- 1Université Paris Descartes, Sorbonne Paris Cité, Paris, France
- 2INSERM U1151, Institut Necker-Enfants Malades, Paris, France
- 3CNRS UMR 8253, Institut Necker-Enfants Malades, Paris, France
Despite significant progress achieved in transplantation, immunosuppressive therapies currently used to prevent graft rejection are still endowed with severe side effects impairing their efficiency over the long term. Thus, the development of graft-specific, non-toxic innovative therapeutic strategies has become a major challenge, the goal being to selectively target alloreactive effector T cells while sparing CD4+Foxp3+ regulatory T cells (Tregs) to promote operational tolerance. Various approaches, notably the one based on monoclonal antibodies or fusion proteins directed against the TCR/CD3 complex, TCR coreceptors, or costimulatory molecules, have been proposed to reduce the alloreactive T cell pool, which is an essential prerequisite to create a therapeutic window allowing Tregs to induce and maintain allograft tolerance. In this mini review, we focus on the differential sensitivity of Tregs and effector T cells to the depleting and inhibitory effect of these immunotherapies, with a particular emphasis on CD3-specific antibodies that beyond their immunosuppressive effect, also express potent tolerogenic capacities.
Introduction
The clinical success of transplantation depends on a life-long use of immunosuppressive drugs that depress the immune system in a global manner. These non-specific therapies significantly reduced the incidence of acute rejection but the benefit in terms of chronic rejection or long-term graft survival is uncertain. Indeed, the induction of a permanent immunosuppressive state, which may be excessive in some patients, is associated with significant toxicity and morbidity with increased incidence of cancer, opportunistic infections, and cardiovascular diseases. Therefore, alternative approaches have been developed with the objective of targeting the unwanted immune responses to the transplanted organ while sparing the beneficial functions of the immune system, i.e., inducing operational tolerance. Strategies include the use of monoclonal antibodies (Abs) or fusion proteins that block T cell activation, the infusion of donor antigens (DST), the induction of hematopoietic chimerism (bone marrow transplantation), the use of immunomodulatory cytokines or cell therapy (dendritic cells, regulatory T cells). These approaches utilize various mechanisms of peripheral tolerance such as deletion, activation-induced cell death (AICD, apoptosis), anergy, immune deviation, and/or induction of regulatory T cells (Tregs).
When looking at the data more closely, notably the one gained from studies using monoclonal antibodies or fusion proteins, we can observe that most treatments differentially affect T cell populations. In particular, activated effector T cells and CD4+Foxp3+ Tregs react differently to the inhibitory or depleting effect of these biological agents. This is of importance as induction of transplant tolerance across full MHC histocompatibility barriers is thought to require the presence of Foxp3+ Tregs as well as the physical elimination of effector T cells. In this mini review, we will discuss the differential sensitivity of T cell subsets to immunointervention with a particular focus on CD3 antibody-based therapy in transplantation.
Differential Sensitivity of Effector and Regulatory T Cells to Immunotherapy
Alloreactive T cells are present in naive recipients and are thus able to recognize alloantigens and mount efficient immune responses against the transplanted organ (1). Therefore, depletion or inhibition of these alloreactive T cells is necessary to promote graft survival (2). However, there is compelling data derived from experimental models suggesting that this step is not sufficient per se to induce peripheral tolerance and must be implemented with mechanisms that maintain effective Treg function to control both remaining alloreactive T cells and new thymic emigrants (3). Therapeutic approaches should combine these two capacities to induce permanent allograft acceptance and antigen-specific tolerance.
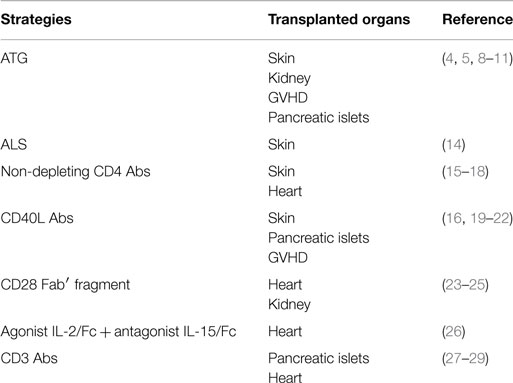
In most experimental models, the success of therapeutic strategies is associated with the fact they target effector T cells while preserving CD4+Foxp3+ Tregs from deletion or functional inhibition (Table 1). Indeed, reports both in mice and humans showed that Foxp3+ Tregs are relatively spared from the lymphodepleting effect of anti-thymocyte globulin (ATG) (4–11). ATG treatment increased the frequency and the functional activity of Tregs and, in some models, de novo generation of antigen-specific Tregs has been demonstrated (12). Similarly, Treg number and activity were not significantly affected by treatment with anti-lymphocyte serum (ALS) (13).
In the same vein, short course of non-depleting CD4 and CD8 Abs (combined with CD40L Abs in some MHC-mismatched conditions) efficiently promoted tolerance by inhibiting alloreative T cells while promoting regulatory mechanisms (30). The use of transgenic models revealed that non-depleting CD4 Abs leave intact the proliferation of allospecific Tregs while abrogating the expansion of allospecific effector T cells in vitro and in vivo (15, 16). Natural and induced CD4+Foxp3+ Tregs play crucial role in inducing and maintaining transplant tolerance through mechanisms of “linked suppression” and “infectious tolerance” (17, 30–34). This occurs when a Foxp3+ Treg and a Foxp3− conventional T cell recognize their respective alloantigens presented by the same APC, thereby inducing a tolerizing signal within the conventional T cells which acquire regulatory properties instead of differentiating into alloreactive effector T cells. These “secondary” Tregs can propagate the tolerance state by inducing “tertiary” Tregs. The infectious nature of the process and the critical role of CD4+Foxp3+ Tregs have been confirmed in a B6.Foxp3hCD2 transgenic mouse model (18). Importantly, the continuous presence of the alloantigens is required to ensure dominance by Tregs, which accumulate in tolerated grafts where they efficiently control alloreactive effectors (18, 35). Immunoregulatory cytokines also contribute to CD4/CD8 Ab-induced tolerance, notably TGF-β, which has been detected in tolerated grafts and can promote the de novo generation of Foxp3+ Tregs (35–37).
Costimulatory blockade is another well-established strategy that targets mature peripheral T cells and manipulates the immune system in a manner that favors Treg development and abrogates alloreactive responses (16, 19–21). It has been reproducibly shown that administration of the fusion protein CTLA-4Ig and/or antibodies to CD40L (CD154), BTLA, ICOS, OX-40, or CD28 efficiently inhibits allogeneic effector T cell activation and expansion (38). Combined with each other, with DST or immunosuppressive drugs such as rapamycin, these biological agents induce long-term acceptance of allogeneic organs and tissues in mice and non-human primates (23, 24, 39–43). Interestingly, besides promoting T cell unresponsiveness, some of these agents also induce T cell apoptosis, which is partial and transient yet mandatory for tolerance induction (40, 44–47). This is well illustrated by the fact that recipient mice transgenic for the anti-apoptotic molecule Bcl-xL are refractory to the therapeutic effect of CTLA-4Ig and CD40L Abs and thereby reject skin or heart allografts (40, 44). Importantly, several reports show that alloreactive effector T cells are the primary targets of these therapeutic agents. For instance, in a major MHC-mismatched skin graft model, combination of CD40L Ab to DST promoted transplant tolerance through the selective depletion of alloantigen-specific CD8+ T cells (47). Another study revealed that CD40L Ab can fix complement through its Fc fragment and mediates selective depletion of activated T cells, which, in association with rapamycin, induce long-term graft survival (46). The use of CD40L F(ab′)2 fragments or C3- and Fc receptor-deficient recipients completely abrogated the therapeutic effect. Clonal deletion was illustrated by the disappearance of effector T cells expressing specific TCR Vβ families after treatment with CD40L Ab, CTLA-4Ig, and rapamycin (48).
Regulatory T cells resist the inhibitory/depleting effect of costimulation blockade and they play a crucial role in the development and the maintenance of transplant tolerance (38). Indeed, their deletion abrogates tolerance and leads to graft rejection (22, 49). Expansion of thymus-derived Tregs as well as de novo generation of Tregs have been reported and infectious tolerance has been proposed as a key mechanisms allowing long-term allograft acceptance (30, 50). This positive effect on Tregs is particularly observed when rapamycin is combined to costimulatory blockade, which is not the case when calcineurin inhibitors (CNIs: cyclosporine A, tacrolimus) are used (20, 48, 51). It has been suggested that CNIs have a detrimental effect on CD4+Foxp3+ Treg homeostasis as they block NFAT activation and IL-2 production in contrast to rapamycin which promotes Treg function and expansion (52–55). Using endoscopic confocal microscopy and color-coded T cell subsets, Fan et al. showed that the number of Tregs detected within the allografts and peripheral blood of mice treated with CD40L Abs and rapamycin was similar to that of untreated recipients but the number of effector T cells was drastically reduced after treatment (20). In a graft-versus-host disease (GVHD) model, CTLA-4Ig/CD40L Ab treatment prevented the expansion of host-reactive donor T cells but promoted proliferation of Foxp3+ Tregs (22). Similar features were observed in vitro (21). In the same vein, recent studies in non-human primates showed that treatment with selective CD28 antagonists did not reduce Foxp3+ Treg number after transplantation and favored their accumulation within the graft (23, 24). Interestingly, selective CD28 blockade prevented formation of the immune synapse between alloreative effector T cells and APC by inhibiting the TCR Stop signal while it increased Treg contact time with APC and induced calcium mobilization which translated into enhanced Treg suppressive activity (25). Such effects were not reported for CD80/86 antagonists. As CD28-B7 interaction is mandatory for Treg development and homeostasis, these results together with the data from the phase III BENEFIT study, reporting an increased occurrence of acute graft rejection episode and lymphoproliferative disorders in renal transplant patients treated with belatacept, have raised concerns about the negative impact of CD28-B7 blockade on regulatory mechanisms and graft survival (56–58).
Preservation of Tregs homeostasis and function has been demonstrated in other tolerance-promoting protocols. Tregs were resistant to the lytic effect of a regimen combining rapamycin, agonist IL-2/Fc, and antagonist mutant IL-15/Fc fusion proteins that depleted activated effector T cells through apoptosis and antibody-dependent pathway and promoted long-term engraftment of allogeneic skins and pancreatic islets (26). However, application of this treatment in non-human primates only permitted a modest prolongation of cardiac allograft survival and did not reduce the pool of effector or memory CD8+ T cells although Tregs were found in increased numbers in the blood of treated animals (59).
A Distinct Effect of CD3-Specific Antibodies on Tregs and Effector T Cells
CD3-specific monoclonal Abs act through distinct mechanisms that are not mutually exclusive (60). The antibodies transiently deplete T cells although they display no or little complement-dependent and antibody-dependent cellular cytotoxicity. Mechanism of redirected cell lysis due to the ability to crosslink CD3 molecules expressed by two different cells (cytotoxic CD8+ T cells on one side and other target T cells on the other side) has been demonstrated (61). However, T cell depletion mostly results from AICD. Indeed, through its F(ab′)2 portion, CD3 Ab can not only induce antigenic modulation (i.e., internalization or shedding of the TCR/CD3 complex) but also can transduce signals into T cells, which activate them and promotes their apoptosis (AICD) or anergy. Importantly, non-FcR binding human or mouse CD3 Abs are not passive blocker of TCRs, they can deliver partial TCR signaling and retain their full therapeutic properties without the toxicity associated with the parental CD3 Ab (62–65). This has been the rationale of using humanized CD3 antibodies in the clinic in type 1 diabetes patients (66–68).
We and other accumulated evidence showing that CD3 Abs preferentially target and deplete activated effector T cells while preserving CD4+Foxp3+ Tregs. This finding was initially suggested by indirect observations in autoimmunity showing that CD3 Abs exert their tolerogenic capacities only when applied at the time of ongoing autoreactive responses or established disease (60, 62, 69). These data highlighted the importance of the immune activation status toward the relevant antigen(s) at the time of treatment. We obtained the same results when translating CD3 Ab therapy to the transplantation field. In experimental models of fully mismatched pancreatic islets or cardiac allografts, low-dose treatment of recipient mice at the time of transplantation prolonged graft survival but rejection occurred systematically (27, 28). By contrast, when postponing CD3 Ab administration after transplantation, in a defined therapeutic window, long-term survival and immune tolerance were observed. This therapeutic window corresponded to the time of effector T cell priming to the alloantigens characterized by the occurrence of anti-donor T cell responses and infiltration of allografts by significant number of alloreative T cells (27, 28).
Several experimental data argue for the preferential elimination of activated effector T cells by CD3 antibodies. First, using a transfer model where OVA-specific OT-I CD8+ T cells were primed in vivo by SIINFEKL(OVA)-loaded dendritic cells, we showed that only highly dividing OT-I cells entered apoptosis after CD3 Ab administration applied 6 days after cell infusion. Resting endogenous CD8+ T cells were not affected by the treatment (27). Second, in a murine model of GVHD, injection of CD3 F(ab′)2 fragments selectively depleted activated donor T cells that underwent cell division upon recipient antigens recognition (70). Third, using another non-Fc receptor binding CD3 Ab (145-2C11-IgG3), Penaranda et al. showed that adoptively transferred effector Th1 cells were much more sensitive to the depleting effect of CD3 Abs than endogenous naïve T cells (71). Fourth, in vitro experiments also showed that non-Fc receptor binding humanized CD3 Abs displayed an increased ability to deplete activated human T cells through AICD (63, 72).
In contrast to effector T cells, Foxp3+ Tregs appear resistant to CD3 Ab-mediated cell death. Indeed, in terms of absolute number, the decrease in Foxp3+ T cells is always much less pronounced than that observed for conventional Foxp3− T cells and, in some situations, is not altered at all (27, 71, 73, 74). Consequently, the frequency of Tregs significantly increases in peripheral blood and secondary lymphoid organs after CD3 Ab treatment. Similar results were reported for CD4+CD25+CD62L+ T cells (75). In most reports, no evidence of conversion of conventional T cells into Tregs as well as significant expansion of natural Tregs were reported except in a recent article by Valle et al. showing an increase in Treg count in the blood of treated mice resulting from in vivo proliferation (73). The reduced sensitivity of CD4+Foxp3+ Tregs to CD3 Abs was illustrated at the molecular level by a transcriptome analysis showing that gene expression profile (related to the TCR signaling pathway) of Tregs was much less impacted than the one of CD4+Foxp3− T cells after in vivo treatment with CD3 Abs (73). All these data concur to show that Tregs resist CD3 Ab-induced AICD.
This feature of Tregs is crucial for CD3 Ab-induced transplant tolerance. Both in the pancreatic islet and the cardiac allograft models, we observed that the proportion of CD4+ and CD8+ T cells significantly decreased at the periphery and within the grafted organs after administration of CD3 Ab F(ab′)2 fragments (27, 28, 76). However, depletion of Foxp3+ Tregs was very limited as compared to that of CD8+ T cells or CD4+Foxp3− T cells (27, 28). Consequently, peripheral and intragraft Treg proportion increased after CD3 Ab therapy (27–29, 76). Foxp3+ Tregs isolated from CD3 Ab-treated tolerant mice and adoptively transferred into a RAG−/− recipients were able to prevent graft rejection mediated by naïve spleen cells toward islet allografts from the same donor but not from a third-party donor, thereby indicating that these Tregs play a key role in sustained antigen-specific transplant tolerance over long term (27).
Mechanisms of Resistance
All these data concur to show that Foxp3+ Tregs are less sensitive than effector T cells to the apoptotic or inhibitory effect of therapeutic antibodies. The mechanisms underlying this resistance are not completely elucidated. In the context of ALS treatment, it has been proposed that this resistance to cell death is dependent on the costimulatory molecule OX-40 as well as the anti-apoptotic molecule Bcl-xL that was detected at higher levels in Tregs than in conventional T cells (13, 14). Another mechanism that may contribute to Treg resistance to apoptosis is related to studies suggesting that they express lower levels of FasLigand (CD95L) upon stimulation as compared to conventional Foxp3− T cells (77). Abrogation of Foxp3 expression (in Scurfy mice) rescues FasL expression at the level comparable to those of conventional T cells, suggesting that Foxp3 controls, at least in part, the expression of FasL and AICD. Accordingly, naïve Tregs has been shown to be more resistant to Fas-induced cell death than conventional T cells (78, 79).
In the context of CD3 antibody therapy, the differential effect of the antibody on effector versus regulatory T cells appears paradoxical as the CD3 molecular complex is expressed on all T cells. The reasons explaining this differential effect are still not elucidated. However, recent studies suggested that both human and mouse Tregs harbor less CD3 molecules (comprising the CD3ε chain, which is that target of the therapeutic antibodies) on their surface than do CD4+Foxp3− T cells and that this variable expression level correlated with different susceptibility to CD3 Ab-mediated cell death (73, 80). In addition, it has been suggested that Tregs (CD4+CD25+) possess distinct isoforms of the CD3epsilon chain characterized by an undegraded N-terminal sequence of negatively charged amino acid residues that is associated with a higher activation threshold (80). A diminished expression of signaling molecules downstream of the TCR/CD3 complex has also been reported in Tregs (CD3ζ, ZAP-70, LcK, Vav, and PI3K p110α) as well as a reduced signaling after TCR engagement in response to CD3 antibodies (phospho-ZAP-70, -Akt, -Erk1/2, -PLCγ1) as compared to conventional T cells (CD4+CD25−) (80, 81). Lastly, the fact that Foxp3+ Tregs may express lower levels of FasL upon stimulation than Foxp3− T cells may account for their resistance to the depleting effect of CD3 antibodies that is at least partially mediated by the Fas/FasL pathway (60, 77).
Concluding Remarks
Several potentially important biological agents have been used to prevent transplant rejection with the aim of inducing transplant tolerance. The intrinsic resistance of Tregs to the depleting effect of these therapeutic tools, which preferentially target effector alloreative T cells, encourages clinical translation of these strategies. Unfortunately, so far, these tolerance-promoting protocols are often disappointing when applied to human transplantation (82). To move faster and to achieve successful translation to the clinical arena in a not too distant future, clinicians and researchers need to build on the drugs currently in clinical development to design new tolerogenic protocols. The challenge is to use these biological tools in a defined therapeutic window to reveal their tolerogenic capacities (i.e., deleting effector/memory alloreactive T cells while harnessing Tregs) in order to minimize/withdraw immunosuppressive drugs without increased risks of acute rejection but in a manner that positively impact on long-term graft survival through the induction of operational tolerance. In that situation, CD3-specific Abs may represent one promising approach as they afford long-term therapeutic effect following a short-term and low-dose administration. Finally, these features are further emphasized by the growing interest on Treg cell therapy in transplantation (83, 84). Indeed, the use of in vitro expanded Tregs requires the selection of therapeutic agents that enhance Treg action to enable efficacy of the combined therapy and application to clinical transplantation (85, 86).
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Lucienne Chatenoud for critical reading of the manuscript. Funding: This work was supported by grants from the RISET consortium (Reprogramming the Immune System for the Establishment of Tolerance, 512090) from the European Commission (FP6), the Juvenile Diabetes Research Foundation (JDRF, 1-2011-654), Fondation CENTAURE and Institutional funding from INSERM and University Paris Descartes.
References
1. Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol (2001) 166:973–81. doi:10.4049/jimmunol.166.2.973
2. Li XC, Strom TB, Turka LA, Wells AD. T cell death and transplantation tolerance. Immunity (2001) 14:407–16. doi:10.1016/S1074-7613(01)00121-2
3. Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest (2004) 114:1398–403. doi:10.1172/JCI200423238
4. D’Addio F, Boenisch O, Magee CN, Yeung MY, Yuan X, Mfarrej B, et al. Prolonged, low-dose anti-thymocyte globulin, combined with CTLA4-Ig, promotes engraftment in a stringent transplant model. PLoS One (2013) 8:e53797. doi:10.1371/journal.pone.0053797
5. D’Addio F, Yuan X, Habicht A, Williams J, Ruzek M, Iacomini J, et al. A novel clinically relevant approach to tip the balance toward regulation in stringent transplant model. Transplantation (2010) 90:260–9. doi:10.1097/TP.0b013e3181e64217
6. Chung DT, Korn T, Richard J, Ruzek M, Kohm AP, Miller S, et al. Anti-thymocyte globulin (ATG) prevents autoimmune encephalomyelitis by expanding myelin antigen-specific Foxp3+ regulatory T cells. Int Immunol (2007) 19:1003–10. doi:10.1093/intimm/dxm078
7. Simon G, Parker M, Ramiya V, Wasserfall C, Huang Y, Bresson D, et al. Murine antithymocyte globulin therapy alters disease progression in NOD mice by a time-dependent induction of immunoregulation. Diabetes (2008) 57:405–14. doi:10.2337/db06-1384
8. Valdez-Ortiz R, Bestard O, Llaudo I, Franquesa M, Cerezo G, Torras J, et al. Induction of suppressive allogeneic regulatory T cells via rabbit antithymocyte polyclonal globulin during homeostatic proliferation in rat kidney transplantation. Transpl Int (2015) 28:108–19. doi:10.1111/tri.12448
9. Hire K, Ngo DK, Stewart-Maynard KM, Hering B, Bansal-Pakala P. FoxP3+, and not CD25+, T cells increase post-transplant in islet allotransplant recipients following anti-CD25+ rATG immunotherapy. Cell Immunol (2012) 274:83–8. doi:10.1016/j.cellimm.2012.01.008
10. Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant (2010) 10:2132–41. doi:10.1111/j.1600-6143.2010.03210.x
11. Crepin T, Carron C, Roubiou C, Gaugler B, Gaiffe E, Simula-Faivre D, et al. ATG-induced accelerated immune senescence: clinical implications in renal transplant recipients. Am J Transplant (2015) 15:1028–38. doi:10.1111/ajt.13092
12. Lu Y, Suzuki J, Guillioli M, Umland O, Chen Z. Induction of self-antigen-specific Foxp3+ regulatory T cells in the periphery by lymphodepletion treatment with anti-mouse thymocyte globulin in mice. Immunology (2011) 134:50–9. doi:10.1111/j.1365-2567.2011.03466.x
13. Minamimura K, Gao W, Maki T. CD4+ regulatory T cells are spared from deletion by antilymphocyte serum, a polyclonal anti-T cell antibody. J Immunol (2006) 176:4125–32. doi:10.4049/jimmunol.176.7.4125
14. Kroemer A, Xiao X, Vu MD, Gao W, Minamimura K, Chen M, et al. OX40 controls functionally different T cell subsets and their resistance to depletion therapy. J Immunol (2007) 179:5584–91. doi:10.4049/jimmunol.179.8.5584
15. Oliveira V, Sawitzki B, Chapman S, Appelt C, Gebuhr I, Wieckiewicz J, et al. Anti-CD4-mediated selection of Treg in vitro – in vitro suppression does not predict in vivo capacity to prevent graft rejection. Eur J Immunol (2008) 38:1677–88. doi:10.1002/eji.200737562
16. Nagahama K, Fehervari Z, Oida T, Yamaguchi T, Ogawa O, Sakaguchi S. Differential control of allo-antigen-specific regulatory T cells and effector T cells by anti-CD4 and other agents in establishing transplantation tolerance. Int Immunol (2009) 21:379–91. doi:10.1093/intimm/dxp005
17. Francis RS, Feng G, Tha-In T, Lyons IS, Wood KJ, Bushell A. Induction of transplantation tolerance converts potential effector T cells into graft-protective regulatory T cells. Eur J Immunol (2011) 41:726–38. doi:10.1002/eji.201040509
18. Kendal AR, Chen Y, Regateiro FS, Ma J, Adams E, Cobbold SP, et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med (2011) 208:2043–53. doi:10.1084/jem.20110767
19. Meng L, Wu Z, Wang Y, Lassman C, Busuttil RW, Zhai Y, et al. Differential impact of CD154 costimulation blockade on alloreactive effector and regulatory T cells in murine renal transplant recipients. Transplantation (2008) 85:1332–8. doi:10.1097/TP.0b013e31816c4f2b
20. Fan Z, Spencer JA, Lu Y, Pitsillides CM, Singh G, Kim P, et al. In vivo tracking of ‘color-coded’ effector, natural and induced regulatory T cells in the allograft response. Nat Med (2010) 16:718–22. doi:10.1038/nm.2155
21. Vogel I, Verbinnen B, Maes W, Boon L, Van Gool SW, Ceuppens JL. Foxp3+ regulatory T cells are activated in spite of B7-CD28 and CD40-CD40L blockade. Eur J Immunol (2013) 43:1013–23. doi:10.1002/eji.201242737
22. Verbinnen B, Billiau AD, Vermeiren J, Galicia G, Bullens DM, Boon L, et al. Contribution of regulatory T cells and effector T cell deletion in tolerance induction by costimulation blockade. J Immunol (2008) 181:1034–42. doi:10.4049/jimmunol.181.2.1034
23. Poirier N, Azimzadeh AM, Zhang T, Dilek N, Mary C, Nguyen B, et al. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transl Med (2010) 2:17ra0. doi:10.1126/scitranslmed.3000116
24. Poirier N, Dilek N, Mary C, Ville S, Coulon F, Branchereau J, et al. FR104, an antagonist anti-CD28 monovalent fab’ antibody, prevents alloimmunization and allows calcineurin inhibitor minimization in nonhuman primate renal allograft. Am J Transplant (2015) 15:88–100. doi:10.1111/ajt.12964
25. Dilek N, Poirier N, Hulin P, Coulon F, Mary C, Ville S, et al. Targeting CD28, CTLA-4 and PD-L1 costimulation differentially controls immune synapses and function of human regulatory and conventional T-cells. PLoS One (2013) 8:e83139. doi:10.1371/journal.pone.0083139
26. Zheng XX, Sanchez-Fueyo A, Sho M, Domenig C, Sayegh MH, Strom TB. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity (2003) 19:503–14. doi:10.1016/S1074-7613(03)00259-0
27. You S, Zuber J, Kuhn C, Baas M, Valette F, Sauvaget V, et al. Induction of allograft tolerance by monoclonal CD3 antibodies: a matter of timing. Am J Transplant (2012) 12:2909–19. doi:10.1111/j.1600-6143.2012.04213.x
28. Goto R, You S, Zaitsu M, Chatenoud L, Wood KJ. Delayed anti-CD3 therapy results in depletion of alloreactive T cells and the dominance of Foxp3(+)CD4(+) graft infiltrating cells. Am J Transplant (2013) 13:1655–64. doi:10.1111/ajt.12272
29. Baas MC, Besançon A, Sawitzki B, Mangez C, Valette F, Chatenoud L, et al. Intragraft mechanisms associated with the immunosuppressive versus the tolerogenic effect of CD3 antibodies in a mouse model of islet allografts. Transplant Proc (2013) 45:1895–8. doi:10.1016/j.transproceed.2013.01.054
30. Waldmann H, Adams E, Fairchild P, Cobbold S. Regulation and privilege in transplantation tolerance. J Clin Immunol (2008) 28:716–25. doi:10.1007/s10875-008-9249-5
31. Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, et al. “Infectious” transplantation tolerance. Science (1993) 259:974–7. doi:10.1126/science.8094901
32. Onodera K, Lehmann M, Akalin E, Volk HD, Sayegh MH, Kupiec-Weglinski JW. Induction of “infectious” tolerance to MHC-incompatible cardiac allografts in CD4 monoclonal antibody-treated sensitized rat recipients. J Immunol (1996) 157:1944–50.
33. Karim M, Kingsley CI, Bushell AR, Sawitzki BS, Wood KJ. Alloantigen-induced CD25+CD4+ regulatory T cells can develop in vivo from CD25-CD4+ precursors in a thymus-independent process. J Immunol (2004) 172:923–8. doi:10.4049/jimmunol.172.2.923
34. Davies JD, Leong LY, Mellor A, Cobbold SP, Waldmann H. T cell suppression in transplantation tolerance through linked recognition. J Immunol (1996) 156:3602–7.
35. Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol (2004) 172:6003–10. doi:10.4049/jimmunol.172.10.6003
36. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med (2003) 198:1875–86. doi:10.1084/jem.20030152
37. Daley SR, Ma J, Adams E, Cobbold SP, Waldmann H. A key role for TGF-beta signaling to T cells in the long-term acceptance of allografts. J Immunol (2007) 179:3648–54. doi:10.4049/jimmunol.179.6.3648
38. Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev (2009) 229:271–93. doi:10.1111/j.1600-065X.2009.00781.x
39. Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tuckerburden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature (1996) 381:434–8. doi:10.1038/381434a0
40. Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med (1999) 5:1298–302. doi:10.1038/15256
41. Nanji SA, Hancock WW, Luo B, Schur CD, Pawlick RL, Zhu LF, et al. Costimulation blockade of both inducible costimulator and CD40 ligand induces dominant tolerance to islet allografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes (2006) 55:27–33. doi:10.2337/diabetes.55.01.06.db04-1154
42. Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliveira M, Wagner JL, et al. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci USA (1999) 96:8132–7. doi:10.1073/pnas.96.14.8132
43. Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates [see comments]. Nat Med (1999) 5:686–93. doi:10.1038/9536
44. Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med (1999) 5:1303–7. doi:10.1038/8466
45. Truong W, Plester JC, Hancock WW, Merani S, Murphy TL, Murphy KM, et al. Combined coinhibitory and costimulatory modulation with anti-BTLA and CTLA4Ig facilitates tolerance in murine islet allografts. Am J Transplant (2007) 7:2663–74. doi:10.1111/j.1600-6143.2007.01996.x
46. Monk NJ, Hargreaves RE, Marsh JE, Farrar CA, Sacks SH, Millrain M, et al. Fc-dependent depletion of activated T cells occurs through CD40L-specific antibody rather than costimulation blockade. Nat Med (2003) 9:1275–80. doi:10.1038/nm931
47. Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol (2000) 164:512–21. doi:10.4049/jimmunol.164.1.512
48. Domenig C, Sanchez-Fueyo A, Kurtz J, Alexopoulos SP, Mariat C, Sykes M, et al. Roles of deletion and regulation in creating mixed chimerism and allograft tolerance using a nonlymphoablative irradiation-free protocol. J Immunol (2005) 175:51–60. doi:10.4049/jimmunol.175.1.51
49. Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J Immunol (2005) 175:771–9. doi:10.4049/jimmunol.175.2.771
50. Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J Immunol (2000) 165:4783–6. doi:10.4049/jimmunol.165.9.4783
51. Smiley ST, Csizmadia V, Gao W, Turka LA, Hancock WW. Differential effects of cyclosporine A, methylprednisolone, mycophenolate, and rapamycin on CD154 induction and requirement for NFkappaB: implications for tolerance induction. Transplantation (2000) 70:415–9. doi:10.1097/00007890-200008150-00005
52. Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood (2005) 105:4743–8. doi:10.1182/blood-2004-10-3932
53. Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood (2006) 107:1018–23. doi:10.1182/blood-2005-07-3032
54. Ruggenenti P, Perico N, Gotti E, Cravedi P, D’Agati V, Gagliardini E, et al. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation (2007) 84:956–64. doi:10.1097/01.tp.0000284808.28353.2c
55. Segundo DS, Ruiz JC, Izquierdo M, Fernandez-Fresnedo G, Gomez-Alamillo C, Merino R, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation (2006) 82:550–7. doi:10.1097/01.tp.0000229473.95202.50
56. Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity (2000) 12:431–40. doi:10.1016/S1074-7613(00)80195-8
57. Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science (2008) 322:271–5. doi:10.1126/science.1160062
58. Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant (2010) 10:535–46. doi:10.1111/j.1600-6143.2009.03005.x
59. Millington T, Koulmanda M, Ng C, Boskovic S, Nadazdin OM, Benichou G, et al. Effects of an agonist interleukin-2/Fc fusion protein, a mutant antagonist interleukin-15/Fc fusion protein, and sirolimus on cardiac allograft survival in non-human primates. J Heart Lung Transplant (2012) 31:427–35. doi:10.1016/j.healun.2012.01.864
60. Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol (2007) 7:622–32. doi:10.1038/nri2134
61. Wong JT, Eylath AA, Ghobrial I, Colvin RB. The mechanism of anti-CD3 monoclonal antibodies. Mediation of cytolysis by inter-T cell bridging. Tranplantation (1990) 50:683–9.
62. Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol (1997) 158:2947–54.
63. Carpenter PA, Pavlovic S, Tso JY, Press OW, Gooley T, Yu XZ, et al. Non-Fc receptor-binding humanized anti-CD3 antibodies induce apoptosis of activated human T cells. J Immunol (2000) 165:6205–13. doi:10.4049/jimmunol.165.11.6205
64. Friend PJ, Hale G, Chatenoud L, Rebello P, Bradley J, Thiru S, et al. Phase I study of an engineered aglycosylated humanized CD3 antibody in renal transplant rejection. Transplantation (1999) 68:1632–7. doi:10.1097/00007890-199912150-00005
65. Smith JA, Tso JY, Clark MR, Cole MS, Bluestone JA. Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J Exp Med (1997) 185:1413–22. doi:10.1084/jem.185.8.1413
66. Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes (2013) 62:3766–74. doi:10.2337/db13-0345
67. Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med (2005) 352:2598–608. doi:10.1056/NEJMoa043980
68. Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet (2011) 6736:60931–8. doi:10.1016/S0140-6736(11)60931-8
69. Kohm AP, Williams JS, Bickford AL, McMahon JS, Chatenoud L, Bach JF, et al. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol (2005) 174:4525–34. doi:10.4049/jimmunol.174.8.4525
70. Yu XZ, Bidwell SJ, Martin PJ, Anasetti C. Anti-CD3 epsilon F(ab’)2 prevents graft-versus-host disease by selectively depleting donor T cells activated by recipient alloantigens. J Immunol (2001) 166:5835–9. doi:10.4049/jimmunol.166.9.5835
71. Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol (2011) 187:2015–22. doi:10.4049/jimmunol.1100713
72. Wesselborg S, Janssen O, Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol (1993) 150:4338–45.
73. Valle A, Barbagiovanni G, Jofra T, Stabilini A, Perol L, Baeyens A, et al. Heterogeneous CD3 expression levels in differing T cell subsets correlate with the in vivo anti-CD3-mediated T cell modulation. J Immunol (2015) 194:2117–27. doi:10.4049/jimmunol.1401551
74. Nishio J, Feuerer M, Wong J, Mathis D, Benoist C. Anti-CD3 therapy permits regulatory T cells to surmount T cell receptor-specified peripheral niche constraints. J Exp Med (2010) 207:1879–89. doi:10.1084/jem.20100205
75. Yang W, Hussain S, Mi QS, Santamaria P, Delovitch TL. Perturbed homeostasis of peripheral T cells elicits decreased susceptibility to anti-CD3-induced apoptosis in prediabetic nonobese diabetic mice. J Immunol (2004) 173:4407–16. doi:10.4049/jimmunol.173.7.4407
76. Baas M, Kuhn C, Valette F, Mangez C, Segovia-Duarte M, Hill M, et al. Combining autologous dendritic cell therapy with CD3 antibodies promotes regulatory T cells and permanent islet allograft acceptance. J Immunol (2014) 193:4696–703. doi:10.4049/jimmunol.1401423
77. Weiss EM, Schmidt A, Vobis D, Garbi N, Lahl K, Mayer CT, et al. Foxp3-mediated suppression of CD95L expression confers resistance to activation-induced cell death in regulatory T cells. J Immunol (2011) 187:1684–91. doi:10.4049/jimmunol.1002321
78. Banz A, Pontoux C, Papiernik M. Modulation of Fas-dependent apoptosis: a dynamic process controlling both the persistence and death of CD4 regulatory T cells and effector T cells. J Immunol (2002) 169:750–7. doi:10.4049/jimmunol.169.2.750
79. Fritzsching B, Oberle N, Pauly E, Geffers R, Buer J, Poschl J, et al. Naive regulatory T cells: a novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood (2006) 108:3371–8. doi:10.1182/blood-2006-02-005660
80. Rojo JM, Ojeda G, Acosta YY, Montes-Casado M, Criado G, Portoles P. Characteristics of TCR/CD3 complex CD3{varepsilon} chains of regulatory CD4+ T (Treg) lymphocytes: role in Treg differentiation in vitro and impact on Treg in vivo. J Leukoc Biol (2014) 95:441–50. doi:10.1189/jlb.1112584
81. Carson BD, Ziegler SF. Impaired T cell receptor signaling in Foxp3+ CD4 T cells. Ann N Y Acad Sci (2007) 1103:167–78. doi:10.1196/annals.1394.022
82. Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev (2009) 229:294–306. doi:10.1111/j.1600-065X.2009.00776.x
83. Tang Q, Bluestone JA. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb Perspect Med (2013) 3:a01552. doi:10.1101/cshperspect.a015552
84. Putnam AL, Safinia N, Medvec A, Laszkowska M, Wray M, Mintz MA, et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am J Transplant (2013) 13:3010–20. doi:10.1111/ajt.12433
85. Lim DG, Koo SK, Park YH, Kim Y, Kim HM, Park CS, et al. Impact of immunosuppressants on the therapeutic efficacy of in vitro-expanded CD4+CD25+Foxp3+ regulatory T cells in allotransplantation. Transplantation (2010) 89:928–36. doi:10.1097/TP.0b013e3181d3c9d4
Keywords: immunotherapy, tolerance, monoclonal antibodies, depletion, Foxp3+ Tregs, resistance, CD3
Citation: You S (2015) Differential sensitivity of regulatory and effector T cells to cell death: a prerequisite for transplant tolerance. Front. Immunol. 6:242. doi: 10.3389/fimmu.2015.00242
Received: 26 March 2015; Accepted: 06 May 2015;
Published: 19 May 2015
Edited by:
Philippe Saas, Etablissement Français du Sang BFC, FranceReviewed by:
Nuala Mooney, Centre National de la Recherche Scientifique, FranceMarcella Franquesa, Erasmus Medisch Centrum, Netherlands
Copyright: © 2015 You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sylvaine You, INSERM U1151 – Hôpital Necker, Bâtiment Hamburger, 5ème étage, 149 rue de Sèvres, Paris 75015, France, sylvaine.you@inserm.fr
 Sylvaine You
Sylvaine You