
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 18 April 2017
Sec. T Cell Biology
Volume 8 - 2017 | https://doi.org/10.3389/fimmu.2017.00419
For protection against pathogens, it is essential that naïve CD4+ T cells differentiate into specific effector T helper (Th) cell subsets following activation by antigen presented by dendritic cells (DCs). Next to T cell receptor and cytokine signals, membrane-bound Notch ligands have an important role in orchestrating Th cell differentiation. Several studies provided evidence that DC activation is accompanied by surface expression of Notch ligands. Intriguingly, DCs that express the delta-like or Jagged Notch ligands gain the capacity to instruct Th1 or Th2 cell polarization, respectively. However, in contrast to this model it has also been hypothesized that Notch signaling acts as a general amplifier of Th cell responses rather than an instructive director of specific T cell fates. In this alternative model, Notch enhances proliferation, cytokine production, and anti-apoptotic signals or promotes co-stimulatory signals in T cells. An instructive role for Notch ligand expressing DCs in the induction of Th cell differentiation is further challenged by evidence for the involvement of Notch signaling in differentiation of Th9, Th17, regulatory T cells, and follicular Th cells. In this review, we will discuss the two opposing models, referred to as the “instructive” and the “unbiased amplifier” model. We highlight both the function of different Notch receptors on CD4+ T cells and the impact of Notch ligands on antigen-presenting cells.
Following signals from both antigen-presenting cells (APCs) and the micro-environment, activated CD4+ T cells are triggered to initiate secretion of specific effector cytokines. Since the original observation in 1986 upon antigenic stimulation naive CD4+ T cells can differentiate into T helper 1 (Th1) or Th2 effector T cells depending on polarizing cytokine signals (1), various additional Th subsets have been recognized. These include Th9, Th17, Th22, follicular T helper cells (Tfh), and regulatory T cells (Tregs), each characterized by a unique cytokine production profile and a key transcription factor [see for recent review Ref. (2)]. These Th subsets play a crucial role in appropriate immune responses during host defense, but are also involved in the pathogenesis of inflammatory diseases (3, 4).
Th1 cells mainly produce IFN-γ and TNF-α and are associated with the elimination of intracellular pathogens. Th1 development is facilitated either by IL-12 and STAT4 or by IFN-γ, STAT1, and the key Th1 transcriptional regulator T-box-containing protein (T-bet), encoded by Tbx21 (5). Th2 cells control helminth infections and are implicated in allergic immune responses such as allergic asthma. They are potent producers of Th2 cytokines that induce IgE synthesis (IL-4), recruit eosinophils (IL-5), and cause smooth muscle hyperreactivity and goblet cell hyperplasia (IL-13). Therefore, Th2 cells are central in the orchestration and amplification of inflammatory events in allergic asthma. The master transcription factor Gata3 is necessary and sufficient for Th2 cytokine gene expression in Th2 cells (6). Because Th2 differentiation is driven by IL-4, this raises the paradox that IL-4 is required to generate the cell type that is its major producer. But the origin of the first IL-4 required for Th2 cell induction remains unclear. While a range of cell types are able to produce IL-4, Th2 cell responses can still be generated when only T cells can make IL-4, arguing against an essential role for an external source of IL-4 (7, 8).
An accumulating number of studies suggest that the Notch signaling pathway, which also plays a crucial role in early hematopoietic development and at multiple steps of T lineage development, is essential for Th cell differentiation [for recent review see Ref. (9)]. Currently, two opposing models have been proposed that explain how Notch ligands can influence Th subset differentiation. According to the “instructive” model, Jagged and delta-like ligands (DLL) on APCs induce Th2 and Th1 differentiation, respectively (10). Alternatively, the “unbiased amplifier” model proposes that Notch ligands are not instructive but rather function to generally amplify Th cell responses (11). In this review, we will discuss these two contrasting hypotheses on the role of Notch signaling. We will focus on both Notch receptor expressing T cells and Notch ligand-expressing cells.
There are five Notch ligands: two Jagged (Jagged1 and Jagged2) and three DLL (DLL1, DLL3, and DLL4), which are bound by four receptors, Notch1–4. For these ligands to be functional, their ubiquitination by Mindbomb1 or Neuralized within the cell is required (12). Details of the Notch signaling pathway are discussed in various excellent reviews (13, 14). Briefly, following ligand–receptor binding, the Notch intracellular domain (NICD) is cleaved by a γ-secretase complex and translocates to the nucleus and binds to the transcription factor recombination signal binding protein for immunoglobulin Jκ region (RBPJκ; Figure 1). Finally, additional co-activating proteins are recruited, such as mastermind-like proteins (MAML1-3) and p300 to induce transcription of target genes. Notch signaling does not only induce Th lineage-defining transcription factors and cytokines (described below) but also general pathways critical for T cell activation, including IL-2 production, upregulation of the IL-2 receptor, and glucose uptake (15–18). Notch signaling potentiates phosphatidylinositol 3-kinase-dependent signaling downstream of the T cell receptor (TCR) and CD28 by inducing activation of Akt kinase and mammalian target of rapamycin, which enhances T cell effector functions and survival and allows them to respond to lower antigen doses (16, 19, 20). Notch signaling can be enhanced by the protein kinase PKCθ, which is crucial for TCR and CD28 signaling and regulation of the actin cytoskeleton (21). Moreover, upon TCR stimulation NICD interacts with other proteins in the cell in a non-canonical, RBPJκ-independent pathway that leads to NFκB activation (22, 23).
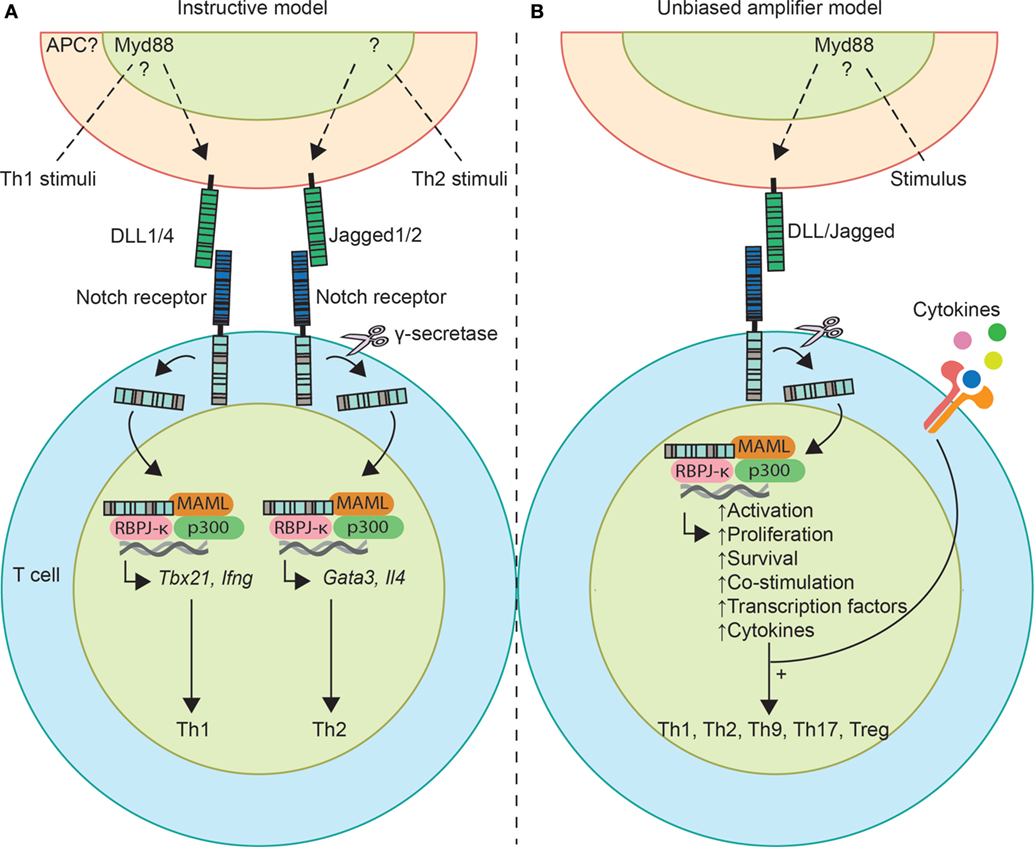
Figure 1. Schematic overview of the two models describing the role of Notch signaling in T helper (Th) cell differentiation. (A) According to the instructive model, Th1-stimuli and Th2-stimuli induce delta-like ligands (DLL) and Jagged ligand expression on antigen-presenting cells (APCs), respectively. Upon receptor–ligand binding, Th1 differentiation is induced by Notch intracellular domain binding and activating transcription of the Th1 transcription factor gene Tbx21 and signature cytokine Ifng. For Th2 differentiation, Notch induces transcription of Gata3 and Il4. (B) Notch ligands act as an unbiased amplifier, thereby sensitizing cells to the environment to ensure that activated CD4+ T cells overcome a Th cell commitment threshold. Notch induces activation, proliferation, enhances anti-apoptotic signals, and is simultaneously recruited to Th1, Th2, and Th17 genes. So, in this hypothesis Notch acts as an enabler of differentiation, whereby the outcome depends on signals of the environment, such as cytokines.
T helper 2-promoting stimuli including helminth eggs, prostaglandin E2, cholera toxin, and allergens, such as house dust mite (HDM), birch pollen, and cockroach allergens, were shown to induce Jagged expression on APCs, as summarized in Table 1. Conversely, microbial Th1-inducing stimuli, e.g., dengue virus, respiratory syncytial virus (RSV), bacterial LPS, and the TRL9 ligand CpG, up regulate the Notch ligands DLL1/DLL4 on APCs (Table 1). Other studies, however, do not show exclusive upregulation of either DLL or Jagged molecules, but rather upregulation of Notch ligands of both families upon stimulation (10, 24–33). Interestingly, whereas surface induction of DLL requires MyD88, this is not the case for Jagged induction (10, 34–38). LPS can promote both Th1 and Th2 responses, which are MyD88 dependent and Myd88 independent, respectively, but the molecular mechanisms responsible for Jagged induction by LPS are unknown (39–41). Together, although there is also evidence that particular stimuli can induce both Th1 and Th2 differentiations, many studies support an instructive role of DLL and Jagged expression on APCs.
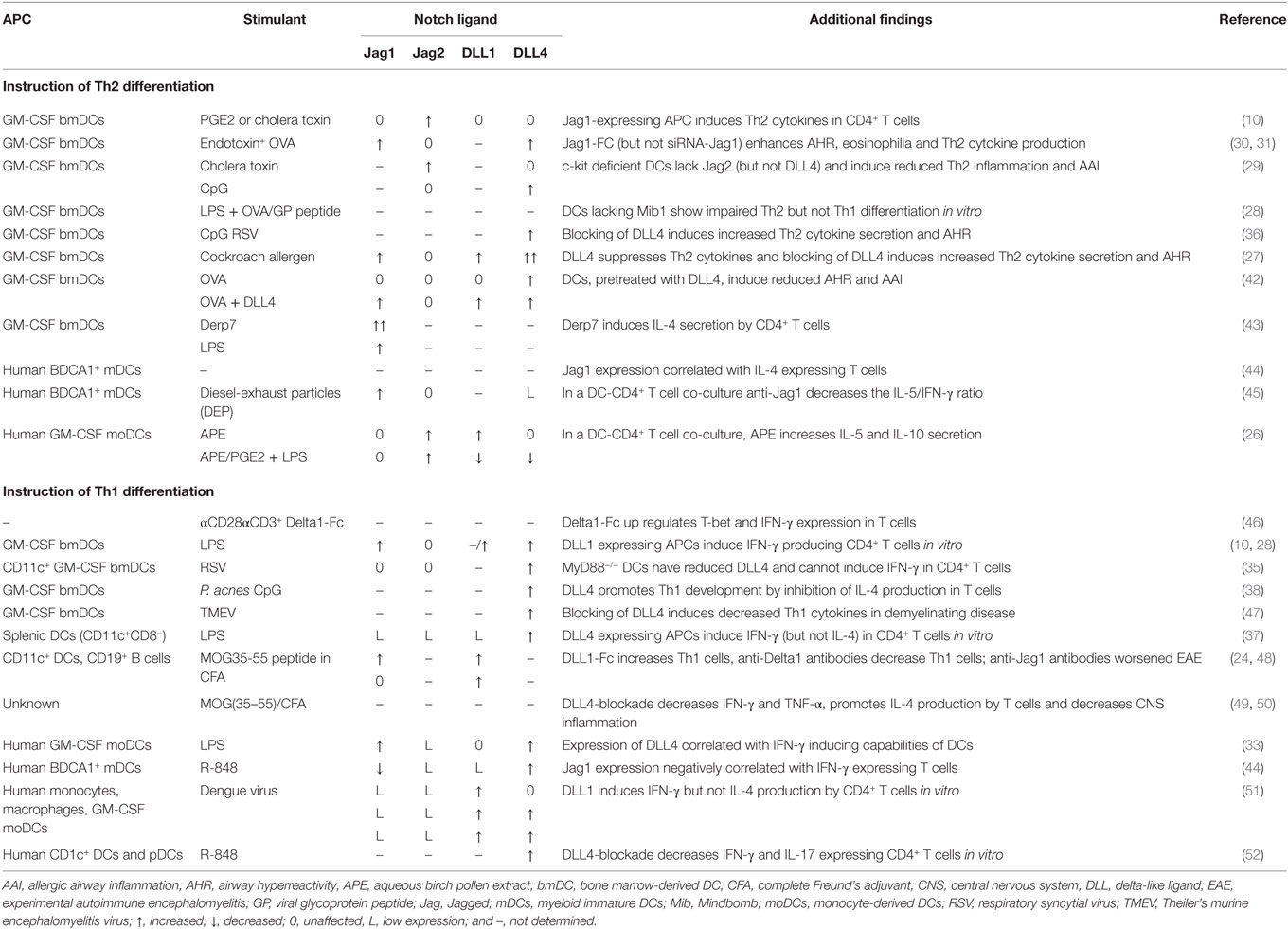
Table 1. Evidence supporting instructive roles for Jagged and DLL in Th2 and Th1 cell differentiation, respectively.
Notch signaling can initiate Th2 cell differentiation by direct activation of (i) a 3′ enhancer of the Il4 gene and (ii) an upstream promoter of Gata3 (10, 53–55). Several studies using mice expressing a dominant negative (DN) MAML transgene have demonstrated that Notch signaling is essential for Th2 cell differentiation and function (54, 56). When γ-secretase inhibitors (GSI) were used to block Notch signaling in OVA-induced asthma or food allergy models, Th2 cytokine production by T cells was inhibited while IFN-γ production was increased (57–59). Moreover, upon gene ablation of Notch1/Notch2 or RBPJκ, IL-4 production was abrogated and functional responses against parasitical pathogens were reduced (10). At the same time, IFN-γ expression was unaffected, supporting an instructive role for Notch signaling. In line with an instructive model, DLL4 was demonstrated to have a regulatory role in Th2 responses to cockroach allergen, OVA, RSV, or Schistosoma mansoni egg antigen (Table 1) and in an experimental autoimmune encephalomyelitis (EAE) model (49). A protective Th1 response to RSV in the lungs was converted into an allergic Th2 response by DLL4-neutralization in vivo (36).
However, defective Th2 responses against the intestinal helminth Trichuris muris in DN-MAML transgenic mice were restored when mice received anti-IFN-γ antibodies, indicating that Notch functions to optimize rather than to initiate the Th2 response (11). Moreover, decreased Th2 responses were found when DLL4 was blocked in a mouse model for RSV-mediated allergic asthma exacerbations (60). Finally, we very recently found that whereas mice with RBPJκ-deficient T cells failed to develop HDM-driven allergic airway inflammation (AAI) and airway hyperreactivity, mice with a DC-specific conditional deficiency of both Jagged1 and Jagged2 developed normal AAI following in vivo HDM-exposure (32). Although most studies using bmDCs would support an instructive role for Jagged in the induction of Th2 cell differentiation and function (Table 1), our studies indicate that induction of Th2 responses in HDM-driven AAI is dependent on Jagged expression on other cell types than DCs or alternatively on cooperation between Jagged and DLL on DCs.
Taken together, although several lines of evidence indicate that DCs use the Notch pathway to instruct Th cell fates, Notch may also act as an unbiased amplifier of Th cell differentiation.
The signature Th1 genes Ifng and Tbx21 were identified as direct Notch targets (11, 61). Mice in which T cells were Notch1/Notch2 double-deficient showed impaired IFN-γ secretion by Th1 cells during in vivo Leishmania major parasite infection, but reports employing DN-MAML transgenic or conditional RBPJκ knockout mice demonstrated that Th1 cell function was unaffected (32, 53, 54, 56, 62). Therefore, these findings suggest that signals that regulate Th1 differentiation involve RBPJκ-independent functions of Notch. Studies using GSI showed that Th1 differentiation was impaired in an in vivo EAE model (11, 61). By contrast, an increase in Th1 differentiation (and a concomitant decrease in Th2 cytokine production) was seen in an OVA-driven AAI model (58). The interpretation of these apparently conflicting findings remains complicated, because effects of GSI are not limited to Notch signaling and, e.g., also involve HLA-A2 expression and cadherins (63).
The capacity of DLL1/DLL4 to induce Th1 cell differentiation is supported by many in vitro and in vivo experiments, as outlined in Table 1. For example, anti-DLL4 antibodies reduced IFN-γ and TNF-α secretion by T cells in vivo (47, 49, 50). DLL1-blockade decreased Th1 cell numbers in an allograft model (64). Conversely, Jagged1-Fc had no effect and anti-Jagged1 antibodies worsened EAE disease (24, 48). Gene ablation of Jagged1 or Mindbomb1, which is critical for expression of functional Notch ligands, did not affect Th1 differentiation in vitro (28, 30).
In conclusion, although most studies would support an instructive role for DLL1/DLL4 in Th1 induction, the role of Notch signaling in Th1 cell differentiation remains incompletely understood.
Given the increasing complexity of T cell subset biology, it is not unexpected that the bipotential instructional model is not sufficient to fully explain the function of Notch signaling in Th cell differentiation. For example, Notch signaling cooperates with TGF-β to induce Th9 cell differentiation and IL-9 expression via Jagged2 ligation (65). The Rorc, Il17, and Il23r gene promoters are direct Notch targets and, accordingly, Th17 cell differentiation is impaired when Notch signaling is blocked (66–70). Hereby, DLL1, DLL3, and DLL4 ligands were found to be essential (49, 50, 52, 60, 71), but a role for Jagged1 remains controversial (72–74). Remarkably, addition of DLL3 enhanced Th17 differentiation in vitro (75), although it was shown that DLL3 cannot activate Notch in adjacent cells, but inhibits signaling when expressed in the same cell as the Notch receptor (76). Differentiation and function of Tregs require Notch signaling in T cells (77–80), whereby both DLL and Jagged ligands can promote Treg expansion (81–88). Although the key Treg transcription factor Foxp3 is a direct Notch target (89), the role of Notch in Tregs seems rather complex, because targeting of DLL4 or Treg-specific components of the Notch pathway was associated with an increase of Tregs in in vivo autoimmune models (49, 90, 91). Moreover, hepatocytes and plasmacytoid DCs can induce IL-10 production in T cells via Jagged1 and DLL4, respectively (85, 92, 93). Finally, the finding that the absence of Notch receptors on T cells or DLL4 on lymph node stromal cells resulted in a deficiency of Tfh cells (94, 95), implicates Notch signaling in Tfh cell differentiation.
As summarized in Table 1, considerable evidence supports an “instructive model” whereby pathogens direct Th1 and Th2 differentiation via upregulation of DLL or Jagged ligands on DCs (Figure 1). This implies that different Notch ligands induce distinct cellular responses in T cells, largely by the same signaling components. Although it has been speculated that different ligands might induce qualitatively different signals, e.g., RBPJκ-dependent or independent, or signals that differ in strength or kinetics (96), the molecular mechanisms involved are currently unknown.
It has been shown that DLL4 induces a stronger Notch signal than DLL1 or Jagged1 (86). Also, the ability of ligands to induce Notch signaling is dependent on the glycosylation status of the extracellular domain of Notch: Notch receptors carrying N-acetylglucosamine preferentially signal via delta ligands, while Jagged binding is inhibited (97). Absence or overexpression of Fringe glycosyltransferase proteins alters Th1 and Th2 differentiation (60, 98). Another possibility would be that different ligands preferentially activate different Notch receptors, which may each have unique downstream nuclear targets to induce distinct cellular programs. Indeed, it has been reported that whereas Notch1 and Notch2 activate Th2 differentiation, Notch3 promotes Th1 differentiation and IFN-γ production (46, 53). The expression of all these Notch receptors is induced on T cells upon TCR stimulation (62, 99, 100). Because different NICDs have different target gene preferences (101), distinct ligand–receptor combinations may produce quantitatively or qualitatively distinct signals (102). However, this is not supported by the findings that both Th1 and Th2 differentiation is affected in T cells that are Notch1/Notch2 double-deficient (53, 62) and that retroviral expression of Notch1 as well as Notch3 was associated with increased Th1 responses (46, 61). This issue is further complicated by the observation that individual Notch receptors are up regulated with different kinetics (103). It is, therefore, conceivable that they have distinct functions depending on the phase of the response.
Several studies are in apparent conflict with the “instructive model.” For example, DLL were reported to promote Th2 responses or Jagged ligands were implicated in Th1 induction (60, 104). Neither Jagged1 nor DLL1 could instruct Th2 or Th1 cytokine differentiation in vitro in the absence of polarizing cytokines (105). Importantly, Bailis et al. showed that Notch signaling simultaneously induced Th1, Th2, and Th17 gene transcription, also under polarizing conditions that were described to favor only one of the differentiation outcomes (11). In addition, Notch signaling via DLL4 was shown to boost antigen sensitivity of CD4+ T cells via promoting co-stimulatory signals in T cells (16). Together, this would suggest that Notch acts as a co-stimulating factor that orchestrates multiple Th cell programs by sensitizing cells to exogenous cytokines, thereby ensuring that activated CD4+ T cells overcome a Th cell commitment threshold. In support of a role for Notch as an unbiased amplifier (Figure 1), Notch signaling was shown to be required for optimal T cell expansion, CD25 and IL-2 induction in vitro of both Th1 and Th2 cells (15, 16, 18, 105). Finally, Notch signals promote survival by enhancing anti-apoptotic signals and glucose uptake (17, 106).
It is conceivable that minor differences in experimental design or conditions form the basis of the discrepant results that support one of the two opposing models for Notch function in Th differentiation. Many studies on Notch ligands on APCs have employed GM-CSF cultured bmDCs (Table 1), which were recently shown to contain not only DCs, but also monocyte-derived macrophages (107). In our own studies, we found that Jagged expression was required for the induction of a Th2 response in the lung when in vitro HDM-pulsed bmDCs were used for allergen sensitization, but not when mice were in vivo sensitized by endogenous airway DCs (32). Moreover, studies are complicated by the finding that Notch ligands are not only induced on DCs, but also on macrophages, B and T cells, or lymph node stromal cells (24, 95, 99, 108). Stimulation via CD46 and CD3 was shown to up regulate Jagged1 on human T cells (109), suggesting that T cells can provide Notch signals to each other. However, it is of note that normally several mechanisms, including lateral inhibition, are used to regulate Notch activity when similar cell types express both ligand and receptor. By lateral inhibition signal-sending cells actively repress their Notch signaling pathway (110), which would hamper concerted Notch-mediated differentiation and polarization of adjacent T helper cells. Finally, Notch receptors can become activated independent of ligand binding (111). Indeed, spontaneous Notch cleavage has been observed upon TCR triggering (15, 18, 22). Ligand-independent Notch signaling would also be supported by the recent identification of a PKCθ-dependent mechanism that enhances Notch activation (21). More experiments targeting Notch ligands in various cells types are required to determine how the Notch signaling pathway is activated in T cell subsets in vivo.
Another concern is that some gain-of-function approaches, involving overexpression of Notch receptors or ligands, may be associated with strong or prolonged, less physiological Notch signals. In this context, it is interesting that variable Notch signal strength allows induction of distinct responses by the same signaling pathway (112, 113), paralleling previous experiments demonstrating Th1 or Th2 cells are induced by strong or weak TCR signals, respectively (114, 115). Therefore, in studies on the effects of Notch ligands on Th differentiation, it may be critical to use a range of antigen doses. Finally, since it has recently been shown that Th2 inflammation also crucially involves IL-4-producing Tfh cells (116, 117), findings of impaired in vivo Th2 cell differentiation may point at Tfh rather than Th2 defects and should, therefore, be interpreted with care.
Given the increasing number of characterized Th subsets, it is unlikely that Notch signaling simply acts as a bimodal molecular switch for the induction of either Th1 and Th2 differentiation, based on DLL and Jagged expression on DCs, respectively. Nevertheless, many studies described above support the notion that individual Notch ligands have differential effects on Th cell differentiation, which cannot be explained by the unbiased amplifier model. The two models, however, may not necessarily be mutually exclusive. Effects of Notch signaling could be quite different during induction and during maintenance of Th subset differentiation. Moreover, the finding that there is quite some plasticity between Th subsets (2) and that Th2 differentiation may involve a Tfh phase has further complicated the role of Notch signaling in Th differentiation. We also conclude that the elucidation of the role of Notch ligands on particular cell types requires comprehensive in vivo studies, using cell-specific knockout of individual Notch ligands or combinations.
Since Notch signaling is involved in the differentiation of basically all Th subsets, it could serve as a potential therapeutic target, for example, by inhibiting Th2 responses in allergies or Th1/Th17 responses in autoimmune diseases. However, because effects of GSI are not limited to Notch signaling, it will be valuable to develop more specific compounds targeting Notch signaling components. Indeed synthetic, cell-permeable stabilized peptides that target a critical protein–protein interface in the Notch transactivation complex (118–120) as well as specific antibodies that target Notch receptors (121–123) or Notch ligands (24, 124) have been designed. Promising results were obtained with Notch pathway blocking antibodies in cancer patients (125) and future studies should explore whether these antibodies are beneficial for allergic or autoimmune patients.
Interestingly, GSI administration during only the challenge in asthma models was sufficient to decrease Th2 cytokine production (58, 59). These findings imply that Notch signaling is not likely critical to initiate IL-4 production in activated T cells and thus the initial source of IL-4, for example in AAI, remains unclear. While several cells including basophils, Tfh cells, NKT cells, and ILC2 are capable of producing IL-4 (55, 116, 126–131), mice deficient for NKT cells, ILC2, or basophils are still capable of inducing Th2 responses (132–134), suggesting that IL-4 production by Tfh cells could be crucial for Th2 cell induction. Nevertheless, the finding that in animal models allergic disease symptoms are reduced by GSI administration during challenge only indicates that Notch signaling is important in maintaining rather than inducing Th2 cell responses. This makes Notch signaling an interesting target for development of therapeutic strategies in allergic asthma.
IT and MP performed the literature research, wrote the paper, and designed the figure. RH critically revised the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study is partly supported by Lung Foundation Netherlands Grants 3.2.12.087 and 3.2.12.067.
1. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol (1986) 136:2348–57.
2. DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol (2016) 16:149–63. doi: 10.1038/nri.2015.18
3. Hirahara K, Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol (2016) 28:163–71. doi:10.1093/intimm/dxw006
4. Li P, Spolski R, Liao W, Leonard WJ. Complex interactions of transcription factors in mediating cytokine biology in T cells. Immunol Rev (2014) 261:141–56. doi:10.1111/imr.12199
5. Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol (2013) 13:777–89. doi:10.1038/nri3536
6. Tindemans I, Serafini N, Di Santo JP, Hendriks RW. GATA-3 function in innate and adaptive immunity. Immunity (2014) 41:191–206. doi:10.1016/j.immuni.2014.06.006
7. Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol (2000) 164:3047–55. doi:10.4049/jimmunol.164.6.3047
8. Schmitz J, Thiel A, Kuhn R, Rajewsky K, Muller W, Assenmacher M, et al. Induction of interleukin 4 (IL-4) expression in T helper (Th) cells is not dependent on IL-4 from non-Th cells. J Exp Med (1994) 179:1349–53. doi:10.1084/jem.179.4.1349
9. Amsen D, Helbig C, Backer RA. Notch in T cell differentiation: all things considered. Trends Immunol (2015) 36:802–14. doi:10.1016/j.it.2015.10.007
10. Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell (2004) 117:515–26. doi:10.1016/S0092-8674(04)00451-9
11. Bailis W, Yashiro-Ohtani Y, Fang TC, Hatton RD, Weaver CT, Artis D, et al. Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals. Immunity (2013) 39:148–59. doi:10.1016/j.immuni.2013.07.006
12. Guo B, McMillan BJ, Blacklow SC. Structure and function of the Mind bomb E3 ligase in the context of Notch signal transduction. Curr Opin Struct Biol (2016) 41:38–45. doi:10.1016/j.sbi.2016.05.012
13. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell (2009) 137:216–33. doi:10.1016/j.cell.2009.03.045
14. Yuan JS, Kousis PC, Suliman S, Visan I, Guidos CJ. Functions of notch signaling in the immune system: consensus and controversies. Annu Rev Immunol (2010) 28:343–65. doi:10.1146/annurev.immunol.021908.132719
15. Adler SH, Chiffoleau E, Xu L, Dalton NM, Burg JM, Wells AD, et al. Notch signaling augments T cell responsiveness by enhancing CD25 expression. J Immunol (2003) 171:2896–903. doi:10.4049/jimmunol.171.6.2896
16. Laky K, Evans S, Perez-Diez A, Fowlkes BJ. Notch signaling regulates antigen sensitivity of naive CD4+ T cells by tuning co-stimulation. Immunity (2015) 42:80–94. doi:10.1016/j.immuni.2014.12.027
17. Maekawa Y, Ishifune C, Tsukumo S, Hozumi K, Yagita H, Yasutomo K. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nat Med (2015) 21:55–61. doi:10.1038/nm.3758
18. Palaga T, Miele L, Golde TE, Osborne BA. TCR-mediated Notch signaling regulates proliferation and IFN-gamma production in peripheral T cells. J Immunol (2003) 171:3019–24. doi:10.4049/jimmunol.171.6.3019
19. Perumalsamy LR, Nagala M, Sarin A. Notch-activated signaling cascade interacts with mitochondrial remodeling proteins to regulate cell survival. Proc Natl Acad Sci U S A (2010) 107:6882–7. doi:10.1073/pnas.0910060107
20. Sade H, Krishna S, Sarin A. The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J Biol Chem (2004) 279:2937–44. doi:10.1074/jbc.M309924200
21. Britton GJ, Ambler R, Clark DJ, Hill EV, Tunbridge HM, McNally KE, et al. PKCtheta links proximal T cell and Notch signaling through localized regulation of the actin cytoskeleton. Elife (2017) 6. doi:10.7554/eLife.20003
22. Dongre A, Surampudi L, Lawlor RG, Fauq AH, Miele L, Golde TE, et al. Non-canonical Notch signaling drives activation and differentiation of peripheral CD4(+) T cells. Front Immunol (2014) 5:54. doi:10.3389/fimmu.2014.00054
23. Shin HM, Tilahun ME, Cho OH, Chandiran K, Kuksin CA, Keerthivasan S, et al. NOTCH1 can initiate NF-kappaB activation via cytosolic interactions with components of the T cell signalosome. Front Immunol (2014) 5:249. doi:10.3389/fimmu.2014.00249
24. Elyaman W, Bradshaw EM, Wang Y, Oukka M, Kivisakk P, Chiba S, et al. JAGGED1 and delta1 differentially regulate the outcome of experimental autoimmune encephalomyelitis. J Immunol (2007) 179:5990–8. doi:10.4049/jimmunol.179.9.5990
25. Fukuyama Y, Tokuhara D, Sekine S, Kataoka K, Markham JD, Irwin AR, et al. Notch-ligand expression by NALT dendritic cells regulates mucosal Th1- and Th2-type responses. Biochem Biophys Res Commun (2012) 418:6–11. doi:10.1016/j.bbrc.2011.12.046
26. Gilles S, Beck I, Lange S, Ring J, Behrendt H, Traidl-Hoffmann C. Non-allergenic factors from pollen modulate T helper cell instructing notch ligands on dendritic cells. World Allergy Organ J (2015) 8:2. doi:10.1186/s40413-014-0054-8
27. Jang S, Schaller M, Berlin AA, Lukacs NW. Notch ligand delta-like 4 regulates development and pathogenesis of allergic airway responses by modulating IL-2 production and Th2 immunity. J Immunol (2010) 185:5835–44. doi:10.4049/jimmunol.1000175
28. Jeong HW, Kim JH, Kim JY, Ha SJ, Kong YY. Mind bomb-1 in dendritic cells is specifically required for Notch-mediated T helper type 2 differentiation. PLoS One (2012) 7:e36359. doi:10.1371/journal.pone.0036359
29. Krishnamoorthy N, Oriss TB, Paglia M, Fei M, Yarlagadda M, Vanhaesebroeck B, et al. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat Med (2008) 14:565–73. doi:10.1038/nm1766
30. Okamoto M, Matsuda H, Joetham A, Lucas JJ, Domenico J, Yasutomo K, et al. Jagged1 on dendritic cells and Notch on CD4+ T cells initiate lung allergic responsiveness by inducing IL-4 production. J Immunol (2009) 183:2995–3003. doi:10.4049/jimmunol.0900692
31. Okamoto M, Takeda K, Lucas JJ, Joetham A, Yasutomo K, Gelfand EW. Low-dose lipopolysaccharide affects lung allergic responses by regulating Jagged1 expression on antigen-pulsed dendritic cells. Int Arch Allergy Immunol (2012) 157:65–72. doi:10.1159/000324836
32. Tindemans I, Lukkes M, de Bruijn MJ, Li BW, van Nimwegen M, Amsen D, et al. Notch signaling in T cells is essential for allergic airway inflammation, but expression of Notch ligands Jagged1 and Jagged2 on dendritic cells is dispensable. J Allergy Clin Immunol (2017). doi:10.1016/j.jaci.2016.11.046
33. Wakui M, Nakano K, Matsushita S. Notch ligand mRNA levels of human APCs predict Th1/Th2-promoting activities. Biochem Biophys Res Commun (2007) 358:596–601. doi:10.1016/j.bbrc.2007.04.175
34. Krawczyk CM, Sun J, Pearce EJ. Th2 differentiation is unaffected by Jagged2 expression on dendritic cells. J Immunol (2008) 180:7931–7. doi:10.4049/jimmunol.180.12.7931
35. Rudd BD, Schaller MA, Smit JJ, Kunkel SL, Neupane R, Kelley L, et al. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J Immunol (2007) 178:5820–7. doi:10.4049/jimmunol.178.9.5820
36. Schaller MA, Neupane R, Rudd BD, Kunkel SL, Kallal LE, Lincoln P, et al. Notch ligand Delta-like 4 regulates disease pathogenesis during respiratory viral infections by modulating Th2 cytokines. J Exp Med (2007) 204:2925–34. doi:10.1084/jem.20070661
37. Skokos D, Nussenzweig MC. CD8- DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med (2007) 204:1525–31. doi:10.1084/jem.20062305
38. Sun J, Krawczyk CJ, Pearce EJ. Suppression of Th2 cell development by Notch ligands Delta1 and Delta4. J Immunol (2008) 180:1655–61. doi:10.4049/jimmunol.180.3.1655
39. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med (2002) 196:1645–51. doi:10.1084/jem.20021340
40. Kaisho T, Hoshino K, Iwabe T, Takeuchi O, Yasui T, Akira S. Endotoxin can induce MyD88-deficient dendritic cells to support T(h)2 cell differentiation. Int Immunol (2002) 14:695–700. doi:10.1093/intimm/dxf039
41. Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol (2001) 2:947–50. doi:10.1038/ni712
42. Huang HM, Hsiao G, Fan CK, Lin CL, Leu SJ, Chiang BL, et al. Notch ligand delta-like 4-pretreated dendritic cells alleviate allergic airway responses by enhancing IL-10 production. PLoS One (2013) 8:e63613. doi:10.1371/journal.pone.0063613
43. Tsai JJ, Wang HC, Chiu CL, Liao EC. The effect of Dermatophagoides pteronyssinus group 7 allergen (Der p 7) on dendritic cells and its role in T cell polarization. Immunobiology (2016) 221(11):1319–28. doi:10.1016/j.imbio.2016.04.002
44. Liotta F, Frosali F, Querci V, Mantei A, Fili L, Maggi L, et al. Human immature myeloid dendritic cells trigger a TH2-polarizing program via Jagged-1/Notch interaction. J Allergy Clin Immunol (2008) 121:1000.e–5.e. doi:10.1016/j.jaci.2008.01.004
45. Bleck B, Tse DB, Gordon T, Ahsan MR, Reibman J. Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol (2010) 185:6636–45. doi:10.4049/jimmunol.1000719
46. Maekawa Y, Tsukumo S, Chiba S, Hirai H, Hayashi Y, Okada H, et al. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity (2003) 19:549–59. doi:10.1016/S1074-7613(03)00270-X
47. Takeichi N, Yanagisawa S, Kaneyama T, Yagita H, Jin YH, Kim BS, et al. Ameliorating effects of anti-Dll4 mAb on Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Int Immunol (2010) 22:729–38. doi:10.1093/intimm/dxq059
48. Jiao Z, Wang W, Xu H, Wang S, Guo M, Chen Y, et al. Engagement of activated Notch signalling in collagen II-specific T helper type 1 (Th1)- and Th17-type expansion involving Notch3 and Delta-like1. Clin Exp Immunol (2011) 164:66–71. doi:10.1111/j.1365-2249.2010.04310.x
49. Bassil R, Zhu B, Lahoud Y, Riella LV, Yagita H, Elyaman W, et al. Notch ligand delta-like 4 blockade alleviates experimental autoimmune encephalomyelitis by promoting regulatory T cell development. J Immunol (2011) 187:2322–8. doi:10.4049/jimmunol.1100725
50. Eixarch H, Mansilla MJ, Costa C, Kunkel SL, Montalban X, Godessart N, et al. Inhibition of delta-like ligand 4 decreases Th1/Th17 response in a mouse model of multiple sclerosis. Neurosci Lett (2013) 541:161–6. doi:10.1016/j.neulet.2013.02.038
51. Li Y, Wu S, Pu J, Huang X, Zhang P. Dengue virus up-regulates expression of notch ligands Dll1 and Dll4 through interferon-beta signalling pathway. Immunology (2015) 144:127–38. doi:10.1111/imm.12357
52. Meng L, Bai Z, He S, Mochizuki K, Liu Y, Purushe J, et al. The Notch ligand DLL4 defines a capability of human dendritic cells in regulating Th1 and Th17 differentiation. J Immunol (2016) 196:1070–80. doi:10.4049/jimmunol.1501310
53. Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity (2007) 27:89–99. doi:10.1016/j.immuni.2007.05.021
54. Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity (2007) 27:100–10. doi:10.1016/j.immuni.2007.04.018
55. Tanaka S, Tsukada J, Suzuki W, Hayashi K, Tanigaki K, Tsuji M, et al. The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity (2006) 24:689–701. doi:10.1016/j.immuni.2006.04.009
56. Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, et al. Notch signaling is an important regulator of type 2 immunity. J Exp Med (2005) 202:1037–42. doi:10.1084/jem.20050923
57. Jiang S, Han S, Chen J, Li X, Che H. Inhibition effect of blunting Notch signaling on food allergy through improving TH1/TH2 balance in mice. Ann Allergy Asthma Immunol (2017) 118:94–102. doi:10.1016/j.anai.2016.10.024
58. Kang JH, Kim BS, Uhm TG, Lee SH, Lee GR, Park CS, et al. Gamma-secretase inhibitor reduces allergic pulmonary inflammation by modulating Th1 and Th2 responses. Am J Respir Crit Care Med (2009) 179:875–82. doi:10.1164/rccm.200806-893OC
59. Zhou M, Cui ZL, Guo XJ, Ren LP, Yang M, Fan ZW, et al. Blockade of Notch signalling by gamma-secretase inhibitor in lung t cells of asthmatic mice affects T cell differentiation and pulmonary inflammation. Inflammation (2015) 38:1281–8. doi:10.1007/s10753-014-0098-5
60. Mukherjee S, Rasky AJ, Lundy PA, Kittan NA, Kunkel SL, Maillard IP, et al. STAT5-induced lunatic fringe during Th2 development alters delta-like 4-mediated Th2 cytokine production in respiratory syncytial virus-exacerbated airway allergic disease. J Immunol (2014) 192:996–1003. doi:10.4049/jimmunol.1301991
61. Minter LM, Turley DM, Das P, Shin HM, Joshi I, Lawlor RG, et al. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat Immunol (2005) 6:680–8. doi:10.1038/ni1209
62. Auderset F, Schuster S, Coutaz M, Koch U, Desgranges F, Merck E, et al. Redundant Notch1 and Notch2 signaling is necessary for IFNgamma secretion by T helper 1 cells during infection with Leishmania major. PLoS Pathog (2012) 8:e1002560. doi:10.1371/journal.ppat.1002560
63. Beel AJ, Sanders CR. Substrate specificity of gamma-secretase and other intramembrane proteases. Cell Mol Life Sci (2008) 65:1311–34. doi:10.1007/s00018-008-7462-2
64. Riella LV, Ueno T, Batal I, De Serres SA, Bassil R, Elyaman W, et al. Blockade of Notch ligand delta1 promotes allograft survival by inhibiting alloreactive Th1 cells and cytotoxic T cell generation. J Immunol (2011) 187:4629–38. doi:10.4049/jimmunol.1004076
65. Elyaman W, Bassil R, Bradshaw EM, Orent W, Lahoud Y, Zhu B, et al. Notch receptors and Smad3 signaling cooperate in the induction of interleukin-9-producing T cells. Immunity (2012) 36:623–34. doi:10.1016/j.immuni.2012.01.020
66. Coutaz M, Hurrell BP, Auderset F, Wang H, Siegert S, Eberl G, et al. Notch regulates Th17 differentiation and controls trafficking of IL-17 and metabolic regulators within Th17 cells in a context-dependent manner. Sci Rep (2016) 6:39117. doi:10.1038/srep39117
67. Keerthivasan S, Suleiman R, Lawlor R, Roderick J, Bates T, Minter L, et al. Notch signaling regulates mouse and human Th17 differentiation. J Immunol (2011) 187:692–701. doi:10.4049/jimmunol.1003658
68. Meyer Zu Horste G, Wu C, Wang C, Cong L, Pawlak M, Lee Y, et al. RBPJ controls development of pathogenic Th17 cells by regulating IL-23 receptor expression. Cell Rep (2016) 16:392–404. doi:10.1016/j.celrep.2016.05.088
69. Mukherjee S, Schaller MA, Neupane R, Kunkel SL, Lukacs NW. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J Immunol (2009) 182:7381–8. doi:10.4049/jimmunol.0804322
70. Zhang W, Zhang X, Sheng A, Weng C, Zhu T, Zhao W, et al. gamma-Secretase inhibitor alleviates acute airway inflammation of allergic asthma in mice by downregulating Th17 cell differentiation. Mediators Inflamm (2015) 2015:258168. doi:10.1155/2015/258168
71. Jiang Y, Zhao S, Yang X, Liu Y, Wang C. Dll4 in the DCs isolated from OVA-sensitized mice is involved in Th17 differentiation inhibition by 1,25-dihydroxyvitamin D3 in vitro. J Asthma (2015) 52:989–95. doi:10.3109/02770903.2015.1056349
72. Higashi T, Hashimoto K, Takagi R, Mizuno Y, Okazaki Y, Tanaka Y, et al. Curdlan induces DC-mediated Th17 polarization via Jagged1 activation in human dendritic cells. Allergol Int (2010) 59:161–6. doi:10.2332/allergolint.09-OA-0103
73. Wang Y, Xing F, Ye S, Xiao J, Di J, Zeng S, et al. Jagged-1 signaling suppresses the IL-6 and TGF-beta treatment-induced Th17 cell differentiation via the reduction of RORgammat/IL-17A/IL-17F/IL-23a/IL-12rb1. Sci Rep (2015) 5:8234. doi:10.1038/srep08234
74. You P, Xing F, Mao C, Chen Z, Zhang H, Wang Y, et al. Jagged-1-HES-1 signaling inhibits the differentiation of TH17 cells via ROR gammat. J Biol Regul Homeost Agents (2013) 27:79–93.
75. Jiao Z, Wang W, Hua S, Liu M, Wang H, Wang X, et al. Blockade of Notch signaling ameliorates murine collagen-induced arthritis via suppressing Th1 and Th17 cell responses. Am J Pathol (2014) 184:1085–93. doi:10.1016/j.ajpath.2013.12.010
76. Ladi E, Nichols JT, Ge W, Miyamoto A, Yao C, Yang LT, et al. The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J Cell Biol (2005) 170:983–92. doi:10.1083/jcb.200503113
77. Anastasi E, Campese AF, Bellavia D, Bulotta A, Balestri A, Pascucci M, et al. Expression of activated Notch3 in transgenic mice enhances generation of T regulatory cells and protects against experimental autoimmune diabetes. J Immunol (2003) 171:4504–11. doi:10.4049/jimmunol.171.9.4504
78. Barbarulo A, Grazioli P, Campese AF, Bellavia D, Di Mario G, Pelullo M, et al. Notch3 and canonical NF-kappaB signaling pathways cooperatively regulate Foxp3 transcription. J Immunol (2011) 186:6199–206. doi:10.4049/jimmunol.1002136
79. Fu T, Zhang P, Feng L, Ji G, Wang XH, Zheng MH, et al. Accelerated acute allograft rejection accompanied by enhanced T-cell proliferation and attenuated Treg function in RBP-J deficient mice. Mol Immunol (2011) 48:751–9. doi:10.1016/j.molimm.2010.11.016
80. Samon JB, Champhekar A, Minter LM, Telfer JC, Miele L, Fauq A, et al. Notch1 and TGFbeta1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood (2008) 112:1813–21. doi:10.1182/blood-2008-03-144980
81. Cahill EF, Tobin LM, Carty F, Mahon BP, English K. Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther (2015) 6:19. doi:10.1186/s13287-015-0021-5
82. Gopisetty A, Bhattacharya P, Haddad C, Bruno JC Jr, Vasu C, Miele L, et al. OX40L/Jagged1 cosignaling by GM-CSF-induced bone marrow-derived dendritic cells is required for the expansion of functional regulatory T cells. J Immunol (2013) 190:5516–25. doi:10.4049/jimmunol.1202298
83. Kared H, Adle-Biassette H, Fois E, Masson A, Bach JF, Chatenoud L, et al. Jagged2-expressing hematopoietic progenitors promote regulatory T cell expansion in the periphery through notch signaling. Immunity (2006) 25:823–34. doi:10.1016/j.immuni.2006.09.008
84. Mota C, Nunes-Silva V, Pires AR, Matoso P, Victorino RM, Sousa AE, et al. Delta-like 1-mediated Notch signaling enhances the in vitro conversion of human memory CD4 T cells into FOXP3-expressing regulatory T cells. J Immunol (2014) 193:5854–62. doi:10.4049/jimmunol.1400198
85. Rutz S, Janke M, Kassner N, Hohnstein T, Krueger M, Scheffold A. Notch regulates IL-10 production by T helper 1 cells. Proc Natl Acad Sci U S A (2008) 105:3497–502. doi:10.1073/pnas.0712102105
86. Rutz S, Mordmuller B, Sakano S, Scheffold A. Notch ligands Delta-like1, Delta-like4 and Jagged1 differentially regulate activation of peripheral T helper cells. Eur J Immunol (2005) 35:2443–51. doi:10.1002/eji.200526294
87. Vigouroux S, Yvon E, Wagner HJ, Biagi E, Dotti G, Sili U, et al. Induction of antigen-specific regulatory T cells following overexpression of a Notch ligand by human B lymphocytes. J Virol (2003) 77:10872–80. doi:10.1128/JVI.77.20.10872-10880.2003
88. Yvon ES, Vigouroux S, Rousseau RF, Biagi E, Amrolia P, Dotti G, et al. Overexpression of the Notch ligand, Jagged-1, induces alloantigen-specific human regulatory T cells. Blood (2003) 102:3815–21. doi:10.1182/blood-2002-12-3826
89. Ou-Yang HF, Zhang HW, Wu CG, Zhang P, Zhang J, Li JC, et al. Notch signaling regulates the FOXP3 promoter through RBP-J- and Hes1-dependent mechanisms. Mol Cell Biochem (2009) 320:109–14. doi:10.1007/s11010-008-9912-4
90. Billiard F, Lobry C, Darrasse-Jeze G, Waite J, Liu X, Mouquet H, et al. Dll4-Notch signaling in Flt3-independent dendritic cell development and autoimmunity in mice. J Exp Med (2012) 209:1011–28. doi:10.1084/jem.20111615
91. Charbonnier LM, Wang S, Georgiev P, Sefik E, Chatila TA. Control of peripheral tolerance by regulatory T cell-intrinsic Notch signaling. Nat Immunol (2015) 16:1162–73. doi:10.1038/ni.3288
92. Burghardt S, Erhardt A, Claass B, Huber S, Adler G, Jacobs T, et al. Hepatocytes contribute to immune regulation in the liver by activation of the Notch signaling pathway in T cells. J Immunol (2013) 191:5574–82. doi:10.4049/jimmunol.1300826
93. Kassner N, Krueger M, Yagita H, Dzionek A, Hutloff A, Kroczek R, et al. Cutting edge: plasmacytoid dendritic cells induce IL-10 production in T cells via the Delta-like-4/Notch axis. J Immunol (2010) 184:550–4. doi:10.4049/jimmunol.0903152
94. Auderset F, Schuster S, Fasnacht N, Coutaz M, Charmoy M, Koch U, et al. Notch signaling regulates follicular helper T cell differentiation. J Immunol (2013) 191:2344–50. doi:10.4049/jimmunol.1300643
95. Fasnacht N, Huang HY, Koch U, Favre S, Auderset F, Chai Q, et al. Specific fibroblastic niches in secondary lymphoid organs orchestrate distinct Notch-regulated immune responses. J Exp Med (2014) 211:2265–79. doi:10.1084/jem.20132528
96. Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol (2009) 9:116–24. doi:10.1038/nri2488
97. Haines N, Irvine KD. Glycosylation regulates Notch signalling. Nat Rev Mol Cell Biol (2003) 4:786–97. doi:10.1038/nrm1228
98. Gu W, Xu W, Ding T, Guo X. Fringe controls naive CD4(+)T cells differentiation through modulating notch signaling in asthmatic rat models. PLoS One (2012) 7:e47288. doi:10.1371/journal.pone.0047288
99. Koyanagi A, Sekine C, Yagita H. Expression of Notch receptors and ligands on immature and mature T cells. Biochem Biophys Res Commun (2012) 418:799–805. doi:10.1016/j.bbrc.2012.01.106
100. Maekawa Y, Minato Y, Ishifune C, Kurihara T, Kitamura A, Kojima H, et al. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol (2008) 9:1140–7. doi:10.1038/ni.1649
101. Ong CT, Cheng HT, Chang LW, Ohtsuka T, Kageyama R, Stormo GD, et al. Target selectivity of vertebrate notch proteins. Collaboration between discrete domains and CSL-binding site architecture determines activation probability. J Biol Chem (2006) 281:5106–19. doi:10.1074/jbc.M506108200
102. Arnett KL, Hass M, McArthur DG, Ilagan MX, Aster JC, Kopan R, et al. Structural and mechanistic insights into cooperative assembly of dimeric Notch transcription complexes. Nat Struct Mol Biol (2010) 17:1312–7. doi:10.1038/nsmb.1938
103. Fiorini E, Merck E, Wilson A, Ferrero I, Jiang W, Koch U, et al. Dynamic regulation of notch 1 and notch 2 surface expression during T cell development and activation revealed by novel monoclonal antibodies. J Immunol (2009) 183:7212–22. doi:10.4049/jimmunol.0902432
104. Xia M, Viera-Hutchins L, Garcia-Lloret M, Noval Rivas M, Wise P, McGhee SA, et al. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J Allergy Clin Immunol (2015) 136:441–53. doi:10.1016/j.jaci.2015.02.014
105. Ong CT, Sedy JR, Murphy KM, Kopan R. Notch and presenilin regulate cellular expansion and cytokine secretion but cannot instruct Th1/Th2 fate acquisition. PLoS One (2008) 3:e2823. doi:10.1371/journal.pone.0002823
106. Helbig C, Gentek R, Backer RA, de Souza Y, Derks IA, Eldering E, et al. Notch controls the magnitude of T helper cell responses by promoting cellular longevity. Proc Natl Acad Sci U S A (2012) 109:9041–6. doi:10.1073/pnas.1206044109
107. Helft J, Bottcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity (2015) 42:1197–211. doi:10.1016/j.immuni.2015.05.018
108. Hoyne GF, Le Roux I, Corsin-Jimenez M, Tan K, Dunne J, Forsyth LM, et al. Serrate1-induced notch signalling regulates the decision between immunity and tolerance made by peripheral CD4(+) T cells. Int Immunol (2000) 12:177–85. doi:10.1093/intimm/12.2.177
109. Le Friec G, Sheppard D, Whiteman P, Karsten CM, Shamoun SA, Laing A, et al. The CD46-Jagged1 interaction is critical for human TH1 immunity. Nat Immunol (2012) 13:1213–21. doi:10.1038/ni.2454
110. Cabrera CV. Lateral inhibition and cell fate during neurogenesis in Drosophila: the interactions between scute, Notch and Delta. Development (1990) 110:733–42.
111. Palmer WH, Deng WM. Ligand-independent mechanisms of Notch activity. Trends Cell Biol (2015) 25:697–707. doi:10.1016/j.tcb.2015.07.010
112. Mazzone M, Selfors LM, Albeck J, Overholtzer M, Sale S, Carroll DL, et al. Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc Natl Acad Sci U S A (2010) 107:5012–7. doi:10.1073/pnas.1000896107
113. Van de Walle I, Waegemans E, De Medts J, De Smet G, De Smedt M, Snauwaert S, et al. Specific Notch receptor-ligand interactions control human TCR-alphabeta/gammadelta development by inducing differential Notch signal strength. J Exp Med (2013) 210:683–97. doi:10.1084/jem.20121798
114. Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med (1995) 182:1591–6. doi:10.1084/jem.182.5.1591
115. Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol (1997) 159:5956–63.
116. Ballesteros-Tato A, Randall TD, Lund FE, Spolski R, Leonard WJ, Leon B. T follicular helper cell plasticity shapes pathogenic T helper 2 cell-mediated immunity to inhaled house dust mite. Immunity (2016) 44:259–73. doi:10.1016/j.immuni.2015.11.017
117. Coquet JM, Schuijs MJ, Smyth MJ, Deswarte K, Beyaert R, Braun H, et al. Interleukin-21-producing CD4(+) T cells promote type 2 immunity to house dust mites. Immunity (2015) 43:318–30. doi:10.1016/j.immuni.2015.07.015
118. Lafkas D, Shelton A, Chiu C, de Leon Boenig G, Chen Y, Stawicki SS, et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature (2015) 528:127–31. doi:10.1038/nature15715
119. Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, et al. Direct inhibition of the NOTCH transcription factor complex. Nature (2009) 462:182–8. doi:10.1038/nature08543
120. Tran IT, Sandy AR, Carulli AJ, Ebens C, Chung J, Shan GT, et al. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest (2013) 123:1590–604. doi:10.1172/JCI65477
121. Aste-Amezaga M, Zhang N, Lineberger JE, Arnold BA, Toner TJ, Gu M, et al. Characterization of Notch1 antibodies that inhibit signaling of both normal and mutated Notch1 receptors. PLoS One (2010) 5:e9094. doi:10.1371/journal.pone.0009094
122. Li K, Li Y, Wu W, Gordon WR, Chang DW, Lu M, et al. Modulation of Notch signaling by antibodies specific for the extracellular negative regulatory region of NOTCH3. J Biol Chem (2008) 283:8046–54. doi:10.1074/jbc.M800170200
123. Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, et al. Therapeutic antibody targeting of individual Notch receptors. Nature (2010) 464:1052–7. doi:10.1038/nature08878
124. Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell (2009) 5:168–77. doi:10.1016/j.stem.2009.05.019
125. Groth C, Fortini ME. Therapeutic approaches to modulating Notch signaling: current challenges and future prospects. Semin Cell Dev Biol (2012) 23:465–72. doi:10.1016/j.semcdb.2012.01.016
126. Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med (2003) 9:582–8. doi:10.1038/nm851
127. Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med (2004) 200:507–17. doi:10.1084/jem.20040590
128. Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol (2016) 138:801.e–11.e. doi:10.1016/j.jaci.2016.02.030
129. Pelly VS, Kannan Y, Coomes SM, Entwistle LJ, Ruckerl D, Seddon B, et al. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol (2016) 9:1407–17. doi:10.1038/mi.2016.4
130. Seder RA, Paul WE, Dvorak AM, Sharkis SJ, Kagey-Sobotka A, Niv Y, et al. Mouse splenic and bone marrow cell populations that express high-affinity Fc epsilon receptors and produce interleukin 4 are highly enriched in basophils. Proc Natl Acad Sci U S A (1991) 88:2835–9. doi:10.1073/pnas.88.7.2835
131. Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med (1994) 179:1285–95. doi:10.1084/jem.179.4.1285
132. Brown DR, Fowell DJ, Corry DB, Wynn TA, Moskowitz NH, Cheever AW, et al. Beta 2-microglobulin-dependent NK1.1+ T cells are not essential for T helper cell 2 immune responses. J Exp Med (1996) 184:1295–304. doi:10.1084/jem.184.4.1295
133. De Grove KC, Provoost S, Hendriks RW, McKenzie AN, Seys LJ, Kumar S, et al. Dysregulation of type 2 innate lymphoid cells and TH2 cells impairs pollutant-induced allergic airway responses. J Allergy Clin Immunol (2017) 139:246–57. doi:10.1016/j.jaci.2016.03.044
Keywords: allergy, cytokines, delta-like ligand, Jagged, Gata3, notch signaling, Th1 immunity, Th2 immunity
Citation: Tindemans I, Peeters MJW and Hendriks RW (2017) Notch Signaling in T Helper Cell Subsets: Instructor or Unbiased Amplifier? Front. Immunol. 8:419. doi: 10.3389/fimmu.2017.00419
Received: 23 January 2017; Accepted: 24 March 2017;
Published: 18 April 2017
Edited by:
Loretta Tuosto, Sapienza University of Rome, ItalyReviewed by:
Lucio Miele, LSU Health Sciences Center New Orleans, USACopyright: © 2017 Tindemans, Peeters and Hendriks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rudi W. Hendriks, ci5oZW5kcmlrc0BlcmFzbXVzbWMubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.