
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 16 December 2014
Sec. T Cell Biology
Volume 5 - 2014 | https://doi.org/10.3389/fimmu.2014.00630
This article is part of the Research Topic CD4+ T cell differentiation in infection: amendments to the Th1/Th2 axiom View all 11 articles
 Jens Geginat*
Jens Geginat* Moira Paroni
Moira Paroni Stefano Maglie
Stefano Maglie Johanna Sophie Alfen
Johanna Sophie Alfen Ilko Kastirr
Ilko Kastirr Paola Gruarin
Paola Gruarin Marco De Simone
Marco De Simone Massimiliano Pagani
Massimiliano Pagani Sergio Abrignani*
Sergio Abrignani*Human beings are exposed to a variety of different pathogens, which induce tailored immune responses and consequently generate highly diverse populations of pathogen-specific T cells. CD4+ T cells have a central role in adaptive immunity, since they provide essential help for both cytotoxic T cell- and antibody-mediated responses. In addition, CD4+ regulatory T cells are required to maintain self-tolerance and to inhibit immune responses that could damage the host. Initially, two subsets of CD4+ helper T cells were identified that secrete characteristic effector cytokines and mediate responses against different types of pathogens, i.e., IFN-γ secreting Th1 cells that fight intracellular pathogens, and IL-4 producing Th2 cells that target extracellular parasites. It is now well established that this dichotomy is insufficient to describe the complexity of CD4+ T cell differentiation, and in particular the human CD4 compartment contains a myriad of T cell subsets with characteristic capacities to produce cytokines and to home to involved tissues. Moreover, it has become increasingly clear that these T cell subsets are not all terminally differentiated cells, but that the majority is plastic and that in particular central memory T cells can acquire different properties and functions in secondary immune responses. In addition, there is compelling evidence that helper T cells can acquire regulatory functions upon chronic stimulation in inflamed tissues. The plasticity of antigen-experienced human T cell subsets is highly relevant for translational medicine, since it opens new perspectives for immune-modulatory therapies for chronic infections, autoimmune diseases, and cancer.
Human CD4+ T cells are critical regulators of the immune system, as drastically demonstrated by HIV-infected individuals that develop susceptibility to opportunistic infections and cancer when virus-dependent depletion reduces CD4+ T cell counts below critical thresholds (1). CD4+ T cells are very heterogeneous in human adults, because they have been generated in response to a high number of different pathogens and belong to a progressively increasing number of different subsets with specialized functions (2). Helper T cell subsets are defined by the production of cytokines and/or the expression of characteristic lineage-defining transcription factors (Table 1). Five principal subsets or lineages of CD4+ T cells have been identified so far: T helper (Th)1, Th2, and Th17 cells that target specific classes of pathogens (3–5), regulatory T cells that are required to maintain self-tolerance (6) and follicular helper T cells (TFH) that provide help to B cells for antibody production (7). Heterogeneity is generated upon T cell priming, since naïve T cells have stem-cell-like properties and can differentiate into virtually all different types of effector, memory, or regulatory cells (Table 1). Antigen-experienced T cells are less flexible, but many subsets retain some plasticity and can acquire additional cytokine producing capacities upon antigenic re-stimulation, while others appear to be terminally differentiated (8). In some cases, T cell functions can even completely change from helper to regulatory functions (9) or vice versa (10). A caveat of these findings in particular in humans is the enormous heterogeneity of T cells (2), making it difficult to exclude a selective outgrowth of rare pre-existing precursor cells. Several excellent reviews on the plasticity of mouse T cells have been published in recent years (11–13), while human T cell plasticity is less understood, but highly relevant for new therapeutic strategies in immune-mediated diseases (14).
Seminal studies have established that CD4+ T cells can differentiate into two types of effector cells with different cytokine producing capacities and functions in humans and mice (3, 4). Uncommitted naïve T cells that are activated by specialized dendritic cells that produce IL-12 (15, 16) acquire IFN-γ producing capacities. These so-called T helper 1 cells (Th1) are induced upon infections with intracellular pathogens like bacteria or viruses and can activate macrophages to destroy intracellular bacteria. In contrast, naïve T cells primed in the presence of IL-4 undergo a different fate and start to produce IL-4, IL-5, IL-10, and IL-13, but not IFN-γ. These Th2 cells are required to fight extracellular parasites like helminths, but since they induce IgE from B cells they are also involved in allergies (17). Importantly, it was shown that Th1 versus Th2 differentiation was a crucial decision to resist infections, since BL/6 mice that mount a Th1 response to leishmania were protected, while BALB/c mice that instead induce a Th2 response were highly susceptible (18). The characteristic cytokines produced by Th1 and Th2 cells, IFN-γ, and IL-4, were further shown to inhibit the differentiation to the opposite differentiation lineage and thus reinforced the original fate decision. The capacity to produce either IFN-γ or IL-4 is stably imprinted by epigenetic modifications like DNA methylation and histone acetylations, ensuring that the cytokine profile of T helper cells is preserved upon cellular division independently of the inducing polarizing cues (19–21). Moreover, the generation of Th1 and Th2 cells was shown to depend on the “master” transcription factors T-bet and GATA-3, which induced not only the characteristic cytokines of Th1 and Th2 cells, but also inhibited the differentiation to the alternative lineage. Based on this evidence, it was initially assumed that the differentiation to Th1 and Th2 cells are mutually exclusive and irreversible fate decisions.
Early studies with human T cell clones showed that IFN-γ and IL-4 production were not necessarily two exclusive features, since some T cells co-produced IFN-γ and IL-4 (22). Notably, human Th1 memory cells are responsive to IL-4 stimulation, and acquire IL-4 producing capacities upon TCR stimulation in the presence of IL-4 without losing IFN-γ production in vitro (23).
In addition, some T cells in human blood co-express the Th1 and Th2 markers CXCR3 and CCR4 (24) or CRTh2 as well as the lineage-defining transcription factors GATA-3 and T-bet (25). Consistently, it was shown in mice that histones of these transcription factor genes had both repressive and permissive marks in opposing T cell lineages (13, 26). In mice, in vivo primed Th2 cells can acquire IFN-γ producing capacities in addition to IL-4 in response to IFN and IL-12 (27), while human blood Th2 cells seem to be less plastic (23). Moreover, the pathogens and the physiological conditions that induce Th1/2 cells in humans and their role in immune responses remain to be fully defined (25).
Another early finding that did not fit well into the fixed Th1/Th2 paradigma was the fact that IL-12 could induce IL-10 in Th1 cell clones (28). IL-10 has potent anti-inflammatory functions and inhibits maturation and T cell stimulatory capacities of APC (29), thus the concomitant expression of both IFN-γ and IL-10 by T cells was unexpected (30). Later it was shown that IL-10 produced by T-bet+ Th1 cells was required to inhibit lethal immunopathology upon infections with intracellular parasites (31, 32), indicating that IL-10-producing Th1 cells prevent overshooting immune responses and the resulting tissue damage in a negative feedback loop (9). Interestingly, although these IL-10 producing Th1 cells inhibited IL-12 production by APC, they were also able to restrict parasite growth via IFN-γ (31). However, IFN-γ has also been shown to have some negative effects on T cell responses (33, 34), providing a possible alternative explanation for IFN-γ production by regulatory T cells. Importantly, IFN-γ/IL-10 co-producing T cells with regulatory functions are present at low frequencies in peripheral blood of healthy donors and respond selectively to persistent pathogens (35), suggesting that similar to their mouse counterparts they inhibit overshooting immune responses in chronic infections. Thus, Th1 cells can switch from pro-inflammatory effector cells to IL-10 producing type 1 regulatory (Tr1)-like T cells (36, 37), and this switch is necessary to maintain the integrity of infected tissues in some infections. Complement receptor stimulation (38), production of IL-27 (39) or IL-12 (28) by myeloid cells (40), or generation of AHR ligands (41) are possible inductive cues, but also chronic or repetitive antigenic stimulation seems to be required to induce IL-10 production in Th1 cells (35, 42, 43). Interestingly, a recent paper suggests that IL-10/IFN-γ co-producing T cells can also be generated from Th17 cells under the influence of IL-12 or IL-27 in mice (44). If IFN-γ/IL-10 co-producing regulatory T cells are stably maintained or are short-lived, if they progressively lose IFN-γ production upon chronic stimulation or revert to Th1 cells upon pathogen clearance is currently unclear (Figure 1).
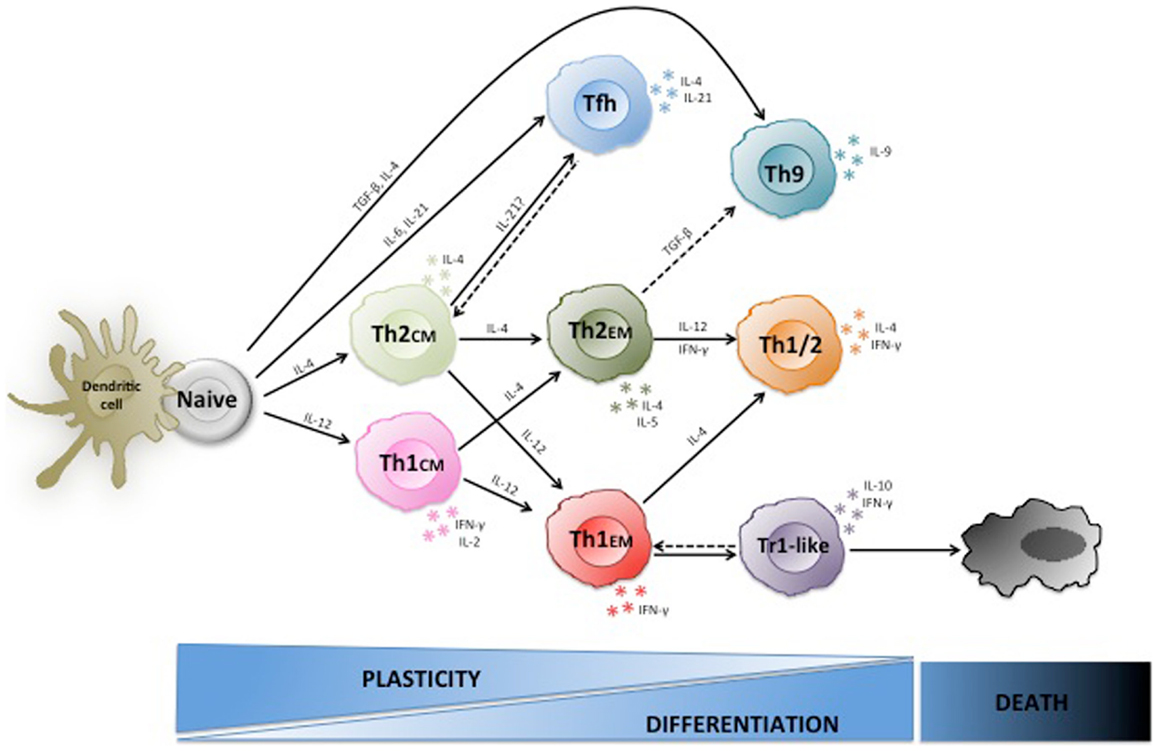
Figure 1. Plasticity of human Th1 and Th2 cells. Naive CD4+ T cells are stem-cell-like cells that under the influence of different cytokines can differentiate to various types of effector cells including Th1, Th2, Th9, and TFH cells. Th1 and Th2 central memory cells are arrested at an early stage of differentiation, are highly plastic and some can still switch lineage. Conversely, effector memory cells are more differentiated, less plastic, and rather become polyfunctional. Moreover, Th1 effector cells can acquire IL-10 producing capacities and regulatory functions in chronically inflammed tissues.
More recently, additional plasticity of Th2 cells was documented. Thus it was shown that TFH cells were derived from Th2 precursor cells in mouse models of helminth infections (45). This finding is relevant for Th2 stability, because TFH cells are professional B helper T cells that secrete IL-21 in B cell follicles, express the transcriptional repressor BCL-6 and are thus distinct from conventional Th1 and Th2 cells (7, 46, 47). Also in human tonsils a fraction of TFH cells express the Th2 marker CRTH2 and produce IL-4 (48). The relationship of Th1 cells with TFH cells is less clear in particular in humans (49, 50). Some murine TFH cells produce IFN-γ (51), which induces IgG2a production by B cells (52), but TFH cells from human tonsils lack IFN-γ production.
Mouse Th2 cells can also switch from IL-4 to IL-9 production upon stimulation with TGF-β (53). These Th9 cells express the PU.1 transcription factor (54) and can also be directly induced from naïve and memory T cells upon stimulation with TGF-β and IL-4 in humans and mice (55, 56). Th9 cells can have a pro-inflammatory role in allergic asthma (57) and respond to helminth antigens and allergens in humans (58, 59). However, IL-9 induction by TGF-β is not restricted to Th2 cells (60).
Collectively, these findings indicate that both Th1 and Th2 cells can acquire different cytokine producing capacities and functional properties upon antigenic re-stimulation under the influence of cytokines, and are thus much more flexible than originally thought (Figure 1).
CD25+ regulatory T cells are required to maintain self-tolerance. They were first identified in mice (61) and later in humans (62), and the Foxp3 transcription factor was shown to be required for their generation and function (63, 64). Consistently, IPEX patients, who suffer from a devastating autoimmune disease, were found to have mutations in the Foxp3 gene (65). Although so-called natural or thymic Foxp3+ Tregs acquire regulatory lineage commitment already upon maturation in the thymus (66), adaptive, or peripheral Foxp3+ Tregs can be induced from mature CD4+ helper T cells in the periphery under the influence of TGF-β (67, 68). The transcription factor Helios was proposed to distinguish between these two subsets of natural and induced Foxp3+ Treg, but this concept was not confirmed by others (69–71). In humans, CD45RA+CD25+Foxp3+ cells represent a population of bona fide “naïve” and thus thymus-derived Tregs, while CD45RA−Tregs are a mixed population that contain antigen-experienced Tregs of both thymic and peripheral origin (72). The stability of Foxp3+ Tregs is debated (73). Lineage tracing of Foxp3+ T cells in mice has lead to conflicting interpretations, since in several studies only very small fractions of Foxp3+ Tregs were found to lose Foxp3 and regulatory functions in vivo (74). In humans, CD45RA+ but not CD45RA− Tregs could be stably expanded in vitro (72, 75), suggesting different stabilities of thymic and peripheric Tregs. However, since human Tregs have to be purified according to surface marker expression, it is difficult to exclude a selective outgrowth of Foxp3− cells or of activated effector T cells that have transiently up-regulated Foxp3 upon stimulation (73).
The functional specialization of Foxp3+ Treg is shaped by the tissue microenvironment (76), and the induction of transcription factors characteristic for helper T cell lineages in mice allows Tregs to suppress the corresponding T helper cell responses (74). Thus, STAT3 in Tregs is required to suppress Th17 cells (77), IRF4 to control Th2 responses (78) while Tregs that regulate TFH cells and antibody responses express BCL-6 (79, 80). Foxp3+ Tregs also acquire T-bet and IFN-γ producing capacities upon stimulation with IL-12, and these Th1regs might be specialized to suppress Th1 responses (14, 74). Tregs also inhibit anti-tumor CTL responses (81), and interestingly they can acquire cytotoxic properties in tumor-draining lymph nodes in mice (82) and in vitro in humans (83), and tumor-infiltrating Tregs are consequently cytotoxic (84). Similar to helper T cells, Tregs that secrete different types of effector cytokines can be identified according to chemokine receptor expression (2), and these Treg subsets might specifically suppress different types of immune responses (85). Human Foxp3+ T cells that produce IL-17 or IFN-γ can be isolated (86, 87), but while IL-17 producing Treg cells were normally suppressive (88), IFN-γ producing Tregs had reduced suppressive functions (87). The conditions that induce human Foxp3+ Tregs to secrete different effector cytokines and the role of these cells in infections, cancer, and autoimmune diseases remain to be fully established.
The discovery of IL-17 producing helper T cells (Th17) in mice (89, 90) and humans (91) and their relative instability (11, 92) has led to a profound re-evaluation of the concept of two terminally differentiated helper T cell subsets. The fact that human CD4+ T cells produce IL-17 was known for a long time (93). However, it took a decade to realize that these cells represented an independent differentiation lineage (89, 90), which have unique differentiation requirements and express the lineage-defining transcription factor ROR-γt in mice and RORC2 in humans (94, 95). Th17 cells are important to fight extracellular bacteria and fungi, since patients that lack Th17 cells have uncontrolled infections with Candida albicans (C. albicans) and Staphylococcus aureus (96). The discovery of Th17 cells has been complicated by the fact that T cell differentiation to Th1 and Th17 cells relies on shared components of cytokines and their receptors. Thus, it was known that IL-12p40 and IL-12Rβ1 hetero-dimerize with respectively IL-12p35 and IL-12Rβ2 to induce Th1 cells, but later it was realized that they can also associate with respectively IL-23p19 and the IL-23R to promote Th17 responses (97). The IL-23/IL-23R pathway is involved in many different autoimmune diseases (98–100) and IL-23-induced Th17 cells are thought play a prominent pathogenic role (101–104). Conversely, the contribution of Th1 cells, which were initially thought to drive autoimmune diseases, is now debated. The requirements for Th17 differentiation are more complex than for Th1 and Th2 cells, because IL-17 production in CD4+ T cells can be induced by different cytokine combinations. Initially, TGF-β plus IL-6 was identified in mice (105), while IL-1β, IL-6, and/or IL-23 were proposed in humans (106, 107). The de novo Th17 differentiation is very inefficient in humans, and therefore it was suggested that only a cocktail with all four cytokines induces significant Th17 differentiation (108). Although the role of TGF-β in human Th17 differentiation has been a subject of debate (109), it was shown in mice that TGF-β induces ROR-γt, while pro-inflammatory cytokines are required to inhibit TGF-β-induced Foxp3 expression and thus Treg generation (110). The presence of CD4+ T cells co-expressing Foxp3, RORC2, and/or IL-17 in humans is consistent with a role for TGF-β in human Th17 and Treg development (86, 88). An alternative explanation for the positive role of TGF-β in Th17 differentiation is that TGF-β indirectly favors Th17 cell differentiation by inhibiting Th1 cell development (111). Indeed, in the absence of TGF-β1 (106, 107, 112), or in the presence of TGF-β3 in mice (113), pathogenic Th17 cells that co-produce IL-17 and IFN-γ are generated. These Th1/17 cells co-express RORC2 and T-bet, are enriched in autoimmune patients and are specific for both Th1 and Th17-inducing pathogens (114, 115).
In vitro stability experiments and fate reporter mice suggested that Th17 cells are partially unstable and can switch completely from IL-17 producing Th17 to IFN-γ producing Th1 cells in chronic immune responses (92, 116). IL-12 can induce this Th17-to-Th1 switch (117), and CD161 was proposed as a marker that distinguishes these ex-Th17 cells from conventional Th1 cells in humans (118). However, ex vivo isolated human Th17 cells exhibited stable epigenetic marks at cytokine and transcription factor loci (119), suggesting that in vivo generated human Th17 cells are not necessarily unstable. Finally, also a very rare population of human T cells that co-produces IL-17 and IL-4 was identified (120). These Th2/17 cells were proposed to be highly pro-inflammatory in allergic asthma, but their role in immune responses against pathogens remains to be understood.
Th17 cells are highly heterogeneous and produce several effector cytokines besides IL-17. IL-22, a cytokine that promotes epithelial proliferation and barrier function (121), is produced by some Th17 cells (122, 123), and IL-22 and IL-17 co-operate to control gram-negative bacteria in the lung (124). However, a subset of human skin-homing IL-22 producing cells was identified that were distinct from Th17 cells (125, 126). Indeed, in contrast to IL-17, IL-22 is inhibited by TGF-β (127) and thus how Th17 cells acquire IL-22 producing capacities and if they can even switch from IL-17 to IL-22 production is unclear. Some Th17 and Th22 cells also produce IL-26, a pro-inflammatory cytokine that is not expressed in mice (128) and that also acts selectively on non-hematopoietic cells. A particular relevant cytokine in the pathogenesis of experimental autoimmunity is GM-CSF, which is induced by IL-1β, IL-23, and ROR-γt in mice (102, 129). Conversely, GM-CSF is inhibited by IL-1β and IL-23 in humans, and is produced by both Th1 and Th17 cells (130, 131).
Th17 cells also produce high levels of IL-21. IL-6 induces IL-21 in naive T cells upon priming (132), and IL-21 can induce its own expression (133) and promotes Th17 differentiation in an autocrine manner (131, 134–136). Importantly however, IL-21 inhibits GM-CSF and IFN-γ production and promotes instead IL-10 secretion in developing Th17 cells. Consequently, IL-21 promotes the generation of conventional (137) or regulatory Th17 cells (138), but inhibits the generation of pathogenic Th1/17 cells (131). Finally, a subset of skin-homing T cells produces IL-9 and responds to C. albicans (139). Some of these cells co-produce IL-9 and IL-17 (60), while others appear to represent Th9 cells. IL-9 production seems however to be transient, suggesting that these skin-homing Th9 cells are largely unstable (139).
In summary, the current knowledge indicates that human Th17 cells are highly heterogeneous and partially unstable (Figure 2), and much remains to be learned on the role of different Th17 subsets in immune-mediated diseases.
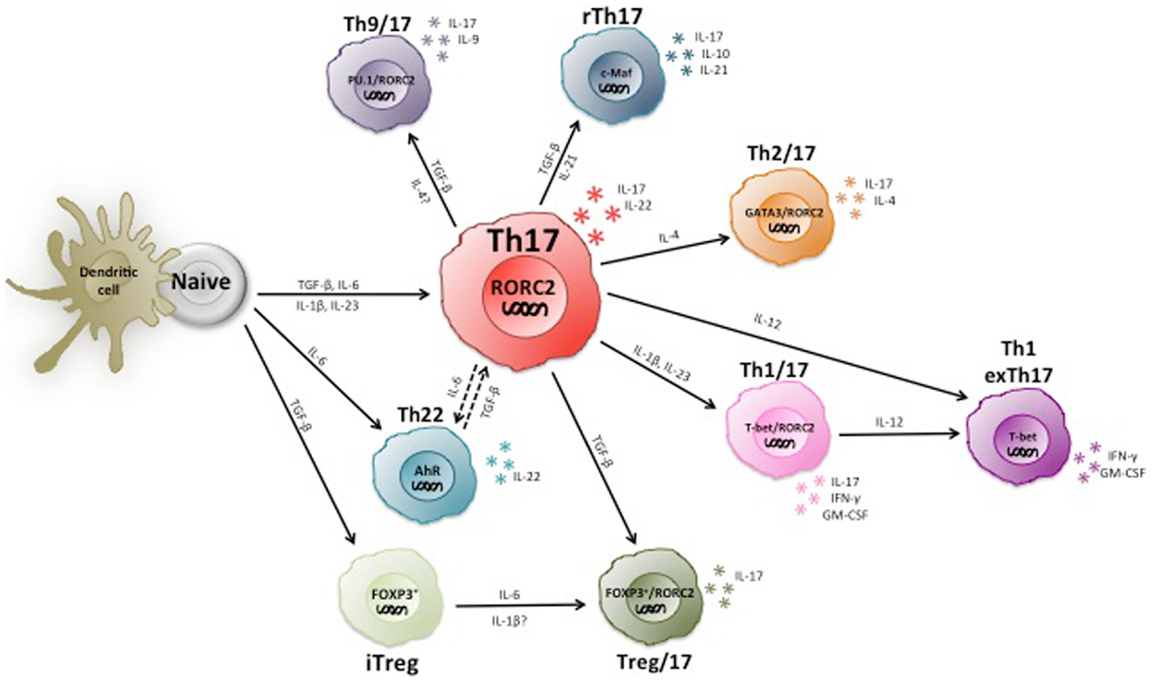
Figure 2. Heterogeneity and plasticity of human Th17 cells. Th17 cells are highly heterogeneous and produce various types of other cytokines in addition to IL-17, including the Th1 and Th2 marker cytokines IFN-γ and IL-4. Some IL-17 producing T cells express Foxp3 and/or IL-10 and are suppressive. Moreover, Th17 cells are partially unstable and can become Th1 cells upon chronic inflammation.
The complex regulation of T helper subsets by cytokines raises the questions where T cells are re-educated and also why this might be important to successfully resist pathogens, since this was a major evolutionary pressure that shaped the human immune system. It was soon realized that stable T cell differentiation often requires repetitive in vitro TCR stimulation in the appropriate cytokine condition, suggesting that immature T cells might be more plastic than more differentiated ones (12, 140). In vivo primed T cells that are at an intermediate stage of differentiation are central memory T cells (TCM), which similar to naïve T cells have maintained the capacity to home to lymph nodes, produce only low levels of effector cytokines, but produce high levels of IL-2 and IL-21 (131), and expand rapidly to generate secondary waves of effector cells (8). Conversely, effector memory T cells (TEM) are more differentiated cells since they produce high levels of effector cytokines and home preferentially to inflamed non-lymphoid tissues (8). Consistent with the view that plasticity is progressively reduced upon T cell differentiation, pre-committed Th1CM cells are more plastic than fully differentiated Th1EM cells, since Th1CM cells generate a substantial population of bona fide Th2 cells upon re-stimulation with IL-4, while Th1EM cells do not revert to Th2 cells, but some acquire IL-4 in addition to IFN-γ producing capacities (24). This plasticity requires TCR stimulation, since antigen-independent proliferation with homeostatic cytokines resulted exclusively in the generation of Th1 effector cells (24). Based on these findings it can be speculated that pre-committed TCM cells that cross-react with a different pathogen can be still partially re-educated to a different lineage in lymph nodes, while TEM cells do not easily switch cytokine production, but rather become polyfunctional (Figure 1). Another example of functional plasticity in lymphoid organs is the generation of follicular Foxp3+BCL-6+ Tregs, which are specialized Tregs that control B cell responses (79, 80). Also Tregs in non-lymphoid tissues acquire tissue-specific properties that are important for their functions (76). TEM helper cells that are activated by antigen in non-lymphoid tissues can up-regulate CCR7 (141) and home to inflamed lymph nodes (142) where they can influence the secondary immune response and are exposed to a different cytokine milieu. Conversely, tissue-resident memory (TRM) cells have lost sphingosine-1 phosphate receptors and thus also the capacity to re-circulate through the blood to secondary lymphoid organs (143). TRM belong predominantly to the CD8 compartment, but influenza virus-specific CD4+ TRM can be identified in the lung of humans and mice (144). If tissue-resident CD4+ T cells are terminally differentiated effector cells or still possess the plasticity to acquire additional cytokine producing capacities remains to be established (145).
A central organ for the generation of different subsets of Th17 cells is the intestine (146). Thus, upon self-limiting colitis induced by anti-CD3 injections in mice predominantly IL-10 producing Th17 cells with regulatory functions are induced (138). Conversely, under conditions that induce IL-23 in the intestine pathogenic IFN-γ and GM-CSF producing Th17 cells are generated that induce colitis (147, 148). IFN-γ and IL-17 co-producing Th1/17 cells have also been observed in patients with IBD (92), but very little is known about the regulation of Th17 responses in the human intestine. Th1/17 cells that produce IL-17, IFN-γ, and GM-CSF also drive central nervous system (CNS) inflammation in EAE, a standard mouse model of multiple sclerosis (MS) (149). The CNS is separated from pro-inflammatory T cells by the blood–brain barrier (150), but spontaneous JC Virus re-activations and progressive multifocal leukoencephalopathy in MS patients treated with anti-VLA-4 antibodies, which block lymphocyte extravasation to the CNS, suggest nevertheless a constant immune surveillance by T cells (151). How the microenvironment of the CNS influences the properties of CD4+ T cells is the focus of intensive research in mice, but is largely unknown in humans given the difficulties to analyze T cells in the human CNS.
Thus, accumulating evidence underlines the role of the tissue microenvironment in T cell plasticity, and the identification of tissue-specific factors that control T cell functions is likely to have a major impact on translational medicine.
The original concept of two terminally differentiated subsets of Th1 and Th2 cells has been substituted by the view that many different T cell subsets with specific cytokine profiles are required to protect us from the different pathogenic insults that were are continuously exposed to. These various T cell subsets possess different degrees of plasticity to acquire new characteristics and functions in secondary or chronic immune responses. In particular, while the stability of Tregs is debated, it is widely accepted that Th17 cells are largely unstable, although exceptions might exist. In addition, human Th17 cells are highly heterogeneous, but the functions of all these different types of Th17 effector cells in protective immune responses and their roles in autoimmune diseases remain to be understood. Another important but poorly understood aspect of T cell plasticity is how different tissue microenvironments impact on human T cell differentiation and stability. The definition of the relative plasticities or stabilities of human T cell subsets in different tissues is highly relevant for future therapeutic interventions in so different immune-related pathologies as chronic viral infections, cancer, and autoimmune diseases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Jens Geginat, Massimiliano Pagani, and Sergio Abrignani are supported by the Cariplo foundation and Sergio Abrignani and Massimiliano Pagani by an ERC grant. The INGM is supported by the Romeo ed Enrica Invernizzi foundation.
1. Jung AC, Paauw DS. Diagnosing HIV-related disease: using the CD4 count as a guide. J Gen Intern Med (1998) 13(2):131–6. doi:10.1046/j.1525-1497.1998.00031.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Geginat J, Paroni M, Facciotti F, Gruarin P, Kastirr I, Caprioli F, et al. The CD4-centered universe of human T cell subsets. Semin Immunol (2013) 25(4):252–62. doi:10.1016/j.smim.2013.10.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol (1989) 7:145–73. doi:10.1146/annurev.iy.07.040189.001045
4. Romagnani S. The Th1/Th2 paradigm. Immunol Today (1997) 18(6):263–6. doi:10.1016/S0167-5699(97)80019-9
5. Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol (2007) 19(6):362–71. doi:10.1016/j.smim.2007.10.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol (2005) 6(4):345–52. doi:10.1038/ni1178
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol (2011) 29:621–63. doi:10.1146/annurev-immunol-031210-101400
8. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol (2004) 22:745–63. doi:10.1146/annurev.immunol.22.012703.104702
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol (2007) 7(6):425–8. doi:10.1038/nri2097
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol (2009) 21(3):281–5. doi:10.1016/j.coi.2009.05.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity (2009) 30(5):646–55. doi:10.1016/j.immuni.2009.05.001
12. Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res (2010) 20(1):4–12. doi:10.1038/cr.2009.138
13. O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science (2010) 327(5969):1098–102. doi:10.1126/science.1178334
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol (2013) 25(4):305–12. doi:10.1016/j.smim.2013.10.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Nizzoli G, Krietsch J, Weick A, Steinfelder S, Facciotti F, Gruarin P, et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T cell responses. Blood (2013) 122(6):932–42. doi:10.1182/blood-2013-04-495424
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev (2010) 234(1):18–31. doi:10.1111/j.0105-2896.2009.00870.x
17. Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med (1992) 326(5):298–304. doi:10.1056/NEJM199201303260504
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Guler ML, Gorham JD, Hsieh CS, Mackey AJ, Steen RG, Dietrich WF, et al. Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development [see comments]. Science (1996) 271(5251):984–7. doi:10.1126/science.271.5251.984
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Kanno Y, Vahedi G, Hirahara K, Singleton K, O’Shea JJ. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu Rev Immunol (2012) 30:707–31. doi:10.1146/annurev-immunol-020711-075058
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Allan RS, Zueva E, Cammas F, Schreiber HA, Masson V, Belz GT, et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature (2012) 487(7406):249–53. doi:10.1038/nature11173
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Tumes DJ, Onodera A, Suzuki A, Shinoda K, Endo Y, Iwamura C, et al. The polycomb protein Ezh2 regulates differentiation and plasticity of CD4(+) T helper type 1 and type 2 cells. Immunity (2013) 39(5):819–32. doi:10.1016/j.immuni.2013.09.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Maggi E, Parronchi P, Manetti R, Simonelli C, Piccinni MP, Rugiu FS, et al. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol (1992) 148(7):2142–7.
23. Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat Immunol (2003) 4(1):78–86. doi:10.1038/ni872
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med (2004) 200(6):725–35. doi:10.1084/jem.20040774
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Peine M, Rausch S, Helmstetter C, Frohlich A, Hegazy AN, Kuhl AA, et al. Stable T-bet(+)GATA-3(+) Th1/Th2 hybrid cells arise in vivo, can develop directly from naive precursors, and limit immunopathologic inflammation. PLoS Biol (2013) 11(8):e1001633. doi:10.1371/journal.pbio.1001633
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity (2009) 30(1):155–67. doi:10.1016/j.immuni.2008.12.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Hegazy AN, Peine M, Helmstetter C, Panse I, Frohlich A, Bergthaler A, et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity (2010) 32(1):116–28. doi:10.1016/j.immuni.2009.12.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli MT, Aste-Amezaga M, et al. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J Exp Med (1996) 183(6):2559–69. doi:10.1084/jem.183.6.2559
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev (2010) 21(5):331–44. doi:10.1016/j.cytogfr.2010.09.002
30. Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol (1993) 150(2):353–60.
31. Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, et al. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med (2007) 204(2):273–83. doi:10.1084/jem.20062175
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med (2007) 204(2):285–97. doi:10.1084/jem.20061886
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med (2002) 196(7):999–1005. doi:10.1084/jem.20020666
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Feuerer M, Eulenburg K, Loddenkemper C, Hamann A, Huehn J. Self-limitation of Th1-mediated inflammation by IFN-gamma. J Immunol (2006) 176(5):2857–63. doi:10.4049/jimmunol.176.5.2857
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Haringer B, Lozza L, Steckel B, Geginat J. Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. J Exp Med (2009) 206(5):1009–17. doi:10.1084/jem.20082238
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Cope A, Le Friec G, Cardone J, Kemper C. The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends Immunol (2011) 32(6):278–86. doi:10.1016/j.it.2011.03.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev (2001) 182:68–79. doi:10.1034/j.1600-065X.2001.1820105.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol (2010) 11(9):862–71. doi:10.1038/ni.1917
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol (2009) 183(4):2435–43. doi:10.4049/jimmunol.0900568
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Ansari NA, Kumar R, Gautam S, Nylen S, Singh OP, Sundar S, et al. IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. J Immunol (2011) 186(7):3977–85. doi:10.4049/jimmunol.1003588
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol (2010) 11(9):846–53. doi:10.1038/ni.1915
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O’Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity (2009) 31(2):209–19. doi:10.1016/j.immuni.2009.05.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Parish IA, Marshall HD, Staron MM, Lang PA, Brustle A, Chen JH, et al. Chronic viral infection promotes sustained Th1-derived immunoregulatory IL-10 via BLIMP-1. J Clin Invest (2014) 124(8):3455–68. doi:10.1172/JCI66108
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
44. Heinemann C, Heink S, Petermann F, Vasanthakumar A, Rothhammer V, Doorduijn E, et al. IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1. Nat Commun (2014) 5:3770. doi:10.1038/ncomms4770
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med (2009) 206(5):991–9. doi:10.1084/jem.20090303
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
46. Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol (2004) 173(1):68–78. doi:10.4049/jimmunol.173.1.68
47. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science (2009) 325(5943):1001–5. doi:10.1126/science.1176676
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
48. Johansson-Lindbom B, Ingvarsson S, Borrebaeck CA. Germinal centers regulate human Th2 development. J Immunol (2003) 171(4):1657–66. doi:10.4049/jimmunol.171.4.1657
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity (2011) 35(4):583–95. doi:10.1016/j.immuni.2011.09.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
50. Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity (2013) 38(4):805–17. doi:10.1016/j.immuni.2013.02.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
51. Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol (2009) 10(4):385–93. doi:10.1038/ni.1715
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
52. Croft M, Swain SL. B cell response to T helper cell subsets. II. Both the stage of T cell differentiation and the cytokines secreted determine the extent and nature of helper activity. J Immunol (1991) 147(11):3679–89.
53. Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol (2008) 9(12):1341–6. doi:10.1038/ni.1659
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
54. Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol (2010) 11(6):527–34. doi:10.1038/ni.1867
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
55. Putheti P, Awasthi A, Popoola J, Gao W, Strom TB. Human CD4 memory T cells can become CD4+IL-9+ T cells. PLoS One (2010) 5(1):e8706. doi:10.1371/journal.pone.0008706
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
56. Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol (2008) 9(12):1347–55. doi:10.1038/ni.1677
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity (2010) 33(2):192–202. doi:10.1016/j.immuni.2010.07.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
58. Anuradha R, George PJ, Hanna LE, Chandrasekaran V, Kumaran P, Nutman TB, et al. IL-4-, TGF-beta-, and IL-1-dependent expansion of parasite antigen-specific Th9 cells is associated with clinical pathology in human lymphatic filariasis. J Immunol (2013) 191(5):2466–73. doi:10.4049/jimmunol.1300911
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
59. Xie J, Lotoski LC, Chooniedass R, Su RC, Simons FE, Liem J, et al. Elevated antigen-driven IL-9 responses are prominent in peanut allergic humans. PLoS One (2012) 7(10):e45377. doi:10.1371/journal.pone.0045377
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
60. Beriou G, Bradshaw EM, Lozano E, Costantino CM, Hastings WD, Orban T, et al. TGF-beta induces IL-9 production from human Th17 cells. J Immunol (2010) 185(1):46–54. doi:10.4049/jimmunol.1000356
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
61. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol (1995) 155(3):1151–64.
62. Stephens LA, Mottet C, Mason D, Powrie F. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol (2001) 31(4):1247–54. doi:10.1002/1521-4141(200104)31:4<1247::AID-IMMU1247>3.0.CO;2-M
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
63. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science (2003) 299(5609):1057–61. doi:10.1126/science.1079490
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
64. Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity (2005) 22(3):329–41. doi:10.1016/j.immuni.2005.01.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
65. Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol (2003) 15(4):430–5. doi:10.1097/00002281-200307000-00010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
66. Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev (2006) 212:8–27. doi:10.1111/j.0105-2896.2006.00427.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
67. Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P, et al. Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut (2006) 55(5):671–80. doi:10.1136/gut.2005.072801
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
68. Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood (2007) 110(8):2983–90. doi:10.1182/blood-2007-06-094656
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
69. Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol (2012) 188(3):976–80. doi:10.4049/jimmunol.1102964
70. Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans. J Immunol (2013) 190(5):2001–8. doi:10.4049/jimmunol.1201379
71. Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol (2010) 184(7):3433–41. doi:10.4049/jimmunol.0904028
72. Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood (2006) 108(13):4260–7. doi:10.1182/blood-2006-06-027409
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
73. Hori S. Regulatory T cell plasticity: beyond the controversies. Trends Immunol (2011) 32(7):295–300. doi:10.1016/j.it.2011.04.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
74. Sawant DV, Vignali DA. Once a Treg, always a Treg? Immunol Rev (2014) 259(1):173–91. doi:10.1111/imr.12173
75. Schmidl C, Hansmann L, Andreesen R, Edinger M, Hoffmann P, Rehli M. Epigenetic reprogramming of the RORC locus during in vitro expansion is a distinctive feature of human memory but not naive Treg. Eur J Immunol (2011) 41(5):1491–8. doi:10.1002/eji.201041067
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
76. Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol (2013) 14(10):1007–13. doi:10.1038/ni.2683
77. Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science (2009) 326(5955):986–91. doi:10.1126/science.1172702
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
78. Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature (2009) 458(7236):351–6. doi:10.1038/nature07674
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
79. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med (2011) 17(8):975–82. doi:10.1038/nm.2425
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
80. Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med (2011) 17(8):983–8. doi:10.1038/nm.2426
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
81. Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol (2005) 174(5):2591–601. doi:10.4049/jimmunol.174.5.2591
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
82. Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, Valet F, Kissenpfennig A, et al. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity (2010) 32(2):266–78. doi:10.1016/j.immuni.2009.11.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
83. Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood (2004) 104(9):2840–8. doi:10.1182/blood-2004-03-0859
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
84. Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity (2007) 27(4):635–46. doi:10.1016/j.immuni.2007.08.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
85. Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood (2012) 119(19):4430–40. doi:10.1182/blood-2011-11-392324
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
86. Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A (2009) 106(21):8635–40. doi:10.1073/pnas.0900621106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
87. Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med (2011) 17(6):673–5. doi:10.1038/nm.2389
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
88. Voo KS, Wang YH, Santori FR, Boggiano C, Arima K, Bover L, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A (2009) 106(12):4793–8. doi:10.1073/pnas.0900408106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
89. Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol (2005) 6(11):1133–41. doi:10.1038/ni1261
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
90. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol (2005) 6(11):1123–32. doi:10.1038/ni1254
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
91. Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol (2007) 8(6):639–46. doi:10.1038/ni1467
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
92. Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med (2007) 204(8):1849–61. doi:10.1084/jem.20070663
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
93. Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol (1995) 155(12):5483–6.
94. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell (2006) 126(6):1121–33. doi:10.1016/j.cell.2006.07.035
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
95. Unutmaz D. RORC2: the master of human Th17 cell programming. Eur J Immunol (2009) 39(6):1452–5. doi:10.1002/eji.200939540
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
96. Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med (2008) 205(7):1551–7. doi:10.1084/jem.20080218
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
97. McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol (2006) 27(1):17–23. doi:10.1016/j.it.2005.10.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
98. Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science (2006) 314(5804):1461–3. doi:10.1126/science.1135245
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
99. Sarin R, Wu X, Abraham C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc Natl Acad Sci U S A (2011) 108(23):9560–5. doi:10.1073/pnas.1017854108
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
100. Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet (2009) 41(2):199–204. doi:10.1038/ng.311
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
101. McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol (2009) 10(3):314–24. doi:10.1038/ni.1698
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
102. El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol (2011) 12(6):568–75. doi:10.1038/ni.2031
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
103. McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol (2007) 8(12):1390–7. doi:10.1038/ni1539
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
104. Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest (2006) 116(5):1310–6. doi:10.1172/JCI21404
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
105. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity (2006) 24(2):179–89. doi:10.1016/j.immuni.2006.01.001
106. Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol (2007) 8(9):942–9. doi:10.1038/ni1496
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
107. Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med (2008) 205(8):1903–16. doi:10.1084/jem.20080397
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
108. Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol (2008) 9(6):641–9. doi:10.1038/ni.1610
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
109. Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol (2008) 9(6):650–7. doi:10.1038/ni.1613
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
110. Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature (2008) 453(7192):236–40. doi:10.1038/nature06878
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
111. Santarlasci V, Maggi L, Capone M, Frosali F, Querci V, De Palma R, et al. TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol (2009) 39(1):207–15. doi:10.1002/eji.200838748
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
112. Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature (2010) 467(7318):967–71. doi:10.1038/nature09447
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
113. Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol (2012) 13(10):991–9. doi:10.1038/ni.2416
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
114. Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature (2012) 484(7395):514–8. doi:10.1038/nature10957
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
115. Duhen T, Campbell DJ. IL-1beta promotes the differentiation of polyfunctional human CCR6+CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. J Immunol (2014) 193(1):120–9. doi:10.4049/jimmunol.1302734
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
116. Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol (2011) 12(3):255–63. doi:10.1038/ni.1993
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
117. Lexberg MH, Taubner A, Albrecht I, Lepenies I, Richter A, Kamradt T, et al. IFN-gamma and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur J Immunol (2010) 40(11):3017–27. doi:10.1002/eji.201040539
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
118. Maggi L, Santarlasci V, Capone M, Rossi MC, Querci V, Mazzoni A, et al. Distinctive features of classic and nonclassic (Th17 derived) human Th1 cells. Eur J Immunol (2012) 42(12):3180–8. doi:10.1002/eji.201242648
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
119. Cohen CJ, Crome SQ, MacDonald KG, Dai EL, Mager DL, Levings MK. Human Th1 and Th17 cells exhibit epigenetic stability at signature cytokine and transcription factor loci. J Immunol (2011) 187(11):5615–26. doi:10.4049/jimmunol.1101058
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
120. Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol (2010) 125(1):e1–4. doi:10.1016/j.jaci.2009.10.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
121. Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin’ through a glass onion. Eur J Immunol (2008) 38(12):3265–8. doi:10.1002/eji.200838655
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
122. Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med (2006) 203(10):2271–9. doi:10.1084/jem.20061308
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
123. Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest (2008) 118(2):597–607. doi:10.1172/JCI33263
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
124. Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med (2008) 14(3):275–81. doi:10.1038/nm1710
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
125. Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol (2009) 10(8):857–63. doi:10.1038/ni.1767
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
126. Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol (2009) 10(8):864–71. doi:10.1038/ni.1770
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
127. Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, et al. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nat Immunol (2011) 12(12):1238–45. doi:10.1038/ni.2134
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
128. Dambacher J, Beigel F, Zitzmann K, De Toni EN, Goke B, Diepolder HM, et al. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut (2009) 58(9):1207–17. doi:10.1136/gut.2007.130112
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
129. Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol (2011) 12(6):560–7. doi:10.1038/ni.2027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
130. Noster R, Riedel R, Mashreghi MF, Radbruch H, Harms L, Haftmann C, et al. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci Transl Med (2014) 6(241):241ra80. doi:10.1126/scitranslmed.3008706
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
131. Kastirr I, Maglie S, Paroni M, Alfen JS, Nizzoli G, Sugliano E, et al. IL-21 is a central memory T cell-associated cytokine that inhibits the generation of pathogenic Th1/17 effector cells. J Immunol (2014) 193(7):3322–31. doi:10.4049/jimmunol.1400775
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
132. Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol (2007) 8(9):967–74. doi:10.1038/ni1488
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
133. Caprioli F, Sarra M, Caruso R, Stolfi C, Fina D, Sica G, et al. Autocrine regulation of IL-21 production in human T lymphocytes. J Immunol (2008) 180(3):1800–7. doi:10.4049/jimmunol.180.3.1800
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
134. Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature (2007) 448(7152):480–3. doi:10.1038/nature05969
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
135. Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature (2007) 448(7152):484–7. doi:10.1038/nature05970
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
136. Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature (2008) 454(7202):350–2. doi:10.1038/nature07021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
137. Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol (2011) 23(6):702–6. doi:10.1016/j.coi.2011.08.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
138. Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, et al. Control of TH17 cells occurs in the small intestine. Nature (2011) 475(7357):514–8. doi:10.1038/nature10228
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
139. Schlapbach C, Gehad A, Yang C, Watanabe R, Guenova E, Teague JE, et al. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med (2014) 6(219):219ra8. doi:10.1126/scitranslmed.3007828
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
140. Lohning M, Richter A, Radbruch A. Cytokine memory of T helper lymphocytes. Adv Immunol (2002) 80:115–81. doi:10.1016/S0065-2776(02)80014-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
141. Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, Qin S, et al. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol (1999) 29(6):2037–45. doi:10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.3.CO;2-M
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
142. Guarda G, Hons M, Soriano SF, Huang AY, Polley R, Martin-Fontecha A, et al. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol (2007) 8(7):743–52. doi:10.1038/ni1469
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
143. Shin H, Iwasaki A. Tissue-resident memory T cells. Immunol Rev (2013) 255(1):165–81. doi:10.1111/imr.12087
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
144. Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol (2014) 7(3):501–10. doi:10.1038/mi.2013.67
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
145. Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity (2013) 38(1):187–97. doi:10.1016/j.immuni.2012.09.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
146. Huber S, Gagliani N, Flavell RA. Life, death, and miracles: Th17 cells in the intestine. Eur J Immunol (2012) 42(9):2238–45. doi:10.1002/eji.201242619
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
147. Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med (2006) 203(11):2485–94. doi:10.1084/jem.20061082
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
148. Griseri T, McKenzie BS, Schiering C, Powrie F. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity (2012) 37(6):1116–29. doi:10.1016/j.immuni.2012.08.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
149. Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol (2010) 162(1):1–11. doi:10.1111/j.1365-2249.2010.04143.x
150. Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol (2012) 33(12):579–89. doi:10.1016/j.it.2012.07.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
151. Berger JR, Houff S. Progressive multifocal leukoencephalopathy: lessons from AIDS and natalizumab. Neurol Res (2006) 28(3):299–305. doi:10.1179/016164106X98198
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: CD4 T cells, cytokines, differentiation, tissue homing, plasticity
Citation: Geginat J, Paroni M, Maglie S, Alfen JS, Kastirr I, Gruarin P, De Simone M, Pagani M and Abrignani S (2014) Plasticity of human CD4 T cell subsets. Front. Immunol. 5:630. doi: 10.3389/fimmu.2014.00630
Received: 03 October 2014; Accepted: 25 November 2014;
Published online: 16 December 2014.
Edited by:
Dragana Jankovic, National Institutes of Health (NIH), USAReviewed by:
António Gil Castro, University of Minho, PortugalCopyright: © 2014 Geginat, Paroni, Maglie, Alfen, Kastirr, Gruarin, De Simone, Pagani and Abrignani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jens Geginat and Sergio Abrignani, Fondazione Istituto Nazionale di Genetica Molecolare “Romeo ed Enrica Invernizzi” INGM, Via Sforza 35, Milano 20122, Italy e-mail:Z2VnaW5hdEBpbmdtLm9yZw==;YWJyaWduYW5pQGluZ20ub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.