
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 21 June 2016
Sec. HIV and AIDS
Volume 7 - 2016 | https://doi.org/10.3389/fimmu.2016.00243
A correction has been applied to this article in:
Corrigendum: Recent Advances in Lentiviral Vaccines for HIV-1 Infection
The development of an effective HIV vaccine to prevent and/or cure HIV remains a global health priority. Given their central role in the initiation of adaptive immune responses, dendritic cell (DC)-based vaccines are being increasingly explored as immunotherapeutic strategies to enhance HIV-specific T cells in infected individuals and, thus, promote immune responses that may help facilitate a functional cure. HIV-1-based lentiviral (LV) vectors have inherent advantages as DC vaccine vectors due to their ability to transduce non-dividing cells and integrate into the target cell genomic DNA, allowing for expression of encoded antigens over the lifespan of the cell. Moreover, LV vectors may express additional immunostimulatory and immunoregulatory proteins that enhance DC function and direct antigen-specific T cells responses. Recent basic and clinical research efforts have broadened our understanding of LV vectors as DC-based vaccines. In this review, we provide an overview of the pre-clinical and clinical LV vector vaccine studies for treating HIV to date. We also discuss advances in LV vector designs that have enhanced DC transduction efficiency, target cell specificity, and immunogenicity, and address potential safety concerns regarding LV vector-based vaccines.
Although antiretroviral therapy has dramatically improved the outcome of HIV infection (1, 2), it is not curative. The failure to cure HIV is due to a reservoir of latently infected T cells formed in the acute phase of infection that re-establishes virus loads upon treatment interruption, thus necessitating lifelong therapy. The development of a vaccine to prevent or cure HIV is, therefore, a major public health priority to fight the global epidemic.
There have been extensive efforts to develop an effective HIV vaccine; however, only one of the preventive vaccine candidates tested in human efficacy trials afforded even modest protection (3–8). Therapeutic vaccine trials for HIV have also failed to show any sustainable impact on viral load and/or viral reservoirs (9–14). Vaccination strategies that generate sustained, high-quality, HIV-specific, adaptive immune responses may be a key component to future success (15–18).
Human trials of HIV vaccines that utilize vector-based antigen delivery approaches have primarily included Adenoviral and Pox vector platforms (3–11, 13). Additional viral and bacterial vectors, derived from cytomegalovirus (CMV), listeria monocytogenes, and HIV lentiviral (LV) constructs have shown potential in pre-clinical studies, but safety concerns have slowed their development (19–22).
In this review, we focus on recent advances in the design of HIV-derived LV vectors for vaccines to induce and augment adaptive immune responses. LV vectors have been explored extensively for gene therapy applications, including for treatment of HIV-1 infection (23, 24). By contrast, the field is considerably less developed regarding their use as HIV vaccines, though pre-clinical and early clinical data show promise (20, 21, 25–30). It is important to highlight certain distinctions between these two applications of LV vectors for HIV, which in large part reflect the genes delivered and desired target cell. LV vectors integrate and replicate in both dividing and non-dividing cells making them efficient vehicles to deliver therapeutic genes with long-lived expression. LV vector-based gene therapies against HIV aim to confer host resistance to infection through delivery of genetic information that interferes with HIV entry or replication and, therefore, primarily target hematopoietic stem cells (HPSCs) or T cells. By contrast, LV vaccines for HIV target antigen-presenting cells (APCs) to deliver HIV antigens that are efficiently presented on MHC molecules. Dendritic cells (DCs) are the ideal LV vaccine targets as they are the most potent APCs and uniquely able to initiate primary immune responses and stimulate robust and durable antigen-specific T cell responses (31). Moreover, the use of LV vectors as DC vaccines offers several advantages over other DC antigen-loading strategies, including more sustained antigen expression following integration into the target cell genomic DNA, endogenous production of antigen for more efficient MHC presentation, the ability to encode immunostimulatory genes and check point inhibitors to enhance T cell responses, and minimal vector immunity when using certain pseudotyped constructs [e.g., LV vectors pseudotyped with different vesicular stomatitis virus G envelope (VSV-G) serotypes] (20, 29).
Here, we review the pre-clinical and early clinical data regarding the use of LV vectors for DC vaccination strategies against HIV-1, and discuss ongoing research to overcome challenges relating to LV vector safety and efficacy. In particular, we focus on recent advances regarding improved LV vector targeting, transduction, and activation of DCs and other APCs to improve their immunostimulatory capacity and mitigate safety concerns.
Several pre-clinical studies have demonstrated that LV vectors are able to induce strong HIV-specific adaptive immune responses (20, 21, 25–29), and preliminary data from an early phase study in HIV-infected individuals support these findings (30). LV vectors encoding HIV-1 or SIV epitopes induced strong HIV-specific cytotoxic T lymphocytes (CTLs), as well as humoral responses in both mouse models and human in vitro studies (21, 25, 26, 28, 29). Notably, LV vectors have demonstrated superior immunogenicity when compared with other vaccine platforms in mice (27, 29, 32). An HIV-1-based LV vector encoding HIV Gag, Pol, and Rev (VRX1023) stimulated more potent and more durable mucosal and systemic cellular and humoral responses than administration of Ad5 HIV vectors (29). Additionally, no anti-vector immunity was detected against the HIV-1-based LV vector, allowing the use of a multiple injection approach to augment immune responses (29). In other disease models, LV vectors were similarly found superior in generating antigen-specific T cell responses in terms of both magnitude and longevity when compared to peptide-pulsed DC vaccines and peptide-adjuvant combinations (27, 32).
In non-human primate models of SIV, macaques that had been immunized with two doses of a VSV-G pseudotyped LV vector expressing SIVmac239 Gag and then challenged with rectal SIVmac251 were protected throughout the acute phase of infection (20). A single injection of the LV vector elicited strong and diverse Gag-specific T cell responses that peaked at 16 days post priming regardless of the dose used, whereas a second dose of non-cross-reactive VSV-G pseudotyped LV vectors administered 11 weeks later raised more robust and rapid Gag-specific T cell responses, albeit similar in breadth. Subsequent challenge of high-dose SIVmac251 resulted in infection in all animals; however, the acute phase of infection showed a reduction in viral replication by more than two orders of magnitude and protection against CD4 T cell (CD28+ CD95+) loss. Lower viral set points in vaccinated animals were also observed, though these were not statistically significant by day 49 post-infection (20).
A first-in-human, phase I/II randomized, controlled study in 38 HIV-infected individuals receiving antiretroviral therapy is currently underway (NCT02054286) (30). In this study, a non-replicative and self-inactivating LV vector encoding portions of HIV Gag, Pol, Nef proteins (TV01, Theravectys) is administered via two intramuscular (IM) injections spaced 8 weeks apart. Initial analysis revealed that TV01 is safe and highly immunogenic with CD4+ and CD8+ T cell responses to multiple vaccine-associated epitopes. These CD4+ and CD8+ T cell responses were found to be polyfunctional as evidenced by production of multiple cytokines and sustained for up to 24 weeks (30). Evaluation of ART interruption is reported to be underway, in addition to plans for a Phase II study.
Taken together, these pre-clinical and now early clinical findings support the potential therapeutic application of LV vectors as HIV vaccines. To improve upon the potency and safety of such vectors, it will be critical to use strategies that enhance the targeting, transduction efficiency, and stimulation of DCs and other APCs.
Initial HIV-1-based LV vectors were generated as VSV-G glycoprotein pseudotypes to allow for production of highly infectious virus with a broad tropism for target cell transduction, including non-dividing cells (Figure 1A) (33). The use of other glycoproteins for pseudotyping has been explored with the intent of minimizing off-target effects, improving safety, and enhancing potency. LV vectors pseudotyped with a mutated Sindbis virus glycoprotein (SVGmu) confer a natural tropism toward human DCs as SVGmu selectively binds to the DC surface protein DC-SIGN (CD209) (Figure 1B) (34). Unlike standard laboratory-adapted Sindbis virus envelopes that target ubiquitously expressed heparan sulfate in addition to DC-SIGN, SVGmu contains mutations in the heparan sulfate binding site that prevent heparan sulfate-mediated cell entry. A single injection of an SVGmu-pseudotyped LV vector encoding tumor antigen or HIV-1 Gag in mice stimulated their DCs to induce a durable antitumor or HIV-1-specific immune response, respectively, while inducing only low levels of anti-vector neutralizing antibodies (34, 35). Moreover, prime/boost regimens consisting of either a heterologous DNA prime/SVGmu-LV-Gag boost or successive SVGmu-LV-Gag injections enhanced cellular and humoral responses and proved superior to a DNA prime/adenoviral vector boost immunization in terms of both the breadth and polyfunctionality of the vaccine-induced Gag-specific CD8+ and CD4+ T cells. Subsequent modifications of SVGmu, through amino acid substitutions in the receptor-binding site and restoration of the wild-type furin cleavage, improved proteolytic processing and virus maturation and led to enhanced DC-SIGN specificity and production yields (36).
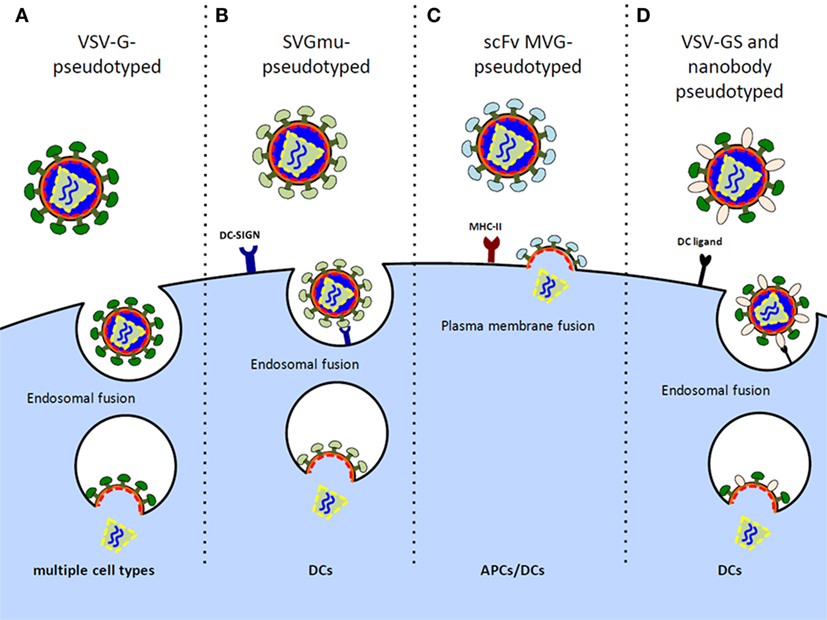
Figure 1. Design of LV vectors to preferentially target DCs/APCs. (A) Vesicular stomatitis virus G (VSV-G) glycoprotein-pseudotyped LV vectors possess diverse cell tropism, including transduction of non-dividing cells. (B) Sindbis virus glycoprotein (SVGmu)-pseudotyped LV vectors selectively target DCs by binding the endocytic receptor, DC-SIGN, while containing mutations in the heparan sulfate binding site to prevent transduction of other cell types. (C) LV vectors pseudotyped with measles virus glycoprotein (MVG) mediate direct cell entry via membrane fusion at the plasma membrane. These vectors can be engineered to display single-chain antibody (scFV) directed at MHC II to target transduction of APCs. (D) VSV-GS, a binding-defective, fusion competent VSV-G, and nanobody-pseudotyped LV virions are engineered to bind various DC ligands for targeted fusion.
Measles virus glycoproteins (MVGs), hemagglutinin (H) and fusion (F), have also been used to pseudotype LV vectors for DC targeting. A potential advantage over SVGmu and VSV-G-pseudotyped lentiviruses that require endocytosis for viral membrane fusion and cell entry (37) is that MVG-pseudotyped lentiviruses fuse at the plasma membrane for direct cell entry. The H glycoprotein of the measles virus is responsible for receptor recognition and confers a natural tropism for both the CD46 receptor expressed on all nucleated cells and the signaling lymphocyte activation molecule (SLAM) receptor constitutively expressed on memory T cells, thymocytes, select B cells, monocytes, and DCs (38). Remarkably, measles virus-pseudotyped vectors were shown to be fourfold more infectious in DCs compared to VSV-G-pseudotyped vectors at an MOI of 10 (39). Moreover, unlike VSV-G-pseudotyped lentiviruses that induced DC activation, pseudotyping with MVGs did not affect the maturation and activation status of the transduced DCs, thereby minimizing any unintended stimulation. In effort to develop APC-specific MVG-pseudotyped LV vectors, mutations were made in the H glycoprotein’s CD46 and SLAM recognition sites, and the glycoprotein was further engineered to display single-chain antibody (scFv) directed against major histocompatibility complex class II (MHC II) (Figure 1C) (40–42). Resultant LV vectors showed high in vivo DC specificity and induced significant CD4+ and CD8+ T cell responses, albeit, inferior to the VSV-G-pseudotyped vectors, likely owing to the impaired transduction efficiency and stability of the chimeric constructs. Mice immunized with a single injection of the HIV-1-derived LV vector pseudotyped with MHC II-targeted MVGs did, however, mount antigen-specific effector CD4+ and CD8+ T cells and establish T cell immune memory, suggesting their potential for clinical use (41).
An alternative approach to generating DC-specific LV vectors is to take advantage of the natural LV budding process and incorporate cell-targeting moieties at the viral surface. To this end, LV vectors were pseudotyped with a binding-defective, fusion-competent VSV-G glycoprotein (VSV-GS) and DC-specific single variable regions derived from camel IgG sequences, called nanobodies. The resultant virions selectively bind DC receptors to allow DC-specific membrane fusion (Figure 1D). This nanobody display technology has proven effective at targeting LV vectors to murine DCs both in vitro and in situ and has previously been well reviewed (43, 44).
A comparison of the different strategies used to develop LV vectors for DC-targeted delivery is summarized (Figure 1). These approaches carry inherent safety advantages over broadly tropic vectors, and it is likely that continued improvements in their stability, DC specificity, and transduction efficiency will advance their readiness for clinical testing.
DC-targeting aside, the development of HIV-1-based LV vectors as DC vaccines has been limited by the low efficiency with which DCs are transduced. DCs express SAMHD1, a phosphohydrolase that depletes the cell of deoxynucleotide triphosphates, thereby halting infection of HIV-1-based vectors at the level of reverse transcription (45–47). HIV-2 and some SIV isolates encode the accessory protein Vpx that counteracts this block to infection by binding to SAMHD1 and recruiting an E3 ubiquitin ligase complex, CRL4, to mediate its proteasomal degradation (48, 49). However, HIV-1 does not encode Vpx and lacks a similar mechanism to counteract SAMHD1. Various strategies have, therefore, been attempted to deliver Vpx with HIV-1-based LV vectors in order to improve their ability to transduce DCs.
Initial studies co-administered LV virions with virus-like particles (VLPs) containing Vpx to facilitate SAMHD1 degradation and improve LV vector DC transduction efficiency (50). More recently, Sunseri et al. identified the 10 amino acid packaging motif in the P6 protein of SIVmac Gag that is required to package Vpx. Introduction of this motif into P6 of the HIV-1 Gag/Pol packaging vector allowed for the production of HIV-1 virions that contained a high copy number of Vpx molecules in its native location (51). The Vpx-packaged HIV-1 vectors infected DCs with a two-log increase in titer and allowed for the stable expression of transgenes or shRNA knock-down of target genes (52). In the absence of the Vpx-packaging motif, HIV-1-based LV vectors packaged only trace quantities of Vpx and the virus had much lower titers on DCs (21, 51, 52). An alternative approach to allow Vpx packaging was to fuse Vpx to a cSrc membrane-targeting domain (53). Co-transfection of the Vpx-cSrc expression plasmid during LV production allowed Vpx to be re-localized from the nucleus to the plasma membrane where it could be packaged into virion particles during virion assembly. Resultant Vpx-packaged lentiviruses encoding antigenic epitopes were shown to efficiently transduce DCs and stimulate expansion of antigen-specific CD8 T cells (54). It has been reported that Vpx-packaging is possible without any modification to the HIV-1 capsid or fusion to cSrc (36), though the Vpx-packaging efficiency is likely to be considerably improved by these systems and allow for more efficient DC transduction.
In chronic infections, such as HIV, continuous antigen stimulation along with other factors leads to immunologic tolerance and CTL exhaustion. An advantage of using viral vectors for DC transduction is that the vectors can accommodate large gene inserts and express immunostimulatory proteins to serve as adjuvants for enhancing or regulating antigen-specific immune responses. Effective stimulation of antigen-specific T cells requires three signals: MHC-peptide complex recognition by antigen-specific T cell receptors (TCR), costimulation by DC surface molecules and their T cell ligands (i.e., DC CD80 and T cell CD28), and cytokine priming mediated by DC secretion of soluble cytokines. We previously showed that Vpx-packaged LV vectors expressing the DC stimulatory protein CD40 ligand (CD40L) fused to a single influenza or HIV viral epitope efficiently transduced DCs, causing the cells to mature and secrete proinflammatory and Th1 skewing cytokines (e.g., TNF-α, IL-12, and IL-6) that enhanced antigen presentation, and thus stimulated robust expansion of influenza or HIV-specific CTLs (21). Furthermore, the proinflammatory cytokines transiently released by DCs transduced with Vpx-vectors expressing CD40L reactivated latent HIV-1 provirus in latency models, supporting the use of such vectors in a two-pronged, “shock and kill” approach for reducing the latent reservoir and facilitating a functional cure (21). Goyvaerts et al. similarly capitalized on the ability of IL-12 to enhance expansion CD4+ Th1 cells and cytotoxic effector cells and showed that DCs transduced with LV vectors expressing IL-12-enhanced antigen-specific CTLs in vivo (55). Others developed LV vectors expressing antigenic epitopes in tandem with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) or GM-CSF and IFN-α to enhance the longevity of transduced DCs and promote a more durable immune response (56–59).
In addition to enhancing immune responses, reversing immunologic tolerance by interfering with the programed death 1 (PD-1) pathway has been a well-studied means of restoring the function of exhausted antigen-specific CD8 T cells (60, 61). PD-1 is upregulated during chronic infection with HIV and its increased binding to its ligand, PD-1 ligand (PD-L1), mediates virus-specific CD8 T cell exhaustion by impairing the ability of T cells to become activated, proliferate, and produce cytokines. Blocking the PD-1/PD-1L inhibitory signal with an anti-PD-L1 antibody led to an enhanced HIV-1–specific CD8+ T cell response in mice immunized with DCs transduced with a LV vector expressing HIV-1 Gag (62). Interfering with PD-L1/PD-1 costimulation via LV vectors expressing soluble PD-1 or shRNA against PD-L1 also led to enhanced antigen-specific CTL responses (63–66), further highlighting the potential feasibility and importance of disrupting the PD-1/PD-L1 interaction in future LV vaccine strategies.
Although DC targeting can minimize the off-target transduction of LV vectors, the use of HIV-derived LV vectors raises inherent safety concerns. These risks primarily relate to the formation of infectious virus and potential for host mutagenesis, and have been addressed in multiple ways to maximize safety. Several aspects regarding the design of LV vectors render the formation of replication-competent virus and subsequent infection exceedingly low (20). The risk of reconstituting pathogenic parental HIV-1 virus is eliminated by providing the only HIV-1 structural and enzymatic proteins required for the formation of LV virions in trans and using heterologous envelopes (e.g., VSV-G) such that LV vectors support only a single round infection. Furthermore, LV vector constructs generally lack accessory and regulatory genes, such as vif, vpr, vpu, and nef, that are required for efficient replication in vivo (67). Finally, self-inactivating vectors can also be designed that lack the 3′-LTR region of the viral promotor and enhancer.
The other major safety concern with LV vectors is the risk for potential mutagenesis caused by insertion of the viral genome into the host genome. The risk of insertional mutagenesis remains controversial but is likely very low as it has not been described with HIV-derived LV vectors in gene therapy trials to date (20, 23, 24). However, these concerns were highlighted in studies that used a retroviral vector derived from the Murine Moloney Leukemia Virus (MoMLV) in two SCID-X1 gene therapy trials (68, 69). In these studies, several cases of leukemia occurred post-treatment, thought to be caused by the transactivation of a proto-oncogene by a viral enhancer rather than a direct effect of integration (69). Options to offset this possibility include using a promotor that lacks enhancer activity, or the use of integration-deficient LV vectors (IDLVs) that cannot insert themselves into the host genome. It should also be highlighted that in the case of LV vectors for vaccination purposes as opposed the gene therapy, the likelihood of a detrimental effect caused by insertional mutagenesis is reduced as the transduced APCs do not proliferate and will likely be cleared by the subsequent CTLs that are formed.
The use of IDLVs generated by the expression of a catalytically inactive integrase is the most fail-safe means to mitigate any potential risk for insertional mutagenesis (70). Instead of inserting into the host genome, episomal DNA accumulates in the nucleus, resulting in transcription and efficient expression of the target gene (71). IDLV have been shown to be immunogenic with the ability to elicit both humoral and cell-mediated responses after IM and mucosal administration, although somewhat weaker than integrating LV vectors (72, 73). It remains poorly understood why IDLV have been less potent as vaccine vectors in pre-clinical studies compared with integrative LV vectors. Unlike integrated DNA, episomal DNA may be diluted in dividing cells over time. However, given that APCs/DCs are non-proliferating, decreased expression of target genes over time would seem less likely to impact their efficacy as vaccine vectors. Despite the weaker potency that has been observed when compared with integrative LV vectors, IDLV have been effective in stable transduction following IM administration in mice (74, 75) and has proven effective in multiple models of infection, including West Nile virus (76), HPV-associated tumors (77), and malaria (78). In terms of pathogens acquired via a mucosal route, including HIV, both mucosal immune responses and systemic immune responses are desirable for protection. Mucosal immunization using IDLV by various routes has shown promise in achieving protection at these surfaces in mouse models (79, 80). CD8+ T cell responses were observed in the gut in the lamina propria following IM vaccination with IDLV, but an adjuvanted sublingual protein boost was required in order to induce mucosal IgA responses (80). Intranasal (IN) vaccination with an IDLV expressing influenza nucleoprotein (NP) was compared to IM vaccination and both were found to induce NP-specific B and T cell responses, however, only the IN route protected from influenza virus infection (79).
A human vaccine trial combining these many safety measures in a phase I dose-escalation study in solid cancers is currently under way (NCT02122861) (81). This trial uses replication-incompetent, IDLV vectors expressing full-length NY-ESO-1, engineered to target DC-SIGN (LV305, Immune Design) and induce tumor-specific CTLs in patients with advanced NY-ESO-1 expressing melanoma, sarcoma, breast, lung, or ovarian cancers. Early results from dose escalation in 12 patients with sarcoma demonstrated safety up through the highest dose (1010 vg) administered intradermally (ID), with only grade I/II adverse events. The early immunogenicity from only the lowest dose group (108 vg, N = 6) has been reported following receipt of 3–4 ID injections q3weeks, and found to generate strong T cell responses and some early evidence of antitumor effect (81).
HIV-1-derived LV vectors are an effective tool for targeting DCs and inducing potent HIV-specific adaptive immune responses. The best means to preferentially target DCs to maximize both safety and immunogenicity of LV vectors is a matter of ongoing investigation and remains a key issue. A better understanding of HIV restriction factors, such as SAMHD1, and their counteraction by viral proteins, such as Vpx, has lead to the development of vectors with high DC transduction efficiencies and reinvigorated the DC vaccine field. Incorporation of immunostimulatory genes into LV vectors as adjuvants has further improved their immunogenicity. Additionally, the safety of LV vectors has been enhanced through developments in IDLVs and substantiated by their exploration in clinical trials. Taking together, therapeutic LV vector-based DC vaccines have the potential to alter the traditional vaccine landscape and may provide a means to contribute to functional cure strategies for HIV, as well as other diseases where antigenic targets have been identified.
EM and TN contributed equally to this mini-review in both research and writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We can thank Drs. Ned Landau and Nicolin Bloch for manuscript review.
NIH/NIAID K08AI120898 to TN and Pfizer Young Investigator Award in Vaccine Development IDSA/NFID to TN.
1. Mouton Y, Alfandari S, Valette M, Cartier F, Dellamonica P, Humbert G, et al. Impact of protease inhibitors on AIDS-defining events and hospitalizations in 10 French AIDS reference centres. Federation National des Centres de Lutte contre le SIDA. AIDS (1997) 11:F101–5. doi:10.1097/00002030-199712000-00003
2. Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med (1998) 338:853–60. doi:10.1056/NEJM199803263381301
3. Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet (2008) 372:1881–93. doi:10.1016/S0140-6736(08)61591-3
4. Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF, et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis (2005) 191:654–65. doi:10.1086/428404
5. Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis (2011) 11:507–15. doi:10.1016/S1473-3099(11)70098-6
6. Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med (2013) 369:2083–92. doi:10.1056/NEJMoa1310566
7. Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis (2006) 194:1661–71. doi:10.1086/508748
8. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med (2009) 361:2209–20. doi:10.1056/NEJMoa0908492
9. Achenbach CJ, Assoumou L, Deeks SG, Wilkin TJ, Berzins B, Casazza JP, et al. Effect of therapeutic intensification followed by HIV DNA prime and rAd5 boost vaccination on HIV-specific immunity and HIV reservoir (EraMune 02): a multicentre randomised clinical trial. Lancet HIV (2015) 2:e82–91. doi:10.1016/S2352-3018(15)00026-0
10. Autran B, Murphy RL, Costagliola D, Tubiana R, Clotet B, Gatell J, et al. Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452). AIDS (2008) 22:1313–22. doi:10.1097/QAD.0b013e3282fdce94
11. Gandhi RT, O’Neill D, Bosch RJ, Chan ES, Bucy RP, Shopis J, et al. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine (2009) 27:6088–94. doi:10.1016/j.vaccine.2009.05.016
12. Garcia F, Climent N, Guardo AC, Gil C, Leon A, Autran B, et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Transl Med (2013) 5:166ra2. doi:10.1126/scitranslmed.3004682
13. Kinloch-de Loes S, Hoen B, Smith DE, Autran B, Lampe FC, Phillips AN, et al. Impact of therapeutic immunization on HIV-1 viremia after discontinuation of antiretroviral therapy initiated during acute infection. J Infect Dis (2005) 192:607–17. doi:10.1086/432002
14. Routy JP, Boulassel MR, Yassine-Diab B, Nicolette C, Healey D, Jain R, et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin Immunol (2010) 134:140–7. doi:10.1016/j.clim.2009.09.009
15. Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature (2012) 487:482–5. doi:10.1038/nature11286
16. Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity (2012) 36:491–501. doi:10.1016/j.immuni.2012.01.014
17. Xing S, Bullen CK, Shroff NS, Shan L, Yang HC, Manucci JL, et al. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J Virol (2011) 85:6060–4. doi:10.1128/JVI.02033-10
18. Xing S, Siliciano RF. Targeting HIV latency: pharmacologic strategies toward eradication. Drug Discov Today (2013) 18(11–12):541–51. doi:10.1016/j.drudis.2012.12.008
19. Hansen SG, Piatak M Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, et al. Immune clearance of highly pathogenic SIV infection. Nature (2013) 502:100–4. doi:10.1038/nature12519
20. Beignon AS, Mollier K, Liard C, Coutant F, Munier S, Riviere J, et al. Lentiviral vector-based prime/boost vaccination against AIDS: pilot study shows protection against Simian immunodeficiency virus SIVmac251 challenge in macaques. J Virol (2009) 83:10963–74. doi:10.1128/JVI.01284-09
21. Norton TD, Miller EA, Bhardwaj N, Landau NR. Vpx-containing dendritic cell vaccine induces CTLs and reactivates latent HIV-1 in vitro. Gene Ther (2015) 22:227–36. doi:10.1038/gt.2014.117
22. Miller EA, Spadaccia MR, Norton T, Demmler M, Gopal R, O’Brien M, et al. Attenuated Listeria monocytogenes vectors overcome suppressive plasma factors during HIV infection to stimulate myeloid dendritic cells to promote adaptive immunity and reactivation of latent virus. AIDS Res Hum Retroviruses (2015) 31:127–36. doi:10.1089/AID.2014.0138
23. Symonds GP, Johnstone HA, Millington ML, Boyd MP, Burke BP, Breton LR. The use of cell-delivered gene therapy for the treatment of HIV/AIDS. Immunol Res (2010) 48:84–98. doi:10.1007/s12026-010-8169-7
24. Wolstein O, Boyd M, Millington M, Impey H, Boyer J, Howe A, et al. Preclinical safety and efficacy of an anti-HIV-1 lentiviral vector containing a short hairpin RNA to CCR5 and the C46 fusion inhibitor. Mol Ther Methods Clin Dev (2014) 1:11. doi:10.1038/mtm.2013.11
25. Buffa V, Negri DR, Leone P, Bona R, Borghi M, Bacigalupo I, et al. A single administration of lentiviral vectors expressing either full-length human immunodeficiency virus 1 (HIV-1)(HXB2) Rev/Env or codon-optimized HIV-1(JR-FL) gp120 generates durable immune responses in mice. J Gen Virol (2006) 87:1625–34. doi:10.1099/vir.0.81706-0
26. Buffa V, Negri DR, Leone P, Borghi M, Bona R, Michelini Z, et al. Evaluation of a self-inactivating lentiviral vector expressing simian immunodeficiency virus gag for induction of specific immune responses in vitro and in vivo. Viral Immunol (2006) 19:690–701. doi:10.1089/vim.2006.19.690
27. Esslinger C, Chapatte L, Finke D, Miconnet I, Guillaume P, Levy F, et al. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. J Clin Invest (2003) 111:1673–81. doi:10.1172/JCI200317098
28. Iglesias MC, Mollier K, Beignon AS, Souque P, Adotevi O, Lemonnier F, et al. Lentiviral vectors encoding HIV-1 polyepitopes induce broad CTL responses in vivo. Mol Ther (2007) 15:1203–10. doi:10.1038/sj.mt.6300135
29. Lemiale F, Asefa B, Ye D, Chen C, Korokhov N, Humeau L. An HIV-based lentiviral vector as HIV vaccine candidate: immunogenic characterization. Vaccine (2010) 28:1952–61. doi:10.1016/j.vaccine.2009.10.089
30. Toussaint H, Sarry E, Bejanariu A, Agaugue S, Rodriguez M, Sabbah-Petrover E, et al. A first-in-human phase I/II trial demonstrates the safety and the immunogenicity of a lentiviral based therapeutic HIV vaccine eliciting potent polyfunctional multispecific CD8 and CD4 Tcell responses in HIV-infected individuals. Towards an HIV Cure Symposium. Vancouver, BC: International Aids Society (2015). Abstract E62LB p.
31. Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med (1973) 137:1142–62. doi:10.1084/jem.137.5.1142
32. He Y, Zhang J, Mi Z, Robbins P, Falo LD Jr. Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol (2005) 174:3808–17. doi:10.4049/jimmunol.174.6.3808
33. Naldini L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr Opin Biotechnol (1998) 9:457–63. doi:10.1016/S0958-1669(98)80029-3
34. Yang L, Yang H, Rideout K, Cho T, Joo KI, Ziegler L, et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol (2008) 26:326–34. doi:10.1038/nbt1390
35. Dai B, Yang L, Yang H, Hu B, Baltimore D, Wang P. HIV-1 Gag-specific immunity induced by a lentivector-based vaccine directed to dendritic cells. Proc Natl Acad Sci USA (2009) 106:20382–7. doi:10.1073/pnas.0911742106
36. Tareen SU, Kelley-Clarke B, Nicolai CJ, Cassiano LA, Nelson LT, Slough MM, et al. Design of a novel integration-deficient lentivector technology that incorporates genetic and posttranslational elements to target human dendritic cells. Mol Ther (2014) 22:575–87. doi:10.1038/mt.2013.278
37. Smit JM, Bittman R, Wilschut J. Low-pH-dependent fusion of Sindbis virus with receptor-free cholesterol- and sphingolipid-containing liposomes. J Virol (1999) 73:8476–84.
38. Funke S, Maisner A, Muhlebach MD, Koehl U, Grez M, Cattaneo R, et al. Targeted cell entry of lentiviral vectors. Mol Ther (2008) 16:1427–36. doi:10.1038/mt.2008.128
39. Humbert JM, Frecha C, Amirache Bouafia F, N’Guyen TH, Boni S, Cosset FL, et al. Measles virus glycoprotein-pseudotyped lentiviral vectors are highly superior to vesicular stomatitis virus G pseudotypes for genetic modification of monocyte-derived dendritic cells. J Virol (2012) 86:5192–203. doi:10.1128/JVI.06283-11
40. Ageichik A, Buchholz CJ, Collins MK. Lentiviral vectors targeted to MHC II are effective in immunization. Hum Gene Ther (2011) 22:1249–54. doi:10.1089/hum.2010.184
41. Cire S, Da Rocha S, Yao R, Fisson S, Buchholz CJ, Collins MK, et al. Immunization of mice with lentiviral vectors targeted to MHC class II+ cells is due to preferential transduction of dendritic cells in vivo. PLoS One (2014) 9:e101644. doi:10.1371/journal.pone.0101644
42. Anliker B, Abel T, Kneissl S, Hlavaty J, Caputi A, Brynza J, et al. Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat Methods (2010) 7:929–35. doi:10.1038/nmeth.1514
43. Goyvaerts C, De Groeve K, Dingemans J, Van Lint S, Robays L, Heirman C, et al. Development of the Nanobody display technology to target lentiviral vectors to antigen-presenting cells. Gene Ther (2012) 19:1133–40. doi:10.1038/gt.2011.206
44. Goyvaerts C, Kurt de G, Van Lint S, Heirman C, Van Ginderachter JA, De Baetselier P, et al. Immunogenicity of targeted lentivectors. Oncotarget (2014) 5:704–15. doi:10.18632/oncotarget.1680
45. Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature (2011) 480:379–82. doi:10.1038/nature10623
46. Powell RD, Holland PJ, Hollis T, Perrino FW. Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J Biol Chem (2011) 286:43596–600. doi:10.1074/jbc.C111.317628
47. Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol (2012) 13:223–8. doi:10.1038/ni.2236
48. Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature (2011) 474:658–61. doi:10.1038/nature10195
49. Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature (2011) 474:654–7. doi:10.1038/nature10117
50. Goujon C, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. With a little help from a friend: increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC). Gene Ther (2006) 13:991–4. doi:10.1038/sj.gt.3302753
51. Sunseri N, O’Brien M, Bhardwaj N, Landau NR. Human immunodeficiency virus type 1 modified to package Simian immunodeficiency virus Vpx efficiently infects macrophages and dendritic cells. J Virol (2011) 85:6263–74. doi:10.1128/JVI.00346-11
52. Bobadilla S, Sunseri N, Landau NR. Efficient transduction of myeloid cells by an HIV-1-derived lentiviral vector that packages the Vpx accessory protein. Gene Ther (2013) 20:514–20. doi:10.1038/gt.2012.61
53. Durand S, Nguyen XN, Turpin J, Cordeil S, Nazaret N, Croze S, et al. Tailored HIV-1 vectors for genetic modification of primary human dendritic cells and monocytes. J Virol (2013) 87:234–42. doi:10.1128/JVI.01459-12
54. Negri DR, Rossi A, Blasi M, Michelini Z, Leone P, Chiantore MV, et al. Simian immunodeficiency virus-Vpx for improving integrase defective lentiviral vector-based vaccines. Retrovirology (2012) 9:69. doi:10.1186/1742-4690-9-69
55. Goyvaerts C, Broos K, Escors D, Heirman C, Raes G, De Baetselier P, et al. The transduction pattern of IL-12-encoding lentiviral vectors shapes the immunological outcome. Eur J Immunol (2015) 45(12):3351–61. doi:10.1002/eji.201545559
56. Daenthanasanmak A, Salguero G, Borchers S, Figueiredo C, Jacobs R, Sundarasetty BS, et al. Integrase-defective lentiviral vectors encoding cytokines induce differentiation of human dendritic cells and stimulate multivalent immune responses in vitro and in vivo. Vaccine (2012) 30:5118–31. doi:10.1016/j.vaccine.2012.05.063
57. Salguero G, Daenthanasanmak A, Munz C, Raykova A, Guzman CA, Riese P, et al. Dendritic cell-mediated immune humanization of mice: implications for allogeneic and xenogeneic stem cell transplantation. J Immunol (2014) 192:4636–47. doi:10.4049/jimmunol.1302887
58. Sundarasetty BS, Kloess S, Oberschmidt O, Naundorf S, Kuehlcke K, Daenthanasanmak A, et al. Generation of lentivirus-induced dendritic cells under GMP-compliant conditions for adaptive immune reconstitution against cytomegalovirus after stem cell transplantation. J Transl Med (2015) 13:240. doi:10.1186/s12967-015-0599-5
59. Sundarasetty BS, Singh VK, Salguero G, Geffers R, Rickmann M, Macke L, et al. Lentivirus-induced dendritic cells for immunization against high-risk WT1(+) acute myeloid leukemia. Hum Gene Ther (2013) 24:220–37. doi:10.1089/hum.2012.128
60. Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol (2009) 182:5891–7. doi:10.4049/jimmunol.0803771
61. Velu V, Shetty RD, Larsson M, Shankar EM. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology (2015) 12:14. doi:10.1186/s12977-015-0144-x
62. Dai B, Xiao L, Bryson PD, Fang J, Wang P. PD-1/PD-L1 blockade can enhance HIV-1 Gag-specific T cell immunity elicited by dendritic cell-directed lentiviral vaccines. Mol Ther (2012) 20:1800–9. doi:10.1038/mt.2012.98
63. He YF, Zhang GM, Wang XH, Zhang H, Yuan Y, Li D, et al. Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. J Immunol (2004) 173:4919–28. doi:10.4049/jimmunol.173.8.4919
64. Karwacz K, Bricogne C, MacDonald D, Arce F, Bennett CL, Collins M, et al. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol Med (2011) 3:581–92. doi:10.1002/emmm.201100165
65. Pen JJ, Keersmaecker BD, Heirman C, Corthals J, Liechtenstein T, Escors D, et al. Interference with PD-L1/PD-1 co-stimulation during antigen presentation enhances the multifunctionality of antigen-specific T cells. Gene Ther (2014) 21:262–71. doi:10.1038/gt.2013.80
66. Song MY, Park SH, Nam HJ, Choi DH, Sung YC. Enhancement of vaccine-induced primary and memory CD8(+) T-cell responses by soluble PD-1. J Immunother (2011) 34:297–306. doi:10.1097/CJI.0b013e318210ed0e
67. Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol (1997) 15:871–5. doi:10.1038/nbt0997-871
68. Gaspar HB, Cooray S, Gilmour KC, Parsley KL, Adams S, Howe SJ, et al. Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Sci Transl Med (2011) 3:97ra79. doi:10.1126/scitranslmed.3002715
69. Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science (2003) 302:415–9. doi:10.1126/science.1088547
70. Banasik MB, McCray PB Jr. Integrase-defective lentiviral vectors: progress and applications. Gene Ther (2010) 17:150–7. doi:10.1038/gt.2009.135
71. Vargas J Jr, Gusella GL, Najfeld V, Klotman ME, Cara A. Novel integrase-defective lentiviral episomal vectors for gene transfer. Hum Gene Ther (2004) 15:361–72. doi:10.1089/104303404322959515
72. Hu B, Dai B, Wang P. Vaccines delivered by integration-deficient lentiviral vectors targeting dendritic cells induces strong antigen-specific immunity. Vaccine (2010) 28:6675–83. doi:10.1016/j.vaccine.2010.08.012
73. Karwacz K, Mukherjee S, Apolonia L, Blundell MP, Bouma G, Escors D, et al. Nonintegrating lentivector vaccines stimulate prolonged T-cell and antibody responses and are effective in tumor therapy. J Virol (2009) 83:3094–103. doi:10.1128/JVI.02519-08
74. Apolonia L, Waddington SN, Fernandes C, Ward NJ, Bouma G, Blundell MP, et al. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol Ther (2007) 15:1947–54. doi:10.1038/sj.mt.6300281
75. Negri DR, Bona R, Michelini Z, Leone P, Macchia I, Klotman ME, et al. Transduction of human antigen-presenting cells with integrase-defective lentiviral vector enables functional expansion of primed antigen-specific CD8(+) T cells. Hum Gene Ther (2010) 21:1029–35. doi:10.1089/hum.2009.200
76. Iglesias MC, Frenkiel MP, Mollier K, Souque P, Despres P, Charneau P. A single immunization with a minute dose of a lentiviral vector-based vaccine is highly effective at eliciting protective humoral immunity against West Nile virus. J Gene Med (2006) 8:265–74. doi:10.1002/jgm.837
77. Grasso F, Negri DR, Mochi S, Rossi A, Cesolini A, Giovannelli A, et al. Successful therapeutic vaccination with integrase defective lentiviral vector expressing nononcogenic human papillomavirus E7 protein. Int J Cancer (2013) 132:335–44. doi:10.1002/ijc.27676
78. Coutant F, Sanchez David RY, Felix T, Boulay A, Caleechurn L, Souque P, et al. A nonintegrative lentiviral vector-based vaccine provides long-term sterile protection against malaria. PLoS One (2012) 7:e48644. doi:10.1371/journal.pone.0048644
79. Fontana JM, Christos PJ, Michelini Z, Negri D, Cara A, Salvatore M. Mucosal immunization with integrase-defective lentiviral vectors protects against influenza virus challenge in mice. PLoS One (2014) 9:e97270. doi:10.1371/journal.pone.0097270
80. Rossi A, Michelini Z, Leone P, Borghi M, Blasi M, Bona R, et al. Optimization of mucosal responses after intramuscular immunization with integrase defective lentiviral vector. PLoS One (2014) 9:e107377. doi:10.1371/journal.pone.0107377
Keywords: dendritic cells, lentiviral vectors, HIV-1, HIV-1 vaccines, SAMHD1, Vpx
Citation: Norton TD and Miller EA (2016) Recent Advances in Lentiviral Vaccines for HIV-1 Infection. Front. Immunol. 7:243. doi: 10.3389/fimmu.2016.00243
Received: 18 March 2016; Accepted: 08 June 2016;
Published: 21 June 2016
Edited by:
Mario Clerici, University of Milan, ItalyReviewed by:
Lucia Lopalco, San Raffaele Scientific Institute, ItalyCopyright: © 2016 Norton and Miller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth A. Miller, ZWxpemFiZXRoLmEubWlsbGVyQG1zc20uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.