
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 14 February 2013
Sec. Molecular Innate Immunity
Volume 4 - 2013 | https://doi.org/10.3389/fimmu.2013.00029
This article is part of the Research TopicDeciphering new molecular mechanisms of mast cell activationView all 11 articles
The relationship between mast cells (MCs) and pregnancy is a controversially discussed topic. The presence and quantitative distribution of MCs in the reproductive tract was confirmed in different species. A phase-dependent oscillation of MCs during the hormonal regulated estrous cycle was suggested and on this basis, MCs were assumed to play a positive role in implantation because of their ability to secrete histamine. At later pregnancy stages, they were proposed to have rather a negative role, as their exacerbated activation is associated with pre-term delivery. The present review is intended to provide an overview about uterine MCs that bring to light their unexpected relevance for reproductive processes.
Already in the early 60s and 70s the presence and quantitative distribution of mast cells (MCs) in the reproductive tract in general and during the estrous cycle in particular was described in different species like rat (Gibbons and Chang, 1972), hamster (Harvey, 1964), and cow (Likar and Likar, 1964). The phase-dependent oscillation of MC numbers during the hormone-regulated estrous cycle was described and assumed that they are important for implantation because they secrete substances that promote tissue remodeling necessary for this process. The functional importance of these data was however unclear as the results were based on the methodological simplicity at that time. Later on, a role for MCs in reproduction was dismissed because of the apparently normal pregnancy outcome of mice lacking MCs. We have recently investigated the importance of MC deficiency in pregnancy outcome using a mouse model. The present review is intended to provide an overview about the available as well as novel data from us and many others, that bring to light the unexpected relevance of MCs for reproductive processes.
Based on their tissue specificity, murine MCs are typically classified as either mucosal or connective tissue-type MCs (Metcalfe et al., 1997). Thereby, this classification is attributed, i.a. to their histochemical staining patterns especially to those of the granule-stored proteases. Already in 1960, Spicer suggested that uterine MCs (uMCs) represent a divergent phenotype composed of mucosal as well as connective tissue-type MCs (Spicer, 1960). The histochemical observations were done by using a combined Alcian blue–Safranin stain whereby mucosal MCs are Alcian blue-positive and Safranin-negative and connective tissue-type MCs are Alcian blue-negative and Safranin-positive, respectively. In our laboratory we could confirm Spicer’s findings on the presence of both MC types. uMCs were positive either for Alcian blue or for safranin. In addition, we found a third MC population that was positive for both dyes (Woidacki et al., 2013). These cells were already described and reportedly reflect different stages of differentiation (Reynolds et al., 1988; Kitamura, 1989). Moreover, they can derive from or represent an ongoing transdifferentiation process, during which mature MCs may change their content in proteoglycans, amines, peptides, etc. (Michaloudi and Papadopoulos, 1999). These conversion processes might be induced as a response to local inflammatory processes (Kitamura, 1989; Tsuji et al., 1990; van Overveld, 1990; Moon et al., 2010) or the presence of fibroblasts (Levi-Schaffer et al., 1986). Here, the most important growth factor for MCs, stem cell factor (SCF), could serve as a pivotal stimulus. It was described that MC proliferation and differentiation in the uterus is regulated by SCF secretion from uterine smooth muscle cells (Mori et al., 1997b). Furthermore, it is known that heparin is essential by controlling the levels of specific granule proteases inside MCs (Humphries et al., 1999). Thereby, the tissue-specific MC phenotype can vary in different mouse strains. In BALB/c, ear MCs express MC protease 7 (Mcpt-7), the analog of human tryptase α/β 1. In contrast, ear MCs from C57BL/6J contain no detectable protein levels of Mcpt-7 (Ghildyal et al., 1994). During pregnancy, uMCs seem to maintain their heterogeneity as in the pregnant human uterus tryptase-positive and chymase-negative (MCT) as well as tryptase-positive and chymase-positive (MCTC) MCs are present (Garfield et al., 2006). In pregnant rats, uMCs have been identified likewise as mucosal and connective tissue-type MCs based on their specific protease content (Salamonsen et al., 1996). It is therefore clear that uMCs represent a heterogeneous population of cells and they can change their phenotype according to local stimuli. Thus, uMCs constitute a special population with unique characteristics and a high plasticity, which is important to consider when designing experiments to analyze their role in utero.
The presence of uMCs and their menstrual or estrous cycle-related variations in number and structure were described in humans (Drudy et al., 1991a,b; Mori et al., 1997a) and other mammals like mouse (Padilla et al., 1990), rat (Aydin et al., 1998), hamster (Harvey, 1964), and goat (Karaca et al., 2008). In mouse, uMCs seem to reach their highest level during the receptive phase of the female, namely in estrus (Woidacki et al., 2013), when the uterus is prepared for nidation. This is in line with results in rats reported by Aydin et al. (1998). They detected the highest number of MCs in estrus as well. If fecundation did not occur the MC number in metestrus was decreased. After pregnancy establishment, uMC numbers became even higher (Woidacki et al., 2013). This might be due to the interplay of the sexual hormones 17β-estradiol and progesterone. In mice, maximum 17β-estradiol levels were observed at estrus whereas progesterone levels were lowest at this phase (Fata et al., 2001). Estradiol is known to potentiate the degranulation of MCs in vitro (Cocchiara et al., 1992). All these observations indicate a hormone-dependent regulation of uMCs. This is further reinforced by our recent observations. We found that not only MCs express the receptors for estrogens and progesterone but these hormones in combination can attract MCs in vitro and in vivo to uterine cells (Jensen et al., 2010).
The fundament of a successful pregnancy outcome in mammals is the maternal tolerance of the semi-allogenic fetus based on a well-orchestrated modulation of the maternal immune system and the functionality of the hormonal system. A variety of innate and adaptive immune cells are participating in this concert especially locally at the feto-maternal interface including uterine natural killer cells (Greenwood et al., 2000; Bilinski et al., 2008), dendritic cells (Blois et al., 2004; Plaks et al., 2008), and regulatory T cells (Aluvihare et al., 2004; Zenclussen et al., 2006; Schumacher, 2013), whereas the function of MCs in maternal tolerance is uncertain. High amounts of MCs were detected in the uterus during pregnancy (Menzies et al., 2012) and MC density was significantly higher in tissue from pregnant women than those of non-pregnant women (Garfield et al., 2006). We confirmed high numbers of uMCs in early pregnancy stages in a mouse model. uMCs were mainly distributed between implantation sites. Implantations from MC-deficient C57BL/6J-KitW-sh/W-sh (W-sh), whose MC deficiency is caused by a defective c-Kit signaling, showed a delayed kinetic of development with a significantly diminished size in comparison to wild-type, MC sufficient controls (Woidacki et al., 2013). The transfer of bone marrow-derived MCs (BMMCs) into W-sh mice positively influenced the size of the implantation sites and restored them to normal levels (Woidacki et al., 2013). It is important to remark that a delayed implantation might have a fatal impact in pregnancy outcome (Song et al., 2002). This is further evidenced by our findings on insufficient placentation and remodeling of spiral arteries in W-sh mice (Woidacki et al., 2013). The embryo itself could act as the stimulus for the implantation process. Here, the embryo-derived histamine-releasing factor (EHRF) might be one of the first signals from the embryo to the uterus. The EHRF-induced local secretion of histamine by uMCs could play a role in preventing maternal immune rejection at the implantation stage (Cocchiara et al., 1986).
Some studies are based on histamine as an important MC-specific mediator for the initiation of blastocyst implantation processes and decidual cell responses (Shelesnyak et al.,1957, 1959; Nalbandov et al., 1971). However, the increment of the uterine histamine levels in MC-deficient WBB6F1-W/WV (W/WV) after steroid treatment (Wordinger et al., 1985) suggests an alternative source of histamine like endothelial cells (Robinson-White et al., 1982) or/and decidual cells which have been shown to release histamine upon stimulation (Schrey et al., 1995). A study from Wordinger et al. (1986) makes the discussion even more controversial. The sterility of MC-deficient WBB6F-W/WV is mainly due to atrophic ovaries with a hyperplastic stroma and absence of follicles and distinct corpora lutea (Wordinger et al., 1985). To determine whether implantation and live births occurred in the absence of uMCs, Wordinger et al. (1985) employed a model of ovariectomized female W/WV mice. After the transplantation of one ovary obtained from normal female littermates (+/+) the authors transferred blastocysts from +/+ into pseudopregnant W/WV. They could not find any differences between W/WV and +/+ in the implantation rate after blastocyst transfer or in the number of live births. Because of these observations, Wordinger et al. (1985) excluded any requirement of uMCs in these processes. However, the transplanted ovaries were obtained from +/+ and should therefore contain a remarkable amount of already mature and differentiated MCs that could then migrate to the surrounding tissue and expand. We recently observed that locally transferred MCs into one single uterine horn were located in the other, untreated, uterine horn shortly thereafter (Woidacki et al., 2013). In the Wordinger study, no information is available regarding the presence of MCs in the ovary before and after transplantation. Additionally, the recipients were treated with steroids and MCs are known for their susceptibility to the action of hormones like estradiol and progesterone (Wordinger et al., 1985; Cocchiara et al., 1992; Rudolph et al., 2004; Jensen et al., 2010) which probably induced the expansion of the MCs present in the ovaries and their migration to the uterus. We detected high amounts of MCs within the ovaries (unpublished observations) that coincides with observations done in different species like mouse (Skalko et al., 1968), rat (Jones et al., 1980; Gaytan et al., 1991; Aydin et al., 1998; Batth and Parshad, 2000), hamster (Shinohara et al., 1987; Krishna and Terranova, 1991), cow (Reibiger and Spanel-Borowski, 2000), goat (Karaca and Simşek, 2007; Karaca et al., 2008), and chicken (Parshad and Kathpalia, 1993). Furthermore, we could not find alterations in the number of follicles as well as corpora lutea between MC-deficient W-sh and control mice (Woidacki et al., 2013). As early pregnancy is highly dependent on the presence of corpora lutea and the progesterone they secrete, we conclude that the establishment of pregnancy does not seem to depend on MCs but implantation and embryo development surely does (Woidacki et al., 2013).
On day 10 of murine normal pregnancies, MCs were present in the decidua, the maternal part of the feto-maternal interface and located closed to blood vessels (Woidacki et al., 2013). In pregnant rats, the degranulation of MCs positively influenced angiogenesis (Varayoud et al., 2004; Bosquiazzo et al., 2007). Nevertheless, some studies exclude MCs as important mediators of pregnancy-relevant processes. Salamonsen et al. (1996) applied to syngeneically mated female rats a highly potent MC stabilizer (FPL 55618). They claimed no differences in the number of implantation sites. This is remarkable, but taking a closer look at the data, as e.g., two of a total of five rats receiving FPL intraperitoneally failed to show any embryo implantation (Salamonsen et al., 1996), meaning only 60% of the animals, got pregnant compared to more than 80% in the control group. Furthermore, the low number of animals employed it is questionable to make such strong conclusions based only in a subgroup of the studied group. Menzies et al. (2012) recently suggested that the absence of MCs had no discernible impact on pregnancy. In this study, MC-deficient C57BL/6J-KitW-sh/W-sh and their wild-type counterparts, both syngeneically mated, had similar offspring birth weights and no difference in fetal–placental index. However, neither the kinetic nor the occurrence of implantations was analyzed or reported by these authors. That survivor animals develop normally does not discard that the first stages of pregnancy are dependent on MCs. Despite that, this study concentrated on those pregnancies that succeed after implantation and no data is provided as to how many females were pregnant after plug detection and how many blastocysts could be implanted, these authors concentrated on syngeneic matings. These two publications denying a role of MCs in pregnancy share one aspect: they are based on syngeneic and therefore biologically questionable matings. Naturally occurring pregnancies in natura are predominantly allogeneic to maintain the genetical variability of a species. That allows the adaptation of the fetus to its later environment at the best. Matings with genetically related and even worse among identic individuals has to be avoided because of the partially tremendous consequences of inbreeding. Syngeneic matings are exclusively necessary to maintain inbreeding colonies in the laboratories. Even there, after some generations mostly a backcross to wild-types has to be done as a result of the genetic impoverishment. Madeja et al. (2011) could show that murine allogeneic fetuses and placentas were heavier at term compared with syngeneic controls. This consequence was based on impaired decidual vascularization as well as placental and fetal growth after syngeneic matings. They supposed that allogeneic placentas are much more sufficient in supporting fetal growth by adequate modulation of spiral arteries. It seems reasonable to assume that paternal allo-antigens are important for stimulating maternal immune cells, which is not further discussed as it would go beyond the scope of this article. In this context, the role of MCs is worth to be studied and critically analyzed in efficient, relevant allogeneic pairings as the results obtained in syngeneic ones are limited by the already mentioned factors.
MC-deficient C57BL/6J-KitW-sh/W-sh (W-sh) mice implanted significantly less blastocysts than their wild-type counterparts after allogeneic mating. Uteri from W-sh mice were either very thick, swollen, and reddish with no visible implantations or contained few implantations. Accordingly, their litter size was significantly reduced as compared to wild-type controls. The systemic and local reconstitution with BMMC completely restored the reproductive phenotype of W-sh mice. Moreover, the few implanted blastocysts in W-sh mice developed significantly smaller placentas and insufficient modifications of the spiral arteries that are responsible for supplying oxygen and nutrients to the fetus. BMMC transfer normalized all parameters and therefore contributed to a normal pregnancy outcome by mediating placental development and spiral artery remodeling (Woidacki et al., 2013).
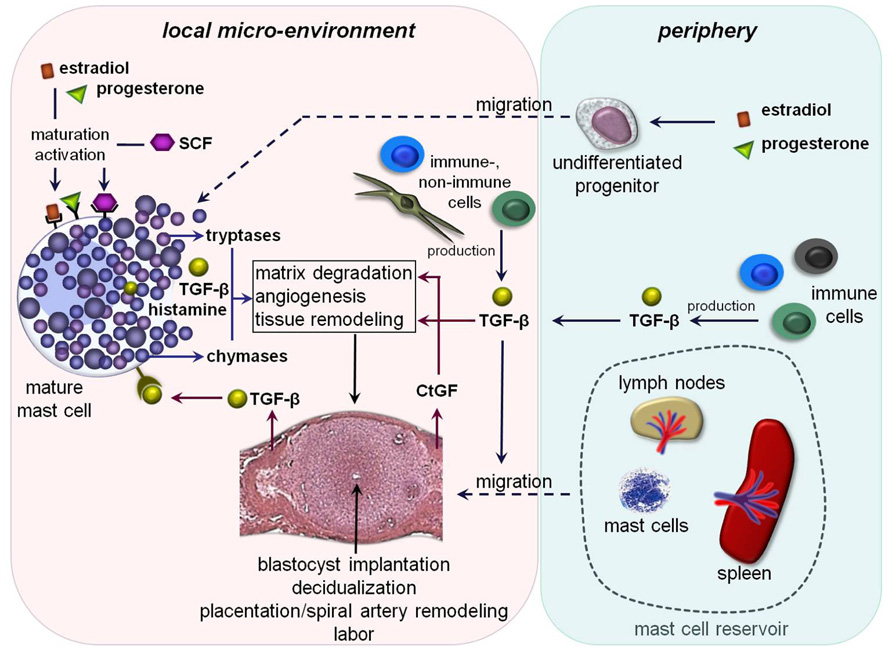
FIGURE 1. Hypothetical scenario of MC-impact on pregnancy-related processes: undifferentiated MC-precursors migrate from the periphery into the uterus due to the simultaneous influence of estradiol and progesterone. Their local maturation and differentiation occurs through SCF. Amongst other mediators estradiol and progesterone could bind locally on uMCs that further lead to their activation. The MC-activation results in a simultaneous release of pre-formed and/or de novo synthesized mediators including different tryptases and chymases as well as transforming growth factor-β (TGF-β) and histamine. These mediators could directly or indirectly affect important processes like blastocyst’s implantation, decidualization, placentation, spiral artery remodeling, and later on labor. Trophoblast-derived and/or peripheral TGF-β binds on TGF-β-receptors expressed on the mast cell surface that would be a possible further mechanism for their activation. TGF-β likewise stimulates the recruitment of other MCs from the periphery into the fetal–maternal interface. Here, lymph nodes and spleen could serve as a MC-reservoir in the periphery. Fibroblast- and later on trophoblast-derived connective tissue growth factor (CtGF) is involved in matrix degradation, angiogenesis as well as tissue remodeling.
Inadequate placental development mainly due to discrepancies in trophoblast differentiation and invasion, respectively can lead to intrauterine growth retardation (IUGR) and pre-eclampsia amongst other complications. In placentas obtained from IUGR-pregnancies the number of MCs was markedly decreased while hypoxia could intensify MC degranulation (Szukiewicz et al., 1999b). The degranulation of MCs resulted in a greater increase of the vascular resistance in pre-eclampsia likely due to the vasoconstrictive function of histamine (Szukiewicz et al., 1999a) and asthmatic pregnant women are at increased risk to develop this disease (Siddiqui et al., 2008; Murphy et al., 2011). In severe pre-eclampsia, the number of human MC chymase-positive cells was significantly higher compared to normal pregnant women (Mitani et al., 2002) and MC-chymase is known to be more potent than angiotensin-converting enzyme to convert angiotensin I to angiotensin II (Wintroub et al., 1984). Whether the secreted histamine, that inhibits the apoptotic activity in trophoblast cells via H(1) receptor (Pyzlak et al., 2010) and further influences the process of trophoblast differentiation (Szewczyk et al., 2005) and invasion (Liu et al., 2004) is derived exclusively from MCs has to be still determined. Thus, the heterogenicity of uMCs is also depicted here: low numbers of uMCs is associated with pathologies as IUGR which would predict a positive role of uMCs on fetal growth while their exacerbated activation is related to pre-eclampsia and pre-term birth. Hence, uMCs represent a heterogenous population, which shows also a high plasticity to respond differently to different stimuli.
There are strong hints for the relevance of MCs in mediating the implantation of the blastocyst as discussed above. As pregnancy advances, MCs exert an influence on the maintenance of pregnancy by allowing the unrestricted development of the placenta and remodeling of the spiral arteries (Woidacki et al., 2013). Interestingly, there are vast evidences that MCs also influence perinatal processes. The degranulation of MCs can lead to substantial changes in the myometrial contractility (Martínez et al., 1999; Garfield et al., 2006). Resident MCs increased uterine contractility in pregnant guinea pigs through multiple mediators including histamine and serotonin. Uterine responses to these mediators are dependent on gestational age (Bytautiene et al., 2008). Pregnant women affected by systemic mastocytosis exhibit manifestations of pre-term labor and delivery. This disease is accompanied by an unexplained and pathologic increase in MCs in specific tissue (Metcalfe and Akin, 2001). The allergic activation of MCs results in a substantial increase in uterine contractility (Garfield et al., 2006) and could be therefore responsible for the allergy-associated induction of pre-term labor (Habek et al., 2000; Bytautiene et al., 2004). This is in line with the fact that pregnant women with asthma are at a higher risk to pre-term delivery (Perlow et al., 1992; Sorensen et al., 2003; Murphy et al., 2006). However, Menzies et al. (2012) concluded that MCs have no impact on initiation of labor because the time of labor initiation in MC-deficient mice was indistinguishable from wild-type controls. Nevertheless, the number of MCs within the mouse cervix doubled from non-pregnant to day 18 of pregnancy, with a further 1.5-fold increase with labor (Menzies et al., 2012). This relevant question is worth to be tested in a mouse model for pre-term delivery and remains highly up-to-date.
In summary our data as well as data from the literature show that MCs accompany and deeply affect many steps of reproduction. MCs are modified and attracted by hormones, uMCs are essential for allowing implantation of allogeneic embryos, and positively influence placentation and thus, embryo development. Later on, an exacerbated number or function of uMCs can negatively influence pregnancy and foster pre-term delivery. It is clear that uMCs are not only different from other MCs because of their unique markers but also seem to secrete different mediators at different pregnancy stages and upon different stimuli. This makes these cells an extremely interesting target of study for both, reproductive biologists and MC researchers. Based on the data discussed in this review, we propose following hypothetical scenario for the impact of MCs on pregnancy-related processes.
Mast cells vitally influence reproductive processes and in particular the pregnancy itself by modulating non-immunological responses like tissue remodeling, angiogenesis, optimal placentation, and spiral artery modifications as well as labor. They further play a rather negative role in parturition as the excessive secretion of MC-mediators may lead to pre-term delivery. MCs may act not only as mediators of the innate immune system but also as cellular switch points between innate and adaptive immune responses. Their activity is regulated by endocrine and physiological signals and based on their granule-stored array of biologically active products. All these well-orchestrated mechanisms allow the non-restrictive development of the semi-allogeneic fetus within the maternal uterus and therefore fetal survival. The understanding of the paradoxon “pregnancy” is of fundamental importance for helping couples to realize their often unfulfilled desire to have children. In this context, the data in regard to the mast cell-associated positive pregnancy outcome might serve as a further puzzle piece to answer these questions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aluvihare, V. R., Kallikourdis, M., and Betz, A. G. (2004). Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271.
Aydin, Y., Tunccel, N., Gürer, F., Tuncel, M., Koşar, M., and Oflaz, G. (1998). Ovarian, uterine and brain mast cells in female rats: cyclic changes and contribution to tissue histamine. Comp. Biochem. Physiol. 120, 255–262.
Batth, B. K., and Parshad, R. K. (2000). Mast cell dynamics in the house rat (Rattus rattus) ovary during estrus cycle, pregnancy and lactation. Eur. J. Morphol. 38, 17–23.
Bilinski, M. J., Thorne, J. G., Oh, M. J., Leonard, S., Murrant, C., Tayade, C., et al. (2008). Uterine NK cells in murine pregnancy. Reprod. Biomed. Online 16, 218–226.
Blois, S. M., Alba Soto, C. D., Tometten, M., Klapp, B. F., Margni, R. A., and Arck, P. C. (2004). Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol. Reprod. 70, 1018–1023.
Bosquiazzo, V. L., Ramos, J. G., Varayoud, J., Muñoz-de-Toro, M., and Luque, E. H. (2007). Mast cell degranulation in rat uterine cervix during pregnancy correlates with expression of vascular endothelial growth factor mRNA and angiogenesis. Reproduction 133, 1045–1055.
Bytautiene, E., Romero, R., Vedernikov, Y. P., El-Zeky, F., Saade, G. R., and Garfield, R. E. (2004). Induction of premature labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. Am. J. Obstet. Gynecol. 191, 1356–1361.
Bytautiene, E., Vedernikov, Y. P., Saade, G. R., Romero, R., and Garfield, R. E. (2008). IgE-independent mast cell activation augments contractility of nonpregnant and pregnant guinea pig myometrium. Int. Arch. Allergy Immunol. 147, 140–146.
Cocchiara, R., Albeggiani, G., Di Trapani, G., Azzolina, A., Lampiasi, N., Rizzo, F., et al. (1992). Oestradiol enhances in vitro the histamine release induced by embryonic histamine-releasing factor (EHRF) from uterine mast cells. Hum. Reprod. 7, 1036–1041.
Cocchiara, R., Di Trapani, G., Azzolina, A., Albeggiani, G., and Geraci, D. (1986). Early embryonic histamine-releasing factor: a new model for human implantation. Hum. Reprod. 1, 445–447.
Drudy, L., Sheppard, B., and Bonnar, J. (1991a). Mast cells in the normal uterus and in dysfunctional uterine bleeding. Eur. J. Obstet. Gynecol. Reprod. Biol. 39, 193–201.
Drudy, L., Sheppard, B. L., and Bonnar, J. (1991b). The ultrastructure of mast cells in the uterus throughout the normal menstrual cycle and the postmenopause. J. Anat. 175, 51–63.
Fata, J. E., Chaudhary, V., and Khokha, R. (2001). Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta-estradiol during the estrous cycle. Biol. Reprod. 65, 680–688.
Garfield, R. E., Irani, A. M., Schwartz, L. B., Bytautiene, E., and Romero, R. (2006). Structural and functional comparison of mast cells in the pregnant versus nonpregnant human uterus. Am. J. Obstet. Gynecol. 194, 261–267.
Gaytan, F., Aceitero, J., Bellido, C., Sánchez-Criado, J. E., and Aguilar, E. (1991). Estrous cycle-related changes in mast cell numbers in several ovarian compartments in the rat. Biol. Reprod. 45, 27–33.
Ghildyal, N., Friend, D. S., Freelund, R., Austen, K. F., McNeil, H. P., Schiller, V., et al. (1994). Lack of expression of the tryptase mouse mast cell protease 7 in mast cells of the C57BL/6J mouse. J. Immunol. 153, 2624–2630.
Gibbons, A. F., and Chang, M. C. (1972). Number of mast cells in the rat uterus with special reference to its relation to hormonal treatment and decidual response. Biol. Reprod. 6, 193–203.
Greenwood, J. D., Minhas, K., di Santo, J. P., Makita, M., Kiso, Y., and Croy, B. A. (2000). Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta 21, 693–702.
Habek, D., Cerkez-Habek, J., and Jalsovec, D. (2000). Anaphylactic shock in response to wasp sting in pregnancy. Zentralbl. Gynäkol. 122, 393–394.
Harvey, E. B. (1964). Mast cell distribution in the uterus of cycling and pregnant hamsters. Anat. Rec. 148, 507–516.
Humphries, D. E., Wong, G. W., Friend, D. S., Gurish, M. F., Qiu, W. T., Huang, C., et al. (1999). Heparin is essential for the storage of specific granule proteases in mast cells. Nature 400, 769–772.
Jensen, F., Woudwyk, M., Teles, A., Woidacki, K., Taran, F., Costa, S., et al. (2010). Estradiol and progesterone regulate the migration of mast cells from the periphery to the uterus and induce their maturation and degranulation. PLoS ONE 5:e14409. doi: 10.1371/journal.pone.0014409
Jones, R. E., Duvall, D., and Guillette, L. J. Jr. (1980). Rat ovarian mast cells: distribution and cyclic changes. Anat. Rec. 197, 489–493.
Karaca, T., Arikan, S., Kalender, H., and Yoruk, M. (2008). Distribution and heterogeneity of mast cells in female reproductive tract and ovary on different days of the oestrus cycle in Angora goats. Reprod. Domest. Anim. 43, 451–456.
Karaca, T., and Simşek, N. (2007). Effects of spirulina on the number of ovary mast cells in lead-induced toxicity in rats. Phytother. Res. 21, 44–46.
Kitamura, Y. (1989). Heterogeneity of mast cells and phenotypic change between subpopulations. Annu. Rev. Immunol. 7, 59–76.
Krishna, A., and Terranova, P. F. (1991). Compartmentalized mast cell degranulations in the ovarian hilum, fat pad, bursa and blood vessel regions of the cyclic hamster: relationships to ovarian histamine and blood flow. Acta Anat. (Basel) 141, 18–25.
Levi-Schaffer, F., Austen, K. F., Gravallese, P. M., and Stevens, R. L. (1986). Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc. Natl. Acad. Sci. U.S.A. 83, 6485–6488.
Likar, I. N., and Likar, L. J. (1964). Acid mucopolysaccharides and mast cells in the bovine uterus at different stages of the sexual cycle. Acta Endocrinol. 46, 493–506.
Liu, Z., Kilburn, B. A., Leach, R. E., Romero, R., Paria, B. C., and Armant, D. R. (2004). Histamine enhances cytotrophoblast invasion by inducing intracellular calcium transients through the histamine type-1 receptor. Mol. Reprod. Dev. 68, 345–353.
Madeja, Z., Yadi, H., Apps, R., Boulenouar, S., Roper, S. J., Gardner, L., et al. (2011). Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc. Natl. Acad. Sci. U.S.A. 108, 4012–4017.
Martínez, L., Gimeno, M. F., Villán, L., Villar, M., and Rudolph, M. I. (1999). Nitroprusside stimulates contractility and the synthesis of 14C-acetylated PAF-like substances in estrogen primed-mouse uterine horns. Prostaglandins Other Lipid Mediat. 57, 49–62.
Menzies, F. M., Higgins, C. A., Shepherd, M. C., Nibbs, R. J., and Nelson, S. M. (2012). Mast cells reside in myometrium and cervix, but are dispensable in mice for successful pregnancy and labor. Immunol. Cell Biol. 90, 321–329.
Metcalfe, D. D., and Akin, C. (2001). Mastocytosis: molecular mechanisms and clinical disease heterogeneity. Leuk. Res. 25, 577–582.
Michaloudi, H. C., and Papadopoulos, G. C. (1999). Mast cells in the sheep, hedgehog and rat forebrain. J. Anat. 195, 577–586.
Mitani, R., Maeda, K., Fukui, R., Endo, S., Saijo, Y., Shinohara, K., et al. (2002). Production of human mast cell chymase in human myometrium and placenta in cases of normal pregnancy and preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 101, 155–160.
Moon, T. C., St Laurent, C. D., Morris, K. E., Marcet, C., Yoshimura, T., Sekar, Y., et al. (2010). Advances in mast cell biology: new understanding of heterogeneity and function. Mucosal Immunol. 3, 111–128.
Mori, A., Zhai, Y. L., Toki, T., Nikaido, T., and Fujii, S. (1997a). Distribution and heterogeneity of mast cells in the human uterus. Hum. Reprod. 12, 368–372.
Mori, A., Nakayama, K., Suzuki, J., Nikaido, T., Isobe, M., and Fujii, S. (1997b). Analysis of stem cell factor for mast cell proliferation in the human myometrium. Mol. Hum. Reprod. 3, 411–418.
Murphy, V. E., Clifton, V. L., and Gibson, P. G. (2006). Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax 61, 169–176.
Murphy, V. E., Namazy, J. A., Powell, H., Schatz, M., Chambers, C., Attia, J., et al. (2011). A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 118, 1314–1323.
Nalbandov, E. V. (1971). “Endocrine control of implantation,” in Biology of the Blastocyst. ed. R. J. Blandau (Chicago: University of Chicago Press).
Padilla, L., Reinicke, K., Montesino, H., Villena, F., Asencio, H., Cruz, M., et al. (1990). Histamine content and mast cells distribution in mouse uterus: the effect of sexual hormones, gestation and labor. Cell. Mol. Biol. 36, 93–100.
Parshad, R. K., and Kathpalia, K. (1993). Distribution and characteristics of mast cells in the chick ovary. Br. Poult. Sci. 34, 65–71.
Perlow, J. H., Montgomery, D., Morgan, M. A., Towers, C. V., and Porto, M. (1992). Severity of asthma and perinatal outcome. Am. J. Obstet. Gynecol. 167, 963–967.
Plaks, V., Birnberg, T., Berkutzki, T., Sela, S., BenYashar, A., Kalchenko, V., et al. (2008). Uterine DCs are crucial for decidua formation during embryo implantation in mice. J. Clin. Invest. 118, 3954–3965.
Pyzlak, M., Szewczyk, G., Szukiewicz, D., and Szczesniak, A. (2010). Histamine influence on apoptosis in trophoblast cell cultures. Inflamm. Res. 59(Suppl. 2), S213–S215.
Reibiger, I., and Spanel-Borowski, K. (2000). Difference in localization of eosinophils and mast cells in the bovine ovary. J. Reprod. Fertil. 118, 243–249.
Reynolds, D. S., Serafin, W. E., Faller, D. V., Wall, D. A., Abbas, A. K., Dvorak, A. M., et al. (1988). Immortalization of murine connective tissue-type mast cells at multiple stages of their differentiation by coculture of splenocytes with fibroblasts that produce Kirsten sarcoma virus. J. Biol. Chem. 263, 12783–12791.
Robinson-White, A., and Beaven, M. A. (1982). Presence of histamine and histamine-metabolizing enzyme in rat and guinea-pig microvascular endothelial cells. J. Pharmacol. Exp. Ther. 223, 440–445.
Rudolph, M. I., Rojas, I. G., and Penissi, A. B. (2004). Uterine mast cells: a new hypothesis to understand how we are born. Biocell 28, 1–11.
Salamonsen, L. A., Jeziorska, M., Newlands, G. F., Dey, S. K., and Woolley, D. E. (1996). Evidence against a significant role for mast cells in blastocyst implantation in the rat and mouse. Reprod. Fertil. Dev. 8, 1157–1164.
Schrey, M. P., Hare, A. L., Ilson, S. L., and Walters, M. P. (1995). Decidual histamine release and amplification of prostaglandin F2 alpha production by histamine in interleukin-1 beta-primed decidual cells: potential interactive role for inflammatory mediators in uterine function at term. J. Clin. Endocrinol. Metab. 80, 648–653.
Schumacher, A. (2013). Human Chorionic Gonadotropin as a central regulator of pregnancy immune tolerance. J. Immunol. (in press).
Shelesnyak, M. C. (1957). Some experimental studies on the mechanism of ova-implantation in the rat. Recent Prog. Horm. Res. 13, 269–317.
Shelesnyak, M. C. (1959). Fall in uterine histamine associated with ovum implantation in pregnant rat. Proc. Soc. Exp. Biol. Med. 100, 380–381.
Shinohara, H., Nakatani, T., Morisawa, S., Matsuda, T., and Naruse, Y. (1987). Mast cells in the ovarian bursa of the golden hamster. Biol. Reprod. 36, 445–450.
Siddiqui, S., Goodman, N., McKenna, S., Goldie, M., Waugh, J., and Brightling, C. E. (2008). Pre-eclampsia is associated with airway hyperresponsiveness. BJOG 115, 520–522.
Skalko, R. G., Ruby, J. R., and Dyer, R. F. (1968). Demonstration of mast cells in the postnatal mouse ovary. Anat. Rec. 161, 459–463.
Song, H., Lim, H., Paria, B. C., Matsumoto, H., Swift, L. L., Morrow, J., et al. (2002). Cytosolic phospholipase A2alpha is crucial [correction of A2alpha deficiency is crucial] for ‘on-time’ embryo implantation that directs subsequent development. Development 129, 2879–2889.
Sorensen, T. K., Dempsey, J. C., Xiao, R., Frederick, I. O., Luthy, D. A., and Williams, M. A. (2003). Maternal asthma and risk of preterm delivery. Ann. Epidemiol. 13, 267–272.
Spicer, S. S. (1960). Siderosis associated with increased lipofuscins and mast cells in aging mice. Am. J. Pathol. 37, 457–475.
Szewczyk, G., Szukiewicz, D., Klimkiewicz, J., Pyzlak, M., Szewczyk, A., and Krajewska, K. (2005). Influence of histamine on the process of human trophoblast differentiation. Inflamm. Res. 54(Suppl. 1), S78–S79.
Szukiewicz, D., Szukiewicz, A., Maslinska, D., Gujski, M., Poppe, P., and Mazurek-Kantor, J. (1999a). Mast cell number, histamine concentration and placental vascular response to histamine in preeclampsia. Inflamm. Res. 48(Suppl. 1), S39–S40.
Szukiewicz, D., Szukiewicz, A., Maslinska, D., Poppe, P., Gujski, M., and Olszewski, M. (1999b). Mast cells and histamine in intrauterine growth retardation–relation to the development of placental microvessels. Inflamm. Res. 48(Suppl. 1), S41–S42.
Tsuji, K., Nakahata, T., Takagi, M., Kobayashi, T., Ishiguro, A., Kikuchi, T., et al. (1990). Effects of interleukin-3 and interleukin-4 on the development of “connective tissue-type” mast cells: interleukin-3 supports their survival and interleukin-4 triggers and supports their proliferation synergistically with interleukin-3. Blood 75, 421–427.
van Overveld, F. J. (1990). Some aspects of mast cell subtypes from human lung tissue. Agents Actions 30, 24–29.
Varayoud, J., Ramos, J. G., Bosquiazzo, V. L., Muñoz-de-Toro, M., and Luque, E. H. (2004). Mast cells degranulation affects angiogenesis in the rat uterine cervix during pregnancy. Reproduction 127, 379–387.
Wintroub, B. U., Schechter, N. B., Lazarus, G. S., Kaempfer, C. E., and Schwartz, L. B. (1984). Angiotensin I conversion by human and rat chymotryptic proteinases. J. Invest. Dermatol. 83, 336–339.
Woidacki, K., Popovic, M., Metz, M., Schumacher, A., Linzke, N., Teles, A., et al. (2013). Mast cells rescue implantation defects caused by c-kit deficiency. Cell Death Dis. 4, e462.
Wordinger, R. J., Jackson, F. L., and Morrill, A. (1986). Implantation, deciduoma formation and live births in mast cell-deficient mice (W/Wv). J. Reprod. Fertil. 77, 471–476.
Wordinger, R. J., Orr, E. L., Pace, K., Oakford, L., and Morrill, A. (1985). An assessment of mast-cell deficient mice (W/Wv) as a model system to study the role of histamine in implantation and deciduoma formation. J. Reprod. Fertil. 73, 451–456.
Keywords: mast cells, placenta, pregnancy, uterus, implantation
Citation: Woidacki K, Jensen F and Zenclussen AC (2013) Mast cells as novel mediators of reproductive processes. Front. Immun. 4:29. doi: 10.3389/fimmu.2013.00029
Received: 13 March 2012; Accepted: 25 January 2013;
Published online: 14 February 2013.
Edited by:
Ulrich Blank, Inserm U699, Université Paris Diderot-Paris 7, FranceReviewed by:
Ulrich Blank, Inserm U699, Université Paris Diderot-Paris 7, FranceCopyright: © 2013 Woidacki, Jensen and Zenclussen. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Ana C. Zenclussen, Department of Experimental Obstetrics and Gynecology, Medical Faculty, Otto-von-Guericke University, Gerhart-Hauptmann-Straße 35, 39108 Magdeburg, Germany. e-mail:YW5hLnplbmNsdXNzZW5AbWVkLm92Z3UuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.