
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 02 March 2017
Sec. Inflammation
Volume 8 - 2017 | https://doi.org/10.3389/fimmu.2017.00198
Microglia are essential for the development and function of the adult brain. Microglia arise from erythro-myeloid precursors in the yolk sac and populate the brain rudiment early during development. Unlike monocytes that are constantly renewed from bone marrow hematopoietic stem cells throughout life, resident microglia in the healthy brain persist during adulthood via constant self-renewal. Their ontogeny, together with the absence of turnover from the periphery and the singular environment of the central nervous system, make microglia a unique cell population. Supporting this notion, recent genome-wide transcriptional studies revealed specific gene expression profiles clearly distinct from other brain and peripheral immune cells. Here, we highlight the breakthrough studies that, over the last decades, helped elucidate microglial cell identity, ontogeny, and function. We describe the main techniques that have been used for this task and outline the crucial milestones that have been achieved to reach our actual knowledge of microglia. Furthermore, we give an overview of the “microgliome” that is currently emerging thanks to the constant progress in the modern profiling techniques.
Until the beginning of the XXI century, the central nervous system (CNS) was seen as an immune-privileged site sealed by the blood–brain barrier, a barrier that was thought to prevent peripheral immune cells infiltration (1). Over the past years, thanks to rapid progress in concepts and new techniques, the idea of the brain as an immune-isolated organ has changed. Specifically, the recent discovery of a meningeal lymphatic system as a pathway allowing the trafficking of immune cells in the brain was the missing link between the brain and the immune system (2, 3).
Distinct populations of resident macrophages colonize almost all the tissues in the body, including the CNS (4, 5). The CNS macrophage populations comprise microglia, perivascular macrophages, meningeal macrophages, and choroid plexus macrophages, though microglia are the only myeloid cells residing in the healthy CNS parenchyma (6, 7). Although sharing a common lineage with monocyte-derived macrophages, microglia’s unique ontogeny clearly distinguishes them from other myeloid cells. Microglia arise from erythro-myeloid precursors in the embryonic yolk sac and populate the embryonic brain early during development (embryonic day 9.5) (8). Unlike monocytes, which are constantly renewed from bone marrow hematopoietic stem cells throughout life, resident microglial cells in the healthy adult brain persist during adulthood via constant self-renewal without turnover from circulating blood progenitors (Figure 1) (8–10). Recent genomic and transcriptomic analysis additionally revealed the uniqueness of microglia, which possess specific genetic signatures that are clearly distinct from other brain and peripheral immune cells (11–20).
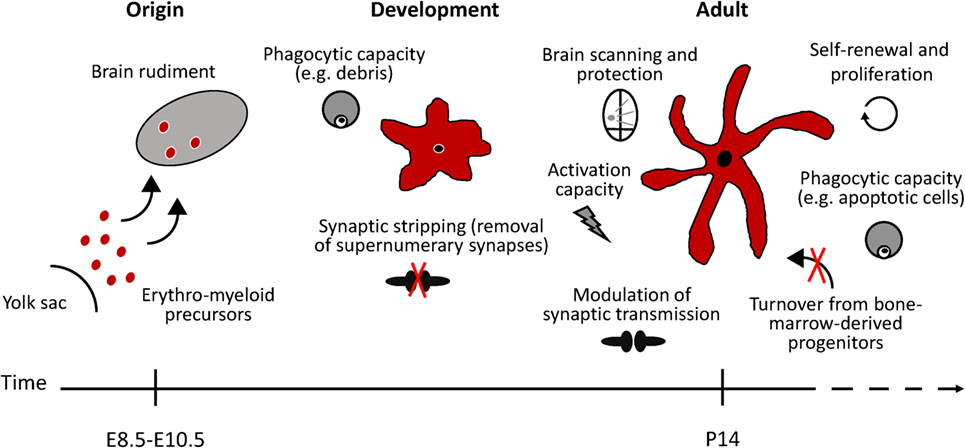
Figure 1. Schematic representation of microglial functional states in the healthy murine brain. Microglia arise from erythro-myeloid precursors in the embryonic yolk sac and populate the brain rudiment early during development. Microglial cell population is maintained by self-renewal, without the contribution of bone marrow-derived progenitors. In the adult healthy brain, microglia continuously survey the brain and readily react to any potential threat to the CNS homeostasis. Phagocytic microglia can detect and quickly remove damaged or dying neurons, preventing further damage to neighboring cells. During developmental stages, microglia phagocytic capacity is particularly important to prune supernumerary synapses. Microglia has also been suggested to modulate neuronal activity by influencing synapse transmission (synaptic stripping). Under specific conditions, microglia are able to remove dysfunctional synapses by physically interacting with functional neurons.
Microglia are non-neuronal cells belonging to the glial population of CNS cells. They comprise between 5 and 20% of the glial cells, approximately 10% of the cells in the brain, being as numerous as neurons (6, 21). Under physiological conditions, microglial cells play fundamental roles during neuronal development, adult neurogenesis, and in modulating synaptic transmission (Figure 1) (22–27). As the resident immune cells of the brain parenchyma, microglia act as central communicators between the nervous and the immune system, as they are the first sentinels protecting against invading pathogens and tissue damage (Figure 1) (28). The rise of innovative imaging, genetic, and immunological tools brought to light the remarkable high dynamism and plasticity of microglial cells under physiological conditions unmasking their crucial role in maintaining brain homeostasis. In the healthy mammalian brain, the so-called “resting” microglia are characterized by a ramified morphology, small cellular bodies, almost no cytoplasm, and slim branching processes bounded in fine protrusions. The majority of microglia occupies their own territory that does not overlap with the neighboring cells (29). Microglia are ubiquitously distributed throughout the adult CNS, yet they show regional diversities as they follow differences of high (such as substantia nigra) and low (such as cerebellum) densities (21). Two-photon imaging in vivo studies revealed that these ramified microglia are highly active, continuously extending and retracting their fine processes, and scanning the CNS microenvironment without disturbing the neuronal fine wire. This notable movement activity sets microglia as the fastest moving structures in the adult healthy brain, monitoring the entire brain parenchyma in less than four hours (30, 31).
Equipped with their branched morphology, microglia readily react to any potential threat to the CNS homeostasis, such as pathogens, trauma, or neuronal dysfunctions by undergoing morphological, genetic, and functional changes, usually referred as microglia “activation.” “Activated” microglia exhibit migratory, proliferative, and phagocytic properties as well as the capacity to release chemokines, cytokines, neurotrophic factors and to present antigens (28). Consequently, a proper and effective microglial function is crucial for CNS homeostasis not only under healthy conditions, but also during threatening events. Similarly to macrophages, in an attempt to simplify the intrinsic spectrum of microglial activation states, it has been assessed for several years that, under defined environmental stimuli, microglia adopt a “classical” (M1-like) or an “alternative” (M2-like) activation state, depending on the nature of the stimulus they encounter. As their corresponding states in macrophages, “classical” activated microglia have been associated with antimicrobial activity through a classical inflammatory reaction driven by the production of proinflammatory mediators, whereas “alternative” polarized microglia have been related to tissue repair and homeostasis restoration (7). However, such dichotomous paradigm represents the extremes of a large spectrum of activation states and is often related to inflammatory reactions and morphological changes, rather than reflecting the microglial physiological and functional status (32). Furthermore, at present, it is becoming more and more evident that “classical” or “alternative” activated microglia per se are barely present in vivo under healthy or diseased conditions (12, 33). In line with these evidences, concepts such as “resting” and “activated” microglia are currently considered simplistic and archaic as they do neither reflect microglia movement dynamism nor their functional plasticity [reviewed in Ref. (34–37)]. In this context, the microglia classification is currently reshaped in order to identify microglial cell phenotypes based, for example, on their inducing stimuli, such as MLPS or MIL4 when stimulated, respectively, with LPS or IL4, instead of relying on microglia/macrophages pre-defined states (38, 39).
In order to follow this rapid advancement of microglia understanding and having in mind the magnitude to which accurate methodologies and innovative techniques can impact our knowledge, we aim here to review the breakthrough steps achieved in microglia research, from the past to the present days. This article is not meant to cover the history of microglia groundwork, but it is rather projected to highlight the steps that contributed to elucidate their identity, ontogeny, and function. We will specifically discuss the impact of the recent genome-wide expression profiling data, which revealed transcriptional microglial cells uniqueness when compared to peripheral immune cells.
How important is the historical context in shaping our current understanding of microglial biology? From the original challenge of “placing glial cells in the conceptual picture of the brain,” it is astonishing to realize that the same work conducted almost a century ago is still directing our present-day view, understanding and research of microglia (40). A timeline of the main tools and methods that revolutionized and critically contributed to elucidate microglial cells identity, ontogeny, and function is listed on Figure 2.
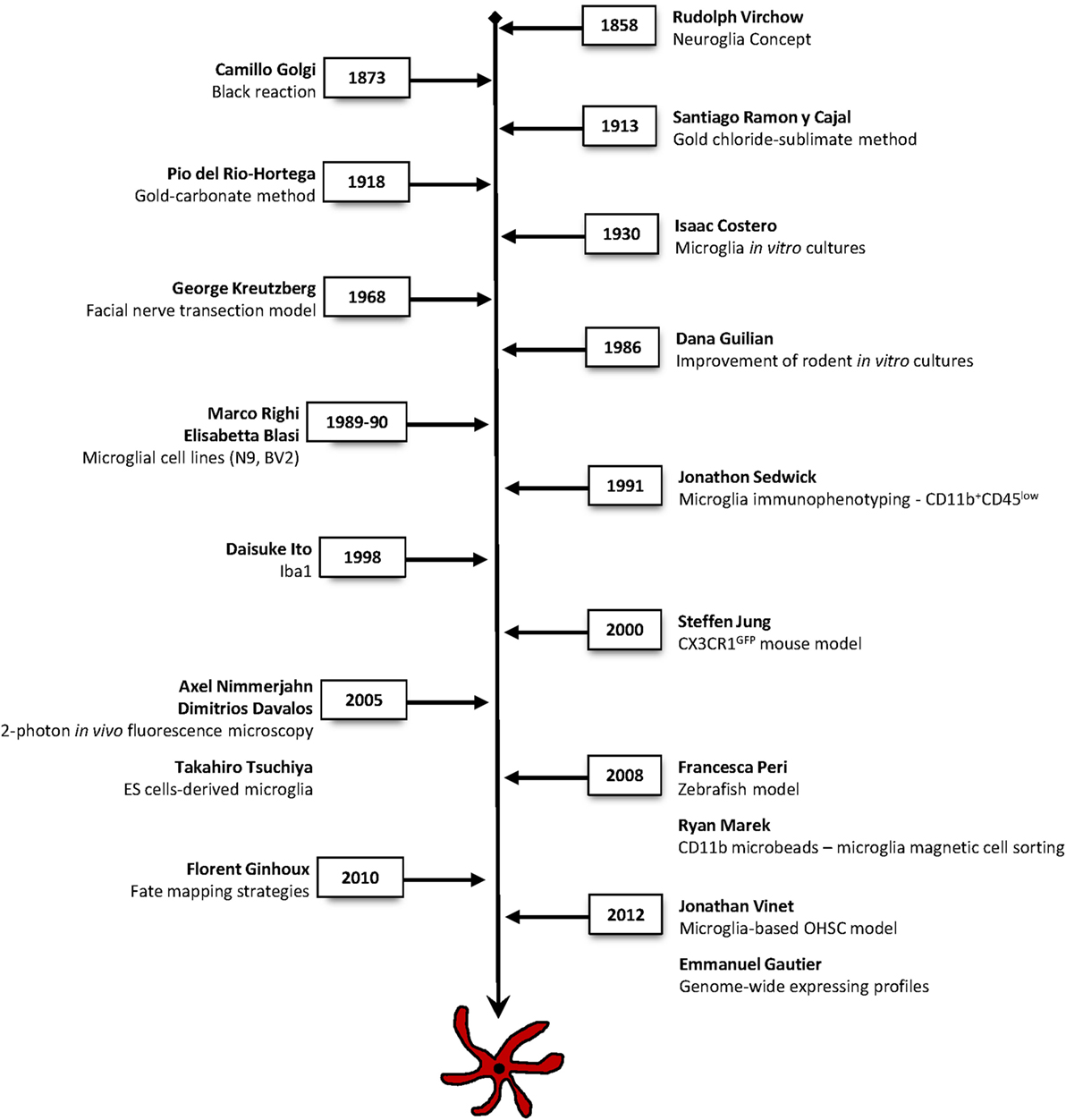
Figure 2. Timeline of the main techniques and methodologies used in microglia research. Major approaches that have contributed to breakthrough findings to elucidate microglial cells identity, ontogeny, and function.
This journey has started in the mid-nineteenth century with the introduction of the concept of neuroglia (“Nervenkitt,” meaning nerve-glue) by Rudolph Virchow in 1856 [for historical review, see Ref. (40–43)]. Neuroglia consisted of a mesodermal connective tissue, the interstitial matter, likely of acellular origin, which major function was to fill in the spaces around the neurons and keep them together (“Zwischenmasse”). Such concept excluded any cellular elements within the neuronal tissue. Nevertheless, the first description of a neuroglial cell was a radial cell of the retina made by Heinrich Müller in 1851 (today known as the retinal Müller cell), years before Virchow introduced the concept of neuroglia (40, 41). Noteworthy, Virchow, Müller, and many others strikingly detailed neuroglia in their drawings using unstained tissue. Prominent discoveries regarding the morphological characterization and the cellular origin of glial cells arose from the inspiring efforts of histologists and anatomists such as Camillo Golgi (1843–1926), Santiago Ramón y Cajal (1852–1934), and Pio Del Rio Hortega (1882–1945).
In the early 1870s, a major breakthrough in neuroglia was empowered by Camillo Golgi and his famous “Reazione nera” (Black reaction) (44) [for more details, see Ref. (45)]. The paraffin sections and their microscopic observation allowed for the first time to see the whole neuronal and neuroglial tissue stained in black against a light yellow background. Although the staining did not permit to differentiate between neurons and neuroglia, it allowed to obtain the best images so far of neurons and neuroglia. Golgi was the first to represent the glial cells as a distinct cellular population from neurons (41). He also reported a diversity of glial cells in the white and gray matter and found that the glial processes contacted both blood vessels and nerve cells. The latter discovery led him to postulate the first theory regarding the glial function, that neuroglia was mostly responsible for the metabolic support and substance exchange of neurons. These functions were later on assigned to astrocytes (41, 45, 46).
Further progress in the study of neuroglia was made possible by Santiago Ramon y Cajal and his pupils. Although the name of Cajal is often associated with the “Neuron doctrine” of the brain structure using the Golgi staining to characterize neurons morphology of the neuronal circuits, Cajal laboratory arduously worked in deciphering the glial enigma by developing new staining and microscopic tools that allowed to identify, classify, and functionally characterize the glial cells. In 1913, Cajal introduced an improved technique, the gold chloride-sublimate staining, which significantly enhanced microscopic visualization under a light microscope of neuroglial cells specifically (47). Cajal’s method improved the visualization of the nucleus and other cellular elements, in contrast with the Golgi’s black reaction that did not distinguish these elements from the dark staining of the background. This method was selectively staining both protoplasmic and fibrous astrocytes. Using the gold chloride-sublimate staining and considering solely the morphological visualization, Cajal reported a new class of cells describing them as “corpuscles without processes,” thus introducing the controversial “third element” within the CNS to further discriminate this group of cells from neurons and astroglia, respectively the first and the second elements [for historical details, see Ref. (40–43, 45)].
Camillo Golgi and Santiago Ramon y Cajal were brilliant pathologists, and their merit and contributions to science were so notable that both were honored with the Nobel Prize in Physiology or Medicine in 1906 (48, 49).
The “third element” postulated by Cajal was later refined by one of his pupils, Pio Del Rio Hortega. As “what we observe is not nature itself, but nature exposed to our method of questioning” (Werner Heisenberg), a student of the Cajal school, Rio Hortega learned the staining methods of Golgi, Cajal, Achúcarro and others, and developed his own method, a modification of Achúcarro’s ammoniacal silver staining (43, 50). This approach allowed to selectively stain microglia and permitted to remarkably visualize glial cells under a light microscope (51). Using his famous silver-carbonate method, Rio Hortega has been able to deeply characterize the morphology of two independent cell types, which he called microglia and interstitial cells, later renamed by him as oligodendrocytes. This method allowed the visualization of the finer micelles that impregnated with prime detail the broad morphology of such cells (40, 41). Moreover, Rio Hortega realized that what Cajal reported as a new class of cells depicted as “corpuscles without processes” was in fact a limitation of his gold chloride-sublimate method that did not allow the complete observation of these cell processes (41). Henceforth, based on morphological and functional differences, Rio Hortega re-categorized Cajal’s “third element” as microglia, “the true third element,” placing oligodendrocytes together with astrocytes as the second element of the CNS. The level of details of his morphological observations as well as the sharpness of his drawings are amazingly resembling the ones that are obtained with the current strategies used to label microglia, such as immuno or transgenic labeling. Notably, Rio Hortega’s detailed cytological observations and statements are still rather valid today (28, 41, 52). Rio Hortega was one of the most important figures in microglia research; his visionary studies and technical brilliance have undoubtedly paved the foundations for the modern research of microglia.
In 1930, Isaac Costero, a prominent student of Rio Hortega, implemented the first in vitro culture method of microglial cells from human brain and recorded their activity using time-lapse cinemicroscopy [(53) for historical details, see Ref. (40, 42)]. His findings were particularly notable in that time since they already echoed microglia motile ability, a process that would only be clearly demonstrated almost one century later (30, 31).
A modern era in microglia research occurred in the sixties, when George Kreutzberg in 1968 implemented the facial nerve lesion model in rodents to study axonal regeneration. The novel insights this model made possible included the study of microglial responses to injury without blood–brain barrier disruption, as well as the first observational differentiation of microglial responses from that of infiltrating mononuclear phagocytes. By using light and electron microscopic and auto-radiographic ultrastructure techniques, Kreutzberg aimed to identify the cells implicated in the regenerative process after the disruption of the facial nerve (54). Using this model, microglia and astrocytes were pointed as major players involved in peripheral nerve regeneration and degeneration. Thus, a major turning point came with the revelation that activated microglia physically interact with neurons by removing synaptic inputs. This feature, known as “synaptic stripping,” has been further described later in the cerebral cortex also by 3D electron microscopy (55, 56). These findings uncovered for the first time a potential neuroprotective role for microglia in neuronal regeneration. In the period preceding the introduction of this concept, microglial function was thought to be merely related to phagocytosis and to the engulfment of damaged neuronal cellular bodies (54, 57).
In the late 1980s, the immunohistochemical methods were replacing the conventional histochemical staining, which allowed not only to study microglial cell phenotypes and distribution, but also enabled the characterization of other cell types within the CNS (21, 58, 59). To specifically analyze microglial cells, it became urgent to be able to discriminate between microglia from brain monocyte-derived macrophages, since the main markers are shared between these cells. In 1991, Jonathon Sedgwick proposed an immunophenotypic discrimination through fluorescence-activated cell sorting (FACS) to distinguish parenchymal microglia (CD11b+CD45low) from other CNS macrophages (CD11b+CD45high) (60). The significance of this method was exceptional since it allowed for the first time to target microglia in their biological environment. Although not new, this approach quickly propagated the usage of discontinuous percoll gradients for isolating microglial cells from adult brains (60). Noteworthy, this technique is still the most used approach to specifically isolate resident microglia from CNS macrophages and infiltrating peripheral immune cells. In this context, a few years later, ionized calcium binding adaptor molecule 1 (Iba1) was reported to be expressed by mononuclear phagocytes and, within the CNS, by microglial cells. Iba1 was detected in all morphological and functional forms of microglia in both rodents and human (61, 62) and, since then, it has been widely used as a microglial marker in vitro and in vivo. Yet, recent expression profile studies revealed the expression of Iba1 in CNS macrophages, neutrophils, and monocytes (12), thus making the discrimination between recruited or infiltrating macrophages from CNS-resident microglia difficult.
Although microglia cultures dated already from 1930 (53), it took 50 years to develop and improve in vitro methods to obtain and culture high numbers of microglia derived from rodent brains and to deeply study microglia biology (63), shortly after enriched cultures of astroglia and oligodendroglia being described (64). Henceforth, studies were developed to culture fetal and adult microglia (65–68). The development of in vitro cultures was indubitably an innovative tool that contributed to a greater characterization of microglia at several levels, which were not possible to be studied in vivo. This included the identification of polarization states, the interaction with other CNS cell types, the expression of neurotrophic and neurotoxic factors, pro- and anti-inflammatory cytokines, neurotransmitter receptors, and mitogen receptors (69–79). It was in this context that both microglia morphological transformation and immune functions were studied, and the concept of microglia as “pathological sensor” of the CNS environment emerged (80).
In the 1990s, protocols for isolating microglia from both neonatal and adult origin were developed. However, they were time-consuming and resulted in low yield of cells, which limited the functional study of microglia at the cellular and biochemical level. The most widely used method for the isolation of glial cells was the mechanical shake-off (64), which is based on differential adherence properties exhibited by the cells into the culture dish. Although several variations of this protocol were used worldwide, the introduction of a magnetic cell sorting using CD11b microbeads improved not only the yield of microglial cells in comparison to the shake-off method but also allowed the accurate separation of microglia from astrocytes (81, 82).
In an attempt to overcome the low yield and time-consuming approaches, new in vitro models, such as immortalized cell lines, started to emerge. In 1985, Blasi and colleagues successfully immortalized murine bone marrow-derived macrophages by infecting the cells with two retroviral oncogenes, raf and myc (83). Five years later, the same approach was adopted to develop the BV2 microglia cell line. The BV2 cells were shown to share several biochemical features of microglial cells (84). Chronologically, the N9 microglial cell line was the first to be generated via infecting primary microglial cells with the v-myc or v-mil oncogenes (85). Overall, the generation of microglial cell lines represented a new and limitless in vitro model for studying microglia properties. Since then, a variety of microglial cell lines from mouse, rat, or human have been developed and used for microglia research (Table 1). However, despite their universal usage, immortalized cell lines are prone to an increased inflammatory status and are sensitive to genetic drift and morphological changes. Additionally, recent studies have being pointing critical differences between these cell lines and primary microglial cells (86) as well as acutely isolated microglial cells (15, 87).
An alternative strategy to cell lines and primary cultures generated from neonatal or adult rodent brains took advantage of embryonic stem (ES) cells. Tsuchiya and colleagues described the first method to obtain microglial cells from ES cells in vitro Takahiro with Tsuchiya. Using a classical protocol to induce ES cells differentiation into tyrosine hydroxylase positive neurons, the authors succeeded in isolating a subpopulation of Iba1+ and CD45+ cells based on a density gradient (100, 101). The isolated population was shown to expand in high number and form mature microglial cells, to be functionally and morphologically identical to primary microglia, and to specifically migrate to the brain rather than to the periphery after transplantation. Of interest, this classical protocol of neuronal differentiation from ES cells displays neurogenesis (101) and yolk sac-like hematopoiesis (102), thus reflecting microglia development in vivo. Since these ES cells-derived microglia were not proven to proliferate or survive under culture systems, new improved protocols to explore the potential of these microglial precursor cells have been developed in the following years (38, 103–105).
Culture-based systems have provided the majority of the knowledge about microglial biology. However, it is becoming unequivocal that, under these conditions, microglial cells loose much of their singularities, thus resembling more to a macrophage-like cell (15, 16). Therefore, elucidating the effective roles of microglia in the CNS requires the development of tools that allow their study and manipulation in their biological environment. Such approaches include, for example, genetic modification strategies. In the CNS, the expression of the fractalkine receptor (CX3CR1) is restricted to microglial cells, whereas the expression of its ligand, the fractalkine, is specific to neurons. Taking advantage of the specificity of CX3CR1, Jung and colleagues developed the first genetic animal model allowing to target microglia, the Cx3cr1GFP knock-in mouse model (106). The murine Cx3cr1 gene was replaced by a reporter gene encoding enhanced green fluorescent protein to generate a Cx3cr1-null locus. However, once more, gene-profiling data have recently shown that Cx3cr1 is also expressed by other mononuclear phagocytes and that it is less microglia specific when compared with other recently identified microglia signature genes (15, 107). Moreover, since the insertion of the reporter gene in Cx3cr1GFP mice generates a Cx3cr1-null locus, this approach resulted in mice heterozygotes or homozygotes for the fractalkine receptor (106). However, although homozygous CX3CR1 deficiency dysregulates microglial responses resulting in neurotoxicity (108), no microglial phenotype has been so far reported for heterozygote animals when compared to mice harboring the GFP transgene under the Iba1 promoter (109, 110). Taken together, the use of this animal model is still having a tremendous impact, and opened the doors to the development of new genetic strategies to target microglia (111).
Another approach to circumvent the disadvantage of in vitro methods was the development of in vivo/ex vivo culture systems mimicking microglial behavior in their biological environment. Mouse organotypic hippocampal slice cultures (OHSC) were implemented in 1981 and, since then, they have been used and optimized as a model recapitulating an in vivo-like situation, thus allowing the study of the intrinsic functional and physiological mechanisms of the nervous system (112, 113). Taking advantage of the OHSC model, the laboratory of Knut Biber in Freiburg expanded their use and implemented this culture system to study adult microglia physiology (114, 115). In the OHSC, ramified microglia was recently reported to exert a neuroprotective function against N-methyl-d-aspartate-induced excitotoxicity (115). Most of the in vitro culture systems are based on the isolation of microglial cells from the brain, meaning that microglia is being studied outside of their biological context. In fact, much of the controversies relying on microglia behavior and function are likely the result of using experimental methods that are far from the physiological conditions. To overcome this obstacle, Masuch and colleagues improved their OHSC methodology by using microglial cells isolated from adult mice to replenish OHSC depleted of endogenous microglia. This model creates an in vivo-like environment that allows the functional study of different microglia phenotypes to be easily accessed in vitro (115, 116).
For decades, microglia were seen as static cells displaying a “resting” phenotype under homeostatic conditions with the capacity to become “activated” when reacting to external stimuli (28, 35). As “the real voyage of discovery consists not only in seeking new landscapes, but in having new eyes” (Marcel Proust), this view has dramatically changed with the advance of microscopic tools that permit the “real-time” in vivo imaging of microglia in their physiological environment. Using a combination of the most recent tools at that time, such as the Cx3cr1GFP mouse model and 2-photon in vivo fluorescence microscopy (a mildly non-invasive technique which achieves an imaging resolution of 100–200 μm of the mouse cerebral cortex), one of the most extraordinary findings that changed our knowledge about microglia biology was uncovered: microglial cells display a highly dynamic cell motility and plasticity in their fully ramified forms within the healthy brain, features that are unique to this cell type within the CNS (30, 31). Moreover, in vivo laser-induced CNS damage confirmed microglia morphological transformation and responses to local brain injury (30). It is worth to mention that, already in 1930, Costero suggested the motile nature of microglia, although the rudimental technology of his time limited definitive conclusions. It took 75 years since then to develop sophisticated microscopic imaging techniques to actually visualize microglia in their physiological environment, in vivo, and in three dimensions at the same time (31). Imaging studies were previously widely performed in brain slices to characterize microglial electrophysiological, morphological, biochemical, and pharmacological properties. Nevertheless, histological approaches did not allow to capture the interactions occurring within and between cells, the responses to stimuli, injury, or disease as well as the motile behavior of microglia (42).
Exploring the combined application of the 2-photon microscopy with microglia expressing fluorescent proteins in vivo will greatly continue to contribute to unveil crucial roles regarding the function of microglia (and other CNS cell types) in the healthy adult brain, but also in the developing and aged brain, and in several disease models (117).
As active surveyors of their environment, microglia serve critical functions in the homeostatic brain and have been shown to influence neuronal activity (30, 31). Therefore, dissecting the functional aspects of such processes requires a real-time observation of microglia performance in the living brain. In that regard, advances in imaging technologies associated with sophisticated transgenic cell labeling methods have greatly contributed to the understanding of microglial interactions with other cells. Because of their transparency, the zebrafish larvae and embryos offer a powerful in vivo high-resolution imaging of the dynamic interactions occurring within cellular and subcellular mechanisms (118). Interestingly, it also enables the visualization of the entire microglial cell network (25–30 cells) (119). In 2008, Francesca Peri, by generating lines of transgenic zebrafish that allowed differential labeling of various nervous system cells, developed a model for studying neuronal–microglial interactions. This approach demonstrated for the first time the dynamism of neuronal phagocytosis by microglia, in real time, and revealed the involvement of v0-ATPase proton pump in phagosomal-mediating vesicles fusion (120).
Microglial ectodermal vs mesodermal origin was a matter of extensive debate until recently. Upon Rio Hortega concept of “microglia” to distinguish oligodendrocytes from the true mesodermal elements, the ectodermal origin of glial cells was generally accepted (52). Given the phenotypical semblance of microglia to other macrophage populations, their myeloid origin was readily accepted. Even Rio Hortega himself believed that microglia could derive from blood circulating monocytes that invade the CNS, replacing the embryonic microglial cells. However, it was only recently demonstrated that microglia belong to the myeloid lineage (8, 10, 121). Using elegant transgenic fate mapping strategies to trace microglia precursors, it was revealed that microglia derive from primitive yolk sac myeloid progenitors that enter the CNS between embryonic days 8.5 and 9.5, rather than hematopoietic-derived cells. Briefly, mice expressing tamoxifen-inducible MER-Cre-MER recombinase gene under the control of the runt-related transcription factor 1 (Runx1) locus were crossed with a Cre-reporter mouse strain. A single injection of 4-hydroxytamoxifen given to pregnant females induces recombination in a 12-hour period, which leads to irreversible expression of fluorescent protein in RUNX1+ cells and their progeny in the knock-in embryos (122). Despite the fact that yolk sac, but also fetal liver progenitors express Runx1, at the embryonic day 7.5 only yolk sac progenitors are expressing Runx1, therefore, the tamoxifen-induced recombinase will only irreversibly tag yolk sac’s concomitant progeny. Moreover, the authors showed that adult microglia are maintained independently of definitive hematopoietic progenitors. To corroborate the notion that microglia are a distinct immune cell population independent from peripheral monocytes circulation, fate mapping strategies coupled with 12-color flow cytometry analysis were recently used to show that retinal microglia and monocyte-derived macrophages exhibit a distinct phenotypic signature. Moreover, this retinal microglia-specific profile (CD45low CD11clow F4/80low I-A/I-E−) is stable under physiological or injury conditions (123). Such sophisticated methodologies are contributing greatly to decipher the unique features and functions of microglial cells under physiological and pathological conditions.
Similarly to microglia, it was recently shown that non-parenchymal CNS meningeal, perivascular, and choroid plexus macrophages are also derived from hematopoietic precursors, establishing stable populations throughout life span with self-renewal capacities (107). Among non-parenchymal CNS macrophages, meningeal and perivascular macrophages do not rely on circulating blood monocytes, while choroid plexus macrophages, which display a dual origin and a shorter turnover, partially depend on circulating blood cells (107). Yet, it is important to highlight that despite their ontogeny similarities, microglia are the only “immune” cells populating the CNS parenchyma, thus displaying unique features to serve critical functions associated to it. Nevertheless, non-parenchymal macrophages are strategically located at the brain boundaries and represent distinct and specialized populations of macrophages serving as key mediators for brain homeostasis and immune responses (107, 124, 125). Although it has been arduous to specifically target the different brain immune cells, perivascular macrophages have been recently discriminated from microglia by their expression of the mannose receptor (CD206) (107, 124, 125). Conversely, the P2Y12 receptor has been shown to be expressed by parenchymal microglia, yet absent in perivascular and meningeal macrophages (107, 126). Taking advantage of CD206 differential expression, it was recently demonstrated that both perivascular macrophages and microglia are the primary source of the chemokine CCL2, which mediates the infiltration of CCR2+ monocytes to the brain, exacerbating the neuroinflammatory environment occurring after status epilepticus (125). Moreover, it has been shown that perivascular macrophages mediate neurovascular and cognitive dysfunction induced by hypertension through their capacity to produce reactive oxygen species and inflammatory cytokines. Intriguingly, perivascular macrophages depletion with clodronate was sufficient to recover from neurovascular impairments (124). Specifically, Faraco and colleagues reported 60–65% reduction of CD206+CD45hiCD11b+ perivascular macrophages, yet the number of microglia and blood lymphocytes remained constant (124). As pharmacological strategies to efficiently deplete microglia are reported and used worldwide (127), such observations underline the need for discriminating between microglia and CNS-resident macrophages to understand their specific roles and clarify their truly homeostatic, neuroprotective, and neurotoxic roles.
In the last decade, genome-wide sequencing technologies have become quite attractive for researchers and started to be widely used as powerful tools to decipher microglial unknown and unique physiological roles in either isolated or cultured microglia (11, 12, 14–20, 128–130). The Immunological Genome (ImmGen) Project was the first systematic study covering the expression profiles of murine macrophages from different organs. Using these data, Gautier and colleagues observed a high diversity among tissue-resident macrophage populations, suggesting their flexibility to adapt to their environment, but also revealed a unique expression profile intrinsic to microglial cells (11). This work opened the door for more sophisticated strategies allowing for the first time to identify a microglia-specific gene signature (15). This approach not only opens up the potential to understand how microglial cells behave in health and disease but also provides opportunities for the discovery and generation of genetic tools that could specifically target microglia. Moreover, applying such techniques to identify unique molecular signatures in both microglia and non-parenchymal macrophages are expected to challenge and shape our current understanding regarding the immune cell populations residing in the brain (107).
As the immune effector cells of the CNS, microglia are central in shaping homeostatic, neuroprotective, degenerative, and regenerative outcomes under different conditions. Recently, the use of modern sequencing technologies coupled with improved methods to enrich and to acutely purify microglia and other types of brain cells, greatly empowered the awareness of microglial uniqueness, properties, and complexity (Figure 3). These recent studies reveal a unique repertoire of transcripts selectively and specifically expressed by CNS-resident microglia that distinguish them from other CNS and peripheral cells, exposing other non-immune functions hitherto unforeseen of microglial cells (Table 2) (11, 12, 14–20, 128–130). The description of microglia gene signatures as well as their transcriptional changes associated with several brain diseases has also been recently accurately revised (131, 132). These systematic transcriptome datasets are fundamental to understand the gene expression patterns of both healthy and diseased tissues, thus allowing studies of brain cellular types aiding in elucidating the mechanisms associated to different CNS pathological processes, such as neurodegenerative diseases. For example, the microglia-unique gene signature is specifically modulated in normal brain aging compared to the young brain or under different neurological diseases (12, 14, 18).
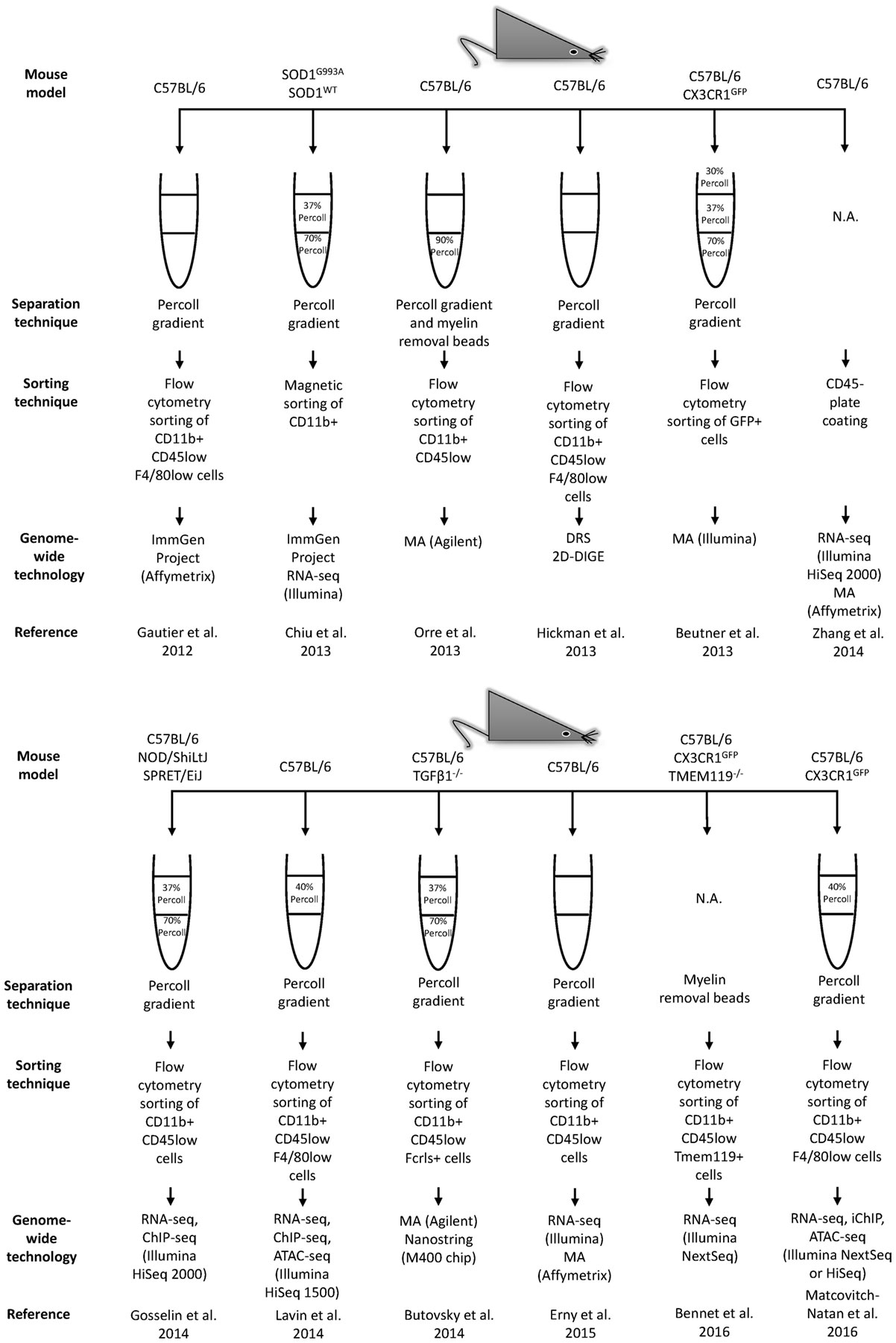
Figure 3. Continued Workflow illustrating technical and methodological details used in the main genome-wide gene expression profiling studies. The related results are shown in Table 2. MA, microarrays; RNA-seq, RNA-sequencing; DRS, direct RNA sequencing; 2D-DIGE, two-dimensional difference gel electrophoresis; ChIP-seq, chromatin immunoprecipitation sequencing; ATAC-seq, assay for transposase accessible chromatin; iChIP, indexing-first chromatin immunoprecipitation.
In the present context, to allow a fast and easy comparison of these genome-wide transcriptome profiles, new databases, such as Glia Open Access Database, available via www.goad.education have been established (133).
Driven by technological advancements, high-throughput -omics methods, such as genomics, transcriptomics, proteomics, and metabolomics seek the thorough description of changes in genes, transcripts, proteins, and metabolites, respectively. These system approaches have emerged as precious ways to interrogate several features of CNS uniqueness under healthy and diseased conditions. More importantly, they have made the molecular study of critical features of the different cell types that populate the brain possible.
Taking advantage of the data generated by these high-throughput studies, the “microgliome” is starting to emerge (Table 3). Here, we define the “microgliome” as the different -omics that characterize microglia under specific conditions, such as homeostasis, injury, or disease.
At the epigenetic level, the gene expression profile of the environment-triggered signal-dependent specific factors are critical for microglia identity and are able to alter gene expression and gene enhancer profiles (16, 17, 130). Specifically, distinct tissue environments drive divergent programs of gene expression by differentially activating a common enhancer repertoire and by inducing the expression of differing transcription factors that collaborate with the macrophage lineage-determining factor PU.1 to establish tissue-specific enhancers (16). As previously mentioned, the microglia transcriptome is the unique set of transcripts expressed by microglial cells when compared to other brain and peripheral immune cells. The microglia proteome is defined as the unique array of proteins selectively expressed by microglial cells under homeostatic conditions (14, 15).
In some of the cited studies, the authors have started to list microglia-specific genes based on their cellular localization or function. In this regard, for example, Hickman and colleagues described a “microglial toolset for sensing changes in the brain’s milieu,” namely the “microglia sensome” (Table 4) (14). Similarly, the microglia “surfaceome” has been designated as the microglia-unique expression of cell surface molecules (15, 128). The “microglia sensome” is required to maintain the CNS homeostatic status and defines the threshold to which critical transitions between CNS homeostasis and pathology can be identified and compared. Highlighting the unique adaptation of microglia to the CNS parenchyma, the “macrophage sensome” is distinct from the one described for microglial cells (Table 4) (14).
Furthermore, emerging data have been highlighting a brain-gut crosstalk that is critically modulated by the gut microbiota (134). The microbiota consists in the specific microflora that colonizes an established microenvironment, such as the gut. Recently, the impact of the host gut microbiota on microglia homeostasis has started to be addressed. Such studies support a critical role for host microbiota in shaping microglia maturation and immune function. The microglia-specific microbiome is defined as the host bacteria that maintain and shape microglia maturation, development, and functions, encompassing microorganism-induced transcriptomic changes in microglial cells (129, 130). These studies address the impact of host microbiota in microglia under specific conditions, such as germ-free and specific-pathogen-free environments. Specific genes that are precisely affected by the host microbiota conditions have been identified (Bcl, Ccnd3, Cdk9, Csf1, Ddit4, Nfkbi-α, Sfpi1) (129, 130).
As a perspective, the “microgliome” will be built based on the data generated at different -omics level in order to associate specific microglial phenotypes to each defined condition, such as homeostasis, injury, or disease. Moreover, the fact that microglia exist under several forms, are highly dynamic and highly sensitive to specific cues, emphasize the importance of compare whether their profile is closely reflected when they are cultivated in vitro. Thus, in vivo vs in vitro “microgliomes” are also foreseen.
Since the description of Rio Hortega’s staining method to distinguish microglia from the surrounding cells, advanced techniques and tools over years and decades have contributed to elucidate the origin of microglial cells as well as their functions during development and in the adult brain under healthy or diseased conditions. Recently, molecular profiling of freshly isolated adult microglial cells have once and for all shown that microglia are distinct from other immune cells of the CNS as well as from other mononuclear phagocytes. The unique features of these cells are essential to fulfill their critical functions in their environment. The distinction of microglia from other myeloid cells is fundamental for understanding their specificity in brain development, function, and disease. Difficulties in addressing microglia functions were mostly related to limitations in isolation protocols and lack of techniques or specific markers to specifically target these cells. Genome-wide profiling of acutely isolated cell populations have opened up new opportunities for the identification of microglia-specific markers distinct from other myeloid cells to precisely address microglia functions (20). For these ex vivo techniques, the significance of isolating microglia from their context (135) as well as the fact that microglia processes, which possess fundamental functions, might be lost during their isolation procedure (131) must be still taken into account.
It is believed that many CNS diseases are strengthened by inappropriate microglial cell functions, therefore understanding the specific molecular triggers and analyzing the resultant gene expression signatures that characterize microglial cell phenotypes is a fundamental step. Supporting this hypothesis, the generated genome-wide profiles suggest that microglia are likely to display unique and characteristic gene signatures depending on the CNS disease [for revision, see Ref. (131, 132).]. In this context, transcriptomic profiles of mouse microglia from distinct brain regions revealed a considerable regional immune-phenotypic diversity across the adult lifespan and an interregional dependency on microglial aging phenotype (136). Microglial expression profiles were also shown to be subtly distinct between different rat brain regions (137). Yet, microglia maintain a specific core signature that, independently of the brain regions, differentiates them from other macrophages (136). This unique microglia transcriptional profile has been recently described in zebrafish (138) and in human microglial cells (20, 126, 139). Taken together, these genome-wide studies are deeply contributing to uncovering the involvement of microglia in different CNS processes and are opening the doors to the identification of microglial specific targets that may have potential therapeutic values (140).
In summary, it has been shown in vivo that microglial activity is critical for normal brain development (27). Ex vivo microglial gene expression profiles from different physiological or pathological conditions show that they scarcely resemble to those classified as the classical polarization states (12, 33), while in vitro microglia studies highlight their immune properties (7). Overall, the notion that microglia share a myeloid origin with other macrophage populations, thus inheriting features such as phagocytosis abilities, lead to the questioning about the real meaning of experimental observations on microglia as the “immune effectors” and “professional phagocytes” residing in the adult CNS. Are microglia immune features a result of their myeloid origin or an outcome of in vitro observations? Are microglial cells essential for CNS host defense or are they professional phagocytes fundamental for brain development? Which cells are the real immune cells of the brain? Further investigations, taking advantage of the technological progress, need to be carried out to properly address these open questions. For example, single-cell transcriptomic and proteomic technologies are gaining their momentum to directly access gene and protein expression profiles (141–143). Their application to individual microglial cells will certainly uncover new magnitudes of cell heterogeneity and will contribute to further characterize microglial phenotypes and functions under physiological and disease contexts.
CS and AM conceived and wrote the manuscript. CS prepared the tables. CS and AM created the pictures. CS, KB, and AM critically revised and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Dr. Manuel Buttini for editing the manuscript. The authors acknowledge the Fondation du Pélican de Mie et Pierre Hippert-Faber under the aegis of Fondation de Luxembourg for supporting CS.
CS was supported by the Fonds National de la Recherche, Luxembourg (AFR project reference 6916713). KB was supported by the BMBF-funded competence network of neurodegenerative disease (KNDD), BMBF project ReelinSys, and DFG grants BI 668/5-1 and BI 668/2-2.
1. Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol (2012) 12:623–35. doi: 10.1038/nri3265
2. Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science (2016) 353:766–71. doi:10.1126/science.aag2638
3. Louveau A, Da Mesquita S, Kipnis J. Lymphatics in neurological disorders: a neuro-lympho-vascular component of multiple sclerosis and Alzheimer’s disease? Neuron (2016) 91:957–73. doi:10.1016/j.neuron.2016.08.027
4. Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol (2010) 6:193–201. doi:10.1038/nrneurol.2010.17
5. Gordon S, Pluddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev (2014) 262:36–55. doi:10.1111/imr.12223
6. Katsumoto A, Lu H, Miranda AS, Ransohoff RM. Ontogeny and functions of central nervous system macrophages. J Immunol (2014) 193:2615–21. doi:10.4049/jimmunol.1400716
7. Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci (2014) 15:300–12. doi:10.1038/nrn3722
8. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (2010) 330:841–5. doi:10.1126/science.1194637
9. Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci (2007) 10:1538–43. doi:10.1038/nn2014
10. Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci (2013) 16:273–80. doi:10.1038/nn.3318
11. Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol (2012) 13:1118–28. doi:10.1038/ni.2419
12. Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP, et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep (2013) 4:385–401. doi:10.1016/j.celrep.2013.06.018
13. Freilich RW, Woodbury ME, Ikezu T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS One (2013) 8:e79416. doi:10.1371/journal.pone.0079416
14. Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci (2013) 16:1896–905. doi:10.1038/nn.3554
15. Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci (2014) 17:131–43. doi:10.1038/nn.3599
16. Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell (2014) 159:1327–40. doi:10.1016/j.cell.2014.11.023
17. Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell (2014) 159:1312–26. doi:10.1016/j.cell.2014.11.018
18. Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I, et al. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging (2014) 35:1–14. doi:10.1016/j.neurobiolaging.2013.07.008
19. Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci (2014) 34:11929–47. doi:10.1523/JNEUROSCI.1860-14.2014
20. Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A (2016) 113:E1738–46. doi:10.1073/pnas.1525528113
21. Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience (1990) 39:151–70. doi:10.1016/0306-4522(90)90229-W
22. Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci (2009) 29:3974–80. doi:10.1523/JNEUROSCI.4363-08.2009
23. Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell (2010) 7:483–95. doi:10.1016/j.stem.2010.08.014
24. Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol (2010) 8:e1000527. doi:10.1371/journal.pbio.1000527
25. Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science (2011) 333:1456–8. doi:10.1126/science.1202529
26. Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron (2012) 74:691–705. doi:10.1016/j.neuron.2012.03.026
27. Casano AM, Peri F. Microglia: multitasking specialists of the brain. Dev Cell (2015) 32:469–77. doi:10.1016/j.devcel.2015.01.018
28. Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev (2011) 91:461–553. doi:10.1152/physrev.00011.2010
29. Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep (2017) 18:391–405. doi:10.1016/j.celrep.2016.12.041
30. Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci (2005) 8:752–8. doi:10.1038/nn1472
31. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science (2005) 308:1314–8. doi:10.1126/science.1110647
32. Biber K, Owens T, Boddeke E. What is microglia neurotoxicity (not)? Glia (2014) 62:841–54. doi:10.1002/glia.22654
33. Szulzewsky F, Pelz A, Feng X, Synowitz M, Markovic D, Langmann T, et al. Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS One (2015) 10:e0116644. doi:10.1371/journal.pone.0116644
34. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci (2007) 10:1387–94. doi:10.1038/nn1997
35. Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol (2009) 27:119–45. doi:10.1146/annurev.immunol.021908.132528
36. Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci (2011) 14:1227–35. doi:10.1038/nn.2923
37. Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron (2013) 77:10–8. doi:10.1016/j.neuron.2012.12.023
38. Beins E, Ulas T, Ternes S, Neumann H, Schultze JL, Zimmer A. Characterization of inflammatory markers and transcriptome profiles of differentially activated embryonic stem cell-derived microglia. Glia (2016) 64:1007–20. doi:10.1002/glia.22979
39. Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci (2016) 19:987–91. doi:10.1038/nn.4338
40. Rezaie P, Male D. Mesoglia & microglia – a historical review of the concept of mononuclear phagocytes within the central nervous system. J Hist Neurosci (2002) 11:325–74. doi:10.1076/jhin.11.4.325.8531
43. Tremblay ME, Lecours C, Samson L, Sanchez-Zafra V, Sierra A. From the cajal alumni achucarro and rio-hortega to the rediscovery of never-resting microglia. Front Neuroanat (2015) 9:45. doi:10.3389/fnana.2015.00045
44. Golgi C. Sulla Fina Anatomia Degli Organi Centrali Del Sistema Nervoso. Reggio Emilia: Tipografia di Stefano Calderini e Figlio (1885).
45. De Carlos JA, Borrell J. A historical reflection of the contributions of Cajal and Golgi to the foundations of neuroscience. Brain Res Rev (2007) 55:8–16. doi:10.1016/j.brainresrev.2007.03.010
46. Lenhossék MV. Der feinere Bau des Nervensystems im Lichte neuester Forschung. Berlin: Fischer’s Medicinische Buchhandlung H. Kornfield (1893).
47. Cajal SR. Contribucion al conocimiento de la neuroglia del cerebro humano. Trab Lab Invest Biol (1913) 11:255–315.
48. Cajal SR. Santiago Ramón y Cajal – Nobel Lecture: The Structure and Connexions of Neurons. Nobelprize.org. Nobel Media AB 2014 (1906). Available from: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1906/cajal-lecture.html
49. Golgi C. Camillo Golgi – Nobel Lecture: The Neuron Doctrine. Theory and Facts. Nobelprize.org. Nobel Media AB 2014 (1906). Available from: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1906/golgi-lecture.html
50. Achúcarro N. Nuevo método para el estudio de la neuroglía y del tejido conjuntivo. Bol Soc Esp Biol (1911) I:139–41.
52. Sierra A, de Castro F, Del Rio-Hortega J, Rafael Iglesias-Rozas J, Garrosa M, Kettenmann H. The “Big-Bang” for modern glial biology: translation and comments on Pio del Rio-Hortega 1919 series of papers on microglia. Glia (2016) 64:1801–40. doi:10.1002/glia.23046
54. Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat (1968) 85:145–57. doi:10.1007/BF00325030
55. Trapp BD, Wujek JR, Criste GA, Jalabi W, Yin X, Kidd GJ, et al. Evidence for synaptic stripping by cortical microglia. Glia (2007) 55:360–8. doi:10.1002/glia.20462
56. Chen Z, Jalabi W, Hu W, Park HJ, Gale JT, Kidd GJ, et al. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat Commun (2014) 5:4486. doi:10.1038/ncomms5486
57. Miyamoto A, Wake H, Moorhouse AJ, Nabekura J. Microglia and synapse interactions: fine tuning neural circuits and candidate molecules. Front Cell Neurosci (2013) 7:70. doi:10.3389/fncel.2013.00070
58. Perry VH, Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci (1988) 11:273–7. doi:10.1016/0166-2236(88)90110-5
59. Imamura K, Ito M, Suzumura A, Asai J, Takahashi A. Generation and characterization of monoclonal antibodies against rat microglia and ontogenic distribution of positive cells. Lab Invest (1990) 63:853–61.
60. Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A (1991) 88:7438–42. doi:10.1073/pnas.88.16.7438
61. Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun (1996) 224:855–62. doi:10.1006/bbrc.1996.1112
62. Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res (1998) 57:1–9. doi:10.1016/S0169-328X(98)00040-0
63. Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci (1986) 6:2163–78.
64. McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol (1980) 85:890–902. doi:10.1083/jcb.85.3.890
65. Hayes GM, Woodroofe MN, Cuzner ML. Characterisation of microglia isolated from adult human and rat brain. J Neuroimmunol (1988) 19:177–89. doi:10.1016/0165-5728(88)90001-X
66. Grenier Y, Ruijs TC, Robitaille Y, Olivier A, Antel JP. Immunohistochemical studies of adult human glial cells. J Neuroimmunol (1989) 21:103–15. doi:10.1016/0165-5728(89)90166-5
67. Lee SC, Liu W, Brosnan CF, Dickson DW. Characterization of primary human fetal dissociated central nervous system cultures with an emphasis on microglia. Lab Invest (1992) 67:465–76.
68. Lauro GM, Babiloni D, Buttarelli FR, Starace G, Cocchia D, Ennas MG, et al. Human microglia cultures: a powerful model to study their origin and immunoreactive capacity. Int J Dev Neurosci (1995) 13:739–52. doi:10.1016/0736-5748(95)00059-3
69. Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia (1993) 7:111–8. doi:10.1002/glia.440070117
70. Nakajima K, Kohsaka S. Functional roles of microglia in the brain. Neurosci Res (1993) 17:187–203. doi:10.1016/0168-0102(93)90047-T
71. Nakajima K, Kohsaka S. Characterization of brain microglia and the biological significance in the central nervous system. Adv Neurol (1993) 60:734–43.
72. Raivich G, Gehrmann J, Moreno-Floros M, Kreutzberg GW. Microglia: growth factor and mitogen receptors. Clin Neuropathol (1993) 12:293–5.
73. Banati RB, Graeber MB. Surveillance, intervention and cytotoxicity: is there a protective role of microglia? Dev Neurosci (1994) 16:114–27. doi:10.1159/000112098
74. Giulian D, Li J, Leara B, Keenen C. Phagocytic microglia release cytokines and cytotoxins that regulate the survival of astrocytes and neurons in culture. Neurochem Int (1994) 25:227–33. doi:10.1016/0197-0186(94)90066-3
75. Nakajima K, Kohsaka S. Functional roles of microglia in the central nervous system. Hum Cell (1998) 11:141–55.
76. Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem (2001) 130:169–75. doi:10.1093/oxfordjournals.jbchem.a002969
77. Hanisch UK. Microglia as a source and target of cytokines. Glia (2002) 40:140–55. doi:10.1002/glia.10161
78. Nakajima K, Kohsaka S. Microglia: neuroprotective and neurotrophic cells in the central nervous system. Curr Drug Targets Cardiovasc Haematol Disord (2004) 4:65–84. doi:10.2174/1568006043481284
79. Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci (2007) 30:527–35. doi:10.1016/j.tins.2007.07.007
80. Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia (1988) 1:301–7. doi:10.1002/glia.440010502
81. Marek R, Caruso M, Rostami A, Grinspan JB, Das Sarma J. Magnetic cell sorting: a fast and effective method of concurrent isolation of high purity viable astrocytes and microglia from neonatal mouse brain tissue. J Neurosci Methods (2008) 175:108–18. doi:10.1016/j.jneumeth.2008.08.016
82. Losciuto S, Dorban G, Gabel S, Gustin A, Hoenen C, Grandbarbe L, et al. An efficient method to limit microglia-dependent effects in astroglial cultures. J Neurosci Methods (2012) 207:59–71. doi:10.1016/j.jneumeth.2012.03.010
83. Blasi E, Mathieson BJ, Varesio L, Cleveland JL, Borchert PA, Rapp UR. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature (1985) 318:667–70. doi:10.1038/318667a0
84. Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol (1990) 27:229–37. doi:10.1016/0165-5728(90)90073-V
85. Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, et al. Monokine production by microglial cell clones. Eur J Immunol (1989) 19:1443–8. doi:10.1002/eji.1830190815
86. Das A, Kim SH, Arifuzzaman S, Yoon T, Chai JC, Lee YS, et al. Transcriptome sequencing reveals that LPS-triggered transcriptional responses in established microglia BV2 cell lines are poorly representative of primary microglia. J Neuroinflammation (2016) 13:182. doi:10.1186/s12974-016-0644-1
87. Melief J, Sneeboer MA, Litjens M, Ormel PR, Palmen SJ, Huitinga I, et al. Characterizing primary human microglia: a comparative study with myeloid subsets and culture models. Glia (2016) 64:1857–68. doi:10.1002/glia.23023
88. Hosaka Y, Kitamoto A, Shimojo M, Nakajima K, Imai Y, Handa H, et al. Generation of microglial cell lines by transfection with simian virus 40 large T gene. Neurosci Lett (1992) 141:139–42. doi:10.1016/0304-3940(92)90880-G
89. Janabi N, Peudenier S, Heron B, Ng KH, Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci Lett (1995) 195:105–8. doi:10.1016/0304-3940(94)11792-H
90. Walker WS, Gatewood J, Olivas E, Askew D, Havenith CE. Mouse microglial cell lines differing in constitutive and interferon-gamma-inducible antigen-presenting activities for naive and memory CD4+ and CD8+ T cells. J Neuroimmunol (1995) 63:163–74. doi:10.1016/0165-5728(95)00146-8
91. Alliot F, Marty MC, Cambier D, Pessac B. A spontaneously immortalized mouse microglial cell line expressing CD4. Brain Res Dev Brain Res (1996) 95:140–3. doi:10.1016/0165-3806(96)00101-0
92. Ohsawa K, Imai Y, Nakajima K, Kohsaka S. Generation and characterization of a microglial cell line, MG5, derived from a p53-deficient mouse. Glia (1997) 21:285–98. doi:10.1002/(SICI)1098-1136(199711)21:3<285::AID-GLIA4>3.3.CO;2-1
93. Zhou W, Cayabyab FS, Pennefather PS, Schlichter LC, DeCoursey TE. HERG-like K+ channels in microglia. J Gen Physiol (1998) 111:781–94. doi:10.1085/jgp.111.6.781
94. Sawada M, Imai F, Suzuki H, Hayakawa M, Kanno T, Nagatsu T. Brain-specific gene expression by immortalized microglial cell-mediated gene transfer in the mammalian brain. FEBS Lett (1998) 433:37–40. doi:10.1016/S0014-5793(98)00879-5
95. Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia (2001) 35:53–62. doi:10.1002/glia.1070
96. Nagai A, Nakagawa E, Hatori K, Choi HB, McLarnon JG, Lee MA, et al. Generation and characterization of immortalized human microglial cell lines: expression of cytokines and chemokines. Neurobiol Dis (2001) 8:1057–68. doi:10.1006/nbdi.2001.0437
97. Takenouchi T, Ogihara K, Sato M, Kitani H. Inhibitory effects of U73122 and U73343 on Ca2+ influx and pore formation induced by the activation of P2X7 nucleotide receptors in mouse microglial cell line. Biochim Biophys Acta (2005) 1726:177–86. doi:10.1016/j.bbagen.2005.08.001
98. Nagamoto-Combs K, Kulas J, Combs CK. A novel cell line from spontaneously immortalized murine microglia. J Neurosci Methods (2014) 233:187–98. doi:10.1016/j.jneumeth.2014.05.021
99. McCarthy RC, Lu DY, Alkhateeb A, Gardeck AM, Lee CH, Wessling-Resnick M. Characterization of a novel adult murine immortalized microglial cell line and its activation by amyloid-beta. J Neuroinflammation (2016) 13:21. doi:10.1186/s12974-016-0484-z
100. Tsuchiya T, Park KC, Toyonaga S, Yamada SM, Nakabayashi H, Nakai E, et al. Characterization of microglia induced from mouse embryonic stem cells and their migration into the brain parenchyma. J Neuroimmunol (2005) 160:210–8. doi:10.1016/j.jneuroim.2004.10.025
101. Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol (2000) 18:675–9. doi:10.1038/76536
102. Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol (1993) 13:473–86. doi:10.1128/MCB.13.1.473
103. Napoli I, Kierdorf K, Neumann H. Microglial precursors derived from mouse embryonic stem cells. Glia (2009) 57:1660–71. doi:10.1002/glia.20878
104. Beutner C, Roy K, Linnartz B, Napoli I, Neumann H. Generation of microglial cells from mouse embryonic stem cells. Nat Protoc (2010) 5:1481–94. doi:10.1038/nprot.2010.90
105. Muffat J, Li Y, Yuan B, Mitalipova M, Omer A, Corcoran S, et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med (2016) 22:1358–67. doi:10.1038/nm.4189
106. Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol (2000) 20:4106–14. doi:10.1128/MCB.20.11.4106-4114.2000
107. Goldmann T, Wieghofer P, Jordao MJ, Prutek F, Hagemeyer N, Frenzel K, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol (2016) 17:797–805. doi:10.1038/ni.3423
108. Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci (2006) 9:917–24. doi:10.1038/nn1715
109. Hirasawa T, Ohsawa K, Imai Y, Ondo Y, Akazawa C, Uchino S, et al. Visualization of microglia in living tissues using Iba1-EGFP transgenic mice. J Neurosci Res (2005) 81:357–62. doi:10.1002/jnr.20480
110. Wolf Y, Yona S, Kim KW, Jung S. Microglia, seen from the CX3CR1 angle. Front Cell Neurosci (2013) 7:26. doi:10.3389/fncel.2013.00026
111. Wieghofer P, Knobeloch KP, Prinz M. Genetic targeting of microglia. Glia (2015) 63:1–22. doi:10.1002/glia.22727
112. Gahwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods (1981) 4:329–42. doi:10.1016/0165-0270(81)90003-0
113. Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods (1991) 37:173–82. doi:10.1016/0165-0270(91)90128-M
114. Vinet J, Weering HR, Heinrich A, Kalin RE, Wegner A, Brouwer N, et al. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J Neuroinflammation (2012) 9:27. doi:10.1186/1742-2094-9-27
115. Masuch A, van der Pijl R, Funer L, Wolf Y, Eggen B, Boddeke E, et al. Microglia replenished OHSC: a culture system to study in vivo like adult microglia. Glia (2016) 64:1285–97. doi:10.1002/glia.23002
116. Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci (2016) 19:995–8. doi:10.1038/nn.4325
117. Wieghofer P, Prinz M. Genetic manipulation of microglia during brain development and disease. Biochim Biophys Acta (2016) 1862:299–309. doi:10.1016/j.bbadis.2015.09.019
118. Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio). Eugene, OR: University of Oregon Press (2000).
119. Sieger D, Peri F. Animal models for studying microglia: the first, the popular, and the new. Glia (2013) 61:3–9. doi:10.1002/glia.22385
120. Peri F, Nusslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell (2008) 133:916–27. doi:10.1016/j.cell.2008.04.037
121. Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science (2012) 336:86–90. doi:10.1126/science.1219179
122. Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature (2007) 446:1056–61. doi:10.1038/nature05725
123. O’Koren EG, Mathew R, Saban DR. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Sci Rep (2016) 6:20636. doi:10.1038/srep20636
124. Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest (2016) 126:4674–89. doi:10.1172/JCI86950
125. Varvel NH, Neher JJ, Bosch A, Wang W, Ransohoff RM, Miller RJ, et al. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc Natl Acad Sci U S A (2016) 113:E5665–74. doi:10.1073/pnas.1604263113
126. Mildner A, Huang H, Radke J, Stenzel W, Priller J. P2Y12 receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia (2016) 65(2):375–87. doi:10.1002/glia.23097
127. Waisman A, Ginhoux F, Greter M, Bruttger J. Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol (2015) 36:625–36. doi:10.1016/j.it.2015.08.005
128. Beutner C, Linnartz-Gerlach B, Schmidt SV, Beyer M, Mallmann MR, Staratschek-Jox A, et al. Unique transcriptome signature of mouse microglia. Glia (2013) 61:1429–42. doi:10.1002/glia.22524
129. Erny D, de Angelis Hrabe AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci (2015) 18:965–77. doi:10.1038/nn.4030
130. Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science (2016) 353:aad8670. doi:10.1126/science.aad8670
131. Crotti A, Ransohoff RM. Microglial physiology and pathophysiology: insights from genome-wide transcriptional profiling. Immunity (2016) 44:505–15. doi:10.1016/j.immuni.2016.02.013
132. Wes PD, Holtman IR, Boddeke EW, Moller T, Eggen BJ. Next generation transcriptomics and genomics elucidate biological complexity of microglia in health and disease. Glia (2016) 64:197–213. doi:10.1002/glia.22866
133. Holtman IR, Noback M, Bijlsma M, Duong KN, van der Geest MA, Ketelaars PT, et al. Glia open access database (GOAD): a comprehensive gene expression encyclopedia of glia cells in health and disease. Glia (2015) 63:1495–506. doi:10.1002/glia.22810
134. Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol (2015) 28:203–9.
135. Neiva I, Malva JO, Valero J. Can we talk about microglia without neurons? A discussion of microglial cell autonomous properties in culture. Front Cell Neurosci (2014) 8:202. doi:10.3389/fncel.2014.00202
136. Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci (2016) 19:504–16. doi:10.1038/nn.4222
137. Doorn KJ, Breve JJ, Drukarch B, Boddeke HW, Huitinga I, Lucassen PJ, et al. Brain region-specific gene expression profiles in freshly isolated rat microglia. Front Cell Neurosci (2015) 9:84. doi:10.3389/fncel.2015.00084
138. Oosterhof N, Holtman IR, Kuil LE, van der Linde HC, Boddeke EW, Eggen BJ, et al. Identification of a conserved and acute neurodegeneration-specific microglial transcriptome in the zebrafish. Glia (2016) 65(1):138–49. doi:10.1002/glia.23083
139. Satoh J, Kino Y, Asahina N, Takitani M, Miyoshi J, Ishida T, et al. TMEM119 marks a subset of microglia in the human brain. Neuropathology (2016) 36:39–49. doi:10.1111/neup.12235
140. Biber K, Moller T, Boddeke E, Prinz M. Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat Rev Drug Discov (2016) 15:110–24. doi:10.1038/nrd.2015.14
141. Darmanis S, Sloan SA, Zhang Y, Enge M, Caneda C, Shuer LM, et al. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci U S A (2015) 112:7285–90. doi:10.1073/pnas.1507125112
142. Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science (2015) 347:1138–42. doi:10.1126/science.aaa1934
Keywords: microglia history, Rio Hortega, technology, genome-wide, microgliome
Citation: Sousa C, Biber K and Michelucci A (2017) Cellular and Molecular Characterization of Microglia: A Unique Immune Cell Population. Front. Immunol. 8:198. doi: 10.3389/fimmu.2017.00198
Received: 30 November 2016; Accepted: 09 February 2017;
Published: 02 March 2017
Edited by:
Fabrice Cognasse, The Rhone-Alpes-Auvergne Regional Branch of the French National Blood System, FranceReviewed by:
Sophie Laye, Centre Bordeaux-Aquitaine (INRA), FranceCopyright: © 2017 Sousa, Biber and Michelucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Michelucci, YWxlc3NhbmRyby5taWNoZWx1Y2NpQGxpaC5sdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.