
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 28 January 2016
Sec. T Cell Biology
Volume 7 - 2016 | https://doi.org/10.3389/fimmu.2016.00018
This article is part of the Research TopicMolecular dynamics at the immunological synapseView all 11 articles
The generation of phagocytic cups and immunological synapses are crucial events of the innate and adaptive immune responses, respectively. They are triggered by distinct immune receptors and performed by different cell types. However, growing experimental evidence shows that a very close series of molecular and cellular events control these two processes. Thus, the tight and dynamic interplay between receptor signaling, actin and microtubule cytoskeleton, and targeted vesicle traffic are all critical features to build functional phagosomes and immunological synapses. Interestingly, both phagocytic cups and immunological synapses display particular spatial and temporal patterns of receptors and signaling molecules, leading to the notion of “phagocytic synapse.” Here, we discuss both types of structures, their organization, and the mechanisms by which they are generated and regulated.
Immunological synapses are organized cell–cell contacts shaped at the interface between T cells and antigen-presenting cells (APCs) (Figure 1). They are triggered by the binding of T cell antigen receptors (TCR) to their ligands, peptide antigens associated with major histocompatibility complex molecules (pMHC) expressed on the surface of APCs. TCR engagement induces the polarization of the T cell toward the APC and a coordinated reorganization of various T cell components, including receptors, signaling and adhesion molecules, the actin and microtubule cytoskeleton, and intracellular vesicle traffic. Thus, the TCR and its proximal signaling molecules (e.g., protein kinases and phosphatases, signaling adapters, and effectors molecules) form dynamic signaling complexes at the immunological synapse that drive T cell activation. Moreover, TCR signaling triggers the fine reorganization of the actin and microtubule cytoskeleton that ensures synapse architecture and signaling complex dynamics, critical for TCR signal regulation. Finally, various intracellular compartments polarize toward the immunological synapse, including the Golgi apparatus, early and late endosomes, and mitochondria. Importantly, the TCR signaling machinery, actin and microtubule cytoskeleton, and intracellular vesicle traffic interplay at the synapse to sustain and regulate T cell activation (1).
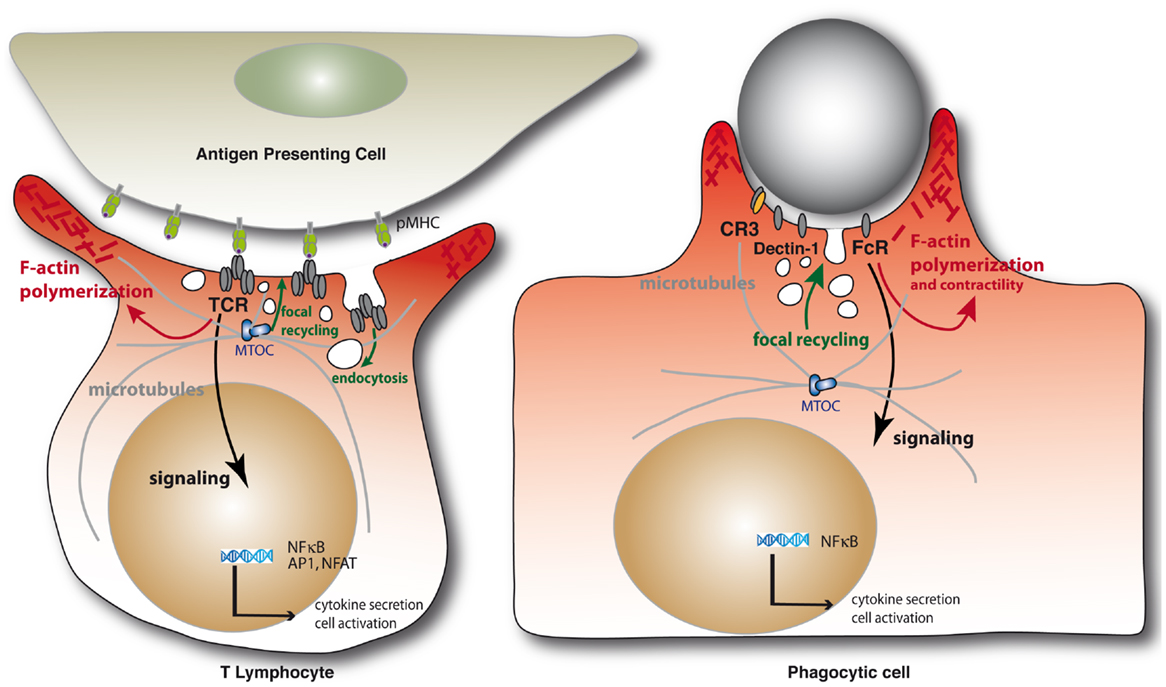
Figure 1. Schematic representation of the immunological synapse and the phagocytic cup formation. Immunological synapse formation is initiated by the engagement of TCRs on the surface of the T lymphocytes by peptide antigen–MHC complexes on the APC (left). Similarly, engagement of phagocytic receptors by multiple ligand binding on a target particle drives the formation of phagocytic synapses (right). In both settings, receptor engagement leads to F-actin polymerization and membrane deformation at contact sites. Polarization of the MTOC and microtubule network toward at the IS are important for the delivery of vesicles containing cytokines or lytic enzymes in helper or cytotoxic T cells, respectively, but also to deliver TCR-signaling components during immunological synapse formation. Microtubules also contribute to F-actin remodeling in complement-mediated phagocytosis. Internalization of cell surface TCRs by endocytosis and their focal recycling participate in the regulation of T cell activation. Finally, in either system, triggering of multiple signaling pathways downstream of the surface receptors leads to de novo transcriptional programs controlling cell survival, activation, and cytokine production.
Phagocytic cup formation mirrors a large number of events occurring during immunological synapse formation, before leading to a productive engulfment of the target (Figure 1). First, clustering of phagocytic receptors induced by particle-associated ligands triggers signal transduction pathways similar to those engaged by the TCR. In particular, a similar spatial and temporal segregation of tyrosine kinases and phosphatases was observed at both immunological synapses and phagocytic cups, leading to the notion of “phagocytic synapse” (2). Second, phagocytic receptor signaling triggers a profound reorganization of the actin cytoskeleton that is similar to the one induced by the TCR, generating large membrane extensions rich in filamentous (F)-actin. Third, microtubule dynamics are also important for some receptor-mediated phagocytosis. Fourth, intracellular traffic involving several vesicular compartments reorients toward the phagocytic cup. Fifth, internalization of the triggered receptors together with their ligands occurs and may lead to their degradation or recycling back to the plasma membrane. Interestingly, the TCRs may be phagocytosed from the immunological synapse internalizing with them their pMHC ligands together with portions of the APC membrane (3). Finally, a series of downstream signaling events lead to cytokine gene activation in both cases.
We review here the molecular and cellular events taking place in both phagocytic and immunological synapses, highlighting their mechanisms of regulation.
T cell receptor engagement induces a series of molecular reorganization events that stabilize T cell–APC interactions and optimize signal transduction. Several other receptors are recruited to the immunological synapse and contribute to the activation process. These include the co-receptors CD4 and CD8, co-stimulatory receptors such as CD28, or adhesion proteins such as the integrins αLβ2 (LFA1) or α4β1 (VLA4) [reviewed in Ref. (4)].
One of the earliest events elicited by antigen recognition is the sequential activation of protein tyrosine kinases belonging to the Src and Syk families. The Src-family kinases Lck and/or Fyn, phosphorylate several TCR complex subunits, namely CD3 (ɛ, γ, and δ) and ζ (5). These subunits are all endowed with one or more consensus sequences called immunoreceptor tyrosine-based activation motif (ITAM), each containing two phosphorylatable tyrosine residues. Doubly phosphorylated ITAMs then recruit Syk-family kinases, either ZAP-70 or Syk (6), whose tandem SH2 domains provide specific, high-affinity binding to ITAM phosphotyrosines. Src kinases may be further required to phosphorylate and activate Syk kinases, in particular ZAP-70. The interplay between these two families of tyrosine kinases is crucial for transmitting downstream signals. Thus, Syk family kinases phosphorylate adaptor proteins, such as LAT and SLP-76 that in turn gather signaling effectors within multiprotein complexes, or signalosomes (6). Moreover, both Src- and Syk-family kinases activate several enzymes recruited in these signalosomes that are responsible for the generation of intracellular second messengers, such as Ca2+ or phosphoinositides. Collectively, these early steps, induced within seconds after TCR engagement, initiate a cascade of downstream events leading to cytoskeletal rearrangement and cellular polarization. Concomitantly, various serine–threonine kinases, including MAP kinases, are activated, regulating the activation of several transcription factors that will drive in turn T cell growth and differentiation and the production of effector cytokines (7).
Detection and engulfment of bacteria or fungi by phagocytic cells are triggered by a similar sequence of early events. However, multiple unrelated ligands trigger phagocytosis by engaging distinct receptors. Indeed, phagocytic receptors can recognize their target by binding either to specific molecules expressed on the target’s surface or to opsonizing antibodies or complement subunits previously bound to the target. For instance, phagocytosis of IgG-coated pathogens is triggered upon antibody recognition by Fcγ receptor (FcγR), whereas integrins, such as αMβ2 (also known as Mac-1 or CR3), can recognize complement-coated particles. Finally, phagocytosis of fungi expressing β-glucans on their cell wall is triggered by Dectin-1 receptor (8).
Phagocytic Fc receptors (FcγRII and FcγRIII) belong to the immunoreceptor family and are structurally related to antigen receptors. Importantly, they transmit activating signals using ITAM motifs that are either built in the receptor intracellular tail or in the associated common γ-chain (9). Hence, early signaling events involve Src- and Syk-family kinases, similarly to what explained above for the TCR. In macrophages, the Src kinases Lyn, Hck, and Fgr are involved in FcR-induced phagocytosis. However, phagocytosis was significantly reduced but not abolished in cells of triple knockout mice, suggesting the existence of further redundancy or alternative triggering mechanisms (10). In contrast, Syk knockout resulted in a complete block of phagocytosis, indicating the indispensable role of this kinase (11). Since Syk, but not ZAP-70, has been shown to phosphorylate ITAM motifs (12), it can be envisaged that Syk can trigger some phagocytic activity in the absence of Src kinases.
The β-glucan receptor Dectin-1, a member of the C-type lectin receptor (CLR) family, also induces sequential activation of Src and Syk kinases. Dectin-1 displays in its cytoplasmic domain ITAM-like sequences named hem-ITAM, each containing a single tyrosine-based motif. Once phosphorylated by Src kinases, they are able to bind Syk and trigger downstream activation (13). Since Dectin-1 is a dimer, it has been proposed that Syk binds in trans to two phosphorylated hem-ITAMs on adjacent subunits in order to be recruited to the activated receptor (13). However, this model has not been validated experimentally. Furthermore, a potential alternative mechanism for Syk recruitment has been revealed recently, implying a scaffolding role of the protein tyrosine phosphatase SHP-2 in bridging Syk to Dectin-1 and other CLRs (14).
The molecular mechanisms underlying integrin-dependent phagocytosis, such as that elicited by complement-coated particles binding to CR3, are more complex than those described for FcRs and Dectin-1. Importantly, integrin binding to their ligand requires prior activation via a conformational change induced by “inside-out signaling.” This priming phase is induced by inflammatory or pathogen-specific signals, such as those triggered by G-protein-coupled (GPCRs) or toll-like receptors (TLRs). These proteins initiate different signaling cascades converging on a common effector, the GTPase Rap1 (15). Active Rap1 induces the recruitment of RapL, RIAM, and talin to integrin cytoplasmic tails, thus promoting the switch of integrins to their extended conformation that can bind ligands with high affinity (16). Then, ligand-bound integrins transmit “outside-in” signals that drive actin polymerization and downstream activation. These steps involve several effectors including the protein kinases FAK (or Pyk2) and ILK, non-muscle myosin II, and Rho GTPases (17). Nonetheless, the fact that Syk inhibition impairs CR3-mediated phagocytosis demonstrates the existence of some crosstalk between integrin activation and ITAM-bearing receptors or adaptors (18). Interestingly, FcRs have confined mobility in the plasma membrane, in fenestrated cortical actin structures that depend on the activity of Src- and Syk-family kinases (19). Integrins or pattern recognition receptors, such as the scavenger receptor CD36, are potentially initiating Syk activation, leading to FcR increased mobility and engagement (8). However, further work is needed to define the molecular basis of integrin interplay with ITAM-dependent signaling.
How early signals are elicited by antigen or phagocytic receptors engagement is still a matter of debate. One model proposed for TCR activation postulates that initial triggering is achieved when key inhibitory proteins, such as the tyrosine phosphatase CD45, are segregated away from the engaged TCR and the proximal tyrosine kinase Lck. This segregation is mainly driven by the size of membrane protein ectodomains. Indeed, the length of the TCR–pMHC pairs is relatively small (7 nm) compared to that of CD45 ectodomain (28–50 nm); hence, TCR engagement by pMHC induces the formation of areas of close juxtaposition of T cell and APC membranes from which phosphatases are excluded (20, 21). As a consequence, local activity of tyrosine kinases would be favored, leading to an increase in net phosphorylation of TCR downstream effectors and T cell activation. Interestingly, a similar mechanism was observed during Dectin-1-dependent phagocytosis, leading to the “phagocytic synapse” model. Indeed, Dectin-1 engagement by β-glucan-bearing particles results in local exclusion of phosphotyrosine phosphatases CD45 and CD148 from receptor-enriched areas containing phosphotyrosine, thus triggering downstream signaling (e.g., Syk phosphorylation) and phagocytic cup formation (2). Importantly, several results suggest that this mechanism also concerns FcRs (22, 23); hence, it may be relevant for all phagocytic receptors.
Concomitantly to initial kinase and phosphatase segregation, T cell receptor subunits, the tyrosine kinases Lck and ZAP70, and the adapters LAT and SLP76 associate into dynamic signaling complexes that nucleate at the periphery of immunological synapses and then migrate toward its center, where they concentrate or vanish (24–26). Interestingly, centripetal dynamics of signaling complexes at the immunological synapse and their concentration in the center is a regulatory mechanism that depends on actin and microtubule cytoskeleton and is meant to downregulate proximal TCR signaling (27–29). Various mechanisms have been proposed for TCR signal downregulation at the synapse. These include relocalization to membrane regions containing the tyrosine phosphatase CD45 (28), internalization and degradation of TCR and signaling complexes (30–32), post-translational modification of signaling adapters leading to signalosome disassembly (33), or the extracellular release of vesicles containing TCR (34). Of note, in FcR-mediated phagocytosis, receptors are engaged sequentially in a receptor-guided, zipper-like advance of the membrane over the particle surface, and there is no evidence for a movement of the receptors toward the base of the phagocytic cup. Receptors are downregulated from the surface with the engulfment of the particle. Thus, the late events in the mature immunological synapse differ from those observed in phagosome completion and closure.
Signaling downstream of the TCR and phagocytic receptors leads to intense and transient actin polymerization that relies on the activation of Rho family GTPases (35). In T cells and phagocytes, Rho GTPase activation occurs to a large extent via tyrosine phosphorylation and activation of the Rac1 and Cdc42 guanine exchange factor (GEF) Vav (36, 37). In addition, Rac1 can be activated by other GEFs, including DOCK2, DOCK8, Tiam1, and Trio. DOCK2 is involved in Rac1 activation downstream of the TCR and in lymphocyte migration in response to chemokines. DOCK2 and DOCK8 physiological relevance has been underscored by the discovery of human-inherited immunodeficiencies caused by DOCK2 or DOCK8 gene mutations. B and T cells from these patients display impaired actin polymerization and migration in response to chemokines, as well as impaired lytic granule release by NK cells (38, 39). DOCK family proteins are also involved in phagocytosis as regulators of Rac1 (40).
In phagocytes, the pioneering description of the involvement of Rho family proteins initially led to the classification of type I phagocytosis implicating Rac1 and Cdc42 downstream of FcR and type II phagocytosis relying on RhoA downstream of CR3 (41). More recently, RhoG has been shown to act as regulator for both FcR and CR3-mediated phagocytosis (42). As RhoG is also critical for phagocytosis of apoptotic bodies (43), and for the nibbling of MHC-associated portions of APC membrane by T cells (3), it could well act as a still ill-defined “master regulator” in immunological synapse and phagosome formation. Dynamic studies by fluorescence resonance energy transfer (FRET) revealed different patterns of activation for Rac and Cdc42 downstream of FcR. Active GTP-Cdc42 is present at the tip of the advancing pseudopod where it colocalizes with polymerizing actin, while Rac1 activation is biphasic. GTP-Rac1 is induced at a low level early after particle binding and peaked at the time of pseudopod fusion (44). Cdc42 activation and phosphatidylinositol-4,5-bisphosphate PI(4,5)P2 accumulation in the nascent phagocytic cup activate effectors among which the actin nucleation promoting factor (NPF) N-WASP that acts on the Arp2/3 actin nucleation complex. Rac1 is then essential for F-actin polymerization to complete extension and closure, through activation of another NPF, the WAVE complex. In CR3-mediated phagocytosis, RhoA is critical for the signaling to actin polymerization as it activates the Rho-Kinase (ROCK), the formin mDia1, and myosin II that are implicated in polymerization and contraction of F-actin around the particles (41, 45–47). The microtubules are important for this pathway, and CLIP1 (CLIP-170), a microtubule plus-end protein, is especially required for efficient recruitment of mDia1 downstream of CR3 and therefore for efficient phagocytosis (48, 49), showing crosstalk between microtubules and actin.
Immunological synapse formation and function require the coordinated activation of RhoA after initial LFA-1 clustering and Rac1 and Cdc42 activation downstream of the TCR (35). Active Cdc42 and its effector WASP are independently recruited to the synapse. WASP seems not to be necessary for broad actin polymerization at the synapse, but rather for the generation of dynamically polymerizing actin foci that facilitate PLCγ activation and calcium flux (50). Consistently, WASP is necessary for efficient IL2 production (51, 52). In contrast, WAVE2, Arp2/3, and the cortactin homolog HS1 are required for T cells to regulate actin polymerization at the synapse (53–55). In turn, actin dynamics is necessary for triggering and sustaining T cell activation (56). This occurs in various concomitant ways, including the regulation of T cell–APC conjugate formation via integrin clustering (57), the interplay between actin cytoskeleton regulators and the calcium second messenger (58), or the regulation of immunological synapse architecture and its interplay with the TCR signaling machinery (59). Finally, the formation of signaling microclusters around the synapse periphery and their convergence toward the center depends on actin dynamics and F-actin inward flows (24, 60).
Cortical actin-associated proteins, such as ezrin and moesin, play important roles in building an activation competent immunological synapse. These proteins connect the cortical cytoskeleton with membrane components. Thus, moesin supports CD43 exclusion from the center of the synapse, a mechanism proposed to remove the CD43-dependent steric inhibition and to facilitate synapse formation (61–63). Moreover, ezrin and moesin contribute to the architecture of the immunological synapse, cell cortex rigidity, and T cell activation as well as differentially regulate early and late activation events (64–67).
Microtubules are finely reorganized at the immunological synapse bringing the microtubule-organizing center (MTOC) close to T cell–APC contact (67–69). Microtubule polarization depends on TCR-induced signaling (70, 71) and the microtubule-driven molecular motor dynein (72). Interestingly, ezrin plays a critical role in driving the MTOC close to the synapse, in controlling microtubule network organization, and in signaling microcluster dynamics at the synapse. Ezrin does so via its association with the polarity regulator Dlg1 (67). Moreover, the actin-nucleating proteins Diaphanous 1 (mDia1) and formin-like 1 (FMNL1) are also necessary to polarize MTOC to the synapse (53). The involvement of ezrin and formins in MTOC polarization highlights that actin and microtubule network organization at the synapse are tightly connected. Microtubule stability modulated by the HDAC6 deacetylase is also regulated during immunological synapse formation and necessary for synapse formation and T cell activation (73). Actin and microtubule interplay is also critical for T cell effector function, such as polarized secretion of helper cytokines, since it is necessary for Golgi complex polarization toward the APC (74).
As mentioned above, microtubule–actin interplay is also necessary for efficient phagocytosis (48). Of note, the MTOC has also been reported to be relocated at the site of phagosome formation (75), but given that multiple targets are often phagocytosed at the same time, how this applies to uptake in physiological situations is uncertain. Similarly, when a cytotoxic T cell is engaged in multiple contacts, the antigen-specific delivery of lytic granules occurs independently of centrosome positioning (76).
Microtubule dynamics and organization ensure the delivery of TCRs and signaling molecules to the synapse via recycling endosomes (77–79). Moreover, microtubules, together with actin flows, drive signaling microcluster centripetal movement at the synapse (67, 80). Therefore, microtubules drive the arrival and removal of TCRs and signaling molecules in a way to sustain and regulate TCR signaling at the synapse.
Actin polymerization is crucial to achieve efficient pseudopod extension and phagosome formation, but actin turnover and depolymerization is as important. This turnover, which occurs at the base of the phagocytic cup (81), is directly dependent on the hydrolysis of PI(4,5)P2 (82), which is mediated by several effectors including phosphatases that hydrolyze PI(4,5)P2, such as phospholipase C, PI3 kinase, and 5′ phosphatases, such as Inpp5b or oculocerebrorenal syndrome of Lowe (OCRL) (81, 83–85). In addition, the severing protein cofilin is recruited to the site of phagocytosis and its activity is regulated by LIM kinase (86). Interestingly, the presence of OCRL at sites of phagocytosis was shown to depend on vesicular recruitment of AP1 and EpsinR adaptors, which is under the unexpected control of the NF-kB signaling protein Bcl10 (81), showing how interconnected the signaling and trafficking events are. Inactivation of Rho GTPases is also achieved by several Rho GAP proteins, such as ARHGAP12, ARHGAP25, and SH3BP1, that are recruited under the dependence of PI3K and synergistically inactivate Rac and Cdc42 (87). Actin clearance from the base of the phagocytic cup, which is required for large but not small particle internalization (87), is then necessary for vesicles to make their way to the plasma membrane (81).
Actin clearance is also observed in immunological synapses, and it is thought to be important to facilitate vesicle fusion at the synapse, particularly in cytotoxic T cells, which destroy target cells by the polarized secretion of lytic granules (88). F-actin relocalization at the immunological synapse depends on PI(3,4,5)P3 (89) and modulates cytotoxicity. Actin and PI(4,5)P2 are cleared from the site of secretion, indicating a tight interplay between actin cytoskeleton reorganization and phospholipid second messenger at the synapse (68, 90).
Therefore, the reorganization of the actin and microtubule cytoskeleton is triggered by TCR and phagocytic receptors and is the key to maintain the structure and function of phagocytic cups and immunological synapses.
Phagocytic cup formation generates membrane protrusions capable of engulfing particles of different sizes. Instead of a decrease in membrane surfaces after internalization of the phagosomes, an increase in cell surface was reported during phagosome formation using capacitance measurements (91). This is in agreement with the concept of membrane remodeling and “focal delivery” of intracellular compartments at the site of phagosome formation (92, 93). The requirement for focal vesicle fusion in optimal phagocytosis of large targets came from studies interfering with the fusion machineries composed of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs). These are membrane fusion regulatory proteins that form a tri-party complex composed of one vesicle (v)-SNARE and two target membrane (t)-SNAREs. SNARE complex formation helps bringing together the two membranes to facilitate their fusion. SNAREs act with various regulatory proteins, such as Rab GTPases, Munc proteins, and the calcium sensors synaptotagmins to bring together, dock, tether, and fuse vesicles with target membranes, either the plasma membrane or other vesicles (94). Several intracellular vesicular compartments have been implicated in focal recruitment and fusion concomitant with phagosome formation (95–97). These include recycling endosomes bearing the v-SNARE VAMP3 on their surface (98–100) and late endocytic compartments displaying the v-SNARE VAMP7 or lysosomes (101, 102). The endocytic compartments also harbor the adaptor proteins AP1 and EpsinR, both implicated in efficient phagosome formation, while the AP2 complexes and the clathrin-related endocytic machinery are not involved (81, 100). Interestingly, VAMP3+/AP1+ endosomes also partially colocalize with TNFα, a cytokine that is delivered at the site of forming phagosomes (103).
Similarly, different endosomal compartments and vesicle traffic regulators are involved in immunological synapse formation. These compartments differentially transport TCRs, the tyrosine kinase Lck, and the adapter LAT to the synapse by recycling these proteins back and forth between their plasma membrane location and endosomes. These endosomal compartments display different traffic regulators, such as Rab GTPases (i.e., Rab4, Rab8, Rab11, Rab27, and Rab35), transport proteins (i.e., MAL, intraflagellar transport proteins), or vesicle fusion regulators (i.e., VAMP3, VAMP7, Synaptotagmin-7, and Munc13) (77, 78, 104–108). The immunological synapse clusters the t-SNAREs SNAP23 and syntaxin 4 preparing the zone for active vesicle fusion activity. It is still a matter of debate whether vesicles transporting the signaling adapter LAT only dock and stay as subsynaptic vesicles (106, 109, 110) or fuse with the plasma membrane driving LAT clustering at the synapse (77, 78, 111, 112). The regulated exocytosis of vesicular compartments in T cells might also be important during the early stages of synapse formation when a large lamellipodium-like membrane structure is formed over the APC. Finally, vesicle traffic is important for T cell effector functions, such as polarized secretion of cytokines or cytotoxic granules in helper and cytotoxic cells, respectively (88, 113).
During phagosome formation, the recruitment of compartments and their fusion are regulated by small GTPases of the Rab and ARF families. Rab11, localized on the recycling compartments, is implicated in efficient phagocytosis (114–116). ARF6 was shown to be activated during phagosome formation and to control the delivery of VAMP3+ recycling endosomes (99, 117, 118). Rab35 regulates actin-dependent phagosome formation by recruiting ACAP2, an ARF6 GTPase-activating protein (119), or by regulating the localization of Rac1 and Cdc42 (120). In addition, Rab11 and ARF6 activities might be coordinated via common effectors, such as the Rab11-FIP3/4/RIP/RCP (Rab-coupling proteins), also named arfophilins, which were implicated in phagosome formation and maturation (115). Rab31 (Rab22b) recruits the adaptor APPL2 that participates in PI3K/Akt signaling and phagosome completion (121). As Rab35 recruits the OCRL phosphatase during cytokinesis (122), it could also be implicated together with Rab5 (85) in OCRL recruitment during phagocytosis, although this has not been demonstrated. There are therefore multiple levels of regulation that implicate tight coordination between the signaling platforms and their subcellular localization, and further investigations are required to dissect them both in the context of the immunological synapse and the phagocytic cup.
Although we have largely progressed in our understanding of the mechanisms underlying the membrane and cytoskeletal reorganization that support phagosome and immunological synapse formation, there are still a number of issues that need further in-depth investigation. These issues may be different in the phagocytosis and the immunological synapse fields, but a comparison of the two systems may help solve these different questions faster. These include how some phagocytic receptors get engaged and the type of signals they generate? What is the phospholipid chemistry of each of the systems and its influence on cytoskeleton organization? What is the precise time and space organization of signaling complexes and vesicular compartments? Interestingly, we have recently described several examples of “ménage à trois” between receptor signals, vesicle traffic, and cytoskeletal structures in both processes; for instance, the involvement of the proinflammatory signaling pathway NFκB in the control of vesicle trafficking and actin clearance in nascent phagosomes via the signaling protein Bcl10 (81), or the orchestrated action of the TCR signaling machinery, the actin and microtubule cytoskeleton, and intracellular vesicle traffic in ensuring immunological synapse architecture and function in T cell activation and effector functions, such as polarized secretion of cytokines or cytotoxic granules (1). Collectively, the vast majority of data presented here emphasize the similarities between immunological and phagocytic synapses formation and suggest a possible evolutionary link between these two structures, whereby the phagocytic synapse of innate immune cells would be an ancestor of the immunological synapse in the adaptive immune system (123).
FN, VDB, and AA contributed equally to this review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Work in the laboratory of FN was supported by CNRS, INSERM, and Université Paris Descartes, and grants from Agence Nationale de la Recherche, ANR (2011 BSV3 025 02), Fondation pour la Recherche Médicale (FRM, INE20041102865), and l’Agence Nationale pour la Recherche sur le SIDA et les Hépatites Virales (ANRS). Work in the laboratory of AA and VDB was supported by grants from Institut Pasteur, CNRS, ANR (2011 BSV3 025 02), Fondation ARC pour la Recherche sur le Cancer, La Ligue Contre le Cancer, and ANRS.
1. Soares H, Lasserre R, Alcover A. Orchestrating cytoskeleton and intracellular vesicle traffic to build functional immunological synapses. Immunol Rev (2013) 256(1):118–32. doi:10.1111/imr.12110
2. Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature (2011) 472(7344):471–5. doi:10.1038/nature10071
3. Martinez-Martin N, Fernandez-Arenas E, Cemerski S, Delgado P, Turner M, Heuser J, et al. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity (2011) 35(2):208–22. doi:10.1016/j.immuni.2011.06.003
4. Dustin ML. The immunological synapse. Cancer Immunol Res (2014) 2(11):1023–33. doi:10.1158/2326-6066.CIR-14-0161
5. Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev (2009) 228(1):9–22. doi:10.1111/j.1600-065X.2008.00745.x
6. Au-Yeung BB, Deindl S, Hsu LY, Palacios EH, Levin SE, Kuriyan J, et al. The structure, regulation, and function of ZAP-70. Immunol Rev (2009) 228(1):41–57. doi:10.1111/j.1600-065X.2008.00753.x
7. Navarro MN, Cantrell DA. Serine-threonine kinases in TCR signaling. Nat Immunol (2014) 15(9):808–14. doi:10.1038/ni.2941
8. Freeman SA, Grinstein S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev (2014) 262(1):193–215. doi:10.1111/imr.12212
9. Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity (2006) 24(1):19–28. doi:10.1016/j.immuni.2005.11.010
10. Fitzer-Attas CJ, Lowry M, Crowley MT, Finn AJ, Meng F, DeFranco AL, et al. Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med (2000) 191(4):669–82. doi:10.1084/jem.191.4.669
11. Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, et al. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med (1997) 186(7):1027–39. doi:10.1084/jem.186.7.1027
12. Zoller KE, MacNeil IA, Brugge JS. Protein tyrosine kinases Syk and ZAP-70 display distinct requirements for Src family kinases in immune response receptor signal transduction. J Immunol (1997) 158(4):1650–9.
13. Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity (2005) 22(4):507–17. doi:10.1016/j.immuni.2005.03.004
14. Deng Z, Ma S, Zhou H, Zang A, Fang Y, Li T, et al. Tyrosine phosphatase SHP-2 mediates C-type lectin receptor-induced activation of the kinase Syk and anti-fungal TH17 responses. Nat Immunol (2015) 16(6):642–52. doi:10.1038/ni.3155
15. Caron E, Self AJ, Hall A. The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr Biol (2000) 10(16):974–8. doi:10.1016/S0960-9822(00)00641-2
16. Dupuy AG, Caron E. Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci (2008) 121(Pt 11):1773–83. doi:10.1242/jcs.018036
17. Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci (2000) 113(Pt 20):3563–71.
18. Shi Y, Tohyama Y, Kadono T, He J, Miah SM, Hazama R, et al. Protein-tyrosine kinase Syk is required for pathogen engulfment in complement-mediated phagocytosis. Blood (2006) 107(11):4554–62. doi:10.1182/blood-2005-09-3616
19. Jaumouille V, Farkash Y, Jaqaman K, Das R, Lowell CA, Grinstein S. Actin cytoskeleton reorganization by syk regulates fcgamma receptor responsiveness by increasing its lateral mobility and clustering. Dev Cell (2014) 29(5):534–46. doi:10.1016/j.devcel.2014.04.031
20. van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nat Rev Immunol (2011) 11(1):47–55. doi:10.1038/nri2887
21. Anton van der Merwe P, Davis SJ, Shaw AS, Dustin ML. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin Immunol (2000) 12(1):5–21. doi:10.1006/smim.2000.0203
22. Yamauchi S, Kawauchi K, Sawada Y. Myosin II-dependent exclusion of CD45 from the site of Fcgamma receptor activation during phagocytosis. FEBS Lett (2012) 586(19):3229–35. doi:10.1016/j.febslet.2012.06.041
23. Zhu JW, Brdicka T, Katsumoto TR, Lin J, Weiss A. Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling. Immunity (2008) 28(2):183–96. doi:10.1016/j.immuni.2007.11.024
24. Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med (2005) 202(8):1031–6. doi:10.1084/jem.20051182
25. Yokosuka T, Saito T. The immunological synapse, TCR microclusters, and T cell activation. Curr Top Microbiol Immunol (2010) 340:81–107. doi:10.1007/978-3-642-03858-7_5
26. Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol (2002) 158(7):1263–75. doi:10.1083/jcb.200203043
27. Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, et al. The immunological synapse balances T cell receptor signaling and degradation. Science (2003) 302(5648):1218–22. doi:10.1126/science.1086507
28. Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity (2006) 25(1):117–27. doi:10.1016/j.immuni.2006.04.010
29. Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science (2005) 310(5751):1191–3. doi:10.1126/science.1119238
30. Niedergang F, Dautry-Varsat A, Alcover A. Peptide antigen or superantigen-induced down-regulation of TCRs involves both stimulated and unstimulated receptors. J Immunol (1997) 159:1703–10.
31. Penna D, Muller S, Martinon F, Demotz S, Iwashima M, Valitutti S. Degradation of ZAP-70 following antigenic stimulation in human T lymphocytes: role of calpain proteolytic pathway. J Immunol (1999) 163(1):50–6.
32. Balagopalan L, Ashwell BA, Bernot KM, Akpan IO, Quasba N, Barr VA, et al. Enhanced T-cell signaling in cells bearing linker for activation of T-cell (LAT) molecules resistant to ubiquitylation. Proc Natl Acad Sci U S A (2011) 108(7):2885–90. doi:10.1073/pnas.1007098108
33. Lasserre R, Cuche C, Blecher-Gonen R, Libman E, Biquand E, Danckaert A, et al. Release of serine/threonine-phosphorylated adaptors from signaling microclusters down-regulates T cell activation. J Cell Biol (2011) 195(5):839–53. doi:10.1083/jcb.201103105
34. Choudhuri K, Llodra J, Roth EW, Tsai J, Gordo S, Wucherpfennig KW, et al. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature (2014) 507(7490):118–23. doi:10.1038/nature12951
35. Rougerie P, Delon J. Rho GTPases: masters of T lymphocyte migration and activation. Immunol Lett (2012) 142(1–2):1–13. doi:10.1016/j.imlet.2011.12.003
36. Hornstein I, Alcover A, Katzav S. Vav proteins, masters of the world of cytoskeleton organization. Cell Signal (2004) 16(1):1–11. doi:10.1016/S0898-6568(03)00110-4
37. Patel JC, Hall A, Caron E. Vav regulates activation of Rac but not Cdc42 during FcgammaR-mediated phagocytosis. Mol Biol Cell (2002) 13(4):1215–26. doi:10.1091/mbc.02-01-0002
38. Dobbs K, Dominguez Conde C, Zhang SY, Parolini S, Audry M, Chou J, et al. Inherited DOCK2 deficiency in patients with early-onset invasive infections. N Engl J Med (2015) 372(25):2409–22. doi:10.1056/NEJMoa1413462
39. Aydin SE, Kilic SS, Aytekin C, Kumar A, Porras O, Kainulainen L, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options – a review of 136 patients. J Clin Immunol (2015) 35(2):189–98. doi:10.1007/s10875-014-0126-0
40. Cote JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol (2007) 17(8):383–93. doi:10.1016/j.tcb.2007.05.001
41. Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science (1998) 282(5394):1717–21. doi:10.1126/science.282.5394.1717
42. Tzircotis G, Braga VM, Caron E. RhoG is required for both FcgammaR- and CR3-mediated phagocytosis. J Cell Sci (2011) 124(Pt 17):2897–902. doi:10.1242/jcs.084269
43. Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J Biol Chem (2006) 281(13):8836–42. doi:10.1074/jbc.M510972200
44. Hoppe AD, Swanson JA. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell (2004) 15(8):3509–19. doi:10.1091/mbc.E03-11-0847
45. Colucci-Guyon E, Niedergang F, Wallar BJ, Peng J, Alberts AS, Chavrier P. A role for mammalian diaphanous-related formins in complement receptor (CR3)-mediated phagocytosis in macrophages. Curr Biol (2005) 15(22):2007–12. doi:10.1016/j.cub.2005.09.051
46. Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med (1997) 186(9):1487–94. doi:10.1084/jem.186.9.1487
47. Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr Biol (2002) 12(16):1413–8. doi:10.1016/S0960-9822(02)01069-2
48. Binker MG, Zhao DY, Pang SJ, Harrison RE. Cytoplasmic linker protein-170 enhances spreading and phagocytosis in activated macrophages by stabilizing microtubules. J Immunol (2007) 179(6):3780–91. doi:10.4049/jimmunol.179.6.3780
49. Lewkowicz E, Herit F, Le Clainche C, Bourdoncle P, Perez F, Niedergang F. The microtubule-binding protein CLIP-170 coordinates mDia1 and actin reorganization during CR3-mediated phagocytosis. J Cell Biol (2008) 183:1287–98. doi:10.1083/jcb.200807023
50. Kumari S, Depoil D, Martinelli R, Judokusumo E, Carmona G, Gertler FB, et al. Actin foci facilitate activation of the phospholipase C-gamma in primary T lymphocytes via the WASP pathway. Elife (2015) 4:e04953. doi:10.7554/eLife.04953
51. Cannon JL, Burkhardt JK. Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production. J Immunol (2004) 173(3):1658–62. doi:10.4049/jimmunol.173.3.1658
52. Cannon JL, Labno CM, Bosco G, Seth A, McGavin MH, Siminovitch KA, et al. Wasp recruitment to the T cell:APC contact site occurs independently of Cdc42 activation. Immunity (2001) 15(2):249–59. doi:10.1016/S1074-7613(01)00178-9
53. Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity (2007) 26(2):177–90. doi:10.1016/j.immuni.2007.01.008
54. Gomez TS, McCarney SD, Carrizosa E, Labno CM, Comiskey EO, Nolz JC, et al. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity (2006) 24(6):741–52. doi:10.1016/j.immuni.2006.03.022
55. Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, et al. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol (2006) 16(1):24–34. doi:10.1016/j.cub.2005.11.036
56. Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med (1995) 181:577–84. doi:10.1084/jem.181.2.577
57. Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev (2007) 218:65–81. doi:10.1111/j.1600-065X.2007.00527.x
58. Babich A, Burkhardt JK. Coordinate control of cytoskeletal remodeling and calcium mobilization during T-cell activation. Immunol Rev (2013) 256(1):80–94. doi:10.1111/imr.12123
59. Lasserre R, Alcover A. Cytoskeletal cross-talk in the control of T cell antigen receptor signaling. FEBS Lett (2010) 584(24):4845–50. doi:10.1016/j.febslet.2010.09.001
60. Nguyen K, Sylvain NR, Bunnell SC. T cell costimulation via the integrin VLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity (2008) 28(6):810–21. doi:10.1016/j.immuni.2008.04.019
61. Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity (2001) 15(5):739–50. doi:10.1016/S1074-7613(01)00224-2
62. Tong J, Allenspach EJ, Takahashi SM, Mody PD, Park C, Burkhardt JK, et al. CD43 regulation of T cell activation is not through steric inhibition of T cell-APC interactions but through an intracellular mechanism. J Exp Med (2004) 199(9):1277–83. doi:10.1084/jem.20021602
63. Delon J, Kaibuchi K, Germain RN. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity (2001) 15(5):691–701. doi:10.1016/S1074-7613(01)00231-X
64. Roumier A, Olivo-Marin JC, Arpin M, Michel F, Martin M, Mangeat P, et al. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity (2001) 15(5):715–28. doi:10.1016/S1074-7613(01)00225-4
65. Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VL, Bismuth G, Trautmann A, et al. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol (2004) 5(3):272–9. doi:10.1038/ni1039
66. Shaffer MH, Dupree RS, Zhu P, Saotome I, Schmidt RF, McClatchey AI, et al. Ezrin and moesin function together to promote T cell activation. J Immunol (2009) 182(2):1021–32. doi:10.4049/jimmunol.182.2.1021
67. Lasserre R, Charrin S, Cuche C, Danckaert A, Thoulouze MI, de Chaumont F, et al. Ezrin tunes T-cell activation by controlling Dlg1 and microtubule positioning at the immunological synapse. EMBO J (2010) 29(14):2301–14. doi:10.1038/emboj.2010.127
68. Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature (2006) 443(7110):462–5. doi:10.1038/nature05071
69. Kupfer A, Dennert G, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci U S A (1983) 80(23):7224–8. doi:10.1073/pnas.80.23.7224
70. Lowin-Kropf B, Shapiro VS, Weiss A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J Cell Biol (1998) 140(4):861–71. doi:10.1083/jcb.140.4.861
71. Martin-Cofreces NB, Sancho D, Fernandez E, Vicente-Manzanares M, Gordon-Alonso M, Montoya MC, et al. Role of Fyn in the rearrangement of tubulin cytoskeleton induced through TCR. J Immunol (2006) 176(7):4201–7. doi:10.4049/jimmunol.176.7.4201
72. Martin-Cofreces NB, Robles-Valero J, Cabrero JR, Mittelbrunn M, Gordon-Alonso M, Sung CH, et al. MTOC translocation modulates IS formation and controls sustained T cell signaling. J Cell Biol (2008) 182(5):951–62. doi:10.1083/jcb.200801014
73. Serrador JM, Cabrero JR, Sancho D, Mittelbrunn M, Urzainqui A, Sanchez-Madrid F. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity (2004) 20(4):417–28. doi:10.1016/S1074-7613(04)00078-0
74. Ueda H, Zhou J, Xie J, Davis MM. Distinct roles of cytoskeletal components in immunological synapse formation and directed secretion. J Immunol (2015) 195(9):4117–25. doi:10.4049/jimmunol.1402175
75. Eng EW, Bettio A, Ibrahim J, Harrison RE. MTOC reorientation occurs during Fc{gamma}R-mediated phagocytosis in macrophages. Mol Biol Cell (2007) 18(7):2389–99. doi:10.1091/mbc.E06-12-1128
76. Faroudi M, Utzny C, Salio M, Cerundolo V, Guiraud M, Muller S, et al. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold. Proc Natl Acad Sci U S A (2003) 100(24):14145–50. doi:10.1073/pnas.2334336100
77. Soares H, Henriques R, Sachse M, Ventimiglia L, Alonso MA, Zimmer C, et al. Regulated vesicle fusion generates signaling nanoterritories that control T cell activation at the immunological synapse. J Exp Med (2013) 210(11):2415–33. doi:10.1084/jem.20130150
78. Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, et al. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity (2004) 20(5):577–88. doi:10.1016/S1074-7613(04)00106-2
79. Martin-Cofreces NB, Baixauli F, Lopez MJ, Gil D, Monjas A, Alarcon B, et al. End-binding protein 1 controls signal propagation from the T cell receptor. EMBO J (2012) 31(21):4140–52. doi:10.1038/emboj.2012.242
80. Hashimoto-Tane A, Yokosuka T, Sakata-Sogawa K, Sakuma M, Ishihara C, Tokunaga M, et al. Dynein-driven transport of T cell receptor microclusters regulates immune synapse formation and T cell activation. Immunity (2011) 34(6):919–31. doi:10.1016/j.immuni.2011.05.012
81. Marion S, Mazzolini J, Herit F, Bourdoncle P, Kambou-Pene N, Hailfinger S, et al. The NF-kappaB signaling protein Bcl10 regulates actin dynamics by controlling AP1 and OCRL-bearing vesicles. Dev Cell (2012) 23(5):954–67. doi:10.1016/j.devcel.2012.09.021
82. Scott CC, Dobson W, Botelho RJ, Coady-Osberg N, Chavrier P, Knecht DA, et al. Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J Cell Biol (2005) 169(1):139–49. doi:10.1083/jcb.200412162
83. Bohdanowicz M, Balkin DM, De Camilli P, Grinstein S. Recruitment of OCRL and Inpp5B to phagosomes by Rab5 and APPL1 depletes phosphoinositides and attenuates Akt signaling. Mol Biol Cell (2012) 23(1):176–87. doi:10.1091/mbc.E11-06-0489
84. Kuhbacher A, Dambournet D, Echard A, Cossart P, Pizarro-Cerda J. Phosphatidylinositol 5-phosphatase oculocerebrorenal syndrome of Lowe protein (OCRL) controls actin dynamics during early steps of Listeria monocytogenes infection. J Biol Chem (2012) 287(16):13128–36. doi:10.1074/jbc.M111.315788
85. Sarantis H, Balkin DM, De Camilli P, Isberg RR, Brumell JH, Grinstein S. Yersinia entry into host cells requires Rab5-dependent dephosphorylation of PI(4,5)P(2) and membrane scission. Cell Host Microbe (2012) 11(2):117–28. doi:10.1016/j.chom.2012.01.010
86. Matsui S, Matsumoto S, Adachi R, Kusui K, Hirayama A, Watanabe H, et al. LIM kinase 1 modulates opsonized zymosan-triggered activation of macrophage-like U937 cells. Possible involvement of phosphorylation of cofilin and reorganization of actin cytoskeleton. J Biol Chem (2002) 277(1):544–9. doi:10.1074/jbc.M110153200
87. Schlam D, Bagshaw RD, Freeman SA, Collins RF, Pawson T, Fairn GD, et al. Phosphoinositide 3-kinase enables phagocytosis of large particles by terminating actin assembly through Rac/Cdc42 GTPase-activating proteins. Nat Commun (2015) 6:8623. doi:10.1038/ncomms9623
88. de Saint Basile G, Menasche G, Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol (2010) 10(8):568–79. doi:10.1038/nri2803
89. Le Floc’h A, Tanaka Y, Bantilan NS, Voisinne G, Altan-Bonnet G, Fukui Y, et al. Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse. J Exp Med (2013) 210(12):2721–37. doi:10.1084/jem.20131324
90. Ritter AT, Asano Y, Stinchcombe JC, Dieckmann NM, Chen BC, Gawden-Bone C, et al. Actin depletion initiates events leading to granule secretion at the immunological synapse. Immunity (2015) 42(5):864–76. doi:10.1016/j.immuni.2015.04.013
91. Holevinsky KO, Nelson DJ. Membrane capacitance changes associated with particle uptake during phagocytosis in macrophages. Biophys J (1998) 75(5):2577–86. doi:10.1016/S0006-3495(98)77703-3
92. Hackam DJ, Rotstein OD, Bennett MK, Klip A, Grinstein S, Manolson MF. Characterization and subcellular localization of target membrane soluble NSF attachment protein receptors (t-SNAREs) in macrophages. Syntaxins 2, 3, and 4 are present on phagosomal membranes. J Immunol (1996) 156(11):4377–83.
93. Hackam DJ, Rotstein OD, Sjolin C, Schreiber AD, Trimble WS, Grinstein S. v-SNARE-dependent secretion is required for phagocytosis. Proc Natl Acad Sci U S A (1998) 95(20):11691–6. doi:10.1073/pnas.95.20.11691
94. Sudhof TC, Rizo J. Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol (2011) 3(12):a005637. doi:10.1101/cshperspect.a005637
95. Braun V, Niedergang F. Linking exocytosis and endocytosis during phagocytosis. Biol Cell (2006) 98(3):195–201. doi:10.1042/BC20050021
96. Deschamps C, Echard A, Niedergang F. Phagocytosis and cytokinesis: do cells use common tools to cut and to eat? Highlights on common themes and differences. Traffic (2013) 14(4):355–64. doi:10.1111/tra.12045
97. Murray RZ, Stow JL. Cytokine secretion in macrophages: SNAREs, Rabs, and membrane trafficking. Front Immunol (2014) 5:538. doi:10.3389/fimmu.2014.00538
98. Bajno L, Peng X-R, Schreiber AD, Moore H-P, Trimble WS, Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol (2000) 149:697–705. doi:10.1083/jcb.149.3.697
99. Niedergang F, Colucci-Guyon E, Dubois T, Raposo G, Chavrier P. ADP ribosylation factor 6 is activated and controls membrane delivery during phagocytosis in macrophages. J Cell Biol (2003) 161:1143–50. doi:10.1083/jcb.200210069
100. Braun V, Deschamps C, Raposo G, Benaroch P, Benmerah A, Chavrier P, et al. AP-1 and ARF1 control endosomal dynamics at sites of FcR mediated phagocytosis. Mol Biol Cell (2007) 18(12):4921–31. doi:10.1091/mbc.E07-04-0392
101. Braun V, Fraisier V, Raposo G, Hurbain I, Sibarita JB, Chavrier P, et al. TI-VAMP/VAMP7 is required for optimal phagocytosis of opsonised particles in macrophages. EMBO J (2004) 23(21):4166–76. doi:10.1038/sj.emboj.7600427
102. Czibener C, Sherer NM, Becker SM, Pypaert M, Hui E, Chapman ER, et al. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol (2006) 174(7):997–1007. doi:10.1083/jcb.200605004
103. Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science (2005) 310(5753):1492–5. doi:10.1126/science.1120225
104. Finetti F, Patrussi L, Galgano D, Cassioli C, Perinetti G, Pazour GJ, et al. The small GTPase Rab8 interacts with VAMP-3 to regulate the delivery of recycling T-cell receptors to the immune synapse. J Cell Sci (2015) 128(14):2541–52. doi:10.1242/jcs.171652
105. Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, et al. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat Cell Biol (2009) 11(11):1332–9. doi:10.1038/ncb1977
106. Larghi P, Williamson DJ, Carpier JM, Dogniaux S, Chemin K, Bohineust A, et al. VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites. Nat Immunol (2013) 14(7):723–31. doi:10.1038/ni.2609
107. Menager MM, Menasche G, Romao M, Knapnougel P, Ho CH, Garfa M, et al. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol (2007) 8(3):257–67. doi:10.1038/ni1431
108. Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell (2003) 115(4):461–73. doi:10.1016/S0092-8674(03)00855-9
109. Purbhoo MA, Liu H, Oddos S, Owen DM, Neil MA, Pageon SV, et al. Dynamics of subsynaptic vesicles and surface microclusters at the immunological synapse. Sci Signal (2010) 3(121):ra36. doi:10.1126/scisignal.2000645
110. Williamson DJ, Owen DM, Rossy J, Magenau A, Wehrmann M, Gooding JJ, et al. Pre-existing clusters of the adaptor Lat do not participate in early T cell signaling events. Nat Immunol (2011) 12(7):655–62. doi:10.1038/ni.2049
111. Balagopalan L, Barr VA, Kortum RL, Park AK, Samelson LE. Cutting edge: cell surface linker for activation of T cells is recruited to microclusters and is active in signaling. J Immunol (2013) 190(8):3849–53. doi:10.4049/jimmunol.1202760
112. Batista A, Millan J, Mittelbrunn M, Sanchez-Madrid F, Alonso MA. Recruitment of transferrin receptor to immunological synapse in response to TCR engagement. J Immunol (2004) 172(11):6709–14. doi:10.4049/jimmunol.172.11.6709
113. Hivroz C, Chemin K, Tourret M, Bohineust A. Crosstalk between T lymphocytes and dendritic cells. Crit Rev Immunol (2012) 32(2):139–55. doi:10.1615/CritRevImmunol.v32.i2.30
114. Cox D, Lee DJ, Dale BM, Calafat J, Greenberg S. A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc Natl Acad Sci U S A (2000) 97(2):680–5. doi:10.1073/pnas.97.2.680
115. Damiani MT, Pavarotti M, Leiva N, Lindsay AJ, McCaffrey MW, Colombo MI. Rab coupling protein associates with phagosomes and regulates recycling from the phagosomal compartment. Traffic (2004) 5(10):785–97. doi:10.1111/j.1600-0854.2004.00220.x
116. Patel PC, Harrison RE. Membrane ruffles capture C3bi-opsonized particles in activated macrophages. Mol Biol Cell (2008) 19(11):4628–39. doi:10.1091/mbc.E08-02-0223
117. Beemiller P, Hoppe AD, Swanson JA. A phosphatidylinositol-3-kinase-dependent signal transition regulates ARF1 and ARF6 during Fcgamma receptor-mediated phagocytosis. PLoS Biol (2006) 4(6):e162. doi:10.1371/journal.pbio.0040162
118. Zhang Q, Calafat J, Janssen H, Greenberg S. ARF6 is required for growth factor- and Rac-mediated membrane ruffling in macrophages at a stage distal to Rac membrane targeting. Mol Cell Biol (1999) 19(12):8158–68. doi:10.1128/MCB.19.12.8158
119. Egami Y, Fukuda M, Araki N. Rab35 regulates phagosome formation through recruitment of ACAP2 in macrophages during FcgammaR-mediated phagocytosis. J Cell Sci (2011) 124(Pt 21):3557–67. doi:10.1242/jcs.083881
120. Shim J, Lee SM, Lee MS, Yoon J, Kweon HS, Kim YJ. Rab35 mediates transport of Cdc42 and Rac1 to the plasma membrane during phagocytosis. Mol Cell Biol (2010) 30(6):1421–33. doi:10.1128/MCB.01463-09
121. Yeo JC, Wall AA, Luo L, Stow JL. Rab31 and APPL2 enhance FcgammaR-mediated phagocytosis through PI3K/Akt signaling in macrophages. Mol Biol Cell (2015) 26(5):952–65. doi:10.1091/mbc.E14-10-1457
122. Dambournet D, Machicoane M, Chesneau L, Sachse M, Rocancourt M, El Marjou A, et al. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat Cell Biol (2011) 13(8):981–8. doi:10.1038/ncb2279
Keywords: phagocytosis, immunological synapse, immune receptor, signal transduction, actin, microtubules, exocytosis, endocytosis
Citation: Niedergang F, Di Bartolo V and Alcover A (2016) Comparative Anatomy of Phagocytic and Immunological Synapses. Front. Immunol. 7:18. doi: 10.3389/fimmu.2016.00018
Received: 19 November 2015; Accepted: 14 January 2016;
Published: 28 January 2016
Edited by:
Nick Gascoigne, National University of Singapore, SingaporeCopyright: © 2016 Niedergang, Di Bartolo and Alcover. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florence Niedergang, ZmxvcmVuY2UubmllZGVyZ2FuZ0BpbnNlcm0uZnI=;
Vincenzo Di Bartolo, dmluY2Vuem8uZGktYmFydG9sb0BwYXN0ZXVyLmZy;
Andrés Alcover, YW5kcmVzLmFsY292ZXJAcGFzdGV1ci5mcg==
†All three authors contributed equally to this review.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.