Response: Commentary: Memory CD8+ T Cells Colocalize with IL-7+ Stromal Cells in Bone Marrow and Rest in Terms of Proliferation and Transcription
- Institute of Molecular Biology and Pathology, Consiglio Nazionale delle Ricerche, c/o Department of Molecular Medicine, Sapienza University, Rome, Italy
A commentary on
Memory CD8+ T cells colocalize with IL-7+ stromal cells in bone marrow and rest in terms of proliferation and transcription
by Sercan Alp O, Durlanik S, Schulz D, McGrath M, Grun JR, Bardua M, et al. Eur J Immunol (2015) 45:975–87. doi: 10.1002/eji.201445295
Several studies have shown that the bone marrow (BM) is implicated in the long-lasting persistence of memory CD8 T cells [see Ref. (1) and references therein]. Generally, it has been thought that the BM accomplishes this by sustaining a higher level of homeostatic proliferation of recirculating memory CD8 T cells than do spleen and lymph nodes (LN) in the steady state. This slow intermittent cell division would counteract cell death, thus contributing to the stable maintenance of memory T cell numbers over time. In a recent article entitled “Memory CD8+ T cells colocalize with IL-7+ stromal cells in bone marrow and rest in terms of proliferation and transcription,” Sercan Alp and coworkers challenge this view (2). They emphasize that results on memory CD8 T cell proliferation are discrepant and propose that the BM instead provides survival signals for resident memory CD8 T cells, as it does for plasma cells (3–5). They show that BM memory CD8 T cells colocalize with stromal cells, expressing the prosurvival cytokine IL-7. Moreover, they demonstrate that CD69, i.e., a typical marker of tissue-resident memory T cells, is expressed by a higher proportion of memory CD8 T cells in the BM than in the spleen. Finally, they show that freshly isolated BM memory CD8 T cells have a predominant resting transcriptional profile, in comparison with in vitro-activated CD8 T cells (2).
Starting from the article by Sercan Alp et al., this commentary revisits the data published so far on memory CD8 T cell proliferation in the BM and suggests that apparent discrepancies can be reconciled by a detailed analysis (see Table 1 and references therein). In respect to the interplay between memory CD8 T cells and other cells within BM niches and the possibility that BM memory T cells represent a pool of tissue-resident memory T cells, the reader is referred to another article in this issue (6).
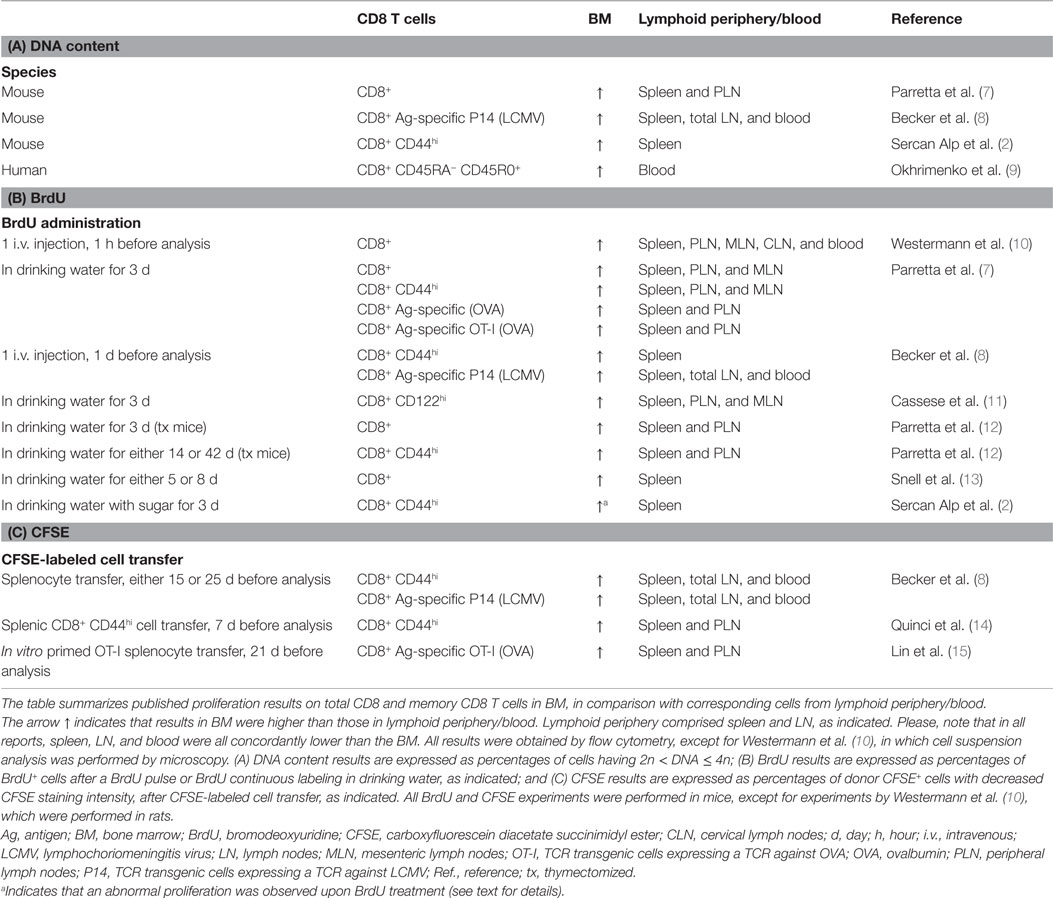
Table 1. Summary of published results on total CD8 and memory CD8 T cell proliferation in bone marrow, grouped according to experimental methods.
Sercan Alp and coworkers examined memory CD8 T cell proliferation or quiescence in mice by three methods, i.e., DNA content analysis, bromodeoxyuridine (BrdU) incorporation, and Ki67 staining (2).
DNA content analysis measures the percentage of cells in S/G2/M phase of cell cycle at a given time, thus providing a static index of proliferation in untreated individuals (16). By this method, Sercan Alp et al. found that the frequency of dividing cells within memory-phenotype CD44high CD8 T cells in the BM was only about 0.4%. However, this low percentage was still three to eight times higher than that found in corresponding spleen samples [(2), BM 0.32–0.47% and spleen 0.05–0.10%; see Figures 4E and S3], in line with what has been seen in other studies by comparing CD8 T cells from BM with those from blood, spleen, or LN (7–9).
Sercan Alp et al. showed that assessment of CD8 T cell proliferation by BrdU incorporation may be misleading (2). BrdU is a thymidine analog that labels cells during S phase, thus marking the cells undergoing division in the course of BrdU treatment. Depending on dose and length of treatment, BrdU may have toxic effects, potentially leading to artifacts (17). In mice, BrdU is either injected or administered in drinking water, sometimes with sugar addition, a stratagem used to overcome the unpleasant taste of the analog (18, 19). Sugar can increase water consumption, e.g. in 4 hours mice drink 0.5–1.5 ml tap water and 2–4 times more water containing 10% sugar (20). However, total water intake is not usually measured in BrdU experiments, leaving actual BrdU dose undetermined. In the study by Sercan Alp et al., the mice were treated with 1 mg/ml BrdU plus 10% sugar in drinking water for 3 days, and there was an artificial rise – especially in the BM – of dividing memory CD8 T cell frequency, as measured by a BrdU-independent method [(2), Figure 4E]. Based on these results, the authors suggest that previous studies had greatly overestimated the extent of memory CD8 T cell proliferation (2).
However, other authors have used lower doses of BrdU without sugar (7, 12, 21–23), and Parretta et al. found little difference in proliferation (when tested by a BrdU-independent assay) between mice treated with BrdU or not (12). In more details, to compare the two groups of mice, Parretta et al. measured CD8 T cell proliferation by carboxyfluorescein diacetate succinimidyl ester (CFSE), a cytoplasmic dye that is equally distributed between daughter cells upon division. They reported that the proportion of dividing (i.e., CFSElow) CD8 T cells in spleen, LN, and BM in response to PolyI:C injection was similar when mice were either untreated or treated with 0.8 mg/ml BrdU in drinking water for 3 days (12), a standard protocol (24). PolyI:C treatment might have masked the toxic effects of BrdU (12); nevertheless, the dose of BrdU plus sugar is a major difference between the Sercan Alp et al.’s study and those of other groups. Indeed, the percentage of BrdU+ cells within spleen CD44high CD8 T cells reported by Sercan Alp et al., i.e., about 30%, was definitely higher in comparison with previous data reported by several authors, all obtained with 0.8 mg/ml BrdU in drinking water for 3 days and no sugar (7, 21, 23). For example, Parretta et al. found that the fraction of BrdU+ cells within CD44high CD8 T cells was 6% in spleen, 5% in LN, and 13% in BM, on average (7). Taking everything into account, it could be argued that the confounding effect of BrdU on BM CD8 T cell proliferation was dose dependent and limited to the study by Sercan Alp and coworkers (2).
Finally, Sercan Alp et al. analyzed CD8 T cells by intracellular staining for Ki67, a cell-cycle marker. They showed that on average, 93–95% of the memory CD8 T cells are negative for Ki67 (i.e., in G0 phase) in the BM and 88–94% in the spleen [(2), Figures 4B,D]. This indicates that the vast majority of the cells are quiescent and non-dividing at a given time, with a slight difference between BM and spleen. However, it should not be overlooked that the Ki67 assay does not give any information on frequency of dividing cells (i.e., in S/G2/M), since all cells in G1/S/G2/M score positively for Ki67. It appears that rather than being in contrast with previous findings on proliferation obtained by other methods (see Table 1 and references therein), the Ki67 results in the Sercan Alp et al.’s study simply report on a different aspect of the cell cycle.
Table 1 is a summary of published findings on total CD8 and memory CD8 T cell proliferation in BM, grouped according to the experimental methods. In addition to DNA content and BrdU, some authors used CFSE. For example, Quinci et al. found that in 1 week the fraction of CFSElow cells within CD44high CD8 T cells was 17% in spleen, 17% in LN, and 27% in BM, on average (14). All data in Table 1 show a higher percentage of proliferating cells within memory CD8 T cells in the BM than in lymphoid periphery, i.e., spleen, LN, and blood.
Thus, the data on proliferation are in agreement, while discrepancies remain in interpretations (25, 26). The main point of contention is how much the proliferation occurring in the BM contributes to the long-term maintenance of memory CD8 T cells. Sercan Alp et al. focused their attention on the paucity of proliferating cells in their BM samples (2), ignoring that this is, nevertheless, a higher proportion than that found in spleen and LN. It could be argued that such difference in proliferating cell frequencies should not be neglected, in light of the fact that BM memory CD8 T cells are a large population. Indeed, the BM is a huge organ and, moreover, after the peak of an acute response, antigen-specific CD8 T cell contraction is often less pronounced in the BM than in other organs, resulting in a high number of memory CD8 T cells in the BM in the memory phase (7, 8, 27, 28). For example, in the contraction phase of the response against the M-45 epitope of murine cytomegalovirus (MCMV), the frequency of antigen-specific CD8 T cells dropped 14–20 times in the blood, liver, and lung, and only about five times in the BM (28). Moreover, at late times (6–10 weeks) after immunization against the model antigen ovalbumin, the number of antigen-specific memory CD8 T cells in the BM was 2–3 times higher than that in the spleen and 3–11 times higher than that in total LN (7). However, enrichment of antigen-specific CD8 T cells in the BM in the memory phase was not observed in other types of responses. For example, at late times after infection, antigen-specific memory CD8 T cell frequency in the BM was not higher than that in blood, liver, or lung in the inflationary response against the M-38 epitope of MCMV (28) or in the response against vaccinia virus induced by skin scarification, which mostly elicited antigen-specific tissue-resident memory CD8 T cells in the skin (29).
Taking everything into account, the BM plays a preferential role in sustaining the homeostatic proliferation of antigen-specific memory CD8 T cells following classical acute responses resulting in the long-term systemic memory (1, 7, 8, 30).
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I thank T. Gebhardt, A. Hayday, J. Hiscott, R. Pabst, A. Santoni, S. Simonetti, and A. Soriani for discussion. A special thanks to P. Matzinger for discussion and for the manuscript reading.
Funding
FD is supported by CTN01_00177_962865 (Medintech) grant from Ministero dell’Università e delle Ricerca (MIUR).
References
1. Di Rosa F. T-lymphocyte interaction with stromal, bone and hematopoietic cells in the bone marrow. Immunol Cell Biol (2009) 87:20–9. doi:10.1038/icb.2008.84
2. Sercan Alp O, Durlanik S, Schulz D, McGrath M, Grun JR, Bardua M, et al. Memory CD8(+) T cells colocalize with IL-7(+) stromal cells in bone marrow and rest in terms of proliferation and transcription. Eur J Immunol (2015) 45:975–87. doi:10.1002/eji.201445295
3. Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature (1997) 388(6638):133–4. doi:10.1038/40540
4. Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity (1998) 8:363–72. doi:10.1016/S1074-7613(00)80541-5
5. Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol (2003) 171:1684–90. doi:10.4049/jimmunol.171.4.1684
6. Di Rosa F, Gebhardt T. Bone marrow T cells and the integrated functions of recirculating and tissue-resident memory T cells. Front Immunol (2016) 7:51. doi:10.3389/fimmu.2016.00051
7. Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J Immunol (2005) 174:7654–64. doi:10.4049/jimmunol.174.12.7654
8. Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol (2005) 174:1269–73. doi:10.4049/jimmunol.174.3.1269
9. Okhrimenko A, Grun JR, Westendorf K, Fang Z, Reinke S, von Roth P, et al. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc Natl Acad Sci U S A (2014) 111:9229–34. doi:10.1073/pnas.1318731111
10. Westermann J, Ronneberg S, Fritz FJ, Pabst R. Proliferation of lymphocyte subsets in the adult rat: a comparison of different lymphoid organs. Eur J Immunol (1989) 19:1087–93. doi:10.1002/eji.1830190619
11. Cassese G, Parretta E, Pisapia L, Santoni A, Guardiola J, Di Rosa F. Bone marrow CD8 cells down-modulate membrane IL-7R{alpha} expression and exhibit increased STAT-5 and p38 MAPK phosphorylation in the organ environment. Blood (2007) 110:1960–9. doi:10.1182/blood-2006-09-045807
12. Parretta E, Cassese G, Santoni A, Guardiola J, Vecchio A, Di Rosa F. Kinetics of in vivo proliferation and death of memory and naive CD8 T cells: parameter estimation based on 5-bromo-2′-deoxyuridine incorporation in spleen, lymph nodes, and bone marrow. J Immunol (2008) 180:7230–9. doi:10.4049/jimmunol.180.11.7230
13. Snell LM, Lin GH, Watts TH. IL-15-dependent upregulation of GITR on CD8 memory phenotype T cells in the bone marrow relative to spleen and lymph node suggests the bone marrow as a site of superior bioavailability of IL-15. J Immunol (2012) 188:5915–23. doi:10.4049/jimmunol.1103270
14. Quinci AC, Vitale S, Parretta E, Soriani A, Iannitto ML, Cippitelli M, et al. IL-15 inhibits IL-7Ralpha expression by memory-phenotype CD8(+) T cells in the bone marrow. Eur J Immunol (2012) 42:1129–39. doi:10.1002/eji.201142019
15. Lin GH, Snell LM, Wortzman ME, Clouthier DL, Watts TH. GITR-dependent regulation of 4-1BB expression: implications for T cell memory and anti-4-1BB-induced pathology. J Immunol (2013) 190:4627–39. doi:10.4049/jimmunol.1201854
16. Hellerstein MK. Measurement of T-cell kinetics: recent methodologic advances. Immunol Today (1999) 20:438–41. doi:10.1016/S0167-5699(99)01529-7
17. Reome JB, Johnston DS, Helmich BK, Morgan TM, Dutton-Swain N, Dutton RW. The effects of prolonged administration of 5-bromodeoxyuridine on cells of the immune system. J Immunol (2000) 165:4226–30. doi:10.4049/jimmunol.165.8.4226
18. Astrakhan A, Sather BD, Ryu BY, Khim S, Singh S, Humblet-Baron S, et al. Ubiquitous high-level gene expression in hematopoietic lineages provides effective lentiviral gene therapy of murine Wiskott-Aldrich syndrome. Blood (2012) 119:4395–407. doi:10.1182/blood-2011-03-340711
19. Kolhatkar NS, Brahmandam A, Thouvenel CD, Becker-Herman S, Jacobs HM, Schwartz MA, et al. Altered BCR and TLR signals promote enhanced positive selection of autoreactive transitional B cells in Wiskott-Aldrich syndrome. J Exp Med (2015) 212:1663–77. doi:10.1084/jem.20150585
20. Bulwa ZB, Sharlin JA, Clark PJ, Bhattacharya TK, Kilby CN, Wang Y, et al. Increased consumption of ethanol and sugar water in mice lacking the dopamine D2 long receptor. Alcohol (2011) 45:631–9. doi:10.1016/j.alcohol.2011.06.004
21. Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med (1994) 179:1127–35. doi:10.1084/jem.179.4.1127
22. Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity (1998) 8:591–9. doi:10.1016/S1074-7613(00)80564-6
23. Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nat Immunol (2007) 8:162–71. doi:10.1038/ni1418
24. Tough DF, Sprent J. Measurement of T and B cell turnover with bromodeoxyuridine. Curr Protoc Immunol (1996) Chapter 4:Unit4.7.
25. Di Rosa F. Maintenance of memory T cells in the bone marrow: survival or homeostatic proliferation? Nat Rev Immunol (2016). doi:10.1038/nri.2016.31
26. Alp OS, Radbruch A. The lifestyle of memory CD8+ T cells. Nat Rev Immunol (2016). doi:10.1038/nri.2016.32
27. Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science (2001) 291:2413–7. doi:10.1126/science.1058867
28. Bolinger B, Sims S, Swadling L, O’Hara G, de Lara C, Baban D, et al. Adenoviral vector vaccination induces a conserved program of CD8(+) T cell memory differentiation in mouse and man. Cell Rep (2015) 13:1578–88. doi:10.1016/j.celrep.2015.10.034
29. Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature (2012) 483:227–31. doi:10.1038/nature10851
Keywords: immunological memory, CD8 T cells, bone marrow, homeostatic proliferation, bromodeoxyuridine
Citation: Di Rosa F (2016) Commentary: Memory CD8+ T cells colocalize with IL-7+ stromal cells in bone marrow and rest in terms of proliferation and transcription. Front. Immunol. 7:102. doi: 10.3389/fimmu.2016.00102
Received: 26 November 2015; Accepted: 07 March 2016;
Published: 31 March 2016
Edited by:
Stephen Philip Schoenberger, La Jolla Institute for Allergy and Immunology, USAReviewed by:
Charles Surh, The Scripps Research Institute, USAKimberly Sue Schluns, University of Texas MD Anderson Cancer Center, USA
Copyright: © 2016 Di Rosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Di Rosa, francesca.dirosa@uniroma1.it
 Francesca Di Rosa
Francesca Di Rosa