 31:3121–7. doi:10.1002/1521-4141(2001010)31:10<3121::AID-IMMU3121>3.0.CO;2-4
22. Verma S, King A, Loke YW. Expression of killer cell inhibitory receptors on human uterine natural killer cells. Eur J Immunol (1997) 27:979–83. doi:10.1002/eji.1830270426
23. Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, et al. p46, a novel natural killer cell–specific surface molecule that mediates cell activation. J Exp Med (1997) 186:1129–36. doi:10.1084/jem.186.7.1129
24. Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol (1989) 143:3183–91.
25. Lanier LL, Buck DW, Rhodes L, Ding A, Evans E, Barney C, et al. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J Exp Med (1988) 167:1572–85. doi:10.1084/jem.167.5.1572
26. Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood (2001) 97:3146–51. doi:10.1182/blood.V97.10.3146
27. Chen Z, Zhang J, Hatta K, Lima PD, Yadi H, Colucci F, et al. DBA-lectin reactivity defines mouse uterine natural killer cell subsets with biased gene expression. Biol Reprod (2012) 87:81. doi:10.1095/biolreprod.112.102293
28. Paffaro VA Jr, Bizinotto MC, Joazeiro PP, Yamada AT. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta (2003) 24:479–88. doi:10.1053/plac.2002.0919
29. Croy BA, Esadeg S, Chantakru S, Van Den Heuvel M, Paffaro VA, He H, et al. Update on pathways regulating the activation of uterine natural killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J Reprod Immunol (2003) 59:175–91. doi:10.1016/S0165-0378(03)00046-9
30. Parr EL, Young LH, Parr MB, Young JD. Granulated metrial gland cells of pregnant mouse uterus are natural killer-like cells that contain perforin and serine esterases. J Immunol (1990) 145:2365–72.
31. Zheng LM, Ojcius DM, Liu CC, Kramer MD, Simon MM, Parr EL, et al. Immunogold labeling of perforin and serine esterases in granulated metrial gland cells. FASEB J (1991) 5:79–85.
32. Croy BA, Reed N, Malashenko BA, Kim K, Kwon BS. Demonstration of YAC target cell lysis by murine granulated metrial gland cells. Cell Immunol (1991) 133:116–26. doi:10.1016/0008-8749(91)90184-D
33. Doisne JM, Balmas E, Boulenouar S, Gaynor LM, Kieckbusch J, Gardner L, et al. Composition, development, and function of uterine innate lymphoid cells. J Immunol (2015) 195:3937–45. doi:10.4049/jimmunol.1500689
34. Tagliani E, Shi C, Nancy P, Tay CS, Pamer EG, Erlebacher A. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J Exp Med (2011) 208:1901–16. doi:10.1084/jem.20110866
35. Kather A, Chantakru S, He H, Minhas K, Foster R, Markert UR, et al. Neither lymphotoxin alpha nor lymphotoxin beta receptor expression is required for biogenesis of lymphoid aggregates or differentiation of natural killer cells in the pregnant mouse uterus. Immunology (2003) 108:338–45. doi:10.1046/j.1365-2567.2003.01586.x
36. Kieckbusch J, Gaynor LM, Moffett A, Colucci F. MHC-dependent inhibition of uterine NK cells impedes fetal growth and decidual vascular remodelling. Nat Commun (2014) 5:3359. doi:10.1038/ncomms4359
37. Madeja Z, Yadi H, Apps R, Boulenouar S, Roper SJ, Gardner L, et al. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natl Acad Sci U S A (2011) 108:4012–7. doi:10.1073/pnas.1005342108
38. Mallidi TV, Craig LE, Schloemann SR, Riley JK. Murine endometrial and decidual NK1.1+ natural killer cells display a B220+CD11c+ cell surface phenotype. Biol Reprod (2009) 81:310–8. doi:10.1095/biolreprod.109.076448
39. Moffett A, Colucci F. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol Rev (2015) 267:283–97. doi:10.1111/imr.12323
40. Yadi H, Burke S, Madeja Z, Hemberger M, Moffett A, Colucci F. Unique receptor repertoire in mouse uterine NK cells. J Immunol (2008) 181:6140–7. doi:10.4049/jimmunol.181.9.6140
41. Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b). J Exp Med (1998) 188:1841–8. doi:10.1084/jem.188.10.1841
42. Vance RE, Jamieson AM, Raulet DH. Recognition of the class Ib molecule Qa-1b by putative activating receptors Cd94/NKg2c and Cd94/NKg2e on mouse natural killer cells. J Exp Med (1999) 190:1801–12. doi:10.1084/jem.190.12.1801
43. Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol (2000) 1:119–26. doi:10.1038/77793
44. Carayannopoulos LN, Barks JL, Yokoyama WM, Riley JK. Murine trophoblast cells induce NK cell interferon-gamma production through KLRK1. Biol Reprod (2010) 83:404–14. doi:10.1095/biolreprod.110.084509
45. Yokoyama WM. Natural killer cell receptors specific for major histocompatibility complex class I molecules. Proc Natl Acad Sci U S A (1995) 92:3081–5. doi:10.1073/pnas.92.8.3081
46. Biassoni R, Pessino A, Bottino C, Pende D, Moretta L, Moretta A. The murine homologue of the human NKp46, a triggering receptor involved in the induction of natural cytotoxicity. Eur J Immunol (1999) 29:1014–20. doi:10.1002/(SICI)1521-4141(199903)29:03<1014::AID-IMMU1014>3.0.CO;2-O
47. Felker AM, Chen Z, Foster WG, Croy BA. Receptors for non-MHC ligands contribute to uterine natural killer cell activation during pregnancy in mice. Placenta (2013) 34:757–64. doi:10.1016/j.placenta.2013.06.004
48. Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife (2014) 3:e01659. doi:10.7554/eLife.01659
49. Arase H, Saito T, Phillips JH, Lanier LL. Cutting edge: the mouse NK cell-associated antigen recognized by DX5 moncoclonal antibody is CD49b (α2 integrin, very late antigen-2). J Immunol (2001) 167:1141–4. doi:10.4049/jimmunol.167.3.1141
50. Karlhofer FM, Yokoyama WM. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK1.1 antigen. IL-2-activated NK cells possess additional specific stimulation pathways. J Immunol (1991) 146:3662–73.
51. Croy BA, Chen Z, Hofmann AP, Lord EM, Sedlacek AL, Gerber SA. Imaging of vascular development in early mouse decidua and its association with leukocytes and trophoblasts. Biol Reprod (2012) 87:125. doi:10.1095/biolreprod.112.102830
52. Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli-Esposti MA, et al. NK Cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol (2007) 178:4764–70. doi:10.4049/jimmunol.178.8.4764
53. Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol (2004) 22:405–29. doi:10.1146/annurev.immunol.22.012703.104711
54. Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med (2000) 192:259–70. doi:10.1084/jem.192.2.259
55. Wang C, Umesaki N, Nakamura H, Tanaka T, Nakatani K, Sakaguchi I, et al. Expression of vascular endothelial growth factor by granulated metrial gland cells in pregnant murine uteri. Cell Tissue Res (2000) 300:285–93. doi:10.1007/s004410000198
56. Ljunggren HG, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med (1985) 162:1745–59. doi:10.1084/jem.162.6.1745
57. Stewart IJ, Peel S. Mouse metrial gland cells do not kill Yac-1 myeloma cells. J Reprod Immunol (1993) 24:165–71. doi:10.1016/0165-0378(93)90018-D
58. Boulenouar S, Doisne JM, Sferruzzi-Perri A, Gaynor LM, Kieckbusch J, Balmas E, et al. The residual innate lymphoid cells in NFIL3-deficient mice support suboptimal maternal adaptations to pregnancy. Front Immunol (2016) 7:43. doi:10.3389/fimmu.2016.00043
59. Montaldo E, Vacca P, Chiossone L, Croxatto D, Loiacono F, Martini S, et al. Unique Eomes+ NK cell subsets are present in uterus and decidua during early pregnancy. Front Immunol (2016) 6:646. doi:10.3389/fimmu.2015.00646
60. Eberl G, Colonna M, Di Santo JP, Mckenzie AN. Innate lymphoid cells: a new paradigm in immunology. Science (2015) 348:aaa6566. doi:10.1126/science.aaa6566
61. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science (2011) 331:44–9. doi:10.1126/science.1198687
62. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells – a proposal for uniform nomenclature. Nat Rev Immunol (2013) 13:145–9. doi:10.1038/nri3365
63. Artis D, Spits H. The biology of innate lymphoid cells. Nature (2015) 517:293–301. doi:10.1038/nature14189
64. Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol (2016) 17:765–74. doi:10.1038/ni.3489
65. Tait Wojno ED, Artis D. Emerging concepts and future challenges in innate lymphoid cell biology. J Exp Med (2016) 213:2229–48. doi:10.1084/jem.20160525
66. Juelke K, Romagnani C. Differentiation of human innate lymphoid cells (ILCs). Curr Opin Immunol (2016) 38:75–85. doi:10.1016/j.coi.2015.11.005
67. Simoni Y, Fehlings M, Kloverpris HN, Mcgovern N, Koo SL, Loh CY, et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity (2017) 46:148–61. doi:10.1016/j.immuni.2016.11.005
68. Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol (2011) 12:21–7. doi:10.1038/ni.1962
69. Male V, Hughes T, Mcclory S, Colucci F, Caligiuri MA, Moffett A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol (2010) 185:3913–8. doi:10.4049/jimmunol.1001637
70. Vacca P, Montaldo E, Croxatto D, Loiacono F, Canegallo F, Venturini PL, et al. Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol (2015) 8:254–64. doi:10.1038/mi.2014.63
71. Vacca P, Vitale C, Montaldo E, Conte R, Cantoni C, Fulcheri E, et al. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci U S A (2011) 108:2402–7. doi:10.1073/pnas.1016257108
72. Hughes T, Briercheck EL, Freud AG, Trotta R, Mcclory S, Scoville SD, et al. The transcription Factor Ahr prevents the differentiation of a stage 3 innate lymphoid cell subset to natural killer cells. Cell Rep (2014) 8:150–62. doi:10.1016/j.celrep.2014.05.042
73. Croy BA. Hasn’t the time come to replace the term metrial gland? J Reprod Immunol (1999) 42:127–9; discussion 131–4.
74. Croy BA, Van Den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev (2006) 214:161–85. doi:10.1111/j.1600-065X.2006.00447.x
75. Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol (2005) 174:21–5. doi:10.4049/jimmunol.174.1.21
76. Klose CSN, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell (2014) 157:340–56. doi:10.1016/j.cell.2014.03.030
77. Male V, Nisoli I, Kostrzewski T, Allan DS, Carlyle JR, Lord GM, et al. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J Exp Med (2014) 211:635–42. doi:10.1084/jem.20132398
78. Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol (2003) 3:413–25. doi:10.1038/nri1088
79. Fathman JW, Bhattacharya D, Inlay MA, Seita J, Karsunky H, Weissman IL. Identification of the earliest natural killer cell–committed progenitor in murine bone marrow. Blood (2011) 118:5439–47. doi:10.1182/blood-2011-04-348912
80. Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP. Identification of committed NK cell progenitors in adult murine bone marrow. Eur J Immunol (2001) 31:1900–9. doi:10.1002/1521-4141(200106)31:6<1900::AID-IMMU1900>3.0.CO;2-M
81. Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol (2005) 174:1213–21. doi:10.4049/jimmunol.174.3.1213
82. Kim S, Iizuka K, Kang H-SP, Dokun A, French AR, Greco S, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol (2002) 3:523–8. doi:10.1038/ni796
83. Vosshenrich CA, Samson-Villeger SI, Di Santo JP. Distinguishing features of developing natural killer cells. Curr Opin Immunol (2005) 17:151–8. doi:10.1016/j.coi.2005.01.005
84. Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood (2009) 113:5488–96. doi:10.1182/blood-2008-10-187179
85. Vosshenrich CAJ, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Goff OR-L, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol (2006) 7:1217–24. doi:10.1038/ni1395
86. Seillet C, Huntington ND, Gangatirkar P, Axelsson E, Minnich M, Brady HJ, et al. Differential requirement for Nfil3 during NK cell development. J Immunol (2014) 192:2667–76. doi:10.4049/jimmunol.1302605
87. Cortez VS, Fuchs A, Cella M, Gilfillan S, Colonna M. Cutting edge: salivary gland NK cells develop independently of Nfil3 in steady-state. J Immunol (2014) 192:4487–91. doi:10.4049/jimmunol.1303469
88. Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest (2013) 123:1444–56. doi:10.1172/JCI66381
89. Takeda K, Cretney E, Hayakawa Y, Ota T, Akiba H, Ogasawara K, et al. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood (2005) 105:2082–9. doi:10.1182/blood-2004-08-3262
90. Warren HS, Smyth MJ. NK cells and apoptosis. Immunol Cell Biol (1999) 77:64–75. doi:10.1046/j.1440-1711.1999.00790.x
91. Renoux VM, Zriwil A, Peitzsch C, Michaelsson J, Friberg D, Soneji S, et al. Identification of a human natural killer cell lineage-restricted progenitor in fetal and adult tissues. Immunity (2015) 43:394–407. doi:10.1016/j.immuni.2015.07.011
92. Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity (2005) 22:295–304. doi:10.1016/j.immuni.2005.01.013
93. Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev (2006) 214:56–72. doi:10.1111/j.1600-065X.2006.00451.x
94. Knox JJ, Cosma GL, Betts MR, Mclane LM. Characterization of T-bet and eomes in peripheral human immune cells. Front Immunol (2014) 5:217. doi:10.3389/fimmu.2014.00217
95. Marquardt N, Beziat V, Nystrom S, Hengst J, Ivarsson MA, Kekalainen E, et al. Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells. J Immunol (2015) 194:2467–71. doi:10.4049/jimmunol.1402756
96. Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity (2013) 38:769–81. doi:10.1016/j.immuni.2013.02.010
97. Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol (2010) 63:434–44. doi:10.1111/j.1600-0897.2009.00794.x
98. Peel S, Stewart I. The differentiation of granulated metrial gland cells in chimeric mice and the effect of uterine shielding during irradiation. J Anat (1984) 139(Pt 4):593–8.
99. Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, et al. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol (2002) 168:22–8. doi:10.4049/jimmunol.168.1.22
100. Lysiak JJ, Lala PK. In situ localization and characterization of bone marrow-derived cells in the decidua of normal murine pregnancy. Biol Reprod (1992) 47:603–13. doi:10.1095/biolreprod47.4.603
101. Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA (2004) 292:81–5. doi:10.1001/jama.292.1.81
102. Cerdeira AS, Rajakumar A, Royle CM, Lo A, Husain Z, Thadhani RI, et al. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol (2013) 190:3939–48. doi:10.4049/jimmunol.1202582
103. Verma S, Hiby SE, Loke YW, King A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod (2000) 62:959–68. doi:10.1095/biolreprod62.4.959
104. Matsuura-Sawada R, Murakami T, Ozawa Y, Nabeshima H, Akahira J, Sato Y, et al. Reproduction of menstrual changes in transplanted human endometrial tissue in immunodeficient mice. Hum Reprod (2005) 20:1477–84. doi:10.1093/humrep/deh783
105. Chiossone L, Vacca P, Orecchia P, Croxatto D, Damonte P, Astigiano S, et al. In vivo generation of decidual natural killer cells from resident hematopoietic progenitors. Haematologica (2014) 99:448–57. doi:10.3324/haematol.2013.091421
106. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol (2001) 22:633–40. doi:10.1016/S1471-4906(01)02060-9
107. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol (2004) 75:163–89. doi:10.1189/jlb.0603252
108. Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med (1989) 320:1731–5. doi:10.1056/NEJM198906293202605
109. Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol (2013) 132:515–26. doi:10.1016/j.jaci.2013.07.020
110. Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A (2000) 97:2731–6. doi:10.1073/pnas.050588297
111. Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature (2001) 409:1055–60. doi:10.1038/35059110
112. Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol (2013) 31:413–41. doi:10.1146/annurev-immunol-032712-095951
113. Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol (2013) 31:227–58. doi:10.1146/annurev-immunol-020711-075005
114. Kelley J, Walter L, Trowsdale J. Comparative genomics of natural killer cell receptor gene clusters. PLoS Genet (2005) 1(2):e27. doi:10.1371/journal.pgen.0010027
115. Beziat V, Hilton HG, Norman PJ, Traherne JA. Deciphering the killer-cell immunoglobulin-like receptor system at super-resolution for natural killer and T-cell biology. Immunology (2016) 150:248–264. doi:10.1111/imm.12684
116. Carrington M, Norman P. The Kir Gene Cluster. The KIR Gene Cluster [Internet]. Bethesda, MD: National Center for Biotechnology Information (US) (2003). Available from: http://www.ncbi.nlm.nih.gov/books/NBK10134
117. Robinson J, Halliwell JA, Hayhurst JD, Flicek P, Parham P, Marsh SG. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res (2015) 43:D423–31. doi:10.1093/nar/gku1161
118. Colonna M, Spies T, Strominger JL, Ciccone E, Moretta A, Moretta L, et al. Alloantigen recognition by two human natural killer cell clones is associated with HLA-C or a closely linked gene. Proc Natl Acad Sci U S A (1992) 89:7983–5. doi:10.1073/pnas.89.17.7983
119. Hilton HG, Guethlein LA, Goyos A, Nemat-Gorgani N, Bushnell DA, Norman PJ, et al. Polymorphic HLA-C receptors balance the functional characteristics of KIR haplotypes. J Immunol (2015) 195:3160–70. doi:10.4049/jimmunol.1501358
120. Proteau MF, Rousselle E, Makrigiannis AP. Mapping of the BALB/c Ly49 cluster defines a minimal natural killer cell receptor gene repertoire. Genomics (2004) 84:669–77. doi:10.1016/j.ygeno.2004.05.004
121. Belanger S, Tai LH, Anderson SK, Makrigiannis AP. Ly49 cluster sequence analysis in a mouse model of diabetes: an expanded repertoire of activating receptors in the NOD genome. Genes Immun (2008) 9:509–21. doi:10.1038/gene.2008.43
122. Colucci F, Di Santo JP, Leibson PJ. Natural killer cell activation in mice and men: different triggers for similar weapons? Nat Immunol (2002) 3:807–13. doi:10.1038/ni0902-807
123. Mason LH, Gosselin P, Anderson SK, Fogler WE, Ortaldo JR, Mcvicar DW. Differential tyrosine phosphorylation of inhibitory versus activating Ly-49 receptor proteins and their recruitment of SHP-1 phosphatase. J Immunol (1997) 159:4187–96.
124. Smith KM, Wu J, Bakker AB, Phillips JH, Lanier LL. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J Immunol (1998) 161:7–10.
125. Schenkel AR, Kingry LC, Slayden RA. The ly49 gene family. A brief guide to the nomenclature, genetics, and role in intracellular infection. Front Immunol (2013) 4:90. doi:10.3389/fimmu.2013.00090
126. Daniels KA, Devora G, Lai WC, O’Donnell CL, Bennett M, Welsh RM. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med (2001) 194:29–44. doi:10.1084/jem.194.1.29
127. Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science (2002) 296:1323–6. doi:10.1126/science.1070884
128. Hanke T, Takizawa H, Mcmahon CW, Busch DH, Pamer EG, Miller JD, et al. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity (1999) 11:67–77. doi:10.1016/S1074-7613(00)80082-5
129. Scarpellino L, Oeschger F, Guillaume P, Coudert JD, Levy F, Leclercq G, et al. Interactions of Ly49 family receptors with MHC class I ligands in trans and cis. J Immunol (2007) 178:1277–84. doi:10.4049/jimmunol.178.3.1277
130. Andrews DM, Sullivan LC, Baschuk N, Chan CJ, Berry R, Cotterell CL, et al. Recognition of the nonclassical MHC class I molecule H2-M3 by the receptor Ly49A regulates the licensing and activation of NK cells. Nat Immunol (2012) 13:1171–7. doi:10.1038/ni.2468
131. George TC, Mason LH, Ortaldo JR, Kumar V, Bennett M. Positive recognition of MHC class I molecules by the Ly49D receptor of murine NK cells. J Immunol (1999) 162:2035–43.
132. Lazetic S, Chang C, Houchins JP, Lanier LL, Phillips JH. Human natural killer cell receptors involved in MHC class I recognition are disulfide-linked heterodimers of CD94 and NKG2 subunits. J Immunol (1996) 157:4741–5.
133. Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity (1998) 8:693–701. doi:10.1016/S1074-7613(00)80574-9
134. Orbelyan GA, Tang F, Sally B, Solus J, Meresse B, Ciszewski C, et al. Human NKG2E is expressed and forms an intracytoplasmic complex with CD94 and DAP12. J Immunol (2014) 193:610–6. doi:10.4049/jimmunol.1400556
135. Lohwasser S, Hande P, Mager DL, Takei F. Cloning of murine NKG2A, B and C: second family of C-type lectin receptors on murine NK cells. Eur J Immunol (1999) 29:755–61. doi:10.1002/(SICI)1521-4141(199903)29:03<755::AID-IMMU755>3.3.CO;2-O
136. Fang M, Orr MT, Spee P, Egebjerg T, Lanier LL, Sigal LJ. CD94 is essential for NK cell-mediated resistance to a lethal viral disease. Immunity (2011) 34:579–89. doi:10.1016/j.immuni.2011.02.015
137. Braud VM, Allan DSJ, O’Callaghan CA, Soderstrom K, D’Andrea A, Ogg GS, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature (1998) 391:795–9. doi:10.1038/35869
138. Kaiser BK, Barahmand-Pour F, Paulsene W, Medley S, Geraghty DE, Strong RK. Interactions between NKG2x immunoreceptors and HLA-E ligands display overlapping affinities and thermodynamics. J Immunol (2005) 174:2878–84. doi:10.4049/jimmunol.174.5.2878
139. Raulet DH, Vance RE, Mcmahon CW. Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol (2001) 19:291–330. doi:10.1146/annurev.immunol.19.1.291
140. Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song Y-J, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature (2005) 436:709–13. doi:10.1038/nature03847
141. Yawata M, Yawata N, Draghi M, Partheniou F, Little AM, Parham P. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood (2008) 112:2369–80. doi:10.1182/blood-2008-03-143727
142. Brodin P, Lakshmikanth T, Karre K, Hoglund P. Skewing of the NK cell repertoire by MHC class I via quantitatively controlled enrichment and contraction of specific Ly49 subsets. J Immunol (2012) 188:2218–26. doi:10.4049/jimmunol.1102801
143. Sternberg-Simon M, Brodin P, Pickman Y, Onfelt B, Karre K, Malmberg KJ, et al. Natural killer cell inhibitory receptor expression in humans and mice: a closer look. Front Immunol (2013) 4:65. doi:10.3389/fimmu.2013.00065
144. Johansson S, Johansson M, Rosmaraki E, Vahlne G, Mehr R, Salmon-Divon M, et al. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med (2005) 201:1145–55. doi:10.1084/jem.20050167
145. Brodin P, Lakshmikanth T, Johansson S, Kärre K, Höglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood (2008) 113:2434–41. doi:10.1182/blood-2008-05-156836
146. Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood (2005) 105:4416–23. doi:10.1182/blood-2004-08-3156
147. Champsaur M, Beilke JN, Ogasawara K, Koszinowski UH, Jonjic S, Lanier LL. Intact NKG2D-independent function of NK cells chronically stimulated with the NKG2D ligand Rae-1. J Immunol (2010) 185:157–65. doi:10.4049/jimmunol.1000397
148. Fauriat C, Ivarsson MA, Ljunggren H-G, Malmberg K-J, Michaëlsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood (2010) 115:1166–74. doi:10.1182/blood-2009-09-245746
149. Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (2002) 295:2097–100. doi:10.1126/science.1068440
150. Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest (2014) 124:1872–9. doi:10.1172/JCI68107
151. Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol (1953) 7:320–38.
152. Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (2005) 20:180–93. doi:10.1152/physiol.00001.2005
153. Croy BA, He H, Esadeg S, Wei Q, Mccartney D, Zhang J, et al. Uterine natural killer cells: insights into their cellular and molecular biology from mouse modelling. Reproduction (2003) 126:149–60. doi:10.1530/rep.0.1260149
154. Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta (2002) 23:3–19. doi:10.1053/plac.2001.0738
155. Benirschke K, Kaufmann P, Baergen RN. Characterization of the developmental stages. 5th ed. In: Benirschke K, Kaufmann P, Baergen RN, editors. Pathology of the Human Placenta. New York, NY: Springer (2006). p. 174–90.
156. Benirschke K, Kaufmann P, Baergen RN. Early development of the human placenta. 5th ed. In: Benirschke K, Kaufmann P, Baergen RN, editors. Pathology of the Human Placenta. New York, NY: Springer (2006). p. 42–50.
157. Mu J, Adamson SL. Developmental changes in hemodynamics of uterine artery, utero- and umbilicoplacental, and vitelline circulations in mouse throughout gestation. Am J Physiol Heart Circ Physiol (2006) 291:H1421–8. doi:10.1152/ajpheart.00031.2006
158. Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta (2009) 30:473–82. doi:10.1016/j.placenta.2009.02.009
159. Kam EPY, Gardner L, Loke YW, King A. The role of trophoblast in the physiological change in decidual spiral arteries. Hum Reprod (1999) 14:2131–8. doi:10.1093/humrep/14.8.2131
160. Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol (2009) 174:1959–71. doi:10.2353/ajpath.2009.080995
161. Hazan AD, Smith SD, Jones RL, Whittle W, Lye SJ, Dunk CE. Vascular-leukocyte interactions: mechanisms of human decidual spiral artery remodeling in vitro. Am J Pathol (2010) 177:1017–30. doi:10.2353/ajpath.2010.091105
162. Anacker J, Segerer SE, Hagemann C, Feix S, Kapp M, Bausch R, et al. Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Mol Hum Reprod (2011) 17:637–52. doi:10.1093/molehr/gar033
163. Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta (1983) 4:397–413. doi:10.1016/S0143-4004(83)80043-5
164. Lyall F. The human placental bed revisited. Placenta (2002) 23:555–62. doi:10.1053/plac.2002.0850
165. Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta (2006) 27:939–58. doi:10.1016/j.placenta.2005.12.006
166. Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, et al. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol (2002) 250:358–73. doi:10.1006/dbio.2002.0773
167. Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol (2011) 8:1–11. doi:10.1038/cmi.2010.38
168. Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod (1997) 56:169–79. doi:10.1095/biolreprod56.1.169
169. Greenwood JD, Minhas K, Di Santo JP, Makita M, Kiso Y, Croy BA. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta (2000) 21:693–702. doi:10.1053/plac.2000.0556
170. Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, et al. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol (2003) 171:2937–44. doi:10.4049/jimmunol.171.6.2937
171. Ashkar AA, Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod (1999) 61:493–502. doi:10.1095/biolreprod61.2.493
172. Kaitu’u TJ, Shen J, Zhang J, Morison NB, Salamonsen LA. Matrix metalloproteinases in endometrial breakdown and repair: functional significance in a mouse model. Biol Reprod (2005) 73:672–80. doi:10.1095/biolreprod.105.042473
173. Fontana V, Coll TA, Sobarzo CM, Tito LP, Calvo JC, Cebral E. Matrix metalloproteinase expression and activity in trophoblast-decidual tissues at organogenesis in CF-1 mouse. J Mol Histol (2012) 43:487–96. doi:10.1007/s10735-012-9429-8
174. Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest (2002) 110:625–32. doi:10.1172/JCI0215334
175. Chakraborty D, Rumi MA, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci U S A (2011) 108:16295–300. doi:10.1073/pnas.1109478108
176. Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol (2003) 260:176–90. doi:10.1016/S0012-1606(03)00210-0
177. Kennedy PR, Chazara O, Gardner L, Ivarsson MA, Farrell LE, Xiong S, et al. Activating KIR2DS4 is expressed by uterine NK cells and contributes to successful pregnancy. J Immunol (2016) 197:4292–300. doi:10.4049/jimmunol.1601279
178. Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, et al. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest (2013) 123:4264–72. doi:10.1172/JCI68991
179. Vacca P, Cantoni C, Prato C, Fulcheri E, Moretta A, Moretta L, et al. Regulatory role of NKp44, NKp46, Dnam-1 and NKG2D receptors in the interaction between NK cells and trophoblast cells. Evidence for divergent functional profiles of decidual versus peripheral NK cells. Int Immunol (2008) 20:1395–405. doi:10.1093/intimm/dxn105
180. Jovanović M, Stefanoska I, Radojčić L, Vićovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins α5 and β1. Reproduction (2010) 139:789–98. doi:10.1530/REP-09-0341
181. Apps R, Sharkey A, Gardner L, Male V, Kennedy P, Masters L, et al. Ex vivo functional responses to HLA-G differ between blood and decidual NK cells. Mol Hum Reprod (2011) 17:577–86. doi:10.1093/molehr/gar022
182. Lash GE, Otun HA, Innes BA, Bulmer JN, Searle RF, Robson SC. Inhibition of trophoblast cell invasion by TGFB1, 2, and 3 is associated with a decrease in active proteases. Biol Reprod (2005) 73:374–81. doi:10.1095/biolreprod.105.040337
183. Fraser R, Whitley GS, Johnstone AP, Host AJ, Sebire NJ, Thilaganathan B, et al. Impaired decidual natural killer cell regulation of vascular remodelling in early human pregnancies with high uterine artery resistance. J Pathol (2012) 228:322–32. doi:10.1002/path.4057
184. Lash GE, Otun HA, Innes BA, Percival K, Searle RF, Robson SC, et al. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum Reprod (2010) 25:1137–45. doi:10.1093/humrep/deq050
185. Tayade C, Hilchie D, He H, Fang Y, Moons L, Carmeliet P, et al. Genetic deletion of placenta growth factor in mice alters uterine NK cells. J Immunol (2007) 178:4267–75. doi:10.4049/jimmunol.178.7.4267
186. Croxatto D, Vacca P, Canegallo F, Conte R, Venturini PL, Moretta L, et al. Stromal cells from human decidua exert a strong inhibitory effect on NK cell function and dendritic cell differentiation. PLoS One (2014) 9:e89006. doi:10.1371/journal.pone.0089006
187. El Costa H, Casemayou A, Aguerre-Girr M, Rabot M, Berrebi A, Parant O, et al. Critical and differential roles of NKp46- and NKp30-activating receptors expressed by uterine NK cells in early pregnancy. J Immunol (2008) 181:3009–17. doi:10.4049/jimmunol.181.5.3009
188. Siewiera J, Gouilly J, Hocine HR, Cartron G, Levy C, Al-Daccak R, et al. Natural cytotoxicity receptor splice variants orchestrate the distinct functions of human natural killer cell subtypes. Nat Commun (2015) 6:10183. doi:10.1038/ncomms10183
189. Jauniaux E, Zaidi J, Jurkovic D, Campbell S, Hustin J. Comparison of colour Doppler features and pathological findings in complicated early pregnancy. Hum Reprod (1994) 9:2432–7. doi:10.1093/oxfordjournals.humrep.a138465
190. Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol (1986) 93:1049–59. doi:10.1111/j.1471-0528.1986.tb07830.x
191. Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension (2013) 62:1046–54. doi:10.1161/HYPERTENSIONAHA.113.01892
192. Pijnenborg R. The human decidua as a passage-way for trophoblast invasion: a review. Placenta (1998) 19(Suppl 1):229–41. doi:10.1016/S0143-4004(98)80017-9
193. Burke SD, Barrette VF, Bianco J, Thorne JG, Yamada AT, Pang SC, et al. Spiral arterial remodeling is not essential for normal blood pressure regulation in pregnant mice. Hypertension (2010) 55:729–37. doi:10.1161/HYPERTENSIONAHA.109.144253
194. Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta (2012) 33:244–51. doi:10.1016/j.placenta.2011.11.010
195. Kemp B, Kertschanska S, Handt S, Funk A, Kaufmann P, Rath W. Different placentation patterns in viable compared with nonviable tubal pregnancy suggest a divergent clinical management. Am J Obstet Gynecol (1999) 181:615–20. doi:10.1016/S0002-9378(99)70501-6
196. Hunt JS, Fishback JL, Chumbley G, Loke YW. Identification of class I MHC mRNA in human first trimester trophoblast cells by in situ hybridization. J Immunol (1990) 144:4420–5.
197. Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (Hla) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology (2009) 127:26–39. doi:10.1111/j.1365-2567.2008.03019.x
198. Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, et al. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol (2003) 171:1376–84. doi:10.4049/jimmunol.171.3.1376
199. Van Der Meer A, Lukassen HG, Van Cranenbroek B, Weiss EH, Braat DD, Van Lierop MJ, et al. Soluble HLA-G promotes Th1-type cytokine production by cytokine-activated uterine and peripheral natural killer cells. Mol Hum Reprod (2007) 13:123–33. doi:10.1093/molehr/gal100
200. Moradi S, Berry R, Pymm P, Hitchen C, Beckham SA, Wilce MC, et al. The structure of the atypical killer cell immunoglobulin-like receptor, KIR2DL4. J Biol Chem (2015) 290:10460–71. doi:10.1074/jbc.M114.612291
201. Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med (1999) 189:1093–100. doi:10.1084/jem.189.7.1093
202. Graef T, Moesta AK, Norman PJ, Abi-Rached L, Vago L, Older Aguilar AM, et al. KIR2DS4 is a product of gene conversion with KIR3DL2 that introduced specificity for Hla-A*11 while diminishing avidity for HLA-C. J Exp Med (2009) 206:2557–72. doi:10.1084/jem.20091010
203. Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur J Immunol (2007) 37:1924–37. doi:10.1002/eji.200737089
204. Vacca P, Pietra G, Falco M, Romeo E, Bottino C, Bellora F, et al. Analysis of natural killer cells isolated from human decidua: evidence that 2B4 (CD244) functions as an inhibitory receptor and blocks NK-cell function. Blood (2006) 108:4078–85. doi:10.1182/blood-2006-04-017343
205. Erlebacher A, Lukens AK, Glimcher LH. Intrinsic susceptibility of mouse trophoblasts to natural killer cell-mediated attack in vivo. Proc Natl Acad Sci U S A (2002) 99:16940–5. doi:10.1073/pnas.222652199
206. Philpott KL, Rastan S, Brown S, Mellor AL. Expression of H-2 class I genes in murine extra-embryonic tissues. Immunology (1988) 64:479–85.
207. Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol (2005) 5:459–71. doi:10.1038/nri1635
208. Hiby SE, Walker JJ, O’Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med (2004) 200:957–65. doi:10.1084/jem.20041214
209. Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest (2010) 120:4102–10. doi:10.1172/JCI43998
210. Hiby SE, Regan L, Lo W, Farrell L, Carrington M, Moffett A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum Reprod (2008) 23:972–6. doi:10.1093/humrep/den011
211. Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol (2005) 5:201–14. doi:10.1038/nri1570
212. Hiby SE, Apps R, Chazara O, Farrell LE, Magnus P, Trogstad L, et al. Maternal KIR in combination with paternal HLA-C2 regulate human birth weight. J Immunol (2014) 192:5069–73. doi:10.4049/jimmunol.1400577
213. Lima PD, Tu MM, Rahim MM, Peng AR, Croy BA, Makrigiannis AP. Ly49 receptors activate angiogenic mouse DBA(+) uterine natural killer cells. Cell Mol Immunol (2014) 11:467–76. doi:10.1038/cmi.2014.44
214. Belanger S, Tu MM, Rahim MM, Mahmoud AB, Patel R, Tai LH, et al. Impaired natural killer cell self-education and “missing-self” responses in Ly49-deficient mice. Blood (2012) 120:592–602. doi:10.1182/blood-2012-02-408732
Keywords: uterine natural killer cells, uterine innate lymphoid cells, placenta, pregnancy, arterial remodeling, trophoblast
Citation: Gaynor LM and Colucci F (2017) Uterine Natural Killer Cells: Functional Distinctions and Influence on Pregnancy in Humans and Mice. Front. Immunol. 8:467. doi: 10.3389/fimmu.2017.00467
Received: 30 January 2017; Accepted: 05 April 2017;
Published: 24 April 2017
Edited by:
Sandra Laurence Lopez-Verges, Instituto Conmemorativo Gorgas de Estudios de la Salud, PanamaReviewed by:
Jacques Zimmer, Centre de Recherche Public de la Santé, LuxembourgVeronika Sexl, University of Veterinary Medicine, Austria
Copyright: © 2017 Gaynor and Colucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Colucci, ZmMyODdAbWVkc2NobC5jYW0uYWMudWs=
 Louise M. Gaynor
Louise M. Gaynor Francesco Colucci
Francesco Colucci