
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Gastroenterol. , 01 May 2023
Sec. Therapy in Gastroenterology
Volume 2 - 2023 | https://doi.org/10.3389/fgstr.2023.1170271
This article is part of the Research Topic Advances in Neurogastroenterology and Therapeutics in Gastrointestinal Function Disorders View all 5 articles
Background: Hospital admissions for diverticulitis, a complication of diverticular disease, are very much on the increase. Prevention of diverticulitis could cut costs and save lives.
Aims: To identify whether the risk of the first episode of diverticulitis (primary prevention) or recurrence of diverticulitis (secondary prevention) can be reduced in patients with diverticular disease using non-absorbable antibiotics (mainly rifaximin).
Methods: The studies were identified by searching PubMed and CENTRAL from 1990 to May 2022. The methodological quality of each study was also evaluated. The outcome of the meta-analysis was the occurrence of a first or subsequent episode of diverticulitis. In addition, a trial sequential analysis was performed to evaluate whether the results would be subject to type I or type II errors.
Results: Primary prevention: the risk difference was statistically significant in favor of rifaximin (-0,019, or -1.9%, CI -0,6 to -3,3%). There was no evidence of heterogeneity (I2 0%). At one year, two years, and eight years of age, the NNT was 62, 52, and 42, respectively. The level of evidence had a moderate degree of certainty. Secondary prevention: the risk difference was statistically significant in favor of rifaximin (- 0,24, or -24%, CI -47 to -2%). There was evidence of heterogeneity (I2 92%); NNT resulted in 5. The grade level was low.
Conclusions: Rifaximin can lower the risk of a first episode of diverticulitis. However, the cost-benefit ratio currently appears too high. Rifaximin could also reduce the risk of a second episode, but the quality of the evidence is low.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022379258.
Doctors and patients face several decisions in appropriate management after the first episode of left colon diverticulitis. Episodes of acute diverticulitis are generally uncomplicated (causing only localized inflammation), but complicated diverticulitis, defined as inflammation associated with an abscess, fistula, hemorrhage, or perforation (1–3), occurs in about 12% of cases (4). Relapses occur in about 8% to 36% of patients between 1 and 10 years (2, 3), and prevention is of great importance. Evidence for the use of various pharmacological and surgical interventions to prevent diverticulitis recurrence has evolved over time (5–11).
The purpose of this meta-analysis is to identify whether the risk of the first episode of diverticulitis or of recurrence of diverticulitis in symptomatic uncomplicated diverticular disease (SUDD) can be reduced using non-absorbable antibiotics (mainly rifaximin), through the identification of published randomized and observational studies.
We followed GRADE guidance 24 and used a framework that considers the certainty of evidence from randomized and non-randomized studies then in an integrative fashion (12).
A first analysis of the evidence gathered for the role of non-absorbable antibiotics based only on symptoms in SUDD appeared in 2011 (13). One of the authors is in the editorial team of this paper.
According to the 2022 latest American guidelines for internists, evidence is very uncertain (insufficient) for treatments to prevent recurrence (like probiotics, combinations of mesalamine and rifaximin, combinations of mesalamine and probiotics, and burdock tea) (14).
The most studied drug for preventing diverticulitis is mesalamine. However, the latest American guidelines definitively exclude a role for this agent in preventing relapses (strong recommendation; high-certainty evidence) (14), following the results of the last meta-analysis (15).
This meta-analysis could help figure out if nonabsorbable antibiotics might play a role in lowering the risk of diverticulitis and, if so, what studies should be done.
Details of our systematic review are registered in the PROSPERO database under the number [379258].
The general recommendations of the PRISMA review were considered for the meta-analysis (16, 17).
The studies were identified by searching PubMed, and the Cochrane Central Register of Controlled Trials from 1990 to May 2022.
((((((diverticulitis OR diverticular)))) AND (((recurrence OR relapse OR rehospitalization))))) NOT ((((((diverticulitis OR diverticular)))) AND (((recurrence OR relapse OR rehospitalization)))) AND ((((clinical[Title/Abstract] AND trial[Title/Abstract]) OR clinical trials as topic[MeSH Terms] OR clinical trial[Publication Type] OR random*[Title/Abstract] OR random allocation[MeSH Terms] OR therapeutic use[MeSH Subheading])))).
The identified records were screened by titles, abstracts, and keywords. Papers with potential eligibility were then obtained for full-text review. No language limits were imposed. We supplemented the electronic search by scanning the reference lists of relevant publications, including review articles and guidelines. When published data were insufficient for our analyses, additional details were sought from the investigators of the corresponding clinical trials.
The flow chart of the items identified and those then eliminated was developed with the help of Prisma 20202 software (https://doi.org/10.1002/cl2.1230).
The PICO question format (18) was used to figure out who was eligible first.
● Patients: patients with symptomatic, uncomplicated diverticular disease (SUDD) who have never had diverticulitis or have only had it.
● Intervention: long term administration of rifaximin. The allowable dose was 800 mg per day in cycles of 7–10 consecutive days per month.
● Comparators/controls: standard of care, placebo, or mesalamine.
● Outcome: the occurrence of a diverticulitis episode, whether first-time or recurring.
● Study design: randomized, non-randomized, and observational studies if peer-reviewed and published in full.
All patients suffered from SUDD, defined as a syndrome characterized by recurrent abdominal symptoms attributed to diverticula in the absence of other macroscopically evident alterations other than the presence of diverticula.
Diverticulitis was predefined as abdominal pain attributed to diverticular disease and one of the following findings: (1) requiring hospitalization or surgery; or (2) described as acute and presenting with fever, and/or being evaluated with computed tomography. Prevention of the first episode of diverticulitis was considered “primary prevention” (PP) when the complication had never appeared previously or “secondary prevention” (SP) when it appeared after the first episode. All articles passed through a systematic review by a team of 3 physicians (MK, SC, and AEM), and methodological criteria and the results were recorded. Studies that fulfilled the inclusion criteria were evaluated by a blinded review done independently by the same 3 authors to tabulate subject demographics, study design, definition of outcomes, and frequencies of diverticulitis using a standardized data form. Disagreement was resolved by consensus.
The Newcastle-Ottawa Scale (NOS), a tool for rating the quality of non-randomized research, was used by the same authors to assess the methodological quality of each study (19). NOS has three areas: selection, comparability, and outcome. A maximum of 13 stars can be assigned.
The Robvis web app was used to measure the risk of bias (Rob 2) (20, 21) in randomized controlled trials.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tool was used for a global evaluation of the body of evidence in the systematic review (22). The level of confidence is determined by the study design (high for RCTs, low for observational studies), whereas reasons for lowering confidence are based bias risk, imprecision, or inconsistency (23). Discrepancies in ratings were resolved between the authors.
The DerSimonian and Laird method was used to compare and summarize the outcomes of each study (24). We used the random effect model since it is more conservative. The risk difference (RD), i.e., the difference in event rates between the treatment and control groups, was used to measure the prevention effect. Confidence intervals (CI) were calculated at 95%.
Along with the pooled effect sizes, a prediction interval (PI) was given. This showed how the effects of the treatment changed in different settings, as well as what effects to expect in future patients (25).
The number needed to treat (NNT), i.e., the number of patients who must be treated to obtain one more therapeutic effect compared to the control group, was also calculated (26). NNT is the reciprocal of RD in mathematics, and the 95% confidence intervals for NNT are the reciprocal of the 95% confidence intervals for RD. The NNT tells us the estimated number of patients that need to be treated with the intervention to prevent an unfavorable event compared with the control group. For calculating RD and NNT from meta-analyses, we refer to the methods cited by Palazon-Bru (27).
The alpha level was set at 0.05, for a two-tailed test.
R statistical software version 4.0.3 (R Project for Statistical Computing, Vienna, Austria) was used to do all the calculations for the meta-analysis.
Interstudy heterogeneity was evaluated using the Q statistic of DerSimonian and Laird (24), and the relevance of heterogeneity was measured using the I2 (28, 29).
We decided to follow the recommendations of the international Grade Guidance 24 on integrating of randomized and non-randomized studies (12).
TSA was performed to assess whether the results regarding the primary outcome (primary or secondary prevention of diverticulitis) would be subject to type I or type II errors. TSA combines traditional meta-analysis methodology with repeating significance testing methods applied to accruing data in clinical studies (30). TSA basically calculates the relative risk reduction (RRR). TSA constructs monitoring boundaries to establish when an estimated effect is so convincingly large that the conclusions are unlikely to change with additional evidence. A model of variance-based diversity-adjusted information size was used for the TSA based on α = 0.05 and β = 0.20 (power of 80%) to be more conservative. The cumulative Z-curve of each cumulative meta-analysis was computed and plotted against the above monitoring boundaries. The crossing of the cumulative Z-curve into the trial sequential monitoring boundary for benefit suggests that a sufficient level of evidence has been reached, and no additional studies may be needed to demonstrate the superiority of the intervention. If the cumulative Z-curve does not cross any of the trial sequential monitoring boundaries, there is probably not sufficient evidence to reach a conclusion, and further studies may be needed.
Meta-analyses cannot be merely data pooling exercises, and TSA appears to be a useful tool for drawing non-biased conclusions (31, 32).
TSA was conducted using Trial Sequential Analysis software version 0.9.5.10 beta (Copenhagen Trial Unit, Centre for Clinical Intervention, Copenhagen, Denmark). All p values < 0.05 were considered statistically significant.
The initial combined search identified 1033 reports, and we excluded 305 because of the title or abstract. Of the remaining 726 articles, we excluded 280 for being not pertinent, 46 registered as reviews, 179 for being dedicated to surgery, 68 dealing with acute diverticulitis, and 66 referring to observational studies not fitting with the predefined PICO criteria. In this group, one cross-over study was excluded from the final analysis (33) due to a short follow-up (14 days). Another one was not considered due to a lack of sufficient information on the rate of diverticulitis relapse (34). A third compatible study was excluded due to a regimen of both rifaximin and mesalamine (35). Twenty-four papers were case reports, and 55 concerned the therapy of lower GI hemorrhage.
This review is based on the results of eight studies (36–43) (see Figure 1).
A total of 3013 patients had been enrolled: 1568 were assigned to treatment with rifaximin, and 1445 to no treatment or mesalamine. There were five studies for preventing the first episode of diverticulitis (primary prevention, PP) (36–39, 43) and three for preventing diverticulitis recurrence (secondary prevention, SP) (40–42).
All studies for primary prevention were randomized trials (36–39), except one retrospective (43).
Two studies in the secondary prevention group (41, 42) were retrospective, and one was a prospective trial (40).
The characteristics of the studies are shown in Table 1. In all studies, the antibiotic used was rifaximin 400 mg b.d. for 7–10 days every month; all patients in both the treated group and control group received a standard dietary fiber supplement, unless in the Polish study, where we were not able to extract the information. In only one study, the control group received a placebo (37). In one study, control therapy included mesalamine 2,4 g/day for 10 days per month (41).
All studies followed patients for up to one year, except for the Di Mario study (8 years) (43).
Regarding RCTs, there were some concerns due to possible biases for deviations from the assigned group and to missing data in all 5 RCTs. The randomization process was unclear in two of them (Supplementary Data; Figure 1A).
All 3 observational studies were in an area of low-risk bias (8 stars) (Supplementary Data; Figure 1B).
We found 5 studies: 4 RCTs (36–39) and 1 observational study (43). The pooled risk of primary diverticulitis among SUDD untreated patients showed a moderate increase through the years: 3.0% (CI 95% 1.6–4.4%), 3.2% (CI 95% 1.4–5.0%), and 4.5% (CI 95% 2.6–6.3%) at 1, 2, and 8 years, respectively. The pooled risk difference was significative in favor of rifaximin (-1.9%, CI -0.6% to -3.3%). There was no evidence of heterogeneity (I2 0%) (Figure 2A).
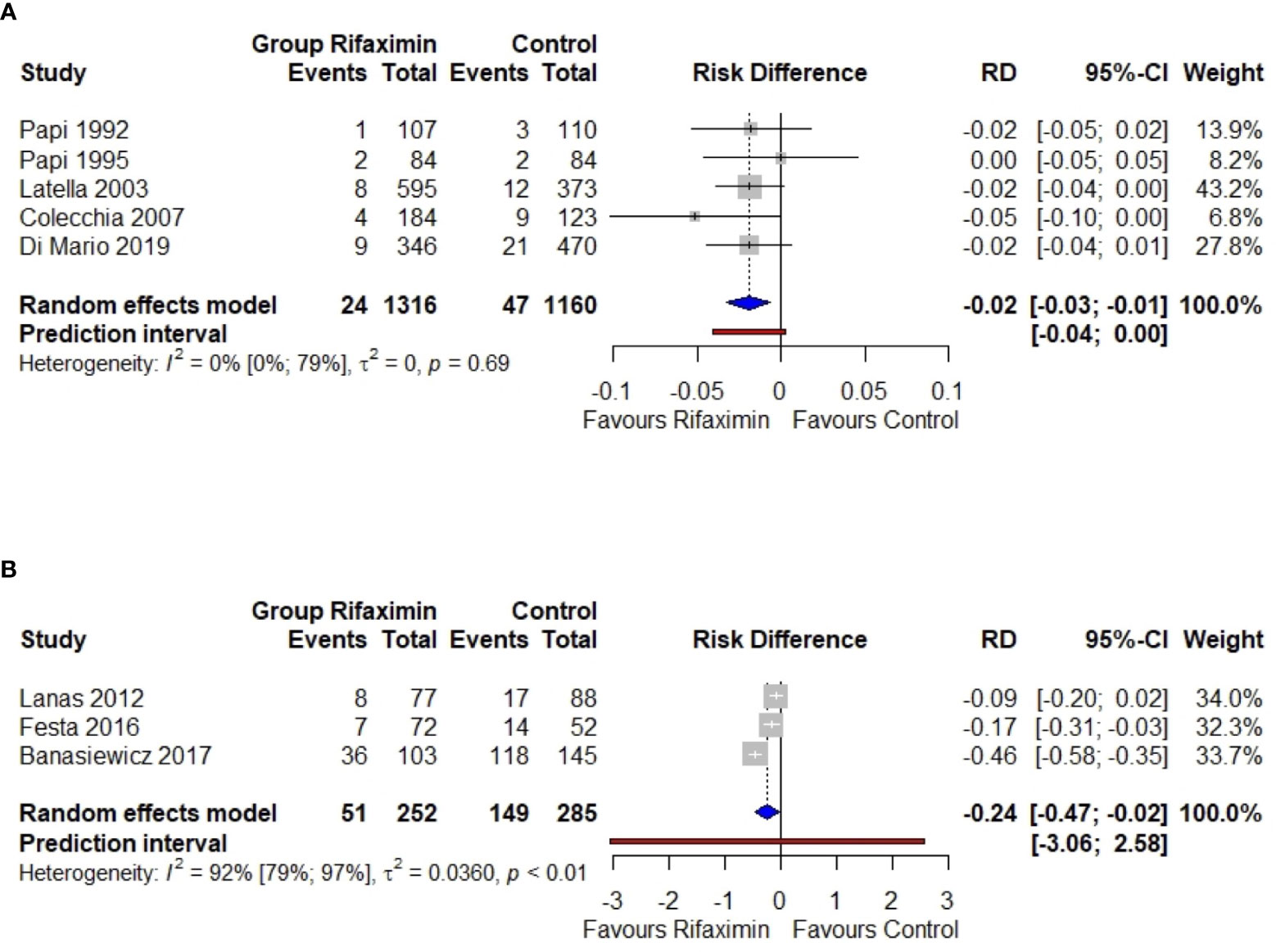
Figure 2 Meta-analysis regarding the risk difference between the rifaximin group and controls as the primary (A) or secondary (B) prevention of diverticulitis. RD, risk difference; 95%-CI, confidence intervals at 95%. The vertical line indicates the ‘no difference’ point between the two options. Squares represent the adjusted risk difference. Diamonds represent the pooled risk difference for all studies. Horizontal lines represent 95% CI.
NNT resulted 62 (CI 95% 42–500), 52 (CI 95% 39–133), and 42 (CI 95% 31–96) at 1, 2, and 8 years, a slight reduction in time (Figure 3) (27). In summary, the use of rifaximin in SUDD patients really reduces the risk of diverticulitis, but the gain is clinically poorly relevant due to the low absolute risk.
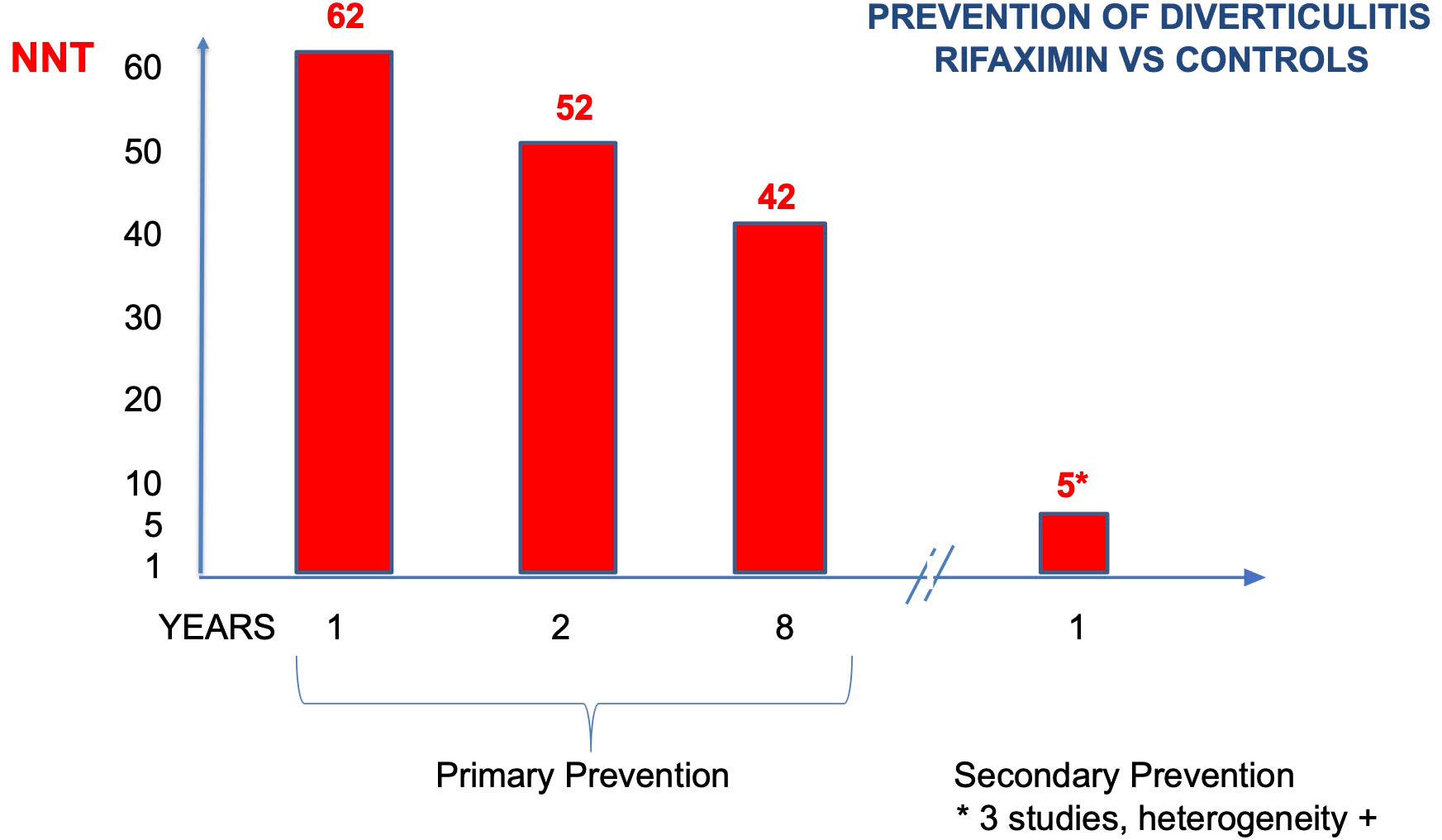
Figure 3 Number of patients who need to be treated to prevent diverticulitis (NNT) (primary and secondary prevention).
The type I error risk in our trial sequential analysis was set at = 0.05, with a power of 0.80 and a 25% expected relative risk reduction (RRR) linked to intervention. Under these premises, the required information size for the meta-analyzed estimate was 1,827. Thus, TSA confirmed the results obtained in the conventional meta-analysis. The Z-score curve (blue line) crossed both the required information size (vertical red line) and the conventional statistical significance boundary corresponding to a two-sided p-value of 0.05 (horizontal red lines), indicating that the observed reduction in the rate of primary diverticulitis in subjects taking rifaximin could be considered conclusive with the existing evidence (Figure 4A).
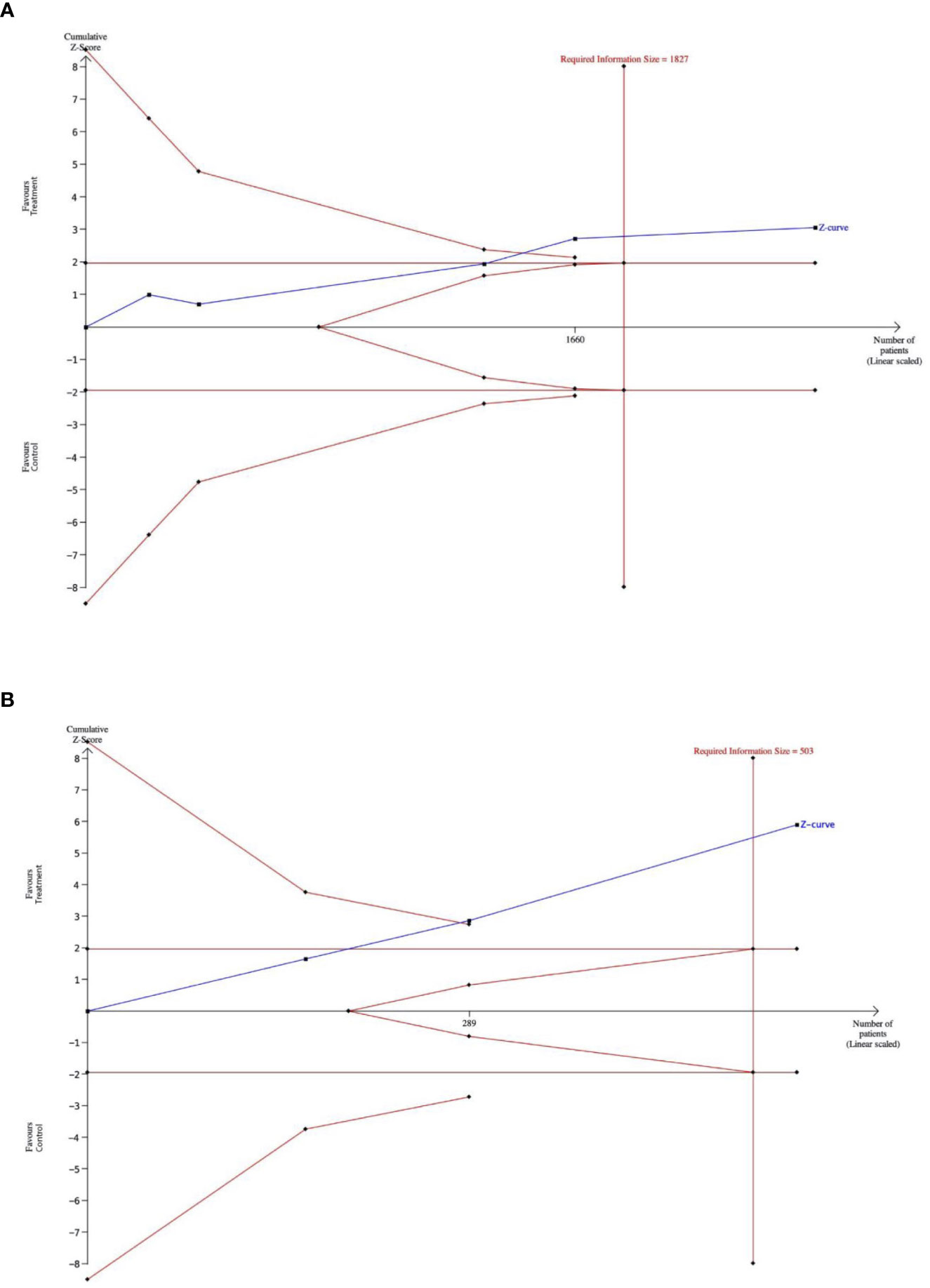
Figure 4 (A). The outcome of a trial-sequential analysis comparing the rates of diverticulitis episodes in patients receiving primary prophylaxis with rifaximin versus patients who did not receive rifaximin. The diversity-adjusted required sample size (1,827 participants) was based on an alpha error of 5% and a beta error of 20. Cumulative z-curves were computed by a fixed effects model. (Alpha-boundaries are the external red lines) (B). The outcome of a trial-sequential analysis comparing diverticulitis relapse rates in patients receiving secondary prophylaxis with rifaximin versus patients not receiving rifaximin. The diversity-adjusted required sample size (503 participants) was based on an alpha error of 5%, a beta error of 20%. Cumulative z-curves were computed by a random effects model. (Alpha-boundaries are the external red lines).
Current certainty of the evidence, when randomized control trials (RCTs) and non-randomized studies (NRS) (12) are included in evidence synthesis, suggests that rifaximin reduces the risk of primary diverticulitis in patients with SUDD. The grade level was moderate (Supplementary Data; Figure 2).
The resulting prediction interval, ranging from −0.04 to 0.00, can be interpreted as the 95% range of true RD expected in similar studies. The prediction interval contains values below zero, corresponding to a decrease in diverticulitis of at best ∼0.04 RD after rifaximin use compared with placebo.
We found 3 studies: 1 RCT (40) and 2 observational (41, 42).
Lanas (40) admitted 165 patients with a previous episode of diverticulitis in a multi-centric clinical trial. The authors randomized them to a group treated cyclically with Rifaximin 400 mg/bid (7 days monthly) and high-fiber supplementation and to a control group with high-fiber supplementation only. The authors followed their patients for one year, registering further episodes of diverticulitis. The recurrence rate of diverticulitis was 19.3% in the control group and 10.4% in the treated patients. Groups were comparable for age, sex, time since their first episode, and disease location.
Festa (41) referred to a retrospective cohort of patients who were followed in a dedicated out-patient clinic (Mal.dive. Clinic, from the Italian acronym Malattia Diverticolare) for 2 years. There were only two clinicians who were dedicated. They applied a pre-constructed form and prescribed 10 days monthly of rifaximin 400 mg/bid and high-fiber supplements, or mesalamine 2.4 g/bid and the same high-fiber supplement, to 124 patients, with a previous episode of diverticulitis. The two groups were comparable, also for ASA and NSAID use. Patients admitted to the study had a mean time of follow-up of 15 months. At one year, the recurrence rate was 26.9% in the control (mesalamine) group and 9.7% in the rifaximin group.
Banasiewic and his co-authors (42) observed retrospectively a large group of patients in the authors’ outpatient clinics. Patients were treated with 7 days of rifaximin 400 mg/bid, monthly, or with other medical therapy (controls). The two groups were comparable for age, sex, and disease duration. The authors registered a high risk of recurrent diverticulitis at one year of follow-up: 60% in the control group and 28% in the study group.
At one year, the risk difference was statistically significant in favor of rifaximin (-0.24, or -24%, CI -47 to -2%). Anyway, there was clear evidence of heterogeneity (I2 92%) (Figure 2B). If calculated, NNT resulted in 5 (CI 95% 4–7) (Figure 3).
In conclusion, the use of rifaximin in SUDD patients with a previous episode of diverticulitis may reduce the risk of a second episode (RD-24%). This finding is hampered by heterogeneity, due to a one-year higher risk of recurrence in the Polish patients.
In our trial sequential analysis, the type I error rate was set at 0.05, with a power of 0.80 and an expected RRR linked to an intervention of 35%. Under these premises, the required sample size for the meta-analyzed estimate was 503 patients. The Z-score curve (blue line) crossed both the required information size (vertical red line) and the conventional statistical significance boundary corresponding to a two-sided p value of 0.05 (horizontal red lines), indicating that the observed reduction in rate of secondary diverticulitis in subjects taking rifaximin could be expected in further studies (Figure 4B).
Certainty of the evidence, when randomized control trials (RCTs) and non-randomized studies (NRS) (12) are included in evidence synthesis, suggests that rifaximin might reduce the risk of diverticulitis relapse in patients with SUDD. Anyway, the GRADE level was considered low (Supplementary Data Figure 3).
The resulting prediction interval, ranging from −3.06 to 2.58, can be interpreted as the 95% range of true RD to be expected in future similar studies. The prediction interval contains values below zero, which correspond to a decrease in diverticulitis of at best ∼3.06 RD after rifaximin use compared with placebo. But it also contains values above zero, which means that the rifaximin may exhibit no or even a harmful effect (RD>0) in some settings, with a 95% worst case increase in RD of 2.58.
Diverticulosis of the colon develops in most individuals in western countries with increasing age and tends to remain asymptomatic (44, 45). Diverticulosis per se cannot be considered a disease. The term “diverticular disease” implies that there are symptoms related to the diverticula.
Most diverticulosis cases remain asymptomatic: only about 4% of patients with an endoscopic diagnosis of diverticulosis develop diverticulitis (46).
However, admissions for diagnosis code diverticulitis are on the increase: in the USA alone, the increase in the admissions rate in 2015 was +21% compared to 2003, with a total aggregate cost of between $2.2 and $2.6 billion and a hospital mortality rate of 0.5% (46).
Two very large European multicenter studies (47, 48) suggest that relapse after diverticulitis is relevant.
Binda’s Italian study reports on a follow-up of 320 patients treated with antibiotics in 17 hospitals after discharge for diverticulitis. In a comparable follow-up period (10.7 years), 25% of patients had relapse of symptoms requiring re-admission. The risk of surgery jumped to 17% (47).
Broderick-Villa reports on the history of 2,366 patients hospitalized for diverticulitis in the Kaiser system. At a median follow-up of 8.9 years, diverticulitis had recurred in 13.3% of patients (48).
A real-world Italian study recently published confirmed an increase in hospital admissions from 2011 to 2014 (+12%) (49). In one year, 8.2% of patients treated non-operatively were readmitted for diverticulitis. Most important, acute episodes of diverticulitis involved a 1.2% risk of mortality in patients over the age of 65.
A recent paper from a national study in Sweden on 97.850 cases shows that diverticulitis strongly elevates mortality vs controls by some 27% (50).
So, any measure to reduce the impact of diverticulitis on hospitalization and mortality should be welcomed.
Relevant evidence indicates that dietary fiber, especially the insoluble fiber found mostly in fruits and vegetables, decreases the risk of diverticula development (51). The protective effect of dietary fiber would make stools bulkier, which would increase the size of the colon, lower intraluminal pressures, and shorten the time it takes for the colon to move (52).
Both experimental and clinical data show that the non-absorbable antibiotic rifaximin has a broad-spectrum antibacterial action, covering gram-positive and gram-negative aerobic and anaerobic bacteria (53, 54).
Dietary fiber and non-absorbable antibiotics, such as rifaximin, interact for the treatment of diverticular disease, as rifaximin has been reported to improve the clinical benefits on symptoms of dietary fiber in SUDD patients. Treatment with rifaximin plus fiber supplementation is effective in obtaining symptom relief at 1 year. The pooled RD for complete symptom relief in favor of the rifaximin group was 29.0% (95% CI 24.5% to 33.6%; P < 0.0001; NNT = 3) (13).
The aim of our meta-analysis was dedicated to evaluating the long-term efficacy of administration of rifaximin in preventing diverticulitis in patients with SUDD. Including both RCTs and non-randomized studies in a systematic review has generated controversy and diverse opinions (55). We followed the GRADE recommendations for evidence syntheses to get the most useful information from the different types of studies used in health syntheses (12).
Rifaximin significantly reduced the risk of the first episode In SUDD patients (-1.9%, CI -6 to -3%). There was no evidence of heterogeneity (I2 0%). GRADE certainty of evidence was moderate. The result is confirmed by TSA, which shows that further investigations in the field are probably useless. Thus, rifaximin reduces the risk of the first episode of diverticulitis in patients with SUDD. Anyway, this finding has a very limited clinical relevance due to the high number of patients to gain a single episode of diverticultis (NNT of 62, 52, and 42 at 1, 2, and 8 years).
Rifaximin could reduce the risk of a second episode in SUDD patients (-24%, CI -47 to -2%). At 1 years, the NNT was 5. However, there was evidence of heterogeneity in pooling (I2 92%), and definite conclusions are blocked. This finding is due to a higher risk of recurrence in the Polish patients (81%). The resulting prediction interval suggests that the effect in a new study may be even the exact opposite of the summary point estimate of the meta-analysis, that is, an increase of 0.24 instead of a decrease of 0.51. So, the GRADE certainty of the evidence resulted in a low, and TSA suggests that more patients should be admitted for further studies (up to 503).
This meta-analysis has some limitations. The study is limited by the quality of the included studies. This could lead to an overestimation of the treatment effect of rifaximin. Three studies were RCTs (36–38), and one was an observational investigation (43). Blinding and a placebo-controlled group were guaranteed in one study only (37). Furthermore, the definition of diverticulitis was not pre-defined in all studies. Consequently, we asked the authors to reconsider including diverticulitis only in cases of hospitalization, and to recalculate the cases accordingly. This occurred in three studies (36, 37, and 39).
Anyway, heterogeneity was not observed. Following the prediction interval, the benefit of rifaximin to prevent diverticulitis can be guaranteed in further studies.
The second meta-analysis includes only 3 studies (40–42), and only one was a randomized trial (40). Heterogeneity was observed, limiting the value of the statistical result. Following the prediction interval, the benefit of rifaximin to prevent recurrent diverticulitis cannot be guaranteed in further studies.
A rifaximin regimen of seven to ten days per month resulted in a consistently better outcome in terms of the appearance of diverticulitis. The evidence is definitive regarding primary prevention: while reducing symptoms (13), rifaximin could reduce the risk of a first episode of diverticulitis. However, the cost-benefit ratio is questionable, and rifaximin cannot be recommended for all patients with SUDD. The quality of the evidence is moderate (i.e.: further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate).
Rifaximin might reduce the risk of a second episode with a good ratio. But the quality of evidence is currently low, due to the low number of studies, and the associated risks of bias and heterogeneity (that is: further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate).
Rifaximin should better be studied in a prospective trial for primary and secondary prevention. The required sample size for further RCTs can be estimated as the difference between the required information size and the number of people already recruited into the previous trials (56).
Furthermore, we need to concentrate the analyses on patients with SUDD who have a higher prior probability of developing diverticulitis. This could be accomplished by identifying patients who have risk factors that may aggravate their clinical history (e.g., obesity, sedentary lifestyle, use of NSAIDs or aspirin, immunosuppressive therapy). Possibly, a subgroup of patients could be identified for whom the cost-benefit ratio f or rifaximin use in prevention could be more favorable (57).
An economic analysis on the long-term prescription of rifaximin must also be performed and is currently under development. In Italy, a monthly cycle of rifaximin costs 16.64 euros and is reimbursed by the National Health System. Preliminary findings have already been published (49).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
MK, AEM, substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work, GN,SC drafting the work or revising it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
We thank The Club for Evidence Based Gastroenterology & Hepatology for technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgstr.2023.1170271/full#supplementary-material
1. Köhler L, Sauerland S, Neugebauer E. Diagnosis and treatment of diverticular disease: results of a consensus development conference. the scientific committee of the European association for endoscopic surgery. Surg Endosc (1999) 13:430–6. doi: 10.1007/s004649901007
2. Feingold D, Steele SR, Lee S, Kaiser A, Boushey R, Buie WD, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum (2014) 57:284–94. doi: 10.1097/DCR.0000000000000075
3. Wilkins T, Embry K, George R. Diagnosis and management of acute diverticulitis. Am Fam Phys (2013) 87:612–20.
4. Bharucha AE, Parthasarathy G, Ditah I, Fletcher JG, Ewelukwa O, Pendlimari R, et al. Temporal trends in the incidence and natural history of diverticulitis: a population-based study. Am J Gastroenterol (2015) 110:1589–96. doi: 10.1038/ajg.2015.302
5. Gavriilidis P, Askari A, Gavriilidis E, de’Angelis N, Di Saverio S, Wheeler J, et al. Appraisal of the current guidelines for the management of diverticular disease using the appraisal of guidelines research and evaluation II (AGREE II) instrument. Coll Surg Engl (2021) 103:471–7. doi: 10.1308/rcsann.2021.0013
6. Schultz JK, Azhar N, Binda GA, Barbara G, Biondo S, Boermeester MA, et al. European Society of coloproctology: guidelines for the management of diverticular disease of the colon. Colorectal Dis (2020) 22(Suppl 2):5–28. doi: 10.1111/codi.15140
7. Francis NK, Sylla P, Abou-Khalil M, Arolfo S, Berler D, Curtis NJ, et al. EAES and SAGES 2018 consensus conference on acute diverticulitis management: evidence-based recommendations for clinical practice. Surg Endosc (2019) 33:2726–41. doi: 10.1007/s00464-019-06882-z
8. Stollman N, Smalley W, Hirano I. AGA institute clinical guidelines committee. American gastroenterological association institute guideline on the management of acute diverticulitis. Gastroenterology (2015) 149:1944–9. doi: 10.1053/j.gastro.2015.10.003
9. Peery AF, Shaukat A, Strate LL. AGA clinical practice update on medical management of colonic diverticulitis: expert review. Gastroenterology (2021) 160:906–11. doi: 10.1053/j.gastro.2020.09.059
10. Hall J, Hardiman K, Lee S, Lightner A, Stocchi L, Paquette IM, et al. Prepared on behalf of the clinical practice guidelines committee of the American society of colon and rectal surgeons. the American society of colon and rectal surgeons clinical practice guidelines for the treatment of left-sided colonic diverticulitis. Dis Colon Rectum (2020) 63:728–47. doi: 10.1097/DCR.0000000000001679
12. Cuello-Garcia CA, Santesso N, Morgan RL, Verbeek J, Thayer K, Ansari MT, et al. GRADE guidance 24 optimizing the integration of randomized and non-randomized studies of interventions in evidence syntheses and health guidelines. J Clin Epidemiol (2022) 142:200–8. doi: 10.1016/j.jclinepi.2021.11.026
13. Bianchi M, Festa V, Moretti A, Ciaco A, Mangone M, Tornatore V, et al. Meta-analysis: long-term therapy with rifaximin in the management of uncomplicated diverticular disease. Aliment Pharmacol Ther (2011) 33:902–10. doi: 10.1111/j.1365-2036.2011.04606.x
14. Qaseem A, Etxeandia-Ikobaltzeta I, Lin JS, Fitterman N, Shamliyan T, Wilt TJ, et al. Clinical guidelines committee of the American college of physicians. colonoscopy for diagnostic evaluation and interventions to prevent recurrence after acute left-sided colonic diverticulitis: a clinical guideline from the American college of physicians. Ann Intern Med (2022) 175:416–31. doi: 10.7326/M21-2711
15. Khan RMA, Ali B, Hajibandeh S, Hajibandeh S. Effect of mesalazine on recurrence of diverticulitis in patients with symptomatic uncomplicated diverticular disease: a meta-analysis with trial sequential analysis of randomized controlled trials. Colorectal Dis (2018) 20:469–78. doi: 10.1111/codi.14064
16. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
17. Haddaway NR, Pritchard CC, McGuinness LA. PRISMA2020: r package and ShinyApp for producing PRISMA 2020 compliant flow diagrams. Zenodo (2021). doi: 10.5281/zenodo.4287834
18. Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol (2020) 35:49–60. doi: 10.1007/s10654-019-00576-5
19. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses . Ottawa: Ottawa Hospital Research Institute. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 17 feb 2022).
20. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
21. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an r package and shiny web app for visualizing risk-of-bias assessments. Res Syn Meth (2021) 1:55–61. doi: 10.1002/jrsm.1411
22. Granholm A, Alhazzani W, Møller MH. Use of the GRADE approach in systematic reviews and guidelines. Br J Anaesth (2019) 123:554–9. doi: 10.1016/j.bja.2019.08.015
23. Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. rating up the quality of evidence. J Clin Epidemiol (2011) 64:1311–6. doi: 10.1016/j.jclinepi.2011.06.004
24. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
25. IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open (2016) 6:e010247. doi: 10.1136/bmjopen-2015-010247
26. Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med (1988) 318:1728–33. doi: 10.1056/NEJM198806303182605
27. Palazón-Bru A, Moscardo-Descalzo A, Morales-Gabriel S, Folgado-de la Rosa DM, Mares-García E, Carbonell-Torregrosa MLÁ, et al. Clinical relevance of an intervention assessed by a meta-analysis of randomized clinical trials. J Clin Epidemiol (2021) 132:46–50. doi: 10.1016/j.jclinepi.2020.12.010
29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
30. Shah A, Smith AF. Trial sequential analysis: adding a new dimension to meta-analysis. Anaesthesia (2019) 74:793–800. doi: 10.1111/anae.14705
31. Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol (2017) 17(1):39. doi: 10.1186/s12874-017-0315-7
32. Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol (2008) 61:763–9. doi: 10.1016/j.jclinepi.2007.10.007
33. D’Incà R, Pomerri F, Vettorato MG, Dal Pont E, Di Leo V, Ferronato A, et al. Interaction between rifaximin and dietary fibre in patients with diverticular disease. Aliment Pharmacol Ther (2007) 25:771–9. doi: 10.1111/j.1365-2036.2007.03266.x
34. Tursi A, Elisei W, Giorgetti GM, Inchingolo CD, Nenna R, Picchio M, et al. Effectiveness of different therapeutic strategies in preventing diverticulitis recurrence. Eur Rev Med Pharmacol Sci (2013) 17:342–8.
35. Tursi A, Brandimarte G, Daffinà R. Long-term treatment with mesalazine and rifaximin versus rifaximin alone for patients with recurrent attacks of acute diverticulitis of colon. Dig Liver Dis (2002) 34:510–5. doi: 10.1016/S1590-8658(02)80110-4
36. Papi C, Ciaco A, Koch M, Capurso L. Efficacy of rifaximin on symptoms of uncomplicated diverticular disease of the colon. a pilot multicentre open trial. diverticular disease study group. Ital J Gastroenterol (1992) 24:452–6.
37. Papi C, Ciaco A, Koch M, Capurso L. Efficacy of rifaximin in the treatment of symptomatic diverticular disease of the colon. a multicenter double-blind placebo-controlled trial. Aliment Pharmacol Ther (1995) 9:33–9. doi: 10.1111/j.1365-2036.1995.tb00348.x
38. Latella G, Pimpo MT, Sottili S, Zippi M, Viscido A, Chiaramonte M, et al. Rifaximin improves symptoms of acquired uncomplicated diverticular disease of the colon. Int J Colorectal Dis (2003) 18:55–62. doi: 10.1007/s00384-002-0396-5
39. Colecchia A, Vestito A, Pasqui F, Mazzella G, Roda E, Pistoia F, et al. Efficacy of long term cyclic administration of the poorly absorbed antibiotic rifaximin in symptomatic, uncomplicated colonic diverticular disease. World J Gastroenterol (2007) 13:264–9. doi: 10.3748/wjg.v13.i2.264
40. Lanas A, Ponce J, Bignamini A, Mearin F. One year intermittent rifaximin plus fibre supplementation vs. fibre supplementation alone to prevent diverticulitis recurrence: a proof-of-concept study. Dig Liver Dis (2013) 45:104–9. doi: 10.1016/j.dld.2012.09.006
41. Festa V, Spila Alegiani S, Chiesara F, Moretti A, Bianchi M, Dezi A, et al. Retrospective comparison of long-term ten-day/month rifaximin or mesalazine in prevention of relapse in acute diverticulitis. Eur Rev Med Pharmacol Sci (2017) 21:1397–404.
42. Banasiewicz T, Francuzik W, Bobkiewicz A, Krokowicz Ł, Borejsza-Wysocki M, Paszkowski J, et al. The influence of rifaximin on diverticulitis rate and quality of life in patients with diverticulosis. Pol Przegl Chir (2017) 89:22–31. doi: 10.5604/01.3001.0009.6012
43. Di Mario F, Miraglia C, Cambiè G, Violi A, Nouvenne A, Franceschi M, et al. Long-term efficacy of rifaximin to manage the symptomatic uncomplicated diverticular disease of the colon. J Investig Med (2019) 67:767–70. doi: 10.1136/jim-2018-000901
44. Peery AF, Keku TO, Martin CF, Eluri S, Runge T, Galanko JA, et al. Distribution and characteristics of colonic diverticula in a united states screening population. Clin Gastroenterol Hepatol (2016) 14:980–5. doi: 10.1016/j.cgh.2016.01.020
45. Rustom LBO, Sharara AI. The natural history of colonic diverticulosis: much ado about nothing? Inflammation Intest Dis (2018) 3:69–74. doi: 10.1159/000490054
46. Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the united states. Gastroenterology (2015) 149:1731–41. doi: 10.1053/j.gastro.2015.08.045
47. Binda GA, Arezzo A, Serventi A, Bonelli L, Italian Study Group on Complicated Diverticulosis (GISDIC), Facchini M, et al. Multicentre observational study of the natural history of left-sided acute diverticulitis. Br J Surg (2012) 99:276–85. doi: 10.1002/bjs.7723
48. Broderick-Villa G, Burchette RJ, Collins JC, Abbas MA, Haigh PI. Hospitalization for acute diverticulitis does not mandate routine elective colectomy. Arch Surg (2005) 140:576–81. doi: 10.1001/archsurg.140.6.576
49. Mennini FS, Sciattella P, Marcellusi A, Toraldo B, Koch M. Economic burden of diverticular disease: an observational analysis based on real world data from an Italian region. Dig Liver Dis (2017) 49:1003–8. doi: 10.1016/j.dld.2017.05.024
50. Cameron R, Walker MM, Thuresson M, Roelstraete B, Sköldberg F, Olén O, et al. Mortality risk increased in colonic diverticular disease: a nationwide cohort study. Ann Epidemiol (2022) 76:39–49. doi: 10.1016/j.annepidem.2022.10.006
51. Aldoori W, Ryan-Harshman M. Preventing diverticular disease. review of recent evidence on high-fiber diets. Can Fam Phys (2002) 48:1632–7.
52. Lupton JR, Turner ND. Potential protective mechanisms of wheat bran fiber. Am J Med (1999) 106:24–7. doi: 10.1016/S0002-9343(98)00343-X
53. Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy (2005) 51(Suppl 1):36–66. doi: 10.1159/000081990
54. Ojetti V, Lauritano EC, Barbaro F, Migneco A, Ainora ME, Fontana L, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol (2009) 5:675–82. doi: 10.1517/17425250902973695
55. Mueller M, D’Addario M, Egger M, Cevallos M, Dekkers O, Mugglin C, et al. Methods to systematically review and meta-analyze observational studies: a systematic scoping review of recommendations. BMC Med Res Methodol (2018) 18(1):44. doi: 10.1186/s12874-018-0495-9
56. Claire R, Gluud C, Berlin I, Coleman T, Leonardi-Bee J. Using trial sequential analysis for estimating the sample sizes of further trials: example using smoking cessation intervention. BMC Med Res Methodol (2020) 20:284–95. doi: 10.1186/s12874-020-01169-7
Keywords: diverticular disease, meta-analysis, non-absorbable antibiotics, diverticulitis, trial sequantia
Citation: Koch M, Maraolo AE, Natoli G and Corrao S (2023) Preventing acute diverticulitis. any roles for non-absorbable antibiotics? in search of evidence: a systematic review, meta-analysis, and trial sequential analysis. Front. Gastroenterol. 2:1170271. doi: 10.3389/fgstr.2023.1170271
Received: 20 February 2023; Accepted: 07 April 2023;
Published: 01 May 2023.
Edited by:
Michele Di Stefano, IRCCS S.Matteo Hospital Foundation, ItalyReviewed by:
Fabio Pace, Bolognini Hospital, ItalyCopyright © 2023 Koch, Maraolo, Natoli and Corrao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maurizio Koch, a29jaG1hdXJpemlvQGdtYWlsLmNvbQ==
†ORCID: Maurizio Koch, orcid.org/0000-0001-7911-1616, Alberto Enrico Maraolo, orcid.org/0000-0002-7218-7762
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.