
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. ICT, 21 December 2017
Sec. Digital Public Health
Volume 4 - 2017 | https://doi.org/10.3389/fict.2017.00028
There are expectations that stem cell therapy (SCT) will treat many currently untreatable diseases. The Internet is widely used by patients seeking information about new treatments, and hence, analyzing websites is a representative sample of the information available to the public. Our aim was to understand what information the public would find when searching for information on SCT on Google, as this would inform us on how lay people form their knowledge about SCT. We analyzed the content and information quality of the first 200 websites returned by a Google.com search on SCT. Most websites returned were from treatment centers (TCs, 44%) followed by news and medical professional websites. The specialty most mentioned in non-TC websites was “neurological” (67%), followed by “cardiovascular” (42%), while the most frequent indication for which SCT is offered by TCs was musculoskeletal (89%) followed by neurological (47%). 45% of the centers specialized in treating 1 specialty, 10% offer 2, and 45% offered between 3 and 18 different specialties. Of the 78 TCs, 65% were in the USA, 23% in Asia, and 8% in Latin America. None of the centers offered SCT based on embryonic cells. Health information quality (JAMA score, measuring trustworthiness) was lowest for TCs and commercial websites and highest for scientific journals and health portals. This study shows a disconnection between information about SCT and what is actually offered by TCs. The study also shows that TCs, potentially acting in a regulatory gray area, have a high visibility on the Internet.
Stem cell therapy (SCT) offers much potential, and raises significant expectations. Scientific and lay discussion have linked SCT to an extensive spectrum of conditions; however, it is a new and evolving area of medicine that is still largely being investigated. With recent scientific advancements, SCT is moving away from the ethical dilemmas surrounding the use of embryos, and toward the efficacy of treatments (Phinney and Prockop, 2007; Robinton and Daley, 2012; Trounson and McDonald, 2015).
At present, SCs are routinely used as bone marrow transplantation, to treat hematological and immunological cancers and disorders, a practice that has existed for 50 years, and for longer than the term “SCT” been a research topic (Copelan, 2006; Appelbaum, 2007).
The only other SC-related treatment that is currently approved by the U.S. Food & Drug Administration (FDA) is an umbilical cord blood derived product produced by the New York Blood Center and used for specific hematological conditions (FDA, 2012). The European Medicines Agency (EMA) approved its first SCT treatment in 2015 for the use of a corneal SC based therapy for treating corneal diseases (EMA, 2015). Both the FDA and EMA, as well as individual national level agencies, have policies in place for appropriate research and clinical translation of SCs (Bianco et al., 2013a).
Despite these regulations, authorities are increasingly concerned about the rise in availability of SC therapies outside the approved indications or regulated clinical trials, with the potential to harm patients (Lau et al., 2008; Turner and Knoepfler, 2016).
Both the FDA and EMA have issued public and industry guidance recommending that any SCT undertaken should be explicitly approved, or as part of a clinical trial that has been allowed to proceed by the authorities (FDA, 2012; EMA, 2013). In addition, institutional organizations such as The International Society for Stem Cell Research (ISSCR) have produced their own information and recommendations patient handbook (ISSCR, 2017). This is in part due to increased numbers of negative media stories emerging of desperate patients undergoing costly SCT abroad only to go onto develop complications (Berkowitz et al., 2016; Kolata, 2016).
However, more recently, there has been a proliferation of TC closer to home, and the USA is now a major center for TCs offering therapies. A recent study found 351 companies operating 570 TCs in the USA (Turner and Knoepfler, 2016), while there is some evidence of similar practices in Europe (Bianco et al., 2013a). In some cases, these TCs operate in a “gray area” in regulations. FDA guidelines are organized in a three-tier structure for deciding on the level of treatment oversight required (Chirba and Garfield, 2011). The first tier covers non-manipulated SC transplants such as bone marrow; the second tier refers to “minimally manipulated,” “homologous” SCs; and the third tier refers to “more than minimally manipulated,” “non-homologous” SCs, such as embryonic SC (ESC) or induced pluripotent SC (iPS). The description of tier two treatments stipulates that SCs “… that are, minimally manipulated, labeled or advertised for homologous use only, and not combined with a drug or device,” are subject to reduced oversight and FDA approval requirements (Parson, 2006). Similarly, in Europe, SCs are only viewed by the EMA as a drug if they are unmodified cells used for a biologically different function, or modified cells that have been substantially manipulated (including expansion) (Martìn et al., 2014). Cells that require manipulation (e.g., expansion) are legally treated as medicines, rather than transplants, by both the EMA and FDA meaning they have to pass rigorous regulatory requirements (Bianco et al., 2013a). The aforementioned subclause is stated commonly by TCs and is considered to be legal authorization to treat with minimally manipulated SCs outside of a clinical trial protocol. In cases where TCs wish to offer treatments with cultured SCs, which have been expanded in the laboratory, the clause no longer applies, and the TC either has to operate according to approved clinical trial protocols (George, 2011), or to perform the procedure outside of the USA/European oversight. TCs around the world offer treatments for an array of conditions (Lau et al., 2008; Turner and Knoepfler, 2016), with variable levels of evidence, while the majority are not performing these therapies as part of a credible clinical trial (George, 2011).
The Internet is a major resource for information-seeking patients who can find information from different sources. A number of criteria and instruments have been devised to assess health information quality, such as the JAMA score (whether the website shows the main transparency indicators: author, date, references, and ownership) (Silberg et al., 1997) or the presence of the health-on-the-net (HON) code seal, which is provided by a not-for-profit organization, the HON foundation (Boyer et al., 1998).
While most of the studies on health information online focus on quality, in reference to patients seeking specific information on a condition (Chumber et al., 2015; Yaqub and Ghezzi, 2015; Bizzi et al., 2017), the Internet also plays a major role in the diffusion of beliefs. Analyzing the information available on the web can give an idea of how people develop their understanding and knowledge of health-related issues, such as vaccination or supplements (Maki et al., 2015; Aslam et al., 2017).
We have used an approach used in other similar studies to analyze the information on SCT that is available on the Internet (Maki et al., 2015; Aslam et al., 2017). Our aim was to investigate the quality of health information available but also how ordinary people gain knowledge about this topic. For this purpose, we used Google.com, the most widely and internationally used search engine, to gather a significant sample (200 websites) of the existing information. Then, we analyzed the type of website (for instance, whether it is a journalistic or a commercial one) and their content. Finally, we assessed two quality indicators, the presence of HONcode certification and the JAMA score.
We used the same established approach used for previous studies of health information online using Google.com (Maki et al., 2015; Aslam et al., 2017). We forced the use of the international Google.com website using the address www.google.com/ncr (no-country-redirect). To minimize personalization of the results, in particular that due to previous browsing history, the browser’s history and cookies were deleted before searching the string “SCT.” Of note, this will not completely prevent personalization of the results as the IP address would provide our location in the UK. We collected the first 200 websites returned in the search engine result page (SERP). The Google ads on top of the page were not included in the analysis.
21 Websites were excluded from analysis, based on assessments of relevance (e.g., solely relating to veterinary applications or products not including SC), accessibility (e.g., sites that denied access or websites that required membership or payment), and generalization (e.g., website results that when clicked on were not specific to the search topic, or social media pages with frequently changing user content). This left a total of 179 websites included in the final analysis.
There was one instance of two websites from the same treatment center (TC). However, because our aim was to analyze website results available from a Google search, not to identify individual TCs or websites owners, the duplicate was not removed. In addition, we cannot exclude the possibility that multiple websites may be owned by the same parent company.
Websites were individually visited and data recorded according to their typology, whether or not they display the HONcode certification for information quality, and the presence or not of each of the four JAMA criteria (author, date of writing or update, references to evidence statements, and indication of the ownership of the website) (Silberg et al., 1997). For each of these four criteria, we assigned a score of 1 if the information was present, or 0 if absent or unclear.
Website content was then analyzed for an emerging set of criteria. As the structure of the websites varied significantly based on the typology of the site, we adopted a flexible approach to analysis, to obtain relevant data. In some cases, the information was clearly defined on the landing page. In other websites, the landing page was frequently a generic page with little or no information, and analysis of further pages was required to obtain the same level of data. The criteria assessed are listed in Table 1.
Frequencies of website typologies were compared using a two-tailed Fisher’s exact test contingency table using GraphPad Software. When multiple comparisons were performed, we adjusted the P value using the Bonferroni correction. For this purpose, the level of significance was set by dividing the P value of 0.05 by the number of comparisons performed. Multiple comparisons of JAMA score were performed using the Kruskal–Wallis non-parametric test with correction for multiplicity using GraphPad. Hierarchical cluster analysis was used to visualize common patterns in webpage content as previously described (Yaqub and Ghezzi, 2015) was performed using Genesis software version 1.8.1 for Windows (Sturn et al., 2002).
As shown in Figure 1, websites from treatment centers (TCs) offering SCT represent the main typology, with 44% of the search results, followed by news websites (22%). In the top 10 results, however, journalism websites are less visible and professional websites are significantly more represented (30%, 3/10, in the top 10 as compared with 9%, 16/179, in the rest of the SERP).
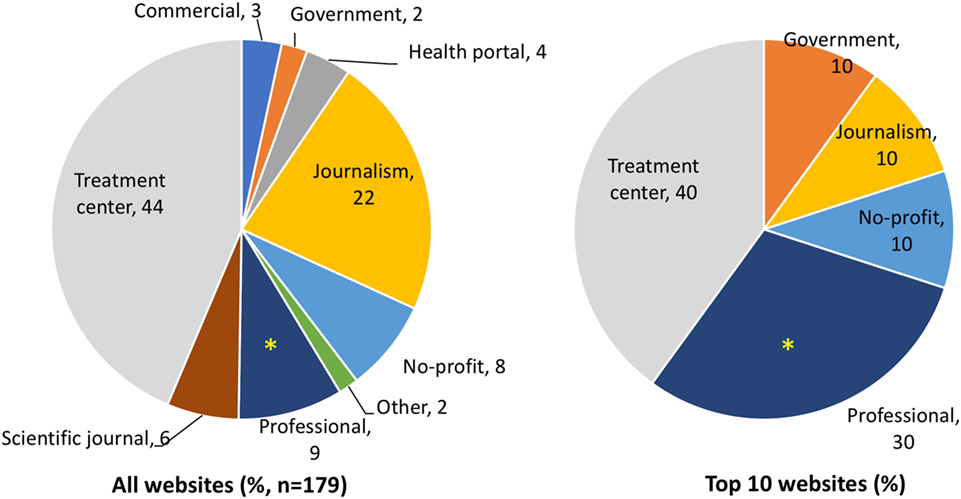
Figure 1. Typologies of websites in the full search engine result page and in the top 10 results by Google. Data are expressed as percentage in the whole search (left) or the top 10 websites returned by Google. *Significantly different (P = 0.048 by two-tailed Fisher’s test).
To investigate whether therapeutic indications (specialties) were discussed differently by different types of websites, we performed a sub-analysis the results of which are shown in Figure 2. There were substantial variations in the types of website discussing each specialty. Neurological treatments were mentioned across all website types, while musculoskeletal treatments were discussed to a similar extent, but predominantly by TCs, commercial, journalism, and professional websites. Musculoskeletal treatment encompassed conditions such as [osteoarthritis, rheumatoid arthritis, and joint (spine, shoulder, hip, knee, ankle, and elbow) injury/damage/degeneration].
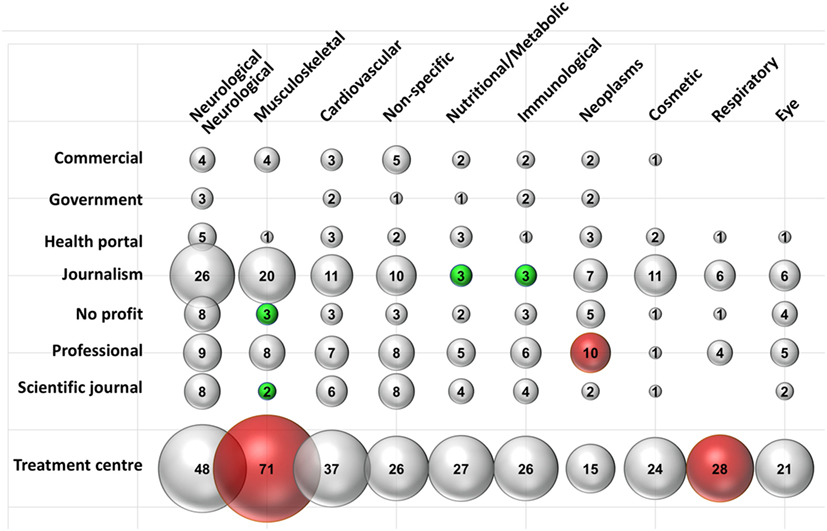
Figure 2. Composition of websites mentioning different disease/specialties. Data are expressed as a percentage of the total number of websites mentioning a type of intervention. Data labels indicate the number of websites. A red or green bubble indicates a disease/specialty, that is; respectively; significantly (P < 0.05) more or less represented in that typology of website; compared with the frequency of the same disease/specialty in the whole search. N in each typology as reported in Table 1. Statistical significance was calculated comparing the observed frequency and the expected one by Fisher’s test.
Within the “musculoskeletal” subcategory the number of those from not-for-profit organizations (observed frequency: 3 out of 14 in total in this category, 21%) and scientific journals (SJs) (2 out of 11, 18%) were underrepresented compared to the whole SERP, where “musculoskeletal” was mentioned in 62% of websites (expected frequency).
Conversely, 71 out of 78 (91%) of the TC discussed “musculoskeletal” compared to the 62% expected from the frequency in the entire SERP. Journalistic websites tended to underrepresent the categories “nutrition and metabolism” (mentioned only in 3 of 40 journalistic websites, 8%) and “immunological” (3 of 40, 8%) compared with their frequency in the whole search (both 27%).
Discussion relating to “neoplasms” was more a common topic in professional websites (mentioned in 10/16 professional websites, 63%, compared to 26% in the whole SERP), while TCs discussed “respiratory” topics more than would be expected (28/78 TC websites, 36% compared to 22% websites mentioning “respiratory” in the whole SERP). Outside of the top 10 most discussed treatment categories, the number of professional websites discussing “wounds and injuries” was also significantly higher (7 of 16, 43% versus 28/179, 16%, in the whole SERP).
We then analyzed each website for the diseases discussed in general terms relating to SCT or as an indication for treatment offered by TCs (according to their websites). Figure 3 shows the list of specialties mentioned by non-TC websites (blue bars), and those for which SCT is offered by TCs (orange bars), both classified using the MESH terms. The specialty mentioned most in non-TC websites was “neurological” (75%), followed by “cardiovascular” (48%), while the most frequent indication for which SCT is offered by TCs was musculoskeletal (89%) followed by neurological (47%).
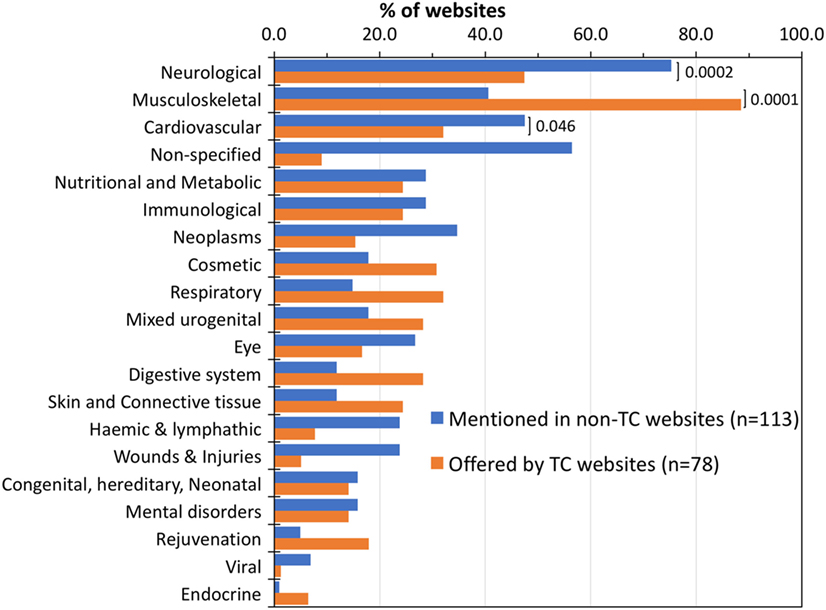
Figure 3. Treatment indications discussed by websites or offered by treatment centers (TCs). Percentage of non-TC websites that discussed each specialty in relation to stem cell therapy (blue bars) compared with treatments offered by TC (orange bars) as detected from their websites. Disease classification as described in Section “Materials and Methods.” Diseases/indications with a value of <5% in both groups are not shown. Brackets show the significance level of the difference in frequency in the two groups by a two-tailed Fisher’s exact test.
Another question we addressed was how many specialties are offered in websites from TCs. A first observation was that we could split TCs as specialist versus generalist. Almost half (n = 35; 45%) of the TCs specialize in only 1 treatment category, 10% offer 2, and the remaining 45% offer between 3 and 18 treatment categories, the average number of categories being 4.8. A hierarchical cluster analysis visualization of the treatment categories offered shows that the majority of TCs that specialize, do so in “musculoskeletal” treatments (Figure 4).
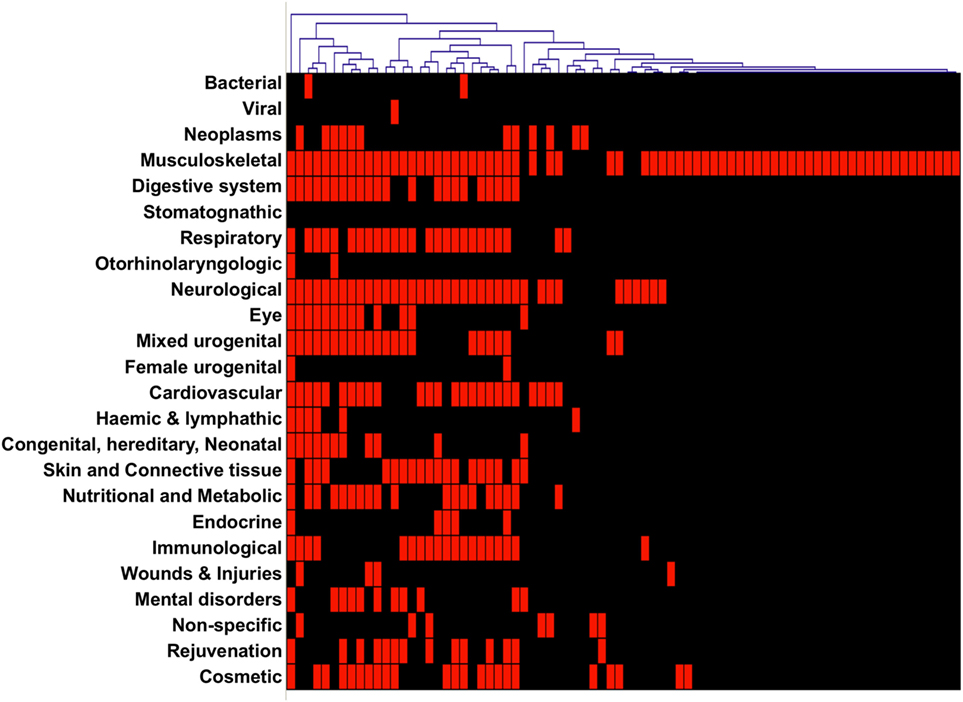
Figure 4. Hierarchical clustering of websites from treatment centers (TCs). Websites were clustered according to the specialties described in their websites as offered by the TC.
Websites of TCs can be used as proxies to study the geographical distribution of TCs offering SCT. TCs identified in our research are focused in the USA, with 65% (n = 66) of all sites globally, while the remaining 35% (n = 36) are spread throughout the world in single figure numbers (Figure 5). Notably, there are no TCs with a website returned by Google.com in Western Europe.
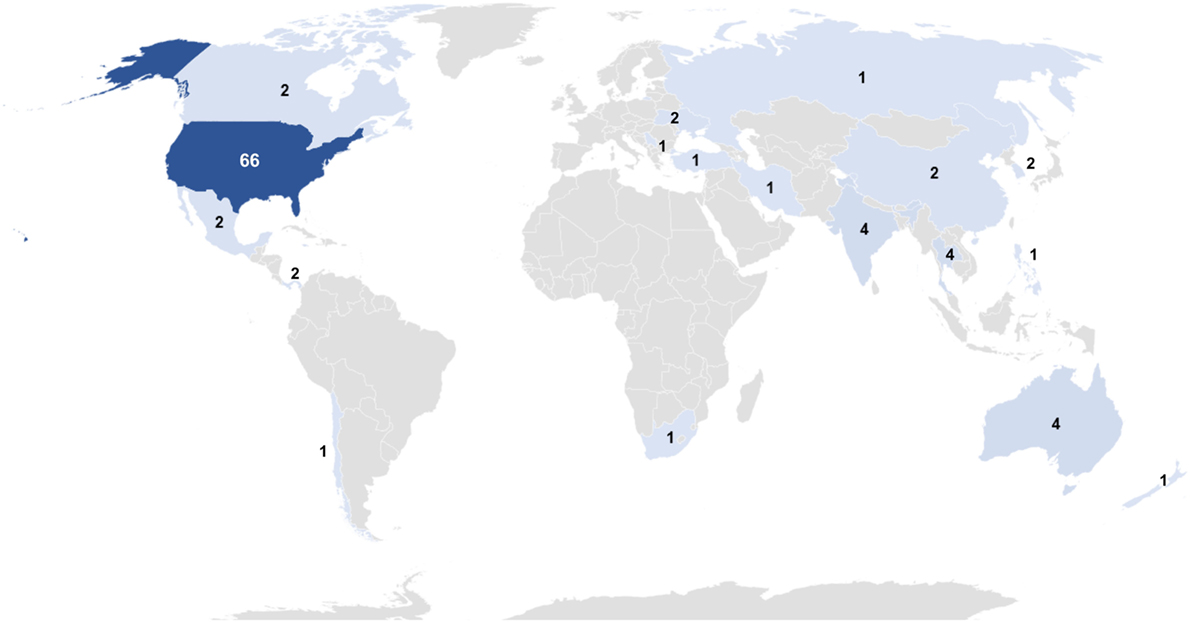
Figure 5. Global distribution of treatment centers with a web page in English. Data are shown as a heat map; darker color indicates higher numbers. Full results: USA, 66; Australia, 4; India, 4; Thailand, 4; Canada, 2; Cayman Islands, 2; China, 2; Mexico, 2; Panama, 2; South Korea, 2; Ukraine, 2; Chile, 1; Iran, 1; Jamaica, 1; Malta, 1; New Zealand, 1; Philippines, 1; Russia, 1, Serbia, 1; South Africa, 1; and Turkey, 1.
Eight websites advertise SC culturing, and an additional three are unclear on the subject. The websites that definitively offer the service are based in USA (2), USA/Cayman Islands (2), USA/Jamaica (1), USA/Mexico (1), Mexico (1), and Thailand (1).
Of the TCs based in the USA, a total of at least 163 TC sites (TCs frequently have multiple sites within each state and/or in interstate locations, the details for which are not always fully detailed) were distributed widely across 40 out of 50 states, as follows: Florida, 16; California, 14; Arizona, 12; Texas, 12; New York, 8; Colorado, 7; Ohio, 6; Pennsylvania, 6; Virginia, 6; Oklahoma, 5; Georgia, 4; Illinois, 4; Maryland, 4; Minnesota, 4; Missouri, 4; Nevada, 4; New Jersey, 4; Utah, 4; Alaska, 3; Indiana, 3; Kentucky, 3; Arkansas, 2; Iowa, 2; Kansas, 2; Louisiana, 2; Michigan, 2; Mississippi, 2; New Mexico, 2; Oregon, 2; Tennessee, 2; Vermont, 2; Washington, 2; Hawaii, 1; Idaho, 1; Massachusetts, 1; Nebraska, 1; North Carolina, 1; West Virginia, 1; Wisconsin, 1; and Wyoming, 1.
When analyzing all websites, not only those from TCs, anecdotal discussion suggests a more widespread availability of SCT. While availability is still predominantly focused in Northern America (103), every geographical region is represented with 45 websites discussing availability in Asia, 28 in Europe, 22 in Central America, 9 in Oceania, 8 in the Caribbean, 4 in Southern America, 3 in the Middle East, and 2 in Africa.
Of the 78 websites from TCs, only 10 explicitly mentioned that SCT was part of a clinical trial (for instance, the websites of the Mayo clinic and that of the MD Anderson Hospital, see Data Sheet 1 in Supplementary Material). Others specifically stated that “treatments offered are not research or a clinical trial”1 or that they “are part of an ongoing FDA clinical trial study and now also offers SCT to patients not enrolled in the study.”2
The first issue we focused on was whether the source of stem cells used was adult stem cells (ASCs) or ESCs. As shown in Figure 6, ASC from bone marrow or adipose tissue is the sources of stem cells predominantly used for treatments by TCs. It should be noted that these are the sources used for treatment. Although 42 (54%) TC websites discuss both ESCs and ASCs as potential sources, none of them uses ESC for their treatments. It should also be noted that multiple sources were frequently used by individual TCs.
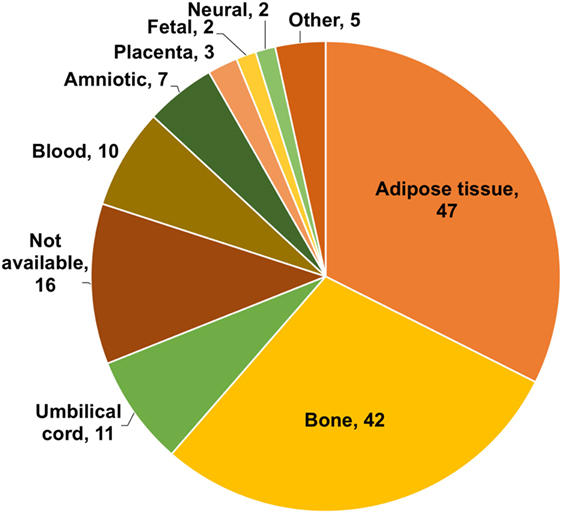
Figure 6. Sources of stem cells used by treatment centers. Numbers indicate the number of treatment centers (TCs). The chart does not include sources of stem cells used by only one TC (these were as follows: endothelial, IPC, dental, insulin producing, and retinal).
We performed a subjective assessment of the tone used by each website, whether positive about SCT, negative or neutral. 100% of commercial and TC websites had a positive outlook when discussing SCT (commercial websites, 6/6 positive; TC, 78/78 positive). Other website typologies had varying ratios of positive and neutral website as follows: professional, 13/16 positive (81%), 3/16 neutral (19%); government, 3/4 (75%) positive, 1/4 (25%) neutral; not-for-profit, 8/14 (57%) positive, 6/14 (43%) neutral; SJ, 6/11 (55%) positive 5/11 (45%) neutral; journalism, 14/40 (35%) positive, 26/40 (65%) neutral; and health portal (HP), 2/7 (29%) positive, 5/7 (71%), neutral. No websites had an overall negative approach to SCT. Two websites had an overtly ethical stance toward the use of ESCs, made clear by direct statements, such as “…100% Pro-life and believe that the harvesting of embryonic stem cells is in fact taking a life…” [About SCT: (Online) Indy Regenerative Medicine; available from: http://indyregen.com/about-stem-cell-therapy/ (accessed 10/2016). Archived at http://www.webcitation.org/6quuhy5Vg] in one case, and by a clear focus on ASCs and an affiliation to a “pro-life” website in the case of the other [Life-Saving Stem Cells: Discover, Learn, share: (Online) Stem Cell Research Facts; available from: http://www.stemcellresearchfacts.org/ (accessed 10/2016). Archived at http://www.webcitation.org/6quwQDBoE].
Another aspect of our research was to look at controversial issues that were discussed across all website typologies. One of these topics was the discussion of the cost of treatments, which is additionally one of the criteria for “health information quality” according to the Association of Health Care Journalists (Schwitzer, 2004). It is important to note that, of the websites from TCs, only 30% talked about the costs of treatment in any way, and most of these still did not directly offer prices on their websites.
Figure 7 reports the JAMA score across different typologies of websites. Commercial and TC websites scored lower than most categories. JAMA score of TC websites was significantly lower than that of HPs, journalism, not-for-profit and SJs (P < 0.0001 by Kruskal–Wallis multiple comparisons test; adjusted P value after correction for multiple comparison using statistical hypothesis testing). Not-for-profit websites had a lower JAMA score than journalism (P = 0.0251) and SJs (P = 0.0004). Professional websites scored lower than SJs (P = 0.0033). A criterion used in health information quality is that the JAMA score should be at least 3, and the analysis with this criterion parallel those obtained for the median score, with TC (0% of websites with JAMA score ≥3) and commercial websites (25%) scoring the lowest and SJs the highest (100%).
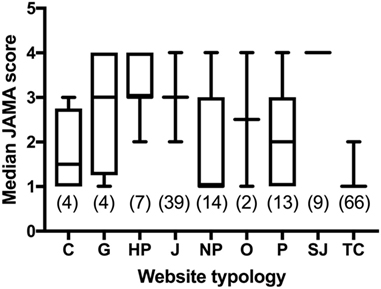
Figure 7. Comparison of JAMA score in websites from different typologies. Data are reported as median and interquartile range. Numbers in parentheses indicate the number of websites in each category that could be scored for the JAMA criteria, excluding those no longer accessible at the time of the analysis. Legend: C, commercial; G, government; HP, health portal; J, journalism; NP, no profit; O, other; P, professional; SJ, scientific journal; TC, treatment center.
Only nine websites (5% of the total) displayed an HON code certificate: five HPs, three journalistic sites, and one professional site.
Our study found that websites from TC are the most common website categories, accounting for just over 40% of the SERP. They were also given high visibility by Google, being present in the same proportion in the top 10 websites returned. This is in contrast with what we observed with commercial websites in this and other studies, which are usually given a low ranking by Google and are never present in the top 10 results.
We were also surprised that SCT was offered by so many TCs, as the scientific literature usually describes SCT as an area of clinical research that has not yet made the final translational step (Bianco et al., 2013b). On the other hand, the prevalence of the musculoskeletal specialty as a treatment offered by TCs is consistent with this indication also being prevalent among those currently addressed by clinical trials (Trounson and McDonald, 2015).
The second most frequent category of websites is journalistic. The majority of these sites are examples of objective journalism; however, we also found a number of cases where the story predominantly focused on sport stars’ treatments or patients’ miraculous recoveries, without going into depth about the realities of SCT (McKean, 2016; Owens, 2016; White, 2016). This media hype about athletes’ use of stem cells had already been highlighted by other studies (Caulfield and McGuire, 2012; Du et al., 2016).
The measure of trustworthiness by the JAMA score only assesses one basic dimension of health information quality, and its main limitation is that it does not take into account the content of the websites. However, within this limitation, we found that TCs have the lowest median score, even lower then commercial websites, which we found to score the lowest in several studies on different health topics (Maki et al., 2015; Aslam et al., 2017; Bizzi et al., 2017). The HON code encompasses more dimensions of information quality (Boyer et al., 1998); however, only nine websites displayed an HON code certification and none of them where websites from TCs.
In theory, some typologies such as not-for-profits, SJs, and HPs should have a more independent and unbiased viewpoint. However, these three typologies only account for 18% of the websites in our research.
Another consideration we can make from the specialties discussed by journalistic and TC websites shown in Figure 1 is that, although neurological applications of SCT is the most newsworthy, musculoskeletal indications are the most commonly offered by TCs, which may indicate a difference between availability of treatments and public expectations.
We did not find TCs offering treatments using ESCs. While it is possible that some TC may not mention this on their websites, it is important to note that a similar study from 2007 showed a greater percentage use of fetal SCs (21%) and ESCs (11%) (Lau et al., 2008). On the other hand, only a few TCs were found in the 2007 study, indicating the expansion and marketization of SCT.
Although SCT may become a commonplace procedure, at present there is a mismatch between capabilities and expectations, and TCs are offering treatments whose efficacy is largely unproven.
Stem cells are a very popular subject and a 2009 study from the Pew Research Center found that 52% of Americans know the definition of stem cell in terms of pluripotentiality (Kohut, 2009). How ordinary people come to know or believe what they do is an important research question (Hardin, 2014). We build knowledge from information available from different and varied sources. A 2015 study analyzed the portrayal of STC in daily English language newspapers and reported that press coverage focuses more on its translational potential rather than on ethical issues (Kamenova and Caulfield, 2015). Press coverage was very optimistic in terms of translational timelines, with an overwhelmingly optimistic view, particularly in the UK.
With the limitation that the study is restricted to websites in English, the present study shows the potential usefulness of studying websites returned by Google to analyze how the public gains an understanding of stem cells. In fact, a Google search returns a wide range of websites representing various types of news outlets, public health organizations, professional and commercial organizations. Given that so many websites were actually from TCs, this might represent an easy way of analyzing their geographical distribution and type of procedures they perform. However, this is an issue where limiting our research to websites in English cannot give a reliable idea of the global distribution of TCs, and this could easily explain the prevalence of TCs in the USA.
Another issue is that of the personalization of the results returned by Google. On one hand, we acknowledge that a search cannot be completely anonymous, and the approach used of deleting cookies and browser’s history avoids personalization based on previous search behavior but does not hide the geolocation of the computer, that is, provided by the IP address of the network. On the other hand, this type of anonymized search may not be representative of the behavior of the average user, who will not take these precautions. However, it must be said that there is little scientific evidence that search personalization affects health search results greatly. The concept of Google creating a “filter bubble” though the personalization of search results provided became popular with the book “The Filter Bubble: What the Internet Is Hiding from You” by the political activist Eli Pariser. While this was taken up by various magazines, there is little empirical evidence for a search bubble in the scientific literature and, specifically in health-related searches, the only published research indicate that health searches are the least affected by personalization (Hannak et al., 2013). A recent study using 1,200 virtual agents performing health searches after different training session of repeated searches on Google could not find any effect of previous searches on search results (Haim et al., 2016).
Of course, having a website displayed in the Google SERP does not mean that this will be read by the users. For instance, some users may then only look at news websites. Analyzing this aspect would require performing studies on human volunteers, for instance using eye-tracking software (Granka et al., 2004). Another important factor in the attention website receive is whether they are in the top 10 results returned by Google. In other studies, we consistently found that Google ranks commercial websites low (Chumber et al., 2015; Maki et al., 2015; Yaqub and Ghezzi, 2015; Aslam et al., 2017), and this is also what we observed here as the first commercial website ranked 47th in the present search, although the small number of commercial websites in the whole search (six) make it impossible to draw conclusions. However, in previous studies on influenza prevention, influenza vaccine (Maki et al., 2015), and migraine (Yaqub and Ghezzi, 2015), the ranking by Google favored evidence-based medicine approaches, giving complementary/alternative medicine and anti-vaccine websites a low ranking. In the present sample, TCs were present with the same frequency in the top 10 websites and the whole search.
Finally, our work should be paralleled by a similar study on the information available on social media, which we did not analyze in this study and where many issues, from information quality and the bubble effect, may well be quantitatively different from websites. Although the most recent study available from the Pew Research Institute, indicated that, in 2013, when seeking health information, users use mainly search engines (77%) and only 1% social network (Fox and Duggan, 2013), these percentages are likely to have changed and, in any case, social media may expose users to health information even if this is not searched for.
In conclusion, this study shows that there is insufficient unbiased, evidence-based information available about SCT. TCs in many countries offer SCT, frequently operating in a “gray area” of regulation, and these have a good visibility on the Internet. Without sufficient regulatory oversight, there is a risk of SCT becoming a form of complementary medicine that is at best unscientific and at worst is unsafe.
DM collected data; PG, IB, and DM analyzed data and wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was carried out as an independent research project of fourth year medical student DM at the Brighton and Sussex Medical School.
The Supplementary Material for this article can be found online at http://www.frontiersin.org/articles/10.3389/fict.2017.00028/full#supplementary-material.
Appelbaum, F. R. (2007). Hematopoietic-cell transplantation at 50. N. Engl. J. Med. 357, 1472–1475. doi: 10.1056/NEJMp078166
Aslam, R., Gibbons, D., and Ghezzi, P. (2017). Online information on antioxidants: information quality indicators, commercial interests, and ranking by Google. Front. Public Health 5:90. doi:10.3389/fpubh.2017.00090
Berkowitz, A. L., Miller, M. B., Mir, S. A., Cagney, D., Chavakula, V., Guleria, I., et al. (2016). Glioproliferative lesion of the spinal cord as a complication of “stem-cell tourism”. N. Engl. J. Med. 375, 196–198. doi:10.1056/NEJMc1600188
Bianco, P., Barker, R., Brüstle, O., Cattaneo, E., Clevers, H., Daley, G. Q., et al. (2013a). Regulation of stem cell therapies under attack in Europe: for whom the bell tolls. EMBO J. 32, 1489–1495. doi:10.1038/emboj.2013.114
Bianco, P., Cao, X., Frenette, P. S., Mao, J. J., Robey, P. G., Simmons, P. J., et al. (2013b). The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat. Med. 19, 35–42. doi:10.1038/nm.3028
Bizzi, I., Ghezzi, P., and Paudyal, P. (2017). Health information quality of websites on periodontology. J. Clin. Periodontol. 44, 308–314. doi:10.1111/jcpe.12668
Boyer, C., Selby, M., Scherrer, J. R., and Appel, R. D. (1998). The health on the net code of conduct for medical and health websites. Comput. Biol. Med. 28, 603–610. doi:10.1016/S0010-4825(98)00037-7
Caulfield, T., and McGuire, A. (2012). Athletes’ use of unproven stem cell therapies: adding to inappropriate media hype? Mol. Ther. 20, 1656–1658. doi:10.1038/mt.2012.172
Chirba, M.A., and Garfield, S.M. (2011). FDA oversight of autologous stem cell therapies: legitimate regulation of drugs and devices or groundless interference with the practice of medicine. J. Health Biomed. Law 7, 233–272.
Chumber, S., Huber, J., and Ghezzi, P. (2015). A methodology to analyze the quality of health information on the Internet: the example of diabetic neuropathy. Diabetes Educ. 41, 95–105. doi:10.1177/0145721714560772
Copelan, E. A. (2006). Hematopoietic stem-cell transplantation. N. Engl. J. Med. 354, 1813–1826. doi:10.1056/NEJMra052638
Du, L., Rachul, C., Guo, Z., and Caulfield, T. (2016). Gordie Howe’s “miraculous treatment”: case study of Twitter users’ reactions to a sport celebrity’s stem cell treatment. JMIR Public Health Surveill. 2, e8. doi:10.2196/publichealth.5264
EMA. (2013). Stem-Cell-Therapy Treatments. European Medicines Agency. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/04/news_detail_001769.jsp&mid=WC0b01ac058004d5c1
EMA. (2015). Holoclar. European Medicines Agency. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002450/human_med_001844.jsp&mid=WC0b01ac058001d124
FDA. (2012). FDA Warns About Stem Cell Claims. US Food and Drug Administration. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm286155.htm
Fox, S., and Duggan, M. (2013). Health Online 2013. Washington, DC: Pew Internet & American Life Project.
George, B. (2011). Regulations and guidelines governing stem cell based products: clinical considerations. Perspect. Clin. Res. 2, 94–99. doi:10.4103/2229-3485.83228
Granka, L. A., Joachims, T., and Gay, G. (2004). “Eye-tracking analysis of user behavior in WWW search,” in Proceedings of the 27th Annual International ACM SIGIR Conference on Research and Development in Information Retrieval (Sheffield, United Kingdom: ACM).
Haim, M., Arendt, F., and Scherr, S. (2016). Abyss or shelter? On the relevance of web search engines’ search results when people google for suicide. Health Commun., 32, 253–258. doi:10.1080/10410236.2015.1113484
Hannak, A., Sapiezynski, P., Molavi Kakhki, A., Krishnamurthy, B., Lazer, D., Mislove, A., et al. (2013). “Measuring personalization of web search,” in Proceedings of the 22nd International Conference on World Wide Web (New York: ACM), 527–538.
ISSCR. (2017). Guidelines for Stem Cell Research and Clinical Application. International Society for Stem Cell Research. Available at: http://www.isscr.org/professional-resources/policy/2016-guidelines/guidelines-for-stem-cell-research-and-clinical-translation
Kamenova, K., and Caulfield, T. (2015). Stem cell hype: media portrayal of therapy translation. Sci. Transl. Med. 7, 278s274. doi:10.1126/scitranslmed.3010496
Kohut, A. (2009). “Public praises science; Scientists fault public, media; scientific achievements less prominent than a decade ago,” in Pew Research Center for the People and the Press, Washington, DC (Washington, DC: Pew Research Center). Available at: http://www.people-press.org/2009/07/09/public-praises-science-scientists-fault-public-media
Kolata, G. (2016). A Cautionary Tale of ‘Stem Cell Tourism’. New York, NY: New York Times. Available at: https://www.nytimes.com/2016/06/23/health/a-cautionary-tale-of-stem-cell-tourism.html?_r=0
Lau, D., Ogbogu, U., Taylor, B., Stafinski, T., Menon, D., and Caulfield, T. (2008). Stem cell clinics online: the direct-to-consumer portrayal of stem cell medicine. Cell Stem Cell 3, 591–594. doi:10.1016/j.stem.2008.11.001
Maki, A., Evans, R., and Ghezzi, P. (2015). Bad news. Analysis of the quality of information on influenza prevention returned by Google in English and Italian. Front. Immunol. 6:616. doi:10.3389/fimmu.2015.00616
Martìn, P. G., Martinez, A. R., Lara, V. G., and Naveros, B. C. (2014). Regulatory considerations in production of a cell therapy medicinal product in Europe to clinical research. Clin. Exp. Med. 14, 25–33. doi:10.1007/s10238-012-0213-6
McKean, M. L. (2016). Northland Woman Believes Stem Cell Therapy Saved Her Leg from Amputation. Fox4 KC News. Available at: http://fox4kc.com/2016/06/24/northland-woman-believes-stem-cell-therapy-saved-her-from-leg-amputation/
Owens, R. (2016). Stem Cell Therapy Going Mainstream. CBS DFW. Available at: http://dfw.cbslocal.com/2016/05/02/stem-cell-therapy-going-mainstream/
Parson, A. (2006). The long journey from stem cells to medical product. Cell 125, 9–11. doi:10.1016/j.cell.2006.03.024
Phinney, D. G., and Prockop, D. J. (2007). Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells 25, 2896–2902. doi:10.1634/stemcells.2007-0637
Robinton, D. A., and Daley, G. Q. (2012). The promise of induced pluripotent stem cells in research and therapy. Nature 481, 295–305. doi:10.1038/nature10761
Schwitzer, G. (2004). A statement of principles for health care journalists. Am. J. Bioeth. 4, W9–W13. doi:10.1080/15265160490908086
Silberg, W. M., Lundberg, G. D., and Musacchio, R. A. (1997). Assessing, controlling, and assuring the quality of medical information on the Internet: Caveant lector et viewor – let the reader and viewer beware. JAMA 277, 1244–1245. doi:10.1001/jama.1997.03540390074039
Sturn, A., Quackenbush, J., and Trajanoski, Z. (2002). Genesis: cluster analysis of microarray data. Bioinformatics 18, 207–208. doi:10.1093/bioinformatics/18.1.207
Trounson, A., and McDonald, C. (2015). Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17, 11–22. doi:10.1016/j.stem.2015.06.007
Turner, L., and Knoepfler, P. (2016). Selling stem cells in the USA: assessing the direct-to-consumer industry. Cell Stem Cell 19, 154–157. doi:10.1016/j.stem.2016.06.007
U.S. National Library of Medicine. (2014). MeSH Tree Structures – 2015. Bethesda, MD: U.S. National Library of Medicine.
White, S. (2016). Man with Multiple Sclerosis Can Walk Again after Radical Stem Cell Therapy. London: The Daily Mirror. Available at: http://www.mirror.co.uk/news/uk-news/man-multiple-sclerosis-can-walk-8491229
Yaqub, M., and Ghezzi, P. (2015). Adding dimensions to the analysis of the quality of health information of websites returned by Google: cluster analysis identifies patterns of websites according to their classification and the type of intervention described. Front. Public Health 3:204. doi:10.3389/fpubh.2015.00204
Keywords: Google, Internet, health information, information quality, stem cell therapy, stem cells
Citation: Meehan D, Bizzi IH and Ghezzi P (2017) Stem Cell Therapy on the Internet: Information Quality and Content Analysis of English Language Web Pages Returned by Google. Front. ICT 4:28. doi: 10.3389/fict.2017.00028
Received: 04 September 2017; Accepted: 04 December 2017;
Published: 21 December 2017
Edited by:
Philip AbdelMalik, Public Health Agency of Canada, CanadaReviewed by:
Laszlop Balkanyi, European Centre for Disease Prevention and Control, SwedenCopyright: © 2017 Meehan, Bizzi and Ghezzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pietro Ghezzi, cC5naGV6emlAYnNtcy5hYy51aw==, cGlldHJvLmdoZXp6aUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.