
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Glob. Womens Health, 25 September 2020
Sec. Maternal Health
Volume 1 - 2020 | https://doi.org/10.3389/fgwh.2020.564560
This article is part of the Research TopicCOVID-19 and Women's HealthView all 41 articles
 Chloe R. McDonald1*†
Chloe R. McDonald1*† Andrea M. Weckman2,3,4†
Andrea M. Weckman2,3,4† Julie K. Wright2,3,4,5
Julie K. Wright2,3,4,5 Andrea L. Conroy6
Andrea L. Conroy6 Kevin C. Kain2,3,4,5
Kevin C. Kain2,3,4,5As the novel coronavirus (SARS-CoV-2) pandemic continues to spread, some predict a disproportionate toll on low- and middle-income countries (LMICs), as it stresses already under-resourced health systems in densely populated regions (1). Two LMICs, Brazil and India, are among the top three countries by number of confirmed COVID-19 cases (2). While the reported prevalence of COVID-19 in sub-Saharan Africa is currently lower than reports from Asia, North America, and Europe, epidemiological modeling suggests that nearly a quarter of a billion people in sub-Saharan Africa may contract SARS-CoV-2 in the first year of the pandemic (3). The UN estimates up to 3 million COVID-19-related deaths in the region (4). Furthermore, the spread of SARS-CoV-2 in LMICs threatens to further increase the burden of adverse birth outcomes among the majority of global pregnancies.
Globally, there are over 213 million pregnancies every year, of which an estimated 190 million (89%) occur in low resource settings where the risk of poor birth outcomes is highest (5). The contributing risk factors for these adverse outcomes are multifactorial: pregnant women in LMICs struggle to access antenatal care (6); an estimated 1 in 10 women in LMICs do not receive adequate nutrition in pregnancy (7); and the majority of pregnant women at risk of, or living with, malaria, HIV, and/or tuberculosis (TB) reside in LMICs (8, 9). High rates of these and other co-morbidities in pregnancy directly translate to adverse birth outcomes: more than 60% of children that are born preterm each year are born in sub-Saharan Africa and south Asia (e.g., India alone accounts for 23.6% of total global preterm births), accounting for over 750,000 deaths within the first month of life (Figure 1) (10).
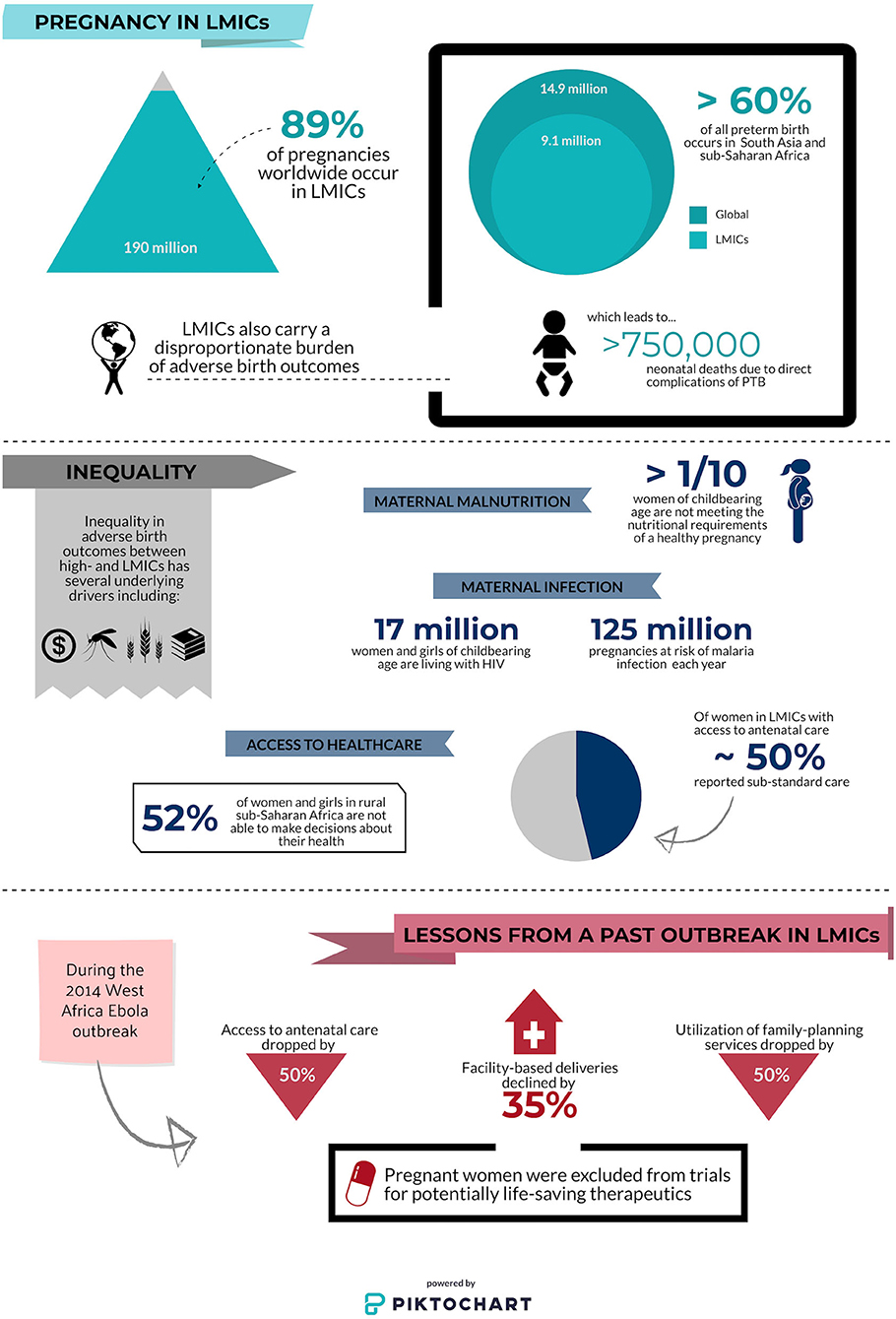
Figure 1. Infographic depicting inequalities in pregnancy outcomes and access to antenatal care at baseline and during a pandemic. Low- and middle-income countries (LMICs); preterm birth (PTB). Data sources (5–7, 9–13).
COVID-19 is likely to influence maternal-child health in profound ways, from the physiological impact of the disease itself, to its indirect impacts on health systems, social, economic, and cultural structures, and by exacerbating pre-existing gender and healthcare access inequalities. Research is needed to identify the risks of COVID-19 in pregnancy, and its interplay with highly prevalent comorbidities already concentrated in LMICs including malnutrition, anemia, HIV, TB, and malaria. Identification and mitigation of both infectious and response-related barriers to health access and information for pregnant women during pandemics is essential for protecting the health of women and their children. Despite the current international focus on COVID-19, there remains an urgent need to direct research attention and resources to the impact of emerging infectious disease threats such as COVID-19 on pregnant women in LMICs.
Very little information is currently available on the impact of COVID-19 in pregnancy. The first joint report published by the WHO and the Chinese Government mentions pregnancy twice in 40 pages (14). Much of the currently published literature contains conflicting information drawn from case reports and case-series with very small sample sizes. Although several original studies and systematic reviews suggest pregnant women are not at increased risk of severe clinical outcomes and that there is low risk of vertical transmission (15–19), one study reported seven maternal deaths out of nine cases in their multi-site COVID-19 case series (20). Increased rates of preterm birth and cesarean-section have also been reported (19, 21, 22). Due to the timing of the COVID-19 outbreak, most studies have reported on infection during the third trimester and there is little to no evidence of its impact in early pregnancy. Based on outcomes of pregnancies complicated by other severe respiratory infections, we hypothesize that more rigorous, pregnancy-focused research will reveal that pregnant women face an increased risk of poor clinical and birth outcomes during this COVID-19 pandemic.
Pregnant women have a higher risk of viral respiratory infection and are more likely to experience severe clinical symptoms (23). Both pandemic (e.g., H1N1) and seasonal influenza in pregnancy have been linked to severe maternal morbidity and increased risk of fetal death and preterm birth (24, 25). Pneumonia is also associated with increased risk of maternal morbidity, mortality, and poor birth outcomes. Co-existing maternal disease increases both the risk of infection as well as the risk of poor clinical outcomes (26). Therefore, as the COVID-19 pandemic continues, overlap with seasonal influenza and resulting co-infections will likely exacerbate morbidity and mortality in pregnancy. A rapidly growing body of evidence further indicates that infections during pregnancy, including respiratory infections such as influenza, are associated with increased risk of neurocognitive and neuropsychiatric disorders in exposed offspring (27). A review and a meta-analysis of coronavirus-spectrum infections reported increased preterm birth, miscarriage, preeclampsia, cesarean-section, and perinatal death in pregnant women with SARS, MERS, or COVID-19 (28, 29). However, the majority of data on coronavirus spectrum infections has been from Europe and North America. In LMICs, there is very little investigation into the impact of coronavirus infections on pregnancy despite the high prevalence of co-existing maternal conditions or co-infections, and barriers to quality antenatal care.
During pregnancy, women's attendance at routine antenatal care visits results in high rates of exposure to health care environments. Consequently, women who continue to observe the recommended antenatal guidelines will be at increased risk for exposure to SARS-CoV-2. Furthermore, as health care systems become over-burdened by COVID-19, access and adherence to prenatal and obstetric care, as well as the quality of care, will suffer. Without timely intervention, pandemic-related restrictions on movement, reduced access to care, and economic constraints could lead to the reversal of important gains made in global antenatal care and maternal-child health. The 2014 Ebola outbreak in west-Africa provides a stark warning of the effect an infectious outbreak may have on already weak maternal-child health systems (11, 30). In Liberia, access to antenatal care plummeted by 50% and healthcare facility-based deliveries were reduced by 35% during the Ebola outbreak (11). Similar declines were also reported in Guinea, and by publication in 2017, had still not recovered to pre-outbreak levels (30). Preliminary evidence from both Uganda and Nepal indicates that even without a high COVID-19 burden, pandemic-related restrictions have already begun to impact maternal-child outcomes in LMICs, showing sharp declines in maternal facility deliveries (by 50% in Nepal), and increased maternal and neonatal mortality (31, 32). Although maternal-child outcomes with Ebola virus infection in pregnancy are more severe than existing evidence suggests for SARS-CoV-2 infection, these studies indicate immediate and lasting effects of emergent infectious diseases on vulnerable maternal-child healthcare systems in LMICs, and highlight the need for research and support to address this issue during the current COVID-19 pandemic.
The prevalence of medically complicated pregnancies is high among women living in LMICs. Women in LMICs carry a higher risk of infection with HIV, malaria, and/or tuberculosis compared to populations in high-income countries. Women of reproductive age in these regions are also more likely to have sickle cell disease, cardiac conditions (for example, rheumatic heart disease), and COPD due to indoor air pollution (33), conditions that increase the risk of developing severe COVID-19 (34). Furthermore, pregnant women in LMICs are at increased risk of having undiagnosed and/or sub-optimally managed gestational hypertension, pre-eclampsia, and gestational diabetes (35, 36). Hypertensive disorders and diabetes are both associated with an increased risk of severe COVID-19 in non-pregnant populations (34), but their impact on COVID-19 severity in pregnant women is not known. Furthermore, emerging data indicates the potential for long-lasting unintended consequences of governmental COVID-19 responses and COVID-19 related interruptions to maternal-child health interventions and critical public health programs (e.g., TB, HIV, and malaria diagnosis and treatment programs; nutritional interventions) on maternal morbidity and mortality in LMICs (32, 37, 38). The interplay of decreased health care access, increased prevalence of medical comorbidities, and the impact of SARS-CoV-2 exposure on pregnancy outcomes in LMICs warrants close surveillance and study in order to guide public health policies in the most at-risk regions.
Beyond antenatal care, pregnant and perinatal women will face psychosocial challenges related to stigma and/or social isolation, a lack of information or misinformation concerning neonatal care (e.g., appropriateness of breastfeeding with SARS-CoV-2 infection or suspected infection), and lack of or reluctance to access facility-based neonatal care services (e.g., for routine immunizations). Countries with strict restrictions on movement (e.g., banning public and private transport, curfews) have seen an impact on the ability of pregnant women to seek routine and/or emergency care, as well as increases in food insecurity and sexual and gender-based violence (32, 39). Challenges to providing antenatal and neonatal care during the COVID-19 outbreak will be further compounded if pregnant women and primary caregivers do not have access to up-to-date and accurate public health messaging to understand risks and recommendations.
Access to contraceptives is often limited in LMICs including sub-Saharan Africa and south Asia and the COVID-19 outbreak is likely to both disrupt global supply chains and prevent women from accessing providers of contraception. Many women will be isolated in domestic environments where they may not have input into family planning (Figure 1). Lessons from the Ebola crisis of 2014 indicate that widespread school closures will disproportionately affect girls of reproductive age, and lead to increased rates of sexual exploitation, sexual and gender-based violence, and forced marriage (39, 40). Compromising the sexual and reproductive health of women and girls means many are likely to experience pregnancy during the COVID-19 pandemic. Modeling estimates published by the Guttmacher Institute suggest that a 10% reduction in short and long-term contraceptive use could result in more than 15 million unintended pregnancies across 132 LMICs (41). As a result of gender inequality, the impact of a pandemic on sexual and reproductive health often goes unnoticed and unaddressed (12). Efforts to provide the means for pregnant women to safely access healthcare and healthcare providers are critical, as are efforts for widespread dissemination of public health policy and recommendations regarding other critical aspects of pregnancy (e.g., breastfeeding, immunization). Public and private health systems should craft responses to COVID-19 that address barriers to access and sexual and reproductive health outcomes in LMICs, in ways that protect the immediate health of pregnant women and inform future pandemic preparedness measures.
Pregnant women are almost uniformly excluded from clinical trials. Protecting vulnerable populations from risks associated with experimental therapies is essential and particularly important in LMICs where limited access to high-quality care creates additional vulnerabilities. However, the most at-risk populations also deserve to benefit from therapeutics that may improve outcomes. Many of the drugs being proposed for the treatment or chemoprophylaxis of COVID-19 have evidence-based safety profiles for use in pregnancy including lopinavir/ritonavir, remdesivir, and hydroxychloroquine (42–45). Yet, large government-funded clinical trials for treatment of COVID-19 (e.g., NIH-funded trials [NCT04280705, NCT04332991]) continue to list pregnancy as an exclusion criterion (46). The WHO Solidarity Trial [ISRCTN83971151] originally listed pregnancy in its exclusion criteria but has since removed it. As the WHO seems to have done, the ethics of excluding women from trials where they may benefit from treatments known to be safe in pregnancy needs to be carefully considered. During the 2013–2016 west-Africa Ebola outbreak, where mortality rates for pregnant women and their unborn children approached 100%, women were actively excluded from clinical trials of novel therapeutics (13). Pregnant women could not participate in clinical trials in the face of a life-threatening infection for which pregnancy increased their risk. This highlights the importance of understanding the unique impact of COVID-19 on pregnancy to assess the risk and benefits associated with novel treatments and vaccines.
As novel treatments and vaccines are developed and employed, we must consider why pregnant women continue to be excluded from trials by default, and when it is or is not appropriate to include them. Given the immediacy of the COVID-19 pandemic and the complexity of pharmacokinetics and pharmacodynamics in pregnancy, studies with therapies already known to be safe in pregnancy should be prioritized. To globally maximize benefits and health equity for pregnant and perinatal women, novel treatments should also be accessible to women living in the regions where most pregnancies occur (e.g., LMICs). These treatments should be effective, inexpensive, and easily accessible. Moreover, studies should also examine the impact of co-morbidities on therapeutic outcomes, including co-infection with HIV, malaria, and TB.
As the global implications of the COVID-19 outbreak in LMICs begin to emerge, it is becoming increasingly clear that vulnerable populations will carry a disproportionate burden. Pregnant women in LMICs can face enormous obstacles to healthy birth outcomes for themselves and their unborn and newborn children and these barriers increase in the face of a global pandemic. As public health systems and the international medical research community focus resources on understanding COVID-19 and identifying therapeutics, the impact of infection in pregnancy and the unique health needs of pregnant women during a pandemic should not be neglected or passed over to be studied retrospectively. Pregnant women, including those in LMICs, deserve an immediate and enhanced focus during the COVID-19 outbreak to protect every woman and every child.
CM and KK: project conception, and oversight. CM, AW, JW, KK, and AC: research, analysis, writing, and editing. All authors: read and approved the final manuscript.
This work was supported by the Canadian Institutes of Health Research (CIHR FDN-148439 to KK), Canada Research Chair (KK), CIHR Doctoral Award GSD-157907 (AW), CIHR Vanier Canada Award (JW), Eliot Phillipson Clinician-Scientist Training Program (JW), and Open Philanthropy. The funders had no role in researching or writing the report, or the decision to submit the article for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Massinga Loembe M, Tshangela A, Salyer SJ, Varma JK, Ouma AEO, Nkengasong JN. COVID-19 in Africa: the spread and response. Nat Med. (2020) 26:999–1003. doi: 10.1038/s41591-020-0961-x
2. World Health Organization. Coronavirus Disease (COVID-19) Dashboard. (2020). https://covid19.who.int/ (accessed August 11, 2020).
3. Cabore JW, Karamagi HC, Kipruto H, Asamani JA, Droti B, Seydi ABW, et al. The potential effects of widespread community transmission of SARS-CoV-2 infection in the World Health Organization African Region: a predictive model. BMJ Global Health. (2020) 5:e002647. doi: 10.1136/bmjgh-2020-002647
4. United Nations Economic Commission for Africa. COVID-19 in Africa: Protecting Lives and Economies. (2020). Available online at: https://www.uneca.org/sites/default/files/PublicationFiles/eca_covid_report_en_24apr_web1.pdf (accessed May 7, 2020).
5. Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann. (2014) 45:301–14. doi: 10.1111/j.1728-4465.2014.00393.x
6. Arsenault C, Jordan K, Lee D, Dinsa G, Manzi F, Marchant T, et al. Equity in antenatal care quality: an analysis of 91 national household surveys. Lancet Glob Health. (2018) 6:e1186–95. doi: 10.1016/S2214-109X(18)30389-9
7. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. (2013) 382:427–51. doi: 10.1016/S0140-6736(13)60937-X
8. Sugarman J, Colvin C, Moran AC, Oxlade O. Tuberculosis in pregnancy: an estimate of the global burden of disease. Lancet Glob Health. (2014) 2:e710–6. doi: 10.1016/S2214-109X(14)70330-4
9. UNAIDS. Women and HIV: A Spotlight on Adolescent Girls and Young Women. (2019). Available online at: https://www.unaids.org/sites/default/files/media_asset/2019_women-and-hiv_en.pdf (accessed May 7, 2020).
10. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. (2012) 379:2162–72. doi: 10.1016/S0140-6736(12)60820-4
11. Shannon FQ 2nd, Horace-Kwemi E, Najjemba R, Owiti P, Edwards J, Shringarpure K, et al. Effects of the 2014 Ebola outbreak on antenatal care and delivery outcomes in Liberia: a nationwide analysis. Public Health Action. (2017) 7:S88–S93. doi: 10.5588/pha.16.0099
12. Camara BS, Delamou A, Diro E, Beavogui AH, Ayadi AME, Sidibe S, et al. Effect of the 2014/2015 Ebola outbreak on reproductive health services in rural district of Guinea: an ecological study. Trans R Soc Trop Med Hyg. (2017) 111:22–9. doi: 10.1093/trstmh/trx009
13. Gomes MF, de la Fuente-Nunez V, Saxena A, Kuesel AC. Protected to death: systematic exclusion of pregnant women from Ebola virus disease trials. Reprod Health. (2017) 14:172. doi: 10.1186/s12978-017-0430-2
14. WHO-China Joint Mission on COVID-19. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). (2019). Available online at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed May 7, 2020).
15. Elshafeey F, Magdi R, Hindi N, Elshebiny M, Farrag N, Mahdy S, et al. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int J Gynaecol Obstet. (2020) 150:47–52. doi: 10.1002/ijgo.13182
16. Ferrazzi E, Frigerio L, Savasi V, Vergani P, Prefumo F, Barresi S, et al. Vaginal delivery in SARS-CoV-2 infected pregnant women in Northern Italy: a retrospective analysis. BJOG. (2020) 127:1116–21. doi: 10.1111/1471-0528.16278
17. Qiancheng X, Jian S, Lingling P, Lei H, Xiaogan J, Weihua L, et al. Coronavirus disease 2019 in pregnancy. Int J Infect Dis. (2020) 95:376–83. doi: 10.1016/j.ijid.2020.04.065
18. Yan J, Guo J, Fan C, Juan J, Yu X, Li J, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. (2020) 223:111.e1–111.e14 doi: 10.1016/j.ajog.2020.04.014
19. Yang Z, Wang M, Zhu Z, Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med. (2020). doi: 10.1080/14767058.2020.1759541. [Epub ahead of print].
20. Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, Seferovic MD, Aski SK, Arian SE, et al. Maternal death due to COVID-19 disease. Am J Obstet Gynecol. (2020) 223:109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030
21. Della Gatta AN, Rizzo R, Pilu G, Simonazzi G. COVID19 during pregnancy: a systematic review of reported cases. Am J Obstet Gynecol. (2020) 223:36–41. doi: 10.1016/j.ajog.2020.04.013
22. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. (2020) 99:823–9. doi: 10.1111/aogs.13867
23. Liu H, Wang LL, Zhao SJ, Kwak-Kim J, Mor G, Liao AH. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Repord Immunol. (2020) 139:103122. doi: 10.1016/j.jri.2020.103122
24. Fell DB, Savitz DA, Kramer MS, Gessner BD, Katz MA, Knight M, et al. Maternal influenza and birth outcomes: systematic review of comparative studies. BJOG. (2017) 124:48–59. doi: 10.1111/1471-0528.14143
25. Regan AK, Feldman B, Azziz-Baumgartner E, Naleway AL, Williams J, Wyant BE, et al. An international cohort study of birth outcomes associated with hospitalized acute respiratory infection during pregnancy. J Infect. (2020) 81:48–56. doi: 10.1016/j.jinf.2020.03.057
26. Goodnight WH, Soper DE. Pneumonia in pregnancy. Crit Care Med. (2005) 33:S390–7. doi: 10.1097/01.ccm.0000182483.24836.66
27. Kepinska AP, Iyegbe CO, Vernon AC, Yolken R, Murray RM, Pollak TA. Schizophrenia and influenza at the centenary of the 1918-1919 Spanish influenza pandemic: mechanisms of psychosis risk. Front Psychiatry. (2020) 11:72. doi: 10.3389/fpsyt.2020.00072
28. Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. (2020) 2:100107. doi: 10.1016/j.ajogmf.2020.100107
29. Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronovirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. (2020) 222:415–26. doi: 10.1016/j.ajog.2020.02.017
30. Delamou A, Ayadi AME, Sidibe S, Delvaux T, Camara BS, Sandouno SD, et al. Effect of Ebola virus disease on maternal and child health services in Guinea: a retrospective observational cohort study. Lancet Glob Health. (2017) 5:e448–e3457. doi: 10.1016/S2214-109X(17)30078-5
31. Ashisk KC, Gurung R, Kinney MV, Sunny AK, Moinuddin M, Basnet O, et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health. (2020). doi: 10.1016/S2214-109X(20)30345-4. [Epub ahead of print].
32. Bell D, Hansen KS, Kiragga AN, Kambugu A, Kissa J, Mbonye AK. Predicting the impact of COVID-19 and the potential impact of the public health response on disease burden in Uganda. Am J Trop Med Hyg. (2020) 103:1191–7. doi: 10.4269/ajtmh.20-0546
33. Amegah AK, Quansah R, Jaakkola JJK. Household air pollution from solid fuel use and risk of adverse pregnancy outcomes: a systematic review and meta-analysis of the empirical evidence. PLoS ONE. (2014) 9:e113920. doi: 10.1371/journal.pone.0113920
34. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed August 11, 2020).
35. Goldenberg RL, McClure EM, Harrison MS, Miodovnik M. Diabetes during pregnancy in low- and middle-income countries. Am J Perinatol. (2016) 33:1227–35. doi: 10.1055/s-0036-1584152
36. Warren CE, Hossain SMI, Ishaku S, Armbruster D, Hillman E. A primary health care model for managing pre-eclampsia and eclampsia in low- and middle-income countries. Reprod Health. (2020) 17:46. doi: 10.1186/s12978-020-0897-0
37. Hogan AB, Jewell BL, Sherrard-Smith E, Vesga JF, Watson OJ, Whittaker C, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. (2020) 8:e1132–41. doi: 10.1016/S2214-109X(20)30288-6
38. Roberton T, Carter ED, Chou VB, Stegmuller AR, Jackson BD, Tam Y, et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health. (2020) 8:e901–8. doi: 10.1016/S2214-109X(20)30229-1
39. Johnson K, Green L, Volpellier M, Kidenda S, McHale T, Naimer K, et al. The impact of COVID-19 on services for people affected by sexual and gender-based violence. Int J Gynaecol Obstet. (2020) 150:285–7. doi: 10.1002/ijgo.13285
40. UNESCO. COVID-19 School Closures Around the World Will Hit Girls Hardest. (2020). Available online at: https://en.unesco.org/news/covid-19-school-closures-around-world-will-hit-girls-hardest (accessed August 11, 2020).
41. Riley T, Sully E, Ahmed Z, Biddlecom A. Estimates of the potential impact of the COVID-19 pandemic on sexual and reproductive health in low- and middle-income countries. Int Perspect Sex Reprod Health. (2020) 46:73–6. doi: 10.1363/46e9020
42. Cohan D, Natureeba P, Koss CA, Plenty A, Luwedde F, Mwesigwa J, et al. Efficacy and safety of lopinavir/ritonavir versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS. (2015) 29:183–91. doi: 10.1097/QAD.0000000000000531
43. Kaplan YC, Ozsarfati J, Nickel C, Koren G. Reproductive outcomes following hydroxychloroquine use for autoimmune diseases: a systematic review and meta-analysis. Br J Clin Pharmacol. (2016) 81:835–48. doi: 10.1111/bcp.12872
44. PREVAIL II Writing Group, Multi-National PREVAIL II Study Team, Davy RT Jr, Dodd L, Proschan MA, Neaton J, et al. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. (2016) 375:1448–56. doi: 10.1056/NEJMoa1604330
45. LaCourse SM, John-Stewart G, Adams Waldorf KM. Importance of inclusion of pregnant and breastfeeding women in COVID-19 therapeutic trials. Clin Infect Dis. (2020) 71:879–81. doi: 10.1093/cid/ciaa444
Keywords: COVID-19, pregnancy, health access equity, low- and middle-income countries, antenatal care, adverse birth outcomes, sexual and reproductive health
Citation: McDonald CR, Weckman AM, Wright JK, Conroy AL and Kain KC (2020) Pregnant Women in Low- and Middle-Income Countries Require a Special Focus During the COVID-19 Pandemic. Front. Glob. Womens Health 1:564560. doi: 10.3389/fgwh.2020.564560
Received: 18 June 2020; Accepted: 24 August 2020;
Published: 25 September 2020.
Edited by:
Marianne Vidler, University of British Columbia, CanadaReviewed by:
Folasade Adenike Bello, University of Ibadan, NigeriaCopyright © 2020 McDonald, Weckman, Wright, Conroy and Kain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chloe R. McDonald, Y2hsb2UubWNkb25hbGRAdXRvcm9udG8uY2E=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.