
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 24 April 2017
Sec. Immunological Tolerance and Regulation
Volume 8 - 2017 | https://doi.org/10.3389/fimmu.2017.00453
 Taylor B. Smallwood1
Taylor B. Smallwood1 Paul R. Giacomin2
Paul R. Giacomin2 Alex Loukas2
Alex Loukas2 Jason P. Mulvenna1,2,3†
Jason P. Mulvenna1,2,3† Richard J. Clark1†
Richard J. Clark1† John J. Miles2,3,4,5*†
John J. Miles2,3,4,5*†
Helminths have evolved to become experts at subverting immune surveillance. Through potent and persistent immune tempering, helminths can remain undetected in human tissues for decades. Redirecting the immunomodulating “talents” of helminths to treat inflammatory human diseases is receiving intensive interest. Here, we review therapies using live parasitic worms, worm secretions, and worm-derived synthetic molecules to treat autoimmune disease. We review helminth therapy in both mouse models and clinical trials and discuss what is known on mechanisms of action. We also highlight current progress in characterizing promising new immunomodulatory molecules found in excretory/secretory products of helminths and their potential use as immunotherapies for acute and chronic inflammatory diseases.
Helminths are large multicellular organisms that can be either free living or parasitic. Parasitic helminths comprise the phyla of roundworms (nematodes), flatworms (platyhelminths), tapeworms (cestodes), and flukes (trematodes) and have plagued humans and archaic humans for hundreds of thousands of years. Today, these parasites remain one of the most successful families of infectious agents on the planet, infecting more than one and a half billion people (1). In humans, heavy infection with parasites can lead to many serious health problems and sometimes even death (2, 3). However, a small worm burden typically has limited or no pathology and has even been suggested to be commensal to the host (4).
Individual hookworms can live in the human intestine for up to 18 years (5). To achieve this impressive feat, the parasites effectively cloak through multipronged immunomodulation. The principal immune subsystem targeted is T cell surveillance (6, 7), which determines self from foreign antigens through a vast yet structured in vivo T cell receptor repertoire (8). Specifically, the parasites stimulate the release of IL-4, IL-5, IL-10, and IL-13, which promotes Th2 polarization (9, 10) (Figure 1). Regulatory T cell (Treg) development is also stimulated during hookworm infection (11) that enhances the cloaking effect through the release of the regulatory cytokines IL-10 and transforming growth factor (TGF) β (12). In addition, hookworms induce activation of parasite-specific and total immunoglobulin E (IgE) and the mobilization of the innate immune systems including mast cells, eosinophils, and basophils (13). Indeed, a recent large-scale community deworming study showed that helminths actively decrease immune responsiveness and modulate immune checkpoint expression in infected individuals (14). The intrinsic talent of parasitic worms to skew the immune response from Th1 to Th2/Treg has led to the idea of using live worms as immunotherapy (helminthic therapy) or, preferably, seeking compounds in helminth secretions for use as immunomodulatory drugs. Indeed, helminthic therapy in animal models and human trials has provided convincing evidence that low-dose inoculation can treat a number of autoimmune diseases.
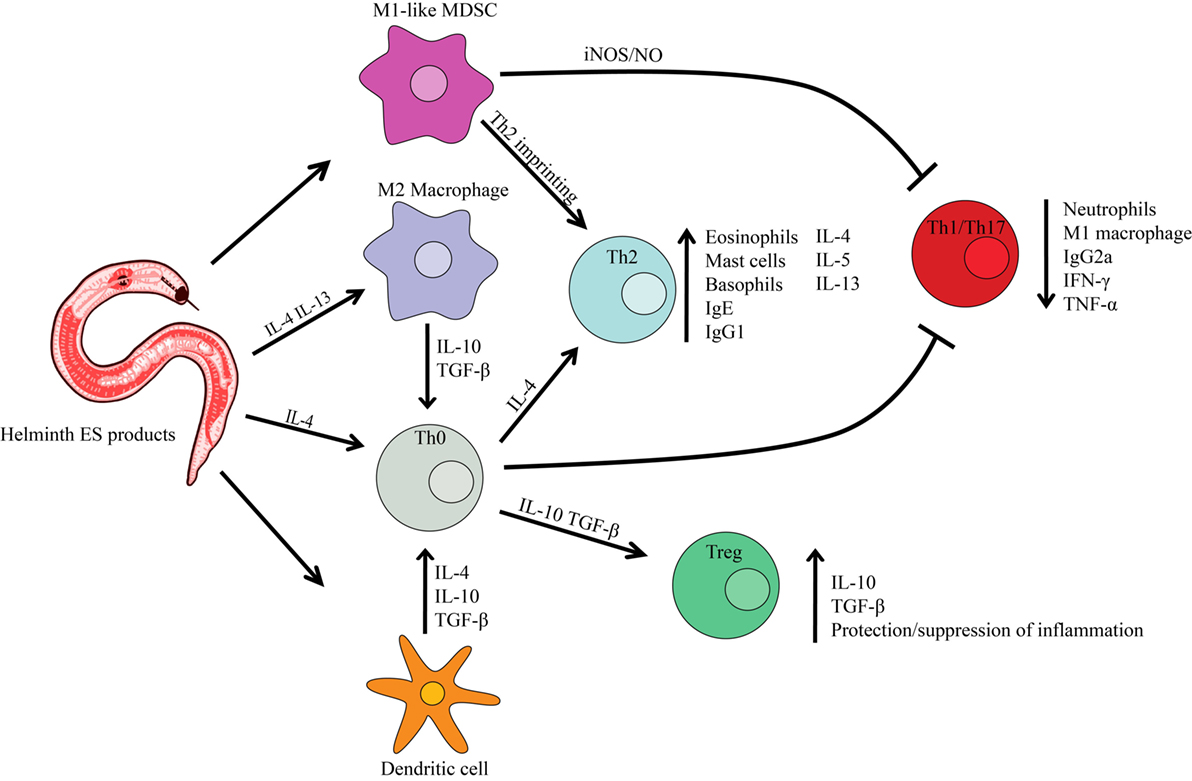
Figure 1. Helminth excretory/secretory (ES) products effect on host immune cells. Infection with parasitic worms causes the host immune system to polarize into a Th2 response (preventing Th1 or Th17 immune response) characterized by Th2 cytokines. Helminth ES products can cause the differentiation of macrophages toward the M2 phenotype, resulting in a Th2 immune response. ES products can also prevent dendritic cell synthesis of pro-inflammatory cytokines and promote the production of immunoregulatory molecules such as IL-10 and TGFβ. A regulatory T cell (Treg) phenotype is also induced, promoting the protection/suppression of inflammation produced by a Th1 autoimmune disease. Myeloid-derived suppressor cells (MDSC) function as immunoregulators, producing reactive oxygen/nitrogen species that inhibit the function of T cells.
Autoimmunity is the failure of the immune system to distinguish pathogens from self-antigens resulting in damage to healthy tissue (15). Today more than 80 autoimmune diseases have been identified, including inflammatory bowel disease (IBD), multiple sclerosis (MS), rheumatoid arthritis (RA), and type 1 diabetes (T1D) (16). Autoimmune diseases are now estimated to affect almost 10% of the world’s population and collectively represent truly massive global disease and financial burdens (17). Most autoimmune diseases have no cures and are not knowingly preventable. Disconcertingly, for several decades, the developed world has seen steady increasing incidence of autoimmune disease (18–21). While genetic predisposition is known to be a key factor in susceptibility (22), the sudden surge in these diseases over a very short time period cannot be explained by genetics alone, but rather points to variations in environment and/or lifestyle (23, 24). Two major theories have been put forward to explain this epidemiology including the “hygiene hypothesis” and the “old friends’ hypothesis” (25, 26).
The hygiene hypothesis, formulated in 1989, proposed that lower intensities of infections during early childhood could explain the emergence of asthma and hay fever later in life (25). The study suggested that declining family size, improvements in household amenities, and increases in personal cleanliness reduced opportunities for cross infections in young families, resulting in a more widespread clinical expression of atopic diseases. Over time, this theory has broadened to include a catalog of chronic inflammatory diseases. Indeed, urban migration, increased access to clean water, and improved sanitation have reduced exposure to many infectious agents including helminths (27). Multiple epidemiological studies have shown an inverse correlation between microorganism exposure and the development of autoimmunity (28–33).
Concordantly, the old friends’ hypothesis suggests that various organisms, including helminths and microbiotas, have long coevolved with their mammalian hosts and act as inducers of immunoregulatory circuits (24, 34). This hypothesis has a sound rationale given that infectious agents, including helminths, are known to be potent modulators of T cell function and that dysregulation of T cell subsets (Th1 and Th17) are fundamental in autoimmune disease processes (35–37) including MS (38), RA (39), and psoriasis (40). Of note, an inverse association has been observed between the prevalence of certain helminths and autoimmune diseases (24).
Over the last decades, there have been numerous animal models used to study hookworm therapy for autoimmune disease (IBD, MS, RA, and T1D). Although these individual animal models do not fully reflect the pathology of human disease, the data obtained can be used for safety and at the very least predictive for therapeutic efficacy in humans. The following sections detail current animal models of helminth therapy and therapy with helminth-derived secretory products.
Inflammatory bowel disease is characterized by a chronic relapsing inflammatory condition of the gastrointestinal tract. IBD primarily encompasses ulcerative colitis (UC) and Crohn’s disease (CD) (41). IBD pathogenesis is thought to involve dysregulation in mucosal immunity (42) and defects at the mucosal barrier, particularly a “leaky” intestinal epithelial barrier with impaired tight-junction formation can cause mucosal inflammation secondary to luminal antigen uptake (43, 44). While both diseases are forms of IBD, the autoimmune T cell responses exhibit different biology (45). CD is driven by a Th1/Th17 response with large amounts of IFNγ, IL-12, and IL-23 playing key roles. In contrast, UC is considered a Th2-mediated disease, where increases in IL-5 and IL-13 drive pathology through chronic inflammation (45).
Similar to CD, mouse models of experimental colitis trigger a Th1 type immune response, reflected by the infiltration of IFNγ-producing T cells in the colon (46). There are three types of animal models of IBD. These are broadly divided into (i) chemically induced models; (ii) models with experimentally altered immune responses; and (iii) models with intestinal epithelial defects (47). Chemically induced colitis models including the trinitrobenzene sulfonic acid (TNBS) model, dinitrobenzene sulfonic acid (DNBS) model, and dextran sodium sulfate (DSS) model are the most common platforms for IBD research. In the TNBS and DNBS models, colitis is induced via intrarectal instillation of the chemicals. In the DSS model, colitis is induced orally. Each model triggers a Th1 pro-inflammatory immune response within the intestine (48). A second broad model for IBD includes varieties of knockout mice (TGFβ1−/−, IL-10−/−, and STAT3−/−) that aid in the study of innate and adaptive immune responses during disease (49). These strains also allow for mechanistic investigations during acute or chronic enteritis. For instance; IL-10−/− mice develop spontaneous colitis that is characterized by histological findings similar to those of human IBD (50). The T cell transfer model has become one of the most widely used models to study pancolitis and chronic transmural inflammation in the intestine (49, 51). This method involves the adoptive transfer of naïve T cells (CD4+CD25−) into immunocompromised mice (52). Advantages of this method include early investigation of immunological events associated with the induction of gut inflammation and the ability to study the role of Tregs in inflammation. The final type of animal model of IBD is defective intestinal epithelial responses (53). Mouse models such as IKK-γ (NEMO), IKK-β, and mdr1a−/− develop spontaneous colitis due to compromised immunity at the epithelial cell wall. Many of these animal models of IBD show that colitis can be attenuated with prior exposure to different helminth species (54–59) (Table 1). Several of the parasites use the same immune regulatory mechanism, such as a Th2 polarization, which suppresses inflammation. These effects are commonly mediated through increases of cytokines including IL-4, IL-10, and IL-13 production, as well as a decrease in the pro-inflammatory cytokines such as IFNγ and TNFα (Table 1).
Characterized by neurodegeneration, MS leads to the severe impairment of mobility, vision, and coordination eventually resulting in paralysis (85). The primary cause of pathology is a misdirected immune response against the myelin sheath. Damage is mediated by immunoglobulin, complement, and T cell immunity (86). Experimental autoimmune encephalomyelitis (EAE) is a mouse model of MS characterized by a pro-inflammatory T cell-mediated disease induced by priming with myelin proteins/peptides (87). CNS autoimmunity in both EAE and MS is mediated by Th1 and Th17 cells (88). Induction is thought to be dependent on the Th1 cytokine IL-12, playing a central role in macrophage activation and nitric oxide production (89). Granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-1 are also considered key cytokines involved in the pathogenesis of EAE. GM-CSF is a key cytokine produced by T cells required for susceptibility to EAE (90). IL-1β/IL-1R signaling in endothelial cells and leukocytes is critical for EAE development (91) and stimulates GM-CSF production. Together the cytokines interact to create a cycle of neuroinflammation in the CNS. Th2 cytokines appear to be protective, suggesting that Th skewing can prevent diseases or decrease disease severity. Akin to IBD, helminthic therapy in the EAE mouse model decreases the progression of EAE through the suppression of Th1 and Th17 cells and induction of Th2 cells, Tregs, and regulatory macrophages (Table 1).
Type 1 diabetes is characterized by a progressive cellular infiltration of the pancreas resulting in the destruction of insulin-producing cells (92). The non-obese diabetic (NOD) mouse provides a model of human disease through mimicking polyuria, glycosuria, weight loss, and lymphocytic infiltration of the islets of Langerhans (75, 93, 94). At 5 weeks of age, immune infiltration of the pancreas begins, ultimately ending in lymphocyte-directed destruction of β-cells (95). Pathology is dependent on CD4+ and CD8+ T cells, with the CD4+ population having a Th1 phenotype (96). Antigen-presenting cells including B cells, dendritic cells (DCs), and macrophages are key mediators of disease through the presentation of self-antigens. Similar to the IBD and EAE models discussed above, helminthic therapy in the NOD mouse also triggers Th2 skewing due to increases in IL-4 and IL-13 expression, ameliorating Th1-mediated disease (Table 1).
Rheumatoid arthritis is characterized by chronic inflammation in the joints and overexpression of the cytokines TNFα, IL-1, and IL-6 (97). Pathogenesis involves both genetic predisposition and environmental trigger(s). A number of induced and spontaneous mouse models have been developed that recapitulate features of human disease (98). Both induced and spontaneous models of RA have been shown to benefit from helminthic therapy through decreasing inflammasome activity at the site of disease and the production of Th1 cytokines such as TNFα, while increasing IL-4 and IgG1 production (Table 1).
Ten clinical trials indicate that controlled, low-dose helminthic therapy is safe in IBD and related GIT diseases, with some trials showing statistically significant efficacy at endpoint (Table 2). In 2003, an open-label phase 1 trial examined safety by exposing CD and UC patients to pig whipworm ova (99). Four patients with active CD and three patients with UC were given a single oral dose of live eggs. Patients were routinely monitored using multiple disease and quality of life indexes over a period of 12 weeks. The trial found that all patients improved clinically without any adverse events. While patients improved for a mean duration of approximately 8 weeks, three patients experienced remission relapse 12 weeks after single helminthic therapy. The study suggested that multiple doses may be required to prolong the benefit of treatment. The study also found that there were no significant clinical complications when patients received multiple doses of live eggs at 3-week intervals for 30 weeks. The group followed up with a placebo-controlled trial of 54 UC patients. The pig whipworm arm received an oral dose of live ova at 3-week intervals for 12 weeks (100). Again, whipworm therapy produced no adverse events. Between the treatment and placebo groups, statistically significant efficacy was observed at 12 weeks in two separate indices in post hoc analysis. One limitation of pig whipworm therapy is that humans are not the natural host and repeated dosing is required to maintain ongoing infection. In addition, given the larvae are invasive, site of infection is unpredictable with potential migration into the lymphatics and/or small blood vessels (101). The problems of repeated inoculation and unpredictable migration motivated an alternative modality. In 2006, a proof-of-concept study explored human hookworm for the treatment of CD (102). While both hookworm and whipworm possess parasite lifecycles that require development in the external environment and therefore unable to proliferate directly in the host; the hookworm is adapted to survive in humans and establish a chronic infection that can last for years from a single inoculation. This makes human hookworm an attractive therapeutic, as a defined dose can be controlled and eliminated via anthelmintic therapy (103). CD patients with longstanding but mostly inactive disease were inoculated with 25 or 50 live hookworm larvae in an initial and reinoculation trial. Disease index for CD patients was unchanged until week 17. After 20 weeks, clinical scores improved and five patients were in remission at week 45.
Two recent human hookworm clinical trials explored the safety and efficacy of hookworm therapy in celiac disease (104, 105). The first double-blind, placebo-controlled study inoculated patients twice with 15 live hookworm larvae followed by an aggressive oral gluten challenge after patient intestinal infection was established (105). Experimental infection proved to be safe but did not result in clinical benefit following gluten challenge. Interestingly, follow-up immunological analysis found that hookworm infection altered cellular immunity (106), through decreasing basal levels of IFNγ and IL-17 in the intestine and altering CD4+ T cell immunity both in the intestine and, interestingly the circulatory system. The second study combined live hookworm larvae inoculation (20 larvae per individual) with desensitization, specifically a sustained gluten microchallenge (104). Of note, no uninfected controls were used in the study. Escalating gluten challenges were well tolerated and resulted in stabilization or improvement across all tested indices of gluten toxicity. IFNγ-producing intestinal T cells were observed to decrease, while Treg numbers in the epithelium increased significantly. Three human clinical trials for IBD that have been completed are yet to post study results (NCT01433471, NCT01576471, and NCT01279577) (Table 2). A larger phase 1b dose-ranging hookworm trial for celiac disease treatment is underway (NCT02754609) (Table 2).
Six clinical trials in MS have been completed or are in progress for helminthic therapy (Table 2). In 2007, a prospective study of MS patients who were recently positive for parasitic infections (and negative for the 2 previous years) were followed over approximately 5 years via disease score and immunomonitoring (108). The study found significantly lower disease scores and lower numbers of disease exacerbations in helminth-infected patients. Compared with uninfected patients, myelin basic protein-specific T cells in the peripheral blood showed increased IL-10 and TGFβ production and decreased IL-12 and IFNγ production. Increased success of in vitro cloning efficacy of Tregs was also noted in infected MS patients when compared with uninfected patients. A succeeding study followed the same relapsing–remitting MS patients with natural parasitic infections from the previous study for approximately 7 years (109). During the course of study, four MS patients received anthelmintic treatment due to worsening symptoms associated with infection. Posttreatment, there was a significant increase in disease score in these individuals accompanied by a permanent alteration of immune phenotype in the circulatory system (decreases in IFNγ-secreting cells and absolute Treg numbers). Asymptomatic, persistently infected patients maintained a significantly lower disease score across the monitoring period. It was speculated that helminths induce regulatory networks that could explain environment-related epidemiology of disease.
The first helminthic therapy trial for MS was published in 2011 (110). Here, five MS patients were given repeated oral doses of pig whipworm for 12 weeks in a baseline versus treatment-controlled exploratory trial. Results revealed that helminthic therapy was well tolerated, and some favorable trends were observed in disease scoring. Increases in serum IL-4 and IL-10 levels were noted in four of the five patients. The second helminthic therapy trial for MS was published in 2015 (111). Here, 10 MS patients were given repeated oral doses of pig whipworm for 12 weeks. Treatment was well tolerated with only mild and self-limiting adverse events. However, no positive effect on disease activity was observed, and there was no alteration in the examined immune biomarkers in the peripheral blood. For both pig whipworm trials, it is currently unknown if the relatively short infection period of 12 weeks is sufficient time to initiate clinical efficacy. Several phase 1/2 clinical trials using pig whipworm or hookworm are currently recruiting or ongoing (Table 2). In addition to IBD and MS, two helminthic therapy trials have been conducted for the treatment of other autoimmune disease such as psoriasis and RA (Table 2). However, a number of trails for MS (NCT01413243 and NCT00630383), psoriasis (NCT01948271 and NCT02011269), and RA (EUCTR2011-006344-71-DE) have been terminated or withdrawn prior to enrollment due to supersession by another study, possessing a lack of efficacy or an unknown cause.
Helminthic therapy is not without controversy. Direct treatment with living worms could cause pathology. Furthermore, the idea of being infected with a living parasite may be a difficult task for many patients. With these limitations in mind, immunomodulatory proteins and peptides secreted by helminths have become a more attractive target for drug development. Here, the use of immunomodulatory drugs derived from helminth molecule “blueprints” would provide a safer and more controllable therapeutic modality.
Excretory/secretory (ES) products are the primary interface between parasitic worms and their hosts (113). ES products contain a mixture of proteins, glycoproteins, and small molecular weight compounds that are secreted from the oral openings or outer body surfaces (114). ES products are essential for helminth survival/propagation, allowing the parasites to evade immune surveillance. While a number of studies have reported the benefits of ES products in treating autoimmune diseases in mouse models, to date, only a few worm-derived immunomodulatory macromolecules and recombinant proteins have been characterized in depth. Likewise, ES proteins investigated to date represent only an infinitely small slice of the bioactive compounds found in the complex fluids of helminths. There have been multiple inventories of ES proteins generated from different types of parasitic worms including Fasciola hepatica (115), Trichinella spiralis (116), Haemonchus contortus (117), Brugia malayi (118), Teladorsagia circumcincta (119), Schistosoma mansoni (120), and Ancylostoma caninum (114). Many studies focus on higher molecular weight proteins (>5 kDa) (114), and there is a notable absence of research on lower MW products (1–5 kDa). Large-scale sequencing projects have revealed the presence of peptides within the genome/transcriptome of Necator americanus and Ancylostoma ceylanicum (121–123). In particular, a group of peptides highly expressed in hookworm species exhibit sequence/structural homology to the Stichodactyla helianthus toxin (ShK) family of peptides (referred to as ShKT domains).
Excretory/secretory-62 is a phosphorylcholine (PC)-containing glycoprotein from the ES of the rodent nematode Acanthocheilonema viteae (124). ES-62 is known to inhibit the activation of B cells and T cells (125, 126) and has also been found to polarize antibody production through increased serum levels of IgG1 but not IgG2a (127). ES-62 affects B cells by stimulating the regulatory cytokine IL-10 and inducing a hyperresponsiveness to antigen (128). Due to its immunomodulatory potential, ES-62 was tested in an induced RA mouse model and was found to reduce disease severity and progression when administered following disease onset (129). ES-62 was also therapeutically effective in a mouse model of systemic lupus erythematosus (SLE) (84). Recently, two small synthetic molecule analogs, based on the active PC-moiety, have been shown to be effective in the mouse models of RA (82) and SLE (84).
Neutrophil inhibitory factor (NIF) is a glycoprotein from the ES of the canine hookworm A. caninum (130). NIF selectively binds the CD11b/CD18 complex, a pattern recognition receptor found on polymorphonuclear leukocytes. When activated, the complex plays an essential role in immune clearance through the facilitation of neutrophil adhesion to the endothelium, transmigration across the epithelia and phagocytosis of opsonized targets (131). Binding of NIF to CD11b/CD18 antagonizes function (132), making the molecule a potential candidate for treating acute and destructive inflammatory processes such as cerebral ischemic injury. In a phase 2 safety study on acute stroke patients, NIF was well tolerated over a wide dose range (133). This led to a study in acute ischemic stroke patients where it was hypothesized that NIF may improve neurological recovery through inhibition of neutrophil migration. However, NIF did not show improved clinical outcome, and the study was terminated (133). Since then there has been a number of animal models demonstrating the potential benefits of NIF in acute inflammatory diseases such as allergic lung inflammation (134) and diabetic retinopathy (79). Interestingly, evidence of homologous NIF proteins has been reported in other parasites including F. hepatica (135).
Macrophage migration inhibitory factor (MIF), a human cytokine homolog, is from the ES of human-tropic nematodes (136). Paradoxically, mammalian MIF is thought to be pro-inflammatory and involved in a number of inflammatory diseases including asthma, RA, IBD, and psoriasis (65). Two secretory MIF homologs have been identified in nematodes: MIF-1 and MIF-2, possessing 40% and 27% identity with the mammalian protein, respectively (137). It has been shown that helminth-derived MIF interacts with the ubiquitously expressed antigen presentation protein CD74, suggesting a role in immunomodulation (138). Mammalian MIF has been found to influence macrophage migration, T cell activation (139), NK cell activation (140), and immunoglobulin synthesis (141), leading to the amplification of inflammatory responses. In contrast, studies on MIF-2, isolated from the nematode Anisakis simplex, have shown amelioration of disease in a DSS-induced colitis model (65) and an allergic airway inflammation model (142). The effect is mediated through Treg induction.
Cystatins are a group of immunomodulatory proteins found in helminth ES products. Cystatins, along with stefins and kininogens, belong to a superfamily of cysteine protease inhibitors found across metazoan and plant taxa. Cysteine protease inhibitors are responsible for various biological and pathological processes including protein catabolism, antigen processing, and inflammation (143). Helminth-derived cystatins have been described in many parasite species including Onchocerca volvulus (144), B. malayi (145), Nippostrongylus brasiliensis (146), and A. viteae (143). These proteins produced by helminths have been found to target monocytes/macrophages both in vivo and in vitro, triggering the release of IL-10 that suppresses inflammatory T cells (147, 148). The cystatin from A. viteae was found to suppress both DSS-induced colitis and allergic lung inflammation in mice (66). In a murine model of asthma, treatment with recombinant cystatin prevented Th2 development of disease. Compared with controls, treated mice has significantly reduced eosinophil recruitment, reduced numbers of autoimmune T cells, reduced IL-4, and reduced total IgE. In a murine model of colitis, cystatin-treated mice showed significant decreases in inflammatory index and reduced epithelial damage compared to controls. The mechanism of action in both disease models was mediated by macrophages and IL-10 dependent. The immunomodulating effects of cystatins have also been examined in pig intestinal inflammation, where pigs treated with transgenic probiotic-secreting A. vitaea cystatin possessed a significantly reduced inflammatory score and reduced infiltration of immune cells in the colon compared with controls (148).
Helminth defense molecules (HDMs) are a newly discovered family of secreted immunomodulatory proteins that share biochemical and structural characteristics with the mammalian “cathelicidin-like” host defense peptides (HDP) (149). HDPs are found in both the animal and plant kingdoms and play important roles in innate immune defense against parasites, fungi, bacteria, and viruses (150). HDMs within helminth ES are thought to minimize excessive inflammation, which helps the survival of the host and in turn survival of the parasite (151). FhHDM-1 is a HDM secreted by the trematode F. hepatica that adopts an α-helical structure (151). FhHDM-1 binds LPS and inhibits interaction with TLRs on macrophages. The protein has been shown to protect mice from LPS-induced inflammation and, when mixed with LPS, significantly reduces TNFα and IL-1β levels in circulation. Mechanistically, FhHDM-1 works by preventing NLRP3 inflammasome activation in macrophages through inhibiting endolysosomal acidification (152).
P28GST is a glutathione S-transferase secreted by the platyhelminth blood fluke S. mansoni (153). P28GST modulates mucosal immunity in mice and humans by increasing Th2 cytokine production (61). Encouragingly, immunization using a recombinant P28GST protein was as effective as helminthic therapy in reducing colitis in the TNBS model; however, a pro-Th2 adjuvant was essential for activity (61). P28GST treatment produced lower local and systemic levels of IL-5 and IL-13 and encouraged eosinophil trafficking, which was crucial for therapeutic effect. P28GST has already successfully undergone phase 1 clinical trials for safety and immunogenicity studies (NCT01512277) (154) and is currently in a phase 2 trial in CD (NCT02281916) (Table 2).
Anti-inflammatory protein-2 (AIP-2) is derived from the ES of the canine hookworm A. caninum. Hookworm ES products have been shown to be protective in mouse models of colitis (58, 59, 155). AIP-2 was found to be one of the most abundant proteins in the hookworm ES proteome (114), and it was recently demonstrated that intranasal delivery of recombinant AIP-2 protein could suppress airway inflammation in a mouse model of asthma and suppress antigen-specific T cell proliferation in human subjects allergic to house dust mite using in vitro stimulation (156). Mechanistic studies showed that AIP-2 is primarily captured by mesenteric DCs and that therapeutic effect was dependent on both DCs and Tregs. In contrast to P28GST, AIP-2 suppressed eosinophil infiltration into the lungs, the site of pathology.
TGFβ is a potent regulatory cytokine important in lymphocyte and myeloid cell differentiation and function system (157). In particular, TGFβ is a key player in the induction of immunological tolerance (158) and production can be influenced by several mechanisms of parasite infection, including host homeostasis, pathogen-triggered TGFβ production, and parasite mimicry (158). TGFβ homologs/orthologs/ligands have been characterized from several species of helminth including A. caninum (159), B. malayi (160, 161), F. hepatica (162), Heligmosomoides polygyrus (163), and the Schistosoma genus (164–166). In the gut, the induction of regulatory cytokines such as TGFβ is important in suppressing colitis. A study using transgenic mice with T cell-specific defects in TGFβ signaling developed spontaneous colitis (166). Here, infection with H. polygyrus did not prevent colitis or dampen mucosal Th1 responsiveness, indicating an essential role of T cell TGFβ signaling in regulating mucosal T cell responses.
Prostaglandin E2 belongs to a family of autocrine and paracrine acting lipids, which in mammals are known to regulate many immune responses. Several reports have described that different helminth species including S. mansoni (167), T. taeniaeformis (168), and B. malayi (169, 170) produce PG homologs. A recent study identified a PGE2 homolog as a major component of Trichuris suis ES and suggests that secretion of this homeostatic factor contributes to protective potential in inflammatory diseases (166). PGE2 directs the immunologic balance away from Th1 responses toward a Th2 type response by modulating DC polarization (171). PGE2 can also promote resolution of inflammation and subsequent tissue repair (172) with evidence showing regeneration of epithelial crypts after DSS intestinal injury (173, 174).
ShkT domains are relatively short peptides, 36–42 amino acids in length, containing 6 conserved cysteines and other conserved residues. ShKT domains adopt a fold with two almost perpendicular stretches of helices that are linked by three disulfide bonds that stabilize the structure (175). ShKTs have been found in both the plant and animal kingdoms suggesting ancient origins (176); however, the largest family of ShKTs are found in helminths (177). ShK from the sea anemone S. helianthus was one of the first immune modulating peptides discovered (178). ShK blocks the voltage-gated potassium channel Kv1.3 at low picomolar concentrations (179) by binding to a shallow vestibule at the outer entrance of the channel, which occludes entrance to the pore. Kv1.3 channels are expressed on the surface of human T cells and are vital for activation by regulating membrane potential and calcium (Ca2+) signaling (180, 181). Kv1.3high IKCallow channel phenotype is found exclusively in activated human effect memory T cells (TEM), whereas naïve and central memory T cells (TCM) remain Kv1.3low upon activation. In MS, myelin-reactive T cells are predominantly TEM cells, exhibiting the Kv1.3high IKCallow phenotype after activation with myelin antigens. Therefore, selective inhibition of autoreactive TEM cells with disulfide rich Kv1.3 blockers could be a valuable new therapeutic lead for the treatment of MS (182). A phase 1 clinical trial was conducted to assess safety, tolerability, and pharmacokinetics of the ShK peptide in healthy volunteers (NCT02446340). Given a satisfactory safety profile, a phase Ib trial was recently conducted in psoriasis patients with results yet to be published (NCT02435342) (Table 2).
A large family of ShK-related peptides have recently been discovered in helminths, including two peptides known as AcK1 and BmK1 (177). AcK1 is a 51-residue peptide found in the ES of the hookworm A. caninum and the human pathogen A. ceylanicum. BmK1 is a C-terminal domain of a metalloprotease from the ES of B. malayi (176). Both peptides have been found to adopt helical structures that closely resemble ShK. To overcome problems in folding during de novo production, a truncated version of AcK1 (AcK1t) was designed lacking the first nine N-terminal residues, and an analog of BmK1 (BmK2) was designed based on the ShK-channel interaction surface, differing from the native peptide by five residues. Both analogs fold without difficulty, yielding a well-resolved, hydrophilic-eluting product. AcK1t and BmK2 were found to block Kv1.3 channels in the low-to-mid nanomolar range, while BmK1 was found to block the channel at low micromolar concentrations. AcK1t and BmK2 were found suppress mouse T cell proliferation in vitro and, in human T cells suppress mitogen stimulation. The results of these studies provide evidence that helminth peptides could potentially replace probiotic worm-based therapies to treat TEM-mediated autoimmune diseases such as RA, MS, T1D, and psoriasis (183–185). This would avoid complications of live worm therapy, providing a safer and more controllable therapeutic for inflammatory diseases.
The potential for helminth-based therapies to treat autoimmune diseases have been demonstrated in animal models and clinical trials highlighted in this review. To date, the majority of clinical trials treat patients with live helminths. Justifiably, there are concerns with this method, including the associated health risks of infection with a live pathogen. However, there is the large potential to harness the specific immunomodulatory ES proteins from helminths to develop more traditional “pill”-based treatments. The synthetic production of ES-derived immunomodulators would alleviate concerns associated with live infection, and they can be produced recombinantly in high quantities at relatively low cost (186). In addition, the molecules could be directly delivered to the site of pathology for diseases such as IBD using probiotic carries that secrete the drug (187). Large-scale technologies such as genomics, proteomics, and metabolomics have increased the rate of discovery of new helminth-derived immunomodulators from the genome, and there is little doubt many more candidates will be discovered in the coming years.
With the accruing global burden of autoimmune disease, helminths have become of heightened scientific interest due to their ability to activate immunoregulatory circuits and control immunity. There is strong evidence in mouse models that helminthic therapy, ES components, and helminth-derived synthetic molecules can treat and/or prevent inflammatory diseases such as IBD, T1D, MS, RA, and asthma. Thus far, human trials in celiac disease, UC, CD, MS, RA, and psoriasis have established that therapy is safe with some evidence of therapeutic effect. However, results in the first wave of human trials are not as striking as mouse disease models. Discordance in mouse/human translation is certainly not unique to this system, as is well known in other settings for a number of reasons (188). Of note, a number of the clinical studies conducted to date were not controlled, comprised small sample sizes, and/or did not use human-tropic helminths. Forthcoming trials will directly address these limitations. Going forward, the concurrent development of helminth-derived anti-inflammatory molecules provides many novel opportunities for safer and more controllable therapeutics against chronic inflammatory diseases. Indeed, inclusive efforts in characterizing and mimicking the full immunomodulating abilities of helminths are only in their infancy and much potential exists in this space.
Drafting and critical revision of the manuscript: TBS, PRG, AL, JPM, RJC, and JJM.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by grants from the Australian Postgraduate Award (TS), Australian Research Council Future Fellowship (RC; grant number FT100100476), Australian Infectious Disease Research Centre (RC), National Cancer Institute (JPM; grant number R01CA155297), and National Health and Medical Research (NHMRC) Senior Principle Research Fellowship (AL; grant number 1117505), and Advance Queensland Fellowship (PG), Career Development Fellowship (JJM and JPM; grant numbers 1031652 and 1051627).
1. World Health Organization. Soil-Transmitted Helminth Infections. (2016). Available from: http://www.who.int/mediacentre/factsheets/fs366/en/
2. Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med (2004) 351:799–807. doi: 10.1056/NEJMra032492
3. Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol (2003) 3:733–44. doi:10.1038/nri1183
4. Navarro S, Ferreira I, Loukas A. The hookworm pharmacopoeia for inflammatory diseases. Int J Parasitol (2013) 43:225–31. doi:10.1016/j.ijpara.2012.11.005
5. Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol (2004) 58:197–288. doi:10.1016/S0065-308X(04)58004-1
6. Miles JJ, McCluskey J, Rossjohn J, Gras S. Understanding the complexity and malleability of T-cell recognition. Immunol Cell Biol (2015) 93:433–41. doi:10.1038/icb.2014.112
7. Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J. T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol (2015) 33:169–200. doi:10.1146/annurev-immunol-032414-112334
8. Miles JJ, Douek DC, Price DA. Bias in the alphabeta T-cell repertoire: implications for disease pathogenesis and vaccination. Immunol Cell Biol (2011) 89:375–87. doi:10.1038/icb.2010.139
9. Pritchard DI. The survival strategies of hookworms. Parasitol Today (1995) 11:255–9. doi:10.1016/0169-4758(95)80206-1
10. Pritchard DI, Brown A. Is Necator americanus approaching a mutualistic symbiotic relationship with humans? Trends Parasitol (2001) 17:169–72. doi:10.1016/S1471-4922(01)01941-9
11. McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev (2012) 25:585–608. doi:10.1128/CMR.05040-11
12. Ricci ND, Fiúza JA, Bueno LL, Cançado GGL, Gazzinelli-Guimarães PH, Martins VG, et al. Induction of CD4(+)CD25(+)FOXP3(+) regulatory T cells during human hookworm infection modulates antigen-mediated lymphocyte proliferation. PLoS Negl Trop Dis (2011) 5:e1383. doi:10.1371/journal.pntd.0001383
13. Loukas A, Hotez PJ, Diemert D, Yazdanbakhsh M, McCarthy JS, Correa-Oliveira R, et al. Hookworm infection. Nat Rev Dis Primers (2016) 2:16088. doi:10.1038/nrdp.2016.88
14. Wammes LJ, Hamid F, Wiria AE, May L, Kaisar MM, Prasetyani-Gieseler MA, et al. Community deworming alleviates geohelminth-induced immune hyporesponsiveness. Proc Natl Acad Sci U S A (2016) 113:12526–31. doi:10.1073/pnas.1604570113
15. Gutierrez-Arcelus M, Rich SS, Raychaudhuri S. Autoimmune diseases – connecting risk alleles with molecular traits of the immune system. Nat Rev Genet (2016) 17:160–74. doi:10.1038/nrg.2015.33
16. Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev (2012) 11:754–65. doi:10.1016/j.autrev.2012.02.001
17. Cooper GS, Bynum MLK, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun (2009) 33:197–207. doi:10.1016/j.jaut.2009.09.008
18. Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G, EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet (2009) 373:2027–33. doi:10.1016/S0140-6736(09)60568-7
19. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology (2011) 140:1785–94. doi:10.1053/j.gastro.2011.01.055
20. Moroni L, Bianchi I, Lleo A. Geoepidemiology, gender and autoimmune disease. Autoimmun Rev (2012) 11:A386–92. doi:10.1016/j.autrev.2011.11.012
21. Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology (2014) 83:1022–4. doi:10.1212/WNL.0000000000000768
22. Gregersen PK, Olsson LM. Recent advances in the genetics of autoimmune disease. Annu Rev Immunol (2009) 27:363–91. doi:10.1146/annurev.immunol.021908.132653
23. Colafrancesco S, Agmon-Levin N, Perricone C, Shoenfeld Y. Unraveling the soul of autoimmune diseases: pathogenesis, diagnosis and treatment adding dowels to the puzzle. Immunol Res (2013) 56:200–5. doi:10.1007/s12026-013-8429-4
24. Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol (2012) 42:5–15. doi:10.1007/s12016-011-8285-8
25. Strachan DP. Hay fever, hygiene, and household size. BMJ (1989) 299:1259–60. doi:10.1136/bmj.299.6710.1259
26. Rook GAW, Brunet LR. Old friends for breakfast. Clin Exp Allergy (2005) 35:841–2. doi:10.1111/j.1365-2222.2005.02112.x
27. Versini M, Jeandel PY, Bashi T, Bizzaro G, Blank M, Shoenfeld Y. Unraveling the hygiene hypothesis of helminthes and autoimmunity: origins, pathophysiology, and clinical applications. BMC Med (2015) 13:81. doi:10.1186/s12916-015-0306-7
28. Godfrey RC. Asthma and IgE levels in rural and urban communities of The Gambia. Clin Allergy (1975) 5:201–7. doi:10.1111/j.1365-2222.1975.tb01853.x
29. Masters S, Barrett-Connor E. Parasites and asthma – predictive or protective? Epidemiol Rev (1985) 7:49–58. doi:10.1093/oxfordjournals.epirev.a036285
30. van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet (2000) 356:1723–7. doi:10.1016/S0140-6736(00)03206-2
31. Yazdanbakhsh M, van den Biggelaar A, Maizels RM. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol (2001) 22:372–7. doi:10.1016/S1471-4906(01)01958-5
32. Scrivener S, Yemaneberhan H, Zebenigus M, Tilahun D, Girma S, Ali S, et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet (2001) 358:1493–9. doi:10.1016/S0140-6736(01)06579-5
33. Gale EA. A missing link in the hygiene hypothesis? Diabetologia (2002) 45:588–94. doi:10.1007/s00125-002-0801-1
34. Rook GAW, Lowry CA, Raison CL. Microbial ‘old friends’, immunoregulation and stress resilience. Evol Med Public Health (2013) 2013:46–64. doi:10.1093/emph/eot004
35. Műzes G, Molnár B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol (2012) 18:5848–61. doi:10.3748/wjg.v18.i41.5848
36. Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, Ilonen J, et al. IL-17 immunity in human type 1 diabetes. J Immunol (2010) 185:1959–67. doi:10.4049/jimmunol.1000788
37. Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KHG. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol (2010) 162:1–11. doi:10.1111/j.1365-2249.2010.04143.x
38. Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler (1999) 5:101–4. doi:10.1191/135245899678847275
39. Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol (1999) 162:1246–51.
40. Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol (1998) 111:645–9. doi:10.1046/j.1523-1747.1998.00347.x
41. Blumberg RS, Strober W. Prospects for research in inflammatory bowel disease. JAMA (2001) 285:643–7. doi:10.1001/jama.285.5.643
42. Khan WI, Blennerhasset PA, Varghese AK, Chowdhury SK, Omsted P, Deng Y, et al. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun (2002) 70:5931–7. doi:10.1128/IAI.70.11.5931-5937.2002
43. Giacomin P, Agha Z, Loukas A. Helminths and intestinal flora team up to improve gut health. Trends Parasitol (2016) 32:664–6. doi:10.1016/j.pt.2016.05.006
44. Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol (2007) 23:379–83. doi:10.1097/MOG.0b013e32816aa392
45. Strober W, Fuss IJ. Pro-inflammatory cytokines in the pathogenesis of IBD. Gastroenterology (2011) 140:1756–67. doi:10.1053/j.gastro.2011.02.016
46. Fuss IJ, Marth T, Neurath MF, Pearlstein GR, Jain A, Strober W. Anti-interleukin 12 treatment regulates apoptosis of Th1 T cells in experimental colitis in mice. Gastroenterology (1999) 117:1078–88. doi:10.1016/S0016-5085(99)70392-6
47. Eri R, McGuckin MA, Wadley R. T cell transfer model of colitis: a great tool to assess the contribution of T cells in chronic intestinal inflammation. Methods Mol Biol (2012) 844:261–75. doi:10.1007/978-1-61779-527-5_19
48. Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protocols (2007) 2:541–6. doi:10.1038/nprot.2007.41
49. Jiminez JA, Uwiera TC, Douglas Inglis G, Uwiera RRE. Animal models to study acute and chronic intestinal inflammation in mammals. Gut Pathog (2015) 7:1–31. doi:10.1186/s13099-015-0076-y
50. Keubler LM, Buettner M, Hager C, Bleich A, Multihit Model A. Colitis lessons from the interleukin-10-deficient mouse. Inflamm Bowel Dis (2015) 21:1967–75. doi:10.1097/MIB.0000000000000468
51. Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity (1994) 1:553–62. doi:10.1016/1074-7613(94)90045-0
52. Song-Zhao GX, Maloy KJ. Experimental mouse models of T cell-dependent inflammatory bowel disease. Methods Mol Biol (2014) 1193:199–211. doi:10.1007/978-1-4939-1212-4_18
53. Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature (2007) 446:557–61. doi:10.1038/nature05698
54. Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF Jr, et al. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol (2003) 284:G385–91. doi:10.1152/ajpgi.00049.2002
55. Moreels TG, Nieuwendijk RJ, De Man JG, De Winter BY, Herman AG, Van Marck EA, et al. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut (2004) 53:99–107. doi:10.1136/gut.53.1.99
56. Zhao Y, Zhang S, Jiang L, Jiang J, Liu H. Preventive effects of Schistosoma japonicum ova on trinitrobenzenesulfonic acid-induced colitis and bacterial translocation in mice. J Gastroenterol Hepatol (2009) 24:1775–80. doi:10.1111/j.1440-1746.2009.05986.x
57. Hang L, Setiawan T, Blum AM, Urban J, Stoyanoff K, Arihiro S, et al. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. J Immunol (2010) 185:3184–9. doi:10.4049/jimmunol.1000941
58. Ruyssers NE, De Winter BY, De Man JG, Loukas A, Pearson MS, Weinstock JV, et al. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis (2009) 15:491–500. doi:10.1002/ibd.20787
59. Ferreira I, Smyth D, Gaze S, Aziz A, Giacomin P, Ruyssers N, et al. Hookworm excretory/secretory products induce interleukin-4 (IL-4)+ IL-10+ CD4+ T cell responses and suppress pathology in a mouse model of colitis. Infect Immun (2013) 81:2104–11. doi:10.1128/IAI.00563-12
60. Sutton TL, Zhao A, Madden KB, Elfrey JE, Tuft BA, Sullivan CA, et al. Anti-inflammatory mechanisms of enteric Heligmosomoides polygyrus infection against trinitrobenzene sulfonic acid-induced colitis in a murine model. Infect Immun (2008) 76:4772–82. doi:10.1128/IAI.00744-07
61. Driss V, El Nady M, Delbeke M, Rousseaux C, Dubuquoy C, Sarazin A, et al. The schistosome glutathione S-transferase P28GST, a unique helminth protein, prevents intestinal inflammation in experimental colitis through a Th2-type response with mucosal eosinophils. Mucosal Immunol (2016) 9:322–35. doi:10.1038/mi.2015.62
62. Mo HM, Liu WQ, Lei JH, Cheng YL, Wang CZ, Li YL. Schistosoma japonicum eggs modulate the activity of CD4+ CD25+ Tregs and prevent development of colitis in mice. Exp Parasitol (2007) 116:385–9. doi:10.1016/j.exppara.2007.02.009
63. Xia C-M, Zhao Y, Jiang L, Jiang J, Zhang S-C. Schistosoma japonicum ova maintains epithelial barrier function during experimental colitis. World J Gastroenterol (2011) 17:4810–6. doi:10.3748/wjg.v17.i43.4810
64. Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie ANJ, van Rooijen N, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol (2007) 178:4557–66. doi:10.4049/jimmunol.178.7.4557
65. Cho MK, Lee CH, Yu HS. Amelioration of intestinal colitis by macrophage migration inhibitory factor isolated from intestinal parasites through toll-like receptor 2. Parasite Immunol (2011) 33:265–75. doi:10.1111/j.1365-3024.2010.01276.x
66. Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, et al. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol (2008) 180:4265–72. doi:10.4049/jimmunol.180.6.4265
67. Reyes JL, Fernando MR, Lopes F, Leung G, Mancini NL, Matisz CE, et al. IL-22 restrains tapeworm-mediated protection against experimental colitis via regulation of IL-25 expression. PLoS Pathog (2016) 12:e1005481. doi:10.1371/journal.ppat.1005481
68. Ramanan D, Bowcutt R, Lee SC, Tang MS, Kurtz ZD, Ding Y, et al. Helminth infection promotes colonization resistance via type 2 immunity. Science (2016) 352:608–12. doi:10.1126/science.aaf3229
69. Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol (2004) 34:2690–8. doi:10.1002/eji.200324833
70. Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, et al. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol (2003) 15:59–69. doi:10.1093/intimm/dxg012
71. La Flamme AC, Ruddenklau K, Backstrom BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun (2003) 71:4996–5004. doi:10.1128/IAI.71.9.4996-5004.2003
72. Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, Stosic-Grujicic S, Milic M, Sofronic-Milosavljevic L. Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in Dark Agouti rats. Parasite Immunol (2010) 32:450–9. doi:10.1111/j.1365-3024.2010.01207.x
73. Walsh KP, Brady MT, Finlay CM, Boon L, Mills KHG. Infection with a helminth parasite attenuates autoimmunity through TGF-β-mediated suppression of Th17 and Th1 responses. J Immunol (2009) 183:1577–86. doi:10.4049/jimmunol.0803803
74. Zheng X, Hu X, Zhou G, Lu Z, Qiu W, Bao J, et al. Soluble egg antigen from Schistosoma japonicum modulates the progression of chronic progressive experimental autoimmune encephalomyelitis via Th2-shift response. J Neuroimmunol (2008) 194:107–14. doi:10.1016/j.jneuroim.2007.12.001
75. Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJ, et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol (1999) 21:169–76. doi:10.1046/j.1365-3024.1999.00213.x
76. Zaccone P, Fehérvári Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol (2003) 33:1439–49. doi:10.1002/eji.200323910
77. Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun (2007) 75:397–407. doi:10.1128/IAI.00664-06
78. Liu Q, Sundar K, Mishra PK, Mousavi G, Liu Z, Gaydo A, et al. Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect Immun (2009) 77:5347–58. doi:10.1128/IAI.01170-08
79. Veenstra AA, Tang J, Kern TS. Antagonism of CD11b with neutrophil inhibitory factor (NIF) inhibits vascular lesions in diabetic retinopathy. PLoS One (2013) 8:e78405. doi:10.1371/journal.pone.0078405
80. Osada Y, Shimizu S, Kumagai T, Yamada S, Kanazawa T. Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol (2009) 39:457–64. doi:10.1016/j.ijpara.2008.08.007
81. He Y, Li J, Zhuang W, Yin L, Chen C, Li J, et al. The inhibitory effect against collagen-induced arthritis by Schistosoma japonicum infection is infection stage-dependent. BMC Immunol (2010) 11:28. doi:10.1186/1471-2172-11-28
82. Rzepecka J, Pineda MA, Al-Riyami L, Rodgers DT, Huggan JK, Lumb FE, et al. Prophylactic and therapeutic treatment with a synthetic analogue of a parasitic worm product prevents experimental arthritis and inhibits IL-1β production via NRF2-mediated counter-regulation of the inflammasome. J Autoimmun (2015) 60:59–73. doi:10.1016/j.jaut.2015.04.005
83. Salinas-Carmona MC, de la Cruz-Galicia G, Perez-Rivera I, Solis-Soto JM, Segoviano-Ramirez JC, Vazquez AV, et al. Spontaneous arthritis in MRL/lpr mice is aggravated by Staphylococcus aureus and ameliorated by Nippostrongylus brasiliensis infections. Autoimmunity (2009) 42:25–32. doi:10.1080/08916930802228290
84. Rodgers DT, Pineda MA, Suckling CJ, Harnett W, Harnett MM. Drug-like analogues of the parasitic worm-derived immunomodulator ES-62 are therapeutic in the MRL/Lpr model of systemic lupus erythematosus. Lupus (2015) 24:1437–42. doi:10.1177/0961203315591031
85. Kahana E. Epidemiologic studies of multiple sclerosis: a review. Biomed Pharmacother (2000) 54:100–2. doi:10.1016/S0753-3322(00)88859-9
86. Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron (1999) 24:511–4. doi:10.1016/S0896-6273(00)81107-1
87. Kuchroo VK, Sobel RA, Laning JC, Martin CA, Greenfield E, Dorf ME, et al. Experimental allergic encephalomyelitis mediated by cloned T cells specific for a synthetic peptide of myelin proteolipid protein. Fine specificity and T cell receptor V beta usage. J Immunol (1992) 148:3776–82.
88. Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol (2009) 9:393–407. doi:10.1038/nri2550
89. Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med (1995) 181:381–6. doi:10.1084/jem.181.1.381
90. El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol (2011) 12:568–75. doi:10.1038/ni.2031
91. Pare A, Mailhot B, Levesque SA, Lacroix S. Involvement of the IL-1 system in experimental autoimmune encephalomyelitis and multiple sclerosis: breaking the vicious cycle between IL-1beta and GM-CSF. Brain Behav Immun (2017) 62:1–8. doi:10.1016/j.bbi.2016.07.146
92. Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A. Parasitic worms and inflammatory diseases. Parasite Immunol (2006) 28:515–23. doi:10.1111/j.1365-3024.2006.00879.x
93. Nakhooda AF, Like AA, Chappel CI, Murray FT, Marliss EB. The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes (1977) 26:100–12. doi:10.2337/diabetes.26.2.100
94. Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu (1980) 29:1–13.
95. Zaccone P, Hall SW. Helminth infection and type 1 diabetes. Rev Diabet Stud (2012) 9:272–86. doi:10.1900/RDS.2012.9.272
96. Healey D, Ozegbe P, Arden S, Chandler P, Hutton J, Cooke A. In vivo activity and in vitro specificity of CD4+ Th1 and Th2 cells derived from the spleens of diabetic NOD mice. J Clin Invest (1995) 95:2979–85. doi:10.1172/JCI118006
97. Garcia-Hernandez MH, Gonzalez-Amaro R, Portales-Perez DP. Specific therapy to regulate inflammation in rheumatoid arthritis: molecular aspects. Immunotherapy (2014) 6:623–36. doi:10.2217/imt.14.26
98. Caplazi P, Baca M, Barck K, Carano RA, DeVoss J, Lee WP, et al. Mouse models of rheumatoid arthritis. Vet Pathol (2015) 52:819–26. doi:10.1177/0300985815588612
99. Summers RW, Elliott DE, Qadir K, Urban JF, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol (2003) 98:2034–41. doi:10.1111/j.1572-0241.2003.07660.x
100. Summers RW, Elliott DE, Urban JF Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology (2005) 128:825–32. doi:10.1053/j.gastro.2005.01.005
101. Van Kruiningen HJ, West AB. Potential danger in the medical use of Trichuris suis for the treatment of inflammatory bowel disease. Inflamm Bowel Dis (2005) 11:515. doi:10.1097/01.MIB.0000160369.47671.a2
102. Croese J, O’Neil J, Masson J, Cooke S, Melrose W, Pritchard D, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut (2006) 55:136–7. doi:10.1136/gut.2005.079129
103. Mortimer K, Brown A, Feary J, Jagger C, Lewis S, Antoniak M, et al. Dose-ranging study for trials of therapeutic infection with Necator americanus in humans. Am J Trop Med Hyg (2006) 75:914–20. doi:10.4269/ajtmh.2006.75.914
104. Croese J, Giacomin P, Navarro S, Clouston A, McCann L, Dougall A, et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J Allergy Clin Immunol (2015) 135:508–16. doi:10.1016/j.jaci.2014.07.022
105. Daveson AJ, Jones DM, Gaze S, McSorley H, Clouston A, Pascoe A, et al. Effect of hookworm infection on wheat challenge in celiac disease – a randomised double-blinded placebo controlled trial. PLoS One (2011) 6:e17366. doi:10.1371/journal.pone.0017366
106. McSorley HJ, Gaze S, Daveson J, Jones D, Anderson RP, Clouston A, et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS One (2011) 6:e24092. doi:10.1371/journal.pone.0024092
107. Sandborn WJ, Elliott DE, Weinstock J, Summers RW, Landry-Wheeler A, Silver N, et al. Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn’s disease. Aliment Pharmacol Ther (2013) 38:255–63. doi:10.1111/apt.12366
108. Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol (2007) 61:97–108. doi:10.1002/ana.21067
109. Correale J, Farez MF. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol (2011) 233:6–11. doi:10.1016/j.jneuroim.2011.01.002
110. Fleming JO, Isaak A, Lee JE, Luzzio CC, Carrithers MD, Cook TD, et al. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult Scler (2011) 17:743–54. doi:10.1177/1352458511398054
111. Voldsgaard A, Bager P, Garde E, Akeson P, Leffers AM, Madsen CG, et al. Trichuris suis ova therapy in relapsing multiple sclerosis is safe but without signals of beneficial effect. Mult Scler (2015) 21:1723–9. doi:10.1177/1352458514568173
112. Fleming JO, Weinstock JV. Clinical trials of helminth therapy in autoimmune diseases: rationale and findings. Parasite Immunol (2015) 37:277–92. doi:10.1111/pim.12175
113. Crompton DW. The public health importance of hookworm disease. Parasitology (2000) 121(Suppl):S39–50. doi:10.1017/S0031182000006454
114. Mulvenna J, Hamilton B, Nagaraj SH, Smyth D, Loukas A, Gorman JJ. Proteomics analysis of the excretory/secretory component of the blood-feeding stage of the hookworm, Ancylostoma caninum. Mol Cell Proteomics (2009) 8:109–21. doi:10.1074/mcp.M800206-MCP200
115. Morphew RM, Wright HA, LaCourse EJ, Woods DJ, Brophy PM. Comparative proteomics of excretory–secretory proteins released by the liver fluke Fasciola hepatica in sheep host bile and during in vitro culture ex host. Mol Cell Proteomics (2007) 6:963–72. doi:10.1074/mcp.M600375-MCP200
116. Robinson MW, Greig R, Beattie KA, Lamont DJ, Connolly B. Comparative analysis of the excretory–secretory proteome of the muscle larva of Trichinella pseudospiralis and Trichinella spiralis. Int J Parasitol (2007) 37:139–48. doi:10.1016/j.ijpara.2006.08.007
117. Yatsuda AP, Krijgsveld J, Cornelissen AW, Heck AJ, de Vries E. Comprehensive analysis of the secreted proteins of the parasite Haemonchus contortus reveals extensive sequence variation and differential immune recognition. J Biol Chem (2003) 278:16941–51. doi:10.1074/jbc.M212453200
118. Hewitson JP, Harcus YM, Curwen RS, Dowle AA, Atmadja AK, Ashton PD, et al. The secretome of the filarial parasite, Brugia malayi: proteomic profile of adult excretory–secretory products. Mol Biochem Parasitol (2008) 160:8–21. doi:10.1016/j.molbiopara.2008.02.007
119. Craig H, Wastling JM, Knox DP. A preliminary proteomic survey of the in vitro excretory/secretory products of fourth-stage larval and adult Teladorsagia circumcincta. Parasitology (2006) 132:535–43. doi:10.1017/S0031182005009510
120. Cass CL, Johnson JR, Califf LL, Xu T, Hernandez HJ, Stadecker MJ, et al. Proteomic analysis of Schistosoma mansoni egg secretions. Mol Biochem Parasitol (2007) 155:84–93. doi:10.1016/j.molbiopara.2007.06.002
121. Cantacessi C, Mitreva M, Jex AR, Young ND, Campbell BE, Hall RS, et al. Massively parallel sequencing and analysis of the Necator americanus transcriptome. PLoS Negl Trop Dis (2010) 4:e684. doi:10.1371/journal.pntd.0000684
122. Schwarz EM, Hu Y, Antoshechkin I, Miller MM, Sternberg PW, Aroian RV. The genome and transcriptome of the zoonotic hookworm Ancylostoma ceylanicum identify infection-specific gene families. Nat Genet (2015) 47:416–22. doi:10.1038/ng.3237
123. Tang YT, Gao X, Rosa BA, Abubucker S, Hallsworth-Pepin K, Martin J, et al. Genome of the human hookworm Necator americanus. Nat Genet (2014) 46:261–9. doi:10.1038/ng.2875
124. Harnett W, Worms MJ, Kapil A, Grainger M, Parkhouse RME. Origin, kinetics of circulation and fate in vivo of the major excretory–secretory product of Acanthocheilonema viteae. Parasitology (1989) 99:229–39. doi:10.1017/S0031182000058686
125. Harnett W, Harnett MM. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory–secretory product. J Immunol (1993) 151:4829–37.
126. Harnett MM, Deehan MR, Williams DM, Harnett W. Induction of signalling anergy via the T-cell receptor in cultured Jurkat T cells by pre-exposure to a filarial nematode secreted product. Parasite Immunol (1998) 20:551–63. doi:10.1046/j.1365-3024.1998.00181.x
127. Houston KM, Wilson EH, Eyres L, Brombacher F, Harnett MM, Alexander J, et al. Presence of phosphorylcholine on a filarial nematode protein influences immunoglobulin G subclass response to the molecule by an interleukin-10-dependent mechanism. Infect Immun (2000) 68:5466–8. doi:10.1128/IAI.68.9.5466-5468.2000
128. Wilson EH, Katz E, Goodridge HS, Harnett MM, Harnett W. In vivo activation of murine peritoneal B1 cells by the filarial nematode phosphorylcholine-containing glycoprotein ES-62. Parasite Immunol (2003) 25:463–6. doi:10.1111/j.1365-3024.2003.00650.x
129. McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W, et al. Approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol (2003) 171:2127–33. doi:10.4049/jimmunol.171.4.2127
130. Anbu KA, Joshi P. Identification of a 55 kDa Haemonchus contortus excretory/secretory glycoprotein as a neutrophil inhibitory factor. Parasite Immunol (2008) 30:23–30. doi:10.1111/j.1365-3024.2007.00995.x
131. Rieu P, Ueda T, Haruta I, Sharma CP, Arnaout MA. The A-domain of beta 2 integrin CR3 (CD11b/CD18) is a receptor for the hookworm-derived neutrophil adhesion inhibitor NIF. J Cell Biol (1994) 127:2081–91. doi:10.1083/jcb.127.6.2081
132. Madden K, Janczak J, McEnroe G, Lim D, Hartman T, Liu D, et al. A peptide derived from neutrophil inhibitory factor (NIF) blocks neutrophil adherence to endothelial cells. Inflamm Res (1997) 46:216–23. doi:10.1007/s000110050176
133. Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo J-M, Ford GA, et al. Acute stroke therapy by inhibition of neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke (2003) 34:2543–8. doi:10.1161/01.STR.0000092527.33910.89
134. Schnyder-Candrian S, Maillet I, Le Bert M, Brault L, Jacobs M, Ryffel B, et al. Neutrophil inhibitory factor selectively inhibits the endothelium-driven transmigration of eosinophils in vitro and airway eosinophilia in OVA-induced allergic lung inflammation. J Allergy (Cairo) (2012) 2012:245909. doi:10.1155/2012/245909
135. Jefferies JR, Turner RJ, Barrett J. Effect of Fasciola hepatica excretory–secretory products on the metabolic burst of sheep and human neutrophils. Int J Parasitol (1997) 27:1025–9. doi:10.1016/S0020-7519(97)00067-2
136. Pastrana DV, Raghavan N, FitzGerald P, Eisinger SW, Metz C, Bucala R, et al. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun (1998) 66:5955–63.
137. Zang X, Taylor P, Wang JM, Meyer DJ, Scott AL, Walkinshaw MD, et al. Homologues of human macrophage migration inhibitory factor from a parasitic nematode: gene cloning, protein activity and crystal structure. J Biol Chem (2002) 277:44261–7. doi:10.1074/jbc.M204655200
138. Cho Y, Jones BF, Vermeire JJ, Leng L, DiFedele L, Harrison LM, et al. Structural and functional characterization of a secreted hookworm macrophage migration inhibitory factor (MIF) that interacts with the human MIF receptor CD74. J Biol Chem (2007) 282:23447–56. doi:10.1074/jbc.M702950200
139. Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, et al. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A (1996) 93:7849–54. doi:10.1073/pnas.93.15.7849
140. Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol (1998) 160:5693–6.
141. Mikayama T, Nakano T, Gomi H, Nakagawa Y, Liu YC, Sato M, et al. Molecular cloning and functional expression of a cDNA encoding glycosylation-inhibiting factor. Proc Natl Acad Sci U S A (1993) 90:10056–60. doi:10.1073/pnas.90.21.10056
142. Park SK, Cho MK, Park H-K, Lee KH, Lee SJ, Choi SH, et al. Macrophage migration inhibitory factor homologs of Anisakis simplex suppress Th2 response in allergic airway inflammation model via CD4+CD25+Foxp3+ T cell recruitment. J Immunol (2009) 182:6907–14. doi:10.4049/jimmunol.0803533
143. Hartmann S, Kyewski B, Sonnenburg B, Lucius R. A filarial cysteine protease inhibitor down-regulates T cell proliferation and enhances interleukin-10 production. Eur J Immunol (1997) 27:2253–60. doi:10.1002/eji.1830270920
144. Lustigman S, Brotman B, Huima T, Prince AM, McKerrow JH. Molecular cloning and characterization of onchocystatin, a cysteine proteinase inhibitor of Onchocerca volvulus. J Biol Chem (1992) 267:17339–46.
145. Manoury B, Gregory WF, Maizels RM, Watts C. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr Biol (2001) 11:447–51. doi:10.1016/S0960-9822(01)00118-X
146. Dainichi T, Maekawa Y, Ishii K, Zhang T, Nashed BF, Sakai T, et al. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen-specific immune response. Infect Immun (2001) 69:7380–6. doi:10.1128/IAI.69.12.7380-7386.2001
147. Klotz C, Ziegler T, Figueiredo AS, Rausch S, Hepworth MR, Obsivac N, et al. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLoS Pathog (2011) 7:e1001248. doi:10.1371/journal.ppat.1001248
148. Whelan RA, Rausch S, Ebner F, Günzel D, Richter JF, Hering NA, et al. A transgenic probiotic secreting a parasite immunomodulator for site-directed treatment of gut inflammation. Mol Ther (2014) 22:1730–40. doi:10.1038/mt.2014.125
149. Alvarado R, O’Brien B, Tanaka A, Dalton JP, Donnelly S. A parasitic helminth-derived peptide that targets the macrophage lysosome is a novel therapeutic option for autoimmune disease. Immunobiology (2015) 220:262–9. doi:10.1016/j.imbio.2014.11.008
150. Mookherjee N, Hancock RE. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci (2007) 64:922–33. doi:10.1007/s00018-007-6475-6
151. Robinson MW, Donnelly S, Hutchinson AT, To J, Taylor NL, Norton RS, et al. A family of helminth molecules that modulate innate cell responses via molecular mimicry of host antimicrobial peptides. PLoS Pathog (2011) 7:e1002042. doi:10.1371/journal.ppat.1002042
152. Alvarado R, To J, Lund ME, Pinar A, Mansell A, Robinson MW, et al. The immune modulatory peptide FhHDM-1 secreted by the helminth Fasciola hepatica prevents NLRP3 inflammasome activation by inhibiting endolysosomal acidification in macrophages. FASEB J (2017) 31(1):85–95. doi:10.1096/fj.201500093R
153. Balloul JM, Sondermeyer P, Dreyer D, Capron M, Grzych JM, Pierce RJ, et al. Molecular cloning of a protective antigen of schistosomes. Nature (1987) 326:149–53. doi:10.1038/326149a0
154. Riveau G, Deplanque D, Remoue F, Schacht AM, Vodougnon H, Capron M, et al. Safety and immunogenicity of rSh28GST antigen in humans: phase 1 randomized clinical study of a vaccine candidate against urinary schistosomiasis. PLoS Negl Trop Dis (2012) 6:e1704. doi:10.1371/journal.pntd.0001704
155. Cancado GG, Fiuza JA, de Paiva NC, Lemos Lde C, Ricci ND, Gazzinelli-Guimaraes PH, et al. Hookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c mice. Inflamm Bowel Dis (2011) 17:2275–86. doi:10.1002/ibd.21629
156. Navarro S, Pickering DA, Ferreira IB, Jones L, Ryan S, Troy S, et al. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci Transl Med (2016) 8:362ra143. doi:10.1126/scitranslmed.aaf8807
157. Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol (1998) 16:137–61. doi:10.1146/annurev.immunol.16.1.137
158. Johnston CJ, Smyth DJ, Dresser DW, Maizels RM. TGF-beta in tolerance, development and regulation of immunity. Cell Immunol (2016) 299:14–22. doi:10.1016/j.cellimm.2015.10.006
159. Brand AM, Varghese G, Majewski W, Hawdon JM. Identification of a DAF-7 ortholog from the hookworm Ancylostoma caninum. Int J Parasitol (2005) 35:1489–98. doi:10.1016/j.ijpara.2005.07.004
160. Gomez-Escobar N, Gregory WF, Maizels RM. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor beta, expressed in microfilarial and adult stages of Brugia malayi. Infect Immun (2000) 68:6402–10. doi:10.1128/IAI.68.11.6402-6410.2000
161. Gomez-Escobar N, Lewis E, Maizels RM. A novel member of the transforming growth factor-beta (TGF-beta) superfamily from the filarial nematodes Brugia malayi and B. pahangi. Exp Parasitol (1998) 88:200–9. doi:10.1006/expr.1998.4248
162. Japa O, Hodgkinson JE, Emes RD, Flynn RJ. TGF-beta superfamily members from the helminth Fasciola hepatica show intrinsic effects on viability and development. Vet Res (2015) 46:29. doi:10.1186/s13567-015-0167-2
163. McSorley HJ, Grainger JR, Harcus Y, Murray J, Nisbet AJ, Knox DP, et al. daf-7-related TGF-beta homologues from trichostrongyloid nematodes show contrasting life-cycle expression patterns. Parasitology (2010) 137:159–71. doi:10.1017/S0031182009990321
164. Freitas TC, Jung E, Pearce EJ. TGF-beta signaling controls embryo development in the parasitic flatworm Schistosoma mansoni. PLoS Pathog (2007) 3:e52. doi:10.1371/journal.ppat.0030052
165. Freitas TC, Jung E, Pearce EJ. A bone morphogenetic protein homologue in the parasitic flatworm, Schistosoma mansoni. Int J Parasitol (2009) 39:281–7. doi:10.1016/j.ijpara.2008.08.001
166. Liu R, Zhao QP, Ye Q, Xiong T, Tang CL, Dong HF, et al. Cloning and characterization of a bone morphogenetic protein homologue of Schistosoma japonicum. Exp Parasitol (2013) 135:64–71. doi:10.1016/j.exppara.2013.05.016
167. Fusco AC, Salafsky B, Kevin MB. Schistosoma mansoni: eicosanoid production by cercariae. Exp Parasitol (1985) 59:44–50. doi:10.1016/0014-4894(85)90055-4
168. Leid RW, McConnell LA. PGE2 generation and release by the larval stage of the cestode, Taenia taeniaeformis. Prostaglandins Leukot Med (1983) 11:317–23. doi:10.1016/0262-1746(83)90043-4
169. Liu LX, Serhan CN, Weller PF. Intravascular filarial parasites elaborate cyclooxygenase-derived eicosanoids. J Exp Med (1990) 172:993–6. doi:10.1084/jem.172.3.993
170. Belley A, Chadee K. Eicosanoid production by parasites: from pathogenesis to immunomodulation? Parasitol Today (1995) 11:327–34. doi:10.1016/0169-4758(95)80185-5
171. Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol (2012) 188:21–8. doi:10.4049/jimmunol.1101029
172. Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol (2013) 35:123–37. doi:10.1007/s00281-012-0342-8
173. Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol (2001) 2:612–9. doi:10.1038/89759
174. Cohn SM, Schloemann S, Tessner T, Seibert K, Stenson WF. Crypt stem cell survival in the mouse intestinal epithelium is regulated by prostaglandins synthesized through cyclooxygenase-1. J Clin Invest (1997) 99:1367–79. doi:10.1172/JCI119296
175. Dauplais M, Lecoq A, Song J, Cotton J, Jamin N, Gilquin B, et al. On the convergent evolution of animal toxins. Conservation of a diad of functional residues in potassium channel-blocking toxins with unrelated structures. J Biol Chem (1997) 272:4302–9. doi:10.1074/jbc.272.7.4302
176. Rangaraju S, Khoo KK, Feng Z-P, Crossley G, Nugent D, Khaytin I, et al. Potassium channel modulation by a toxin domain in matrix metalloprotease 23. J Biol Chem (2010) 285:9124–36. doi:10.1074/jbc.M109.071266
177. Chhabra S, Chang SC, Nguyen HM, Huq R, Tanner MR, Londono LM, et al. Kv1.3 channel-blocking immunomodulatory peptides from parasitic worms: implications for autoimmune diseases. FASEB J (2014) 28:3952–64. doi:10.1096/fj.14-251967
178. Castañeda O, Sotolongo V, Amor AM, Stöcklin R, Anderson AJ, Harvey AL, et al. Characterization of a potassium channel toxin from the Caribbean sea anemone Stichodactyla helianthus. Toxicon (1995) 33:603–13. doi:10.1016/0041-0101(95)00013-C
179. Pennington MW, Byrnes ME, Zaydenberg I, Khaytin I, de Chastonay J, Krafte DS, et al. Chemical synthesis and characterization of ShK toxin: a potent potassium channel inhibitor from a sea anemone. Int J Pept Protein Res (1995) 46:354–8. doi:10.1111/j.1399-3011.1995.tb01068.x
180. DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature (1984) 307:465–8. doi:10.1038/307465a0
181. Chandy KG, DeCoursey TE, Cahalan MD, McLaughlin C, Gupta S. Voltage-gated potassium channels are required for human T lymphocyte activation. J Exp Med (1984) 160:369–85. doi:10.1084/jem.160.2.369
182. Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Beeton C, et al. The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J Clin Invest (2003) 111:1703–13. doi:10.1172/JCI16921
183. Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci U S A (2006) 103:17414–9. doi:10.1073/pnas.0605136103
184. Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de Fougerolles A, et al. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med (2007) 13:836–42. doi:10.1038/nm1605
185. Kivisakk P, Mahad DJ, Callahan MK, Sikora K, Trebst C, Tucky B, et al. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann Neurol (2004) 55:627–38. doi:10.1002/ana.20049
186. Steinfelder S, O’Regan NL, Hartmann S. Diplomatic assistance: can helminth-modulated macrophages act as treatment for inflammatory disease? PLoS Pathog (2016) 12:e1005480. doi:10.1371/journal.ppat.1005480
187. Whelan RA, Rausch S, Ebner F, Gunzel D, Richter JF, Hering NA, et al. A transgenic probiotic secreting a parasite immunomodulator for site-directed treatment of gut inflammation. Mol Ther (2014) 22:1730–40. doi:10.1038/mt.2014.125
Keywords: helminthic therapy, autoimmunity, immunomodulation, excretory/secretory products, immunotherapy
Citation: Smallwood TB, Giacomin PR, Loukas A, Mulvenna JP, Clark RJ and Miles JJ (2017) Helminth Immunomodulation in Autoimmune Disease. Front. Immunol. 8:453. doi: 10.3389/fimmu.2017.00453
Received: 30 January 2017; Accepted: 03 April 2017;
Published: 24 April 2017
Edited by:
Anne Cooke, University of Cambridge, UKReviewed by:
Henry Mcsorley, University of Edinburgh, UKCopyright: © 2017 Smallwood, Giacomin, Loukas, Mulvenna, Clark and Miles. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John J. Miles, am9obi5taWxlc0BqY3UuZWR1LmF1
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.