
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 10 August 2016
Sec. B Cell Biology
Volume 7 - 2016 | https://doi.org/10.3389/fimmu.2016.00296
This article is part of the Research TopicNatural antibodies in health and diseaseView all 17 articles
Natural antibodies (NAbs) are produced in the absence of exogenous antigenic stimulation and circulate in the blood of normal, healthy individuals. These antibodies have been shown to provide one of the first lines of defense against both bacterial and viral pathogens. Conservation of the NAb repertoire reactivity profile is observed both within and across species. One view holds that this conservation of NAb self-reactivities reflects the use of germline antibody sequence, whereas the opposing view holds that the self-reactivities reflect selection driven by key conserved self-antigens. In mice, B-1a B cells are a major source of NAbs. A significant fraction of the B-1a antibody repertoire is devoid of N nucleotides in H chain complementarity determining region 3 (CDR-H3) and, thus, completely germline encoded. To test the role of germline DH sequence on the self-reactivity profile of the NAb repertoire, we examined the composition and self-antigen specificity of NAbs produced by a panel of DH gene-targeted BALB/c mice, each strain of which expresses a polyclonal, altered CDR-H3 repertoire that differs from the wild-type norm. We found that in most cases the same key self-antigens were recognized by the NAbs created by each DH-altered strain. The differences in reactivity appeared to represent the genetic signature of the NAb repertoire of each mouse strain. These findings suggest that although germline CDR-H3 sequence may facilitate the production of certain NAbs, a core set of self-antigens are likely the main force driving the selection of Nab self-specificities.
“Natural antibodies” (NAbs) are immunoglobulins present in the healthy organism in the absence of intentional immunization (1). The stimuli behind the production of NAbs by B cells have been a topic of considerable interest since NAbs were first recognized. Several studies have shown that germ-free (GF) and even antigen-free (AgF) mice produce serum IgM in amounts equivalent to mice raised under normal vivarium conditions, suggesting that NAbs are largely independent of stimulation by external antigens, including antigens derived from the microbiota (2–4). Instead there is considerable support for NAb production being driven by endogenous antigen stimulation (5). Self-antigen recognition appears to play a physiological role in the distribution of B cells within subpopulations, often into distinct anatomical niches (6–8).
B-1a lymphocytes are generated preferentially early in ontogeny and have been implicated as a main source of NAbs. The B-1a cells demonstrate decreased or absent N-region addition at the V-D and D-J junctions and are thus, enriched for entirely germline-encoded Ig variable domains (9–17). Given that B-1a cells are among the first B cells to develop in the fetus, their participation in the secretion of NAbs might provide a reasonable explanation for the conservation of the Nab repertoire and its establishment early in ontogeny (18, 19). However, studies with irradiated chimeras reconstituted with fetal liver and adult bone marrow from wild-type (WT) mice have shown that most NAb reactivities are produced even when B-1a are profoundly reduced in numbers, thus suggesting a role for somatic selection in regenerating the NAb repertoire of specificities (20).
Although previous studies have shown that the reactivity profile of NAbs remains conserved regardless of gut colonization or depletion of B-1 cell compartment (2, 3, 20); the influence of inherited CDR-H3 antigen-binding sites on the NAb repertoire remains unclear. BALB/c mice IgH loci contain 13 functional DH gene segments, each belonging to one of four families (DFL, DSP, DST, and DQ52). Theoretically, each DH gene segment gives the developing B cell access to six reading frames (RFs) of differing peptide sequence. In practice, however, the use of RF1 is preferred, RF2 and RF3 are used less frequently, and the three inverted RF are rarely used (21). We had previously used techniques of cre–loxP-based gene targeting to delete 12 of the 13 DH gene segments in the BALB/c DH locus, retaining only the single DFL16.1 segment (ΔD-DFL mice) (22). We then generated the ΔD-iD strain by replacing the center of the single DFL16.1 segment with an inverted DSP2.2 gene segment (23). DH gene-targeted BALB/c mice express polyclonal, altered CDR-H3 repertoires that differ from the WT norm [reviewed in Ref. (24)].
We have previously observed that the ΔD-iD strain lacks NAbs that protect against infection with Streptococcus pneumoniae while continuing to exhibit protective reactivity against a key altered self-antigen, oxidized LDL (25). In the present work, we sought to investigate, in depth, the nature and extent of the conservation of NAb reactivities with self-antigens from brain tissue. To test the extent to which germline antibody gene content controls the composition of NAbs and the innate antibody response to self-antigens, we examined the NAb repertoire in a panel of WT and DH gene-targeted BALB/c mice raised under specific pathogen-free conditions. We found that most, but not all, of the reactivities against self-antigens were found in the serum of mice irrespective of changes in germline DH content and, thus, CDR-H3 sequence and structure. Our findings suggest that for most self-reactivities the primary force driving the generation of NAbs is exposure to a key set of self-antigens.
Serum IgM repertoire of antigen specificities have been screened using a semi-quantitative immunoblot assay that enables accurate en bloc analysis of the self-reactivity of IgM present in the serum (actual repertoire) of unmanipulated mice (26–28). We used the same assay to analyze the serum IgM reactivities of DH-altered mice with self-antigens from brain tissue. Shown in Figure 1A is a comparison of the pattern of self-reactivity expressed by the WT BALB/c, ΔD-DFL, and ΔD-iD strains. To test whether the reactivity levels correlate with immunoglobulin specificity or with the relative abundance of the proteins, we compared protein staining with immunoreactivity densities in the same membrane. The comparison shown in Figure 1B reveals highly abundant proteins that are not recognized by natural serum IgM, as well as highly IgM-recognized proteins that are not abundant in the tissue extract. With a few notable exceptions, the mean sera IgM reactivity profiles were remarkably conserved among mouse lineages that generate very different primary CDR-H3 repertoires (22, 23) (Figure 1C).
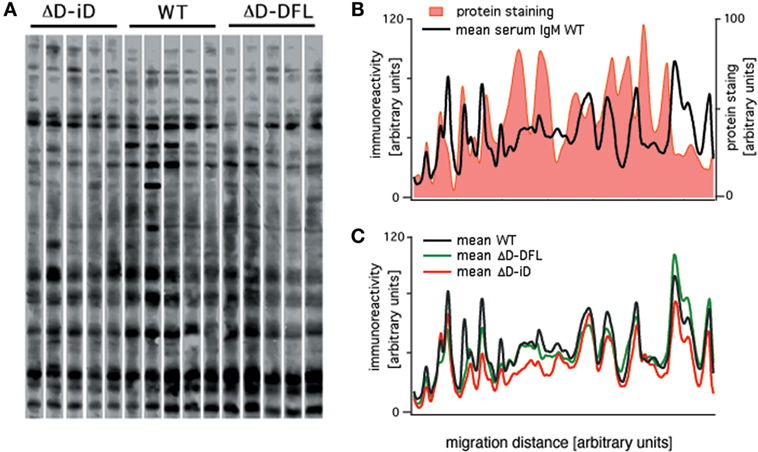
Figure 1. Serum natural IgM pattern of self-reactivity expressed by ΔD-DFL, ΔD-iD, and WT BALB/c mice. (A) Serum IgM reactivities against syngeneic brain extract from five animals of each strain. (B) Total protein staining by colloidal gold densitometric profile was compared to natural IgM recognition profile in the same membrane. (C) Mean serum IgM reactivity profiles of each mouse strain. Raw data adapted from Ref. (25).
It is noteworthy that these data revealing restriction of self-antigen recognition comply with the idea of the immunological homunculus, as proposed by Cohen (29). Furthermore, these data demonstrate that the reactivities found in the immunoblot assay cannot be attributed to simple non-specific interactions.
The results shown above suggest that strong mechanisms of antigen-driven somatic selection may operate at the cellular level to recreate a normal WT NAb repertoire of specificities in genetically modified mice. In mice, the peritoneal cavity (PerC) is the major site for both B-1 and B-2 cells. We, thus, sought to investigate whether these somatic selection pressures would generate similar repertoires among the PerC B-1 and B-2 B cells (available repertoire) from the different mouse strains. The reactivity profiles of these B cell populations provided a privileged opportunity for comparison between different B cell populations that share the same anatomical niche. After sorting each PerC B cell population (B-1a, B-1b, and B-2), we cultured the cells under LPS stimulation to induce polyclonal IgM secretion and tested the supernatants using the immunoblot against brain extract assay.
All three subsets from DH-altered PerC B cells demonstrated frequencies of response to LPS that were similar to their corresponding WT B cell subsets (Figure 2A). Production of IgM in culture was also similar (Figure 2B). Thus, DH alteration and restriction of the CDR-H3 repertoire did not affect the B cell response to polyclonal stimulus from LPS.
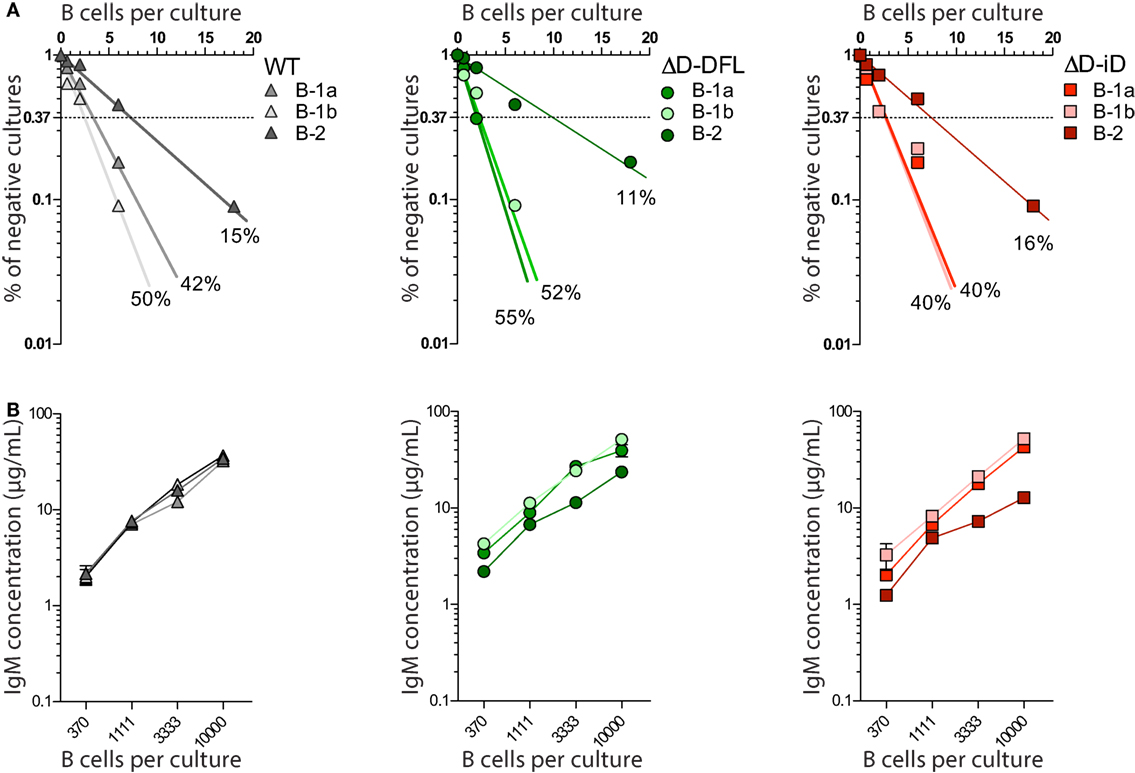
Figure 2. DH-altered B cells response to polyclonal stimuli. (A) Frequencies of response to LPS of PerC B cell subsets of each mouse strain were calculated by limiting dilution analysis. Presence of IgM in culture supernatants of 18, 6, 2, and 0.66 cells/culture on day 7 was assessed by ELISA. (B) IgM production by each PerC B cell subset in culture was measured by ELISA on culture supernatants of increasing number of cells/culture (370, 1111, 3333, and 10,000 cells/culture). PerC B-1a cells were sorted as B220loCD5+, B-1b as B220loCD5− Mac-1lo/+, and PerC B-2 cells were sorted as B220hiCD5−Mac-1−. All cultures received 30 μg/mL of LPS and 5 × 103 S17 feeder cells. Supernatants were collected on day 7.
We then performed the same analysis of self-reactivities shown for serum IgM with the supernatants from the B-1 and B-2 cultures (Figure 3). As there is a difference in the frequency of response to LPS among B cell subsets, we diluted each supernatant to normalize all to the same IgM concentration per growing clone (30). We performed an immunoblot analysis of the reactivities against syngeneic brain extract from seven separate culture supernatants from the three strains of mice, for a total of 21 supernatants for each B cell subset.
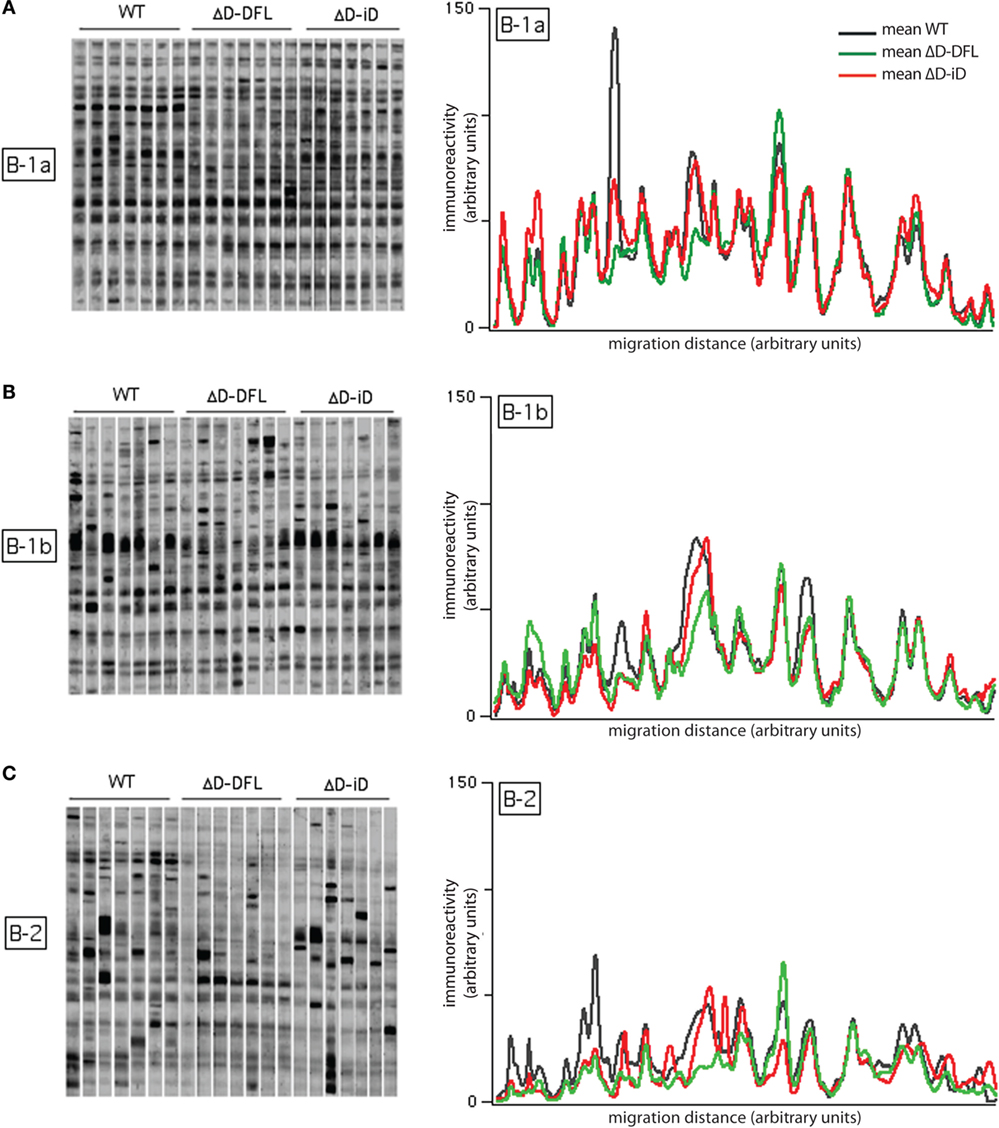
Figure 3. Self-reactivities of the available repertoire in PerC B cell subsets. Left panels (A–C) show 21 B-1a, B-1b, and B-2 cells culture supernatants (~1,500 responding B cell per culture), respectively, reacting with syngeneic brain extract. Right panels show the mean intensity of reactivities displayed by B-1a (A), B-1b (B), and B-2 (C) cells from each mouse genotype. Raw data adapted from Ref. (25).
The mean intensity of reactivities present in B-1a cells culture supernatants remained almost completely invariant despite the genetic alteration of the DH gene sequences (Figure 3A, right). The mean reactivity profiles for B-1b cells supernatants (Figure 3B, right) also demonstrated similarities in self-antigen reactivities irrespective of genotype, although the replicates in the membrane suggested a greater degree of variation around the mean values (Figure 3B, left). Conversely, the mean reactivity profiles of the B-2 cells were divergent both within and across the different mouse strains. This corresponded well with the different sets of reactivities of the B-2 cells supernatants (Figure 3C, right). The individual variability of immunoreactivities of the 21 B-2 cell culture supernatants were also highly increased when compared to the B-1 reactivity profiles (Figure 3C, left). These findings suggested that differences in the variance of the reactivities were intrinsic to the B cell subset, and not dependent on the germline sequence of the immunoglobulin repertoire.
To address the issue of variance in depth, we divided the immunoreactivity profiles into 36 sections, which corresponded to the major bands of reactivity. We plotted the magnitudes of reactivity against these sections for each supernatant from the B-1a, B-1b, and B-2 subsets (Figures 4A–C, respectively). The solid lines indicate the mean profile of all supernatants for each B cell subset. In this format, the variance of the distribution around the mean profile can be properly visualized. Despite few exceptions, we observed that the variance around the mean was much lower for the B-1a supernatants when compared to B-1b and B-2 (Figure 4).
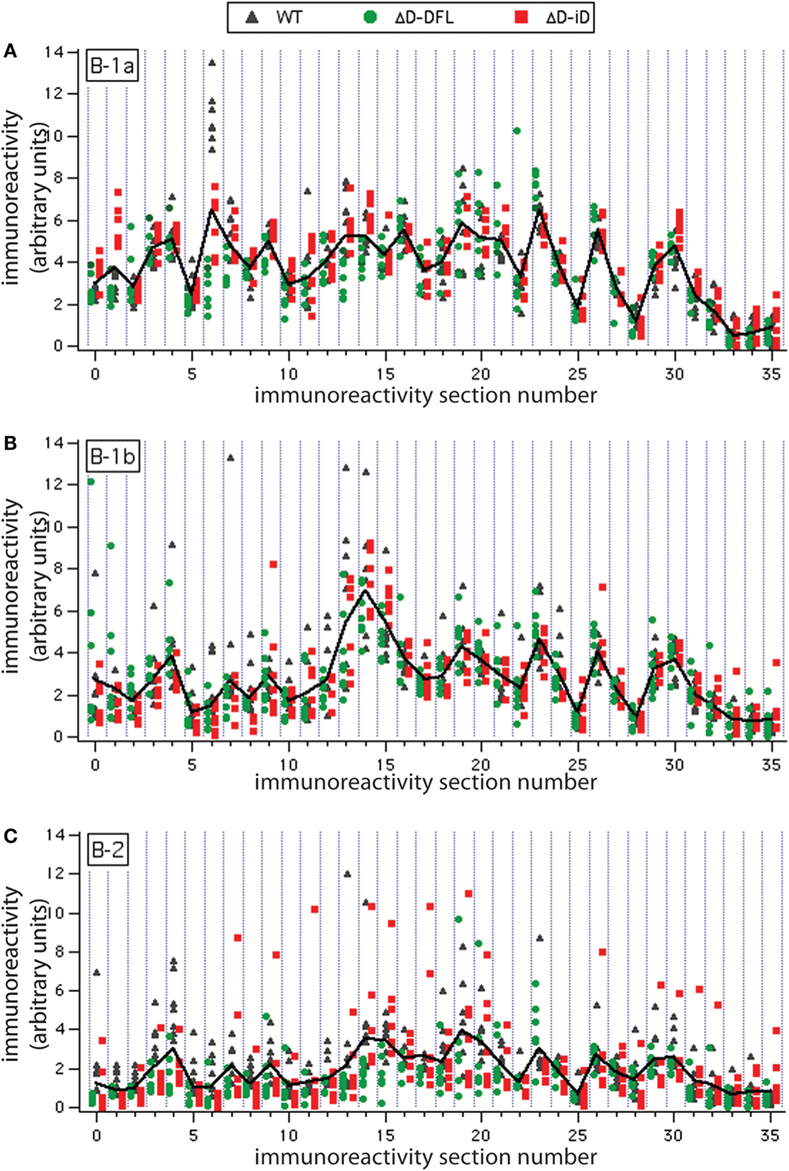
Figure 4. Variance distribution of reactivities around the mean densitometric profile for each PerC B cell subset. Supernatant magnitudes of reactivity against the 36 sections in which reactivity profiles divided are plotted in (A–C). These corresponded to B-1a, B-1b, and B-2 subsets, respectively. Solid lines indicate the mean profile of all supernatants for each B cell subset.
To further confirm that each B cell subset displays a unique repertoire, we analyzed the reactivity profiles within each mouse strain. Irrespective of DH content, the B-1a individual culture supernatants profiles proved very homogeneous, with little variance around their mean (Figure 5A, top; and Figure S1 in Supplementary Material). The reactivity profiles from B-1b subset were observed to differ from the B-1a for all mouse strains (Figure 5A, middle). The B-2 reactivity profiles displayed increased variance of the distribution in WT mice and especially for the ΔD-iD strain (Figure S1 in Supplementary Material), whereas the single normal DH control (ΔD-DFL) displayed less variability and intensity of reactivity (Figure 5A, bottom).
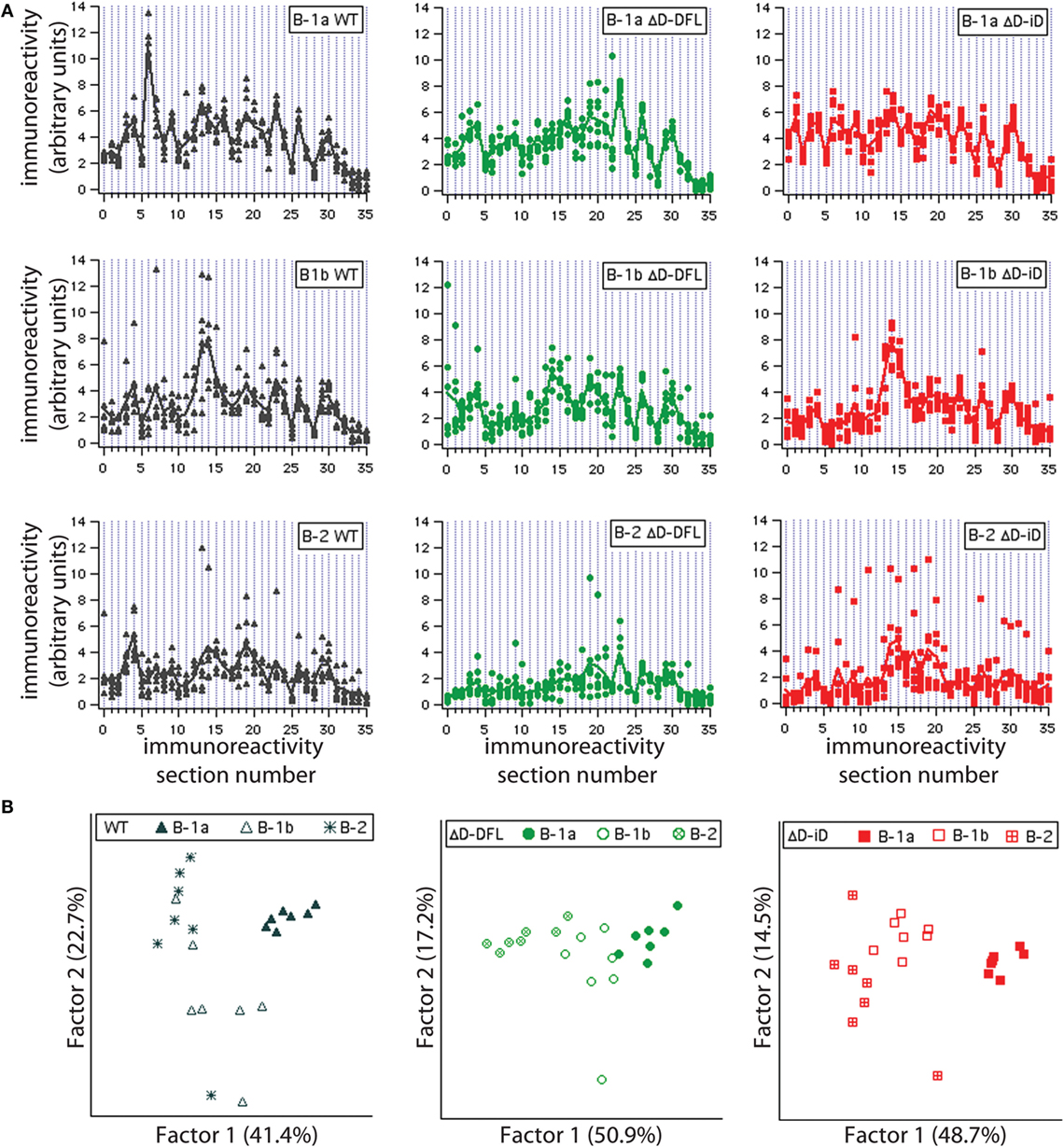
Figure 5. Graphic representation of variance distribution of each B cell subset reactivity profile within each mouse strain. (A) Profiles of reactivities and distribution around the mean profile of each PerC B cell subset from WT, ΔD-DFL, and ΔD-iD are represented graphically. (B) Principal component analysis (PCA) of reactivity profiles from each mouse strain discriminates B cell subsets.
To substantiate these observations, we performed principal component analysis (PCA) for each mouse strain. This multivariate approach takes in consideration all the reactivities together, providing an unbiased evaluation of the data (26, 31). All PerC B cell subset repertoires were discriminated within each mouse strain, suggesting distinct repertoire signatures for each population.
Although the mean reactivity profiles were generally conserved among the three mouse strains (Figure 3A), individual differences between the repertoires were also apparent (Figures 2 and 3). We, thus, performed a PCA of the data presented in Figure 4, which are grouped by B cell subset. This multivariate analysis revealed the impact of the genetic alteration of DH sequences on the B-1a available repertoire of specificities (Figure 6A, left), demonstrating distinct differences between the strains. To better evaluate the contribution of each factor to the resulting B cell subset repertoires, we collected all the data from the reactivity profiles (B-1a, B-1b, and B-2 subsets from the three mouse strains) on a single heat map (Figure 6B). This large data matrix was similarly subjected to PCA.
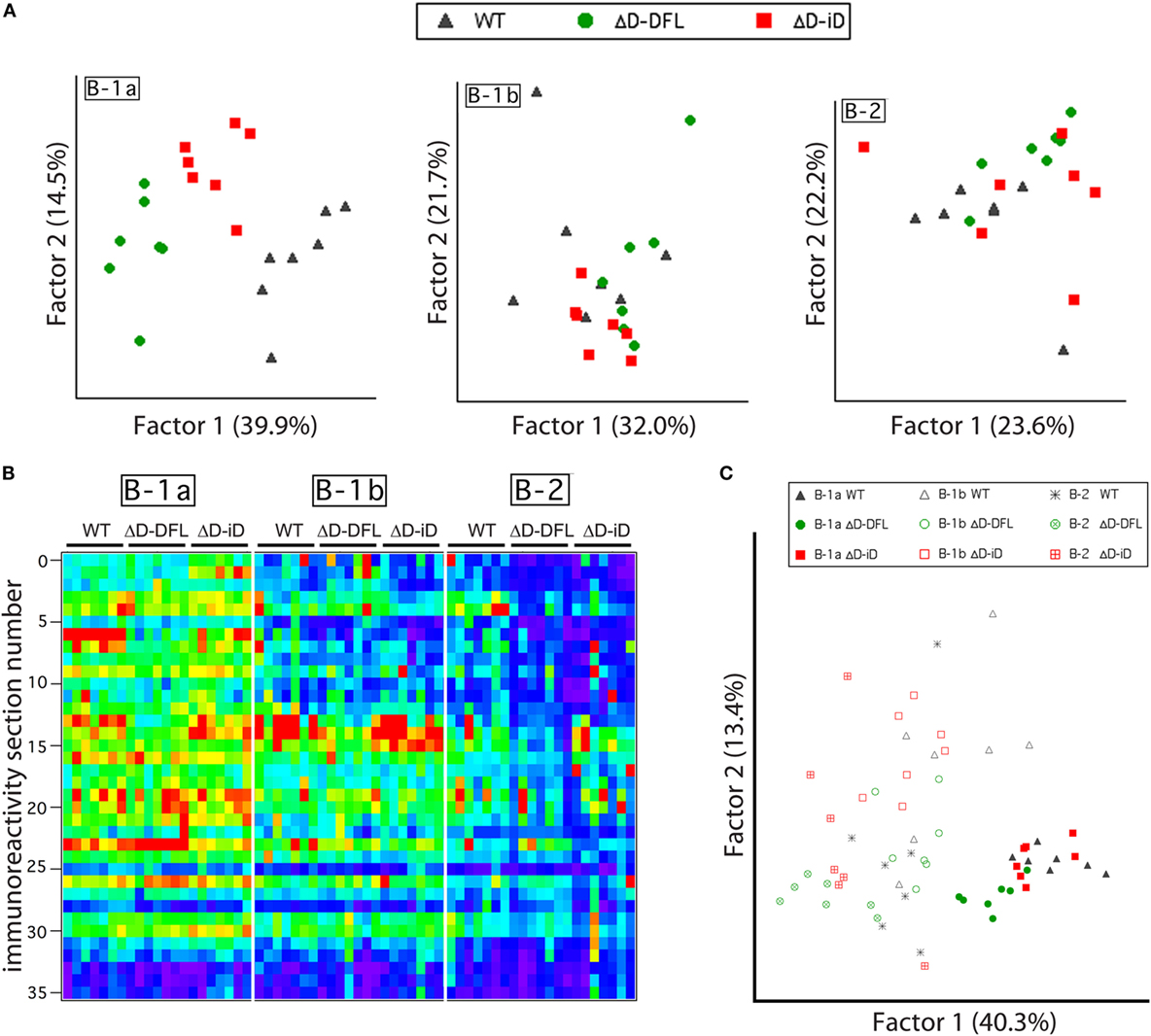
Figure 6. B cell subset-intrinsic and genotype-dependent features of the repertoire. (A) Principal component analysis (PCA) of reactivities for each PerC B cell subset discriminates the B-1a genetic alterations of DH sequences in the available repertoire of specificities but not B-1b or B-2 subsets available repertoire. (B) All data from reactivity profiles (B-1a, B-1b, and B-2 subsets from the three mouse strains) are shown on a single heat map indicating similarities between reactivity profiles. (C) Subjecting all data matrix to PCA results in subset characteristics prevailing over genetic distinctions imposed by DH alterations.
Both the immunoblot assay (Figures 2 and 3) and the heat map of the reactivities (Figure 6B) disclosed a set of major bands that were unique to each strain and were shared among individual mice from each strain. These shared similarities and differences were much less apparent in the B-1b and B-2 subsets (Figure 6A). When analyzed by PCA together, the characteristics common to the subsets appeared to exert a greater effect than the characteristics provided by the differences in DH content (Figure 6C).
Natural antibodies are formed early in ontogeny and are, thus, mostly germline encoded (18, 19). We sought to test whether the specificity of the NAb repertoire is the product of natural selection of germline immunoglobulin sequence or whether self-antigen is the driving force. These two hypotheses are not mutually exclusive, because even if self-antigen were the stimuli, the use of conserved germline sequence could facilitate the development of antibodies with certain specific epitope recognition properties that could provide protection in an anticipatory fashion (25).
In this work, we focused on the role of the sequence encoded by CDR-H3, which is created de novo by VDJ rearrangement and N addition and lies at the center of the antigen-binding site. This central position allows CDR-H3 to often play a major role in defining the epitope specificity of the antibody (32). The diversity gene segment (DH) contributes significantly to the amino acid composition of CDR-H3. The ΔD-DFL strain expresses that portion of the CDR-H3 repertoire that is typically generated through use of the DFL16.1 gene segment, and is thus enriched for tyrosine, serine, and glycine. The ΔD-iD strain forces use of amino acids contributed by DSP gene segment inverted sequence. This repertoire is enriched for arginine, histidine, and asparagine.
Even though the CDR-H3 repertoire differs between the three strains of mice, the global self-antigen specificity of serum IgM in all three strains was largely the same (Figure 1), suggesting that antigen reactivity, and not germline immunoglobulin sequence content, was the driving force. The immunoreactivity profiles were similar in mice missing that portion of the immunoglobulin repertoire that is normally created by the 12 other deleted DH gene segments in the ΔD-DFL mice. The same finding was obtained in the ΔD-iD mice that are not only missing the contribution of 12 DH gene segments but also preferentially use an inverted RF sequence that completely alters the normal contribution of DH encoded amino acids to the repertoire.
In previous studies in WT BALB/c mice, we found evidence of categorical selection of CDR-H3 sequence during B cell development, with B cell subsets, including those from the PerC, often exhibiting characteristic global sequence signatures (15, 33–35). These signatures were in addition to the bias for germline sequence exhibited by B-1a. To test whether the IgM repertoire expressed by these subsets in our panel of mice varied in its self-antigen specificities, we sorted B-1a, B-1b, and B-2 cells from the PerC, cultured them in the presence of LPS, and examined the IgM that was produced (Figure 3). If the self-specificity of the IgM were to reflect the influence of germline sequence, then the prediction was that B-1a IgM expressed by the D-altered B cells would demonstrate the greatest deviation from the WT norm. However, the opposite was observed. The reactivity of the IgM produced was most conserved in B-1a with the least variance, and least conserved in B-2 with the most variance (Figures 3 and 4). The B-1a repertoire, thus, demonstrated major convergent self-antigen-binding selection specificities in the presence of divergent germline sequence, whereas the B-1b and B-2 repertoires did not. PCA confirmed the distinct nature of the repertoire produced by each of the three subsets (Figure 5). The mechanisms behind these differences in variability are unclear, but potentially could reflect B cell subset-intrinsic characteristics, such as self-renewal capacity, clonal size, and repertoire polyreactivity.
The dominance of the biology of the B-1a component of the repertoire over the genetic alteration of CDR-H3 content among the activated PerC B cells, which is revealed by the heat-map PCA (Figure 6C), is in agreement with the substantial homology of the self-reactivities of the IgM in the sera of the WT, ΔD-DFL, and ΔD-iD mice (Figure 1), which has been argued to derive primarily from the B-1a subset (16). Although the biology of each individual subset exerted the greater effect on the reactivity of the IgM produced, a multivariate analysis revealed individual differences between the repertoires as a function of DH sequence (Figure 6). By inspection, it is clear that some of these individual differences reflect the presence or absence of specific bands.
ΔD-DFL mice bear a DH segment that is expressed in 20–30% of B-1a WT; this may result in a large overlap of B-1a repertoires and explain the similarity of their NAb repertoires. However, this explanation does not hold for ΔD-iD mice. One possible mechanism that could explain the convergence of the reactivities would be an antigen-influenced shift in the immunoglobulin repertoire expressed by the D-altered PerC B cells in the use of VH or JH, the extent of N nucleotide insertion or exonucleotide nibbling, or use of alternative DH RFs. Studies are underway in our laboratory to test these hypotheses.
Our findings of similar immunoreactivity profiles between D-altered mice indicate that NAb reactivity is likely driven by a key subset of self-antigens. The nature of these antigens and the epitopes that define them remain largely unknown. In WT animals, they are preferentially recognized by IgM formed by germline-conserved CDR-H3 sequences. In mice forced to use alternative sequences, the majority, but not all, of these self-antigens seems to be able to induce a NAb repertoire with a similar reactivity profile. The exceptions may represent epitopes that require unique, germline-encoded DH sequence in the antigen-binding site (25). These findings strongly suggest that it is the self-antigens, rather than conserved CDR-H3 germline sequence, that play the greater role in driving NAb production and reactivity.
The panel of DH-altered BALB/c mouse strains was bred in our mouse colony at the University of Alabama at Birmingham (UAB). Mice bearing ΔD-DFL (22) or ΔD-iD (23) DH alleles were created by cre–loxP targeting of a BALB/c ES cell line. For each allele, 12 of the 13 BALB/c DH gene segments were deleted and then the single, remaining DFL16.1 gene segment was either retained or altered. All WT and DH-altered mice were studied at from 8 to 10 weeks of age. The mice were maintained in a SPF barrier facility and in a climate-controlled environment with a 12 h light/12 h dark cycle, with diet and water supplied ad libitum. Animal care was conducted in accordance with established guidelines and protocols approved by the UAB Animal Care and Use Committee.
Flow cytometric analysis and cell sorting were performed as previously described (36). Briefly, peritoneal washout cells were obtained from five different mice of each DH-altered strain. The following mAbs were used to isolate PerC B cells into the B-1a, B-1b, and B-2 subpopulations: anti-B220 (RA 3.6B2) (BD Pharmingen) (Southern Biotechnology, Birmingham, AL, USA), anti-Mac-1 (BD Pharmingen, San Diego, CA, USA) and anti-CD5 (BD Pharmingen, San Diego, CA, USA). PerC B-1a cells were sorted as B220loCD5+, B-1b as B220loCD5−Mac-1lo/+, and PerC B-2 cells were sorted as B220hiCD5−Mac-1−. A MoFlo instrument (Dako) was used for cell sorting.
The preparation of brain extract, as well as determination of protein concentration, electrophoretic separation, blotting onto nitrocellulose membranes, and the test of immunoreactivities in the Mini-Cassette System, was performed as previously described (27) with secondary anti-IgM antibody coupled to alkaline phosphatase from Southern Biotechnology (Birmingham, AL, USA). The four gels used for limiting dilution assay (LDA) of reactivities were polymerized and run in parallel, such that profiles of total proteins blotted (revealed with colloidal gold) and the immunoreactivity profiles of lanes containing the standard pooled supernatant matched accurately between the gels, both qualitatively and quantitatively.
The densitometric profiles of immunoreactivities were analyzed as previously described (30). Briefly, profiles were acquired first by scanning (Silverscanner II). This was followed by colloidal gold staining in order to reveal the migration position of the proteins. A comparison between any two immunoreactivity profiles could subsequently be performed by referring to the corresponding protein profiles. Data analysis was performed using the software IGOR Pro (Wavemetrics, Lake Oswego, OR, USA). Special software packages were written by the authors for the analysis and statistical treatment of the data and can be obtained from the authors.
B cell cultures were performed as described previously (36). Sorted B-1a, B-1b, and B-2 cells were cultured in 250 μl of complete RPMI medium in 96 well flat-bottom plates in the presence of 30 μg/ml of LPS (Salmonella typhimurium, Sigma-Aldrich). All cultures contained 5 × 103 S17 feeder cells/well for growth support as described by Ref. (37) with some modifications. Briefly, 1 day before the start of the LDA cultures, 5 × 103 S17 cells were added per well and incubated overnight at 37°C with 5% CO2. The next day, the S17 culture plates were irradiated with 3000 rad and various numbers of the sorted B cells were added to the cultures. Culture supernatants were used to determine the frequency of IgM secreting clones by ELISA according to the Poisson distribution (38, 39) and contained sorted B cells with 22 replicates for each cell number (18, 6, 2, and 0.66 B cells per well). Another culture set was performed to analyze reactivity profiles by immunoblot, with 22 replicates of 10,000; 3333; 1111; and 370 B cells added per well. Culture supernatants were typically harvested on the fifth day of culture, unless indicated otherwise in the text.
To determine IgM concentration in the supernatants, ELISA was performed as previously described (36), using anti mouse IgM-specific reagents (Southern Biotechnology). Standard curves, obtained by using polyclonal, serially threefold diluted, mouse IgM (Southern Biotechnology), were used to quantify IgM.
Differences between populations were assessed by Student’s t-test, two tailed; Fisher’s exact test, two tailed; χ2-test; or Levene’s tests for the homogeneity of variance, as appropriate. Analysis was performed with JMP version 7.0 (SAS Institute, Inc., Cary, NC, USA). Means are reported with the SE of the mean.
AV, AN, and HS designed research; AV performed the experiments; AV, CC, and AN performed the statistical analysis; AV, CC, AN, and HS analyzed data and wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The present study was supported, in part, by the US National Institutes of Health (NIH) AI48115, AI090902, AI090742, and AI117703. Flow cytometry was supported by NIH AR48311 and AI027767. AN was supported by CNPq, FAPERJ, and FINEP. AV was supported in part by CNPq, FAPERJ, and a fellowship from CAPES (CsF-BJT; 024/2012). CC was supported by a fellowship from CNPq.
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2016.00296
Figure S1. The immunoblot reactivity profiles of culture supernatants from LPS-stimulated B cells were subdivided into 36 reactivity sections, as shown in Figure 4. For each mouse lineage and each B cell subpopulation, seven different supernatants were tested in the immunoblot assay for immunoreactivity against self-antigens derived from brain tissue extract (Figure 4). The variance of the magnitude of immunoreactivity for all 36 sections of reactivity was calculated for each mouse lineage and each B cell population. Histograms are shown in the figure. In all cases, most of the reactivity sections presented variance values below 106 units. For comparison between histograms, the number of sections with variance values inferior, or superior, to 106 units was indicated for each case. The differences between histograms were tested for statistical significance with Fischer exact test applied to 2 × 2 table containing the numbers of reactivity sections with variance values inferior, or superior, to 106 units.
ΔD-DFL, depleted DH locus with a single DFL16.1 gene segment; ΔD-iD, depleted DH locus with a single mutated DFL16.1 gene segment containing inverted DSP2.2 sequence; CDR-H3, complementarity determining region 3 of the immunoglobulin heavy chain; LDA, limiting dilution assay; NAb, natural antibody; PCA, principal components analysis; PerC, peritoneal cavity; TdT, terminal deoxynucleotidyl transferase; WT, wild type.
1. Avrameas S. Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton’. Immunol Today (1991) 12(5):154–9. doi: 10.1016/S0167-5699(05)80045-3
2. Hooijkaas H, Benner R, Pleasants JR, Wostmann BS. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered “antigen-free” diet. Eur J Immunol (1984) 14(12):1127–30. doi:10.1002/eji.1830141212
3. Bos NA, Kimura H, Meeuwsen CG, De Visser H, Hazenberg MP, Wostmann BS, et al. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol (1989) 19(12):2335–9. doi:10.1002/eji.1830191223
4. Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, Nobrega A. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol (1997) 27(6):1557–63. doi:10.1002/eji.1830270635
5. Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, et al. Positive selection of natural autoreactive B cells. Science (1999) 285(5424):113–6. doi:10.1126/science.285.5424.113
6. Wen L, Brill-Dashoff J, Shinton SA, Asano M, Hardy RR, Hayakawa K. Evidence of marginal-zone B cell-positive selection in spleen. Immunity (2005) 23(3):297–308. doi:10.1016/j.immuni.2005.08.007
7. Gaudin E, Hao Y, Rosado MM, Chaby R, Girard R, Freitas AA. Positive selection of B cells expressing low densities of self-reactive BCRs. J Exp Med (2004) 199(6):843–53. doi:10.1084/jem.20030955
8. Cancro MP, Kearney JF. B cell positive selection: road map to the primary repertoire? J Immunol (2004) 173(1):15–9. doi:10.4049/jimmunol.173.1.15
9. Hayakawa K, Hardy RR, Honda M, Herzenberg LA, Steinberg AD, Herzenberg LA. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A (1984) 81(8):2494–8. doi:10.1073/pnas.81.8.2494
10. Sidman CL, Shultz LD, Hardy RR, Hayakawa K, Herzenberg LA. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science (1986) 232(4756):1423–5. doi:10.1126/science.3487115
11. Feeney AJ. Junctional sequences of fetal T cell receptor beta chains have few N regions. J Exp Med (1991) 174(1):115–24. doi:10.1084/jem.174.1.115
12. Tornberg UC, Holmberg D. B-1a, B-1b and B-2 B cells display unique VHDJH repertoires formed at different stages of ontogeny and under different selection pressures. EMBO J (1995) 14(8):1680–9.
13. Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J Immunol (1997) 158(3):1175–86.
14. Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity (2005) 23(1):7–18. doi:10.1016/j.immuni.2005.04.011
15. Vale AM, Tanner JM, Schelonka RL, Zhuang Y, Zemlin M, Gartland GL, et al. The peritoneal cavity B-2 antibody repertoire appears to reflect many of the same selective pressures that shape the B-1a and B-1b repertoires. J Immunol (2010) 185(10):6085–95. doi:10.4049/jimmunol.1001423
16. Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol (2011) 11(1):34–46. doi:10.1038/nri2901
17. Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol (2012) 42(1):120–9. doi:10.1002/eji.201141890
18. Mouthon L, Nobrega A, Nicolas N, Kaveri SV, Barreau C, Coutinho A, et al. Invariance and restriction toward a limited set of self-antigens characterize neonatal IgM antibody repertoires and prevail in autoreactive repertoires of healthy adults. Proc Natl Acad Sci U S A (1995) 92(9):3839–43. doi:10.1073/pnas.92.9.3839
19. Merbl Y, Zucker-Toledano M, Quintana FJ, Cohen IR. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest (2007) 117(3):712–8. doi:10.1172/JCI29943
20. Nobrega A, Stransky B, Nicolas N, Coutinho A. Regeneration of natural antibody repertoire after massive ablation of lymphoid system: robust selection mechanisms preserve antigen binding specificities. J Immunol (2002) 169(6):2971–8. doi:10.4049/jimmunol.169.6.2971
21. Ivanov II, Link JM, Ippolito GC, Schroeder HW Jr. Constraints on hydropathicity and sequence composition of HCDR3 are conserved across evolution. In: Zanetti M, Capra JD, editors. The Antibodies. London: Taylor and Francis Group (2002). p. 43–67.
22. Schelonka RL, Ivanov II, Jung DH, Ippolito GC, Nitschke L, Zhuang Y, et al. A single DH gene segment creates its own unique CDR-H3 repertoire and is sufficient for B cell development and immune function. J Immunol (2005) 175(10):6624–32. doi:10.4049/jimmunol.175.10.6624
23. Ippolito GC, Schelonka RL, Zemlin M, Ivanov II, Kobayashi R, Zemlin C, et al. Forced usage of positively charged amino acids in immunoglobulin CDR-H3 impairs B cell development and antibody production. J Exp Med (2006) 203(6):1567–78. doi:10.1084/jem.20052217
24. Schroeder HW Jr, Zemlin M, Khass M, Nguyen HH, Schelonka RL. Genetic control of DH reading frame and its effect on B-cell development and antigen-specifc antibody production. Crit Rev Immunol (2010) 30(4):327–44. doi:10.1615/CritRevImmunol.v30.i4.20
25. Vale AM, Kapoor P, Skibinski GA, Elgavish A, Mahmoud TI, Zemlin C, et al. The link between antibodies to OxLDL and natural protection against pneumococci depends on DH gene conservation. J Exp Med (2013) 210(5):875–90. doi:10.1084/jem.20121861
26. Nobrega A, Haury M, Grandien A, Malanchere E, Sundblad A, Coutinho A. Global analysis of antibody repertoires. II. Evidence for specificity, self-selection and the immunological “homunculus” of antibodies in normal serum. Eur J Immunol (1993) 23(11):2851–9. doi:10.1002/eji.1830231119
27. Haury M, Grandien A, Sundblad A, Coutinho A, Nobrega A. Global analysis of antibody repertoires. 1. An immunoblot method for the quantitative screening of a large number of reactivities. Scand J Immunol (1994) 39(1):79–87. doi:10.1111/j.1365-3083.1994.tb03343.x
28. Mouthon L, Haury M, Lacroix-Desmazes S, Barreau C, Coutinho A, Kazatchkine MD. Analysis of the normal human IgG antibody repertoire. Evidence that IgG autoantibodies of healthy adults recognize a limited and conserved set of protein antigens in homologous tissues. J Immunol (1995) 154(11):5769–78.
29. Cohen IR. The cognitive paradigm and the immunological homunculus. Immunol Today (1992) 13(12):490–4. doi:10.1016/0167-5699(92)90024-2
30. Nobrega A, Grandien A, Haury M, Hecker L, Malanchere E, Coutinho A. Functional diversity and clonal frequencies of reactivity in the available antibody repertoire. Eur J Immunol (1998) 28(4):1204–15. doi:10.1002/(SICI)1521-4141(199804)28:04<1204::AID-IMMU1204>3.0.CO;2-G
31. Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A (1998) 95(25):14863–8. doi:10.1073/pnas.95.25.14863
32. Kabat EA, Wu TT. Identical V region amino acid sequences and segments of sequences in antibodies of different specificities. Relative contributions of VH and VL genes, minigenes, and complementarity-determining regions to binding of antibody-combining sites. J Immunol (1991) 147(5):1709–19.
33. Ivanov II, Schelonka RL, Zhuang Y, Gartland GL, Zemlin M, Schroeder HW Jr. Development of the expressed Ig CDR-H3 repertoire is marked by focusing of constraints in length, amino acid use, and charge that are first established in early B cell progenitors. J Immunol (2005) 174(12):7773–80. doi:10.4049/jimmunol.174.12.7773
34. Schelonka RL, Tanner J, Zhuang Y, Gartland GL, Zemlin M, Schroeder HW Jr. Categorical selection of the antibody repertoire in splenic B cells. Eur J Immunol (2007) 37(4):1010–21. doi:10.1002/eji.200636569
35. Schelonka RL, Szymanska E, Vale AM, Zhuang Y, Gartland GL, Schroeder HW Jr. DH and JH usage in murine fetal liver mirrors that of human fetal liver. Immunogenetics (2010) 62(10):653–66. doi:10.1007/s00251-010-0469-5
36. Vale AM, Hayashi E, Granato A, Schroeder HW Jr, Bellio M, Nobrega A. Genetic control of the B cell response to LPS: opposing effects in peritoneal versus splenic B cell populations. Immunogenetics (2010) 62(1):41–8. doi:10.1007/s00251-009-0404-9
37. Collins LS, Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol (1987) 138(4):1082–7.
38. Andersson J, Coutinho A, Lernhardt W, Melchers F. Clonal growth and maturation to immunoglobulin secretion in vitro of every growth-inducible B lymphocyte. Cell (1977) 10(1):27–34. doi:10.1016/0092-8674(77)90136-2
Keywords: natural antibodies, self-antigen recognition, CDR-H3 repertoire, peritoneal B cell subsets
Citation: Vale AM, Cavazzoni CB, Nobrega A and Schroeder HW Jr (2016) The Global Self-Reactivity Profile of the Natural Antibody Repertoire Is Largely Independent of Germline DH Sequence. Front. Immunol. 7:296. doi: 10.3389/fimmu.2016.00296
Received: 25 May 2016; Accepted: 22 July 2016;
Published: 10 August 2016
Edited by:
Ana María Hernández, Center of Molecular Inmunology, CubaReviewed by:
Patrick C. Wilson, University of Chicago, USACopyright: © 2016 Vale, Cavazzoni, Nobrega and Schroeder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto Nobrega, YWZub2JyZWdhQGdtYWlsLmNvbQ==;
Harry W. Schroeder Jr., aHdzakB1YWIuZWR1
†Alberto Nobrega and Harry W. Schroeder Jr. contributed equally.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.