- 1Rockefeller Branch, St. Giles Laboratory of Human Genetics of Infectious Diseases, The Rockefeller University, New York, NY, USA
- 2The Center for Stem Cell Biology, Developmental Biology Program, Sloan-Kettering Institute for Cancer Research, New York, NY, USA
- 3Department of Microbiology-Immunology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
- 4Division of Immunology, Boston Children’s Hospital and Harvard Medical School, Boston, MA, USA
- 5Howard Hughes Medical Institute, New York, NY, USA
- 6Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U1163, Necker Hospital for Sick Children, Paris, France
- 7Imagine Institute, Paris Descartes University, Paris, France
- 8Pediatric Hematology-Immunology Unit, Necker Hospital for Sick Children, Paris, France
Herpes simplex virus 1 (HSV-1) is a common virus that can rarely invade the human central nervous system (CNS), causing devastating encephalitis. The permissiveness to HSV-1 of the various relevant cell types of the CNS, neurons, astrocytes, oligodendrocytes, and microglia cells, as well as their response to viral infection, has been extensively studied in humans and other animals. Nevertheless, human CNS cell-based models of anti-HSV-1 immunity are of particular importance, as responses to any given neurotropic virus may differ between humans and other animals. Human CNS neuron cell lines as well as primary human CNS neurons, astrocytes, and microglia cells cultured/isolated from embryos or cadavers, have enabled the study of cell-autonomous anti-HSV-1 immunity in vitro. However, the paucity of biological samples and their lack of purity have hindered progress in the field, which furthermore suffers from the absence of testable primary human oligodendrocytes. Recently, the authors have established a human induced pluripotent stem cells (hiPSCs)-based model of anti-HSV-1 immunity in neurons, oligodendrocyte precursor cells, astrocytes, and neural stem cells, which has widened the scope of possible in vitro studies while permitting in-depth explorations. This mini-review summarizes the available data on human primary and iPSC-derived CNS cells for anti-HSV-1 immunity. The hiPSC-mediated study of anti-viral immunity in both healthy individuals and patients with viral encephalitis will be a powerful tool in dissecting the disease pathogenesis of CNS infections with HSV-1 and other neurotropic viruses.
Introduction
Herpes simplex virus 1 (HSV-1) is a double-stranded DNA virus of the α-herpesvirinae subfamily (1). These viruses are characterized by a short replication cycle and their ability to establish latency in sensory neurons (2). The proportion of individuals infected with HSV-1 worldwide increases with age and can reach up to 90% in the elderly (3, 4). Primary infections by this virus lead to a life-long latency (5) although reactivation of the virus can occur in infected individuals, because of stress or exposure to ultraviolet radiation, leading to anterograde spread of HSV-1 to epithelial cells and formation of herpes labialis. Encephalitis is a rare manifestation of HSV-1 infection and has been reported in an estimated 1 per 250,000 cases per year (1). Approximately one-third of cases are caused by primary infection in children (6). Herpes simplex encephalitis (HSE) is the most common sporadic viral encephalitis in the western countries, accounting for 20% of the 20,000 annual cases in the Unites States (7–9). Most patients show positive PCR results for HSV-1 DNA in their cerebral spinal fluid. Although >70% of patients survive the disease with acyclovir treatment, an inhibitor of viral DNA synthesis (10), the majority of them consequently suffer from epilepsy, mental retardation, and other neurological deficits (11).
Herpes simplex virus 1 reaches the central nervous system (CNS) via cranial nerves such as olfactory and trigeminal neurons (1). During the course of HSE, no viremia is detected in affected patients. Within the brain, destruction of glial and neuronal cells is detectable during the first month of disease (12), suggesting a cytotoxic effect of HSV-1 on both neurons and astroglial cell types. The fact that most HSE patients have intact T- and B-cell responses to HSV-1 and are otherwise healthy (13) suggests that intrinsic immunity to HSV-1 by CNS cell types may be a key in controlling viral spread within the CNS. Viral susceptibility and production of protective cytokines, mainly interferons (IFNs), in the CNS of infected hosts during the course of HSV-1 infection have been studied in vivo using mouse models, and reviewed elsewhere (13–15). Interestingly, induction of IFNs and other cytokines because of viral infection differs between human and mice (16). Hence, human CNS cell-based models of anti-HSV-1 immunity have become increasingly relevant. The disease pathogenesis of HSE has long remained unclear, until the recent findings that inborn error of toll-like receptor 3 (TLR3) immunity may underlie the development of HSE in children with mutations in TLR3, UNC93B1, TRIF, TRAF3, and TBK1 (14). The authors herein review the studies of human CNS anti-HSV-1 immunity based on in vitro models of human cell lines and primary cells, as well as their recent study using human induced pluripotent stem cells (hiPSCs)-derived neuronal cells from patients with HSE-causing TLR3 pathway deficiencies.
Anti-HSV-1 Immunity in Neuron Cell Lines and Primary Neurons
The human embryonic carcinoma cell line NT2 has been used as an in vitro model in studies of CNS neurons anti-HSV-1 immunity. Characterized as an equivalent of CNS neuronal progenitors, NT2 cells are capable of differentiating into neuron-like cells in response to retinoic acid (RA) treatment (17, 18). Differentiation of these progenitor cells is very efficient, giving rise to 99% pure populations of “NT2-N” neurons, which morphologically resemble human primary neurons (19). These cells can be infected by HSV-1 which induces mRNA for IFN-α and IFN-λ1 (20). NT2-N cells also express TLR3 mRNA, which is a receptor capable of recognizing double-stranded RNA (dsRNA) generated during HSV-1 infections (21–23). The synthetic dsRNA polyinosinic-polycytidylic acid [poly(I:C)], used as an agonist for TLR3 (24), induces IFN-α and IFN-β (type I IFNs) as well as IFN-λ1 (type III IFN) expression in NT2-N neurons in a dose-dependent manner (19–21). Similar poly(I:C) responses were observed in BE(2)-C/m cells differentiated on RA treatment to mature neurons (25). Pre-treatment with poly(I:C) reduces HSV-1 replication by 80% in NT2-N neurons, down to similar levels as observed in IFN-α, IFN-β, IFN-λ1, and -λ2 pre-treated cells. Interestingly, HSV-1 infection in NT2-N cells induces IFN-α but not -β expression, although the neurons can respond to this cytokine (21). Overall, these data suggested that human CNS neuronal-like cells harbor a functional TLR3/IFN system involved in anti-HSV-1 immunity.
To investigate the role of the TLR/IFNs pathway against HSV-1 in a physiologically more relevant model, human primary CNS neurons have been cultured/isolated from aborted fetuses’ cortical tissues (19, 20, 26). Neuronal cultures ultimately consist of 80–90% neurons and 10–20% astroglial cell types. TLR2 and TLR3 are expressed throughout the CNS (27), including in human primary neurons (28). Like in NT2-N cells, poly(I:C) can induce elevated levels of IFN-α, IFN-β, and IFN-λ1 in primary neurons. Pre-treatment with IFN-λ1 or -λ2 reduces HSV-1 replication in these cells and induces IRF7 expression, a key transcription factor creating a positive feedback loop for IFN production (29). Globally, these results indicated that the TLR3/IFN pathway is also critical for control of HSV-1 virus infection in primary CNS neurons. It would also be important to look at anti-HSV-1 immunity in human peripheral nervous system (PNS) neurons, as HSV-1 spreads along nerves of the PNS on its way to the CNS leading to HSE, as shown by the presence of late viral proteins in PNS neurons (30). There is, however, no reported cell line capable of generating PNS neurons such as trigeminal neurons. Hence, dorsal root ganglia explants from human fetal tissues have been utilized as in vitro models (31–33). Although HSV-1 can infect PNS neurons, their response to infection was not studied. In addition, trigeminal ganglia (TG) removed from cadavers and latently infected by HSV-1 showed elevated transcription of IFN-γ (34). Further studies will need to address the response of purified human TG neurons to primary infection with HSV-1.
Anti-HSV-1 Immunity in Primary Glial Cells
Central nervous system glial cells include astrocytes, oligodendrocytes, and microglial cells. Within the CNS, astrocytes are the most abundant cell type (35). Culture of fetal tissues in medium containing fetal bovine serum generates >90% pure primary human astrocytes population upon passaging (36, 37). These cells express primarily TLR2 and TLR3, the latter mainly on their plasma membrane (27). In vitro poly(I:C) stimulation induces human astrocytes to express IFN-λ1, -λ2/3 (38), and IFN-β, but not TNF-α (36, 39), a cytokine controlling HSV-1 reactivation rate in mice (40). Numerous other IFN stimulated genes can be upregulated through poly(I:C) stimulation of primary human astrocytes, including IL-6, IRF7, IFIT2, and MX1. These cells are also permissible to HSV-1 infection (41). Recombinant IFN-λ1 and -λ2 can block HSV-1 replication in human astrocytes and induce the expression of endogenous IFN-α through which they exert their anti-viral activity (26). These data suggest that IFN-λ and IFN- α have a role in anti-HSV-1 immunity at least in astrocytes.
Although human primary oligodendrocytes are known to primarily express TLR2 and TLR3 similar to astrocytes (27), no human primary oligodendrocyte culture system exists to date for the study of antiviral immunity. Microglia cells clear the CNS of dead neurons via phagocytosis (42). In contrast to neurons and astrocytes, primary human microglia cells express a wide range of TLRs, but presence of TLR3 proteins was only observed in intracellular vesicles as assessed by immunostaining (27). Furthermore, these cells are not permissive to HSV-1 replication, although immediate early viral proteins are present in infected cells (41). Microglia cells also undergo apoptosis quickly following infection. Interestingly, although primary human microglial cells stimulated by HSV-1 do not upregulate IFN-α and -β expression levels (41), they do display elevated levels of NF-κB activation and TNF-α production (43). TNF-α pre-treatment blocks HSV-1 replication in primary human astrocytes but not in primary human neurons suggesting a cell-type specific impact of this microglia-secreted anti-viral cytokine. The potential role of TNF-α in anti-HSV-1 immunity is highlighted by the fact that treatment with inhibitors of TNF-α was associated with HSE susceptibility in several individuals (44).
Anti-HSV-1 Immunity in hiPSC-Derived Neuronal Cells
In order to study the HSE pathogenesis in patients with known disease-causing genetic etiologies, we have recently established an hiPSC-based in vitro model of HSV-1 infection in CNS-resident cells (45). Advances in the field of stem cell biology has allowed for the reprogramming of human somatic cells to a pluripotent state (46). Human pluripotent stem cells have two key properties: they can differentiate into any cells of the three germ layers (ectoderm, endoderm, mesoderm) and self-renew, potentially replicating indefinitely in culture. Hence, we have reprogrammed dermal fibroblasts from HSE patients to iPSCs and tested anti-HSV-1 immunity in CNS-resident cells derived from these iPSCs. After differentiating healthy control and patient-specific iPSCs toward highly enriched populations of forebrain CNS neurons, astrocytes, oligodendrocyte progenitor cells (OPCs), and neural stem cells (NSCs), we have assessed viral susceptibility and IFNs production in each cell type. Poly(I:C) treatment induced elevated levels of IFN-λ1 mRNA in OPCs, and IFN-β as well as -λ1 in neurons and astrocytes from healthy donors. Pre-treatment of poly(I:C) as well as of recombinant IFN-α and -β inhibited HSV-1 replication in these cell types.
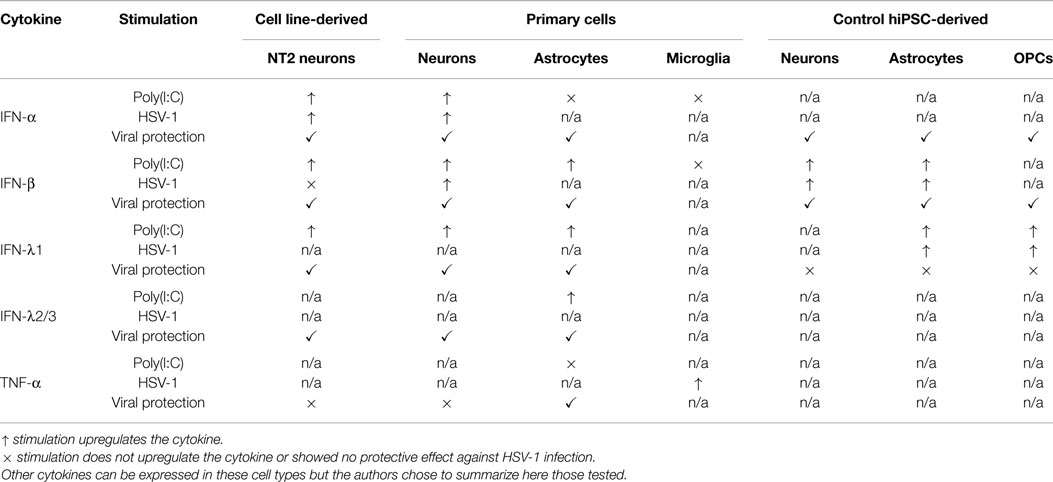
We then focused on cells from two genetically defined HSE patients, harboring an autosomal recessive complete TLR3 (47) or UNC-93B (48) deficiency. UNC-93B is a key factor within the TLR3/IFN pathway required to activate downstream molecular signals (49). Susceptibility to HSV-1 was shown to be cell type and genotype dependent. Indeed, healthy neurons and OPCs could control viral replication, whereas UNC-93B- or TLR3-deficient cells could not. This cellular phenotype was related to the impaired production of IFN-β or -λ1 in response to HSV-1 or poly(I:C) in the same cells. On the other hand, no differences were observed between control and patients’ NSCs and astrocytes. Hence, these in vitro experiments demonstrated that CNS neurons and OPCs control HSV-1 infection intrinsically in a TLR3-dependent manner, although the in vivo protective role of astrocytes and NSCs cannot be excluded. Overall, these iPSC-based experiments are in agreement with studies based on cell lines and primary sources, suggesting that TLR3 and IFN responses play important roles in defense against HSV-1 infection in CNS cells (Table 1). HSE is, to our knowledge, the first example of hiPSC-mediated modeling of an inborn error of immunity, demonstrating a disease-relevant immunological cellular phenotype in disease-relevant CNS cell types.
Conclusion
Human neuronal cell lines as well as primary cells have allowed for the study of anti-HSV-1 immunity of the human CNS. Human cell line-derived and primary CNS neurons express TLR3, display elevated levels of IFN-α, -β, and -λ1 on poly(I:C) treatment, and are permissible to HSV-1 infection. Interestingly, although poly(I:C), IFN-α and -β can block viral replication in primary, cell line-derived and control iPSC-derived human neurons, IFN-λ1 and -λ2 could only do so on primary and cell line-derived neurons. Like oligodendrocytes, human primary astrocytes express TLR3 and were found to express high levels of IFN-λ1, -λ2/3, as well as IFN-β on poly(I:C) stimulation. Primary astrocytes are permissible to HSV-1 infection and are protected from viral replication by IFN-λ1 and -λ2, which was not the case for control iPSC-derived astrocytes. Interestingly, we were able to compare directly the susceptibility to HSV-1 of control hiPSC-derived CNS neurons, OPCs, and astrocytes and demonstrated that neurons and OPCs have an intrinsic resistance to viral replication. This control of HSV-1 replication was lost in neurons and OPCs derived from patient-specific iPSCs with inborn errors of TLR3 pathway immunity. Overall, these data suggest a key role for the TLR3–IFN pathway in cell-autonomous HSV-1 immunity in neurons and OPCs, and a potential role for this pathway in astrocytes, although this may not be the case in microglia cells. Indeed, these cells do not express TLR3 on their surface, are not permissible to HSV-1 replication, and do not upregulate IFN-α and -β on HSV-1 infection, although they do express the cytokine TNF-α, which can block HSV-1 replication in human astrocytes but not in neurons.
Obviously useful, experiments in primary human cells are however limited because of the difficulty to access cell sources, embryos, and cadavers. Furthermore, some cells are either difficult to purify, such as TG neurons, or simply not possible to isolate, such as oligodendrocytes. The use of hiPSCs can alleviate these issues by providing an unlimited source of neuronal cell types for disease modeling (50). Within the last 6 years, at least 190 studies have described iPSC-based human disease models (51). Disease modeling using hiPSCs has benefited from key technical developments including the establishment of differentiation protocols mimicking early embryonic development steps to generate CNS (52, 53) and PNS (54, 55) cells. Furthermore, the advent of non-integrating methods of reprogramming (56) and new technological breakthroughs in the field of gene editing (57, 58) allows for the precise relationship assessment between germline mutations and disease phenotypes. However, some tools are still missing to completely survey the response of all neuronal cells types infected by HSV-1 during the course of HSE. Indeed, to date, no protocol exists for the generation of microglia cells from hiPSCs. Hence, while a work in progress, in vitro hiPSC-based model of HSV-1 infection in CNS cells holds tremendous potential for our understanding of the molecular and cellular basis of cell-autonomous, non-hematopoietic, anti-HSV-1, intrinsic immunity in the CNS.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the past and present members of both branches of the Laboratory of Human Genetics of Infectious Diseases, and our collaborators on HSE, for the human studies discussed in this article, particularly, Itai Pessach. They also thank their colleagues for helpful discussions and critical reading of this manuscript. The authors warmly thank their patients, their families, and physicians. The work was funded by the National Center for Translational Sciences (NCATS) of the National Institutes of Health (NIH) (8UL1TR000043), National Institute of Allergy and Infectious Diseases (R01AI088364), National Institute of Neurological Disorders and Stroke (R01NS072381), the Rockefeller University, the St. Giles Foundation, the French National Research Agency (ANR), Institut National de la Santé et de la Recherche Médicale (INSERM), Paris Descartes University, the March of Dimes, the Thrasher Research Fund, and the New York Stem Cell Foundation (NYSCF). Fabien Gilbert Lafaille was a NYSCF Druckenmiller Fellow.
Author Contributions
FL and SYZ designed the manuscript. FL wrote the initial draft. JLC and SYZ edited the manuscript. All authors read the manuscript and provided comments. LN and LS participated in the design and data interpretation of our original study. LN, LS and GS edited this manuscript. GS gave critical original input on the review’s topic. LN, LS and GS approved the final version. LN, LS and GS acknowledge their accountability in the review’s content.
References
1. Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res (2006) 71(2–3):141–8. doi:10.1016/j.antiviral.2006.04.002
2. Mori I. Herpes simplex virus and varicella-zoster virus: why do these human alpha herpes viruses behave so differently from one another? Rev Med Virol (2005) 15(6):393–406. doi:10.1002/rmv.478
3. Arduino PG. Herpes simplex virus type 1 infection: overview on relevant clinico-pathological features. J Oral Pathol Med (2008) 37(2):107–21. doi:10.1111/j.1600-0714.2007.00586.x
4. Smith JS. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis (2002) 186:S3–S28. doi:10.1086/343739
5. Conrady CD. Herpes simplex type I (HSV-1) infection of the nervous system: is an immune response a good thing? J Neuroimmunol (2010) 220(1–2):1–9. doi:10.1016/j.jneuroim.2009.09.013
6. Abel L. Age-dependent Mendelian predisposition to herpes simplex virus type 1 encephalitis in childhood. J Pediatr (2010) 157(4):623–9. doi:10.1016/j.jpeds.2010.04.020
7. Whitley RJ. Herpes simplex: encephalitis children and adolescents. Semin Pediatr Infect Dis (2005) 16(1):17–23. doi:10.1053/j.spid.2004.09.007
8. Levitz R. Herpes simplex encephalitis: a review. Heart Lung (1998) 27(3):209–12. doi:10.1016/S0147-9563(98)90009-7
9. Whitley RJ. Viral encephalitis. N Engl J Med (1990) 323(4):242–50. doi:10.1056/NEJM199007263230406
10. McGrath N. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry (1997) 63(3):321–26. doi:10.1136/jnnp.63.3.321
12. Studahl M. Difference in pathogenesis between herpes simplex virus type 1 encephalitis and tick-borne encephalitis demonstrated by means of cerebrospinal fluid markers of glial and neuronal destruction. J Neurol (2000) 247(8):636–42. doi:10.1007/s004150070134
13. Sancho-Shimizu V. Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans. Curr Opin Allergy Clin Immunol (2007) 7(6):495–505. doi:10.1097/ACI.ob013e3282f151d2
14. Zhang SY. TLR3 immunity to infection in mice and humans. Curr Opin Immunol (2013) 25(1):19–33. doi:10.1016/j.coi.2012.11.001
15. Kollias CM. Animal models of herpes simplex virus immunity and pathogenesis. J Neurovirol (2014) 21(1):8–23. doi:10.1007/s13365-014-0302
16. Mestas J. Of mice and not men: differences between mouse and human immunology. J Immunol (2004) 172(5):2731–8. doi:10.4049/jimmunol.172.5.2731
17. Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol (1984) 103(2):285–93. doi:10.1016/0012-1606(84)90316-6
18. Trojanowski JQ. Transfectable and transplantable postmitotic human neurons: a potential “platform” for gene therapy of nervous system diseases. Exp Neurol (1997) 144(1):92–7. doi:10.1006/exnr.1996.6393
19. Zhou Y. Activation of toll-like receptors inhibits herpes simplex virus-1 infection of human neuronal cells. J Neurosci Res (2009) 87(13):2916–25. doi:10.1002/jnr.22110
20. Zhou L. Activation of TLR3 induces IFN-λ expression in human neuronal cells. Neuroscience (2009) 159(2):629–37. doi:10.1371/journal.pone.0083149
21. Préhaud C. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol (2005) 79(20):12893–904. doi:10.1128/JVI.79.20.12893-12904.2005
22. Vercammen E. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin Microbiol Rev (2008) 21(1):13–25. doi:10.1128/CMR.00022-07
23. Suh H-S. Toll-like receptors in CNS viral infections. Curr Top Microbiol Immunol (2009) 336:63–81. doi:10.1007/978-3-642-00549-7_4
24. Alexopoulou L. Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature (2001) 413(6857):732–8. doi:10.1038/35099560
25. Peltier DC. Human neuronal cells possess functional cytoplasmic and toll-like receptor-mediated innate immune pathways influenced by phosphatidylinositol-3 kinase signaling. J Immunol (2010) 184(12):7010–21. doi:10.4049/jimmunol.0904133
26. Li J. Interferon lambda inhibits herpes simplex virus type I infection of human astrocytes and neurons. Glia (2011) 59(1):58–67. doi:10.1002/glia.21076
27. Bsibsi M. Broad expression of toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol (2002) 61(11):1013–21.
28. Jackson AC. Expression of toll-like receptor 3 in the human cerebellar cortex in rabies, herpes simplex encephalitis, and other neurological diseases. J Neurovirol (2006) 12(3):229–34. doi:10.1080/13550280600848399
29. Marie I. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J (1998) 17(22):6660–9. doi:10.1093/emboj/17.22.6660
30. Held K. Control of HSV-1 latency in human trigeminal ganglia – current overview. J Neurovirol (2011) 17(6):518–27. doi:10.1007/s13365-011-0063-0
31. Penfold MET. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc Natl Acad Sci U S A (1994) 91(14):6529–33. doi:10.1073/pnas.91.14.6529
32. Holland DJ. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J Virol (1999) 73(10):8503–11.
33. Miranda-Saksena M. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J Virol (2000) 74(4):1827–39. doi:10.1128/JVI.74.4.1827-1839.2000
34. Theil D. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol (2003) 163(6):2179–84. doi:10.1016/S0002-9440(10)63575-4
35. Volterra A. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci (2005) 6(8):626–40. doi:10.1038/nrn1722
36. Jack CS. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol (2005) 175(7):4320–30. doi:10.4049/jimmunol.175.7.4320
37. Lokensgard JR. Glial cell responses to herpesvirus infections: role in defense and immunopathogenesis. J Infect Dis (2002) 186:S171–9. doi:10.1086/344272
38. Li J. Induction of IFN-lambda contributes to TLR3-mediated HSV-1 inhibition in astrocytes. J Neurosci Res (2012) 90(2):399–406. doi:10.1002/jnr.22758
39. Rivieccio MA. TLR3 ligation activates an antiviral response in human fetal astrocytes: a role for Viperin/cig5. J Immunol (2006) 177:7:4735–41. doi:10.4049/jimmunol.177.7.4735
40. Minami M. Role of IFN-gamma and tumor necrosis factor-alpha in herpes simplex virus type 1 infection. J Interferon Cytokine Res (2002) 22(6):671–6. doi:10.1089/10799900260100150
41. Lokensgard JR. Robust expression of TNF-, IL-1, RANTES, and IP-10 by human microglial cells during non-productive infection with herpes simplex virus. J Neurovirol (2001) 7(3):208–19.
42. Brown GC. Microglial phagocytosis of live neurons. Nat Rev Neurosci (2014) 15(4):209–16. doi:10.1038/nrn3710
43. Marques CP. Interleukin-10 attenuates production of HSV-induced inflammatory mediators by human microglia. Glia (2004) 47(4):358–66. doi:10.1002/glia.20045
44. Bradford R. Herpes simplex encephalitis during treatment with tumor necrosis factor-alpha inhibitors. Clin Infect Dis (2009) 49(6):924–7. doi:10.1086/605498
45. Lafaille FG. Impaired intrinsic immunity to HSV-1 in human iPS-derived TLR3-deficient CNS cells. Nature (2012) 491(7426):769–73. doi:10.1038/nature11583
46. Takahashi K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell (2007) 131(5):861–72. doi:10.1016/j.cell.2007.11.019
47. Zhang SY. TLR3 deficiency in patients with herpes simplex encephalitis. Science (2007) 317(5844):1522–7. doi:10.1126/science.1139522
48. Casrouge A. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science (2006) 314(5797):309–12. doi:10.1126/science.1128346
49. Sancho-Shimizu V. Inborn errors of anti-viral interferon immunity in humans. Curr Opin Virol (2011) 1(6):487–96. doi:10.1016/j.coviro.2011.10.016
50. Weinacht KG. The role of induced pluripotent stem cells in research and therapy of primary immunodeficiencies. Curr Opin Immunol (2012) 24(5):617–24. doi:10.1016/j.coi.2012.07.001
51. Inoue H. iPS cells: a game changer for future medicine. EMBO J (2014) 33(5):409–17. doi:10.1002/embj.201387098
52. Chambers S. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol (2009) 27(6):275–80. doi:10.1038/nbt.1529
53. Kriks S. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature (2011) 480:7378:547–51. doi:10.1038/nature10648
54. Dincer Z. Specification of functional cranial placode derivatives from human pluripotent stem cells. Cell Rep (2013) 5(5):1387–1402. doi:10.1016/j.celrep.2013.10.048
55. Zeltner N. Feeder-free derivation of neural crest progenitor cells from human pluripotent stem cells. J Vis Exp (2014) 87. doi:10.3791/51609
56. Robinson DA. The promise of induced pluripotent stem cells in research and therapy. Nature (2012) 481(7381):295–305. doi:10.1038/nature10761
57. Hockemeyer D. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol (2011) 29(8):731–4. doi:10.1038/nbt.1927
Keywords: HSV-1, CNS, disease modeling, antiviral immunity, hiPSCs, HSE, interferons, TLR3
Citation: Lafaille FG, Ciancanelli MJ, Studer L, Smith G, Notarangelo L, Casanova J-L and Zhang S-Y (2015) Deciphering human cell-autonomous anti-HSV-1 immunity in the central nervous system. Front. Immunol. 6:208. doi: 10.3389/fimmu.2015.00208
Received: 03 March 2015; Accepted: 15 April 2015;
Published: 08 May 2015
Edited by:
Mirjam Van Der Burg, Erasmus University Medical Center, NetherlandsReviewed by:
Elham Hossny, Ain Shams University, EgyptHirokazu Kanegane, Tokyo Medical and Dental University, Japan
Copyright: © 2015 Lafaille, Ciancanelli, Studer, Smith, Notarangelo, Casanova and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabien G. Lafaille, 1230 York Avenue, New York, NY, USA,ZmxhZmFpbGxlQHJvY2tlZmVsbGVyLmVkdQ==
 Fabien G. Lafaille
Fabien G. Lafaille Michael J. Ciancanelli
Michael J. Ciancanelli Lorenz Studer2
Lorenz Studer2 Luigi Notarangelo
Luigi Notarangelo Jean-Laurent Casanova
Jean-Laurent Casanova Shen-Ying Zhang
Shen-Ying Zhang