- Department of Neurology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States
Purpose of the review: This review will elucidate reasons to explain why women may be at greater risk for Alzheimer's disease.
Recent findings: Potential mechanisms to explain sex and gender differences in Alzheimer dementia include: differences in risk associated with the apolipoprotein E 4 allele; telomere shortening- which is linked with neurodegeneration, higher incidence of depression and insomnia in women as psychiatric co-morbidities which are linked with an increased Alzheimer disease risk, disorders of pregnancy including gestational hypertension and preeclampsia and psychosocial factors such as educational level which may contribute to differences in cognitive reserve.
Summary: The sex and gender differences in Alzheimer's disease can be explained by biological and psychosocial factors.
Introduction
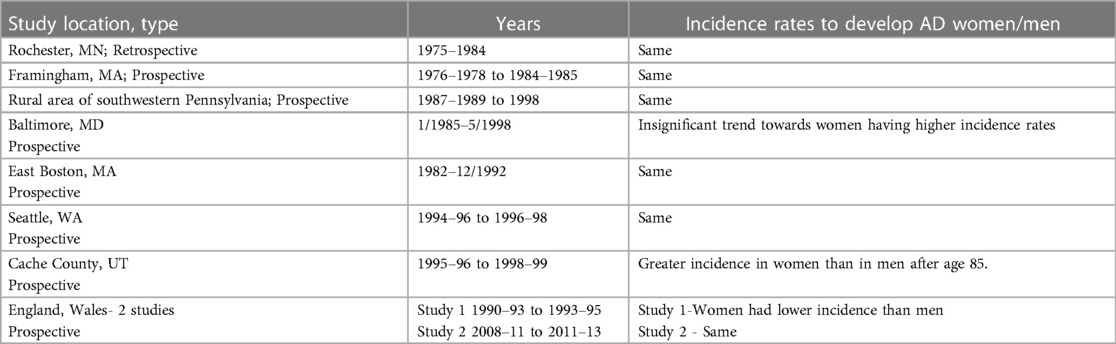
Alzheimer's disease (AD) is the most common cause of dementia affecting more than 5.5 million Americans, two thirds of whom are women (1). Age is a known risk factor for development of AD, with the risk doubling for each decade after age 60. It is a well-known fact that women live longer, thereby explaining the difference in prevalence of the disease. Incidence studies examining sex differences in AD are equivocal. A majority of studies do not demonstrate any sex differences in the incidence of AD. See Table 1 (2–8) A meta-analysis of seven population -based studies looking at the incidence of AD found that the increase in the incidence rate slows after age 85. In contrast to this age effect, the meta-analysis showed a significant effect of sex, where the odds ratio of women developing AD compared to men was 1.56 (9). The Framingham study suggests that the difference in disease prevalence is due to a “survivor bias”, as men who survive beyond age 65 may have lower cardiovascular risk factors which may explain the lower risk of dementia compared to women after the age of 80 years (10).
There are multiple potential biological mechanisms that may explain the sex and gender differences in AD. These include differences in genetic risk, response to aging, hormonal effects, psychiatric and pregnancy co-morbidities as well as lifestyle/psychosocial factors effecting cognitive reserve. This review will explore these mechanisms.
Apolipoprotein E4-genetic risk
The apolipoprotein E4 (APOE4) allele is the most potent genetic risk factor for late onset sporadic AD. The APOE4 allele generates a dose dependent risk of developing AD, where patients with the E4/E4 genotype have an increased risk of AD compared to the E4/E3 genotype (11). The apolipoprotein E (APOE) protein is widely distributed throughout the human body. In the brain, astrocytes primarily produce APOE (12). The APOE protein in AD plays an important role in amyloid-beta protein transcription, production, aggregation and clearance (13).
The risk of developing AD related to the APOE4 allele effects both sexes equally. However, women who carry the APOE4 allele are more likely to develop mild cognitive impairment (MCI) than men. In addition, among patients with MCI, women with either the APOE3/3 or APOE3/4 genotypes, are more likely to develop AD compared to men (14). In a meta-analysis of 27 studies with 58,000 participants, looking at patients with 1 copy of APOE4 allele, women were at a fourfold increased risk to develop AD at younger ages, 65–75 years (15). Further, women carriers had increased total tau in cerebrospinal fluid, a biomarker in AD indicative of neuronal degeneration (16). However, these studies may be confounded by the fact that they did not control for educational level. A study which looked at the longitudinal rates of change from baseline in 398 MCI patients showed women with MCI had greater rates of cognitive and functional progression than men. The effect was greater in APOE4 carriers. In this study educational attainment was statistically greater in men, but the difference was small (17). Available data suggests that the APOE4 may modulate the risk of AD in a sex specific manner.
Telomere shortening and aging
Telomeres are the DNA structures that cap the ends of chromosomes. They are important to protect chromosomes from degradation. Telomere shortening has been associated with limited stem cell function, regeneration and organ maintenance in aging. The enzyme telomerase offsets this reaction by adding repeat telomeres to the terminal DNA. A reduced telomere length is associated with AD especially in females (18). Evidence demonstrates that APOE4 carriers have shorter leukocyte telomere lengths compared to noncarriers (19). This supports the idea that the APOE4 carriers undergo premature aging.
Telomere length demonstrate significant sex differences. In adulthood, women have significantly longer telomere length than men of the same age and this effect appears to be driven by estrogen. Estrogen both increases telomerase activity and decreases oxidative stress (20). In an interesting study, conducted over two years, healthy postmenopausal women who were APOE4 carriers showed significant leucocyte telomere shortening compared to noncarriers. Further the APOE4 carriers who remained on hormonal replacement therapy did not show telomere attrition and this effect was not seen in the noncarriers. Thus, suggesting that hormonal therapy might modulate AD risk for those who are vulnerable (21). Another explanation for this difference may be related to sex differences in educational attainment as this appears to have a protective effect against telomere shortening (22).
Hormonal effects
Initial studies from Cache county Utah supported the idea that there may be a “window of opportunity” for hormonal therapy on cognition. In a population- based study of over 2,000 nondemented women over age 65 (when the covariates of lower education, depression, and APOE ε4 status were controlled) lifetime hormonal replacement therapy (HRT) use was associated with a better baseline mini-mental status exam scores and a slower rate of cognitive decline (23). In another prospective study of 1,889 women from Cache county Utah to look at incidence of dementia those who used HRT had a reduced risk of AD compared with non-HRT users (adjusted HR, 0.59; 95% CI, 0.36–0.96). Risk varied with duration of HRT use, such that the sex-specific increase for women disappeared with more than 10 years of use (24). It appears from these studies that the beneficial effect of HRT is dependent on the timing. However, the generalizability of these studies is unclear as both were performed in a single county and educational and socioeconomic factors may have influenced those who could participate.
In 2003, a large randomized controlled trial, The Women's Health Initiative Memory study (WHIMS) showed that postmenopausal women, ages 65–79, who had not had a hysterectomy, when treated with estrogen and progesterone compared to controls had a doubled risk of dementia. The increased risk of dementia in women treated with hormonal therapy would result in 23 new cases of dementia per 10,000 women/year (25). This well-designed trial clearly demonstrated the negative effects of postmenopausal hormone replacement on cognition.
There have been mixed results in studies to see if there is indeed a therapeutic window for HRT. A prospective cohort study showed that women who used any type of HRT within 5 years of menopause had a 30% less risk of AD. This benefit was also realized in women who used HRT more than 10 years after menopause (26). A 20-year prospective cohort study from Finland following women ages 47–56 did not provide strong evidence that postmenopausal hormone replacement therapy prevents AD. Although, a protective association between long-term (>10 years) self-reported use of HT and AD was observed. This finding indirectly supports the effectiveness of HT if started in the early postmenopausal period (26).
A recent retrospective population- based study demonstrated that women with 5 or more pregnancies had 1.7-fold increased risk of AD compared with women with 1–4 completed pregnancies. In addition, women who had incomplete pregnancies showed half the level of AD risk compared with those who never experienced an incomplete pregnancy (27). Whether these findings are related to hormonal changes, differences in medical comorbidities or education/socioeconomic status remains to be seen.
Pregnancy complications
Retrospective studies have shown that women who had preeclampsia have higher stroke risks even decades later. See Table 2 Pregnancy related complications unmasks those women at risk for cerebrovascular complications. The increased risk of stroke put these women at risk for vascular dementia and increase risks of cognitive decline. Another study documented those women who had hypertensive disorders of pregnancy had an increased risk of cognitive problems primarily associated with poorer working memory and verbal learning 15 years after pregnancy (28).
Vascular dementia can be caused by either a strategically placed stroke or from small vessel disease. The typical cognitive problems found in patients with vascular dementia are slowed processing speed, impairments in executive function and visual memory, whereas verbal learning is not as affected. This is different from the results of the hypertensive disorder of pregnancy study which showed worsened working memory and verbal learning. Problems which are more in those with seen in with AD. Potentially pregnancy complications may impact cognition by contributing to a mixed dementia. Mixed dementia from both AD and vascular causes is common with small vessel disease of different types underpinning both etiologies (29).
Psychiatric co-morbidities-depression and insomnia
Depression increases the risk of AD and women have twice the risk of depression compared to men (30). There is evidence that early life depression can act as a risk factor for later life dementia, and that depression later in life may be a prodromal feature to dementia (31). A meta-analysis of available studies showed a positive correlation between the length of time after a diagnosis of depression and the risk of developing AD, suggesting that depression is a risk factor for AD (30).
In the WHIMS study women with depression were almost twice as likely to develop MCI and AD (32). The risk of developing AD appears to be related to both the severity and timing of the depression. For example, one study demonstrated that patients followed for a mean of 27 months with MCI and active depression within the last 2 years had a 41.7% conversion to AD as compared to 31.6% conversion to AD in patients with a more remote history of depression (33).
Insomnia may also be a risk factor for accelerated cognitive decline and AD. Cognitive ability is sleep dependent, especially memory consolidation. Several longitudinal studies confirmed that patients with insomnia were twice as likely to have cognitive decline or a diagnosis of AD (34, 35). Women have a higher prevalence of insomnia. It is not clear if insomnia is an independent risk factor for AD or linked due to its interplay with stress and depression.
Educational status-cognitive reserve
Lower education levels and occupational attainment pose similar risk for AD in both women and men. Women, older than 65 years of age, have had fewer opportunities for higher education and professional achievement, directly affecting their cognitive resilience, thus putting them at risk. Recent population trends indicate that the education gap between women and men is in decline with more women in the workplace. In addition, women are achieving both greater professional success and financial status (36).
Conclusions
Women bear a larger burden of the Alzheimer's disease epidemic. The difference in risks for this disorder can be explained by sex and gender specific variances in genetics, response to aging, hormonal influences, psychiatric conditions as well as psychosocial factors.
Take Home Points
1. Alzheimer's disease is more prevalent in women
2. The risks associated with the APOE allele are stronger in women
3. There are sex differences in how telomeres respond to aging and hormonal changes
4. There may be a beneficial window where estrogen exposure improves cognition for women at risk for AD
5. Hypertensive disorders of pregnancy can contribute to risks of dementia
6. Gender differences in psychiatric co-morbidities especially depression as well as educational status may also impact AD risk
Author contributions
MO: Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with the author MO. MO does consultant work for Crico Insurance and Teladocs.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimer’s Dement (2017) 13:325–73. doi: 10.1016/j.jalz.2017.02.001
2. Rocca WA, Cha RH, Waring SC, Kokmen E. Incidence of dementia and Alzheimer’s disease: a reanalysis of data from Rochester, Minnesota, 1975–1984. Am J Epidemiol. (1998) 148(1):51–62. doi: 10.1093/oxfordjournals.aje.a009560
3. Bachman DL, Wolf PA, Linn RT, Knoefel JE, Cobb JL, Belanger AJ, et al. Incidence of dementia and probable Alzheimer’s disease in a general population: the Framingham study. Neurology. (1993) 43(3 Pt 1):515–9. doi: 10.1212/WNL.43.3_Part_1.515
4. Ganguli M, Dodge HH, Chen P, Belle S, DeKosky ST. Ten-year incidence of dementia in a rural elderly US community population: the MoVIES project. Neurology. (2000) 54(5):1109–16. doi: 10.1212/WNL.54.5.1109
5. Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of aging. Neurology. (2000) 54(11):2072–7. doi: 10.1212/WNL.54.11.2072
6. Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men? Am J Epidemiol. (2001) 153(2):132–6. doi: 10.1093/aje/153.2.132
7. Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. (2002) 59(11):1737–46. doi: 10.1001/archneur.59.11.1737
8. Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. (2013) 382(9902):1405–12. doi: 10.1016/S0140-6736(13)61570-6
9. Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. (1998) 55:809–15. doi: 10.1001/archpsyc.55.9.809
10. Chêne G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, et al. Gender and incidence of dementia in the Framingham heart study from mid-adult life. Alzheimers Dement. (2015) 11(3):310–20. doi: 10.1016/j.jalz.2013.10.005
11. Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. (2011) 10(3):241–52. doi: 10.1016/S1474-4422(10)70325-2
12. Huang Y, Weisgraber KH, Mucke L, Mahley RW. Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer’s disease. J Mol Neurosci. (2004) 23(3):189–204. doi: 10.1385/JMN:23:3:189
13. Theendakara V, Peters-Libeu CA, Bredesen DE, Rao RV. Transcriptional effects of ApoE4: relevance to Alzheimer’s disease. Mol Neurobiol. (2018) 55:5243–54. doi: 10.1007/s12035-017-0757-2
14. Altmann A, Lu T, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. (2014) 75(4):563–73. doi: 10.1002/ana.24135
15. Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci. (2004) 1019:24–8. doi: 10.1196/annals.1297.005
16. Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. (2017) 74(10):1178–89. doi: 10.1001/jamaneurol.2017.2188
17. Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM. Alzheimer’s disease neuroimaging initiative. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y). (2015) 1(2):103–10. doi: 10.1016/j.trci.2015.07.001
18. Liu M, Huo YR, Wang J, Wang C, Liu S, Liu S, et al. Telomere shortening in Alzheimer’s disease patients. Ann Clin Lab Sci. (2016) 46(3):260–5. PMID: 27312549
19. Honig LS, Schupf N, Lee JH, Tang MX, Mayeux RS. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann Neurol. (2006) 60(2):181–7. doi: 10.1002/ana.20894
20. Bayne S, Jones ME, Li H, Liu JP. Potential roles for estrogen regulation of telomerase activity in aging. Annals NY Acad Sci. (2007) 1114:48–55. doi: 10.1196/annals.1396.023
21. Jacobs EG, Kroenke C, Lin J, Epel ES, Kenna HA, Blackburn EH, et al. Accelerated cell aging in female APOE-ε4 carriers: implications for hormone therapy use. PLoS One. (2013) 8(2):e54713. doi: 10.1371/journal.pone.0054713
22. Adler N, Pantell MS, O'Donovan A, Blackburn E, Cawthon R, Koster A, et al. Educational attainment and late life telomere length in the health, aging and body composition study. Brain Behav Immun. (2013) 27(1):15–21. doi: 10.1016/j.bbi.2012.08.014
23. Carlson MC, Zandi PP, Plassman BL, Tschanz JT, Welsh-Bohmer KA, Steffens DC, et al. Hormone replacement therapy and reduced cognitive decline in older women: the Cache County Study. Neurology. (2001) 57(12):2210–6. doi: 10.1212/WNL.57.12.2210
24. Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. (2002) 288(17):2123–9. doi: 10.1001/jama.288.17.2123
25. Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. (2003) 289(20):2651–62. doi: 10.1001/jama.289.20.2651
26. Imtiaz B, Tuppurainen M, Rikkonen T, Kivipelto M, Soininen H, Kröger H, et al. Postmenopausal hormone therapy and Alzheimer disease: a Prospective Cohort Study. Neurology. (2017) 88(11):1062–8. doi: 10.1212/WNL.0000000000003696
27. Jang H, Bae JB, Dardiotis E, Scarmeas N, Sachdev PS, Lipnicki DM, et al. Differential effects of completed and incomplete pregnancies on the risk of Alzheimer disease. Neurology. (2018) 91(7):e643–51. doi: 10.1212/WNL.0000000000006000
28. Adank MC, Hussainali RF, Oosterveer LC, Ikram MA, Steegers EAP, Miller EC, et al. Hypertensive disorders of pregnancy and cognitive impairment: a Prospective Cohort Study. Neurology. (2021) 96(5):e709–18. doi: 10.1212/WNL.0000000000011363
29. Inoue Y, Shue F, Bu G, Kanekiyo T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol Neurodegener. (2023) 18(1):46. doi: 10.1186/s13024-023-00640-5
30. Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and meta regression analysis. Arch Gen Psychiatry. (2006) 63:530–8. doi: 10.1001/archpsyc.63.5.530
31. Kessing LV. Depression and the risk for dementia. Curr Opin Psychiatry. (2012) 25(6):457–61. doi: 10.1097/YCO.0b013e328356c368
32. Goveas JS, Espeland MA, Woods NF, Wassertheil-Smoller S, Kotchen JM. Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women’s Health Initiative Memory Study. J Am Geriatr Soc. (2011) 59:57–66. doi: 10.1111/j.1532-5415.2010.03233.x
33. Gallagher D, Kiss A, Lanctot K, Herrmann N. Depression and risk of Alzheimer’s dementia: a longitudinal analysis to determine predictors of increased risk among older adults with depression. Am J Geriatr Psychiatry. (2018) 26(8):819–27. doi: 10.1016/j.jagp.2018.05.002
34. Lobo A, López-Antón R, de-la-Cámara C, Quintanilla MA, Campayo A, Saz P, et al. Non-cognitive psychopathological symptoms associated with incident mild cognitive impairment and dementia, Alzheimer’s type. Neurotox Res. (2008) 14(2–3):263–72. doi: 10.1007/BF03033815
Keywords: women, Alzheimer’s disease—AD, sex, risk factor, gender
Citation: O’Neal MA (2024) Women and the Risk of Alzheimer's disease. Front. Glob. Womens Health 4:1324522. doi: 10.3389/fgwh.2023.1324522
Received: 19 October 2023; Accepted: 11 December 2023;
Published: 5 January 2024.
Edited by:
Aleksandra Pikula, University of Toronto, CanadaReviewed by:
Suzana Uzun, Josip Juraj Strossmayer University of Osijek, Croatia© 2024 O’Neal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mary A. O’Neal bWFvbmVhbEBid2guaGFydmFyZC5lZHU=
 Mary A. O’Neal
Mary A. O’Neal