- 1Department of Biomedical Sciences, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
- 2Department of Public Health, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
- 3Department of Internal Medicine College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
Background: Health-related quality of life (HRQoL) reflects an individual’s perception of how disease and treatment affect their physical, functional, emotional, and social well-being. The burden of cirrhosis is high in Ethiopia but reports on HRQoL among cirrhosis patients are lacking. This study aimed to assess HRQoL and associated factors among patients with cirrhosis at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia.
Methods: A cross-sectional study was conducted among 221 patients with cirrhosis at Tikur Anbessa Specialized Hospital. Data were coded and entered into Epi Data 4.6.0.2 and analyzed by STATA software version 17m/p. Bivariable and multivariable linear regression analyses were performed. A p-value lower than 0.05 was used to declare statistical significance.
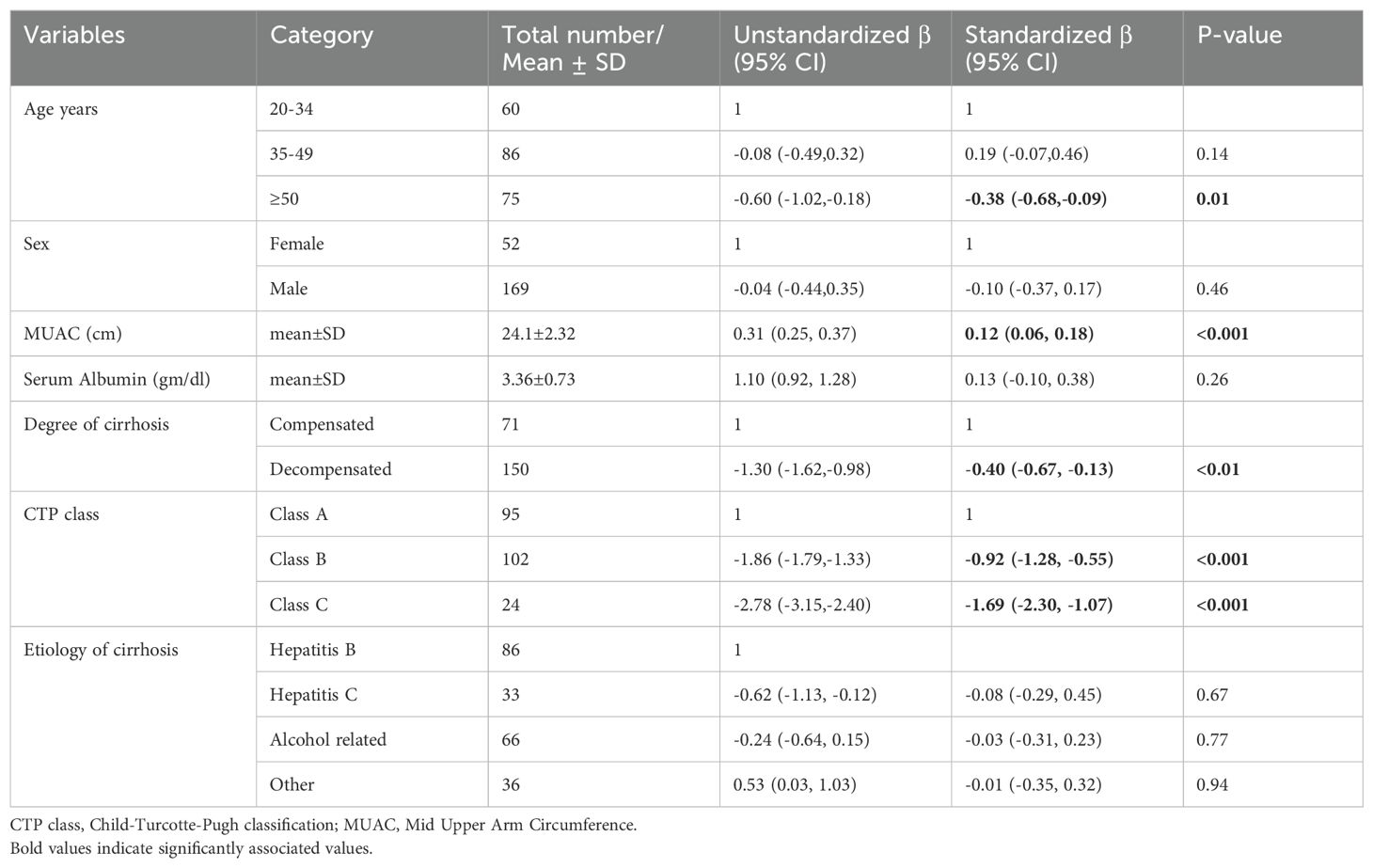
Results: The mean ± SD age was 42.74 ± 12.25. In multivariable linear regression analysis, Mid Upper Arm Circumference (MUAC) (Standardized β.coff (Std.β.coff) = 0.12, 95% CI (0.06, 0.18), age ≥50 (Std.β.coff = -0.38, 95% CI (-0.68,-0.09), Child-Turcotte-Pugh (CTP) class B (Std.β.coff = -0.92, 95% CI (-1.28, -0.55), CTP class C (Std.β.coff = -1.69, 95% CI (-2.30,-1.07) were significantly associated with mean quality of life score.
Conclusion: Quality of life score was significantly associated with age, CTP class, and MUAC. Health-related quality of life should be taken into consideration in the assessment and treatment of patients with cirrhosis. Older patients, patients with decompensated cirrhosis, and patients with lower MUAC measurements should be given special attention.
Introduction
Cirrhosis is the end stage of liver disease characterized by irreversible hepatic fibrosis as a result of repeat injury (1). Cirrhosis is an ever-increasing problem. It has become one of the top 10 leading causes of death in the world (2). Cirrhosis contributed to 2.4% of deaths globally in 2017. Sub-Saharan Africa has the highest mortality from cirrhosis (3, 4). Liver cirrhosis is the seventh leading cause of death in Ethiopia, hepatitis B virus, hepatitis C virus, and alcohol consumption are the major causes (5).
Cirrhosis decreases the overall metabolic ability of the liver, leading to different complications (6) including ascites (7), gastrointestinal bleeding (8), encephalopathy (9), loss of energy, and malnutrition (10). These symptoms and metabolic derangements decrease the overall quality of life of patients.
Health-related quality of life (HRQoL) is a concept about an individual’s perception of their physical, social, emotional, and mental well-being. It reflects a patient’s experience with the disease and treatment (11, 12).
The Chronic Liver Disease Questionnaire (CLDQ) is a widely used method for assessing HRQoL in cirrhosis patientsHRQoL in patients with cirrhosis (13). The questionnaire was developed by Younossi and colleagues in 1999. It has been validated and used by researchers worldwide (14).
Measuring HRQoL in patients with cirrhosis provides information about the extent and the effect of the disease on individuals. Additionally, it can be used to improve treatments, health system delivery (15), determining the prognosis, and decision-making (16, 17).
In countries like Ethiopia, there is a high burden of cirrhosis and low treatment capacity, cirrhosis causes socioeconomic, and health burdens among patients, but there is no information relating to HRQoL in patients with cirrhosis. Therefore, this study aimed to assess HRQoL and associated factors among patients with cirrhosis in Ethiopia.
Methods
Study design and settings
A cross-sectional study was conducted at Tikur Anbessa Specialized Hospital in Addis Ababa, Ethiopia. The hospital is one of the main tertiary care centers in the country and provides services for referral cases from all over the country. Data were collected from September to December 2021.
Study population
The study population were all patients with cirrhosis who visited the gastrointestinal unit of Tikur Anbessa Specialized Hospital during the study period. Two hundred twenty-one patients with cirrhosis were included.
Inclusion and exclusion criteria
All patients (age ≥ 18 years) who were diagnosed with cirrhosis were included. Patients with hepatocellular carcinoma and patients who were unable to communicate were excluded from the study.
Study variables
The dependent variable was health-related quality of life (HRQoL) assessed with the Chronic liver Disease Questionnaire (CLDQ). The CLDQ has six domains: namely abdominal symptoms (AS), fatigue (FA), systemic symptoms (SS), activity (AA), emotional function (EF), and worry (WO). The independent variables were socio-demographic variables (age, sex, marital status, occupation), and clinical variables (cirrhosis degree, Child-Turcotte-Pugh class, etiology of cirrhosis, MUAC, and serum albumin).
Data collection procedure and data quality assurance
Data were collected by face-to-face interview by four interns and supervision was done by three residents. The questionnaire was prepared in English and translated to Amharic, then translated back to English to check the consistency. Three-day training was given to the data collectors. Close supervision was conducted by the investigator and supervisors during the data collection and data entry.
Health-related quality of life (HRQoL) was assessed using the CLDQ (chronic liver disease questionnaire), which is disease disease-specific questionnaire developed for use in CLD patients by Younossi and colleagues (13). The questionnaire consists of 29 questions. The response is assessed on a 7-point Likert scale and the responses range from 1 “all of the time” to 7 “none of the time”. A higher response indicates a good level of functioning, while a lower response indicates a low level of functioning. There are six domains in the questionnaire, which include abdominal symptoms, fatigue, systemic symptoms, activity, emotional functioning, and worry.
Ascites grading was done according to the international ascites grading system. Patients with ascites detectable with ultrasound were grouped as grade 1, patients with moderate ascites were grouped as grade 2, and patients with marked abdominal distention were grouped as grade 3 (18).
Scoring for the Child-Turcotte-Pugh classification was done by incorporating values of encephalopathy, ascites, bilirubin, albumin, and international normalized ratio (INR). Patients were grouped into class A, class B, and class C depending on the scores (19).
Data processing and analysis
Data were coded and entered into Epi Data 4.6.0.2 and analyzed by STATA software version 17m/p. Descriptive statistics with frequency and percentage were used to characterize socio-demographic and clinical variables. Bivariable and multivariable linear regression analyses were conducted, and variables with p-value <0.05 were considered statistically significant. The models showed no influential cases, no multicollinearity, no heteroscedasticity, and residuals were normally distributed showing good fit model.
Ethics approval and consent to participate
The study was conducted after ethical clearance was obtained from the ethics committee of the Department of Internal Medicine, School of Medicine, Addis Ababa University. The objectives of the study were explained to the participants. Verbal and written consent was obtained from each participant.
Results
A total of 221 patients with cirrhosis were included in the study. The mean ± SD age was 42.74 ± 12.25. Among the total patients, 52 (23.53%) were female and 169 (76.47) were male. Thirty two (14.48%) patients were single, 180(81.45%) were married while the remaining 9 (4.07%) were divorced or widowed (Table 1).
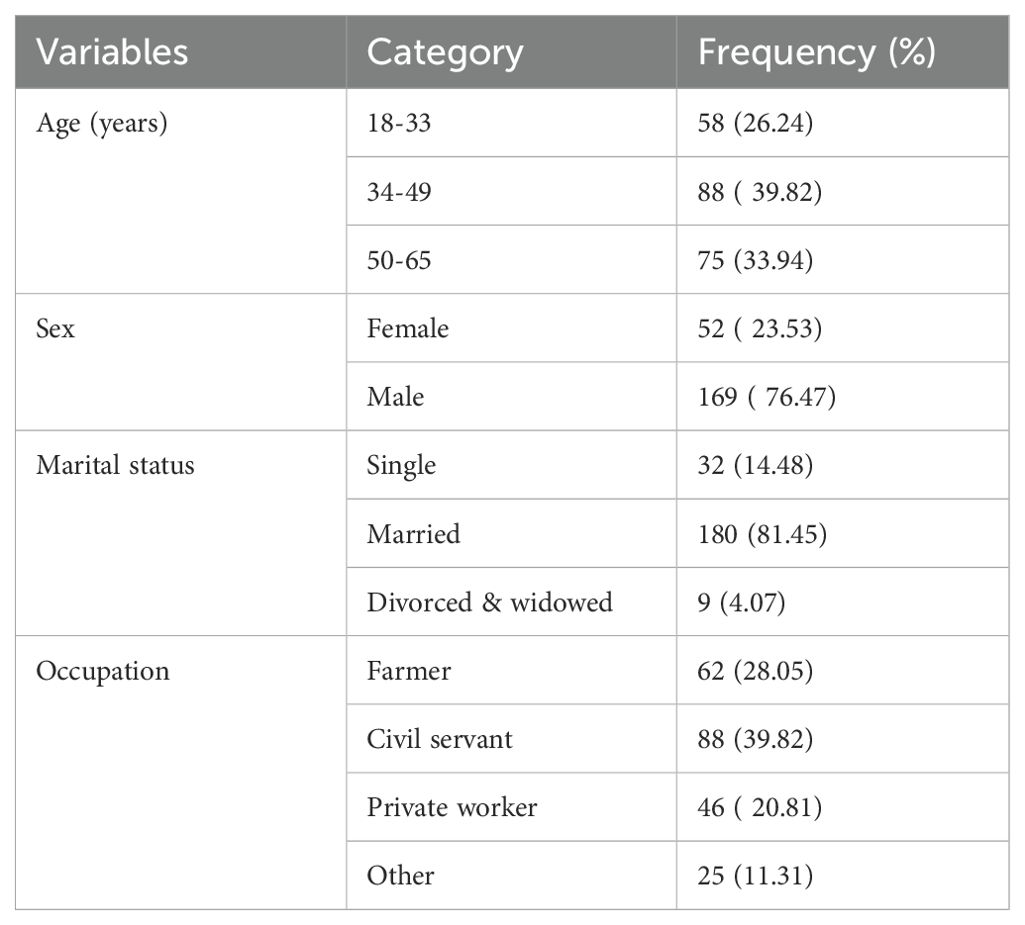
Table 1. Summary of socio-demographic characteristics of patients with cirrhosis at Tikur Anbessa Specialized Hospital, Addis Ababa Ethiopia.
Clinical characteristics
The mean ± SD of serum albumin was 3.36 ± 0.73. Seventy-one (32.13%) of patients had compensated cirrhosis while the remaining 150 (67.87%) had decompensated cirrhosis. According to Child-Turcotte-Pugh classification 95 (42.99%) of patients were class A, 102 (46.15%) were class B, and 24(10.86%) were class C. Regarding ascites 110 (49.77%) patients had grade 1 ascites, 87 (39.37%) had grade 2 ascites, and the remaining 24 (10.86%) had grade 3 ascites (Table 2).
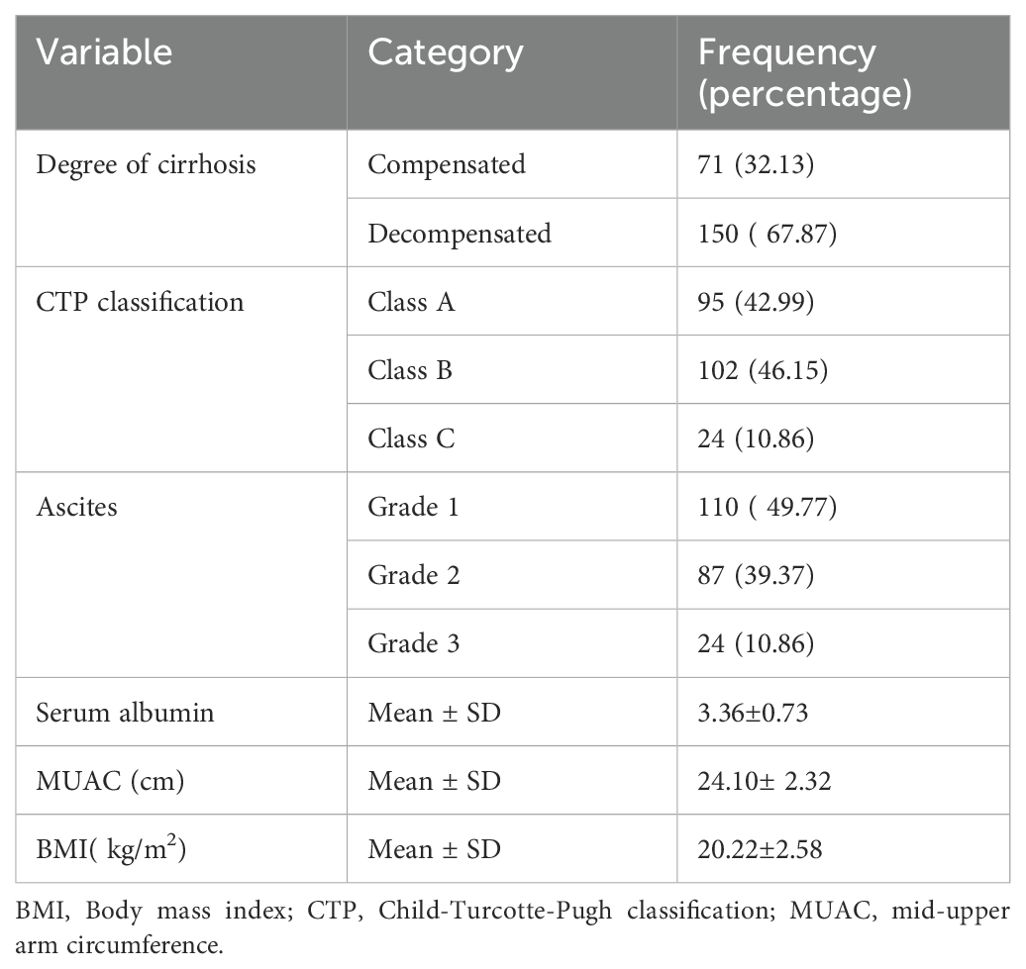
Table 2. Clinical characteristics of cirrhosis patients at Tikur Anbessa Specialized Hospital Addis Ababa Ethiopia.
Quality of life using the chronic liver disease questionnaire
Health Related Quality of life (HRQoL) was assessed using the Chronic Liver Disease Questionnaire (CLDQ) which has 29 items with six domains including abdominal symptoms, fatigue, systemic symptoms, activity, emotional functioning, and worry. The response was scored 0-7. A higher score indicates a higher level of functioning and good quality of life while a lower score indicates lower quality of life.
Among the domains lower mean value was observed in the worry domain Mean ± SD (3.60± 1.21) while a higher mean score was observed in the systemic symptoms domain Mean ± SD (5.63± 1.13) (Table 3).
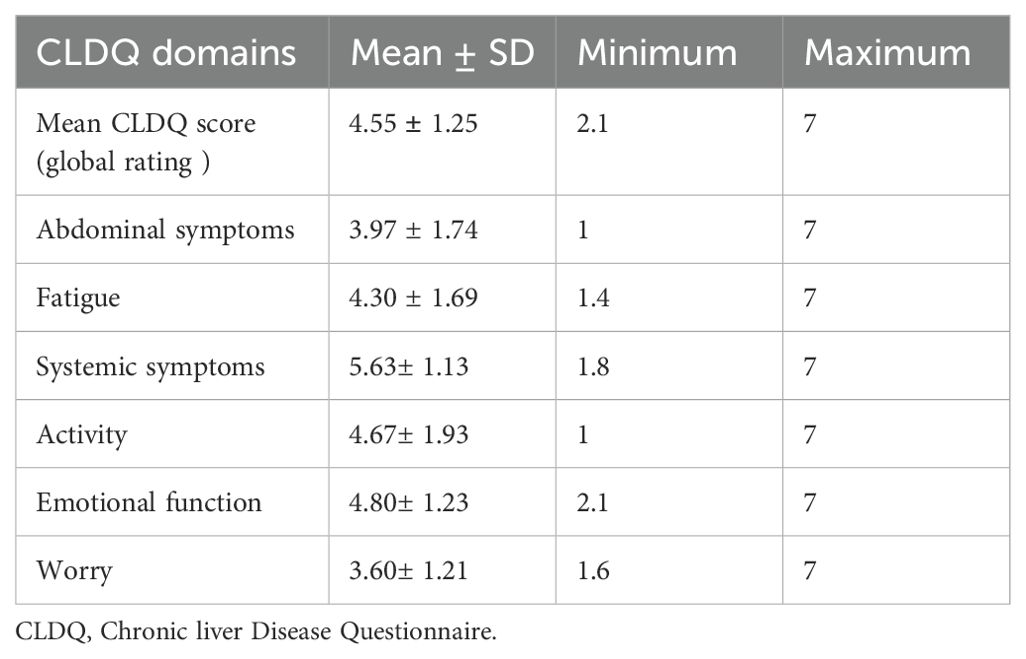
Table 3. Mean score of CLDQ score and domains of patients with cirrhosis at Tikur Anbessa Specialized Hospital.
Health-related quality of life among patients with compensated and decompensated cirrhosis
Independent samples t-test was used to compare differences in mean HRQoL score, and mean scores in HRQoL domains including; abdominal symptoms, fatigue, systemic symptoms, activity, emotional functioning, and worry between patients with decompensated cirrhosis and compensated cirrhosis. Patients with decompensated cirrhosis scored significantly lower in the mean CLDQ score and all the domains (Table 4).
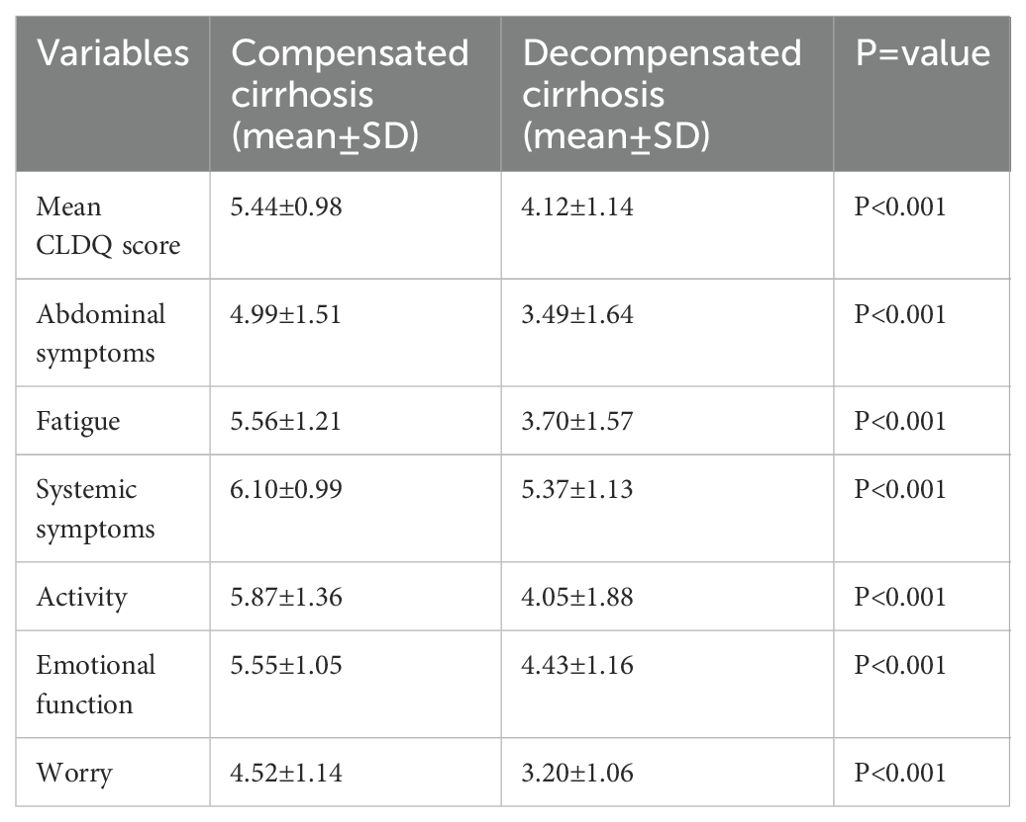
Table 4. Independent samples t-test of mean CLDQ and domains among patients with compensated and decompensated cirrhosis.
Factors associated with health-related quality of life scores
A bivariable linear regression analysis was performed to obtain candidate variables for multivariable linear regression analysis. Variables with a p-value of less than 0.25 were included in the multivariable linear regression analysis. These variables included: Age, Sex, Serum albumin, MUAC, CTP score, degree of cirrhosis, and etiology of cirrhosis.
In multivariable linear regression analysis MUAC (Std.β.coff = 0.12, 95% CI (0.06, 0.18), age ≥ 50 (Std.β.coff = -0.38, 95% CI (-0.68,-0.09), Child- Turcotte Pugh (CTP) class B (Std.β.coff = -0.92, 95% CI (-1.28, -0.55), CTP class C (Std.β.coff = -1.69, 95% CI (-2.30,-1.07) were significantly associated with quality of life score (Table 5).
Discussion
Patients with liver cirrhosis face different complications that reduce their quality of life. HRQoL is important to reflect patients perception on disease progression and treatment. The CLDQ is a disease-specific questionnaire to assess HRQoL in patients with cirrhosis (13).
In this study age is one of the significant predictors of quality of life. Patients older than 50 had worse quality of life. In line with the current study, studies conducted among community-dwelling adults in Spain and China, as well as a study among heart failure patients in Ethiopia, have demonstrated that older adults have lower HRQoL compared to younger adults (20–22). However, there are conflicting findings regarding the impact of age on HRQoL in patients with CLD. A study conducted among CLD patients in Thailand found that older age negatively affects HRQoL (23), while a study conducted in Canada showed younger age negatively affects HRQoL (24). Meanwhile, studies conducted in Nepal (25) and Germany (26) showed no association between age and HRQoL. Methodological differences and use of different tools may be the reason for the discrepancy of results among the studies. According to studies, showing negative impact of aging on HRQoL, aging by itself lowers physical and mental function which decreases the ability to perform daily activities thereby lowering the overall quality of life (27).
In this study patients with Child-Turcotte-Pugh (CTP) score B and C have lower quality of life sores. This is in line with other articles conducted in India (28) Spain (29) and Pakistan (30). Different reports have shown that the severity of disease influences HRQOL including heart failure (21), Lupus (31), and Cancer (32). As the severity of the disease increases, symptoms and complications from the disease cause, limitations in physical, mental, and emotional function which lowers the overall quality of life (33).
Findings from our study indicate that as MUAC increases mean quality of life score also increases. MUAC is a simple non-invasive anthropometric indicator widely used to assess malnutrition among children and adults globally. Studies have shown that MUAC measurement can detect malnutrition with good accuracy similar to Triceps skin fold thickness and Middle arm Muscle circumference (34). MUAC has been used to measure appendicular muscle mass, sarcopenia, and malnutrition in patients with cirrhosis (10, 35). Reports have shown that malnutrition lowers HRQoL in patients with cirrhosis (36).
Limitations
One limitation of this study is the lack of control groups, the researchers couldn’t incorporate control groups due to budget constraints. Additionally, since the data was collected at a tertiary hospital, the results may not be generalizable to all patients with cirrhosis.
Conclusion
In this study, mean HRQoL score was significantly associated with age, CTP classification, and MUAC. Routine assessment HRQoL should be considered in patients with cirrhosis. Older patients, patients with advanced cirrhosis, and patients with lower MUAC scores should be given special attention.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Addis Ababa University Ethical Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was obtained from the participants in accordance with the national legislation and institutional requirements.
Author contributions
EZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. AT: Conceptualization, Formal Analysis, Methodology, Software, Supervision, Writing – review & editing. TA: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – review & editing. AM: Conceptualization, Investigation, Methodology, Software, Visualization, Writing – review & editing. MA: Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. MM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank the staff of Tikur Anbessa Specialized Hospital Gastrointestinal unit for facilitating the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CLDQ, chronic Liver Disease Questionnaire; CTP, Child-Turcotte-Pugh classification; HRQoL, Health health-related quality of life; INR, international normalized ratio; MUAC, Mid Upper Arm Circumference.
References
1. Jagdish RK, Roy A, Kumar K, Premkumar M, Sharma M, Rao PN, et al. Pathophysiology and management of liver cirrhosis: from portal hypertension to acute-on-chronic liver failure. Front Med. (2023) 10. doi: 10.3389/fmed.2023.1060073
2. Ye F, Zhai M, Long J, Gong Y, Ren C, Zhang D, et al. The burden of liver cirrhosis in mortality: Results from the global burden of disease study. Front Public Heal. (2022) 10. doi: 10.3389/fpubh.2022.909455
3. Sepanlou SG, Safiri S, Bisignano C, Ikuta KS, Merat S, Saberifiroozi M, et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2020) 5:245–66. doi: 10.1016/S2468-1253(19)30349-8
4. Vento S, Dzudzor B, Cainelli F, Tachi K. Liver cirrhosis in sub-Saharan Africa: neglected, yet important. Lancet Glob Heal. (2018) 6:e1060–1. doi: 10.1016/S2214-109X(18)30344-9
5. Tesfaye BT, Feyissa TM, Workneh AB, Gudina EK, Yizengaw MA. Chronic liver disease in Ethiopia with a particular focus on the etiological spectrums: A systematic review and meta-analysis of observational studies. Can J Gastroenterol Hepatol. (2021) 2021:6–8. doi: 10.1155/2021/8740157
6. Arakawa Y, Moriyama M, Arakawa Y. Liver cirrhosis and metabolism (sugar, protein, fat and trace elements). Hepatol Res. (2004) 30:46–58. doi: 10.1016/j.hepres.2004.10.009
7. Biecker E. Diagnosis and therapy of ascites in liver cirrhosis. World J Gastroenterol. (2011) 17:1237–48. doi: 10.3748/wjg.v17.i10.1237
8. Biecker E. Gastrointestinal bleeding in cirrhotic patients with portal hypertension. ISRN Hepatol. (2013) 2013:541836. doi: 10.1155/2013/541836
9. Mesejo A, Juan M, Serrano A. Liver cirrhosis and encephalopathy: clinical and metabolic consequences and nutritional support. Nutr Hosp. (2008) 23 Suppl 2:8–18.
10. Sherpa TW, Pathak R, Khadga PK, Sharma S, Hamal R, Jha A. Nutritional assessment of patients with liver cirrhosis by nutrition screening tool and anthropometry at a tertiary care center. J Inst Med Nepal. (2019) 41:21–5. doi: 10.59779/jiomnepal.1038
11. Sitlinger A, Zafar SY. Health-related quality of life: the impact on morbidity and mortality. Surg Oncol Clin N Am. (2018) 27:675–84. doi: 10.1016/j.soc.2018.05.008
12. Basch E, Snyder C, McNiff K, Brown R, Maddux S, Lou SM, et al. Patient-reported outcome performance measures in oncology. J Oncol Pract. (2014) 10:209–11. doi: 10.1200/JOP.2014.001423
13. Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. (1999) 45:295–300. doi: 10.1136/gut.45.2.295
14. Çelik F, Bektaş H. Psychometric properties of the chronic liver disease questionnaire in patients with chronic liver disease. J Basic Clin Heal Sci. (2023) 7:671–83. doi: 10.30621/jbachs.1170430
15. Orr JG, Homer T, Ternent L, Newton J, Mcneil CJ, Hudson M, et al. Review Health related quality of life in people with advanced chronic liver disease. J Hepatol. (2014) 61:1158–65. doi: 10.1016/j.jhep.2014.06.034
16. Montagnese S, Bajaj JS. Impact of hepatic encephalopathy in cirrhosis on quality − of − Life issues. Drugs. (2019) 79:11–6. doi: 10.1007/s40265-018-1019-y
17. Muhamed AN, Chekole B, Tafesse FE, Dessie G, Bantie B, Habtu BF, et al. Quality of life among Ethiopian cancer patients: A systematic review of literatures. SAGE Open Nurs. (2023) 9:6. doi: 10.1177/23779608231202691
18. Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. (2006) 55:1–12. doi: 10.1136/gut.2006.099580
19. Galbois A, Das V, Carbonell N, Guidet B. Prognostic scores for cirrhotic patients admitted to an intensive care unit: Which consequences for liver transplantation? Clinics Res Hepatol Gastroenterol Elsevier Masson SAS. (2013) 37:455–66. doi: 10.1016/j.clinre.2013.05.001
20. Etxeberria I, Urdaneta E, Galdona N. Factors associated with health-related quality of life (HRQoL): differential patterns depending on age. Qual Life Res. (2019) 28:2221–31. doi: 10.1007/s11136-019-02182-0
21. Mulugeta H, Sinclair PM, Wilson A. Health-related quality of life and its influencing factors among people with heart failure in Ethiopia: using the revised Wilson and Cleary model. Sci Rep. (2023) 13:1–11. doi: 10.1038/s41598-023-47567-x
22. Ko H, Cho B, Lim KC, Jang SN, Chang SJ, Yi YM, et al. Changes in the health status and health-related quality of life of community-dwelling older adults living alone: one-year follow-up from a cohort study. Front Public Heal. (2023) 11:1–9. doi: 10.3389/fpubh.2023.1278008
23. Sobhonslidsuk A, Silpakit C, Kongsakon R, Satitpornkul P, Sripetch C, Khanthavit A. Factors influencing health-related quality of life in chronic liver disease. World J Gastroenterol. (2006) 12:7786–91. doi: 10.3748/wjg.v12.i48.7786
24. Kok B, Whitlock R, Ferguson T, James Bailey R, Warren Burak K, Kowalczewski J, et al. Health-related quality of life: A rapid predictor of hospitalization in patients with cirrhosis. Am J Gastroenterol. (2020) 115:575–83. doi: 10.14309/ajg.0000000000000545
25. Pradhan RR, Kafle Bhandari B, Pathak R, Poudyal S, Anees S, Sharma S, et al. The assessment of health-related quality of life in patients with chronic liver disease: A single-center study. Cureus. (2020) 36:7. doi: 10.7759/cureus.10727
26. Häuser W, Holtmann G, Grandt D. Determinants of health-related quality of life in patients with chronic liver diseases. Clin Gastroenterol Hepatol. (2004) 2:157–63. doi: 10.1016/S1542-3565(03)00315-X
27. Noto S. Perspectives on aging and quality of life. Healthc. (2023) 11:3–5. doi: 10.3390/healthcare11152131
28. Mayank Jain JK, Vargese J, Srinivasan V, Harika K, Michael T, Venkataraman J. Health-related quality of life in liver cirrhosis patients using SF-36 and CLDQ questionnaires. Clin Exp Hepatol. (2018) 4:232–9. doi: 10.5114/ceh.2018.80124
29. Pantiga C, López L, Pérez M, Rodríguez M, Linares A, Dieguez LG, et al. Quality of life in cirrhotic patients and liver transplant recipients. Psicothema. (2005) 17:143–7. Available online at: http://hdl.handle.net/10651/26717
30. Gazder DP, Parvez SS, Gazder NP, Muqtadir J. Health-related quality of life assessment for liver cirrhosis patients at a tertiary care hospital in karachi, Pakistan. Cureus. (2024) 16:4–11. doi: 10.7759/cureus.53766
31. Chaiamnuay S, Lomaratana V, Sumransurp S, Phukongchai S, Narongroeknawin P, Asavatanabodee P. Health-related quality of life and disease severity of SLE patients in Phramongkutklao Hospital. J Med Assoc Thai. (2010) 93 (Suppl 6):3–6.
32. Hörnquist JO, Hansson B, Åkerlind I, Larsson J. Severity of disease and quality of life: A comparison in patients with cancer and benign disease. Qual Life Res. (1992) 1:135–41. doi: 10.1007/BF00439721
33. Finlayson TL, Moyer CA, Sonnad SS. Assessing symptoms, disease severity, and quality of life in the clinical context: A theoretical framework. Am J Manag Care. (2004) 10:336–44. doi: c.com/view/may04-1786p336-344
34. Topan MM, Sporea I, Dănilă M, Popescu A, Ghiuchici AM, Lupuşoru R, et al. Comparison of different nutritional assessment tools in detecting malnutrition and sarcopenia among cirrhotic patients. Diagnostics. (2022) 12:4–9. doi: 10.3390/diagnostics12040893
35. Hu FJ, Liu H, Liu XL, Jia SL, Hou LS, Xia X, et al. Mid-upper arm circumference as an alternative screening instrument to appendicular skeletal muscle mass index for diagnosing sarcopenia. Clin Interv Aging. (2021) 16:1095–104. doi: 10.2147/CIA.S311081
Keywords: cirrhosis, Child-Turcotte-Pugh (CTP) classification, health-related quality of life, Ethiopia, MUAC (Mid-Upper Arm Circumference)
Citation: Zewde EA, Teklemariam AB, Ayele TS, Agidew MM, Mulu AT and Melaku MD (2024) Health-related quality of life among patients with cirrhosis at a tertiary care center in Addis Ababa, Ethiopia: a cross-sectional study. Front. Gastroenterol. 3:1455837. doi: 10.3389/fgstr.2024.1455837
Received: 27 June 2024; Accepted: 19 November 2024;
Published: 12 December 2024.
Edited by:
Cinthia G. Goldman, University of Buenos Aires, ArgentinaReviewed by:
Morven Cunningham, University Health Network (UHN), CanadaMaría Cielo Gutiérrez, University of Buenos Aires, Argentina
Copyright © 2024 Zewde, Teklemariam, Ayele, Agidew, Mulu and Melaku. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edgeit Abebe Zewde, ZWRnZXRhYmViZTgyQGdtYWlsLmNvbQ==
 Edgeit Abebe Zewde
Edgeit Abebe Zewde Awgichew Behaile Teklemariam
Awgichew Behaile Teklemariam Tesfaneh Shemeles Ayele
Tesfaneh Shemeles Ayele Melaku Mekonnen Agidew
Melaku Mekonnen Agidew Anemut Tilahun Mulu
Anemut Tilahun Mulu Metages Damtie Melaku
Metages Damtie Melaku