
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Gastroenterol., 03 June 2024
Sec. Therapy in Gastroenterology
Volume 3 - 2024 | https://doi.org/10.3389/fgstr.2024.1335380
Mesalazine is an established and recommended first-line treatment for mild-to-moderate ulcerative colitis (UC). For patients with moderately active UC, the choice to use mesalazine or to initiate treatment with an oral corticosteroid or anti-tumor necrosis factor (TNF) agent is not clearly informed from current guidelines. The use of mesalazine is supported by robust clinical evidence supporting its efficacy at inducing remission in patients with moderately active disease. A key advantage of mesalazine is its tolerability profile being similar to that of placebo, which contrasts with that of the corticosteroids and advanced therapies, where there is the potential for significant toxicities. Mesalazine also has cost advantages over anti-TNFs and other advanced therapies. Evidence supports the consideration of all patients with moderately active UC for first-line mesalazine therapy at an optimized dose of ≥4g/d (± 1g/d rectal). Patients responding to treatment within 2 weeks should continue at ≥4g/d for at least 6 months before a dose reduction is considered, since this then alters the pattern of disease.
Mesalazine, also known as mesalamine or 5-aminosalicylic acid (5-ASA), has been a mainstay of treatment for mild-to-moderate ulcerative colitis (UC) for over three decades. Current clinical guidelines, such as those from the European Crohn´s and Colitis Organisation (ECCO) and American Gastroenterological Association (AGA), recommend mesalazine as first-line treatment of mild-to-moderately active UC (1, 2). These same guidelines also provide recommendations on moderate-to-severely active UC, where oral corticosteroids and anti-tumor necrosis factor (TNF) agents are standard initial therapies (1, 2). When considered together, these recommendations leave it unclear what should be the most appropriate first-line treatment for patients with moderately active UC, particularly as there is no universally applied definition of disease severity. Indeed, the ECCO guidelines state that: “We recognise that these divisions [mildly-to-moderately active disease and moderately-to-severely active disease] are somewhat arbitrary, partially overlapping, and inconsistently defined…” (1).
To aid clinical decision-making, we present the rationale and evidence supporting the continuing role of mesalazine as first-line treatment of moderately active UC.
UC is a chronic inflammatory condition affecting the intestinal mucosa of the colon and rectum, characterized by periods of active disease interspersed by periods of remission (3). In active UC, the most common symptoms exhibited are bloody diarrhea with urgency, increased frequency and abdominal pain. Additional symptoms include fatigue, weight loss, and may include extra-intestinal manifestations, such as anemia, joint inflammation, or mouth ulcers (4). The symptoms of UC can lead to a profound negative impact on multiple aspects of patient quality of life (QoL), including functional, psychological, social and occupational (5, 6). These detrimental impacts on QoL correlate with increasing disease severity (6).
A number of clinical and endoscopic assessment tools exist for the assessment of UC severity, including Truelove & Witts’ criteria, the Mayo Clinic score, the UC Disease Activity Index (UCDAI), Simple Clinical Colitis Activity Index (SCCAI), and the UC Endoscopic Index of Severity (UCEIS) (7–11). However, there is no consensus on the most appropriate tool to use across guidelines or within clinical practice (2, 4, 9, 12). Moderately active UC is typically defined as a stool frequency (SF) of >4 times daily with urgency and visible blood, but without systemic features (8).
The greater part of clinical evidence for mesalazine has focused on it use across the spectrum of mild-to-moderate UC, with Cochrane reviews (13, 14) and other meta-analyses (15, 16) confirming its effectiveness at inducing and maintaining remission in patients with extensive, left-sided, or distal disease. Several studies and a recent meta-analysis have assessed the use of mesalazine specifically in patients with moderately active UC (17–20).
In a study by Kamm et al. (17) of MMX mesalazine 2.4g/d and 4.8g/d, both doses achieved similarly higher rates of combined clinical and endoscopic remission (UCDAI score ≤1, rectal bleeding [RB]=0, SF=0, ≥1 point reduction from baseline in sigmoidoscopy score at week 8) than placebo (38.9% and 36.0% vs 22.8%, respectively; p-value not reported) in patients with moderately active UC (modified UCDAI score 6–10). The study also included 2.4g/d Eudragit-S-coated mesalazine as a reference arm, which had a combined remission rate of 30.2% (17). In contrast to this study, results from the ASCEND II and III studies suggested an advantage when using higher doses of mesalazine (18, 19). In patients with moderately active UC (Physician’s Global Assessment [PGA]=2 points, SF=1, RB=1, ≥2 points in sigmoidoscopy assessment with positive friability assessment), increased rates of treatment success (overall improvement at week 6) were achieved with Eudragit-S-coated mesalazine 4.8g/d than 2.4g/d (ASCEND II: 71.8% vs 59.2%, respectively, p=0.036; ASCEND III: 70.2% vs 65.5%, p=0.17). Rates of clinical (SF=0 and RB=0) and complete remission (no clinical evidence of disease and normal endoscopy) also tended to be higher for the 4.8g/dose, albeit reaching statistical significance only for the former (clinical: 43% vs 35% for 2.4g/d, p=0.04; complete: 20.2% vs 17.7% for 2.4g/d, p-value not reported) (18, 19). Of clinical relevance, the time to cessation of RB was significantly shorter in patients who received 4.8g/day compared with those on 2.4 g/day (9 vs 16 days, respectively; p=0.035) (18). The clinical relevance of this is that if rectal bleeding has not stopped within 10 days on high dose (>4g) mesalazine, then the patient will be a slow or incomplete responder, so treatment can be escalated at that stage.
More recently, a network meta-analysis only in patients with moderately active UC, used data from the Kamm et al. (17) and ASCEND II & III studies (18, 19). This reported that oral prolonged-release mesalazine 4g/d has broadly similar efficacy at inducing combined clinical and endoscopic remission to MMX mesalazine 4.8g/d and Eudragit-S-coated mesalazine 4.8g/d (20). Whilst there was no statistical difference between oral prolonged-release 4g/d and MMX 4.8g/d, there was an approximately 5% difference between oral prolonged-release 4g/d and Eudragit-S-coated mesalazine 4.8g/d in favor of the former (20).
All these studies included patients with left-sided or extensive disease. In patients with moderately active distal disease, the real-world QUARTZ study found that 75.6% and 82.4% of those treated with oral and/or rectal prolonged-release mesalazine were in clinical remission (Mayo clinical subscore [excluding endoscopy] ≤2 with no item >1) at 8 weeks and 12 months, respectively (21). Corresponding results for normal or inactive disease at endoscopy (Mayo endoscopy score <1) were 57.1% at 8 weeks and 61.5% at 12 months (21). The study also assessed the important outcome of health-related QoL (Short Inflammatory Bowel Disease Questionnaire) and this was found to be significantly improved in patients with moderately active disease receiving prolonged-release mesalazine (total score 43.3 at week 8 vs 35.9 at baseline; p<0.001) (21). This 7.4 point difference in scores did not quite reach what is generally considered a clinically meaningful change of 9 points, but was achieved in a patient population with distal disease and a comparatively low baseline QoL (35.9 versus, for example, 44.9 in the EpiCom cohort) (21, 22).
In addition to its proven efficacy, a strong rationale for mesalazine use is its excellent tolerability profile. Different meta-analyses have reported similar adverse event (AE) results for mesalazine and placebo (13, 15). The most commonly reported adverse events associated with mesalazine are headache, nausea, abdominal pain, nasopharyngitis, rash, loss of appetite, flatulence and fever (13). Nevertheless, these events are as common in patients receiving placebo in the randomized controlled trials. The lack of notable AEs is likely in large part due to the topical effect of mesalazine on the intestinal mucosa, since mesalazine acts on, is metabolized by and excreted from intestinal epithelial cells. Unusually for medications with documented efficacy, studies have found no clinically relevant difference in AE profile or rate with higher (>2g) versus lower (<2g) mesalazine doses (15, 17–19).
Alternative treatments for moderately active UC, including oral systemic corticosteroids and advanced therapies (e.g. adalimumab, filgotinib, golimumab, infliximab, tofacitinib, ustekinumab and vedolizumab), are effective and generally well-tolerated (23–26). Nonetheless, salient limitations to their use is their potential for debilitating and serious AEs, particularly with longer-term use. The side effects of systemic corticosteroids are well recognized, including infections, psychological disturbances, weight gain, gastrointestinal (GI) AEs, hirsutism, alopecia, vertigo, venous thromboembolism (VTE), cardiovascular disease, myopathy and osteoporosis (27–30). Although no guidelines recommend the long-term use of corticosteroids, it is recognized that approximately 5–15% of patients with UC may be on chronic steroidal treatment (defined as >3 or 6 months therapy) in clinical practice (31, 32), albeit it would be expected that the majority would have disease at the severer end of the spectrum. Advanced therapies also have the potential to cause AEs such as injection site reactions (ISRs)/infusion-related reactions, serious infections, behavioral disturbances, hypertension, anemia and musculoskeletal disorders, while some increase the risk of malignancy (e.g. lymphoma), cardiovascular events, VTE and serious skin conditions (23, 25, 26). When considering severe UC (≥6 bloody stools per day with additional signs of toxicity (8)) the balance would lean towards the efficacy of advanced therapies exceeding the potential risks of toxicity. However, for patients with moderately active disease, the potential for serious AEs assumes increasing importance, particularly when there is the option to initiate mesalazine treatment with its efficacy and safety in this population. From a healthcare provider perspective, the treatment costs of mesalazine are also advantageous, being lower than that of advanced therapies (33).
To illustrate the potential clinical and cost benefits of mesalazine for patients with moderately active UC, a modeling exercise was undertaken. This used a modified version of a published model that quantified the benefits of optimized mesalazine treatment strategy across the spectrum of mild-to-moderate disease (34). The modeling focused on the induction of remission in patients with moderately active disease and compared two treatment strategies (Supplementary Figure 1):
1. High-dose mesalazine (≥4g/d oral, plus budesonide MMX in left-sided or extensive disease as included in the previous model (34)) as first-line treatment before oral systemic steroids and anti-TNFs; versus,
2. Oral systemic steroids as first-line treatment before anti-TNFs.
The model compared outcomes for a hypothetical population of 10,000 patients who were followed until remission or requirement for anti-TNF treatment (i.e. maintenance treatment was not modeled). Efficacy inputs were derived from the available literature and, data permitting, focused on combined clinical and endoscopic remission (Table 1) (20, 35, 36, 43). Patients are assumed to be equally adherent in both model arms and to be equivalent in adherence to that seen in clinical data. Remission rates for mesalazine were extracted from the most recent meta-analysis in moderate UC (20). The efficacy for budesonide MMX was extracted from the combined analysis of the CORE I and CORE II trials (44, 45), which included a subgroup analysis of patients with moderate UC (35). For systemic steroids, a meta-analysis that included both beclomethasone dipropionate and prednisolone was used (36), albeit the data for the latter were derived from a single study (23). Mesalazine and budesonide MMX were assumed to cause no major AEs (as outlined above) (15, 35), while several notable AEs for systemic corticosteroids and anti-TNFs were assessed (27, 39–42). Treatment acquisition costs were taken from the British National Formulary (October 2023) (33) and administration costs from the UK National Health Service (NHS) reference costs (2021–2022) (37) or Personal Social Services Research Unit data (2022) (38). The benefits of mesalazine as first-line treatment were expressed as the number of patients avoiding systemic corticosteroids and anti-TNF therapy due to remission being achieved without the need to escalate to these therapies. In addition, the potential AEs avoided and cost savings related to this reduced use were also calculated.
The model projected that the use of mesalazine as first-line therapy was associated with 3,311 fewer patients (33.1% of modeled patient population, Figure 1) requiring systemic corticosteroids (relative reduction: 33.1%) and 2,381 (23.8%) requiring anti-TNFs (relative reduction: 33.1%). An alternative visualization of these results, using 100 patients as the input population, is included as Supplementary Figure 2 as this may be more intuitive for some readers. The model calculated that this reduction in exposure to systemic corticosteroids resulted in the potential to avoid up to 430 GI AEs, 357 neurological AEs, 273 dermatological AEs, 166 psychological AEs, 148 infections, and 12 VTEs. With the reduction in use of anti-TNF agents, up to 150 ISRs or 276 infusion-related reactions, 14 serious infections, 64 cases of anemia, and 43 cases of pyrexia would potentially be avoided. Whilst no AEs for mesalazine (or budesonide MMX) were included in this analysis, the toxicities highlighted for the systemic corticosteroids and anti-TNFs were far from exhaustive due to limitations in the data available.
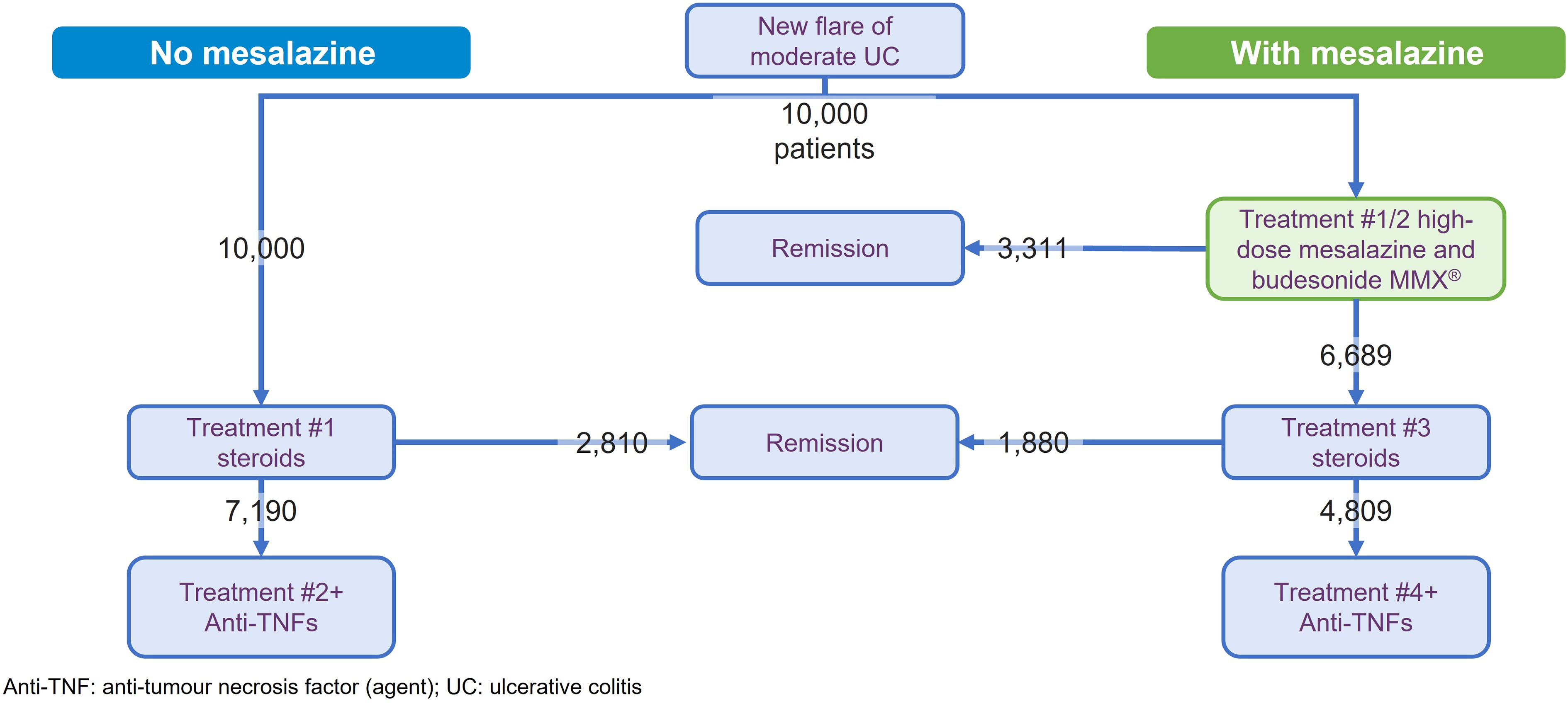
Figure 1 Results of model comparing the first-line treatment with and without mesalazine treatment in the induction of remission for moderately active ulcerative colitis.
Financially, the use of mesalazine was associated with an overall saving of £6,565,382 versus initiating treatment with systemic corticosteroids and progressing to anti-TNFs (saving of £9,736,138 with costs of mesalazine/budesonide MMX treatment of £3,170,756). This equated to a per patient saving of about £656. Additional breakdown by mesalazine formulation found cost savings of £688 per patient for prolonged-release mesalazine, £648 for MMX mesalazine, and £610 for Eudragit S coated mesalazine (Supplementary Table 1), predicated on the efficacy reported in the meta-analysis (remission rate: 34.1%, 32.9% and 31.6%, respectively) (20).
A key limitation of the modeling was the variability in the definition of remission across the studies. This was particularly striking for systemic corticosteroids and budesonide MMX, where the latter used the strictest definition of remission (combined clinical and endoscopic remission, where RB=0 and SF=0, with normal mucosal and no friability on full colonoscopy, and a ≥1 point reduction in endoscopy score, meaning a DAI <1). In contrast studies of systemic steroids defined remission as a DAI score ≤3 ± mucosal healing (22, 43–45). Assuming that stricter assessment criteria would result in decreased remission rates, a sensitivity analysis was performed reducing the efficacy of the systemic corticosteroids by a nominal 10% or 25%. The number of patients avoiding anti-TNFs was 2,473 with a 10% rate and 2,612 with a 25% rate (versus 2,381 in the base case). This corresponded to a cost saving per patient of £694 and £751, respectively (base case: £656.54). As the use of mesalazine leads to fewer patients requiring steroids, the reduction in the steroidal remission rate meant that more patients in the no mesalazine treatment pathway were affected by this change, thus increasing the differential benefit for mesalazine. It is also acknowledged that there is a very small but definite risk of serious AEs with mesalazine, such as interstitial nephritis, nephrotic syndrome, or pneumonitis. Such events remain the subject of case reports, so their inclusion in the model, even with a hypothetical population of 10,000, would not be expected materially to affect the results.
The evidence presented demonstrates that mesalazine has a well-supported history as effective and well-tolerated first-line therapy for patients with moderately active UC. Use of mesalazine should be considered for all patients with moderately active UC, particularly those with newly diagnosed disease or who have relapsed after a long period of remission. An optimized oral dose of mesalazine ≥4g/day (± 1g/d rectal mesalazine for proctitis) is strongly recommended to maximize the chances of treatment success. For patients not responding to mesalazine therapy (e.g. no reduction or cessation of RB within 2 weeks), then escalation to oral corticosteroids or advanced therapies should be explored. For those who do respond to mesalazine, however, the IMPACT study has shown that longer durations of treatment for at least 6 months with mesalazine 4g/d led to a significant reduction in the risk of relapse (46). If a dose reduction is considered following a sustained period of remission, then guidelines recommend that an oral dose of at least 2g/day mesalazine should be maintained (1, 2).
The advantages of using mesalazine as first-line therapy include the avoidance of potentially serious AEs associated with systemic steroids and the advanced therapies, as well as cost savings to the healthcare system, as supported by the modeling. In addition, avoiding the use of anti-TNFs means that if they are required subsequently in the disease course, then the potential issue of immunogenicity (antidrug antibodies) compromising efficacy will be deferred (42, 47). Likewise, the cumulative effects of steroids on, for example, bone density, would be minimized since fewer courses would need to be prescribed (27). When considering long-term maintenance therapy, the cost savings of mesalazine in preference to the anti-TNFs would be considerable. It is clear that some patients with moderately active UC will need steroids and/or advanced therapies. The point is that some – perhaps many – do not. Such patients matter.
In view of an increasing worldwide prevalence of UC, particularly in less industrialized countries (48–50) where restrictions on healthcare budgets are even greater than Western Europe or North America, it is important that the therapeutic armamentarium for UC be used effectively. Optimal mesalazine therapy remains the starting point of treatment for patients with moderately active UC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
MF: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. KP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ST: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare that this study received funding from Ferring Pharmaceuticals. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
KP is an employee of Ferring Pharmaceuticals. MF is an employee of Violicom Medical Limited that has received funding from Ferring for work on various projects. ST has received grants/research support from AbbVie, Buhlmann, Celgene, IOIBD, Janssen, Lilly, Pfizer, Takeda, UCB, Vifor, and Norman Collisson Foundation; Consulting Fees from Abacus, AbbVie, Actial, ai4gi, Alcimed, Allergan, Amgen, Aptel, Arena, Asahi, Aspen, Astellas, Atlantic, AstraZeneca, Barco, Biocare, Biogen, BLPharma, Boehringer Ingelheim, BMS, Buhlmann, Calcico, Celgene, Cellerix, Cerimon, ChemoCentryx, Chiesi, CisBio, ComCast, Coronado, Cosmo, Ducentis, Dynavax, Elan, Endpoint health, Enterome, EQRx, Falk, Ferring, FPRT Bio, Galapagos, Genentech/Roche, Genzyme, Gilead, Glenmark, Grunenthal, GSK, GW Pharmaceuticals, Immunocore, Immunometabolism, Indigo, Janssen, Lexicon, Lilly, Medarex, Medtrix, Merck, Merrimack, Millenium, Neovacs, Novartis, Novo Nordisk, NPSNycomed, Ocera, Optima, Origin, Otsuka, Palau, Pentax, Pfizer, Pharmaventure, Phillips, P&G, Pronota, Proximagen, Resolute, Robarts, Sandoz, Santarus, Satisfai, Sensyne, Shire, SigmoidPharma, Souffinez, Syndermix, Synthon, Takeda, Theravance, Tigenix, Tillotts, Topivert, Tr1X, Trino Therapeutics with Wellcome Trust, TxCell, UCB Pharma, Vertex, VHsquared, Vifor, Warner Chilcott and Zeria; and Speaker fees from AbbVie, Amgen, Biogen, Falk, Ferring, Janssen, Pfizer, Shire, Takeda, UCB. ST holds no stocks or share options.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgstr.2024.1335380/full#supplementary-material
1. Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, et al. ECCO guidelines on therapeutics in ulcerative colitis: Medical treatment. J Crohns Colitis. (2022) 16:2–17. doi: 10.1093/ecco-jcc/jjab178
2. Ko CW, Singh S, Feuerstein JD, Falck-Ytter C, Falck-Ytter Y, Cross RK, et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology. (2019) 156:748–64. doi: 10.1053/j.gastro.2018.12.009
3. Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvancarova M, et al. Clinical course during the first 10 years of ulcerative colitis: Results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. (2009) 44:431–40. doi: 10.1080/00365520802600961
4. Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. (2017) 11:649–70. doi: 10.1093/ecco-jcc/jjx008
5. Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka-Walus A. Quality of life in inflammatory bowel disease: A systematic review and meta-analyses-Part I. Inflammation Bowel Dis. (2018) 24:742–7751. doi: 10.1093/ibd/izx100
6. Panés J, Domènech E, Peris MA, Nos P, Riestra S, de Páramo BJ, et al. Association between disease activity and quality of life in ulcerative colitis: Results from the CRONICA-UC study. J Gastroenterol Hepatol. (2017) 32:1818–24. doi: 10.1111/jgh.13795
7. Caron B, Jairath V, D'Amico F, Paridaens K, Magro F, Danese S, et al. Definition of mild to moderate ulcerative colitis in clinical trials: A systematic literature review. United Eur Gastroenterol J. (2022) 10:854–67. doi: 10.1002/ueg2.12283
8. Peyrin-Biroulet L, Panés J, Sandborn WJ, Vermeire S, Danese S, Feagan BG, et al. Defining disease severity in inflammatory bowel diseases: Current and future directions. Clin Gastroenterol Hepatol. (2016) 14:348–354.e17. doi: 10.1016/j.cgh.2015.06.001
9. Walsh AJ, Bryant RV, Travis SPL. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol. (2016) 13:567–79. doi: 10.1038/nrgastro.2016.128
10. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. (1998) 43:29–32. doi: 10.1136/gut.43.1.29
11. Travis SPL, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. (2013) 145:987–95. doi: 10.1053/j.gastro.2013.07.024
12. Feuerstein JD, Isaacs KL, Schneider Y, Mehmood Siddique S, Falck-Ytter Y, Singh S, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. (2020) 158:1450–61. doi: 10.1053/j.gastro.2020.01.006
13. Murray A, Nguyen TM, Parker CE, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. (2020) 8:CD000543. doi: 10.1002/14651858.CD000543.pub5
14. Murray A, Nguyen TM, Parker CE, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. (2020) 8:CD000544. doi: 10.1002/14651858.CD000544.pub5
15. Paridaens K, Fullarton JR, Travis SPL. Efficacy and safety of oral Pentasa (prolonged-release mesalazine) in mild-to-moderate ulcerative colitis: A systematic review and meta-analysis. Curr Med Res Opin. (2021) 37:1891–900. doi: 10.1080/03007995.2021.1968813
16. Barberio B, Segal JP, Quraishi MN, Black CJ, Savarino EV, Ford AC. Efficacy of oral, topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: Systematic review and network meta-analysis. J Crohns Colitis. (2021) 15:1184–96. doi: 10.1093/ecco-jcc/jjab010
17. Kamm MA, Sandborn WJ, Gassull M, Schreiber S, Jackowski L, Butler T, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. (2007) 132:66–75. doi: 10.1053/j.gastro.2006.10.011
18. Hanauer SB, Sandborn WJ, Kornbluth A, Katz S, Safdi M, Woogen S, et al. Delayed-release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: the ASCEND II trial. Am J Gastroenterol. (2005) 100:2478–85. doi: 10.1111/j.1572-0241.2005.00248.x
19. Sandborn WJ, Regula J, Feagan BG, Belousova E, Jojic N, Lukas M, et al. Delayed-release oral mesalamine 4.8 g/day (800-mg tablet) is effective for patients with moderately active ulcerative colitis. Gastroenterology. (2009) 137:1934–1943.e1-3. doi: 10.1053/j.gastro.2009.08.069
20. Paridaens K, Fullarton JR, Travis SPL. Efficacy of oral prolonged-release mesalazine in moderately active ulcerative colitis. JGH Open. (2023) 7:516–9. doi: 10.1002/jgh3.12935
21. Paupard T, Gonzalez F, Caron B, Siproudhis L, Peyrin-Biroulet L. Real-world evidence of quality of life improvement in patients with distal ulcerative colitis treated by mesalazine: The Quartz study. Eur J Gastroenterol Hepatol. (2022) 34:1203–9. doi: 10.1097/MEG.0000000000002444
22. Burisch J, Weimers P, Pedersen N, Cukovic-Cavka S, Vucelic B, Kaimakliotis I, et al. Health-related quality of life improves during one year of medical and surgical treatment in a European population-based inception cohort of patients with inflammatory bowel disease — An ECCO-EpiCom study. J Crohns Colitis. (2014) 8:1030–42. doi: 10.1016/j.crohns.2014.01.028
23. Van Assche G, Manguso F, Zibellini M, Cabriada Nuño JL, Goldis A, Tkachenko E, et al. Oral prolonged release beclomethasone dipropionate and prednisone in the treatment of active ulcerative colitis: Results from a double-blind, randomized, parallel group study. Am J Gastroenterol. (2015) 110:708–15. doi: 10.1038/ajg.2015.114
24. Leone GM, Mangano K, Petralia MC, Nicoletti F, Fagone P. Past, present and (foreseeable) future of biological anti-TNF alpha therapy. J Clin Med. (2023) 12:1630. doi: 10.3390/jcm12041630
25. Bickston SJ, Behm BW, Tsoulis DJ, Cheng J, MacDonald JK, Khanna R, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. (2014) 8:CD007571. doi: 10.1002/14651858.CD007571.pub2
26. Davies SC, Hussein IM, Nguyen TM, Parker CE, Khanna R, Jairath V. Oral Janus kinase inhibitors for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. (2020) 1:CD012381. doi: 10.1002/14651858.CD012381.pub2
27. Hoes JN, Jacobs JWG, Verstappen SMM, Bijlsma JWJ, van der Heijden GJMG. Adverse events of low- to medium-dose oral glucocorticoids in inflammatory diseases: A meta-analysis. Ann Rheum Dis. (2009) 68:1833–8. doi: 10.1136/ard.2008.100008
28. Fietta P, Fietta P, Delsante G. Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin Neurosci. (2009) 63:613–22. doi: 10.1111/j.1440-1819.2009.02005.x
29. Isidori AM, Minnetti M, Sbardella E, Graziadio C, Grossman AB. Mechanisms in endocrinology: The spectrum of haemostatic abnormalities in glucocorticoid excess and defect. Eur J Endocrinol. (2015) 173:R101–13. doi: 10.1530/EJE-15-0308
30. Johannesdottir SA, Horváth-Puhó E, Dekkers OM, Cannegieter SC, Jørgensen JOL, Ehrenstein V, et al. Use of glucocorticoids and risk of venous thromboembolism: A nationwide population-based case-control study. JAMA Intern Med. (2013) 173:743–52. doi: 10.1001/jamainternmed.2013.122
31. Chhaya V, Saxena S, Cecil E, Subramanian V, Curcin V, Majeed A, et al. Steroid dependency and trends in prescribing for inflammatory bowel disease - a 20-year national population-based study. Aliment Pharmacol Ther. (2016) 44:482–94. doi: 10.1111/apt.13700
32. Farraj KL, Pellegrini JR, Munshi RF, Russe-Russe J, Kaliounji A, Tiwana MS, et al. Chronic steroid use: An overlooked impact on patients with inflammatory bowel disease. JGH Open. (2022) 6:910–4. doi: 10.1002/jgh3.12841
33. British National Formulary (BNF) (2023). National Institute for Health and Care Excellence. Available online at: https://bnf.nice.org.uk/ (Accessed November 08, 2023).
34. Louis E, Paridaens K, Al Awadhi S, Begun J, Cheon JH, Dignass AU, et al. Modelling the benefits of an optimised treatment strategy for 5-ASA in mild-to-moderate ulcerative colitis. BMJ Open Gastroenterol. (2022) 9:e000853. doi: 10.1136/bmjgast-2021-000853
35. Sandborn WJ, Danese S, D'Haens G, Moro L, Jones R, Bagin R, et al. Induction of clinical and colonoscopic remission of mild-to-moderate ulcerative colitis with budesonide MMX 9 mg: Pooled analysis of two phase 3 studies. Aliment Pharmacol Ther. (2015) 41:409–18. doi: 10.1111/apt.13076
36. Manguso F, Bennato R, Lombardi G, Riccio E, Costantino G, Fries W. Efficacy and Safety of oral beclomethasone dipropionate in ulcerative colitis: A systematic review and meta-analysis. PloS One. (2016) 11:e0166455. doi: 10.1371/journal.pone.0166455
37. NHS England. National Cost Collection: National Schedule of NHS costs - Year 2021–22 (2023). Available online at: https://www.england.nhs.uk/national-cost-collection/ (Accessed November 08, 2023).
38. Jones K, Weatherly H, Birch S, Castelli A, Chalkley M, Dargan A, et al. Unit Costs of Health & Social Care 2022. Canterbury, UK: PSSRU, University of Kent (2023). doi: 10.22024/UniKent/01.02.100519
39. Waljee AK, Rogers MAM, Lin P, Singal AG, Stein JD, Marks RM, et al. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ. (2017) 357:j1415. doi: 10.1136/bmj.j1415
40. European Medicines Agency. Humira (adalimumab) EPAR (2020). Available online at: https://www.ema.europa.eu/en/documents/overview/humira-epar-medicine-overview_en.pdf (Accessed November 08, 2023).
41. Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. (2014) 146:85–95. doi: 10.1053/j.gastro.2013.05.048
42. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2005) 353:2462–76. doi: 10.1056/NEJMoa050516
43. Barberio B, Marsilio I, Buda A, Bertin L, Semprucci G, Zanini A, et al. Efficacy and safety of oral beclomethasone dipropionate and budesonide MMX versus 5-aminosalicylates or placebo in ulcerative colitis: A systematic review and meta-analysis. Therap Adv Gastroenterol. (2023) 16:17562848231188549. doi: 10.1177/17562848231188549
44. Sandborn WJ, Travis S, Moro L, Jones R, Gautille T, Bagin R, et al. Once-daily budesonide MMX® extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: Results from the CORE I study. Gastroenterology. (2012) 143:1218–1226.e2. doi: 10.1053/j.gastro.2012.08.003
45. Travis SPL, Danese S, Kupcinskas L, Alexeeva O, D'Haens G, Gibson PR, et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: Results from the randomised CORE II study. Gut. (2014) 63:433–41. doi: 10.1136/gutjnl-2012-304258
46. West R, Russel M, Bodelier A, Kuijvenhoven J, Bruin K, Jansen J, et al. Lower risk of recurrence with a higher induction dose of mesalazine and longer duration of treatment in ulcerative colitis: Results from the Dutch, non-interventional, IMPACT study. J Gastrointestin Liver Dis. (2022) 31:18–24. doi: 10.15403/jgld-3927
47. Moreira Genaro L, Miani Gomes LE, Menezes de Freitas Franceschini AP, Dugolin Ceccato H, Nascimento de Jesus R, Pereira Lima A, et al. Anti-TNF therapy and immunogenicity in inflammatory bowel diseases: A translational approach. Am J Transl Res. (2021) 13:13916–30.
48. Molodecky NA, Shian Soon I, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. (2012) 142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001
49. Ng SC, Yun Shi H, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. (2017) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0
Keywords: aminosalicylates, mesalamine, 5-ASA, inflammatory bowel disease, delayed-release
Citation: Paridaens K, Freddi MJ and Travis SPL (2024) The continuing value of mesalazine as first-line therapy for patients with moderately active ulcerative colitis. Front. Gastroenterol. 3:1335380. doi: 10.3389/fgstr.2024.1335380
Received: 08 November 2023; Accepted: 06 May 2024;
Published: 03 June 2024.
Edited by:
Glen A. Doherty, University College Dublin, IrelandReviewed by:
Elisabeth Schnoy, Augsburg University Hospital, GermanyCopyright © 2024 Paridaens, Freddi and Travis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew J. Freddi, bWF0dEB2aW9saWNvbS5jby51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.