- Shenzhen Hospital, Peking University, Shenzhen, China
Background: Presurgical evaluation of the histopathological grade of soft tissue sarcoma (STS) is important for enacting treatment strategies. In this study, we plan to investigate the correlation of high-output ultrasound (US) radiomic features and the histopathological grade of STS.
Methods: Patients with STS were retrospectively enrolled. The radiomic features were extracted from the US images of the STS lesions. The lesions were graded according to the Fédération Nationale des Centers de Lutte Contre le Cancer (FNCLCC) histopathological grading system. The correlation of the radiomic features and the FNCLCC grades was evaluated. We used the features correlated with the histopathological grades to build a model for predicting high-grade STS (Grade II and III).
Results: A total of 79 patients with STS were enrolled. And 15 radiomic features were found correlated with the FNCLCC grades of STSs, with the correlation coefficient ranging from 0.22 to 0.38. And 8 features showed significant difference among the three grades. The model for predicting high-grade STS based on the 8 radiomic features had an AUC value of 0.80, a sensitivity of 0.73, and a specificity of 0.78.
Conclusion: The US radiomic features were correlated with the FNCLCC grade of STS. The radiomic analysis of US imaging could be potentially helpful for identifying the FNCLCC grades of STS pre-surgically.
Introduction
Soft tissue sarcomas (STSs) are a cohort of malignancies that derive from heterogenous soft tissues in various parts of the entire body. They account for about 1% of cancers in adult and comprise over 80 histological subtypes (Siegel et al., 2019; Choi and Ro, 2021). It is challenging to diagnose and treat STSs properly due to their diverse pathological changes and clinical behaviors. Clinicians attempt to tailor histology-specific treatment algorithms for the patients with STSs instead of a single method for all types of STSs (Demetri et al., 2017). To note, it is important to differentiate G1 (Grade I), the low-grade sarcomas, with G2/G3 (Grade II/Grade III), the high-grade sarcomas, due to their different response to the therapeutic strategies (Peeken et al., 2019, 2018). For those high-grade tumors, neoadjuvant therapy, such as chemotherapy and radiation therapy before tumor resection, could be helpful for improving the prognosis of the patients with STS (Casali et al., 2018; Gradishar et al., 2021). Therefore, a pre-surgical evaluation of STS is of significant importance for decision making.
The malignant degree of STSs is associated with their histological manifestations. A histopathological grading system for STSs has been established by the Fédération Nationale des Centers de Lutte Contre le Cancer (FNCLCC) to determine the malignant degree of STSs histologically (Neuville et al., 2014). The FNCLCC grading system is constituted by three histopathological parameters, including tumor differentiation, tumor necrosis, and mitotic count. And the imaging-guided biopsies of the tumors enable the pre-surgical histological evaluation of STSs. However, the histological scores acquired from pretreatment biopsied samples might be inaccurate. Schneider et al. found that about 68% in 100 patients with leiomyosarcoma showed difference in FNCLCC scores between biopsied specimens and resected specimens. And the scores of biopsied specimens were relatively lower than that of resected ones (Schneider et al., 2017). Weigl et al. reported a concordance rate of 92.5% in FNCLCC scores between ultrasound-guided biopsied samples and resected samples in 74 cases of STSs. And a total of five cases had a lower score in biopsied samples than the resected samples (Weigl et al., 2021). The discrepancy in FNCLCC scores could be explained by that the biopsy-obtained samples are relatively small, which could not be representative for the entire tumors with large sizes and intratumoral heterogenicity. Therefore, a supplementary for assessing the malignant degree of STSs to pretreatment biopsy is necessary.
High-frequency ultrasound (US) and magnetic resonance imaging (MRI) are two major imaging methods for the evaluation of STSs, due to their excellent resolution to soft tissues (Achar et al., 2022; Crombé et al., 2023; Sharon et al., 2022). Apart from diagnosing STSs, studies have shown that STS imaging could also indicates the histopathological information of the tumors. The correlation between MRI indexes and histological characteristics of STSs have been revealed by previous studies (Crombé et al., 2019; Hettler et al., 2022; Teixeira et al., 2016; Chhabra et al., 2019; Nakamura et al., 2017; Zhao et al., 2014). Compared with MRI, US enjoyed a larger popularity in scanning patients with soft tissue tumors clinically as a convenient and rapid imaging tool (Hung et al., 2020; Morii et al., 2018). However, the correlation of US and histopathological features has not been investigated previously.
Although US provides limited image information, the development of radiomics has addressed this issue by employing advanced analytical methods. Radiomics is an emerging methodology in medical imaging analysis that extracts high-throughput characteristics from medical images via computer techniques (Avanzo et al., 2020; Lambin et al., 2017). US radiomics has been utilized to facilitate cancer diagnosis and prognosis prediction in some diseases, such as breast cancer, thyroid cancer, and hepatocarcinoma (Conti et al., 2021; Han et al., 2021; Mao et al., 2021; Yu et al., 2022; Chen, 2021). While the association of US radiomics and STSs has not been reported previously. Given the capacity of US radiomics in implying pathological information, it is of clinical value to investigate the correlation of US radiomics and histopathological characteristics of STS and explore the potential role of radiomics in guiding treatment of STS.
In this study, we recruited patients with STSs from various tumor sites. We made radiomic analysis of US images and applied the FNCLCC criteria to evaluate the histopathological grades of the tumors. The correlation of radiomic features of US imaging and FNCLCC grades was explored subsequently. This pilot study aimed to investigate the association between US radiomics and histopathological grading of STSs and explore the role of radiomics in assessing STSs.
Materials and methods
Ethical approval
The retrospective study was approved by the ethics committee of Hospital (Approval number: 202200901). The informed consent was not waived in this study, for there was no interventional method performed, and only medical information was retrospectively collected. The ethics committee approved the exemption of informed consent.
Patient recruitment
The patients with STSs from July 2013 to December 2021 were recruited consecutively. We collected the basic clinical information, US images and pathological records of the patients for further analysis. The inclusion criteria of the patients were: (1) patients newly diagnosed with STSs by post-surgical histopathological results; (2) patients who received US scanning of the lesion within 1 month before surgery; (3) patients with complete US imaging and pathological results; (4) patients who received no neoadjuvant therapy of STS. The basic clinical information included age, sex, and the site of the STSs.
US examination and US imaging reading
The US scanning of soft tissue tumors was performed by commercial US machines, including Philips IU22, Mindray Resona70, Aplio 500, and GE healthcare EPIQ7, using linear probes with a frequency of 5–15 MHz. The images of longitudinal section showing the largest diameter of the lesions were recorded for radiomic analysis.
Radiomic analysis
Two radiologists with 5 years of experiences in musculoskeletal US (CY.Z. and N.Z.) selected the US images of the patients with STSs from the imaging system. Two radiologists with 5 years of experiences in musculoskeletal US (YS. Z and H.L.) outlined ROI of the selected images of STSs manually. A senior radiologist with 12 years of experiences in musculoskeletal US (HQ.X.) checked the ROI delineation of all the selected images and modified the inappropriate ROIs. The radiologists (YS. Z, H.L., and HQ.X.) were blind to the pathological results of the STSs. ROI segmentation was conducted using ITK-SNAP (Yushkevich et al., 2006). The radiomic features of the US images were extracted by an open-source python package (PyRadiomics) (van Griethuysen et al., 2017). A total of 96 radiomic features were extracted, including first-order statistics (distribution of voxel intensities), two-dimension shape-based statistics (two-dimensional size and shape), gray level cooccurrence matrix (GLCM) (the second-order joint probability function), gray level run length matrix (GLRLM) (the length in number of pixels), gray level size zone matrix (GLSZM), neighboring gray tone difference matrix (NGTDM) (difference between a gray value and the average gray value of its neighbors), gray level dependence matrix (GLDM).
Histopathological assessment
An expert pathologist evaluated the FNCLCC grading of the recruited STS cases based on the standard proposed by the French Sarcoma Group. The FNCLCC grading system includes three pathological parameters, including tumor differentiation, mitotic count, and tumor necrosis, scored as 1–3. For tumor differentiation, Score 1 is the tumors resembling normal mesenchymal tissues; Score 2 represents some certain types of STSs; and embryonal and undifferentiated STSs, synovial sarcomas, doubtful-type STSs were categorized into Score 3. For mitotic count, score 1 is 0–9 mitoses per 10 high-power field (HPF); Score 2 is 10–19 mitoses; Score 3 is more than 19 mitoses. For tumor necrosis, Score 0 represents no necrosis; Score 1 is less than 50% of necrosis; Score 2 is more than 50% or 50%. And the total score of 2–3 is Grade I; total score of 4–5 is Grade II; total score of 6–8 is Grade III (Neuville et al., 2014).
Statistical analysis
According to previous study, the specificity of the MR feature on T1-weighted imaging reflecting the margin of the lesion was 0.69 (Zhao et al., 2014). We calculated the sample size of the study using PASS 2021. A total sample size of 80 (which includes 40 subjects with the disease) could achieve 90% power to detect a change in specificity from 0.69 to 0.88 using a one-sided binomial test. The target significance level is 0.05.
R (http://www.R-project.org) was used for statistical analysis in this study. P < 0.05 was considered statistically significant.
We used the chi-square or Fisher test to investigate the associations between the FNCLCC grade and categorical or ordinal variables. And for each radiomic feature analyzed, we calculated the univariable odds ratio (OR) along with a 95% confidence interval (CI), to assess the strength of the association between radiomics feature and the FNCLCC grading. Continuous variables were compared using either the Wilcoxon test or the student t-test, depending on the results of the Shapiro-Wilk normality test.
We first assessed whether there were the differences among the three pathological types of STSs. Then we made pairwise comparisons between each pair of groups. One-way analysis of variance (ANOVA) for multiple group comparisons were utilized for comparing the imaging parameters of the three grades of STSs. When ANOVA test showed significant differences of the three pathological grades, the student t-test was performed for pairwise comparisons. For the post hoc multiple tests, P-values were adjusted using Bonferroni correction to control the overall probability of making at least one Type I error.
Additionally, the correlation between the imaging indexes and histopathological grades were assessed via Spearman's rank-order correlation coefficient, which could provide insight into the strength of the association between the variables and the pathological grades.
The features showing difference among the three FNCLCC grades were selected for building model for predicting the histopathological grade of STSs. We categorized the STSs into two groups: low-grade (FNCLCC Grade I) and high-grade (FNCLCC Grade II and III) STSs. The backward stepwise variable selection method based on the Akaike information criterion (AIC) was used to establish the model. This process was enhanced by bootstrap resampling of 500 times to ensure robust model performance. Finally, we assessed the diagnostic performance of the model by measuring sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). We also evaluated the diagnostic performance of the model through depicting its receiver operating characteristic (ROC) curve and calculating the area under the ROC curve (AUC).
Results
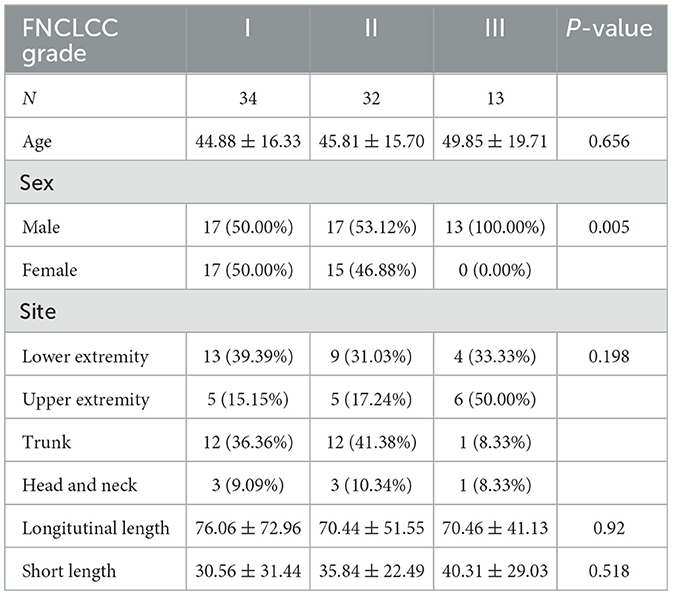
A total of 79 patients with STSs were recruited in this study (47 male and 32 female). The basic clinical information and histopathological types of the enrolled cases is shown in Table 1. A total of 34 (43.0%) were Grade I STS, 32 (40.1%) were Grade II STS, and 13 (16.5%) were Grade III STS. Among the recruited cases, the most common types of STS were fibrosarcoma (34, 43.0%), liposarcoma (16, 20.3%), and rhabdomyosarcoma (6, 7.6%). The lesions were located in the lower extremity (26, 33.0%), the upper extremity (16, 20.3%), the trunk (25, 31.6%), and head and neck (12, 15.2%). The Grade-I STSs were measured 76.06 × 30.56 (longitudinal length × short length) averagely. The Grade-II STSs were measured 70.44 × 35.84 (longitudinal length × short length) averagely. The Grade-III STSs were measured 70.46 × 40.31 (longitudinal length × short length) averagely.
Correlation between the radiomic features and the FNCLCC grading of STSs
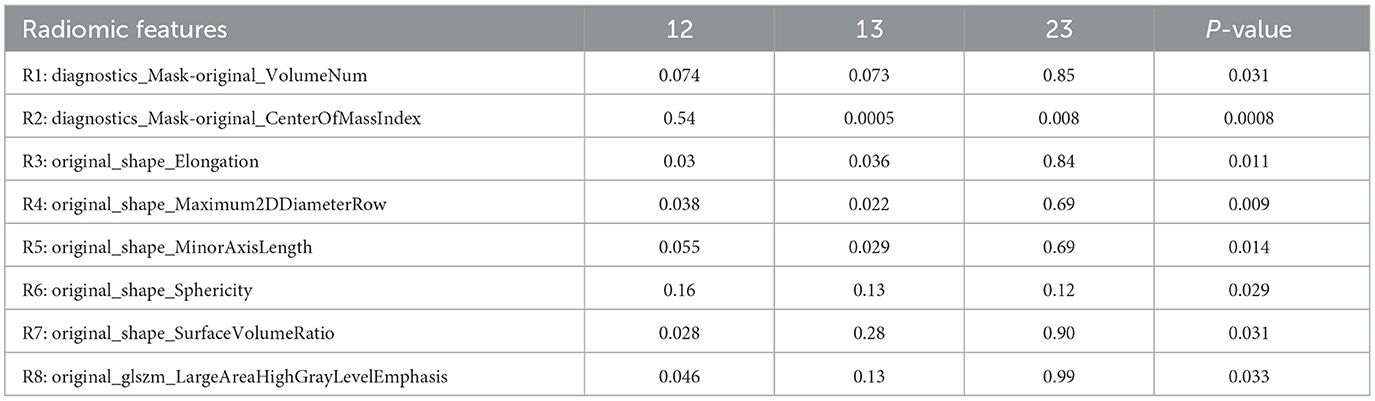
Among the 96 radiomic features, there were a total of 15 features showing significant correlation with the FNCLCC grade, as shown in Table 2 and Figure 1, including 3 features of mask indexes, 6 features of the tumor shape, 3 features of GLCM, one GLDM feature, and one GLSZM feature. The correlation coefficient ranged from 0.23 to 0.38.
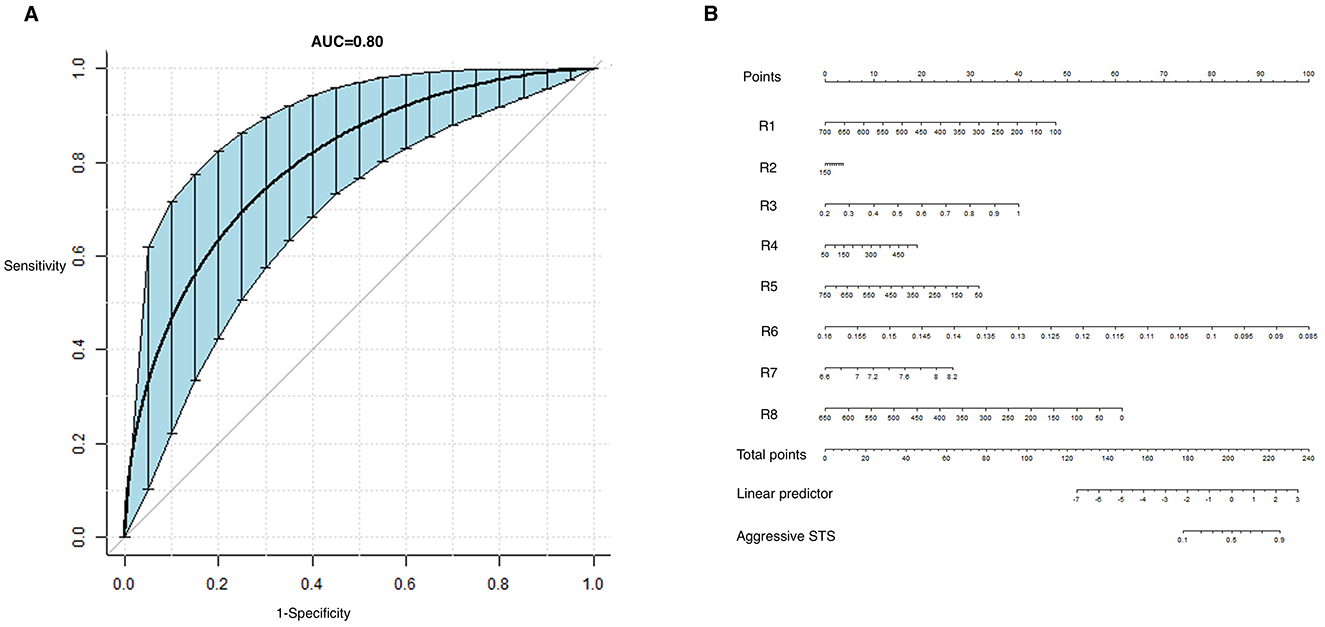
Figure 1. Receiver operating characteristic (ROC) curve of the model predicting aggressive STS (A) and the nomogram for calculating the radiomic scores (B).
Among the 15 features correlated with the FNCLCC grade, a total of 8 features (R1-8) showed significant difference among the three grades, as shown in Table 3. On adjusted pairwise comparison, the difference still exist in the following features: R2 (p = 0.0005 for Grade I vs. III, p = 0.008 for Grade II vs. III), R3 (p = 0.030 for Grade I vs. II, p = 0.036 for Grade I vs. III), R4 (p = 0.038 for Grade I vs. II, p = 0.022 for Grade I vs. III), R5 (p = 0.029 for Grade I vs. III), R7 (p = 0.028 for Grade I vs. II), R8 (p = 0.046 for Grade I vs. II).
Establishing the model for predicting the high-grade STSs based on radiomic features
There are 34 of low-grade STSs (Grade I) and 45 high-grade STSs (Grade II and III). The formula of the predictive model based on ultrasonic radiomic features using stepwise regression is listed as follows:
Logit (FNCLCC) = 3.73799 −0.00723*R1 +0.00084*R2 +4.55453*R3 +0.00346*R4 −0.00413*R5 −121.71883*R6 +1.49956*R7-0.00863*R8.
The predictive model had a sensitivity of 0.73, specificity of 0.78, PPV of 0.87, NPV of 0.71, and AUC of 0.80 (0.71–0.89). The ROC curve and the nomogram representing the formulation of the radiomic model is shown in Figure 1. And an example of predicting the high-grade STS using the model is shown in Figure 2.
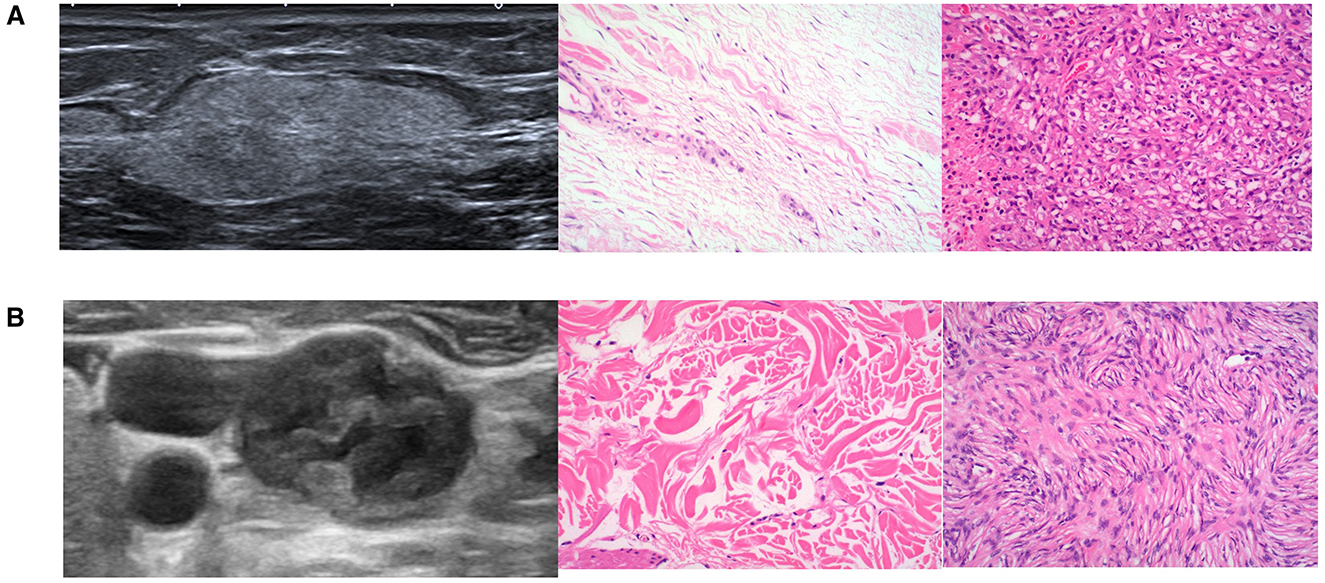
Figure 2. Example of predicting aggressive STS. (A) Gray-scale US image and pathological images of a 23-year-old female with Grade I STS. The radiomic model predicted it as a low-grade STS with a high-grade probability of 0.44. (B) Gray-scale US image and pathological images of a 55-year-old male with Grade III STS. The radiomic model predicted it as a high-grade STS with a probability of 0.78.
Discussion
The histopathological grade is highly predictive to the prognosis of STSs. However, the grades acquired with biopsied samples can be inaccurate, which could not be representative for the whole lesions. In this study, we found that the high-output radiomic features were associated with the FNCLCC grades of STSs, with the correlation coefficient ranging from 0.22 to 0.38. The model for predicting high-grade STS based on radiomic features could reach an AUC value of 0.80. The radiomic analysis of US imaging could be potentially helpful for identifying the FNCLCC grades of STS pre-surgically.
Previous studies have revealed the association of several MRI features with the pathological grade and prognosis of STS. However, the role of US imaging in pre-surgical histopathological evaluation has not been reported previously. This is the first study exploring the correlation of US and histopathological FNCLCC grading of STS. US imaging-based radiomics has emerged as a valuable approach in the diagnosis of malignancies and prognostic prediction for a range of diseases, including breast cancer, thyroid carcinoma, and hepatic cancer (Qi et al., 2022; Wang et al., 2019; Yu et al., 2020). Radiomics captures spatial distributions of pixels or voxels that are imperceptible to the human eye, which could potentially elucidate the associations of imaging with underlying pathological processes. The radiomic analysis of US could acquire more information from the US images, compared with common hand-crafted features, such as boundary, margin, and internal echogenicity. Therefore, it is possible to explore the correlation between ultrasonic radiomic features and the histopathological grades of STS.
The features correlated with the histopathological grades includes mask indexes, tumor shape, gray-level co-occurrence matrix, gray level dependence matrix, and gray level size zone matrix. The mask indexes represent the physical size and voxels of the region of interest. Larger tumors may indicate more aggressive behavior of STSs. The tumor shape features measure the size, the ratio of longitudinal length and short length of the lesion, and the sphericity of the lesion. Irregular shapes can also suggest the aggressiveness of STSs in histopathology. The gray-level co-occurrence matrix, gray-level dependence matrix, and gray-level size zone matrix reflect pixel intensities and the uniformity of pixel distribution within the lesions. Variations in these patterns can indicate differences in tissue composition and cellularity, which are associated with histopathological characteristics. Therefore, physical appearances and components of the STS lesions, including the shape, size, distribution of pixels, are associated with their histopathological characteristics. The quantitative assessment of US features through computer algorithms could be helpful in predicting the histopathological aggressiveness of STSs. To note, the correlation coefficients between the radiomic features and the pathological grades are relatively low in this study, ranging from 0.23 to 0.38. This might be attributed to the limited sample size of the study. Also, the biological behaviors of STSs are relatively complex. The ultrasonic features alone cannot fully reflect the histopathological characteristics of STSs. Further studies with larger sample size and additional imaging variables are needed to explore the association between imaging and histopathological features of STSs.
Grade I and Grade II STSs showed significant differences in radiomic features reflecting the shape and distribution of pixels. However, Grade II and Grade III is hard to differentiate through US radiomic features. Given their histopathological characteristics, we attributed Grade II and Grade III into one category as high-grade STS, and Grade I as low-grade STS. The model based on the radiomic features with significant differences among the three grades had an AUC value of 0.80, sensitivity of 0.73, and specificity of 0.78 for predicting aggressiveness of STS. The diagnostic model provides a presurgical assessment of the lesions, which may guide clinical decisions. For those lesions that may exhibit greater aggressiveness according to the model, additional treatment strategies can be considered, such as neoadjuvant therapy, or larger incision area.
There exist several limitations of the study. Firstly, the number of cases included in this study is relatively small, especially for Grade III STS. More cases will be enrolled for further exploring the association of US radiomic features and histopathological grades of STSs. Moreover, radiomic features of pseudo-color images of Color-Doppler US were not analyzed in this study, which might also provide useful information in assessing histopathological grade of STS. Additionally, we established a model for predicting the high-grade STS based on US radiomic features with internal validation of 500 times of bootstrap resampling. External validation should be performed in further studies.
Conclusions
High output radiomic features of US imaging are correlated with the FNCLCC histopathological grades of STS. The model based on the radiomic features correlated with the histopathological grade showed a good performance in identifying high-grade STS with an AUC value of 0.80.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Peking University Shenzhen Hospital. The studies were conducted in accordance with the local legisla tion and institutional requirements. The Ethics Committee/Institutional Review Board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because there was no interventional method performed, and only medical information was retrospectively collected.
Author contributions
CZ: Data curation, Investigation, Methodology, Software, Writing – original draft. YZ: Data curation, Methodology, Writing – original draft. HL: Data curation, Investigation, Software, Writing – original draft. NZ: Data curation, Writing – original draft. GY: Supervision, Writing – review & editing. YS: Data curation, Writing – original draft. LD: Investigation, Writing – original draft. WW: Data curation, Writing – original draft. LX: Data curation, Investigation, Writing – original draft. YT: Software, Writing – original draft. ZY: Formal analysis, Software, Writing – original draft. DS: Data curation, Formal analysis, Software, Writing – original draft. XW: Supervision, Validation, Writing – review & editing. HX: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by General Program for Clinical Research at Peking University Shenzhen Hospital (LCYJ2021003), the Science Technology and Innovation Commission of Shenzhen Municipality in China (JCYJ20210324110015040), National Natural Science Foundation of China (82302207), Research Foundation of Peking University Shenzhen Hospital (JCYJ2020012), Shenzhen Key Medical Discipline Construction Fund (SZXK051), Shenzhen High-level Hospital Construction Fund, Sanming Project of Medicine in Shenzhen (SZSM202111011), and National Key Research and Development Program of China (Grant no. 2023YFC3402605).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Achar, S., Yamanaka, J., and Oberstar, J. (2022). Soft tissue masses: evaluation and treatment. Am. Fam. Physician 105, 602–612.
Avanzo, M., Wei, L., Stancanello, J., Vallières, M., Rao, A., Morin, O., et al. (2020). Machine and deep learning methods for radiomics. Med. Phys. 47, e185–e202. doi: 10.1002/mp.13678
Casali, P. G., Abecassis, N., Aro, H. T., Bauer, S., Biagini, R., Bielack, S., et al. (2018). Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 29, iv51–iv67. doi: 10.1093/annonc/mdy096
Chen, Y. (2021). Ultrasound radiomics in breast cancer - a literature review. Adv. Ultrasound Diagnosis Ther. 5, 12–17. doi: 10.37015/AUDT.2021.200052
Chhabra, A., Ashikyan, O., Slepicka, C., Dettori, N., Hwang, H., Callan, A., et al. (2019). Conventional MR and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: correlation with histologic grading. Eur. Radiol. 29, 4485–4494. doi: 10.1007/s00330-018-5845-9
Choi, J. H., and Ro, J. Y. (2021). The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv. Anat. Pathol. 28, 44–58. doi: 10.1097/PAP.0000000000000284
Conti, A., Duggento, A., Indovina, I., Guerrisi, M., and Toschi, N. (2021). Radiomics in breast cancer classification and prediction. Semin. Cancer Biol. 72, 238–250. doi: 10.1016/j.semcancer.2020.04.002
Crombé, A., Kind, M., Fadli, D., Miceli, M., Linck, P. A., Bianchi, G., et al. (2023). Soft-tissue sarcoma in adults: Imaging appearances, pitfalls and diagnostic algorithms. Diagn. Interv. Imaging 104, 207–220. doi: 10.1016/j.diii.2022.12.001
Crombé, A., Marcellin, P. J., Buy, X., Stoeckle, E., Brouste, V., Italiano, A., et al. (2019). Soft-tissue sarcomas: assessment of MRI features correlating with histologic grade and patient outcome. Radiology. 291, 710–721. doi: 10.1148/radiol.2019181659
Demetri, G. D., Blay, J. Y., and Casali, P. G. (2017). Advances and controversies in the management of soft tissue sarcomas. Future Oncol. 13:3–11. doi: 10.2217/fon-2016-0498
Gradishar, W. J., Moran, M. S., Abraham, J., Aft, R., Agnese, D., Allison, K. H., et al. (2021). NCCN guidelines® insights: breast cancer, version 4.2021. J Natl. Compr. Canc. Netw. 19, 484–493. doi: 10.6004/jnccn.2021.0023
Han, X., Cao, W., Wu, L., and Liang, C. (2021). Radiomics assessment of the tumor immune microenvironment to predict outcomes in breast cancer. Front. Immunol. 12:773581. doi: 10.3389/fimmu.2021.773581
Hettler, M., Kitz, J., Hosseini, A. S. A., Guhlich, M., Panahi, B., Ernst, J., et al. (2022). Comparing apparent diffusion coefficient and FNCLCC grading to improve pretreatment grading of soft tissue sarcoma-a translational feasibility study on fusion imaging. Cancers (Basel) 14:4331. doi: 10.3390/cancers14174331
Hung, E. H. Y., Griffith, J. F., Yip, S. W. Y., Ivory, M., Lee, J. C. H., Ng, A. W. H., et al. (2020). Accuracy of ultrasound in the characterization of superficial soft tissue tumors: a prospective study. Skeletal. Radiol. 49, 883–892. doi: 10.1007/s00256-019-03365-z
Lambin, P., Leijenaar, R. T. H., Deist, T. M., Peerlings, J., de Jong, E. E. C., van Timmeren, J., et al. (2017). Radiomics: the bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 14, 749–762. doi: 10.1038/nrclinonc.2017.141
Mao, B., Ma, J., Duan, S., Xia, Y., Tao, Y., Zhang, L., et al. (2021). Preoperative classification of primary and metastatic liver cancer via machine learning-based ultrasound radiomics. Eur. Radiol. 31, 4576–4586. doi: 10.1007/s00330-020-07562-6
Morii, T., Kishino, T., Shimamori, N., Motohashi, M., Ohnishi, H., Honya, K., et al. (2018). Differential diagnosis between benign and malignant soft tissue tumors utilizing ultrasound parameters. J. Med. Ultrason. 45, 113–119. doi: 10.1007/s10396-017-0796-3
Nakamura, T., Matsumine, A., Matsubara, T., Asanuma, K., Yada, Y., Hagi, T., et al. (2017). Infiltrative tumor growth patterns on magnetic resonance imaging associated with systemic inflammation and oncological outcome in patients with high-grade soft-tissue sarcoma. PLoS ONE 12:e0181787. doi: 10.1371/journal.pone.0181787
Neuville, A., Chibon, F., and Coindre, J. M. (2014). Grading of soft tissue sarcomas: from histological to molecular assessment. Pathology 46, 113–120. doi: 10.1097/PAT.0000000000000048
Peeken, J. C., Goldberg, T., Knie, C., Komboz, B., Bernhofer, M., Pasa, F., et al. (2018). Treatment-related features improve machine learning prediction of prognosis in soft tissue sarcoma patients. Strahlenther Onkol. 194, 824–834. doi: 10.1007/s00066-018-1294-2
Peeken, J. C., Knie, C., Kessel, K. A., Habermehl, D., Kampfer, S., Dapper, H., et al. (2019). Neoadjuvant image-guided helical intensity modulated radiotherapy of extremity sarcomas - a single center experience. Radiat. Oncol. 14:2. doi: 10.1186/s13014-019-1207-2
Qi, Y., Zhao, T., and Han, M. (2022). The application of radiomics in predicting gene mutations in cancer. Eur. Radiol. 32, 4014–4024. doi: 10.1007/s00330-021-08520-6
Schneider, N., Strauss, D. C., Smith, M. J., Miah, A. B., Zaidi, S., Benson, C., et al. (2017). The adequacy of core biopsy in the assessment of smooth muscle neoplasms of soft tissues: implications for treatment and prognosis. Am. J. Surg. Pathol. 41, 923–931. doi: 10.1097/PAS.0000000000000867
Sharon, C. E., Straker, R. J., and Karakousis, G. C. (2022). The role of imaging in soft tissue sarcoma diagnosis and management. Surg. Clin. North Am. 102, 539–550. doi: 10.1016/j.suc.2022.04.003
Siegel, R. L., Miller, K. D., and Jemal, A. (2019). Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34. doi: 10.3322/caac.21551
Teixeira, P. A. G., Gay, F., Chen, B., Zins, M., Sirveaux, F., Felblinger, J., et al. (2016). Diffusion-weighted magnetic resonance imaging for the initial characterization of non-fatty soft tissue tumors: correlation between T2 signal intensity and ADC values. Skeletal. Radiol. 45, 263–271. doi: 10.1007/s00256-015-2302-6
van Griethuysen, J. J. M., Fedorov, A., Parmar, C., Hosny, A., Aucoin, N., Narayan, V., et al. (2017). Computational radiomics system to decode the radiographic phenotype. Cancer Res. 77, e104–e107. doi: 10.1158/0008-5472.CAN-17-0339
Wang, K., Lu, X., Zhou, H., Gao, Y., Zheng, J., Tong, M., et al. (2019). Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut 68, 729–741. doi: 10.1136/gutjnl-2018-316204
Weigl, H., Hohenberger, P., Marx, A., Vassos, N., Jakob, J., Galata, C., et al. (2021). Accuracy and safety of ultrasound-guided core needle biopsy of soft tissue tumors in an outpatient setting: a sarcoma center analysis of 392 consecutive patients. Cancers (Basel) 13:5659. doi: 10.3390/cancers13225659
Yu, B., Li, Y., Yu, X., Ai, Y., Jin, J., Zhang, J., et al. (2022). Differentiate thyroid follicular adenoma from carcinoma with combined ultrasound radiomics features and clinical ultrasound features. J. Digit. Imaging 35, 1362–1372. doi: 10.1007/s10278-022-00639-2
Yu, J., Deng, Y., Liu, T., Zhou, J., Jia, X., Xiao, T., et al. (2020). Lymph node metastasis prediction of papillary thyroid carcinoma based on transfer learning radiomics. Nat. Commun. 11:4807. doi: 10.1038/s41467-020-18497-3
Yushkevich, P. A., Piven, J., Hazlett, H. C., Smith, R. G., Ho, S., Gee, J. C., et al. (2006). User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116–1128. doi: 10.1016/j.neuroimage.2006.01.015
Keywords: ultrasonography, soft tissue sarcoma, radiomics, histopathological grade, fibrosarcoma
Citation: Zhao C, Zhang Y, Lv H, Zhuang N, Yu G, Shen Y, Dong L, Wu W, Xie L, Tian Y, Yi Z, Sun D, Wang X and Xie H (2025) Exploring the correlation of radiomic features of ultrasound images and FNCLCC Grading of soft tissue sarcoma. Front. Imaging 4:1436275. doi: 10.3389/fimag.2025.1436275
Received: 21 May 2024; Accepted: 17 February 2025;
Published: 05 March 2025.
Edited by:
Paolo Spinnato, Rizzoli Orthopedic Institute (IRCCS), ItalyReviewed by:
Yun Liang, University of Florida, United StatesSalvatore Claudio Fanni, University of Pisa, Italy
Copyright © 2025 Zhao, Zhang, Lv, Zhuang, Yu, Shen, Dong, Wu, Xie, Tian, Yi, Sun, Wang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingen Wang, ZG9jdG9yd2FuZ183N0AxNjMuY29t; Haiqin Xie, eGllaGFpcWluQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Chenyang Zhao†
Chenyang Zhao† Yusen Zhang
Yusen Zhang Desheng Sun
Desheng Sun Haiqin Xie
Haiqin Xie