- 1Boyce Thompson Institute, Cornell University, Ithaca, NY, USA
- 2Department of Plant and Environmental Protection Sciences, University of Hawaii at Manoa, Honolulu, HI, USA
Salicylic acid (SA) is a critical plant hormone that is involved in many processes, including seed germination, root initiation, stomatal closure, floral induction, thermogenesis, and response to abiotic and biotic stresses. Its central role in plant immunity, although extensively studied, is still only partially understood. Classical biochemical approaches and, more recently, genome-wide high-throughput screens have identified more than two dozen plant SA-binding proteins (SABPs), as well as multiple candidates that have yet to be characterized. Some of these proteins bind SA with high affinity, while the affinity of others exhibit is low. Given that SA levels vary greatly even within a particular plant species depending on subcellular location, tissue type, developmental stage, and with respect to both time and location after an environmental stimulus such as infection, the presence of SABPs exhibiting a wide range of affinities for SA may provide great flexibility and multiple mechanisms through which SA can act. SA and its derivatives, both natural and synthetic, also have multiple targets in animals/humans. Interestingly, many of these proteins, like their plant counterparts, are associated with immunity or disease development. Two recently identified SABPs, high mobility group box protein and glyceraldehyde 3-phosphate dehydrogenase, are critical proteins that not only serve key structural or metabolic functions but also play prominent roles in disease responses in both kingdoms.
Introduction
In plants, salicylic acid (SA) was viewed as a relatively unimportant secondary metabolite until the late twentieth century, when Raskin and coworkers revealed its involvement in signaling thermogenesis (1) and our group (2), together with Métraux and colleagues (3), demonstrated its importance in activating disease resistance. Today, a Google search for the “number of papers on salicylic acid and plant disease resistance” lists ~59,000. Many, if not most, of these studies confirm SA’s central role in immunity, principally against biotrophic and hemibiotrophic pathogens.
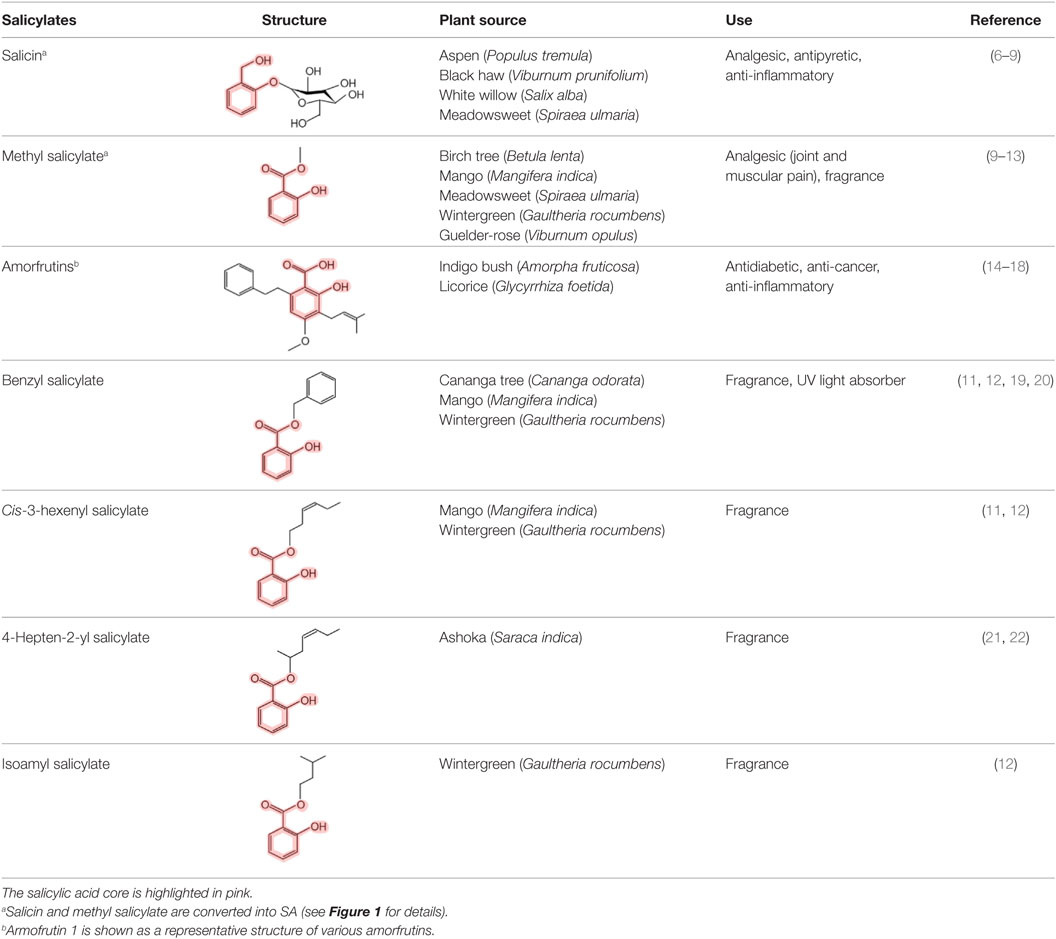
In contrast, the importance of SA and its derivatives (collectively called salicylates) as pharmacological agents has long been appreciated. Salicin, the SA derivative that is the active ingredient in willow bark, was isolated in 1828; however, Hippocrates, the father of medicine, reportedly prescribed willow bark to reduce fever and the pain of childbirth in the fifth millennium B.C. High levels of salicylates have been detected in several plant species besides willow. For example, meadowsweet contains both salicin and methyl salicylate (MeSA), another medicinal derivative that also is known as the highly fragrant oil of wintergreen. In animals/humans, these “prodrugs” are converted to SA upon digestion (Figure 1) (4, 5). The sources and chemical structures of several useful natural salicylates are shown in Table 1. Not only have medicinal plants rich in salicylates been used worldwide in many different cultures for thousands of years but also they continue to be used today. In this regard, the most famous SA derivative, acetyl SA, is a relative “new comer” as it was first synthesized by Bayer and Company in 1897 and subsequently sold under the trade name aspirin. Interestingly, the name SA is derived from the Latin name for white willow (Salix alba), while the term aspirin is derived from meadowsweet (Spiraea ulmaria).
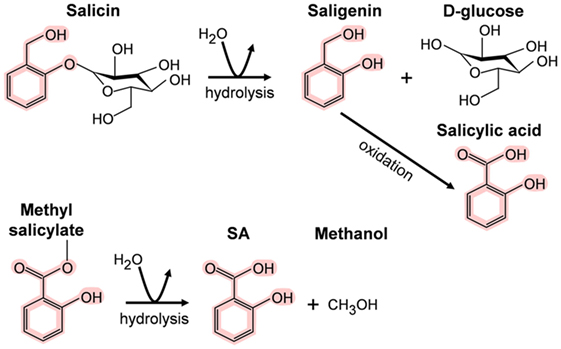
Figure 1. Metabolism of salicin and methyl salicylate to salicylic acid (SA). The SA core is highlighted in pink.
One or a Few Receptors vs. Multiple Targets
The current view is that hormones in plants, as well as in animals, exert their effect(s) by binding to one or a small number of receptors. Is SA’s mechanism(s) of action consistent with this dogma? The answer to this question is currently unclear. One or more members of the non-expressor of pathogenesis-related protein (NPR) family were proposed to function as SA receptors in plants (23, 24). The molecular mechanism(s) through which SA mediates NPR1’s function as a co-activator of immunity-induced transcriptional reprograming is currently unresolved. While the identification of NPR proteins as SA targets is a major step toward elucidating SA’s mechanisms of action in defense against microbial pathogens, the upregulation of some plant immune responses, including expression of a subset of defense-related genes, is mediated via a pathway(s) that is dependent on SA, but independent of NPR1 (25, 26). Moreover, it is not known whether NPR1/NPR3/NPR4 is involved in mediating SA’s effects on other plant processes, including growth and development and/or response to abiotic stress (27). Thus, NPR proteins may not function as SA receptors in the traditional sense.
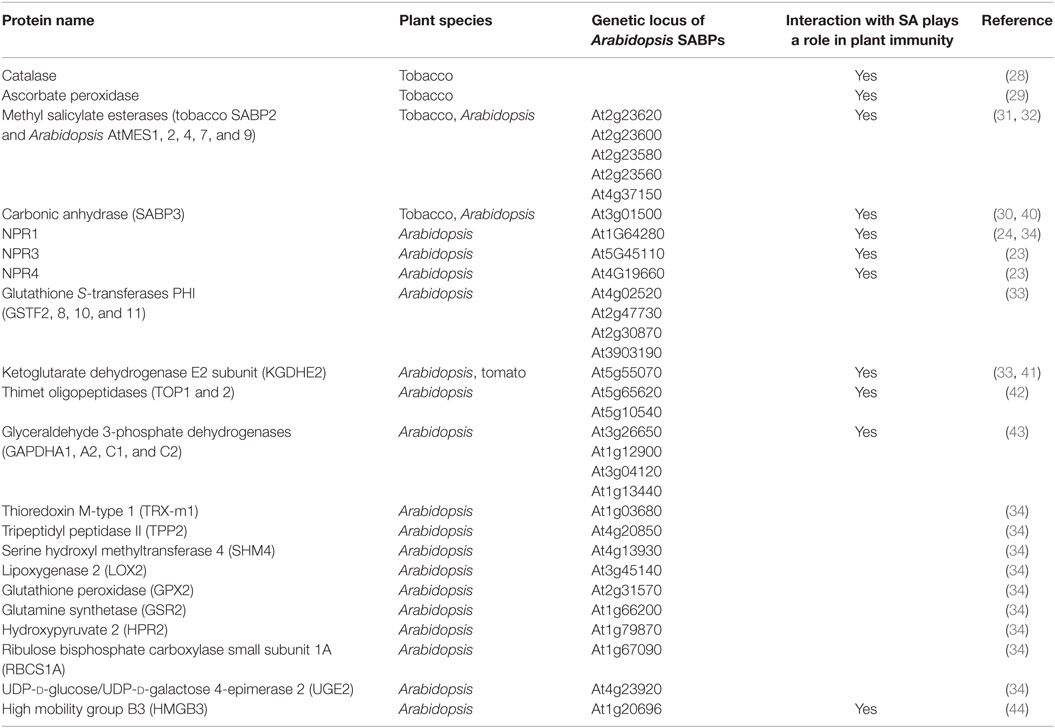
The identification of almost 30 SA-binding proteins (SABPs) using traditional purification approaches (28–32) and genome-wide, high-throughput screens (33, 34) (http://bioinfo.bti.cornell.edu/SA2010/), further argues that SA exerts its effects via more than one or a few receptors. Given that SA-binding alters the activity of many of these SABPs, several difficult questions must be considered: should all identified SABPs listed in Table 2 be promoted to SA receptor status? Alternatively, should the level of SA-binding affinity be used as a criterion, with only those SABPs displaying high affinity qualifying for receptor status? The latter scenario presents additional concerns, as it is unclear what dissociation constant (Kd) value should serve as the cutoff, and who should decide it? In addition, the affinities of the reported NPR receptors overlap those of several SABPs. For example, the MeSA esterase SABP2 from tobacco and its Arabidopsis ortholog MES9 have high affinities for SA (apparent Kd = 0.092 and ~0.200 μM, respectively) (31, 32, 35), which are similar to those of NPR1 (Kd = 0.140–0.190 μM) (24, 34) and NPR4 (Kd = 0.046 μM) (23). By contrast, the SA affinity displayed by NPR3, the other reported SA receptor, is considerably lower (Kd = 1 μM) and approaches those of catalase (Kd = 15.5 μM) (36) and carbonic anhydrase (Kd = 3.7 μM) (30). We propose that proteins which bind hormones (or other ligands) and as a result have altered function or activity be termed “targets” of their corresponding hormone. The term receptor could be applied to a subset of these targets that meet additional criteria. For example, classic receptors for water-soluble hormones, which cannot diffuse through the plasma membrane, span this membrane in order to detect extracellular hormones at the cell surface and initiate downstream intracellular signaling (e.g., G protein-coupled receptors and the enzyme-linked receptors) (37, 38). On the other hands, many receptors for steroids, which readily diffuse through the plasma membrane, are located intracellularly, and directly regulate gene transcription upon complex formation with their cognate hormone (39). Given the many targets through which SA appears to mediate its effects on diverse physiological and pathological plant processes, we suggest that this represents a paradigm shift for how, at least, some hormones function. Furthermore, this novel paradigm may prove applicable to other plant hormones and perhaps even some animal hormones.
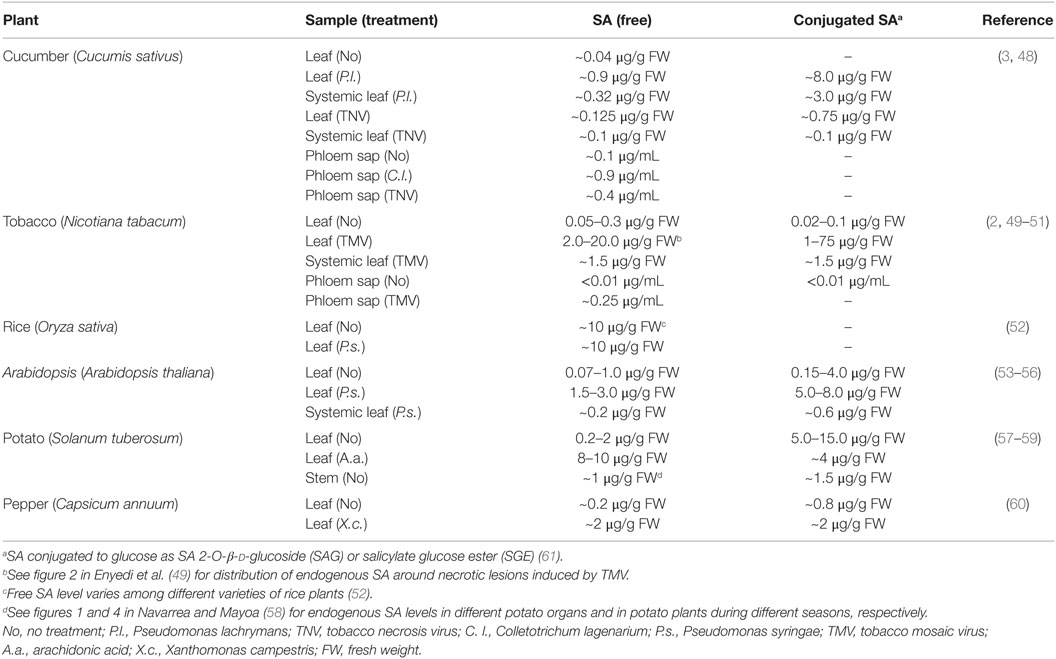
Of the various SABPs whose SA-binding affinities have been determined, their Kd values span from 0.046 to 15.5 μM. Consistent with this 300-fold range, SA levels in plants can vary dramatically (Table 3). Not only do they differ between various plant species but they also can vary within an individual plant depending on the tissue type, subcellular compartment, and developmental stage. In addition, SA levels can vary with respect to the time and/or location after reception of an (a)biotic stress, such as pathogen infection (Table 3). Thus, we hypothesize that SA exerts its multitudinous effects by differentially interacting with various SABPs depending on their affinity for SA, their location, and the local SA concentration. Our analyses of SABP2, a MeSA esterase, and its role in signaling systemic defense responses in tobacco are consistent with this mechanism (45). Following pathogen infection, SA levels increase dramatically in the inoculated leaves, where much of it is converted to biologically inactive MeSA by SA/benzoic acid methyl transferase; once the SA concentration becomes sufficiently high, it binds in the active site of SABP2 and inhibits SABP2’s ability to convert MeSA back into SA (45). The resultant increase in MeSA facilitates its translocation to the distal, uninfected tissue. Since SA levels in the distal tissue are too low to inhibit SABP2, the transported MeSA is converted to active SA, which then induces and/or primes various systemic defense responses. Similarly, the interplay between SA, NPR1, and NPR3/4 fine-tunes NPR1 homeostasis in a SA concentration-dependent manner, which determines the levels and types of plant defense responses during pathogen infection (23, 27). The presence of SABPs exhibiting a wide range of affinities for SA, combined with the varying SA levels found in specific subcellular compartments, in different tissues, at different developmental stages, or during responses to environmental cues, provides tremendous flexibility and multiple mechanisms through which SA can exert its effects. Unfortunately, little is known about the concentrations and distributions of SA at the cellular and/or subcellular levels, because measurements are generally made on total tissue extracts. Thus, there is a pressing need for novel in vivo detection methods of SA (e.g., fluorescent probes) (46, 47) to provide a greater, more detailed understanding of SA functions in mediating the activities of its target or receptor proteins.
Multiple Targets of SA and Its Derivatives in Humans
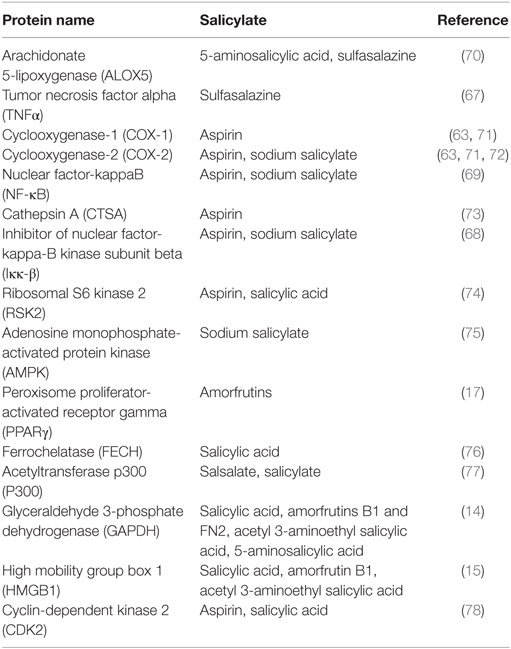
The first SA targets identified in humans were the cyclooxygenases COX1 and COX2. These enzymes convert arachidonic acid, the major plasma membrane fatty acid in animals, into prostaglandins. Prostaglandins have hormone-like activities that induce pain, inflammation, swelling, and fever. Notably, these are the same symptoms that are relieved by ingestion of salicylate-rich medicinal plants, SA, or acetyl SA (aspirin). In the early 1970s, Vane and coworkers discovered that aspirin irreversibly inhibits COX1 and COX2 by acetylating a serine near the active site, which prevents access of the arachidonic acid substrate to the active site (62, 63). This hallmark discovery has dominated the field ever since and supports the prevailing view in the biomedical community of how aspirin works. However, this hypothesis cannot explain how salicylate-rich medicinal plants, which have been used worldwide for millennia, and SA, which was used extensively for half a century before the synthesis of aspirin, are able to treat pain, inflammation, and fever. Naturally occurring salicylates and SA are only weak inhibitors of COX1 and COX2, as they cannot acetylate them (63), and yet SA has most of the same pharmacological effects as aspirin. Moreover, aspirin is rapidly converted to SA in the human body with a half-life of about 20 min (64, 65). In contrast, plasma SA levels after aspirin ingestion rapidly increase and are sustained for more than 12 h (66). These facts argue that there must be additional SA targets besides the cyclooxygenases. During the past three decades, 15 additional potential targets of aspirin, SA, and/or SA prodrugs (Figure 2) have been identified (Table 4). Several of these SA/aspirin targets are associated with inflammation, including tumor necrosis factor alpha (TNFα), nuclear factor-kappa-B (NF-κB), inhibitor of NF-κB kinase subunit beta (Iκκ-β), and high mobility group box 1 (HMGB1), while others regulate energy metabolism, such as adenosine monophosphate-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor gamma (PPARγ). For example, sulfasalazine (see Figure 2 for its structure) blocks TNFα-induced T-cell activation by inhibiting the binding of TNFα to its receptor (67). Aspirin and sodium salicylate are proposed to inhibit transcription factor NF-κB-mediated pro-inflammatory signaling by inhibiting Iκκ-β kinase activity, which induces degradation of inhibitory protein of NF-κB (IκB) by phosphorylation (68, 69). Unfortunately, the levels of aspirin or SA needed to alter the activities of many of these potential targets are very high and are likely to have toxic side effects in humans.
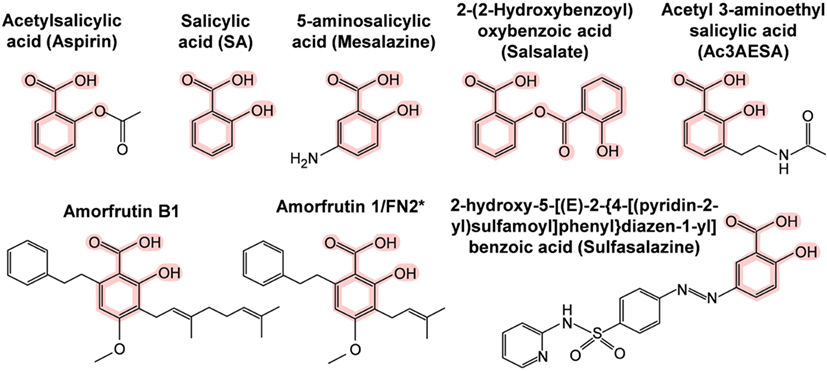
Figure 2. Chemical structures of SA and its synthetic and natural derivatives. The SA core is highlighted in pink. *This amorfrutin was called amorfrutin 1 in Weidner et al. (17) and FN2 in Choi et al. (14).
Plants and Animals Share Several SA Targets
Using a high-throughput screen, we recently identified several members of the Arabidopsis glyceraldehyde 3-phosphate dehydrogenase (GAPDH) family, including GAPDHC1, as SABPs. In both plants and animals, GAPDH plays a central role in glycolysis; in addition, some family members are usurped by invading viruses to facilitate their replication. For example, efficient replication of tomato bushy stunt virus (TBSV) requires binding of GAPDH to the 3′ end of the negative-strand RNA template for synthesis of the positive strand, which is translated or packaged into the virion (79). In collaboration with Peter Nagy’s group, we showed that SA inhibits TBSV replication by binding to GAPDH and thereby preventing its binding to the negative-strand RNA template in the replication complex (Figure 3) (43). Similarly, SA binding to human GAPDH suppresses its ability to bind the poly (U) tract of the 3′ non-coding region of the genome of hepatitis C virus (HCV), which is required for efficient replication and/or translation (Tian and Klessig, unpublished results). It is interesting to note that glycyrrhizin, a compound derived from Glycyrrhiza foetida (common name licorice), also binds to human GAPDH and alters its activities much like SA – see below (14). Moreover, glycyrrhizin has anti-HCV activity and has been used for decades in Japan to treat chronic HCV infection (80, 81). Together, these findings suggest that SA or its more potent derivatives (see below) might be useful treatments for HCV infection.
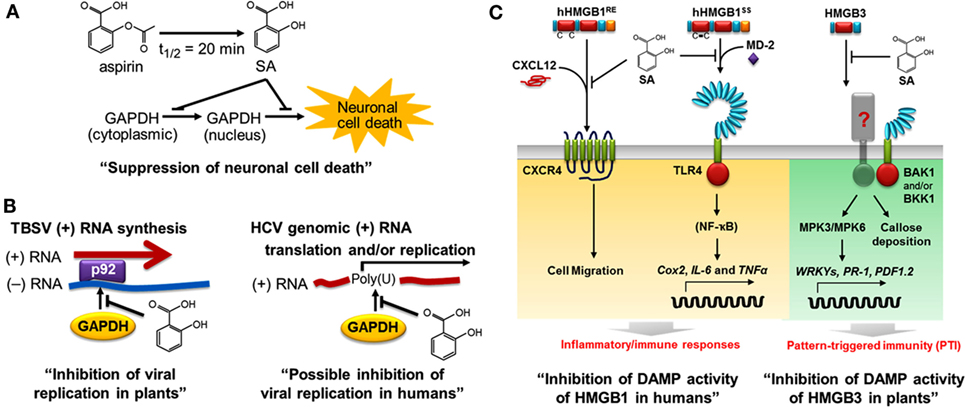
Figure 3. Salicylic acid (SA) affects both plant and human health, in part through common targets such as GAPDH and HMGB proteins. In plants, SA is a key hormone that modulates immune responses; in humans, it is the major metabolite of aspirin. (A) SA binds to human GAPDH and suppresses its translocation from cytoplasm to nucleus and the resulting cell death (14). (B) SA binds to GAPDH and suppresses its participation in viral replication. In plants, GAPDH binding to the minus (−) RNA strand of tomato bush stunt virus (TBSV) promotes plus (+) RNA strand synthesis by the viral RNA-dependent RNA polymerase p92 (82). SA inhibits the interaction between plant GAPDH and the (−) RNA strand of TBSV, thereby reducing viral replication (left panel) (43). In humans, Petrik et al. (83) reported that human GAPDH binds to the poly (U) tract of genomic hepatitis C virus (HCV) RNA, while SA and aspirin were subsequently shown to suppress HCV replication (84, 85). We found that SA inhibits human GAPDH binding to poly (U), suggesting that SA has a similar mechanism of action for inhibition of HCV and TBSV (right panel, Tian and Klessig, unpublished results). (C) SA inhibits the DAMP activities of HMGBs in humans (left panel) (15) and in plants (right panel) (44). Extracellular human HMGB1 functions as a damage-associated molecular pattern (DAMP or alarmin). SA binds to HMGB1, thereby inhibiting the pro-inflammatory activities of reduced and disulfide-bonded HMGB1 (hHMGB1RE and hHMGB1SS, respectively). C–X–C chemokine receptor 4 (CXCR4) recognizes the heterocomplex of hHMGB1RE and C–X–C motif-containing chemokine 12 (CXCL12) to induce cell migration, while the toll-like receptor 4 (TLR4) binds the heterocomplex of hHMGB1SS and myeloid differentiation factor 2 (MD-2) (86) to activate expression of Cox2 and pro-inflammatory cytokine genes (IL-6 and TNFα). SA blocks these pro-inflammatory pathways (15). In plants, HMGB3 functions as a DAMP. Extracellular HMGB3 activates pattern-triggered immunity responses, including MAPK activation (MPK3 and MPK6), defense-related gene expression (WRKYs, PR-1, and PDF1.2), and callose deposition. The regulatory receptor-like kinases BAK1 and/or BKK1 are required for HMGB3 signaling through a yet to be discovered receptor. This figure is modified from Klessig (87).
In addition to GAPDH’s role in viral infection, it is a major suspect in several neurodegenerative diseases in humans, including Huntington’s, Parkinson’s, and Alzheimer’s diseases (88). The central role human GAPDH plays in neurodegeneration was established by the pioneering work of Ishitani and Chuang (89), and later by Snyder and coworkers (90). The latter study also provided evidence for a novel cell death cascade involving GAPDH, nitric oxide, and the E3 ubiquitin ligase called Seven in absentia homolog (Siah) (91). In brief, oxidative stress conditions can lead to elevated levels of nitric oxide, which cause S-nitrosylation of GAPDH’s catalytic cysteine 150. This inactivates GAPDH’s glycolytic activity and induces its interaction with Siah, whose nuclear localization signal enables the complex to enter the nucleus (91). Since the complex between Siah and GAPDH stabilizes this E3 ubiquitin ligase, turnover of Siah’s nuclear target proteins is increased, which in turn leads to cell death. Underscoring the significance of this GAPDH/Siah cell death cascade is the demonstration that the anti-Parkinson’s disease drug deprenyl, which reduces neuronal cell death in both in vitro and in vivo models, prevents S-nitrosylation of GAPDH, blocks the GAPDH–Siah interaction, and inhibits GAPDH nuclear translocation (90).
Using recombinant human GAPDH, we demonstrated that SA not only binds this protein but also suppresses its ability to translocate to the nucleus and induce cell death at low micromolar concentrations (14). Several natural and synthetic derivatives of SA that bind GAPDH more strongly than aspirin/SA also were identified; importantly, their greater binding affinity is correlated with enhanced inhibition of GAPDH’s nuclear translocation and cell death induction. The natural SA derivatives, called amorfrutins, are produced by G. foetida, while the synthetic derivative, acetyl 3-aminoethyl SA, was designed based on the structure of the amorfrutins, as well as the ability of other SA-like compounds to very tightly bind GAPDH and HMGB1, our other newly identified SA/aspirin target (15).
In parallel, our high-throughput screens used to identify human SABPs uncovered HMGB1. HMGB1 is the most abundant non-histone protein in the nucleus. It binds to the minor groove of DNA and plays a central role in condensing DNA, which affects nucleosome packing, transcription, and DNA replication, repair, and recombination. In addition, when HMGB1 is passively released to the extracellular milieu due to tissue damage or necrosis, it functions as a damage-associated molecular pattern (DAMP) to activate the innate immune system (92, 93). Extracellular HMGB1 triggers inflammation by recruiting immune-related cells involved in fighting infection and repairing damaged tissue. In addition, it stimulates these recruited immune-related cells to express genes encoding pro-inflammatory signaling proteins called cytokines. The resulting inflammation protects damaged tissue against infection and promotes healing. In some circumstances, however, inflammation is not properly controlled or it persists (non-resolved); this can contribute to the pathogenesis of many inflammation-associated diseases, such as arthritis, atherosclerosis, lupus, inflammatory bowel disorders, and sepsis, and certain cancers, such as colorectal and mesothelioma cancers.
We have discovered that SA binds to HMGB1, thereby blocking its pro-inflammatory activities (15). It does so at concentrations (low micromolar) far lower than those required to suppress the enzymatic activity of COX1 and COX2. Notably, we found that HMGB1 induces the expression of Cox2, as well as cytokine genes, and that low levels of SA suppress this induction. Thus, SA, such as aspirin, can suppress inflammatory responses mediated by COX2, but SA does so by inhibiting COX2 synthesis, rather than its activity. The discovery that HMGB1’s pro-inflammatory activities are inhibited by low levels of SA provides one likely explanation for the protective effects of low-dose aspirin usage.
Analyses of amorfrutin B1 and acetyl 3-aminoethyl SA revealed that they bind to HMGB1 in the same site as SA but do so with higher affinity. Similar to their greater potency in suppressing GAPDH activity, these compounds were 40- to 70-fold more effective than SA at inhibiting HMGB1’s pro-inflammatory activities. The existence of natural and synthetic SA derivatives that are even more potent than aspirin/SA at suppressing HMGB1’s and GAPDH’s disease-associated activities argues that there is significant potential for the development of SA-based drugs with improved efficacy and, possibly, fewer negative side effects.
All eukaryotic cells, including plants, have HMGB1-related proteins. Arabidopsis has eight HMGB-type proteins, including HMGB3, which is present in the cytoplasm and in the nucleus. Given that human HMGB1 is a prototypic DAMP in animals (92, 93) and that its DAMP activities are inhibited by SA binding (15), we asked whether Arabidopsis HMGB3 (i) functions as a DAMP, (ii) binds SA, and (iii) exhibits reduced DAMP activity following SA binding (44). We found that introduction of HMGB3 into the extracellular space (apoplast) induced innate immune responses, including callose deposition, MAPK activation, defense gene expression, and enhanced resistance to a necrotrophic fungal pathogen. Like its animal counterpart, HMGB3 bound SA and this binding suppressed its ability to induce innate immune responses and protect against pathogen infection.
Why Might Animals have so Many SA Targets
Further research will likely uncover additional SA targets and help clarify which are responsible for SA’s beneficial therapeutic activity, as well as its negative side effects. The potentially large number of SA targets, combined with the multiple pharmacological effects mediated by SA and its prodrug aspirin, and the widespread use of aspirin and/or natural SA derivatives (which our studies suggest are the basis for at least some traditional medicines) suggest that much remains to be done in order to elucidate SA’s mechanisms of action. We predict that SA (and aspirin) exerts its effects in humans via multiple mechanisms of action that are mediated by a variety of targets. Such a scenario would be consistent with our discovery that plants contain more than two dozen proteins through which SA regulates immunity and other plant processes. The majority of animals eat plants, which exposes them to SA and its derivatives on a regular basis. Indeed, vegetarians contain similar levels of SA and its urinary metabolite salicyluric acid as individuals taking low-dose aspirin (94). However, dietary intake of SA appears to account for only a modest portion of the serum and urinary salicylates present in animals. Analyses of germ-free animals indicate that serum SA is not synthesized by gastrointestinal microbes. Rather, studies with 13C-labeled benzoic acid suggest that animals synthesize endogenous SA in large part using this precursor. Benzoic acid and its salts are found in high amounts in some fruits and vegetables, and thus it might contribute to the modest variability in serum SA associated with diet. Also, benzoic acid may be synthesized endogenously in animals using phenylalanine as a precursor. Based on these findings, Paterson and coworkers (94) suggested that it is “increasingly likely that SA is a biopharmaceutical with a central, broadly defensive role in animals as in plants.” Low levels of SA, resulting from dietary intake of SA and endogenous synthesis from benzoic acid/benzoate, might have led to the emergence of multiple SA targets in animals. If future studies confirm this hypothesis, it is highly likely that a variety of SA targets common to both kingdoms will be identified. Their characterization will not only help elucidate the mechanisms through which SA exerts its varied effects but also should provide clues for devising highly effective strategies to control pathological processes in plants and animals.
Conclusion
Salicylic acid acts through many targets, rather than a few receptors, to mediate its many effects on diverse physiological and pathological processes in plants. The presence of SABPs exhibiting a wide range of affinities for SA, combined with the varying SA levels found in specific subcellular compartments, in different tissues, at different developmental stages, or during responses to environmental cues, provides tremendous flexibility and multiple mechanisms through which SA can exert its effects in plants. Animals have multiple targets of SA and its derivatives besides cyclooxygenases COX1 and COX2, which are the two major targets of aspirin. The discovery that HMGB1’s pro-inflammatory activities are inhibited by low levels of SA provides one likely explanation for the protective effects of low-dose aspirin usage. The existence of natural and synthetic SA derivatives that are even more potent than aspirin/SA at suppressing HMGB1’s and GAPDH’s disease-associated activities argues that there is significant potential for the development of SA-based drugs with improved efficacy and, possibly, fewer negative side effects. Low levels of SA, resulting from dietary intake of SA and endogenous synthesis from benzoic acid/benzoate, might have led to the emergence of multiple SA targets in animals, as in plants, some of which are common to both kingdoms.
Author Contributions
DFK, MT, and HWC wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. D’Maris Dempsey for editing the manuscript. The work summarized above, which was carried out by the authors, was funded by the US National Science Foundation grant number IOS-0820405.
References
1. Raskin I, Ehmann A, Melander WR, Meeuse BJD. Salicylic acid – a natural inducer of heat production in Arum lilies. Science (1987) 237:1601–2. doi:10.1126/science.237.4822.1601
2. Malamy J, Carr J, Klessig D, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science (1990) 250:1002–4. doi:10.1126/science.250.4983.1002
3. Métraux JP, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, et al. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science (1990) 250:1004–6. doi:10.1126/science.250.4983.1004
4. Wick JY. Aspirin: a history, a love story. Consult Pharm (2012) 27:322–9. doi:10.4140/TCP.n.2012.322
6. Abreu IN, Ahnlund M, Moritz T, Albrectsen BR. UHPLC-ESI/TOFMS determination of salicylate-like phenolic gycosides in Populus tremula leaves. J Chem Ecol (2011) 37:857–70. doi:10.1007/s10886-011-9991-7
7. Brunke EJ, Hammerschmidt FJ, Schmaus G. Flower scent of some traditional medicinal plants. In: Teranishi R, Buttery RG, Ugisawa H, editors. Bioactive Volatile Compounds from Plants. Washington, DC: American Chemical Society (1993). p. 282–96. doi:10.1021/bk-1993-0525.ch020
8. Chrubasik S, Eisenberg E, Balan E, Weinberger T, Luzzati R, Conradt C. Treatment of low back pain exacerbations with willow bark extract: a randomized double-blind study. Am J Med (2000) 109:9–14. doi:10.1016/S0002-9343(00)00442-3
9. Hedner T, Everts B. The early clinical history of salicylates in rheumatology and pain. Clin Rheumatol (1998) 17:17–25. doi:10.1007/bf01450953
10. Fecka I. Qualitative and quantitative determination of hydrolysable tannins and other polyphenols in herbal products from meadowsweet and dog rose. Phytochem Anal (2008) 20:177–90. doi:10.1002/pca.1113
11. Pino JA, Mesa J, Muñoz Y, Martí MP, Marbot R. Volatile components from mango (Mangifera indica L.) cultivars. J Agric Food Chem (2005) 53:2213–23. doi:10.1021/jf051106m
12. Towers GHN, Tse A, Maass WSG. Phenolic acids and phenolic glycosides of Gaultheria species. Phytochemistry (1966) 5:677–81. doi:10.1016/S0031-9422(00)83646-8
13. Yilmaz N, Yayli N, Misir G, Karaoglu S. Chemical composition and antimicrobial activities of the essential oils of Viburnum opulus, Viburnum lantana and Viburnum oriental. Asian J Chem (2008) 20:3324–30.
14. Choi HW, Tian M, Manohar M, Harraz MM, Park SW, Schroeder FC, et al. Human GAPDH is a target of aspirin’s primary metabolite salicylic acid and its derivatives. PLoS One (2015) 10:e0143447. doi:10.1371/journal.pone.0143447
15. Choi HW, Tian M, Song F, Venereau E, Preti A, Park SW, et al. Aspirin’s active metabolite salicylic acid targets human high mobility group box 1 to modulate inflammatory responses. Mol Med (2015) 21:526–35. doi:10.2119/molmed.2015.00148
16. Fuhr L, Rousseau M, Plauth A, Schroeder FC, Sauer S. Amorfrutins are natural PPARγ agonists with potent anti-inflammatory properties. J Nat Prod (2015) 78:1160–4. doi:10.1021/np500747y
17. Weidner C, de Groot JC, Prasad A, Freiwald A, Quedenau C, Kliem M, et al. Amorfrutins are potent antidiabetic dietary natural products. Proc Natl Acad Sci U S A (2012) 109:7257–62. doi:10.1073/pnas.1116971109
18. Weidner C, Rousseau M, Micikas RJ, Fischer C, Plauth A, Wowro SJ, et al. Amorfrutin C induces apoptosis and inhibits proliferation in colon cancer cells through targeting mitochondria. J Nat Prod (2016) 79:2–12. doi:10.1021/acs.jnatprod.5b00072
19. Boullard O, Leblanc H, Besson B. Salicylic acid. In: Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH (2000). p. 127–34. doi:10.1002/14356007.a23_477
20. Lapczynski A, McGinty D, Jones L, Bhatia S, Letizia CS, Api AM. Fragrance material review on benzyl salicylate. Food Chem Toxicol (2007) 45:S362–80. doi:10.1016/j.fct.2007.09.036
21. Kaiser R. Meaningful Scents around the World: Olfactory, Chemical, Biological, and Cultural Considerations. Zürich: Verlag Helvetica Chimica Acta, Wiley-VCH (2006).
22. Schilling B, Kaiser R, Natsch A, Gautschi M. Investigation of odors in the fragrance industry. Chemoecology (2010) 20:135–47. doi:10.1007/s00049-009-0035-5
23. Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature (2012) 486:228–32. doi:10.1038/nature11162
24. Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, et al. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep (2012) 1:639–47. doi:10.1016/j.celrep.2012.05.008
25. Lu H. Dissection of salicylic acid-mediated defense signaling networks. Plant Signal Behav (2009) 4:713–7. doi:10.4161/psb.4.8.9173
26. An C, Mou Z. Salicylic acid and its function in plant immunity. J Integr Plant Biol (2011) 53:412–28. doi:10.1111/j.1744-7909.2011.01043.x
27. Moreau M, Tian M, Klessig DF. Salicylic acid binds NPR3 and NPR4 to regulate NPR1-dependent defense responses. Cell Res (2012) 22:1631–3. doi:10.1038/cr.2012.100
28. Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science (1993) 262:1883–6. doi:10.1126/science.8266079
29. Durner J, Klessig DF. Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc Natl Acad Sci U S A (1995) 92:11312–6. doi:10.1073/pnas.92.24.11312
30. Slaymaker DH, Navarre DA, Clark D, del Pozo O, Martin GB, Klessig DF. The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc Natl Acad Sci U S A (2002) 99:11640–5. doi:10.1073/pnas.182427699
31. Kumar D, Klessig DF. High-affinity salicylic acid-binding protein 2 is required for plant innate immunity and has salicylic acid-stimulated lipase activity. Proc Natl Acad Sci U S A (2003) 100:16101–6. doi:10.1073/pnas.0307162100
32. Vlot AC, Liu PP, Cameron RK, Park SW, Yang Y, Kumar D, et al. Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana. Plant J (2008) 56:445–56. doi:10.1111/j.1365-313X.2008.03618.x
33. Tian M, von Dahl CC, Liu P-P, Friso G, van Wijk KJ, Klessig DF. The combined use of photoaffinity labeling and surface plasmon resonance-based technology identifies multiple salicylic acid-binding proteins. Plant J (2012) 72:1027–38. doi:10.1111/tpj.12016
34. Manohar M, Tian M, Moreau M, Park SW, Choi HW, Fie Z, et al. Identification of multiple salicylic acid-binding proteins using two high throughput screens. Front Plant Sci (2015) 5:777. doi:10.3389/fpls.2014.00777
35. Du H, Klessig DF. Identification of a soluble, high-affinity salicylic acid-binding protein in tobacco. Plant Physiol (1997) 113:1319–27.
36. Chen Z, Ricigliano JW, Klessig DF. Purification and characterization of a soluble salicylic acid-binding protein from tobacco. Proc Natl Acad Sci U S A (1993) 90:9533–7. doi:10.1073/pnas.90.20.9533
37. Kobilka BK. G protein coupled receptor structure and activation. Biochim Biophys Acta Biomembranes (2007) 1768:794–807. doi:10.1016/j.bbamem.2006.10.021
38. Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol (2010) 6:515–25. doi:10.1038/nrendo.2010.123
39. Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov (2004) 3:27–41. doi:10.1038/nrd1283
40. Wang Y, Feechan A, Yun BW, Shafiei R, Hofmann A, Taylor P, et al. S-nitrosylation of AtSABP3 antagonizes the expression of plant immunity. J Biol Chem (2009) 284:2131–7. doi:10.1074/jbc.M806782200
41. Liao Y, Tian M, Zhang H, Li X, Wang Y, Xia X, et al. Salicylic acid binding of mitochondrial alpha-ketoglutarate dehydrogenase E2 affects mitochondrial oxidative phosphorylation and electron transport chain components and plays a role in basal defense against tobacco mosaic virus in tomato. New Phytol (2015) 205:1296–307. doi:10.1111/nph.13137
42. Moreau M, Westlake T, Zampogna G, Popescu G, Tian M, Noutsos C, et al. The Arabidopsis oligopeptidases TOP1 and TOP2 are salicylic acid targets that modulate SA-mediated signaling and the immune response. Plant J (2013) 76:603–14. doi:10.1111/tpj.12320
43. Tian M, Sasvari Z, Gonzalez PA, Friso G, Rowland E, Liu X, et al. Salicylic acid inhibits the replication of tomato bushy stunt virus by directly targeting a host component in the replication complex. Mol Plant Microbe Interact (2015) 28:379–86. doi:10.1094/MPMI-09-14-0259-R
44. Choi HW, Manohar M, Manosalva P, Tian M, Moreau M, Klessig DF. Activation of plant innate immunity by extracellular high mobility group box 3 and its inhibition by salicylic acid. PLoS Pathog (2016) 12:e1005518. doi:10.1371/journal.ppat.1005518
45. Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science (2007) 318:113–6. doi:10.1126/science.1147113
46. Waadt R, Hsu PK, Schroeder JI. Abscisic acid and other plant hormones: methods to visualize distribution and signaling. Bioessays (2015) 37:1338–49. doi:10.1002/bies.201500115
47. Liu JW, Deng DY, Yu Y, Liu FF, Lin BX, Cao YJ, et al. In situ detection of salicylic acid binding sites in plant tissues. Luminescence (2015) 30:18–25. doi:10.1002/bio.2682
48. Mölders W, Meuwly P, Summermatter K, Métraux JP. Salicylic acid content in cucumber plants infected with Pseudomonas lachrymans and tobacco necrotic virus. Acta Hortic (1994) 381:375–8. doi:10.17660/ActaHortic.1994.381.47
49. Enyedi AJ, Yalpani N, Silverman P, Raskin I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci U S A (1992) 89:2480–4. doi:10.1073/pnas.89.6.2480
50. Malamy J, Hennig J, Klessig DF. Temperature dependent induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell (1992) 4:359–65. doi:10.1105/tpc.4.3.359
51. Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, et al. Requirement of salicylic acid for the induction of systemic acquired resistance. Science (1993) 261:754–6. doi:10.1126/science.261.5122.754
52. Silverman P, Seskar M, Kanter D, Schweizer P, Métraux JP, Raskin I. Salicylic acid in rice: biosynthesis, conjugation, and possible role. Plant Physiol (1995) 108:633–9.
53. Nawrath C, Métraux J. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell (1999) 11:1393–404. doi:10.2307/3870970
54. Lawton K, Weymann K, Friedrich L, Vernooij B, Ukness S, Ryals J. Sytemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Mol Plant Microbe Interact (1995) 8:863–70. doi:10.1094/MPMI-8-0863
55. Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, Klessig DF, et al. Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J (2001) 26:509–22. doi:10.1046/j.1365-313x.2001.01050.x
56. Jagadeeswaran G, Raina S, Acharya B, Maqbool S, Mosher S, Appel H, et al. Arabidopsis GH3-like defense gene 1 is required for accumulation of salicylic acid, activation of defense responses and resistance to Pseudomonas syringae. Plant J (2007) 51:234–46. doi:10.1111/j.1365-313X.2007.03130.x
57. Coquoz JL, Buchala A, Métraux JP. The biosynthesis of salicylic acid in potato plants. Plant Physiol (1998) 117:1095–101. doi:10.1104/pp.117.3.1095
58. Navarrea DA, Mayoa D. Differential characteristics of salicylic acid-mediated signaling in potato. Physiol Mol Plant Pathol (2004) 64:179–88. doi:10.1016/j.pmpp.2004.09.001
59. Yu D, Liu Y, Fan B, Klessig DF, Chen Z. Is the high basal level of salicylic acid important for disease resistance in potato? Plant Physiol (1997) 115:343–9.
60. Choi HW, Kim YJ, Hwang BK. The hypersensitive induced reaction and leucine-rich repeat proteins regulate plant cell death associated with disease and plant immunity. Mol Plant Microbe Interact (2011) 24:68–78. doi:10.1094/MPMI-02-10-0030
61. Dempsey DA, Vlot AC, Wildermuth MC, Klessig DF. Salicylic acid biosynthesis and metabolism. Arab B (2011) 9:e0156. doi:10.1199/tab.0156
62. Ekinci D, Şentürk M, Küfrevioğlu Öİ. Salicylic acid derivatives: synthesis, features and usage as therapeutic tools. Expert Opin Ther Pat (2011) 21:1831–41. doi:10.1517/13543776.2011.636354
63. Vane J. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature (1971) 231:232–5. doi:10.1038/newbio231232a0
64. Proost J, Van Imhoff G, Wesseling H. Plasma levels of acetylsalicylic acid and salicylic acid after oral ingestion of plain and buffered acetylsalicylic acid in relation to bleeding time and thrombocyte function. Pharm Weekbl Sci (1983) 5:22–7. doi:10.1007/bf01959647
65. Higgs GA, Salmon JA, Henderson B, Vane JR. Pharmacokinetics of aspirin and salicylate in relation to inhibition of arachidonate cyclooxygenase and antiinflammatory activity. Proc Natl Acad Sci U S A (1987) 84:1417–20. doi:10.1073/pnas.84.5.1417
66. Cerletti C, Dell’Elba G, Manarini S, Pecce R, Di Castelnuovo A, Scorpiglione N, et al. Pharmacokinetic and pharmacodynamic differences between two low dosages of aspirin may affect therapeutic outcomes. Clin Pharmacokinet (2003) 42:1059–70. doi:10.2165/00003088-200342120-00004
67. Shanahan F, Niederlehner A, Carramanzana N, Anton P. Sulfasalazine inhibits the binding of TNF alpha to its receptor. Immunopharmacology (1990) 20:217–24. doi:10.1016/0162-3109(90)90037-f
68. Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature (1998) 396:77–80. doi:10.1038/23948
69. Kopp E, Ghosh S. Inhibition of NF-κB by sodium salicylate and aspirin. Science (1994) 265:956–9. doi:10.1126/science.8052854
70. Nielsen OH, Bukhave K, Elmgreen J, Ahnfelt-Rønne I. Inhibition of 5-lipoxygenase pathway of arachidonic acid metabolism in human neutrophils by sulfasalazine and 5-aminosalicylic acid. Dig Dis Sci (1987) 32:577–82. doi:10.1007/bf01296156
71. Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A (1993) 90:11693–7. doi:10.1073/pnas.90.24.11693
72. Mitchell JA, Saunders M, Barnes PJ, Newton R, Belvisi MG. Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor κB) activation: role of arachidonic acid. Mol Pharmacol (1997) 912:907–12. doi:10.1124/mol.51.6.907
73. Ostrowska H. Inhibition of human platelet cathepsin A by non-steroidal anti-inflammatory drugs-in vitro study. Pol J Pharmacol (1996) 48:113–6.
74. Stevenson MA, Zhao MJ, Asea A, Coleman CN, Calderwood SK. Salicylic acid and aspirin inhibit the activity of RSK2 kinase and repress RSK2-dependent transcription of cyclic AMP response element binding protein- and NF-kappa B-responsive genes. J Immunol (1999) 163:5608–16.
75. Hawley S, Fullerton M, Ross F, Schertzer J, Chevtzoff C, Walker K, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science (2012) 336:918–22. doi:10.1126/science.1215327
76. Gupta V, Liu S, Ando H, Ishii R, Tateno S, Kaneko Y, et al. Salicylic acid induces mitochondrial injury by inhibiting ferrochelatase heme biosynthesis activity. Mol Pharmacol (2013) 84:824–33. doi:10.1124/mol.113.087940
77. Min SW, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med (2015) 21:1154–62. doi:10.1038/nm.3951
78. Dachineni R, Ai G, Kumar DR, Sadhu SS, Tummala H, Bhat GJ. Cyclin A2 and CDK2 as novel targets of aspirin and salicylic acid: a potential role in cancer prevention. Mol Cancer Res (2015) 14:241–52. doi:10.1158/1541-7786.MCR-15-0360
79. Wang RYL, Nagy PD. Tomato bushy stunt virus co-opts the RNA-binding function of a host metabolic enzyme for viral genomic RNA synthesis. Cell Host Microbe (2008) 3:178–87. doi:10.1016/j.chom.2008.02.005
80. van Rossum TG, Vulto AG, de Man RA, Brouwer JT, Schalm SW. Review article: glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment Pharmacol Ther (1998) 12:199–205. doi:10.1111/apt.12601
81. Ashfaq UA, Masoud MS, Nawaz Z, Riazuddin S. Glycyrrhizin as antiviral agent against hepatitis C virus. J Transl Med (2011) 9:112. doi:10.1186/1479-5876-9-112
82. Nagy PD, Pogany J. The dependence of viral RNA replication on co-opted host factors. Nat Rev Microbiol (2011) 10:137–49. doi:10.1038/nrmicro2692
83. Petrik J, Parker H, Alexander GJM. Human hepatic glyceraldehyde-3-phosphate dehydrogenase binds to the poly(U) tract of the 3’ non-coding region of hepatitis C virus genomic RNA. J Gen Virol (1999) 80:3109–13. doi:10.1099/0022-1317-80-12-3109
84. Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, et al. Cellular cofactors affecting hepatitis C virus infection and replication. Proc Natl Acad Sci U S A (2007) 104:12884–9. doi:10.1073/pnas.0704894104
85. Trujillo-Murillo K, Rincón-Sánchez AR, Martínez-Rodríguez H, Bosques-Padilla F, Ramos-Jiménez J, Barrera-Saldaña HA, et al. Acetylsalicylic acid inhibits hepatitis C virus RNA and protein expression through cyclooxygenase 2 signaling pathways. Hepatology (2008) 47:1462–72. doi:10.1002/hep.22215
86. Yang H, Wang H, Ju Z, Ragab A, Lundbäck P, Long W, et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J Exp Med (2015) 212:5–14. doi:10.1084/jem.20141318
87. Klessig DF. Newly identified targets of aspirin and its primary metabolite, salicylic acid. DNA Cell Biol (2016) 35:163–6. doi:10.1089/dna.2016.3260
88. Chuang D-M, Hough C, Senatorov VV. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu Rev Pharmacol Toxicol (2005) 45:269–90. doi:10.1146/annurev.pharmtox.45.120403.095902
89. Ishitani R, Chuang D. Glyceraldehyde-3-phosphate dehydrogenase antisense oligodeoxynucleotides protect against cytosine arabinonucleoside-induced apoptosis in cultured cerebellar neurons. Proc Natl Acad Sci U S A (1996) 93:9937–41. doi:10.1073/pnas.93.18.9937
90. Hara MR, Thomas B, Cascio MB, Bae B-I, Hester LD, Dawson VL, et al. Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc Natl Acad Sci U S A (2006) 103:3887–9. doi:10.1073/pnas.0511321103
91. Hara MR, Snyder SH. Nitric oxide-GAPDH-Siah: a novel cell death cascade. Cell Mol Neurobiol (2006) 26:527–38. doi:10.1007/s10571-006-9011-6
92. Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol (2011) 29:139–62. doi:10.1146/annurev-immunol-030409-101323
93. Yang H, Wang H, Chavan SS, Andersson U. High mobility group box protein 1 (HMGB1): the prototypical endogenous danger molecule. Mol Med (2015) 21:S6–12. doi:10.2119/molmed.2015.0008
Keywords: salicylic acid, salicylic acid-binding proteins, salicylic acid derivatives, plant immunity, animal immunity and inflammation, disease, common plant and animal targets
Citation: Klessig DF, Tian M and Choi HW (2016) Multiple Targets of Salicylic Acid and Its Derivatives in Plants and Animals. Front. Immunol. 7:206. doi: 10.3389/fimmu.2016.00206
Received: 02 March 2016; Accepted: 12 May 2016;
Published: 26 May 2016
Edited by:
Brigitte Mauch-Mani, Université de Neuchâtel, SwitzerlandReviewed by:
Robin Katrina Cameron, McMaster University, CanadaTaras P. Pasternak, University of Freiburg, Germany
Francesca Granucci, University of Milano-Bicocca, Italy
Copyright: © 2016 Klessig, Tian and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel F. Klessig, ZGZrOEBjb3JuZWxsLmVkdQ==
 Daniel F. Klessig
Daniel F. Klessig Miaoying Tian
Miaoying Tian Hyong Woo Choi
Hyong Woo Choi