
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 13 February 2017
Sec. Inflammation
Volume 8 - 2017 | https://doi.org/10.3389/fimmu.2017.00139
This article is part of the Research TopicRegulation of Inflammation in Chronic DiseaseView all 48 articles
In the last two decades, many studies have focused on whether periodontitis is a risk factor for preterm birth (PTB). However, both epidemiological investigation and intervention trials have reached contradictory results from different studies. What explains the different findings, and how should future studies be conducted to better assess this risk factor? This article reviews recent epidemiological, animal, and in vitro studies as well as intervention trials that evaluate the link between periodontitis and PTB. Periodontitis may act as a distant reservoir of microbes and inflammatory mediators and contribute to the induction of PTB. Animal studies revealed that maternal infections with periodontal pathogens increase levels of circulating IL-1β, IL-6, IL-8, IL-17, and TNF-α and induce PTB. In vitro models showed that periodontal pathogens/byproducts induce COX-2, IL-8, IFN-γ, and TNF-α secretion and/or apoptosis in placental tissues/cells. The effectiveness of periodontal treatment to prevent PTB is influenced by the diagnostic criteria of periodontitis, microbial community composition, severity of periodontitis, treatment strategy, treatment efficiency, and the period of treatment during pregnancy. Although intervention trials reported contradictory results, oral health maintenance is an important part of preventive care that is both effective and safe throughout pregnancy and should be supported before and during pregnancy. As contradictory epidemiological and intervention studies continue to be published, two new ideas are proposed here: (1) severe and/or generalized periodontitis promotes PTB and (2) periodontitis only promotes PTB for pregnant women who are young or HIV-infected or have preeclampsia, pre-pregnancy obesity, or susceptible genotypes.
Each year, about 15 million infants worldwide are born preterm (before 37 weeks of gestation), and these preterm babies typically have low birth weight (LBW, <2,500 g) (1). Preterm birth (PTB) is the leading cause of neonatal mortality, morbidity, and developmental loss (2). Advances in obstetric care have not altered the rates of PTB, and it is estimated that 9.6% of worldwide births are preterm (3). The highest rates of PTB are in Africa (11.9%) and North America (10.6%), and the lowest rates are in Europe (6.2%) (3). However, the underlying causes of PTB are still not entirely clear, thus an accurate identification of risk factors for PTB that are amenable to intervention would have far-reaching and long-lasting impact.
Of the multiple risk factors for PTB, maternal infection is identified consistently. Periodontal disease is a highly prevalent infectious and inflammatory disease of tooth-supporting tissues and if untreated can lead to oral disabilities (Figure 1A) (4). Periodontal disease is caused mainly by gram-negative microaerophilic and anaerobic bacteria that colonize the subgingival area and produce significant amounts of proinflammatory mediators (5). Periodontal disease includes gingivitis and periodontitis. Gingivitis is the presence of gingival inflammation without loss of connective tissue attachment. Periodontitis is the presence of gingival inflammation at sites where there has been apical migration of the epithelial attachment onto the root surfaces accompanied by the loss of connective tissue and alveolar bone (Figure 1B) (6). In the last two decades, many studies have examined the relationship between periodontitis and PTB. Periodontitis may be a risk factor for PTB due to the presence in the bloodstream of bacteria and proinflammatory cytokines during infection that can affect distant organs (7). However, epidemiological studies and intervention trials have reached contradictory conclusions about the relationship between periodontitis and PTB. The aim of this review is to summarize the current evidence from epidemiological, animal, and in vitro studies, as well as intervention trials and to propose new ideas about the link between periodontitis and PTB.
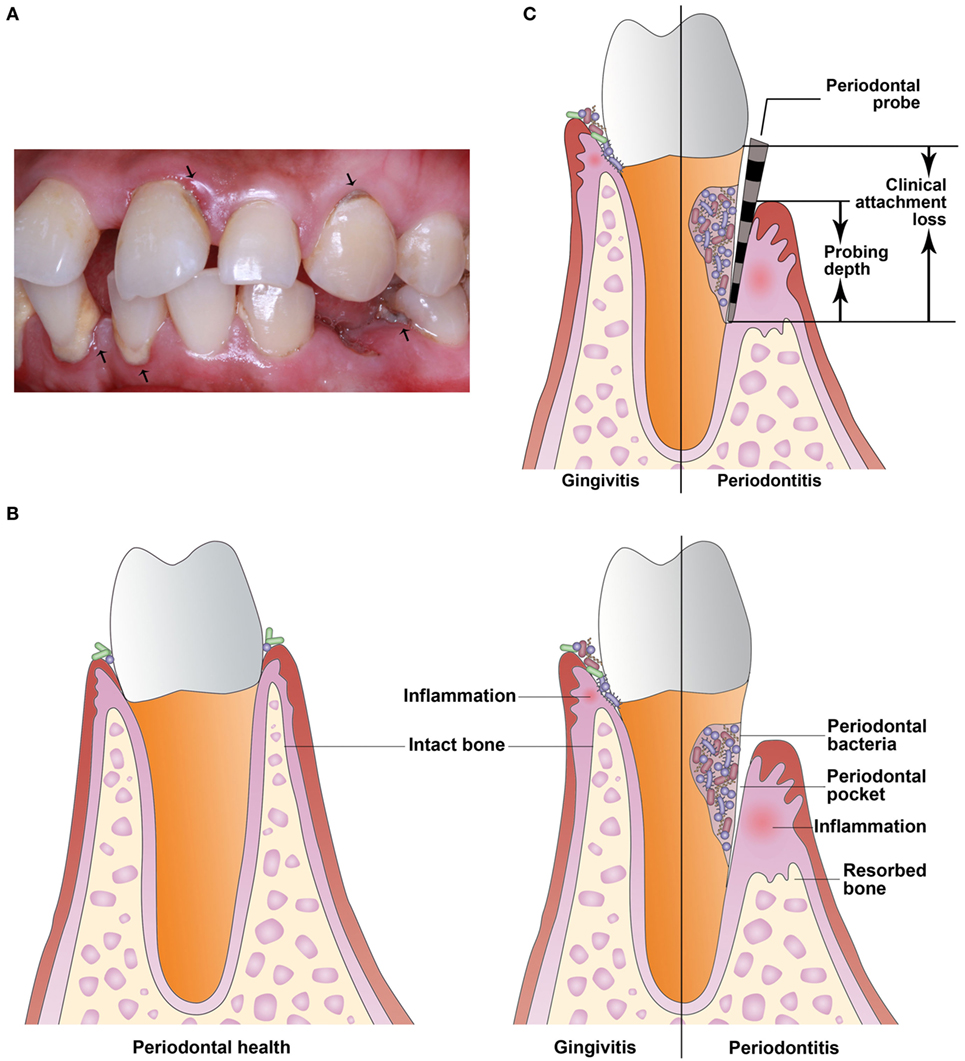
Figure 1. Periodontal disease is a highly prevalent infectious and inflammatory disease of tooth-supporting tissues. (A) The arrowheads indicate periodontal disease. (B) Periodontal disease includes gingivitis and periodontitis. Gingivitis is the presence of gingival inflammation without loss of connective tissue attachment. Periodontitis is the presence of gingival inflammation at sites where there has been apical migration of the epithelial attachment onto the root surfaces accompanied by the loss of connective tissue and alveolar bone. (C) Clinical attachment loss is measured with a periodontal probe and is the distance from the base of the probeable crevice to the cementoenamel junction. Probing depth is defined as the distance between the bottom of the periodontal pocket and the gingival margin.
About one-third of all PTB are caused by preterm labor (uterine contraction) and one-third are due to the premature rupture of membranes (PROM); the remaining cases are due to other pregnancy complications such as induced labor (of which preeclampsia is the major indication) (8). In the last two decades, numerous epidemiological studies have examined the link between periodontitis and PTB, including cross-sectional, case–control, and cohort studies.
In a cross-sectional study, or census, data are collected at a defined time and is used to assess the prevalence of chronic or acute conditions, the results of intervention, or the causes of disease. In the last 5 years, several cross-sectional studies (9–11) reported a correlation between periodontitis and PTB/LBW. In a study published in 2016 (9), women with PTB were found to have worse periodontal parameters and significantly increased gingival crevicular fluid (GCF) levels of IL-6 and prostaglandin E2 (PGE2) compared with women who experienced full-term birth. Based on significant correlations between serum PGE2 level and probing depth, clinical attachment loss (CAL, Figure 1C), and GCF TNF-α in PTB, periodontitis may increase the risk of labor triggers and hence contribute to preterm labor onset. However, in 2016 Martinez-Martinez et al. (12) suggested that PTB is a multifactorial condition and that periodontitis and the presence of periodontal pathogens are not sufficient to trigger PTB.
In case–control studies, mothers with PTB are identified and their periodontitis history is determined and compared with that of healthy control subjects. Of the 14 case–control studies published in the last 5 years, 12 (13–24) reported an association between periodontitis and PTB, LBW, or preterm LBW (PLBW), and 2 (25, 26) found no association. In a study published in 2015 (23), mothers in the periodontitis group with single delivery had an eightfold higher chance of delivering a LBW infant compared to those in the control group. The mothers in the periodontitis group with multiple deliveries delivered PTB infants with an eightfold higher frequency and LBW infants at a 10-fold higher frequency compared to the mothers in the control group.
In cohort studies, investigators monitor pregnant women to determine if those with periodontitis demonstrate a higher incidence of PTB than those without periodontitis. Of the 11 published cohort studies in the last 5 years, 7 (27–33) reported an association between periodontitis and PTB, LBW, or PLBW, and 4 (34–37) revealed no association. A hospital-based prospective study published in 2016 (33) comprising 790 pregnant women found that periodontitis was a risk factor for PTB and an independent risk factor for LBW. Recently periodontitis was also found to be associated with preeclampsia (38) and PROM (39, 40), common causes of PTB.
In the last 5 years, most literature and systematic reviews reported an association between periodontitis and PTB (41–46). A meta-analysis published in 2016 (41) assessed case–control studies reporting periodontal status and pregnancy outcomes. The computed risk ratio for periodontitis was 1.61 for PTB using data from 16 studies, the risk for LBW was 1.65 based on 10 studies, and the risk for PLBW was 3.44 based on 4 studies. A systematic and evidence-based review in 2012 (46) focused on the association of periodontitis and PTB and LBW and found 62 relevant studies that suggested that periodontitis may be a potential risk factor for PTB and LBW.
Different epidemiological data may have reached different conclusions due to the following reasons: (1) many studies used different definitions of periodontitis and adverse pregnancy outcomes, for instance the use of PLBW as a composite outcome, or PTB versus LBW, terms that reflect different disease severities and pathologic entities. (2) The risk factors of PTB may be similar to the risk factors for periodontitis (ethnicity, tobacco use, and socioeconomic and educational levels) and thus may confound the association between periodontitis and PTB. In a prospective case–control study (47), PTB was associated with periodontitis when the USA (48), but not the European (49), definitions were used. Therefore, future studies should employ both continuous and categorical assessment of periodontal status and control for confounding factors. Additionally, the further use of the composite outcome PLBW is not encouraged.
Periodontitis is one of the most common chronic infectious diseases and is caused mainly by gram-negative microaerophilic and anaerobic bacteria that colonize the subgingival area and produce significant amounts of proinflammatory mediators, mainly IL-1β, IL-6, PGE2, and TNF-α (4). Periodontitis may act as a distant reservoir of both microbes and inflammatory mediators that may influence pregnancy and contribute to induction of PTB (Figure 2). These two potential mechanisms for how periodontitis can affect PTB are described more fully below.
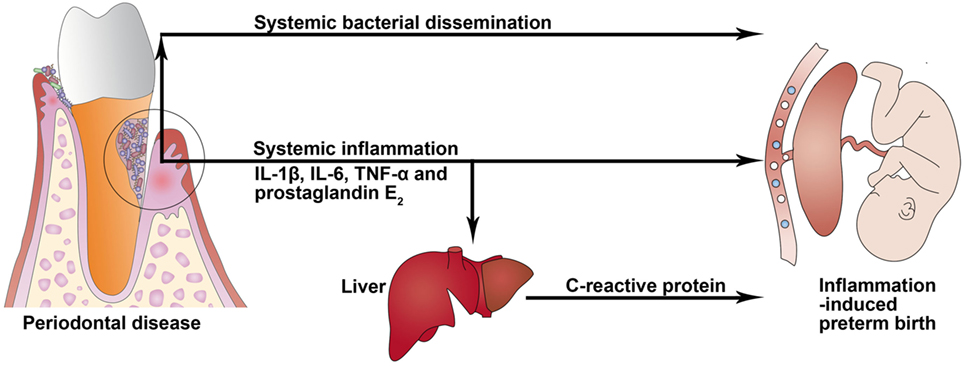
Figure 2. Potential biological mechanisms linking periodontal disease to preterm birth. In periodontitis, gingival ulceration in the periodontal pocket enables egress and systemic bacterial dissemination, and locally produced proinflammatory cytokines can enter systemic circulation and induce an acute-phase response in the liver that is characterized by an increased level of C-reactive protein.
Periodontal microorganisms can act as pathogens not only in the oral cavity but also in other body areas. This is due to the following characteristics of bacteria: (1) the ability to rapidly colonize, (2) the ability to elude the host’s defense mechanisms, and (3) the ability to produce substances that directly contribute to the destruction of tissue. Periodontal pathogens/byproducts may reach the placenta and enter the amniotic fluid and fetal circulation, serving to activate inflammatory signaling pathways.
Porphyromonas gingivalis has been detected in human placenta tissues (50). Interestingly, one study reported that P. gingivalis was only detected within the villous mesenchyme in the preterm cohort, but not the term group (51). Thus, the detection of P. gingivalis in the placenta may be related to PTB (52, 53).
The Fusobacterium nucleatum subsp. polymorphum strain was not detected in vaginal samples, but was found in both neonatal gastric aspirates and oral samples from mothers with PTB and localized periodontal pockets, which strongly indicated that F. nucleatum subsp. polymorphum of oral origin may relate to PTB (54). Bohrer et al. (55) reported a case of acute chorioamnionitis caused by F. nucleatum that progressed to maternal sepsis in a term patient with intact membranes. Cassini et al. (56) reported that the presence of periodontal pathogen Treponema denticola in the vagina, regardless of the levels, increased risk of PTB.
Levels of P. gingivalis, F. nucleatum, Actinomyces actinomycetemcomitans, Tannerella forsythia, T. denticola, Eikenella corrodens, and Capnocytophaga spp. have been reported at significantly higher levels in preterm deliveries as compared to term births (37, 52, 57). In one study (58), when Prevotella intermedia and/or Aggregatibacter actinomycetemcomitans were not detected in maternal periodontal pockets, the infants were more than 129% likely to have a normal birth weight.
The above findings suggest that periodontal bacteria may be normally present in the placenta. However, the levels of certain periodontal pathogens in the placenta may be dependent on the maternal periodontal state (59). Further studies are needed to elucidate the role of microbial load and maternal immune responses in PTB.
Clinical attachment loss, as the main periodontal measure, is associated with plasma levels of IL-1β and TNF-α in pregnant women (60), which may promote labor activation through placental and chorion–amnion production of PGE2 (61). Women with PTB demonstrated significantly increased GCF levels of IL-6 and PGE2 compared with those who had full-term births (9). A systematic review in 2013 (62) reported an association between GCF inflammatory mediator levels and adverse pregnancy outcomes. In a subset of patients with severe periodontitis, locally produced proinflammatory mediators—such as IL-1β, IL-6, and TNF-α—can enter systemic circulation and induce an acute-phase response in the liver that is characterized by an increased level of C-reactive protein (CRP) (63, 64). Serum CRP level was reported to be elevated in subjects with periodontitis (65). An increased CRP level can enhance the risk of cardiovascular disease, cerebrovascular accidents, and PLBW infants (65).
Clinical studies support the association between increased levels of circulating proinflammatory mediators and PTB (66, 67) and have implicated IL-1β and IL-6 as major players in the onset of PTB (68, 69). Moreover, polymorphisms in proinflammatory genes, including the above-mentioned cytokines, have been associated with PTB (70, 71). In addition, elevated amniotic fluid level of IL-6 in the second trimester was associated with the initiation and timing of PTB (72, 73). Therefore, the potential link between periodontitis and PTB may be explained by the following mechanisms. First, periodontal pathogens/byproducts can disseminate toward the placental and fetal tissues. Immune/inflammatory reactions within the placental tissues of the pregnant woman may occur, and the release of proinflammatory mediators in the amniotic fluid may increase and further contribute to PTB. Second, systemic inflammatory changes induced by periodontitis can exacerbate local inflammatory responses within the fetoplacental unit to increase the risk for PTB.
The possible roles of periodontitis in PTB have also been explored using experimental animal models. In separate studies, periodontal bacteria were injected into a small chamber in pregnant animals, allowing the establishment of a site of infection distant to the fetal–placental unit to mimic a periodontal infection in a reproducible and simplified manner. These results revealed that maternal infections with periodontal pathogens increase pregnancy complications.
Dental infection of mice with P. gingivalis significantly increased levels of circulating IL-1β, IL-6, IL-17, and TNF-α (74). Defects in the placental tissues of P. gingivalis-infected mice included degenerative changes in endothelial and trophoblast cells, increased placental detachment, and PROM, and P. gingivalis was detected in placental tissues by PCR and immunohistochemistry (74). The P. gingivalis-infected group delivered at gestational day (gd) 18.25 versus gd 20.45 for the non-infected control group (p < 0.01), with pups exhibiting LBW compared to controls (p < 0.01) (74). In another study (75), mice with periodontitis induced by using an inoculum of P. gingivalis and F. nucleatum exhibited increased circulating levels of IL-6 and IL-8. Similarly, Miyoshi et al. (76) found high levels of contractile-associated proteins and ion channels in the myometrium of PTB model mice with chronic odontogenic P. gingivalis infection. In murine models, F. nucleatum translocated and caused intrauterine infections (77) and Campylobacter rectus significantly decreased fetoplacental weight (78). In a baboon model, a significantly greater frequency of the periodontitis group neonates had decreased gestational age and LBW (79). Spontaneous abortion/stillbirth/fetal demise was increased in the periodontitis group versus the control group (79). Also, combined oral infection of mice with P. gingivalis and C. rectus significantly reduced overall fecundity compared to controls (80). Overall, most animal studies reported a harmful impact of periodontitis on pregnancy outcome. However, a study performed by Fogacci et al. (81) found no increased risk for PTB or LBW in Wistar rats with induced periodontitis.
In addition to the data from animal studies, in vitro experiments have been conducted to explore the molecular mechanisms underlying periodontitis-induced PTB. In most in vitro models, periodontal pathogens/byproducts were used to infect placental tissues or trophoblast cells/cell lines. These experiments were designed to mimic an in vitro periodontal infection in a simplified and reproducible manner to allow investigation of the interaction between periodontal pathogens and placental tissues/cells.
Riewe et al. (82) investigated the transcriptional responses after infection with P. gingivalis in extravillous trophoblast (HTR8) cells derived from the human placenta, and found that over 2,000 genes were differentially regulated. P. gingivalis induced IL-8 and IFN-γ secretion (83), apoptosis, and arrest in the G(1) phase of the cell cycle (84, 85) in HTR8 cells. Increased IFN-γ secretion and Fas expression occurred in P. gingivalis-induced apoptosis of HTR8 cells via the ERK1/2 pathway (86). In normal human term fetal membrane explants, P. gingivalis may significantly increase TLR7 expression (87).
Porphyromonas gingivalis has bioactive components on the cell surface, including lipopolysaccharide, capsules, and fimbriae. P. gingivalis lipopolysaccharide induces the production of IL-6 and IL-8 via TLR2 in chorion-derived cells (50) and increases expression levels of IL-8, TNF-α, and COX-2 in HTR8 cells in an NF-κB-dependent manner (74). Interestingly, Komine-Aizawa et al. (88) reported that although there is limited direct pathogenic effect of P. gingivalis lipopolysaccharide on trophoblast invasion, concurrent smoking reduces trophoblast invasion into the myometrium and thus inhibits maternal vascular reconstruction.
In human placental trophoblast-like BeWo cells, the presence of A. actinomycetemcomitans lipopolysaccharide led to increased levels of cytochrome c, caspase-2, caspase-3, caspase-9, and BCL2-antagonist/killer 1 mRNA and decreased levels of B-cell CLL/lymphoma 2, BCL2-like 1, and catalase mRNA, consistent with the activation of the mitochondria-dependent apoptotic pathway (89). Additionally, C. rectus was reported to effectively invade BeWo cells and upregulate both mRNA and protein levels of IL-6 and TNF-α in a dose-dependent manner (78). Therefore, there is significant evidence that periodontal pathogens and byproducts can induce inflammation and/or apoptosis in placental tissues and cells.
Periodontal treatment usually refers to non-surgical periodontal therapy that can improve periodontal health and is defined as plaque removal, plaque control, supragingival and subgingival scaling, root surface debridement, and the adjunctive use of chemical agents. To evaluate periodontitis as a risk factor for PTB, intervention studies were conducted to evaluate the effect of periodontal treatment on the risk of PTB. In these studies, women with preexisting periodontitis were randomly divided into two groups. One received periodontal treatment during or before pregnancy, and the other did not. In this type of study, the researchers could assess if periodontitis was an independent risk factor for PTB by determining if periodontal treatment decreased the incidence of PTB.
In a study published in 2015 (90), the mean gestational age in the periodontal treatment group (treatment performed during the second trimester of the gestational period) was 35.57 ± 2.40 versus 34.17 ± 2.92 weeks in the non-treated group (p < 0.05). The treatment group showed a statistically significant reduction in mean CRP levels after delivery compared to baseline values; the control group showed no significant reduction in CRP levels. Another study (91) suggested that periodontal treatment during pregnancy is not only safe for both the child and the mother, but also provides beneficial effects for pregnancy and embryo-fetal development, leading to reduced morbidity and mortality in PTB infants. Macedo et al. (92) also reported an association between a low number of daily tooth brushings and PTB. However, in other recent studies (93–96), periodontal treatment performed on pregnant women was not found to be efficacious in reducing PTB or LBW.
Data from recent systematic reviews are also contradictory. Several reviews (97–99) reported that the risks of perinatal outcomes could be potentially reduced by periodontal treatment in pregnant women, but other reviews (100–103) suggested that periodontal treatment during pregnancy was not an efficient way to reduce the incidence of PTB. However, the evidence was not conclusive due to confounding effects and risks of random errors and bias. Thus, further randomized clinical trials are still necessary.
The preventive effectiveness of periodontal treatment for PTB has still not been established, because it is influenced by many factors such as the diagnostic criteria of periodontitis, microbial community composition, severity of disease, treatment strategy, treatment efficiency, and the timing of treatment during pregnancy (the pre-pregnancy period or during the first or second trimester). Jeffcoat et al. (104) confirmed that decreased PTB may depend on the success of periodontal treatment. In this study of 322 pregnant women with periodontitis, 162 were randomly assigned to receive only oral hygiene instruction and served as the untreated control group, whereas the remaining 160 received scaling and root planing treatment as well as oral hygiene instruction. No significant difference was found between the incidence of PTB in the periodontal treatment group and the control group. However, a logistic regression analysis showed a significant and strong relationship between successful periodontal treatment and full-term birth. Subjects refractory to periodontal treatment were significantly more likely to have PTB. Similarly, in another study (105), periodontal treatment during pregnancy reduced the levels of IL-1β, IL-6, IL-10, and IL-12 in GCF and improved dental parameters. Additionally, the severity of periodontitis was significantly associated with an increased risk of babies born small for gestational age, but no changes in pregnancy-related outcomes were observed following periodontal treatment. These studies suggest the need for the next randomized controlled trials to standardize methodological criteria and utilize a more precise definition of periodontitis. Additionally, for better statistical power, studies should preferably be performed as multicenter studies, and include a large number of participants. Finally, the resulting success or failure of periodontal treatment must be considered.
Preterm birth is the leading cause of infant morbidity and mortality, but classical risk factors explain only one-third of PTB cases, and current intervention strategies have not led to an appreciable reduction of PTB. Therefore, it is necessary to explore mechanisms of causality and generate new hypotheses using an integrated approach. This should be done with increased collaboration among research groups, and using more comprehensive theoretical–methodological approaches to formulate more effective intervention strategies and to detect new risk factors. Although intervention during pregnancy has not consistently been correlated with a reduction in PTB rates, oral health maintenance is an important part of preventive care that is both effective and safe throughout pregnancy and should be supported before and during pregnancy.
The severity of periodontitis can be categorized based on CAL as follows: mild = 1–2 mm, moderate = 3–4 mm, and severe ≥5 mm (106). CAL is measured with a periodontal probe and is the distance from the base of the probeable crevice to the cementoenamel junction (107). In a case–control study (92), periodontitis that met definition 1 (four or more teeth with at least one site showing CAL of ≥3 mm and probing depth of ≥4 mm) was not associated with fewer weeks of gestation. However, a significant association was found between PTB and periodontitis that was classified according to definition 2 (four or more teeth with at least one site showing CAL of ≥4 mm and probing depth of ≥4 mm). A cohort study (108) of preeclamptic women showed that 49.3% of patients with mild periodontitis and 82.6% of patients with moderate to severe periodontitis delivered preterm. Several studies (47, 109–111) found a highly significant association between PTB and moderate to severe periodontitis. Other studies (112, 113) also suggested that the strength of the association between periodontitis and PTB incidence is higher with increased severity of periodontitis.
Periodontitis can also be defined according to the extent of the disease. Generalized periodontitis is defined as CAL ≥3 mm and probing depth ≥4 mm on four or more teeth and localized periodontitis is defined as CAL ≥3 mm and probing depth ≥4 mm on two or three teeth (6). In a case–control multi-center study (114) of singleton live births, periodontal examinations after delivery identified generalized and localized periodontitis. Generalized periodontitis was identified in 13.4% of PTB women and in 10.8% of control women, and localized periodontitis was identified in 11.6 and 10.8%, respectively (114). A significant association was observed between generalized periodontitis and PTB (114). A case–control study (115) confirmed that only the presence of gingival recession for more than two teeth increased the risk of PTB. In addition, several studies (108, 111, 116) reported greater risk for PTB for mothers if periodontitis progressed during pregnancy.
Usin et al. (58) reported that the presence of periodontal pathogens in periodontal pockets from pregnant women with different periodontal status was only associated with PLBW infants for young mothers. Pattrapornnan et al. (117) also found a positive risk of PTB, LBW, and PLBW in HIV-infected pregnant women with periodontitis. Nabet et al. (114) demonstrated a significant association between periodontitis and PTB for preeclampsia. Riche et al. (108) and Pattanashetti et al. (118) confirmed that pregnant women with preeclampsia exhibited a greater risk for PTB if periodontitis was present or progressed during pregnancy. Interestingly, Lee et al. (119) reported that pregnant women with periodontitis were 5.56 times more likely to have PTB with preeclampsia than women without periodontitis and that the association was much stronger (odds ratio 15.94) in women with both periodontitis and obesity. In fact, there is a strong association between pre-pregnancy obesity and periodontitis in pregnant females (120).
Genetic factors involved in altered immune response against bacterial infections may also influence the effect of periodontitis in pregnancy. Periodontitis induced by P. gingivalis was found to drive periodontal microbiota dysbiosis and cause systemic disease via an impaired adaptive immune response in mice (121). Jeffcoat et al. (122) reported a significant relation between a specific polymorphism of prostaglandin E receptor 3 (a gene associated with inflammatory response) and both periodontitis treatment failure and spontaneous PTB.
Here, for the first time, we describe four possible models of periodontitis in PTB: (1) periodontitis is an independent risk factor for PTB; (2) severe and/or generalized periodontitis promotes PTB; (3) periodontitis only promotes PTB for pregnant women who are young or HIV-infected or have preeclampsia, pre-pregnancy obesity, or susceptible genotypes; and (4) periodontitis has no significant effect on PTB. Because contradictory epidemiological data continue to emerge (model 1 versus 4), future studies should try to test the second and third models, which may, to some extent, explain the conflicting epidemiological data. Although intervention during pregnancy has not consistently been correlated with a reduction in PTB rates, oral health maintenance is an important part of preventive care that is both effective and safe throughout pregnancy and should be supported before and during pregnancy.
HR contributed to the literature search, interpretation, writing, and proofreading of the manuscript. MD designed the study and made the ultimate decision on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the National Natural Science Foundation of China (NSFC Grant No. 81371145).
1. Zi MY, Longo PL, Bueno-Silva B, Mayer MP. Mechanisms involved in the association between periodontitis and complications in pregnancy. Front Public Health (2014) 2:290. doi: 10.3389/fpubh.2014.00290
2. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet (2008) 371(9606):75–84. doi:10.1016/S0140-6736(08)60074-4
3. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ (2010) 88(1):31–8. doi:10.2471/BLT.08.062554
4. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet (2005) 366(9499):1809–20. doi:10.1016/S0140-6736(05)67728-8
5. Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol (2008) 79(8 Suppl):1585–91. doi:10.1902/jop.2008.080183
6. Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000 (2004) 34:9–21. doi:10.1046/j.0906-6713.2002.003421.x
7. Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect (2007) 13(Suppl 4):3–10. doi:10.1111/j.1469-0691.2007.01798.x
8. Bobetsis YA, Barros SP, Offenbacher S. Exploring the relationship between periodontal disease and pregnancy complications. J Am Dent Assoc (2006) 137:7S–13S. doi:10.14219/jada.archive.2006.0403
9. Perunovic N, Rakic MM, Nikolic LI, Jankovic SM, Aleksic ZM, Plecas DV, et al. The association between periodontal inflammation and labor triggers (elevated cytokine levels) in preterm birth: a cross-sectional study. J Periodontol (2016) 87(3):248–56. doi:10.1902/jop.2015.150364
10. Muwazi L, Rwenyonyi CM, Nkamba M, Kutesa A, Kagawa M, Mugyenyi G, et al. Periodontal conditions, low birth weight and preterm birth among postpartum mothers in two tertiary health facilities in Uganda. BMC Oral Health (2014) 14(1):42. doi:10.1186/1472-6831-14-42
11. Takeuchi N, Ekuni D, Irie K, Furuta M, Tomofuji T, Morita M, et al. Relationship between periodontal inflammation and fetal growth in pregnant women: a cross-sectional study. Arch Gynecol Obstet (2013) 287(5):951–7. doi:10.1007/s00404-012-2660-4
12. Martinez-Martinez RE, Moreno-Castillo DF, Loyola-Rodriguez JP, Sanchez-Medrano AG, Miguel-Hernandez JH, Olvera-Delgado JH, et al. Association between periodontitis, periodontopathogens and preterm birth: is it real? Arch Gynecol Obstet (2016) 294(1):47–54. doi:10.1007/s00404-015-3945-1
13. Chakki BA, Ealla KR, Hunsingi P, Kumar A, Manidanappanavar P. Influence of maternal periodontal disease as a risk factor for low birth weight infants in Indian population. J Contemp Dent Pract (2012) 13(5):676–80. doi:10.5005/jp-journals-10024-1208
14. Khadem N, Rahmani ME, Sanaei A, Afiat M. Association between preterm and low-birth weight with periodontal disease: a case-control study. Iran J Reprod Med (2012) 10(6):561–6.
15. Piscoya MD, Ximenes RA, Silva GM, Jamelli SR, Coutinho SB. Maternal periodontitis as a risk factor for prematurity. Pediatr Int (2012) 54(1):68–75. doi:10.1111/j.1442-200X.2011.03502.x
16. Baig SA, Khan N, Baqai T, Fatima A, Karim SA, Aziz S. Preterm birth and its associated risk factors. A study at tertiary care hospitals of Karachi, Pakistan. J Pak Med Assoc (2013) 63(3):414–8.
17. Mesa F, Pozo E, Blanc V, Puertas A, Bravo M, O’Valle F. Are periodontal bacterial profiles and placental inflammatory infiltrate in pregnancy related to birth outcomes? J Periodontol (2013) 84(9):1327–36. doi:10.1902/jop.2012.120462
18. Jacob PS, Nath S. Periodontitis among poor rural Indian mothers increases the risk of low birth weight babies: a hospital-based case control study. J Periodontal Implant Sci (2014) 44(2):85–93. doi:10.5051/jpis.2014.44.2.85
19. Govindaraju P, Venugopal S, Shivakumar MA, Sethuraman S, Ramaiah SK, Mukundan S. Maternal periodontal disease and preterm birth: a case-control study. J Indian Soc Periodontol (2015) 19(5):512–5. doi:10.4103/0972-124X.164751
20. Kayar NA, Alptekin NO, Erdal ME. Interleukin-1 receptor antagonist gene polymorphism, adverse pregnancy outcome and periodontitis in Turkish women. Arch Oral Biol (2015) 60(12):1777–83. doi:10.1016/j.archoralbio.2015.09.013
21. Kayar NA, Alptekin NO, Haliloglu S. Interleukin-1 receptor antagonist levels in gingival crevicular fluid and serum in nonsmoking women with preterm low birth weight and intrauterine growth retardation. Eur J Dent (2015) 9(1):109–16. doi:10.4103/1305-7456.149655
22. Leal AS, de Oliveira AE, Brito LM, Lopes FF, Rodrigues VP, Lima KF, et al. Association between chronic apical periodontitis and low-birth-weight preterm births. J Endod (2015) 41(3):353–7. doi:10.1016/j.joen.2014.11.018
23. Reza KM, Hamissi JH, Naeini SR, Karimi M. The relationship between maternal periodontal status of and preterm and low birth weight infants in Iran: a case control study. Glob J Health Sci (2015) 8(5):184–8. doi:10.5539/gjhs.v8n5p184
24. Pozo E, Mesa F, Ikram MH, Puertas A, Torrecillas-Martinez L, Ortega-Oller I, et al. Preterm birth and/or low birth weight are associated with periodontal disease and the increased placental immunohistochemical expression of inflammatory markers. Histol Histopathol (2016) 31(2):231–7. doi:10.14670/HH-11-671
25. Sugita N, Kobayashi T, Kikuchi A, Shimada Y, Hirano E, Sasahara J, et al. Immunoregulatory gene polymorphisms in Japanese women with preterm births and periodontitis. J Reprod Immunol (2012) 93(2):94–101. doi:10.1016/j.jri.2012.01.005
26. Abati S, Villa A, Cetin I, Dessole S, Luglie PF, Strohmenger L, et al. Lack of association between maternal periodontal status and adverse pregnancy outcomes: a multicentric epidemiologic study. J Matern Fetal Neonatal Med (2013) 26(4):369–72. doi:10.3109/14767058.2012.733776
27. Al HR, Khader YS, Jabali OA, Alchalabi H. Prediction of preterm and low birth weight delivery by maternal periodontal parameters: receiver operating characteristic (ROC) curve analysis. Matern Child Health J (2013) 17(2):299–306. doi:10.1007/s10995-012-0974-2
28. Alchalabi HA, Al HR, Jabali OA, Khader YS. Association between periodontal disease and adverse pregnancy outcomes in a cohort of pregnant women in Jordan. Clin Exp Obstet Gynecol (2013) 40(3):399–402.
29. Kumar A, Basra M, Begum N, Rani V, Prasad S, Lamba AK, et al. Association of maternal periodontal health with adverse pregnancy outcome. J Obstet Gynaecol Res (2013) 39(1):40–5. doi:10.1111/j.1447-0756.2012.01957.x
30. Wang YL, Liou JD, Pan WL. Association between maternal periodontal disease and preterm delivery and low birth weight. Taiwan J Obstet Gynecol (2013) 52(1):71–6. doi:10.1016/j.tjog.2013.01.011
31. Basha S, Shivalinga SH, Noor MR. Maternal periodontitis as a possible risk factor for preterm birth and low birth weight – a prospective study. Oral Health Prev Dent (2015) 13(6):537–44. doi:10.3290/j.ohpd.a34053
32. Soroye M, Ayanbadejo P, Savage K, Oluwole A. Association between periodontal disease and pregnancy outcomes. Odontostomatol Trop (2015) 38(152):5–16.
33. Tellapragada C, Eshwara VK, Bhat P, Acharya S, Kamath A, Bhat S, et al. Risk factors for preterm birth and low birth weight among pregnant Indian women: a hospital-based prospective study. J Prev Med Public Health (2016) 49(3):165–75. doi:10.3961/jpmph.16.022
34. Ali TB, Abidin KZ. Relationship of periodontal disease to pre-term low birth weight infants in a selected population – a prospective study. Community Dent Health (2012) 29(1):100–5.
35. Harper LM, Parry S, Stamilio DM, Odibo AO, Cahill AG, Strauss JR, et al. The interaction effect of bacterial vaginosis and periodontal disease on the risk of preterm delivery. Am J Perinatol (2012) 29(5):347–52. doi:10.1055/s-0031-1295644
36. Schenkein HA, Koertge TE, Sabatini R, Brooks CN, Gunsolley JC. Birth weight of infants of mothers with aggressive periodontitis. J Periodontol (2012) 83(3):279–86. doi:10.1902/jop.2011.110192
37. Santa CI, Herrera D, Martin C, Herrero A, Sanz M. Association between periodontal status and pre-term and/or low-birth weight in Spain: clinical and microbiological parameters. J Periodontal Res (2013) 48(4):443–51. doi:10.1111/jre.12024
38. Soucy-Giguere L, Tetu A, Gauthier S, Morand M, Chandad F, Giguere Y, et al. Periodontal disease and adverse pregnancy outcomes: a prospective study in a low-risk population. J Obstet Gynaecol Can (2016) 38(4):346–50. doi:10.1016/j.jogc.2016.02.012
39. Lauren M, Minire A, Maldi X, Mirton M, Aferdita M. The impact of periodontitis in the preterm birth and body size of newborns. Mater Sociomed (2012) 24(1):44–7. doi:10.5455/msm.2012.24.44-47
40. Stadelmann PF, Eick S, Salvi GE, Surbek D, Mohr S, Burgin W, et al. Increased periodontal inflammation in women with preterm premature rupture of membranes. Clin Oral Investig (2015) 19(6):1537–46. doi:10.1007/s00784-014-1371-6
41. Corbella S, Taschieri S, Del FM, Francetti L, Weinstein R, Ferrazzi E. Adverse pregnancy outcomes and periodontitis: a systematic review and meta-analysis exploring potential association. Quintessence Int (2016) 47(3):193–204. doi:10.3290/j.qi.a34980
42. Ide M, Papapanou PN. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes – systematic review. J Clin Periodontol (2013) 40(Suppl 14):S181–94. doi:10.1111/jcpe.12063
43. Baskaradoss JK, Geevarghese A, Al DA. Causes of adverse pregnancy outcomes and the role of maternal periodontal status – a review of the literature. Open Dent J (2012) 6:79–84. doi:10.2174/1874210601206010079
44. Corbella S, Taschieri S, Francetti L, De Siena F, Del FM. Periodontal disease as a risk factor for adverse pregnancy outcomes: a systematic review and meta-analysis of case-control studies. Odontology (2012) 100(2):232–40. doi:10.1007/s10266-011-0036-z
45. Konopka T, Paradowska-Stolarz A. Periodontitis and risk of preterm birth and low birthweight – a meta-analysis. Ginekol Pol (2012) 83(6):446–53.
46. Shanthi V, Vanka A, Bhambal A, Saxena V, Saxena S, Kumar SS. Association of pregnant women periodontal status to preterm and low-birth weight babies: a systematic and evidence-based review. Dent Res J (Isfahan) (2012) 9(4):368–80.
47. Martinez DTB, Gayet-Ageron A, Combescure C, Irion O, Baehni P. Association between early preterm birth and periodontitis according to USA and European consensus definitions. J Matern Fetal Neonatal Med (2012) 25(11):2160–6. doi:10.3109/14767058.2012.663827
48. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol (2007) 78(7 Suppl):1387–99. doi:10.1902/jop.2007.060264
49. Tonetti MS, Claffey N. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol (2005) 32(Suppl 6):210–3. doi:10.1111/j.1600-051X.2005.00822.x
50. Hasegawa-Nakamura K, Tateishi F, Nakamura T, Nakajima Y, Kawamata K, Douchi T, et al. The possible mechanism of preterm birth associated with periodontopathic Porphyromonas gingivalis. J Periodontal Res (2011) 46(4):497–504. doi:10.1111/j.1600-0765.2011.01366.x
51. Vanterpool SF, Been JV, Houben ML, Nikkels PG, De Krijger RR, Zimmermann LJ, et al. Porphyromonas gingivalis within placental villous mesenchyme and umbilical cord stroma is associated with adverse pregnancy outcome. PLoS One (2016) 11(1):e146157. doi:10.1371/journal.pone.0146157
52. Chen ZB, He L, Kang J, Huang Z, Sha YQ, Zhu WF, et al. [Relationship between the preterm low birth weight infant and the periodontal pathogen bacteria in maternal saliva]. Beijing Da Xue Xue Bao (2012) 44(1):29–33.
53. Ye C, Katagiri S, Miyasaka N, Bharti P, Kobayashi H, Takeuchi Y, et al. The anti-phospholipid antibody-dependent and independent effects of periodontopathic bacteria on threatened preterm labor and preterm birth. Arch Gynecol Obstet (2013) 288(1):65–72. doi:10.1007/s00404-013-2741-z
54. Gonzales-Marin C, Spratt DA, Allaker RP. Maternal oral origin of Fusobacterium nucleatum in adverse pregnancy outcomes as determined using the 16S-23S rRNA gene intergenic transcribed spacer region. J Med Microbiol (2013) 62(Pt 1):133–44. doi:10.1099/jmm.0.049452-0
55. Bohrer JC, Kamemoto LE, Almeida PG, Ogasawara KK. Acute chorioamnionitis at term caused by the oral pathogen Fusobacterium nucleatum. Hawaii J Med Public Health (2012) 71(10):280–1.
56. Cassini MA, Pilloni A, Condo SG, Vitali LA, Pasquantonio G, Cerroni L. Periodontal bacteria in the genital tract: are they related to adverse pregnancy outcome? Int J Immunopathol Pharmacol (2013) 26(4):931–9. doi:10.1177/039463201302600411
57. Andonova I, Iliev V, Zivkovic N, Susic E, Bego I, Kotevska V. Can oral anaerobic bacteria cause adverse pregnancy outcomes? Pril (Makedon Akad Nauk Umet Odd Med Nauki) (2015) 36(1):137–43.
58. Usin MM, Menso J, Rodriguez VI, Gonzalez A, Tabares S, Parodi R, et al. Association between maternal periodontitis and preterm and/or low birth weight infants in normal pregnancies. J Matern Fetal Neonatal Med (2016) 29(1):115–9. doi:10.3109/14767058.2014.987751
59. Blanc V, O’Valle F, Pozo E, Puertas A, Leon R, Mesa F. Oral bacteria in placental tissues: increased molecular detection in pregnant periodontitis patients. Oral Dis (2015) 21(7):905–12. doi:10.1111/odi.12364
60. Mesa F, Pozo E, O’Valle F, Puertas A, Magan-Fernandez A, Rosel E, et al. Relationship between periodontal parameters and plasma cytokine profiles in pregnant woman with preterm birth or low birth weight. Clin Oral Investig (2016) 20(4):669–74. doi:10.1007/s00784-015-1553-x
61. Cetin I, Pileri P, Villa A, Calabrese S, Ottolenghi L, Abati S. Pathogenic mechanisms linking periodontal diseases with adverse pregnancy outcomes. Reprod Sci (2012) 19(6):633–41. doi:10.1177/1933719111432871
62. Stadelmann P, Alessandri R, Eick S, Salvi GE, Surbek D, Sculean A. The potential association between gingival crevicular fluid inflammatory mediators and adverse pregnancy outcomes: a systematic review. Clin Oral Investig (2013) 17(6):1453–63. doi:10.1007/s00784-013-0952-0
63. Genco RJ, Van Dyke TE. Prevention: reducing the risk of CVD in patients with periodontitis. Nat Rev Cardiol (2010) 7(9):479–80. doi:10.1038/nrcardio.2010.120
64. Tonetti MS. Periodontitis and risk for atherosclerosis: an update on intervention trials. J Clin Periodontol (2009) 36(Suppl 10):15–9. doi:10.1111/j.1600-051X.2009.01417.x
65. Patil VA, Desai MH. Effect of periodontal therapy on serum C-reactive protein levels in patients with gingivitis and chronic periodontitis: a clinicobiochemical study. J Contemp Dent Pract (2013) 14(2):233–7. doi:10.5005/jp-journals-10024-1305
66. Lyon D, Cheng CY, Howland L, Rattican D, Jallo N, Pickler R, et al. Integrated review of cytokines in maternal, cord, and newborn blood: part I – associations with preterm birth. Biol Res Nurs (2010) 11(4):371–6. doi:10.1177/1099800409344620
67. Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol (2009) 24(9):1119–26. doi:10.1177/0883073809338066
68. Tanaka Y, Narahara H, Takai N, Yoshimatsu J, Anai T, Miyakawa I. Interleukin-1beta and interleukin-8 in cervicovaginal fluid during pregnancy. Am J Obstet Gynecol (1998) 179(3 Pt 1):644–9. doi:10.1016/S0002-9378(98)70058-4
69. Jun JK, Yoon BH, Romero R, Kim M, Moon JB, Ki SH, et al. Interleukin 6 determinations in cervical fluid have diagnostic and prognostic value in preterm premature rupture of membranes. Am J Obstet Gynecol (2000) 183(4):868–73. doi:10.1067/mob.2000.109034
70. Annells MF, Hart PH, Mullighan CG, Heatley SL, Robinson JS, Bardy P, et al. Interleukins-1, -4, -6, -10, tumor necrosis factor, transforming growth factor-beta, FAS, and mannose-binding protein C gene polymorphisms in Australian women: risk of preterm birth. Am J Obstet Gynecol (2004) 191(6):2056–67. doi:10.1016/j.ajog.2004.04.021
71. Moura E, Mattar R, de Souza E, Torloni MR, Goncalves-Primo A, Daher S. Inflammatory cytokine gene polymorphisms and spontaneous preterm birth. J Reprod Immunol (2009) 80(1–2):115–21. doi:10.1016/j.jri.2008.11.007
72. Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG (2006) 113(Suppl 3):17–42. doi:10.1111/j.1471-0528.2006.01120.x
73. Goepfert AR, Goldenberg RL, Andrews WW, Hauth JC, Mercer B, Iams J, et al. The preterm prediction study: association between cervical interleukin 6 concentration and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol (2001) 184(3):483–8. doi:10.1067/mob.2001.109653
74. Ao M, Miyauchi M, Furusho H, Inubushi T, Kitagawa M, Nagasaki A, et al. Dental infection of Porphyromonas gingivalis induces preterm birth in mice. PLoS One (2015) 10(8):e137249. doi:10.1371/journal.pone.0137249
75. Stockham S, Stamford JE, Roberts CT, Fitzsimmons TR, Marchant C, Bartold PM, et al. Abnormal pregnancy outcomes in mice using an induced periodontitis model and the haematogenous migration of Fusobacterium nucleatum sub-species to the murine placenta. PLoS One (2015) 10(3):e120050. doi:10.1371/journal.pone.0120050
76. Miyoshi H, Konishi H, Teraoka Y, Urabe S, Furusho H, Miyauchi M, et al. Enhanced expression of contractile-associated proteins and ion channels in preterm delivery model mice with chronic odontogenic Porphyromonas gingivalis infection. Reprod Sci (2016) 23(7):838–46. doi:10.1177/1933719115620497
77. Coppenhagen-Glazer S, Sol A, Abed J, Naor R, Zhang X, Han YW, et al. Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect Immun (2015) 83(3):1104–13. doi:10.1128/IAI.02838-14
78. Arce RM, Diaz PI, Barros SP, Galloway P, Bobetsis Y, Threadgill D, et al. Characterization of the invasive and inflammatory traits of oral Campylobacter rectus in a murine model of fetoplacental growth restriction and in trophoblast cultures. J Reprod Immunol (2010) 84(2):145–53. doi:10.1016/j.jri.2009.11.003
79. Ebersole JL, Holt SC, Cappelli D. Periodontitis in pregnant baboons: systemic inflammation and adaptive immune responses and pregnancy outcomes in a baboon model. J Periodontal Res (2014) 49(2):226–36. doi:10.1111/jre.12099
80. Arce RM, Barros SP, Wacker B, Peters B, Moss K, Offenbacher S. Increased TLR4 expression in murine placentas after oral infection with periodontal pathogens. Placenta (2009) 30(2):156–62. doi:10.1016/j.placenta.2008.11.017
81. Fogacci MF, Barbirato DS, Amaral CS, Da SP, Coelho MO, Bertozi G, et al. No association between periodontitis, preterm birth, or intrauterine growth restriction: experimental study in Wistar rats. Am J Obstet Gynecol (2016) 214(6):741–9. doi:10.1016/j.ajog.2015.12.008
82. Riewe SD, Mans JJ, Hirano T, Katz J, Shiverick KT, Brown TA, et al. Human trophoblast responses to Porphyromonas gingivalis infection. Mol Oral Microbiol (2010) 25(4):252–9. doi:10.1111/j.2041-1014.2010.00573.x
83. Ren H, Li Y, Jiang H, Du M. Porphyromonas gingivalis induces IL-8 and IFN-gamma secretion and apoptosis in human extravillous trophoblast derived HTR8/SVneo cells via activation of ERK1/2 and p38 signaling pathways. Placenta (2016) 45:8–15. doi:10.1016/j.placenta.2016.06.010
84. Inaba H, Kuboniwa M, Bainbridge B, Yilmaz O, Katz J, Shiverick KT, et al. Porphyromonas gingivalis invades human trophoblasts and inhibits proliferation by inducing G1 arrest and apoptosis. Cell Microbiol (2009) 11(10):1517–32. doi:10.1111/j.1462-5822.2009.01344.x
85. Inaba H, Kuboniwa M, Sugita H, Lamont RJ, Amano A. Identification of signaling pathways mediating cell cycle arrest and apoptosis induced by Porphyromonas gingivalis in human trophoblasts. Infect Immun (2012) 80(8):2847–57. doi:10.1128/IAI.00258-12
86. Ren H, Li Y, Jiang H, Du M. Interferon-gamma and Fas are involved in Porphyromonas gingivalis-induced apoptosis of human extravillous trophoblast-derived HTR8/SVneo cells via extracellular signal-regulated kinase 1/2 pathway. J Periodontol (2016) 87(11):e192–9. doi:10.1902/jop.2016.160259
87. Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R. Bacterial modulation of human fetal membrane toll-like receptor expression. Am J Reprod Immunol (2013) 69(1):33–40. doi:10.1111/aji.12016
88. Komine-Aizawa S, Hirohata N, Aizawa S, Abiko Y, Hayakawa S. Porphyromonas gingivalis lipopolysaccharide inhibits trophoblast invasion in the presence of nicotine. Placenta (2015) 36(1):27–33. doi:10.1016/j.placenta.2014.10.015
89. Li Y, Shibata Y, Zhang L, Kuboyama N, Abiko Y. Periodontal pathogen Aggregatibacter actinomycetemcomitans LPS induces mitochondria-dependent-apoptosis in human placental trophoblasts. Placenta (2011) 32(1):11–9. doi:10.1016/j.placenta.2010.10.007
90. Khairnar MS, Pawar BR, Marawar PP, Khairnar DM. Estimation of changes in C-reactive protein level and pregnancy outcome after nonsurgical supportive periodontal therapy in women affected with periodontitis in a rural set up of India. Contemp Clin Dent (2015) 6(Suppl 1):S5–11. doi:10.4103/0976-237X.152930
91. Bilinska M, Osmola K. [Active periodontitis as a potential risk factor of preterm delivery]. Ginekol Pol (2014) 85(5):382–5.
92. Macedo JF, Ribeiro RA, Machado FC, Assis NM, Alves RT, Oliveira AS, et al. Periodontal disease and oral health-related behavior as factors associated with preterm birth: a case-control study in south-eastern Brazil. J Periodontal Res (2014) 49(4):458–64. doi:10.1111/jre.12124
93. Fiorini T, Susin C, Da RJ, Weidlich P, Vianna P, Moreira CH, et al. Effect of nonsurgical periodontal therapy on serum and gingival crevicular fluid cytokine levels during pregnancy and postpartum. J Periodontal Res (2013) 48(1):126–33. doi:10.1111/j.1600-0765.2012.01513.x
94. Pirie M, Linden G, Irwin C. Intrapregnancy non-surgical periodontal treatment and pregnancy outcome: a randomized controlled trial. J Periodontol (2013) 84(10):1391–400. doi:10.1902/jop.2012.120572
95. Weidlich P, Moreira CH, Fiorini T, Musskopf ML, Da RJ, Oppermann ML, et al. Effect of nonsurgical periodontal therapy and strict plaque control on preterm/low birth weight: a randomized controlled clinical trial. Clin Oral Investig (2013) 17(1):37–44. doi:10.1007/s00784-012-0679-3
96. Dasanayake AP. Scaling and root planing performed on pregnant women with mild to moderate periodontal disease is not efficacious in reducing preterm birth, low birth weight, or other poor pregnancy outcomes. J Evid Based Dent Pract (2012) 12(3):135–7. doi:10.1016/j.jebdp.2012.06.007
97. Kim AJ, Lo AJ, Pullin DA, Thornton-Johnson DS, Karimbux NY. Scaling and root planing treatment for periodontitis to reduce preterm birth and low birth weight: a systematic review and meta-analysis of randomized controlled trials. J Periodontol (2012) 83(12):1508–19. doi:10.1902/jop.2012.110636
98. Shah M, Muley A, Muley P. Effect of nonsurgical periodontal therapy during gestation period on adverse pregnancy outcome: a systematic review. J Matern Fetal Neonatal Med (2013) 26(17):1691–5. doi:10.3109/14767058.2013.799662
99. Schwendicke F, Karimbux N, Allareddy V, Gluud C. Periodontal treatment for preventing adverse pregnancy outcomes: a meta- and trial sequential analysis. PLoS One (2015) 10(6):e129060. doi:10.1371/journal.pone.0129060
100. Boutin A, Demers S, Roberge S, Roy-Morency A, Chandad F, Bujold E. Treatment of periodontal disease and prevention of preterm birth: systematic review and meta-analysis. Am J Perinatol (2013) 30(7):537–44. doi:10.1055/s-0032-1329687
101. Condylis B, Le Borgne H, Demoersman J, Campard G, Philippe HJ, Soueidan A. [Interest of periodontitis screening and treatment in pregnancy: systematic review]. J Gynecol Obstet Biol Reprod (Paris) (2013) 42(6):511–7. doi:10.1016/j.jgyn.2012.05.012
102. Michalowicz BS, Gustafsson A, Thumbigere-Math V, Buhlin K. The effects of periodontal treatment on pregnancy outcomes. J Clin Periodontol (2013) 40(Suppl 14):S195–208. doi:10.1111/jcpe.12081
103. Rosa MI, Pires PD, Medeiros LR, Edelweiss MI, Martinez-Mesa J. Periodontal disease treatment and risk of preterm birth: a systematic review and meta-analysis. Cad Saude Publica (2012) 28(10):1823–33. doi:10.1590/S0102-311X2012001000002
104. Jeffcoat M, Parry S, Sammel M, Clothier B, Catlin A, Macones G. Periodontal infection and preterm birth: successful periodontal therapy reduces the risk of preterm birth. BJOG (2011) 118(2):250–6. doi:10.1111/j.1471-0528.2010.02713.x
105. Penova-Veselinovic B, Keelan JA, Wang CA, Newnham JP, Pennell CE. Changes in inflammatory mediators in gingival crevicular fluid following periodontal disease treatment in pregnancy: relationship to adverse pregnancy outcome. J Reprod Immunol (2015) 112:1–10. doi:10.1016/j.jri.2015.05.002
106. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol (1999) 4(1):1–6. doi:10.1902/annals.1999.4.1.1
107. Armitage GC. Clinical evaluation of periodontal diseases. Periodontol 2000 (1995) 7:39–53. doi:10.1111/j.1600-0757.1995.tb00035.x
108. Riche EL, Boggess KA, Lieff S, Murtha AP, Auten RL, Beck JD, et al. Periodontal disease increases the risk of preterm delivery among preeclamptic women. Ann Periodontol (2002) 7(1):95–101. doi:10.1902/annals.2002.7.1.95
109. Sharma R, Maimanuku LR, Morse Z, Pack AR. Preterm low birth weights associated with periodontal disease in the Fiji Islands. Int Dent J (2007) 57(4):257–60. doi:10.1111/j.1875-595X.2007.tb00129.x
110. Offenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG, et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol (2006) 107(1):29–36. doi:10.1097/01.AOG.0000190212.87012.96
111. Kazmierczak W, Fiegler P, Wegrzyn P, Fiegler-Mecik H, Przybylek B, Kaminski K. [Risk assessment for preterm delivery in pregnant women with active periodontitis]. Ginekol Pol (2005) 76(8):632–8.
112. Gomes-Filho IS, Cruz SS, Rezende EJ, Dos SC, Soledade KR, Magalhaes MA, et al. Exposure measurement in the association between periodontal disease and prematurity/low birth weight. J Clin Periodontol (2007) 34(11):957–63. doi:10.1111/j.1600-051X.2007.01141.x
113. Manau C, Echeverria A, Agueda A, Guerrero A, Echeverria JJ. Periodontal disease definition may determine the association between periodontitis and pregnancy outcomes. J Clin Periodontol (2008) 35(5):385–97. doi:10.1111/j.1600-051X.2008.01222.x
114. Nabet C, Lelong N, Colombier ML, Sixou M, Musset AM, Goffinet F, et al. Maternal periodontitis and the causes of preterm birth: the case-control Epipap study. J Clin Periodontol (2010) 37(1):37–45. doi:10.1111/j.1600-051X.2009.01503.x
115. Resende M, Pinto E, Pinto M, Montenegro N. [Periodontal disease, tobacco and preterm delivery]. Acta Med Port (2011) 24(Suppl 2):419–30.
116. Kazmierczak W, Fiegler P, Fiegler-Mecik H, Mecik TJ, Przybylek B. [Influence of the prevention of maternal periodontitis on the risk of preterm delivery]. Wiad Lek (2004) 57(Suppl 1):148–51.
117. Pattrapornnan P, DeRouen TA, Songpaisan Y. Increased risks of preterm birth and a low-birth-weight baby in Thai human immunodeficiency virus-positive pregnant women with periodontitis. J Periodontol (2012) 83(11):1372–81. doi:10.1902/jop.2012.110500
118. Pattanashetti JI, Nagathan VM, Rao SM. Evaluation of periodontitis as a risk for preterm birth among preeclamptic and non-preeclamptic pregnant women – a case control study. J Clin Diagn Res (2013) 7(8):1776–8. doi:10.7860/JCDR/2013/6497.3308
119. Lee HJ, Ha JE, Bae KH. Synergistic effect of maternal obesity and periodontitis on preterm birth in women with pre-eclampsia: a prospective study. J Clin Periodontol (2016) 43(8):646–51. doi:10.1111/jcpe.12574
120. Lee HJ, Jun JK, Lee SM, Ha JE, Paik DI, Bae KH. Association between obesity and periodontitis in pregnant females. J Periodontol (2014) 85(7):e224–31. doi:10.1902/jop.2014.130578
121. Blasco-Baque V, Garidou L, Pomie C, Escoula Q, Loubieres P, Le Gall-David S, et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut (2016). doi:10.1136/gutjnl-2015-309897
Keywords: periodontitis, pregnancy, preterm birth, low birth weight, risk factor
Citation: Ren H and Du M (2017) Role of Maternal Periodontitis in Preterm Birth. Front. Immunol. 8:139. doi: 10.3389/fimmu.2017.00139
Received: 25 December 2016; Accepted: 26 January 2017;
Published: 13 February 2017
Edited by:
Jixin Zhong, Case Western Reserve University, USACopyright: © 2017 Ren and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minquan Du, ZHVtaW5xdWFuQHdodS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.