
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Gastroenterol. , 05 July 2022
Sec. Gastroenterology and Cancer
Volume 1 - 2022 | https://doi.org/10.3389/fgstr.2022.939192
 Kerstin Schütte1,2,3
Kerstin Schütte1,2,3 Juozas Kupčinskas4
Juozas Kupčinskas4 Egidijus Morkunas4
Egidijus Morkunas4 Osman Öcal5
Osman Öcal5 Regina Schinner5
Regina Schinner5 Max Seidensticker5
Max Seidensticker5 Enrico N. De Toni6
Enrico N. De Toni6 Najib Ben Khaled6
Najib Ben Khaled6 Maciej Pech7
Maciej Pech7 Daniel Palmer8
Daniel Palmer8 Thomas Berg9
Thomas Berg9 Christian Sengel10
Christian Sengel10 Bristi Basu11
Bristi Basu11 Juan W. Valle12
Juan W. Valle12 Julia Benckert13
Julia Benckert13 Antonio Gasbarrini14
Antonio Gasbarrini14 Bruno Sangro15
Bruno Sangro15 Peter Malfertheiner6
Peter Malfertheiner6 Jens Ricke5*
Jens Ricke5*Introduction: Prediction of response to treatment in patients with advanced hepatocellular carcinoma (HCC) may assist in the selection of personalized management.
Objective: This exploratory analysis of the palliative arm of the SORAMIC trial (ClinicalTrials.gov NCT01126645) evaluated the prognostic potential of basal and dynamic changes in systemic levels of interleukin 6 (IL-6), interleukin 8 (IL-8), systemic vascular endothelial growth factor (VEGF), and lipopolysaccharide (LPS).
Methods: We evaluated the correlations between overall survival (OS) and concentrations of IL-6, IL-8, VEGF, and LPS at follow-up approximately 7-9 weeks after treatment initialization (FU) compared to baseline (BL) in 90 patients treated either with 90Yttrium (90Y) microspheres combined with sorafenib (n = 44) or with sorafenib (n = 46) alone.
Results: Changes in IL-6 concentration during treatment showed correlations with the outcome. An increase in IL-6 concentration of less than 16.8 pg/mL over baseline readings was associated with better survival [median OS 16.3 months compared with 8.9 months (p = 0.0354)]. Correlations with survival were not observed for VEGF or LPS concentrations at baseline, at FU, or changes between these time points.
Conclusions: Changes in IL 6 serum levels at 7-9 weeks after treatment initialization but not in IL 8, VEGF, or LPS add important information on the outcome of advanced HCC patients treated palliatively within the SORAMIC trial.
The predominant causes of hepatocellular carcinoma (HCC) in Europe are alcoholic liver disease, non-alcoholic steatohepatitis (NASH), and chronic Hepatitis B or C virus infection (1). Development of HCC is regarded as closely linked to a state of chronic inflammation, independent of its cause, and end-stage inflammatory liver disease is commonly associated with liver cirrhosis. Intrahepatic and systemic inflammatory events interact in the development of HCC. The tumor microenvironment (TME) with its local pro-inflammatory and pro-fibrotic elements promotes hepatic carcinogenesis. Several circulating inflammatory cytokines, including interleukin-1α (IL-1α), IL-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α), participate in chronic hepatic inflammation and contribute to the neoplastic transformation of hepatocytes (2, 3).
Within the prospective randomized multicenter SORAMIC clinical study patients with advanced HCC were randomized to receive treatment with sorafenib with or without 90Yttrium (90Y) radioembolization. In previous substudies of the SORAMIC trial we confirmed that baseline IL-6 and IL-8 levels could correlate with survival outcomes of sorafenib-treated patients with HCC (4, 5). In addition, baseline IL-6 showed predictive value for overall survival in patients with advanced HCC undergoing radioembolization (4, 5). This exploratory analysis evaluated whether dynamic changes in systemic levels of the cytokines IL-6 and IL-8 could add prognostic value over basal concentrations. The relevance of VEGF and LPS levels was also assessed in this cohort.
The cohort evaluated in this exploratory sub-analysis comprised patients within the palliative treatment arm of the randomized, controlled, multicenter phase II SORAMIC study, which evaluated the impact of 90Y selective internal radiation therapy (SIRT) combined with sorafenib compared to sorafenib alone on survival in patients with advanced HCC (6).
Patients were included in this analysis if they received study treatment within the palliative arm of SORAMIC and took part in the translational program of the study with blood sample analysis at baseline and at first follow-up at approximately 7-9 weeks of treatment (FU). Of the 424 patients randomized after assignment to the palliative arm, 90 fulfilled these criteria within the intention to treat (ITT) population, comprising 46 patients treated with sorafenib alone and 44 patients treated with the combination of SIRT and sorafenib (Figure 1). No statistically significant differences were present between the two treatment cohorts with respect to age, gender distribution, presence of liver cirrhosis, liver function, or tumor stage according to Barcelona Clinic Liver Cancer (BCLC) stage (patient characteristics in Table 1).
Serum levels of VEGF, IL-6, IL-8, and LPS were measured with enzyme-linked immunosorbent assay (ELISA) from patients’ serum which were obtained at BL and FU. Human VEGF Quantikine ELISA Kit (DVE00; R&D systems, Minneapolis, MN, USA), Human IL-6 Quantikine ELISA KIT (D6050; R&D systems, MN, USA), Human IL-8/CXCL8 Quantikine ELISA Kit (D8000C, R&D systems, MN, USA), and Human Lipopolysaccharides (LPS) ELISA Kit (CSB-E09945h; Cusabio, China) were used in the study. Optical density evaluations were performed at 450 nm and 570 nm (as the reference) using Tecan Sunrise absorbance microplate reader, concentrations were calculated using four parameter logistic regression (4-PL) curve fitting model.
All statistical analyses were performed using SAS (SAS version 9.4 for Windows; Copyright SAS Institute Inc., Cary, NC, USA). Numerical data are presented as means with standard deviations. For categorical data, results are given as absolute numbers with percentages. For comparison of categorical data, chi-square tests were applied. T-tests or Mann-Whitney U tests were used for testing homogeneity of independent samples in continuous data. We used receiver operating characteristic (ROC) curves to determine the cut-off value for differences in concentrations of IL-6, IL-8, and VEGF that could produce the highest sensitivity and specificity to predict individual survival shorter than the median overall survival. Univariate and multivariate Cox regression analyses were performed to identify risk factors for mortality. The Kaplan-Meier method was used for estimates of overall survival, and the log-rank test was used to compare survival groups. All tests were carried out two-sided. The level of significance was set to 0.05 without adjusting for multiplicity.
At data closure for this substudy 72 patients (80%) had died. The median overall survival (OS) of the cohort analyzed within this sub-analysis was 14.5 months: OS was 14.3 months in the sorafenib only arm and 15.0 months in the combined treatment arm (p=0.5964).
IL-6 concentrations at BL (rho = -0.3024, p = 0.0038) and FU (rho = -0.3192, p = 0.0022) negatively correlated with OS.
ROC curve analysis identified a FU concentration of 24.18 pg/mL best distinguished patients with a median survival longer than 12 months (sensitivity 42.9%, specificity 87.5%). Median OS was 17.0 months if FU IL-6 was lower than 24.18 pg/mL and was 7.7 months in the 23 patients (28% of patients) with higher FU IL-6 levels (p < 0.0001) (Table 2 and Figure 2A). Patients with lower IL-6 at FU also had a significantly longer progression-free survival (median PFS: 9.6 months vs. 5.0 months; p=0.0278) (Figure 2C).
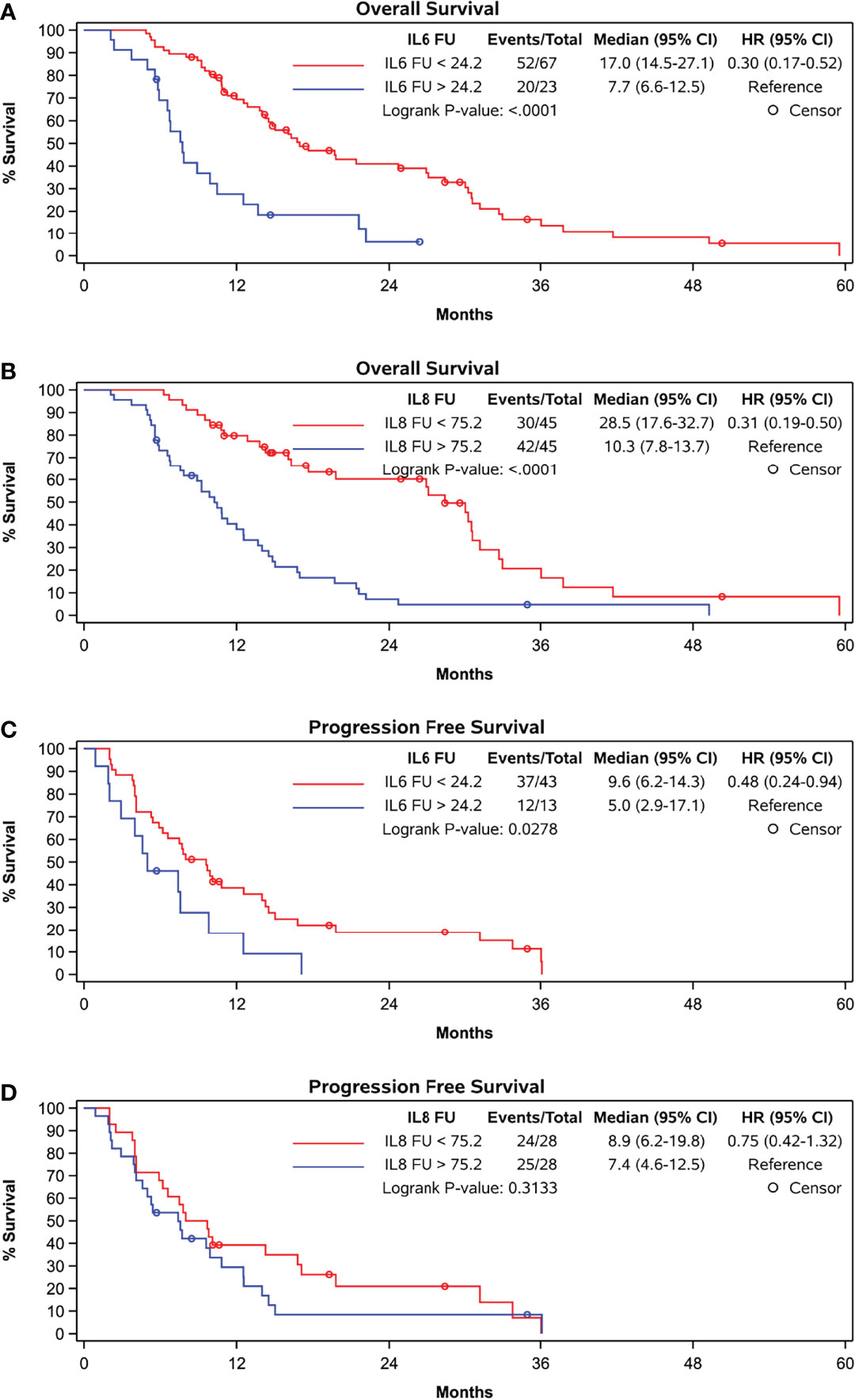
Figure 2 Kaplan-Meier curves grouped by follow-up levels according to cut-off in ROC-curve-analyses (A) OS and IL-6, cut-off at 24.2 pg/ml, (B) OS and IL-8, cut-off at 75.2 pg/ml, (C) PFS and IL-6, cut-off at 24.2 pg/ml, and (D) PFS and IL-8, cut-off at 75.2 pg/ml.
ROC curve analysis identified that absolute increases in IL-6 from BL to FU with a cut-off of 16.8 pg/mL optimally distinguished patients with OS shorter than 12 months from those with an OS exceeding 12 months (sensitivity 38.1%, specificity 85.4%). Patients showing absolute increase in IL-6 concentration less than 16.8 pg/mL had a median OS of 16.3 months versus 8.9 months for those with greater IL-6 increase (p = 0.0354) (Figure 3A).
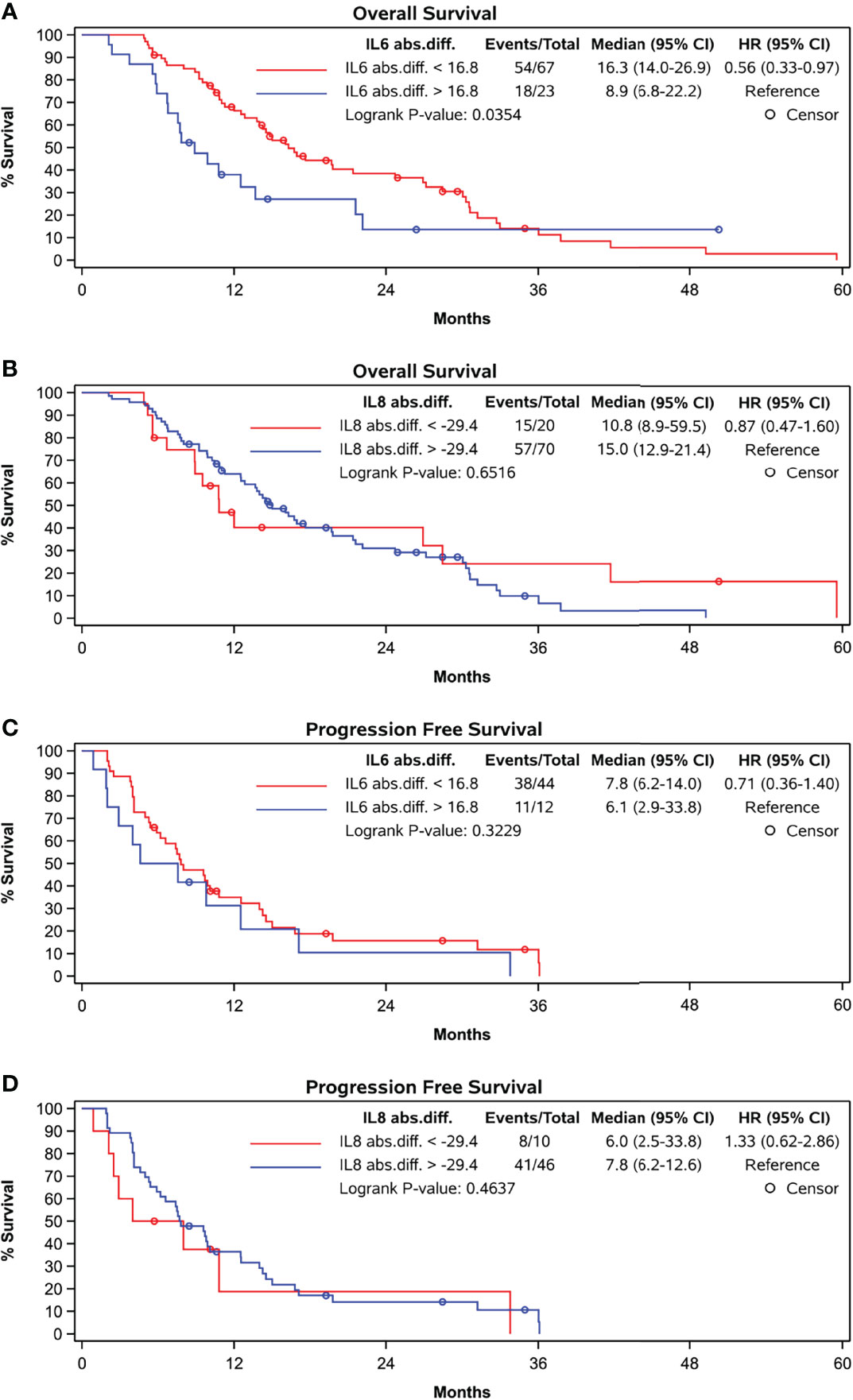
Figure 3 Kaplan-Meier curves grouped by absolute difference between baseline and follow-up levels according to cut-off in ROC-curve-analyses. (A) OS and abs. difference in IL-6, cut-off at 16.8 pg/ml, (B) OS and abs. difference in IL-8, cut-off at -29.4 pg/ml, (C) PFS and abs. difference in IL-6, cut-off at 16.8 pg/ml and (D) PFS and abs. difference in IL-8, cut-off at -29.4 pg/ml.
A Cox regression with BL IL-6 as well as absolute IL-6 change from BL to FU in the model showed significant p-values for both effects (BL: p<0.0001; abs. change BL to FU: p=0.0260).
In patients with a BL IL-6 concentration < 9.7 pg/mL the additional FU IL-6 did not add prognostic value. However, in patients with a BL IL-6 concentration exceeding 9.7 pg/ml (n=39), the additional FU value identified a subgroup with worse expectation of survival. An increase of more than 16.8 pg/mL at FU was associated with a median OS of only 6.8 months compared with median OS of 11.3 months in those showing a less pronounced increase within this subgroup (p = 0.0337) (Figure 4).
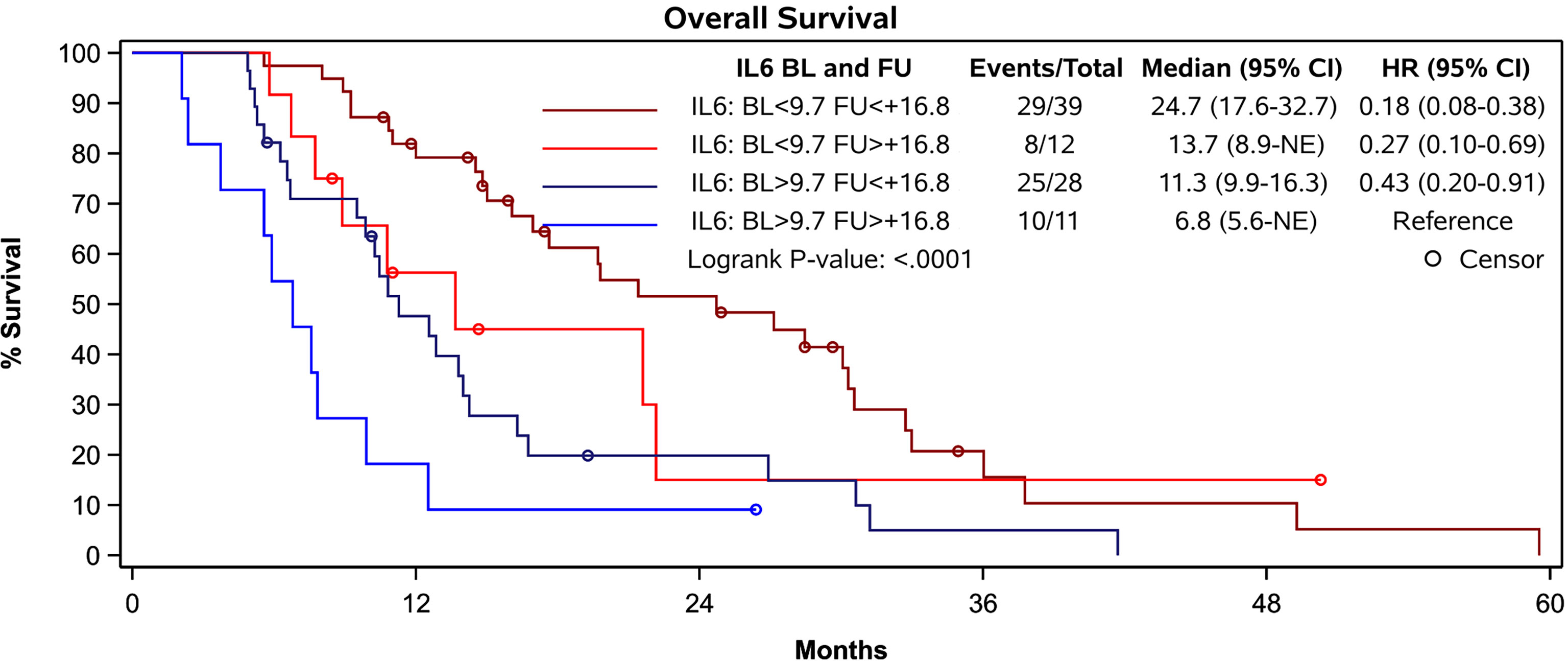
Figure 4 Survival of patients according to their prognostic groups identified by IL-6 levels. Patients with a baseline IL-6 concentration exceeding 9.7 pg/ml and an increase of 16.8 pg/mL or more at FU presented with a median OS of 6.8 months while patients with less pronounced increase showed a median OS of 11.3 months (p = 0.0337). Patients with low IL-6 levels at baseline and increase of less than 16.8 pg/ml at FU had the most favorable prognosis.
The percentage increase in IL-6 concentration did not correlate with OS nor did the absolute or percentage increase in IL-6 concentration correlate with PFS (Figure 3C).
Both baseline (rho = -0.3913, p = 0.0001) and FU concentrations (rho = -0.4370, p = <.0001) of IL-8 significantly correlated with OS (Table 2 zand Figure 2B). At follow-up, patients with IL-8 concentrations below the threshold of 75.25 pg/ml had a significantly longer OS than those above threshold (28.5 months compared to 10.3 months, p < 0.0001). However, there was no correlation with PFS at this cut-off (Figure 2D).
Furthermore, the absolute or percentage difference in IL-8 concentrations between both time points did not correlate with OS (rho = -0.0991, p = 0.3667) (Figure 3B) nor with PFS (Figure 3D).
Multivariate Cox regression analyses integrating also treatment modality, liver function, portal vein thrombosis, and tumor distribution revealed that baseline concentrations of IL-6 and IL-8 as well as liver function and the presence of portal vein thrombosis are independent factors impacting on OS, while absolute differences in IL-6 and IL-8 concentrations are not (Tables 3A, B and Supplementary Table S1).
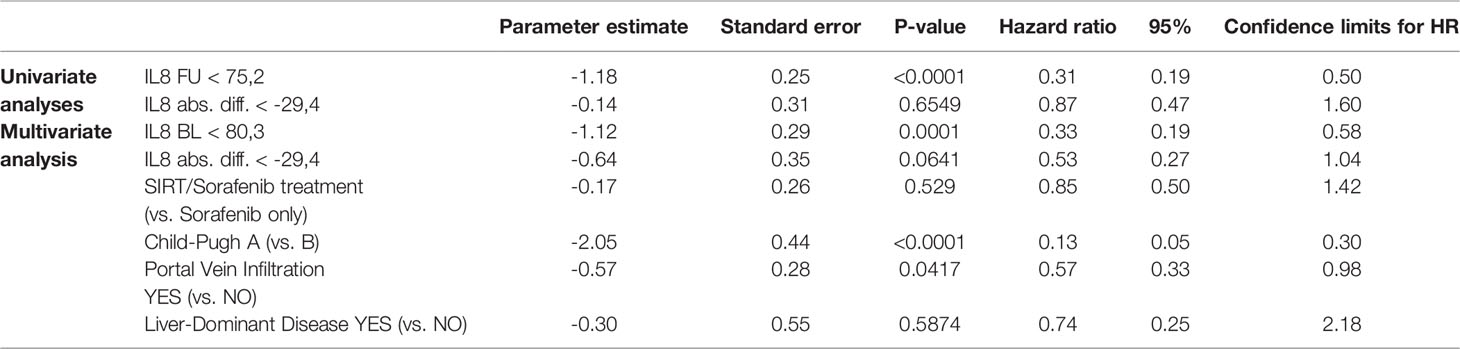
Table 3B Impact of follow-up assessment of IL-8 concentration: univariate and multivariate analysis.
Serum VEGF and LPS concentrations did not show any correlation with survival in this cohort of patients, either at baseline, follow-up level, or the changes between these time points (Table 2).
While baseline IL-6 levels did not correlate with liver decompensation indicated by an increase in ALBI grade at FU (p = 0.2413), baseline IL-8 concentration (p = 0.0275) as well as IL-6 and IL-8 concentration at FU did (p = 0.0267 and p = 0.0109) (Figures 5A–D).
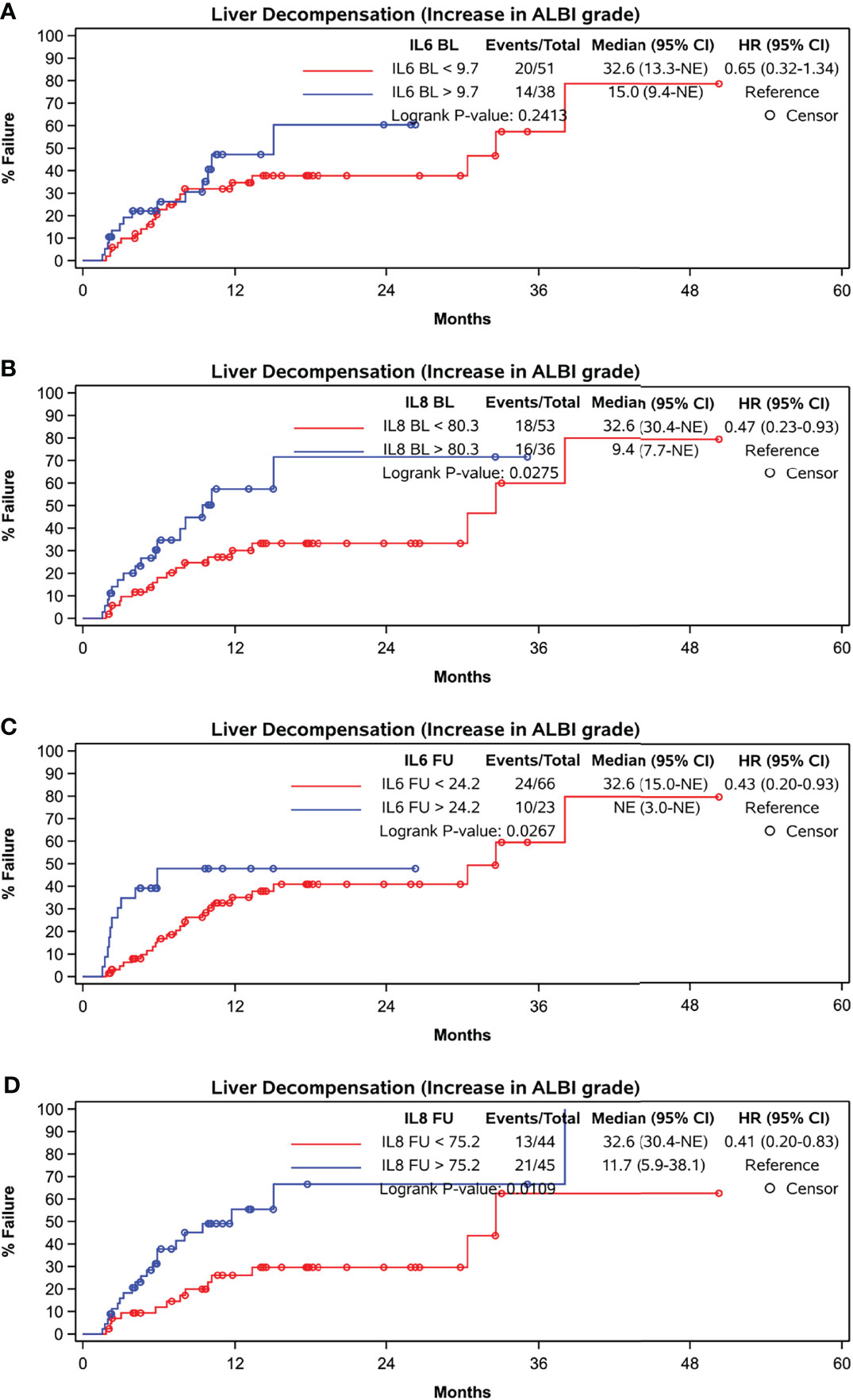
Figure 5 Time to liver function deterioration assessed by ALBI-grade. (A) baseline IL-6, cut-off at 9.7 pg/ml, (B) baseline IL-8, cut-off at 80.3 pg/ml, (C) FU IL-6, cut-off at 24.2 pg/ml, (D) FU IL-8, cut-off at 75.2 pg/ml.
We have previously reported on the prognostic value of baseline IL-6 concentrations in patients treated within the palliative arm of the SORAMIC study (4, 5). This current analysis suggests that repeated measurement of IL-6 7-9 weeks after treatment initialization is of prognostic value in these patients and that dynamic changes in IL-6 concentrations further helps to identify patients with dismal prognosis. An increase of IL-6 levels above 16.8 pg/ml from baseline is associated with poor prognosis. The subgroup of patients with baseline IL-6 readings of 9.7 pg/ml or over and an increase of IL-6 level of more than 16.8 ppg/ml have particularly poor outcomes with median OS of only 6.8 months. Favorable prognosis (median OS of 24.7 months) was associated with low IL-6 levels at baseline and increases of less than 16.8 pg/ml at follow-up. Contrary to initial expectation, no additional value in the prognostic assessment of these palliative HCC patients was conferred by the analysis of the dynamics of IL-8, VEGF, or LPS.
Clinical factors influencing survival of HCC patients in numerous studies including the SORAMIC trial, relate to tumor burden (number and size of HCC nodules within the liver, invasion of portal vein, existence of extrahepatic metastases), clinical performance status, and liver function (1, 6). As previously published, our analysis confirms liver function and portal vein infiltration as prognostic factors in patients with advanced HCC included in the SORAMIC trial (7). A pooled analysis of the two phase III studies that led to approval of sorafenib for treatment of patients with advanced HCC revealed that presence of macrovascular invasion, high alpha-fetoprotein (AFP) levels, and high neutrophil-to-lymphocyte ratio (NLR) were prognostic factors of poorer OS. Patients had a benefit of the treatment with sorafenib irrespective of prognostic factors, although lack of extrahepatic metastases, lower NLR, and chronic hepatitis C virus infection were predictive for greater survival benefit (8, 9). Since the approval of sorafenib, the evaluation of additional systemic biomarkers applicable either in a prognostic or in a predictive manner has been the focus of many studies.
The chronically inflamed microenvironment in the liver induces macrophages to adopt the M2 phenotype and transform into tumor associated macrophages (TAMs) which are responsible for continuous local proinflammatory signaling leading to parenchymal cell transformation (10). TAMs secrete IL-8 which activates the PI3K/AKT/HIF-1a signaling pathway involved in invasiveness and metastatic progress of HCC (10–12). They also secrete further proinflammatory cytokines including IL-6 with antiapoptotic activity in HCC cell lines (12). TAMs stimulate tumor growth also by suppression of the adaptive immune system through expression of high levels of programmed cell death-ligand 1 (PD-L1), and by this mechanism suppress the antitumor cytotoxic T-cell responses (13). Tumor cells also secrete a variety of inflammatory factors and chemokines to recruit TAMs including IL-6, IL-8, and IL-34 (14). Increased levels of proinflammatory cytokines in patients with chronic liver disease are associated with increased risk of HCC and correlate with survival in patients with advanced HCC (15, 16). IL-6 activates signal transducer and activator of transcription 3 (STAT3) which is implicated in induction of sorafenib resistance in patients with HCC (2, 17), but IL-6 levels have also been shown to be a predictor of survival even in patients with liver cirrhosis without HCC (18).
IL-8 in addition to its pro-tumorigenic activity is a potent angiogenic factor and produced by most HCC cell lines (19, 20). The inhibition of IL-8 signaling increases the sensitivity of liver cancer cells to sorafenib (21). We and others have previously reported that baseline IL-8 values predict outcome in patients with advanced HCC treated with sorafenib (5). However, we did not find an add-on value by follow-up measurement or the assessment of dynamics of IL-8 concentration with respect to survival.
The analysis of larger panels focusing on factors relevant to the proinflammatory tumor microenvironment and giving a more holistic view on systemic consequences of chronic tumor associated inflammation might result in more accurate results with respect to individual prognosis in patients with advanced HCC. In recent years, a clinical breakthrough in systemic therapy of HCC was reached with the introduction of checkpoint-inhibitors targeting programmed death-1, programmed death-ligand 1, and cytotoxic T lymphocyte antigen-4. These molecules resolve T-cell activation to maintain inflammatory homeostasis, protect tissue integrity, and prevent unwanted autoimmunity under physiological condition (22). In patients with tumors, the administration of checkpoint inhibitors unleashes tumor-directed cytotoxic T-cells specific against an unknown spectrum of tumor-associated antigens (22, 23). The immune contexture of HCC before treatment is the most promising predictive marker for response to immunotherapies (24). The identification of a subset of patients with high grade of tumor associated inflammation might therefore guide treatment decisions toward early systemic immune-checkpoint inhibition.
Concentrations of IL-6 and IL-8 at FU both were of prognostic value with respect to future deterioration of liver function assessed by the ALBI-Grade (25). This is of special clinical interest, as preserved liver function is the key factor in decisions on applicability of sequential treatments, and the repeated measurement of IL-6 and IL-8 potentially help to identify patients at risk to become untreatable because of impairment of liver function rather than because of tumor progression. In a previous analysis on patients treated within SORAMIC in palliative intent with the combination of radioembolization and sorafenib high IL-6 concentrations at baseline were associated with a significant shorter time to liver dysfunction. Although there was a tendency for a shorter time-to-liver dysfunction in patients with high IL-8 concentrations in that analysis, the result was not significant (4). As in that paper, liver function deterioration was defined by a significant increase in bilirubin levels, cholestasis, and secretory function of the liver are assumed to be the dominant factors in this setting. The current analysis also takes liver synthesis mirrored by albumin concentration into account which might explain differences in the results.
Vascular endothelial growth factor (VEGF), a protein that promotes angiogenesis, supports tumor cell survival, proliferation, and vessel formation has been shown to have prognostic value in HCC patients (26, 27). Sorafenib, a multi-tyrosine kinase inhibitor with inhibitory activity against vascular endothelial growth factor receptor (VEGFR) 2 (28) was the standard treatment in patients with advanced HCC for more than a decade. Baseline concentrations of IL-6 and IL-8 have been demonstrated to be predictive for response to sorafenib monotherapy in patients with advanced HCC (5). Recently, the combination of atezolizumab, a humanized anti-PDL1 antibody to restore antitumor T-cell activity, with bevacizumab, binding to VEGF, has been defined as the new first line treatment standard (29, 30).
Previously, it has been reported that circulating concentrations of VEGF are of prognostic value in patients with HCC undergoing liver resection, liver transplantation, or locoregional therapy (26). A decrease in VEGF levels at week 8 of sorafenib treatment was prognostic for better survival in advanced HCC patients (mostly on the background of chronic viral hepatitis) (31). We however did not observe any prognostic potential of systemic VEGF levels in our cohort. It is unclear whether this discrepancy is related to the patient characteristics within this cohort, with low numbers of patients with viral etiology of HCC.
Alterations in gut microbiota composition are associated with hepatic inflammatory diseases and may play a contributory role in hepatic carcinogenesis (32). Dysbiotic gut microbiota composition leads to increased hepatic exposure with gut-derived microbiota-associated molecular patterns (MAMPs) that include lipopolysaccharides (LPS), a cell wall component of Gram-negative bacteria.
Bacterial compounds reach the liver via the portal blood flow and are eliminated by Kupffer cells under normal conditions (33). In a mouse model, LPS levels increase during the course of chronic liver disease fostering proinflammatory responses in the periphery and in the liver (32, 34). Plasma LPS concentrations correlate with the degree of liver dysfunction (35, 36).
An increased exposure to LPS contributes to hepatocarcinogenesis via activation of Toll-like receptor-4 signaling in mouse models (37). By this, LPS promote hepatic inflammation, fibrosis, proliferation, and the activation of anti-apoptotic signaling (37). In an analysis of fecal samples of patients with HCC and liver cirrhosis LPS‐producing genera were increased compared to patients with liver cirrhosis (38).
The proinflammatory tumor microenvironment in part induced by pathogen-associated molecular patterns (PAMPs) including LPS also promotes tumor progression. High concentrations of infiltrating TAMs are associated with poor prognosis of HCC (12, 39). However, our study does not support the idea of measuring systemic concentrations of LPS for prognostic purposes in patients receiving treatment with sorafenib with or without radioembolization in patients with advanced HCC. It is likely that concentrations of LPS in the portal blood flow and in the liver are of higher significance for progression of HCC than systemic concentrations.
Our analysis is limited by the rather small number of patients that could be included into the analysis compared to the whole study population of SORAMIC.
As availability of effective systemic treatment options for advanced HCC patients increases, sequential treatments are now a realistic strategy for management. Methods to expeditiously identify patient groups who do well on particular treatments, or select patients with more dismal prognosis, may help to guide optimal management. Although clinical factors have previously been utilized in this aim, the baseline assessment of proinflammatory cytokines IL-6 and IL-8 in addition to inclusion of the dynamic changes of IL-6 values at 7-9 weeks after treatment initiation (sorafenib with or without radioembolization used in the SORAMIC trial) appears of value in the prediction of patients who do better on palliative treatments within the SORAMIC study. Early re-assessment of IL-6 and IL-8 may also help to identify patients at risk for deterioration of liver function which potentially precludes further lines of treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethical committee Otto-von-Guericke University Magdeburg, Germany and the local ethics committees. The patients/participants provided their written informed consent to participate in this study.
KS, PM, and JR: Conception and design of the study; Generation, collection, assembly, analysis, and/or interpretation of data; Drafting or revision of the manuscript; Approval of the final version of the manuscript. JK, EM, OÖ, RS, MS, ED, NBK, MP, DP, TB, CS, BB, JV, JB, AG, and BS: Generation, collection, assembly, analysis, and/or interpretation of data; Drafting or revision of the manuscript; Approval of the final version of the manuscript.
SORAMIC is an investigator-initiated trial sponsored by the University of Magdeburg. Financial support was granted by Sirtex Medical and Bayer Healthcare. This research was supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014*).
KS: Personal fees: Bayer. MS: Grants: Sirtex, Bayer; Personal fees: Sirtex, Bayer. ED: ED has served as a paid consultant for AstraZeneca, Bayer, BMS, EISAI, Eli Lilly & Co, Pfizer, IPSEN, and Roche. He has received reimbursement of meeting attendance fees and travel expenses from Arqule, Astrazeneca, BMS, Bayer, Celsion and Roche, and lecture honoraria from BMS and Falk. He has received third-party funding for scientific research from Arqule, AstraZeneca, BMS, Bayer, Eli Lilly, and Roche. NB: NB has received reimbursement of meeting attendance fees and travel expenses from EISAI and lecture honorarium from Falk. MP: Personal fees: Sirtex, Bayer. TB: Receipt of grants/research supports: Abbvie, BMS, Gilead, MSD/Merck, Humedics, Intercept, Merz, Norgine, Novartis, Orphalan, Sequana Medical. Receipt of honoraria or consultation fees/advisory board: Abbvie, Alexion, Bayer, Gilead, GSK, Eisai, Enyo Pharma, Falk Foundation, HepaRegeniX GmbH, Humedics, Intercept, Ipsen, Janssen, MSD/Merck, Novartis, Orphalan, Roche, Sequana Medical, SIRTEX, SOBI, and Shionogi Participation in a company sponsored speaker's bureau: Abbvie, Alexion, Bayer, Gilead, Eisai, Intercept, Ipsen, Janssen, MedUpdate GmbH, MSD/Merck, Novartis, Orphalan, Sequana Medica, SIRTEX, and SOBI. JV: Dr. Valle reports personal fees from Agios, personal fees from AstraZeneca, personal fees from Baxter, personal fees from Genoscience Pharma, personal fees from Hutchison Medipharma, personal fees from Imaging Equipment Ltd (AAA), personal fees from Incyte, personal fees from Ipsen, personal fees from Mundipharma EDO, personal fees from Mylan, grants, personal fees and non-financial support from NuCana, personal fees from QED, personal fees from Servier, personal fees from Sirtex, personal fees from Zymeworks, outside the submitted work. PM: Grants: Bayer, Sirtex. JR: Sirtex, Bayer; Personal fees: Sirtex, Bayer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgstr.2022.939192/full#supplementary-material
Supplementary Figure 1 | Boxplots indicating concentrations of IL-6, IL-8, VEGF and LPS, each comparing absolute levels at baseline and follow-up stratified by treatment, overall survival and a combination of both.
1. European Association for the Study of the Liver, Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
2. Jiang Y, Chen P, Hu K, Dai G, Li J, Zheng D, et al. Inflammatory Microenvironment of Fibrotic Liver Promotes Hepatocellular Carcinoma Growth, Metastasis and Sorafenib Resistance Through STAT3 Activation. J Cell Mol Med (2021) 25(3):1568–82. doi: 10.1111/jcmm.16256
3. Bishayee A. The Role of Inflammation and Liver Cancer. Adv Exp Med Biol (2014) 816:401–35. doi: 10.1007/978-3-0348-0837-8_16
4. Öcal O, Kupcinskas J, Morkuna E, Amthauer H, Schütte K, Malfertheiner P, et al. Prognostic Value of Baseline Interleukin 6 Levels in Liver Decompensation and Survival in HCC Patients Undergoing Radioembolization. EJNMMI Res (2021) 11(1):51. doi: 10.1186/s13550-021-00791-w
5. Öcal O, Schütte K, Kupcinskas J, Morkunas E, Jurkeviciute G, De Toni EN, et al. Baseline Interleukin-6 and -8 Predict Response and Survival in Patients With Advanced Hepatocellular Carcinoma Treated With Sorafenib Monotherapy: An Exploratory Post Hoc Analysis of the SORAMIC Trial. J Cancer Res Clin Oncol (2021) 148(2):475–85. doi: 10.1007/s00432-021-03627-1
6. Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of Combined Selective Internal Radiation Therapy and Sorafenib on Survival in Advanced Hepatocellular Carcinoma. J Hepatol (2019) 71(6):1164–74. doi: 10.1016/j.jhep.2019.08.006
7. Öcal O, Ingrisch M, Ümütlü MR, Peynircioglu B, Loewe C, van Delden O, et al. Prognostic Value of Baseline Imaging and Clinical Features in Patients With Advanced Hepatocellular Carcinoma. Br J Cancer (2022) 126(2):211–8. doi: 10.1038/s41416-021-01577-6
8. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic Factors and Predictors of Sorafenib Benefit in Patients With Hepatocellular Carcinoma: Analysis of Two Phase III Studies. J Hepatol (2017) 67(5):999–1008. doi: 10.1016/j.jhep.2017.06.026
9. Mohr R, Jost-Brinkmann F, Ozdirik B, Lambrecht J, Hammerich L, Loosen SH, et al. Lessons From Immune Checkpoint Inhibitor Trials in Hepatocellular Carcinoma. Front Immunol (2021) 12:652172. doi: 10.3389/fimmu.2021.652172
10. Galun E. Liver Inflammation and Cancer: The Role of Tissue Microenvironment in Generating the Tumor-Promoting Niche (TPN) in the Development of Hepatocellular Carcinoma. Hepatology (2016) 63(2):354–6. doi: 10.1002/hep.28344
11. Huang W, Chen Z, Zhang L, Tian D, Wang D, Fan D, et al. Interleukin-8 Induces Expression of FOXC1 to Promote Transactivation of CXCR1 and CCL2 in Hepatocellular Carcinoma Cell Lines and Formation of Metastases in Mice. Gastroenterology (2015) 149(4):1053–67 e14. doi: 10.1053/j.gastro.2015.05.058
12. Shirabe K, Mano Y, Muto J, Matono R, Motomura T, Toshima T, et al. Role of Tumor-Associated Macrophages in the Progression of Hepatocellular Carcinoma. Surg Today (2012) 42(1):1–7. doi: 10.1007/s00595-011-0058-8
13. Saviano A, Roehlen N, Virzi A, Roca Suarez AA, Hoshida Y, Lupberger J, et al. Stromal and Immune Drivers of Hepatocarcinogenesis. In: Hoshida Y, editor. Hepatocellular Carcinoma: Translational Precision Medicine Approaches. Cham CH: Humana Press (2019). p. 317–31.
14. Ge Z, Ding S. The Crosstalk Between Tumor-Associated Macrophages (TAMs) and Tumor Cells and the Corresponding Targeted Therapy. Front Oncol (2020) 10:590941. doi: 10.3389/fonc.2020.590941
15. Seidensticker M, Powerski M, Seidensticker R, Damm R, Mohnike K, Garlipp B, et al. Cytokines and (90)Y-Radioembolization: Relation to Liver Function and Overall Survival. Cardiovasc Intervent Radiol (2017) 40(8):1185–95. doi: 10.1007/s00270-017-1622-4
16. Jang JW, Oh BS, Kwon JH, You CR, Chung KW, Kay CS, et al. Serum Interleukin-6 and C-Reactive Protein as a Prognostic Indicator in Hepatocellular Carcinoma. Cytokine (2012) 60(3):686–93. doi: 10.1016/j.cyto.2012.07.017
17. Sakurai T, Yada N, Hagiwara S, Arizumi T, Minaga K, Kamata K, et al. Gankyrin Induces STAT3 Activation in Tumor Microenvironment and Sorafenib Resistance in Hepatocellular Carcinoma. Cancer Sci (2017) 108(10):1996–2003. doi: 10.1111/cas.13341
18. Remmler J, Schneider C, Treuner-Kaueroff T, Bartels M, Seehofer D, Scholz M, et al. Increased Level of Interleukin 6 Associates With Increased 90-Day and 1-Year Mortality in Patients With End-Stage Liver Disease. Clin Gastroenterol Hepatol (2018) 16(5):730–7. doi: 10.1016/j.cgh.2017.09.017
19. Sun HC, Tang ZY. Angiogenesis in Hepatocellular Carcinoma: The Retrospectives and Perspectives. J Cancer Res Clin Oncol (2004) 130(6):307–19. doi: 10.1007/s00432-003-0530-y
20. Fousek K, Horn LA, Palena C. Interleukin-8: A Chemokine at the Intersection of Cancer Plasticity, Angiogenesis, and Immune Suppression. Pharmacol Ther (2021) 219:107692. doi: 10.1016/j.pharmthera.2020.107692
21. Kahraman DC, Kahraman T, Cetin-Atalay R. Targeting PI3K/Akt/mTOR Pathway Identifies Differential Expression and Functional Role of IL8 in Liver Cancer Stem Cell Enrichment. Mol Cancer Ther (2019) 18(11):2146–57. doi: 10.1158/1535-7163.MCT-19-0004
22. Pardoll DM. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
23. Sprinzl MF, Galle PR. Current Progress in Immunotherapy of Hepatocellular Carcinoma. J Hepatol (2017) 66(3):482–4. doi: 10.1016/j.jhep.2016.12.009
24. Macek Jilkova Z, Ghelfi J, Decaens T. Immunomodulation for Hepatocellular Carcinoma Therapy: Current Challenges. Curr Opin Oncol (2022) 34(2):155–60. doi: 10.1097/CCO.0000000000000812
25. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of Liver Function in Patients With Hepatocellular Carcinoma: A New Evidence-Based Approach-the ALBI Grade. J Clin Oncol (2015) 33(6):550–8. doi: 10.1200/JCO.2014.57.9151
26. Zhang W, Kim R, Quintini C, Hashimoto K, Fujiki M, Diago T, et al. Prognostic Role of Plasma Vascular Endothelial Growth Factor in Patients With Hepatocellular Carcinoma Undergoing Liver Transplantation. Liver Transpl (2015) 21(1):101–11. doi: 10.1002/lt.24013
27. Duda DG, Dima SO, Cucu D, Sorop A, Klein S. Potential Circulating Biomarkers of Recurrence After Hepatic Resection or Liver Transplantation in Hepatocellular Carcinoma Patients. Cancers (Basel) (2020) 12(5):1275. doi: 10.3390/cancers12051275
28. Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, et al. The Mechanisms of Sorafenib Resistance in Hepatocellular Carcinoma: Theoretical Basis and Therapeutic Aspects. Signal Transduct Target Ther (2020) 5(1):87. doi: 10.1038/s41392-020-0187-x
29. Kulik L, da Fonseca LG, He AR, Rimola J, Wilson Woods A, Zöllner YF, et al. Potential Impact of IMbrave150 Results in the Evolving Treatment Landscape of Advanced Hepatocellular Carcinoma: A Multidisciplinary Expert Opinion. J Hepatocell Carcinoma (2020) 7:423–33. doi: 10.2147/JHC.S274930
30. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
31. Tsuchiya K, Asahina Y, Matsuda S, Muraoka M, Nakata T, Suzuki Y, et al. Changes in Plasma Vascular Endothelial Growth Factor at 8 Weeks After Sorafenib Administration as Predictors of Survival for Advanced Hepatocellular Carcinoma. Cancer (2014) 120(2):229–37. doi: 10.1002/cncr.28384
32. Behary J, Raposo AE, Amorim NML, Zheng H, Gong L, McGovern E, et al. Defining the Temporal Evolution of Gut Dysbiosis and Inflammatory Responses Leading to Hepatocellular Carcinoma in Mdr2 -/- Mouse Model. BMC Microbiol (2021) 21(1):113. doi: 10.1186/s12866-021-02171-9
33. Zhou A, Tang L, Zeng S, Lei Y, Yang S, Tang B. Gut Microbiota: A New Piece in Understanding Hepatocarcinogenesis. Cancer Lett (2020) 474:15–22. doi: 10.1016/j.canlet.2020.01.002
34. Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, et al. Gut–liver Axis: The Impact of Gut Microbiota on non Alcoholic Fatty Liver Disease. Nutr Metab Cardiovasc Dis (2012) 22(6):471–6. doi: 10.1016/j.numecd.2012.02.007
35. Roderburg C, Luedde T. The Role of the Gut Microbiome in the Development and Progression of Liver Cirrhosis and Hepatocellular Carcinoma. Gut Microbes (2014) 5(4):441–5. doi: 10.4161/gmic.29599
36. Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, et al. Endotoxemia in Patients With Chronic Liver Diseases: Relationship to Severity of Liver Diseases, Presence of Esophageal Varices, and Hyperdynamic Circulation. J Hepatol (1995) 22(2):165–72. doi: 10.1016/0168-8278(95)80424-2
37. Yu LX, Schwabe RF. The Gut Microbiome and Liver Cancer: Mechanisms and Clinical Translation. Nat Rev Gastroenterol Hepatol (2017) 14(9):527–39. doi: 10.1038/nrgastro.2017.72
38. Zheng R, Wang G, Pang Z, Ran N, Gu Y, Guan X, et al. Liver Cirrhosis Contributes to the Disorder of Gut Microbiota in Patients With Hepatocellular Carcinoma. Cancer Med (2020) 9(12):4232–50. doi: 10.1002/cam4.3045
Keywords: IL-8, IL-6, biomarker, prognosis, HCC, LPS, VEGF
Citation: Schütte K, Kupčinskas J, Morkunas E, Öcal O, Schinner R, Seidensticker M, De Toni EN, Ben Khaled N, Pech M, Palmer D, Berg T, Sengel C, Basu B, Valle JW, Benckert J, Gasbarrini A, Sangro B, Malfertheiner P and Ricke J (2022) Dynamics in Circulating Proinflammatory Biomarkers for Prognostic Assessment of Patients With Advanced HCC – A Substudy From the SORAMIC Trial. Front. Gastroenterol. 1:939192. doi: 10.3389/fgstr.2022.939192
Received: 08 May 2022; Accepted: 31 May 2022;
Published: 05 July 2022.
Edited by:
Ankur Sharma, Harry Perkins Institute of Medical Research, AustraliaReviewed by:
Giorgio Frega, University of Bologna, ItalyCopyright © 2022 Schütte, Kupčinskas, Morkunas, Öcal, Schinner, Seidensticker, De Toni, Ben Khaled, Pech, Palmer, Berg, Sengel, Basu, Valle, Benckert, Gasbarrini, Sangro, Malfertheiner and Ricke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jens Ricke, amVucy5yaWNrZUBtZWQudW5pLW11ZW5jaGVuLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.