
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Glob. Womens Health , 24 February 2022
Sec. Contraception and Family Planning
Volume 3 - 2022 | https://doi.org/10.3389/fgwh.2022.837358
This article is part of the Research Topic Integrated HIV-Family Planning Delivery View all 7 articles
There is substantial unmet need for family planning (FP) among women living with HIV (WLHIV), leading to unintended pregnancies and may contribute indirectly to increasing the risk of transmission of HIV. This review aims to determine whether integration of FP into HIV testing and care results in increased use of contraception, a reduction in unmet need for FP, improved use of safer conception methods and a reduction in unintended pregnancies in low and middle-income countries. A systematic review was undertaken incorporating studies from PubMed, EMBASE, CINAHL, Web of Science and Global Health, the International AIDS Society Abstract Archive, the World STI & HIV Congress Abstract Archive and the Conference on Retroviruses and Opportunistic Infections Abstract Archive published between 2016 and 2021, updating previous systematic reviews. After screening, 13 studies were included, 11 conducted in sub-Saharan Africa and 2 in India. The primary outcome of the review was contraceptive uptake and secondary outcomes included unmet need for FP, safer conception and unintended pregnancy. Integrated FP-HIV facilities were found to increase dual contraceptive use by at least 8% in five studies and modern contraceptive use by at least 8% in four studies. Findings from two studies suggested integration decreased the unmet need for contraception. Limited data prevented a conclusion from being drawn regarding whether integration increases safer conception. There was no evidence of integration reducing unintended pregnancies. The median quality score of studies was 3/9. Overall, integrated facilities have the potential of improving reproductive health of women accessing HIV services in LMICs. FP may be enhanced by including a safer conception component for WLHIV.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021251008, identifier: CRD42021251008.
The HIV epidemic continues to present an immense global public health challenge with ~37.7 million individuals living with HIV in 2020, two thirds being in sub-Saharan Africa (1). The global scale-up of antiretroviral therapy (ART) has substantially reduced both morbidity and mortality (2). To deliver ART to large numbers of people, many HIV care programmes in low and middle income countries (LMIC) with generalized HIV epidemics use a public health approach using standardized simplified treatment protocols and decentralized service delivery (3). While this approach has enabled the scale-up of ART in overwhelmed health systems, broader health needs of people with HIV are often not addressed.
An example is family planning (FP), which is often provided through separate vertical programmes (4). FP enables women to make decisions regarding the number, timing and spacing of pregnancies and enables better reproductive health outcomes through education about birth spacing and provision of contraception (5). Successive births separated by <2 years are associated with 45% higher infant mortality than with births are separated by two or more years (6, 7). Unintended pregnancies carry a greater risk of poorer health outcomes than planned pregnancies such as low infant birth weight and poor maternal mental health (8, 9). In addition, unintended pregnancies in women living with HIV (WLHIV) are associated with late presentation for antenatal care and therefore delayed access to ART for prevention of mother-to-child transmission (PMTCT) and reduced adherence to ART, both of which increase the risk of mother-to-child HIV transmission (10).
WLHIV have a much higher unmet need for FP than HIV negative women (11). In sub-Saharan Africa, 66–92% of WLHIV reported not wanting another child and yet only 20–43% of these women were utilizing a form of contraception (12). The unmet need for FP among WLHIV is due to multiple factors including social marginalization and poverty (4). Young women and adolescents living with HIV face further barriers including restrictive policies and stigma, preventing access to sexual and reproductive health services (13).
Reducing the unmet need for contraception in WLHIV has the potential to not only improve reproductive health outcomes but also to reduce the risk of mother-to-child HIV transmission (14). Safer conception interventions embedded in family planning services, such as education and counseling, ensuring viral suppression in the mother and/or her partner, timed condom-less intercourse, intravaginal or intrauterine insemination and pre-exposure prophylaxis (PrEP) initiation for HIV-negative partners, may reduce the risk of vertical HIV transmission as well as horizontal transmission when partners are sero-discordant (15).
Integration of FP services into HIV services has been proposed as a method to decrease the unmet need for FP among WLHIV in LMICs (16). Integration of services may improve convenience for patients and strengthen the health systems (14). Three previous systematic reviews have been conducted between 2009 and 2017 on the integration of FP into HIV care (17–19). Spaulding et al. considered integration to be feasible and effective (18). They found some evidence of integration increasing contraceptive use, however, not enough studies reported on this outcome to conclude an effect (18). Wilcher et al. later concluded that while an association between integrated services and increased contraceptive use exists, average study quality was weak (a mean of 3.4 out of 9) (19). The latest review was conducted by Haberlen et al. and included studies up to February 2016 (17). The investigators reported integration increased the use of modern contraception methods and three studies reported an association between integration and dual method use (17). However, limited evidence prevented an evaluation for unintended pregnancy or unmet need for FP (17). Previous reviews did not include safer conception, defined as interventions to reduce the risk of mother-to-child HIV transmission as an outcome of interest. In September 2015, the World Health Organization (WHO) released guidelines recommending all pregnant WLHIV should be provided with lifelong ART, regardless of clinical or immunological status (20). This approach further minimized the risk of mother-to-child transmission of HIV during pregnancy (20). This coincided with an increase in the accessibility of pre-exposure prophylaxis (PrEP) globally (21). Therefore, since the previous review, safer conception has become an even more relevant outcome in relation to provision of FP.
We conducted a systematic review to update evidence presented in previous reviews and investigate whether integration of FP and HIV services increases uptake of contraception. Other examined outcomes included unmet need for FP, safer conception practices and unintended pregnancy.
A systematic review was conducted using PRISMA guidelines and the review was registered with PROSPERO. PubMed, EMBASE, CINAHL, Web of Science and Global Health databases were searched in June 2021. The database search employed a summation of three concepts: family planning, HIV and integration, guided by previous reviews. Terms searched for family planning included “family planning,” “contraception,” “birth control,” “safer conception,” “planned parenthood,” “birth spacing,” and “birth prevention.” HIV terms included “HIV,” “Human immunodeficiency virus,” “AIDS,” and “acquired immunodeficiency syndrome.” Concept three included the terms “integrated,” “integration,” and “linkage.” The search was limited to articles in English published between March 2016 and May 2021 as the previous review included studies up to February 2016. Three conference abstract archives: International AIDS Society, the World STI & HIV Congress and the Conference on Retroviruses and Opportunistic Infections (CROI) were also searched utilizing a keyword search. The Cochrane Central Register of Controlled Trials was searched to identify any unpublished or ongoing trials and references from the bibliographies of the included studies were similarly examined.
Studies with women of reproductive age (15–49 years) accessing HIV testing or care services in any LMIC defined according to the World Bank were included (22). HIV testing and care interventions included provider-initiated and client-initiated testing and counseling, treatment services and PMTCT services. The definition of FP interventions for inclusion in this review included contraception and safer conception education and counseling, provision of contraception methods and referrals for contraception methods not available at the point of the HIV service (including termination of pregnancy). Studies with no detail of the FP intervention were excluded. Studies without a comparator were not eligible for inclusion. Suitable comparators included control groups, comparison groups and before/after designs. Integration was defined as any relevant FP intervention (as defined above) specifically incorporated into any HIV testing and care service (as defined above). This could be, delivered by the same provider in one appointment, on the same site or through an enhanced referral service. The primary outcome of the review was contraception use. This was defined as use or desire to use any contraceptive method. Categories within this outcome included dual method contraception and modern contraception. Use of condoms in addition to another contraceptive is defined as dual contraception use (23). The definition of modern contraceptive methods is dependent on national guidelines (24). All definitions of modern contraception include contraceptive injectables, implants, IUDs, oral contraceptives and vaginal rings. However, while some definitions include condoms, others exclude them due to the inconsistency of use (24). The secondary outcomes in this review were unintended pregnancy, unmet need for FP and safer conception. Unintended pregnancy was defined as when an infant was either not desired or conceived earlier than desired (25). The unmet need for FP was defined as not desiring a pregnancy in a designated period of time and yet not using the contraceptives required to prevent a pregnancy or WLHIV wanting to conceive but not receiving the support to do so safely (26). Finally, safer conception was defined as any intervention to minimize the risk of HIV transmission from mother to child in WLHIV desiring a pregnancy for example through discussions with a healthcare professional or the practicing of safer conception methods (15). Experimental or observational designs were included and qualitative studies, case report, case series and studies proposing models were excluded.
The results of the search strategy were exported into EndNote X9 and one reviewer screened search results against the exclusion criteria first by their titles, then abstracts and full-texts. Duplicates were deleted after examination of their titles, authors and date of publication. Following full-text screening, data was extracted into Excel from all eligible studies. Extracted data included the article title, authors, date, study design, location, study duration, target population, type of FP intervention, type of HIV care, outcomes and results. Data extraction was performed twice to minimize transcription error.
The same 9-item tool, used to assess study quality in the three previous systematic reviews, was used for continuity (27). Spaulding et al. adapted this scale from HIV behavioral intervention reviews (18). One point was allocated for each of the criteria met. The criteria included: pre/post-intervention data availability, control or comparison group, cohort analysis, comparison groups being equivalent at baseline on sociodemographic characteristics, comparison groups being equivalent at baseline on outcome measures, random assignment, participants being randomly selected for assessment, control for potential confounders and having a follow-up rate ≥75%. A score of ≥7/9 was deemed a high-quality study, scores between 4 and 6/9 were considered to carry risk of bias, and those scoring <4/9 were considered weak quality studies and thus at high risk of bias.
Cohort and case-control studies underwent an additional assessment using the Newcastle-Ottawa Scale, which utilizes the allocation of stars in three categories: selection, comparability and outcome. Studies attaining ≥7 stars out of a possible 9 were considered high-quality, those scoring between 4 and 6 stars were considered to be at high risk of bias and those with 0–3 stars had a very high risk of bias.
All included studies were categorized by whether the outcome assessed contraceptive uptake, unintended pregnancy, unmet need for FP or safer conception. Data on these outcomes was extracted into Excel and analyzed. A meta-analysis was considered but given the studies were very heterogeneous with respect to FP intervention, HIV service and the extent of integration, this was not feasible. Instead, a descriptive analysis and narrative synthesis were conducted.
The database search strategy yielded 1,524 results, leaving 796 records following the deletion of duplicates (Figure 1). The remaining records were screened by title, abstract and full-text and 12 studies were deemed suitable for inclusion from the database search (11 full-text articles and one conference abstract). One additional conference abstract was identified through abstract archive searching (28). Efforts to contact the authors of the two included conference abstracts were unsuccessful; therefore, no supplementary information could be obtained (28, 29). No further studies were identified through either The Cochrane Central Register of Controlled Trials or the bibliographies of included studies.
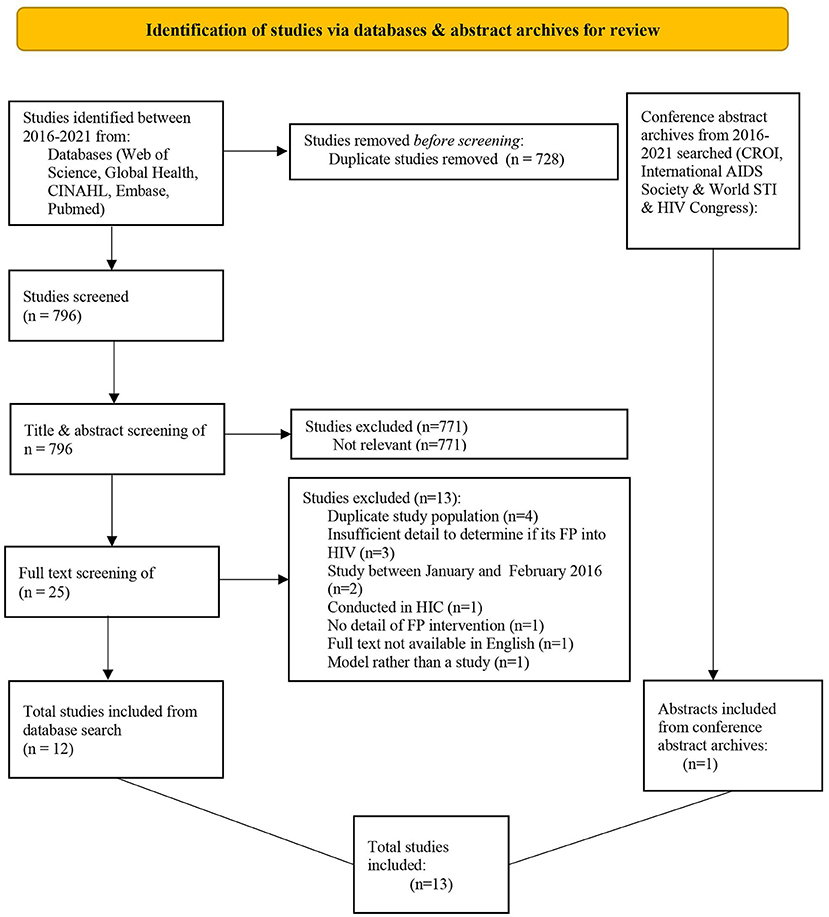
Figure 1. PRISMA flow chart depicting the process leading to the inclusion of studies for the review.
A total of 48,587 women were enrolled across 13 studies (Table 1). Two studies were conducted in India which has experienced a concentrated HIV epidemic (29, 30). The remainder were conducted in countries in sub-Saharan Africa (11/13), which have experienced generalized HIV epidemics, including, Kenya (n = 3), Tanzania (n = 1), Rwanda (n = 1), Botswana (n = 1), South Africa (n = 1), Zambia (n = 1), Uganda (n = 2) and Malawi (n = 1). The studies included two randomized trials, two quasi-experimental studies, four before/after designs, two cross-sectional and three cohort studies. All studies except one included an element of FP counseling in their interventions and 7/13 expanded the onsite provision of contraception.
All studies examined the effect of integration on contraceptive uptake with the most frequently used categories being dual method and modern contraceptive use (Table 2). Out of the six studies investigating dual method contraceptive use, five suggested an association between integration and increased dual method use (29–33). Chen et al., a cross-sectional study, found 30% of individuals attending non-integrated facilities use dual methods compared to 40% at integrated services (p < 0.01) (30). A quasi-experimental study design conducted by Joshi et al. demonstrated that after repeated FP counseling, poster advertisement and a referral mechanism was put in place, uptake of dual contraception was 32.6% in the intervention arm compared to 10.6% in the control group (31). Medley et al. similarly found dual contraceptive use was 9% pre-intervention compared to 18% post-intervention (29). Joshi et al. reported 0% dual contraception uptake pre-intervention compared to 44.6% at the endpoint (33). Mantell et al. observed a 10% absolute increase in use of dual contraceptives in the intervention group compared to the control (32). Dulli et al., found no association between integrated facilities and dual method contraception (34). After introducing the enhanced integration intervention to the experimental arm, Dulli et al. reported an adjusted odds ratio (aOR) of 0.76 (95% CI: 0.69–1.37) for dual method use (34).
Results of four studies suggest an association between integrated services and increased use of modern contraception methods (30, 34–36). Chen et al. observed integrated facilities have a modern contraception prevalence rate of 88% compared to 80% at non-integrated facilities (30). Dulli et al. also presented an aOR of 1.38 (95% CI: 1.04–1.83), suggesting an association between integrated facilities and increased use of modern contraception (34). Additionally, an adjusted prevalence ratio (PR) of 1.21 (95% CI: 1.10–1.33 p < 0.001) reported by Nabirye et al. indicates an association between receiving FP counseling and increased use of modern contraception (35). Guillaine et al. also reported an increase in modern contraception use from baseline to post-intervention (30.3–72%, respectively) (36). Conversely, Wagner et al. found no evidence of integration increasing modern contraceptive use with an aOR of 3.72 (95% CI: 0.37–37.48) when comparing their high intensity intervention to the low intensity intervention. However, this had limited power due to a small sample size (24).
Hawkins et al. reported on women's desire for wanting to utilize LARC pre-intervention compared to post-intervention (37). A positive association between integration and more positive attitudes toward FP was identified with the desire to use LARC at 6% at pre-intervention compared to 29% at the endpoint (p < 0.001) (37).
Two studies appraised this outcomes, both of which provide evidence for integration reducing the unmet need for FP (Table 3). Chen et al. reported integrated facilities had an unmet need of 8% compared to non-integrated facilities having an unmet need of 15% (30). Lastly, Medley et al. observed a reduction from 59% pre-intervention to 46% post-intervention (29). This remained significant after adjustment for facility, age group and time since diagnosis.
Three studies assessed the effect of integrated services on safer conception methods for WLHIV desiring a pregnancy (Table 4) (24, 29, 32). Medley et al. included safer pregnancy counseling in their FP services and consequently observed a 12% absolute increase in women discussing safer pregnancy with their HIV provider (29). Wagner et al. introduced safer conception counseling into HIV care and subsequently observed a 24.1% increase in the correct use of timed condom-less sex or manual self-insemination in the intervention arm compared to usual care accompanied by a covariate aOR of 91.84 (95% CI: 4.94–1709 p < 0.01) (24). Mantell et al. assessed a binary outcome reporting whether safer conception guidelines were being adhered to (32). The result was deemed significant if the difference between the enhanced intervention and standard of care groups was >10%. Mantell et al. observed a difference in adherence to safer conception guidelines of 11% (95% CI −0.04, 0.27) between the study groups, thus meeting this criterion (32). However, this was not statistically significant.
Two studies assessed the association between integrated facilities and unintended pregnancy (Table 5) (30, 31). Chen et al. found no difference in the proportion of unintended pregnancy in non-integrated facility settings (35%) compared to integrated services (36%) (p = 0.81) (30). Joshi et al. reported the number of unintended pregnancies in the intervention and control groups as 13 and 20, respectively (31). Three studies looked at pregnancy incidence, however, in the absence of information regarding if these pregnancies were planned or not, interpreting these figures is challenging.
The median score after applying the 9-point quality scale was 3 out of 9 with a mean of 3.5 making it no different from previous reviews (Table 6). Using the pre-defined criteria, 2/13 were classified as high-quality studies, 3/13 were considered to be at risk of bias and 8/13 were weak quality studies.
The Newcastle-Ottawa scale was used to assess the quality of the three cohort studies. All three studies achieved scores ranging from 4 to 5 and were considered to be at high risk of bias. Tweya et al. achieved a score of 4, Guillaine et al. a score of 5 and Cohen et al. a score of 5 (36, 38, 39). Tweya et al. and Guillaine et al. did not contain a non-exposed cohort and therefore the selection of a non-exposed cohort was denoted as ‘not applicable' (36, 38).
This systematic review suggests that the integration of FP with HIV services improves uptake of and unmet need for FP among WLHIV in LMICs. The review builds on the evidence from previous systematic reviews with regard to contraceptive uptake. While the first two reviews conducted by Spaulding et al. and Wilcher et al. did find some association between integrated services and increased general contraceptive use, they did not report on specific types of contraceptive use (18, 19). Haberlen et al., however, broke down contraceptive use into subsections including modern contraception and dual method use (17). The current review reinforces the finding of Haberlen et al. that integration increases modern contraception use (17).
There was evidence that integration increased dual method use, building on the evidence presented in the review by Haberlen et al. (17). Despite this evidence, in general, dual contraception use was lower than modern contraceptive use. Chen et al. reported that in integrated facilities only 40% of individuals employed dual contraception, despite a modern contraception prevalence rate of 88% (30). The reason for low uptake of dual contraception may be simply an unwillingness to use condoms or to a lack of awareness of the importance of employing condoms in addition to other methods to prevent the acquisition of STIs and HIV. It may also be attributed to gender inequality, specifically unequal gender power dynamics within relationships. While modern contraception forms allow for increased autonomy, frequently, a women's ability to negotiate condom use is limited, particularly in vulnerable groups such as female sex workers (40). It is also important to note that self-reported condom use poses a risk of social-desirability bias, potentially inflating the results for this outcome (41).
This review presents accumulating evidence of an effect of integration decreasing the unmet need for FP. The previous review conducted by Haberlen et al., found three studies evaluated the impact of integration on unmet need (17). Two of these suggested an association between integration and decreased unmet need for FP (42, 43). This was not enough evidence to conclude an effect. However, the two studies from the previous review combined with Chen et al. and Medley et al. in this review, provides further evidence suggesting that integration does decrease the unmet need for FP (29, 30).
In line with previous systematic reviews, there remains insufficient data to conclude an effect on unintended pregnancy. Only Chen et al. and Joshi et al. reported on unintended pregnancy and both reported integration had no effect (30, 31). The limited reporting on this outcome is likely due to this being hard to measure. Nevertheless, contraceptive uptake, unmet need for FP and unintended pregnancy are intrinsically linked. If providing integrated care likely increases modern and dual contraception use while decreasing the unmet need for FP, as this review shows, this is anticipated to lead to a reduction in unintended pregnancies.
Despite not being able to draw a conclusion on safer conception due to limited data, the review included the first two randomized intervention trials assessing this outcome, Mantell et al. and Wagner et al. (24, 32). These high-quality studies reported increases in the use of safer conception methods in their intervention arms compared to the control, however, the finding of Mantell et al. was not statistically significant (32).
Moving forward, this review has revealed the need for further research on the effectiveness and impact of incorporating safer conception interventions for WLHIV within HIV services. Bringing to an end outdated beliefs surrounding WLHIV not being able to safely conceive and interventions that teach and support WLHIV to safely conceive will aid in reducing stigma and empowering PLHIV.
We acknowledge several limitations. Limited information was available from conference abstracts. Attempts to obtain further information from the first authors of the two conference abstracts were unsuccessful. Therefore, some detail regarding methodologies were missing potentially leading to a downgrading of the quality of these studies. Selection and screening of studies was conducted by only one reviewer which may have led to some studies being missed. The outcomes were obtained through self-report which may introduce social desirability bias. Publication bias can also not be excluded. As in the previous reviews, the overall quality of studies remained low and there was substantial heterogeneity across studies, precluding a meta-analysis.
Notwithstanding these limitations, this review builds on existing evidence demonstrating the benefits of integration of FP into HIV services for WLHIV in terms of enhancing modern and dual contraceptive uptake and reducing the unmet need for FP. Therefore, focus should now be on translating the evidence accumulated over the past two decades into practice in LMICs. This review has highlighted the gaps in the research, specifically the scarcity of integrated programmes focusing on safer conception for WLHIV desiring a pregnancy and the importance of such programmes.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
TG-M and RF conceived the study. TG-M conducted the systematic search, extracted the data, and drafted the manuscript. RF and KK edited the full manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
RF is funded by the Wellcome Trust through a senior Fellowship in Clinical Science (Grant No: 206316_Z_17_Z).
1. WHO. HIV/AIDS Fact Sheet. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed August 06, 2021).
2. UNAIDS DATA 2019. (2019). Available online at: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf (accessed August 06, 2021).
3. Pham MD, Romero L, Parnell B, Anderson DA, Crowe SM, Luchters S. Feasibility of antiretroviral treatment monitoring in the era of decentralized HIV care: a systematic review. AIDS Res Ther. (2017) 14:3. doi: 10.1186/s12981-017-0131-5
4. FHI360. Integrating Family Planning into HIV Programs: Evidence-Based Practices. (2013). Available online at: https://www.fhi360.org/sites/default/files/media/documents/fp-hiv-evidence%20based%20practices%202013.pdf (accessed August 06, 2021).
5. Wilcher R, Petruney T, Cates W. The role of family planning in elimination of new pediatric HIV infection. Curr Opin HIV AIDS. (2013) 8:490–7. doi: 10.1097/COH.0b013e3283632bd7
6. The Alan Guttmacher Institute. Family Planning Can Reduce High Infant Mortality Levels: Issues in Brief. (2002). Available online at: https://www.guttmacher.org/sites/default/files/report_pdf/ib_2-02.pdf (accessed January 25, 2022).
7. Rutstein SO. DHS Working Papers - Further Evidence of the Effects of Preceding Birth Intervals on Neonatal, Infant, and Under-Five-Years Mortality and Nutritional Status in Developing Countries: Evidence from the Demographic and Health Surveys. USAID (2008). Available online at: https://dhsprogram.com/pubs/pdf/wp41/wp41.pdf (accessed January 25, 2022).
8. Shah PS, Balkhair T, Ohlsson A, Beyene J, Scott F, Frick C. Intention to become pregnant and low birth weight and preterm birth: a systematic review. Matern Child Health J. (2011) 15:205–16. doi: 10.1007/s10995-009-0546-2
9. Bahk J, Yun S-C, Kim Y-m, Khang Y-H. Impact of unintended pregnancy on maternal mental health: a causal analysis using follow up data of the Panel Study on Korean Children (PSKC). BMC Pregn Childb. (2015) 15:85. doi: 10.1186/s12884-015-0505-4
10. Iyun V, Brittain K, Phillips TK, le Roux S, McIntyre JA, Zerbe A, et al. Prevalence and determinants of unplanned pregnancy in HIV-positive and HIV-negative pregnant women in Cape Town, South Africa: a cross-sectional study. BMJ Open. (2018) 8:e019979. doi: 10.1136/bmjopen-2017-019979
11. MacQuarrie K. DHS Working Papers - HIV/AIDS and Unmet Need for Family Planning. USAID (2015). Available online at: https://dhsprogram.com/pubs/pdf/WP122/WP122.pdf (accessed August 06, 2021).
12. Sarnquist CC, Rahangdale L, Maldonado Y. Reproductive health and family planning needs among HIV-infected women in Sub-Saharan Africa. Curr HIV Res. (2013) 11:160–8. doi: 10.2174/1570162X11311020008
13. Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet. (2019) 394:303–13. doi: 10.1016/S0140-6736(19)31288-7
14. WHO. Strategic Considerations for Strengthening the Linkages between Family Planning and HIV/AIDS Policies, Programs, and Services. Geneva (2009). Available online at: https://www.who.int/reproductivehealth/publications/linkages/fp_hiv_strategic_considerations.pdf (accessed August 06, 2021).
15. Davey JD, West S, Umutoni V, Taleghani S, Klausner H, Farley E, et al. A systematic review of the current status of safer conception strategies for HIV affected heterosexual couples in Sub-Saharan Africa. AIDS Behav. (2018) 22:2916–46. doi: 10.1007/s10461-018-2170-x
16. WHO. It's Time to Strengthen Linkage Between Family Planning (FP) and HIV Interventions. (2017). Available online at: https://www.who.int/reproductivehealth/test/Linkages-FP-HIV.pdf (accessed August 06, 2021).
17. Haberlen SA, Narasimhan M, Beres LK, Kennedy CE. Integration of family planning services into HIV care and treatment services: a systematic review. Stud Fam Plann. (2017) 48:153–77. doi: 10.1111/sifp.12018
18. Spaulding AB, Brickley DB, Kennedy C, Almers L, Packel L, Mirjahangir J, et al. Linking family planning with HIV/AIDS interventions: a systematic review of the evidence. AIDS. (2009) 23(Suppl. 1):S79–88. doi: 10.1097/01.aids.0000363780.42956.ff
19. Wilcher R, Hoke T, Adamchak SE, Cates W Jr. Integration of family planning into HIV services: a synthesis of recent evidence. AIDS. (2013) 27(Suppl. 1):S65–75. doi: 10.1097/QAD.0000000000000051
20. WHO. Guidelines: Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. (2015). Available online at: http://apps.who.int/iris/bitstream/handle/10665/186275/9789241509565_eng.pdf;jsessionid=48F61D2C5390CDA177965A36035DF29A?sequence=1 (accessed December 09, 21).
21. Irungu EM, Baeten JM. PrEP rollout in Africa: status and opportunity. Nat Med. (2020) 26:655–64. doi: 10.1038/s41591-020-0872-x
22. The World Bank. World Bank Country and Lending Groups. (2021). Available online at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed August 06, 2021).
23. Tsuyuki K, Barbosa RM, Pinho AdA. Dual protection and dual methods in women living with HIV: the Brazilian context. J Sexual Transm Dis. (2013) 2013:540789. doi: 10.1155/2013/540789
24. Wagner GJ, Wanyenze RK, Beyeza-Kashesya J, Gwokyalya V, Hurley E, Mindry D, et al. “Our Choice” improves use of safer conception methods among HIV serodiscordant couples in Uganda: a cluster randomized controlled trial evaluating two implementation approaches. Implement Sci. (2021) 16:41. doi: 10.1186/s13012-021-01109-z
25. Santelli J, Rochat R, Hatfield-Timajchy K, Gilbert BC, Curtis KM, Cabral R, et al. The measurement and meaning of unintended pregnancy. Perspect Sexual Reprod Health. (2003) 35:94–101. doi: 10.1363/3509403
26. Darroch J, Audam S, Biddlecom A, Kopplin G, Riley T, Singh S, et al. Adding It Up: Investing in Contraception and Maternal and Newborn Health - Fact Sheet. Guttmacher Institute (2017). Available online at: https://www.srhr-ask-us.org/themencode-pdf-viewer-sc/?file=https://www.srhr-ask-us.org/wp-content/uploads/2018/10/ADDING-IT-UP_-Investing-in-Contraception-and-Maternal-and-Newborn-Health-442017.pdf&settings=001101111&lang=en-US#page=&zoom=auto&pagemode (accessed August 06, 2021).
27. Kennedy C, O'Reilly K, Medley A, Sweat M. The impact of HIV treatment on risk behaviour in developing countries: a systematic review. AIDS Care. (2007) 19:707–20. doi: 10.1080/09540120701203261
28. Casalini C, Basomingera J, Lennemann T, Boyee D, Komba A, Schueller J, et al. Delivering Integrated HIV/Modern Family Planning Services to Female Sex Workers Through Mobile, Community-Based, Integrated Biomedical Services in Five Regions of Tanzania. International Aids Society; Durban, South Africa (2016). Available online at: https://www.abstract-archive.org/Abstract/Share/71701 (accessed August 06, 2021).
29. Medley AM, Pals S, Lasry A, Cain M, Aholou TM, Tsiouris F, et al. An evaluation of an enhanced model of FP/HIV service integration in Lusaka, Zambia. Top Antiviral Med. (2020) 28:400. Available online at: https://www.croiconference.org/abstract/an-evaluation-of-an-enhanced-model-of-fp-hiv-service-integration-in-lusaka-zambia/
30. Chen Y, Begnel E, Muthigani W, Achwoka D, McGrath CJ, Singa B, et al. Higher contraceptive uptake in HIV treatment centers offering integrated family planning services: a national survey in Kenya. Contraception. (2020) 102:39–45. doi: 10.1016/j.contraception.2020.04.003
31. Joshi B, Velhal G, Chauhan S, Kulkarni R, Begum S, Linkage Study Team. Linking HIV & family planning services to improve dual methods of contraception among women infected with HIV in Mumbai, Maharashtra, India. Indian J Med Res. (2016) 143:464–73. doi: 10.4103/0971-5916.184286
32. Mantell JE, Cooper D, Exner TM, Moodley J, Hoffman S, Myer L, et al. Emtonjeni-a structural intervention to integrate sexual and reproductive health into public sector HIV care in Cape Town, South Africa: results of a phase II study. AIDS Behav. (2017) 21:905–22. doi: 10.1007/s10461-016-1562-z
33. Joshi B, Shetty S, Kulkarni R, Begum S, Girase B, Verma V, et al. Improving public health service delivery response to address contraceptive needs of socio-economically disadvantaged HIV positive people in Maharashtra, India. Contracept Reproduct Med. (2021) 6:14. doi: 10.1186/s40834-021-00159-4
34. Dulli L, Field S, Masaba R, Ndiritu J. Addressing broader reproductive health needs of female sex workers through integrated family planning/HIV prevention services: a non-randomized trial of a health-services intervention designed to improve uptake of family planning services in Kenya. PLoS ONE. (2019) 14:e0219813. doi: 10.1371/journal.pone.0219813
35. Nabirye J, Bwanika JB, Makumbi F, Wanyenze RK, Matovu JKB. Missed opportunities for family planning counselling among HIV-positive women receiving HIV Care in Uganda. BMC Womens Health. (2020) 20:91. doi: 10.1186/s12905-020-00942-6
36. Guillaine N, Mwizerwa W, Odhiambo J, Hedt-Gauthier BL, Hirschhorn LR, Mugwaneza P, et al. A novel combined mother-infant clinic to optimize post-partum maternal retention, service utilization, and linkage to services in HIV care in Rural Rwanda. Int J MCH AIDS. (2017) 6:36–45. doi: 10.21106/ijma.186
37. Hawkins L, Sickboy O, Gertz AM, Badubi O, Mussa A, Maotwe T, et al. Integration of family planning services into health care for HIV-positive women in Botswana. Int J Gynecol Obstetr. (2021) 152:208–14. doi: 10.1002/ijgo.13464
38. Tweya H, Feldacker C, Gugsa S, Phiri S. Contraceptive use and pregnancy rates among women receiving antiretroviral therapy in Malawi: a retrospective cohort study. Reproduct Health. (2018) 15:25. doi: 10.1186/s12978-017-0440-0
39. Cohen CR, Blat C, Newmann SJ, Burger RL, Grossman D, Onono M, et al. Integration of family planning services into HIV care clinics: results one year after a cluster randomized controlled trial in Kenya. PLoS ONE. (2017) 12:e0172992. doi: 10.1371/journal.pone.0172992
40. Madiba S, Ngwenya N. Cultural practices, gender inequality and inconsistent condom use increase vulnerability to HIV infection: narratives from married and cohabiting women in rural communities in Mpumalanga province, South Africa. Glob Health Action. (2017) 10:1341597. doi: 10.1080/16549716.2017.1341597
41. Zenilman JM, Weisman CS, Rompalo AM, Ellish N, Upchurch DM, Hook EW III, et al. Condom use to prevent incident STDs: the validity of self-reported condom use. Sex Transm Dis. (1995) 22:15–21. doi: 10.1097/00007435-199501000-00003
42. Baumgartner JN, Green M, Weaver MA, Mpangile G, Kohi TW, Mujaya SN, et al. Integrating family planning services into HIV care and treatment clinics in Tanzania: evaluation of a facilitated referral model. Health Policy Plann. (2013) 29:570–9. doi: 10.1093/heapol/czt043
43. Wanyenze RK, Matovu JK, Kamya MR, Tumwesigye NM, Nannyonga M, Wagner GJ. Fertility desires and unmet need for family planning among HIV infected individuals in two HIV clinics with differing models of family planning service delivery. BMC Womens Health. (2015) 15:5. doi: 10.1186/s12905-014-0158-x
Keywords: family planning, HIV, integration, contraception, safer conception, systematic review
Citation: Grant-Maidment T, Kranzer K and Ferrand RA (2022) The Effect of Integration of Family Planning Into HIV Services on Contraceptive Use Among Women Accessing HIV Services in Low and Middle-Income Countries: A Systematic Review. Front. Glob. Womens Health 3:837358. doi: 10.3389/fgwh.2022.837358
Received: 16 December 2021; Accepted: 31 January 2022;
Published: 24 February 2022.
Edited by:
Chelsea Morroni, University of Edinburgh, United KingdomReviewed by:
Theresa Hoke, Family Health International 360, United StatesCopyright © 2022 Grant-Maidment, Kranzer and Ferrand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tallulah Grant-Maidment, dC5ncmFudC1tYWlkbWVudDFAdW5pLmJzbXMuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.