
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 19 September 2024
Sec. Cancer Genetics and Oncogenomics
Volume 15 - 2024 | https://doi.org/10.3389/fgene.2024.1455502
Introduction: In this study, we aimed to explore the relationship between clinicopathological features and driver gene changes in Chinese NSCLC patients.
Methods: Amplification refractory mutation system PCR was used to detect the aberrations of 10 driver oncogenes in 851 Chinese NSCLC patients, and their correlation with clinicopathological characteristics was also analyzed. Moreover, three models of logistic regression were used to analyze the association between histopathology and EGFR or KRAS mutations.
Results: The top two most frequently aberrant target oncogenes were EGFR (48.06%) and KRAS (9.51%). These were followed by ALK (5.41%), HER2 (2.35%), MET (2.23%), RET (2.11%), ROS1 (1.88%), BRAF (0.47%), NRAS (0.24%), and PIK3CA (0.12%). Additionally, 11 (1.29%) patients had synchronous gene alterations in two genes. The main EGFR mutations were exon 21 L858R and exon 19-Del, which accounted for 45.97% and 42.79% of all EGFR mutations, respectively. Logistic regression analysis showed that the frequency of EGFR mutations was positively correlated with women, non-smokers, lung adenocarcinoma, and invasive non-mucinous adenocarcinoma (IA), and negatively correlated with solid nodule, micro-invasive adenocarcinoma, and solid-predominant adenocarcinoma. KRAS mutations were positively associated with men and longer tumor long diameters and negatively correlated with lung adenocarcinoma (P < 0.05 for all).
Conclusion: Our findings suggest that the EGFR mutation frequency was higher in women, non-smokers, lung adenocarcinoma, and the IA subtype in lung adenocarcinoma patients, while the KRAS mutation rate was higher in men and patients with longer tumor long diameter and lower in lung adenocarcinoma patients.
Lung cancer is one of the common malignant tumors that threaten human life and health, and the most recent global cancer statistics show that lung cancer is the leading cause of cancer death (Lancet, 2024). In recent years, with the rapid development of molecular detection, more and more molecular drivers have been detected as therapeutic targets for non-small cell lung cancer (NSCLC) (Yang et al., 2022), including alterations of anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1), rearranged during transfection (RET), epidermal growth factor receptor (EGFR), v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS), human epidermal growth factor receptor 2 (HER2), B-raf proto-oncogene (BRAF), and mesenchymal-epithelial transition factor (MET), among which the most common is EGFR mutations (Di Maio, 2023). As new molecular-targeted therapies have made substantial progress in NSCLC, the survival rate of NSCLC patients with specific genotypic alterations has been significantly improved (Wu et al., 2024; Planchard et al., 2023). The latest version of NCCN Clinical Practice Guidelines in Oncology recommends that when testing for NSCLC, in addition to all of the classical actionable molecular markers (e.g., EGFR, ALK, BRAF, KRAS, METex14 skipping, NTRK1/2, RET, ROS1), emerging molecular markers, such as high-level MET amplification, and ERBB2 mutation should also be detected (Ettinger et al., 2022).
EGFR tyrosine kinase inhibitors (EGFR-TKIs) can prolong survival and improve the quality of life of patients with NSCLC with EGFR mutations (Ramalingam et al., 2020). At the same time, the continuous activation mutation of KRAS may affect the therapeutic effect of EGFR-TKIs in patients with NSCLC. In addition, mutations in exon 20 of EGFR, such as T790M, may mediate resistance to EGFR-TKIs (Arbour and Riely, 2019; Tan et al., 2024). A comprehensive analysis of pathological types and tumor-specific molecular abnormalities in patients with NSCLC can help patients maximize treatment benefits while reducing treatment-related risks (Eberhardt et al., 2015). Therefore, using accurate and low-cost gene detection methods to identify the alterations of targeted driver genes in NSCLC patients and explore the correlation between them and clinicopathological features is of great significance for the individualized treatment of NSCLC patients. Some studies have suggested that lung adenocarcinoma, women, and non-smokers were positively associated with EGFR mutations (Li et al., 2020; Wang et al., 2020). However, other studies found no significant association between smoking status and gender with EGFR mutations (Ess et al., 2017). Many studies have reported that age was not associated with EGFR mutations (Gao et al., 2020; Zhao et al., 2018; Yang et al., 2014), while some investigators found EGFR mutations were more frequent in younger or older patients with NSCLC (Vallee et al., 2013; Wu et al., 2019). Thus, the role of clinicopathological characteristics, including gender, age, histology types, smoking status, etc., as predictors for the alteration of NSCLC driver genes remains controversial.
In this retrospective study, participants were recruited to undergo the detection of 10 target oncogene aberrations, including EGFR, KRAS, MET, ALK, ROS1, HER2, RET, BRAF, NRAS, and PIK3CA. The chi-squared test, Fisher exact test, and logistic regression analysis were used to analyze the relationship between 10 driver gene alterations and the clinicopathological characteristics of NSCLC patients.
In this study, information from 851 patients with NSCLC seen between October 2019 and September 2023 in the Sixth Affiliated Hospital of Guangzhou Medical University was collected. Patients who met the following criteria were included:
(1) patients with histological or pathological diagnosis of NSCLC;
(2) complete clinical medical records.
The exclusion criterion was patients with concomitant malignant disease of other systems. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Sixth Affiliated Hospital of Guangzhou Medical University. Due to the retrospective nature of the study, the Ethics Committee waived the need for patient informed consent.
Clinical data were obtained from patients’ electronic medical record database, including sex, age, smoking history, drinking status, family history, serum markers of lung tumors, chest computed tomography (CT) features, and TNM stage. Serum tumor markers included carcinoma embryonic antigen (CEA), cytokeratin 19 fragment antigen21-1 (CYFRA21-1), neuron-specific enolase (NSE), pro-gastrin-releasing peptide (ProGRP), and squamous-cell carcinoma antigen (SCCA). Abnormal serum markers were defined as elevated levels of at least one of the above serum markers. TNMs were determined according to the eighth edition of the American Joint Committee on Cancer TNM staging system for lung cancer (Rami-Porta et al., 2015). Histologic subtypes of NSCLCs were classified according to the 5th edition of the World Health Organization Classification of Thoracic Tumours (2021).
We collected three types of specimens, including biopsy (504), surgical specimen (328), and malignant exudate (19). All specimens were fixed with a 10% neutral formalin solution, dehydrated, paraffin-embedded, sliced, and stained by HE. Pathologists with more than 5 years of diagnostic experience evaluated the proportion of tumor cells in the tissues for gene change detection. Tumor specimens with more than 30% tumor cells were selected, and 5–8 wax rolls of 5 μm thickness were cut and placed in an enzyme-free EP tube for DNA and RNA extraction in strict accordance with the FFEP DNA/RNA extraction kit instructions (Amoy Diagnostics Co., Ltd., Xiamen, China). In strict accordance with the kit instructions (Amoy Diagnostics Co., Ltd., Xiamen, China), we used the amplification refractory mutation system PCR (ARMs-PCR) method in the standard PCR laboratory to detect the target oncogene aberrations, including EGFR, KRAS, MET, ALK, ROS1, HER2, RET, BRAF, NRAS, and PIK3CA. (Table 1).
All statistical analyses were performed using SPSS 27.0 software (SPSS, Armonk, NY, United States). A Student’s t-test was used to examine the association of tumor long diameter with the 10 target genes. The chi-squared test or Fisher exact test was used to explore the association of other clinicopathological features with 10 targeted gene alterations. A logistic regression analysis was performed to assess possible confounding factors between driver gene alterations and histology types, subtypes of adenocarcinoma subtypes, and subtypes of invasive adenocarcinoma (Model 1). Preliminary adjustment covariates included gender, age, and smoking history (Model 2), while full adjustment covariates included gender, age, smoking history, drinking status, serum tumor markers, pulmonary nodule types, tumor long diameter, and TNM stage (Model 3). All tests were two-sided, and a P-value < 0.05 was considered statistically significant.
Table 2 summarizes the demographics, clinical characteristics, biochemical parameters, and imaging findings in patients with NSCLC. Originally, 857 patients were included in this study, but six patients were excluded due to concurrent systemic malignancies, and 851 patients were analyzed. The histopathologic diagnosis of the NSCLC cases included in this study was confirmed by one or more thoracic pathologists. Overall, in 851 cases of non-small-cell lung cancer, 492 (57.81%) patients were men, 360 (42.30%) patients were younger than 60 years, 345 (40.54%) patients had a history of smoking, and 95 (11.16%) patients had a history of drinking. Of the complete cohort, 668 (78.50%) patients had abnormal serum tumor markers; 337 (39.60%) patients were in the early stage (including TNM stages 0, I, and II) of lung cancer, and the remaining 514 (60.40%) patients were in the advanced stage (including TNM stages III and IV) of lung cancer.
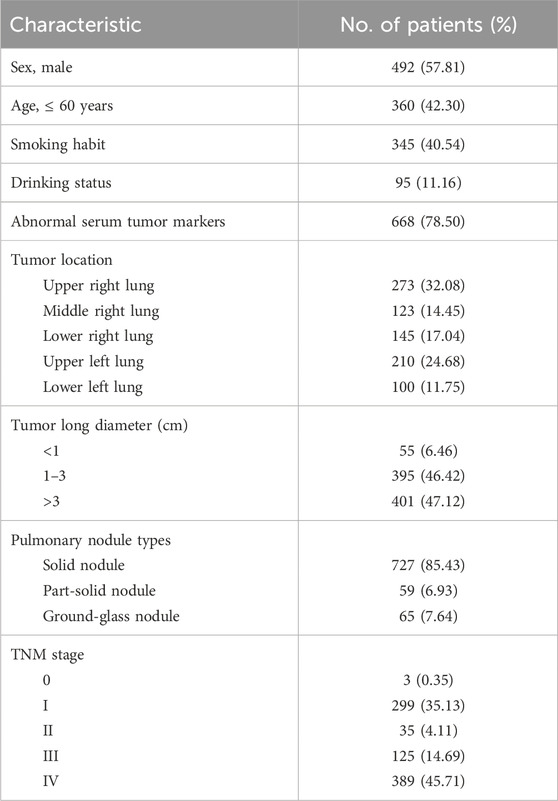
Table 2. Demographics, clinical characteristics, biochemical parameters, and imaging findings in patients with NSCLC.
As shown in Figure 1, among the 851 patients with NSCLC, 771 (90.60%) patients had adenocarcinoma (ADC), 33 (3.88%) patients had squamous-cell carcinoma (SCC), nine (1.06%) patients had adenosquamous carcinoma (ASC) and 38 (4.47%) patients had other subtypes of NSCLC. Of those 771 ADC patients, five (0.65%) patients had adenocarcinoma in situ (AIS), 45 (5.84%) patients had minimally invasive adenocarcinoma (MIA), 714 (92.61%) patients had invasive non-mucinous adenocarcinoma (IA), and seven (0.91%) patients had invasive mucinous adenocarcinoma (IMA). For patients with IA, 249 patients were classified, as only surgical specimens could be further classified. Amongst the 249 IA patients, acinar-predominant adenocarcinoma was the most common (64.26%), followed by lepidic-predominant adenocarcinoma (14.86%), 31 papillary-predominant adenocarcinoma (12.45%), and solid-predominant adenocarcinoma (8.43%).
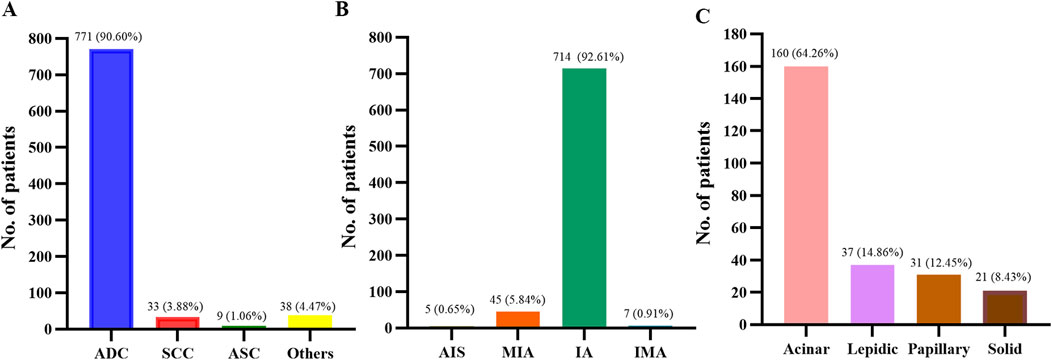
Figure 1. Histopathology of patients. (A) Histology types of 851 patients with NSCLC. (B) Histological subtypes of 771 patients with lung adenocarcinoma. (C) Histological subtypes of 249 patients with lung invasive adenocarcinoma. NSCLC, non-small cell lung cancer; ADC, adenocarcinoma; SCC, squamous cell carcinoma; ASC, adenosquamous carcinoma; AIS, adenocarcinoma in situ; MIA, micro-invasive adenocarcinoma; IA, invasive non-mucinous adenocarcinoma; IMA, invasive mucinous adenocarcinoma.
Among the 851 patients with NSCLC, 627 cases (73.68%) had alterations in at least one of the 10 target genes (Supplementary Figure S1A). As shown in Supplementary Figure S1B, the top two most frequently aberrant target oncogenes were EGFR (48.06%) and KRAS (9.51%). The remaining eight target oncogenes aberrations were ALK (5.41%), HER2 (2.35%), MET (2.23%), RET (2.11%), ROS1 (1.88%), BRAF (0.47%), NRAS (0.24%), and PIK3CA (0.12%). Relationships between 10 target gene alterations and patient characteristics are shown in Table 3. In general, the frequency of alterations in 10 target genes was significantly higher in women, never smokers, never drinkers, patients with normal serum tumor markers, patients in the early stage (p = 0.019), and shorter tumor long diameters. There was a prevalence of more target gene alterations in ADC than in other histology types. Target gene alterations were more common in patients with part-solid nodules than solid nodules and in patients with ground-glass nodules than that of solid nodules. There was no significant correlation between target gene alterations and age or tumor location. Additionally, Table 4 shows that 11 patients (1.29%) had synchronous gene alterations in two genes, 10 of which were EGFR mutations combined with mutations or rearrangements of other nine targeted driver genes, and the remaining case was a KRAS G12C, G12R, G12V, G12A, G13C/MET exon 14 skipping mutation.
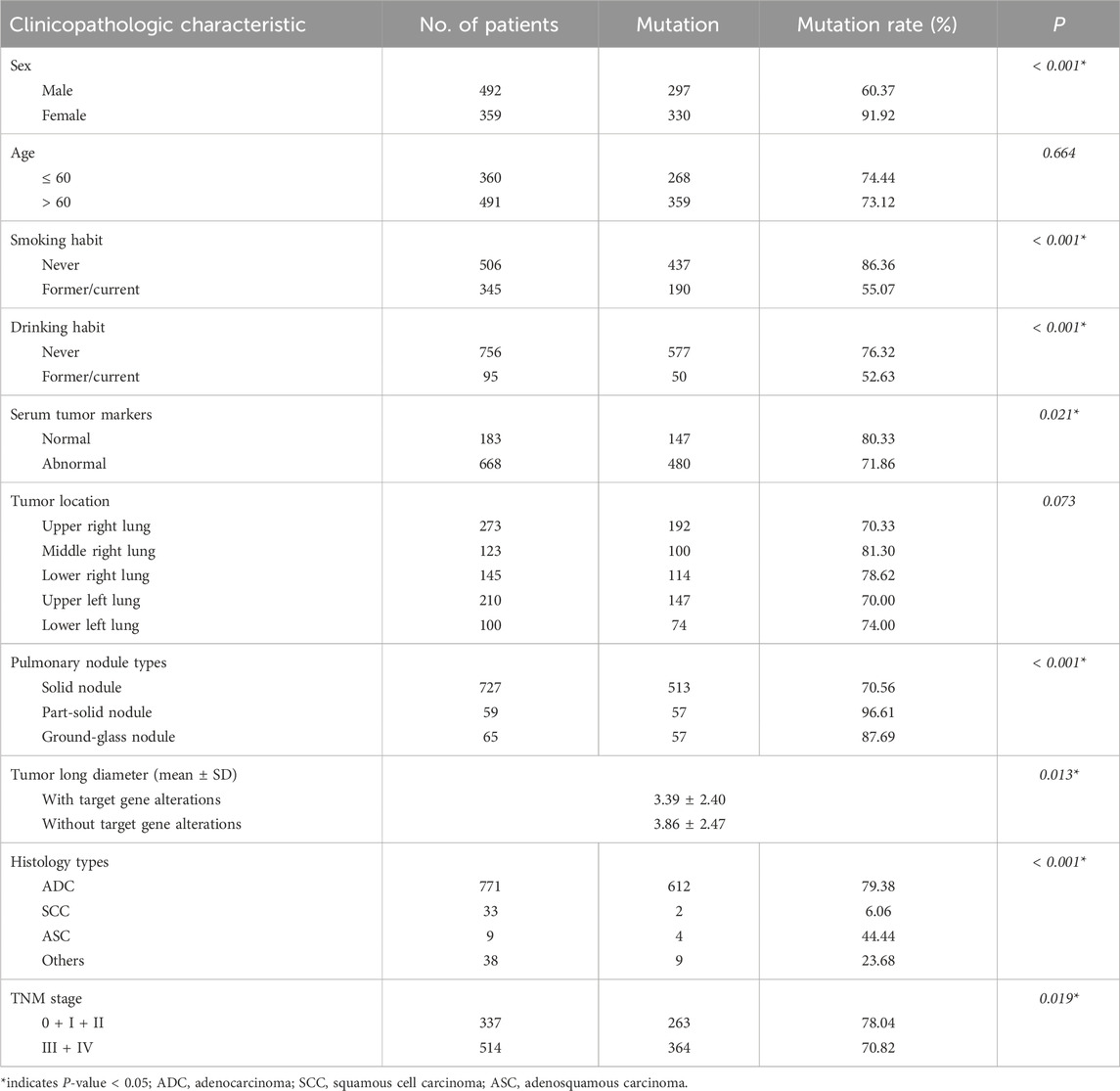
Table 3. Comparison of the mutation rate of 10 targeted genes in different clinicopathologic characteristics.
Among 409 patients with EGFR mutations, the two most prevalent mutation subtypes were exon 21 L858R and exon 19-Del, which accounted for 22.09% and 20.56% of the total 10 targeted gene alterations, and 45.97% and 42.79% of total EGFR mutations, respectively. The other three mutation subtypes were exon 20 ins (14 patients, 3.42%), exon 18 G719X (8 patients, 1.96%), and exon 21 L861Q (6 patients, 1.47%). Moreover, 18 of 409 EGFR mutation patients (4.40%), all of whom were lung adenocarcinoma patients, harbored two coexisting subtypes of EGFR. There were patients with S768I combined with other EGFR mutations, the most common being G719X (6/9), followed by S768I/L858R (2/9) and S768I/19-Del (1/9). Six patients had T790M combined with other EGFR mutations, including five cases of T790M/19-Del mutations and one case of T790M/L858R. The remaining two cases had 19-Del/L858R mutations, and one case had a G719X/L861Q mutation (Supplementary Table S1).
Relationships between EGFR mutations and clinicopathologic characteristics of patients are shown in Table 5. Overall, EGFR mutations were more frequent in women than men (69.36% vs. 32.52%, P< 0.001), more frequent in never smokers than former/current smokers (61.26% vs. 28.70%, P< 0.001), more frequent in never drinkers than drinkers (50.40% vs. 29.47%, P< 0.001), more frequent in patients with normal serum tumor markers than abnormal serum tumor makers (54.64% vs. 46.26%, P = 0.044), and more frequent in patients with early stage than advanced stage (58.16% vs. 41.44%, P< 0.001). The frequency rate of EGFR mutations was lower in patients with solid nodules than in patients with ground-glass nodules (43.60% vs. 69.23%, P< 0.001) and was higher in patients with shorter tumor long diameters than in patients with longer tumor long diameters (3.15 ± 2.23 vs. 3.84 ± 2.56, P< 0.001). Furthermore, the mutation rate of EGFR in 771 adenocarcinoma patients (n = 407, 52.79%) was higher than that in patients with other types of NSCLC (P < 0.001). There was no significant relationship between age and total EGFR mutations.
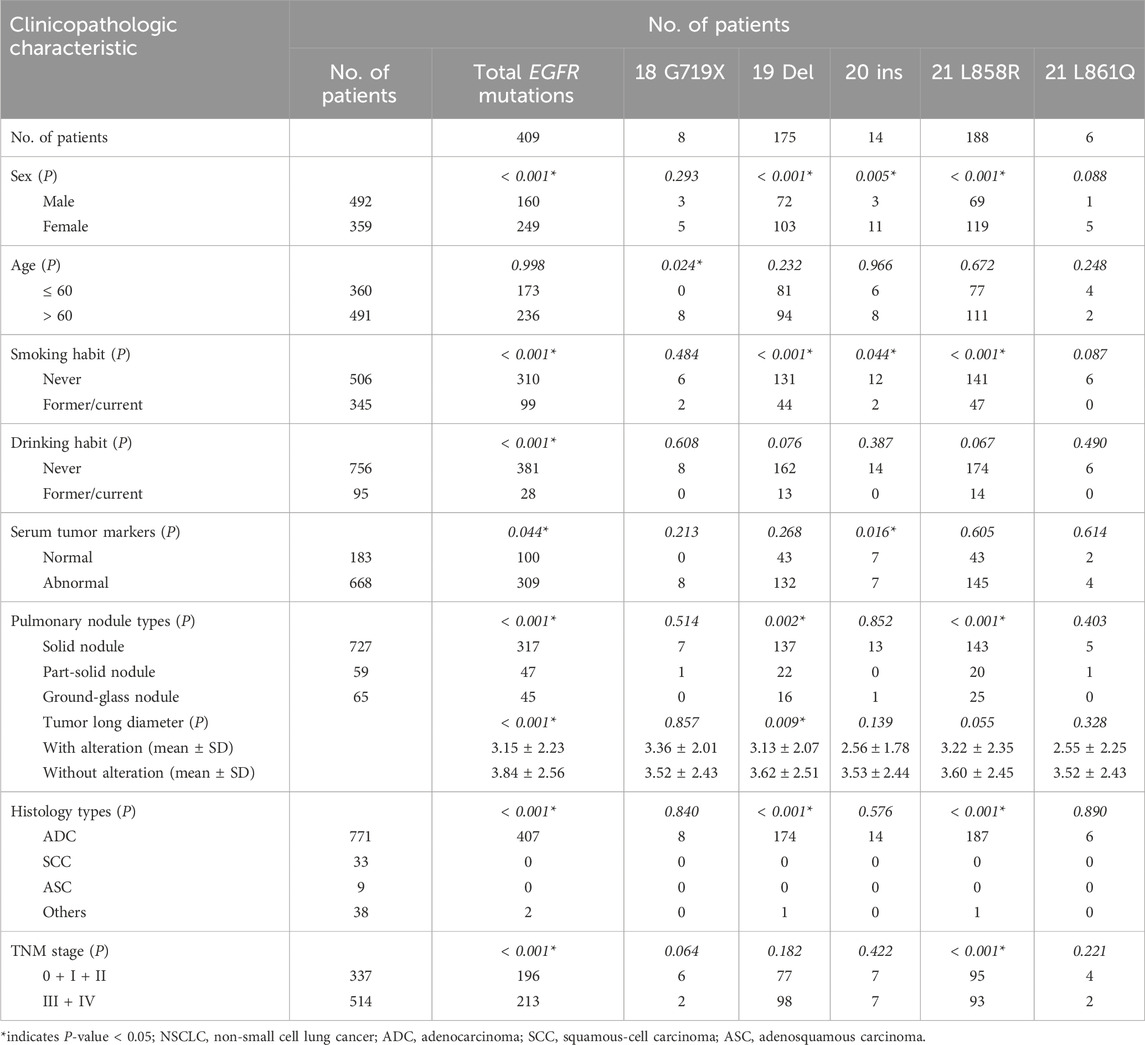
Table 5. Relationship between EGFR gene mutations and clinicopathologic characteristics in patients with NSCLC.
The incidence of EGFR 19 Del mutations, 20 ins mutations, and 21 L858R mutations was higher in women than men (28.69% vs. 14.63, P< 0.001; 3.06% vs. 0.61%, P = 0.005; 33.15% vs. 14.02%, P< 0.001; respectively) and higher in never smokers than former/current smokers (25.89% vs. 12.75%, P< 0.001; 2.37% vs. 0.58%, P = 0.044; 27.87% vs. 13.62%, P< 0.001, respectively). EGFR 18 G719X mutations were more frequent in older patients than in younger patients (1.63% vs. 0.00%, P = 0.024), while other EGFR mutation subtypes were not significantly associated with age. EGFR 20 ins mutations were more common in patients with normal serum tumor markers (3.83% vs. 1.05%, P = 0.037), and EGFR 21 L858R mutations were more frequent in patients with early stage disease (28.19% vs. 18.09%, P< 0.001). The EGFR 19 Del mutation rate was lower in patients with longer tumor long diameters than patients with shorter tumor long diameters (P = 0.009). The frequency rate of EGFR 19 Del mutations was lower in patients with solid nodules than in patients with partial solid nodules (18.84% vs. 37.29%, P = 0.002), while the mutation rate of 21 L858R was higher in patients with solid nodules than in patients with partial solid nodules or ground-glass nodules (45.11% vs. 33.90% and 45.11% vs. 38.46%, P< 0.001). Moreover, the frequency of EGFR 19-Del mutations (22.54%) and EGFR 21 L858R mutations (24.25%) in patients with adenocarcinoma was higher than in patients with other types of NSCLC (all P< 0.001) (Table 5).
Multivariate logistic regression analysis was performed on the gender, age, smoking, and drinking history, histopathologic types, serum tumor markers, tumor long diameter, nodule types, and TNM stage of NSLCL patients, and the results showed that EGFR mutations were correlated with gender (odds ratio [OR] = 0.359, 95% confidence interval [CI]: 0.237–0.543; P< 0.001), smoking history (OR = 1.714, 95% CI: 1.126–2.607; P = 0.012), histopathological type (OR = 0.064, 95% CI: 0.015–0.274; P< 0.001), and nodule types (OR = 1.974, 95% CI: 1.096–3.556, P = 0.023). Meanwhile, EGFR mutations were not correlated with age, drinking history, serum tumor markers, tumor long diameter, or TNM stage (P> 0.05 for all).
Next, we aimed to investigate associations between EGFR mutation status and the histopathology classification. In the histology types, EGFR mutations were most prevalent in ADC (52.79%), while there were no EGFR mutations in patients with SCC and ASC (Figure 2A). Among the 771 cases of ADC, the EGFR mutation rates of AIS, IMA, IA, and IMA were 40.00%, 48.49%, 53.50%, and 0.00%, respectively (Figure 2B). Of those 249 IA patients, EGFR mutations were most common in papillary-predominant (83.87%) and lepidic-predominant adenocarcinoma (83.78%), followed by acinar-predominant (66.88%) and solid-predominant carcinoma (0.00%) (Figure 2C).
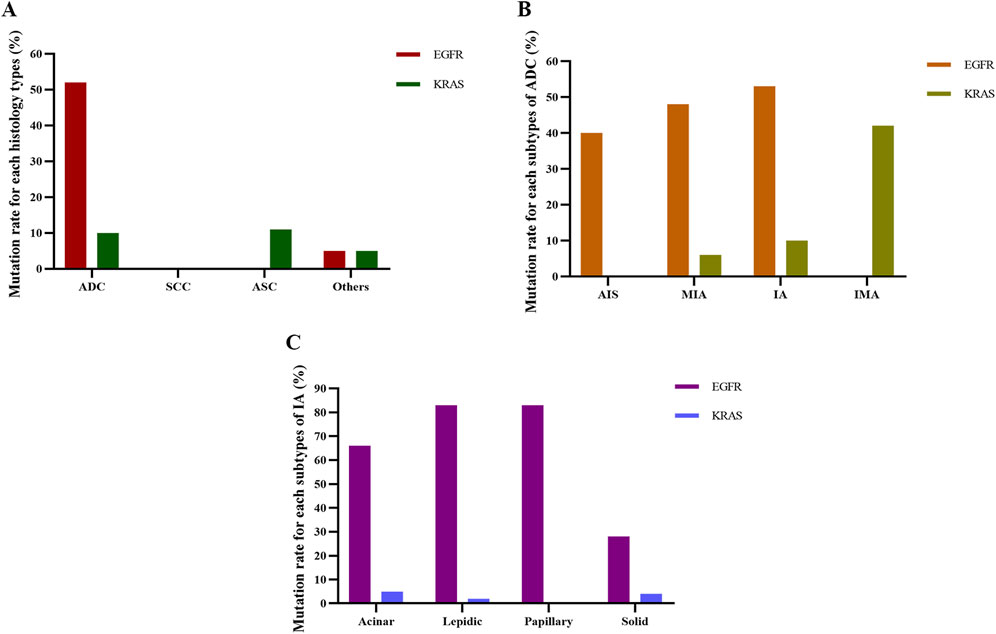
Figure 2. Detection of EGFR and KRAS mutation rates for each histological subtype. (A) Mutation rate for each histology type. (B) Mutation rate for each subtype of ADC. (C) Mutation rate for each subtype of IA. ADC, adenocarcinoma; SCC, squamous cell carcinoma; ASC, adenosquamous carcinoma; AIS, adenocarcinoma in situ; MIA, micro-invasive adenocarcinoma; IA, invasive non-mucinous adenocarcinoma; IMA, invasive mucinous adenocarcinoma.
Three models of logistic regression were used (as shown in statistical analysis) to further analyze the relationship between histopathology and EGFR mutations. The results are shown in Table 6. ADC was associated with an increased probability of EGFR mutations in Model 1, Model 2, and Model 3, and the ORs were 43.607, 36.824, and 32.456, respectively (P< 0.001 for all). Among subtypes of ADC, the frequency of EGFR mutations was positively correlated with IA and negatively correlated with MIA in Model 2 and Model 3. Meanwhile, in Model 1, there was no correlation between EGFR and IA or MIA. In the IA subtypes, EGFR mutations were negatively associated with solid-predominant adenocarcinoma in Model 1, Model 2, and Model 3 (P< 0.001, P< 0.001, P = 0.004, respectively) and positively associated with lepidic-predominant adenocarcinoma in Model 1 and Model 2 (P = 0.033, P = 0.023, respectively). However, in Model 3, EGFR mutations were not significantly associated with lepidic-predominant adenocarcinoma. According to the results of logistic regression analysis, EGFR mutations were positively associated with women, non-smokers, ADC, and IA and negatively correlated with solid nodule, MIA, and solid-predominant adenocarcinoma after adjusting for gender, age, smoking history, drinking status, serum tumor markers, pulmonary nodule types, tumor long diameter, and TNM stage.
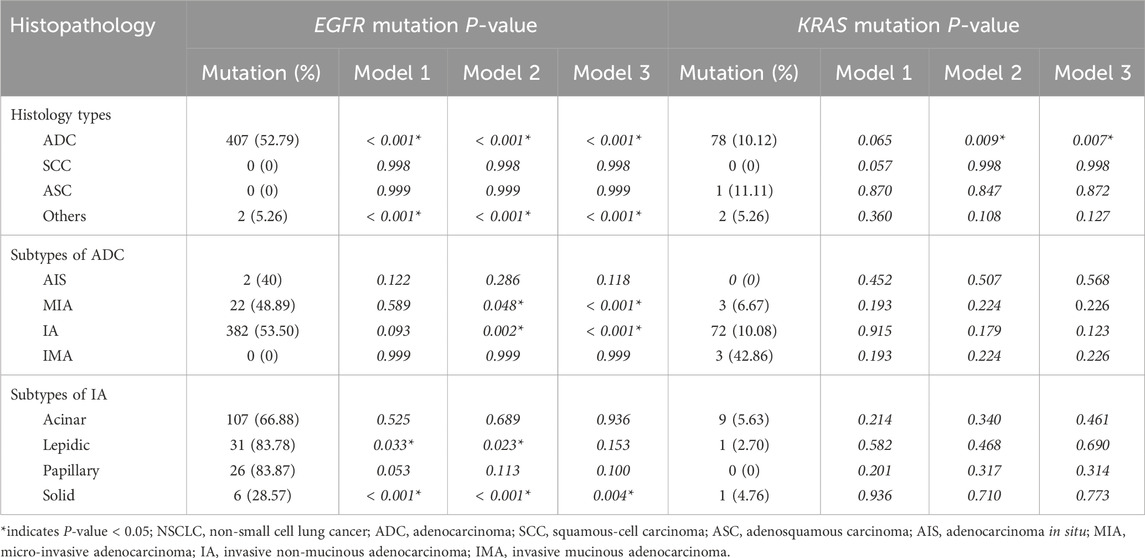
Table 6. Univariate and multivariate regression analysis for the correlation of EGFR and KRAS mutations with histopathologic subtypes of NSCLC.
Among 81 patients with KRAS mutations, the distribution of the mutations was 66 cases in KRAS exon 2 G12C, G12R, G12V, G12A, G13C (81.48%) and 15 cases in KRAS exon 2 G12S, G12D (18.52%). As Table 7 shows, the mutation rate of KRAS was higher in men than women (16.06% vs. 0.56%, P< 0.001), higher in older patients than younger patients (11.41% vs. 6.94%, P = 0.028), higher in current or former smokers than non-smokers (16.81% vs. 4.55%, P< 0.001), higher in abnormal serum tumor markers than normal serum tumor markers (10.78% vs. 4.92%, P = 0.017), and higher in advanced stage than early stage (12.45% vs. 5.04%, P< 0.001). KRAS mutations were more common in patients with longer tumor long diameters than in patients with shorter tumor long diameters (P = 0.006). No significant correlation was observed in total KRAS mutations with drinking habits and histology types.
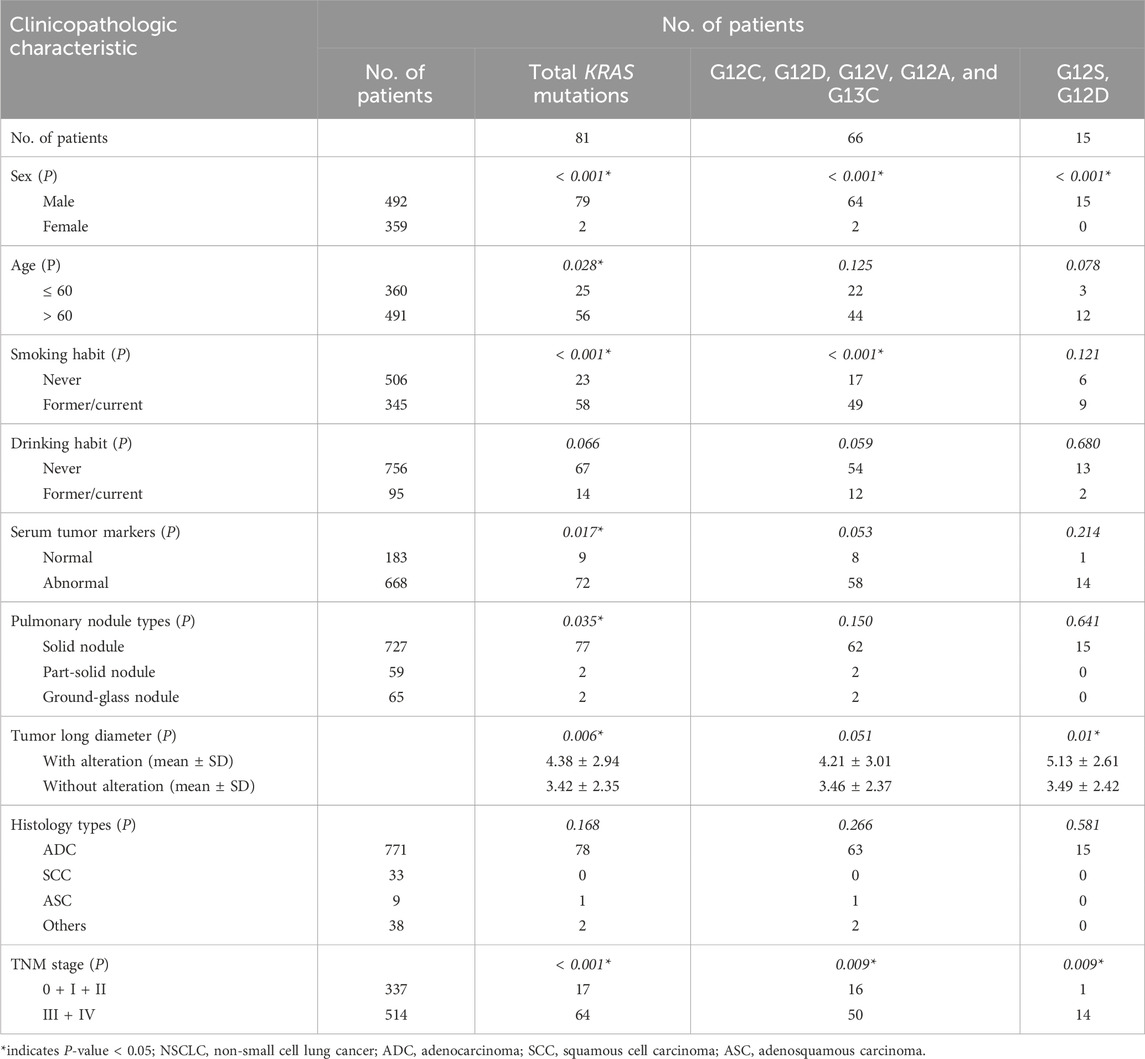
Table 7. Relationship between KRAS gene mutations and clinicopathologic characteristics in patients with NSCLC.
Both KRAS exon 2 G12C, G12R, G12V, G12A, G13C mutations and G12S, G12D mutations were more common in men than women (13.01% vs. 0.56%, 3.05% vs. 0.00%, both P< 0.001), and more in advanced stage than early stage (9.73% vs. 4.75%, 2.72% vs. 0.30%, both P = 0.009). Current or former smokers had a higher KRAS 2 G12C, G12V, G12V, G12A, G12A, G13C mutation rate than non-smokers (14.20% vs. 3.36%, P< 0.001). However, there was no significant association between G12S, G12D mutations and smoking history. In addition, KRAS exon 2 G12S, G12D mutations were significantly associated with longer tumor long diameter (P = 0.01), while there was no correlation between G12C, G12R, G12V, G12A, G13C mutations and tumor long diameter. No significant associations were observed between KRAS exon 2 G12C, G12R, G12V, G12A, G13C mutations and G12S, G12D mutations and age, drinking habit, serum tumor markers, pulmonary nodule types, histology types, or subtypes of invasive adenocarcinoma (Table 7).
Multivariate logistic regression analysis was performed on the gender, age, smoking, and drinking history, histopathologic types, serum tumor markers, tumor long diameter, nodule types, and TNM stage of NSLCL patients, and the results showed that KRAS mutations were correlated with gender (OR = 0.029, 95% CI: 0.007–0.117; P< 0.001) and tumor long diameter (OR = 1.120, 95% CI: 1.025–1.223; P = 0.012). Meanwhile, KRAS mutations were not correlated with age, smoking status, drinking history, serum tumor markers, histological types, nodule types, or TNM stage.
In the histology types, KRAS mutations were most common in ASC (11.11%), followed by ADC (10.12%), other types (5.26%), and SCC (0.00%) (Figure 2A). Among ADC patients, the frequency of KRAS mutation in AIS, IMA, IA, and IMA was 0.00%, 6.67%, 10.08%, and 42.86%, respectively (Figure 2B). Of the IA patients, KRAS mutations were most frequent in acinar-predominant (5.63%), followed by solid-predominant adenocarcinoma (4.76%), lepidic-predominant (2.70%), and papillary-predominant carcinoma (0.00%) (Figure 2C). As with the EGFR analysis, we used Model 1, Model 2, and Model 3 to further analyze the relationship between histopathology and KRAS mutations. The results showed that ADC was significantly associated with a reduced probability of KRAS mutations in Model 2 and Model 3 (P = 0.009 and P = 0.007, respectively) (Table 6). According to the results of logistic regression analysis, KRAS mutations were positively associated with men and larger tumor long diameter and negatively associated with lung adenocarcinoma after adjusting for gender, age, smoking history, drinking status, serum tumor markers, pulmonary nodule types, tumor long diameter, and TNM stage.
As shown in Table 8, the frequency of ALK rearrangements, HER2 mutations, and RET fusions was significantly higher in women than in men (8.08% vs. 3.46%, P = 0.003; 4.18% vs. 1.02%, P = 0.003; 3.34% vs. 1.22%, P = 0.034; respectively). In older patients, there was a significantly higher mutation rate of MET exon 14 skipping mutations (3.67% vs. 0.28%, P< 0.001) and a lower frequency of ALK rearrangements and HER2 mutations (2.85% vs. 8.89%, P< 0.001; 1.22% vs. 3.89%, P = 0.011; respectively). ALK rearrangements and HER2 mutations were more common in non-smokers than current or former smokers (7.31% vs. 2.61%, P = 0.003; 3.56% vs. 0.58%, P = 0.005; respectively). ALK rearrangements were more frequent in non-drinkers (5.95% vs. 1.05%, P = 0.047) and patients with advanced stages (6.81% vs. 3.26%, P = 0.025), and RET fusions were more common in patients with normal tumor markers (4.92% vs. 1.35%, P = 0.007). HER2 mutations were significantly positively associated with shorter tumor long diameters (P = 0.034). MET exon 14 skipping mutations were more common in patients with ASC (1/33, P = 0.018), while PIK3CA mutations were more frequent in patients with SCC (1/33, P = 0.049). No significant associations of these eight target gene alterations were observed with subtypes of invasive adenocarcinoma or nodule types, other than the mutation rate of ALK fusions was higher in solid-predominant adenocarcinoma patients than that of acinar or lepidic-predominant adenocarcinoma patients (23.81% vs. 3.13%, 23.81% vs. 0.00%, P = 0.002).
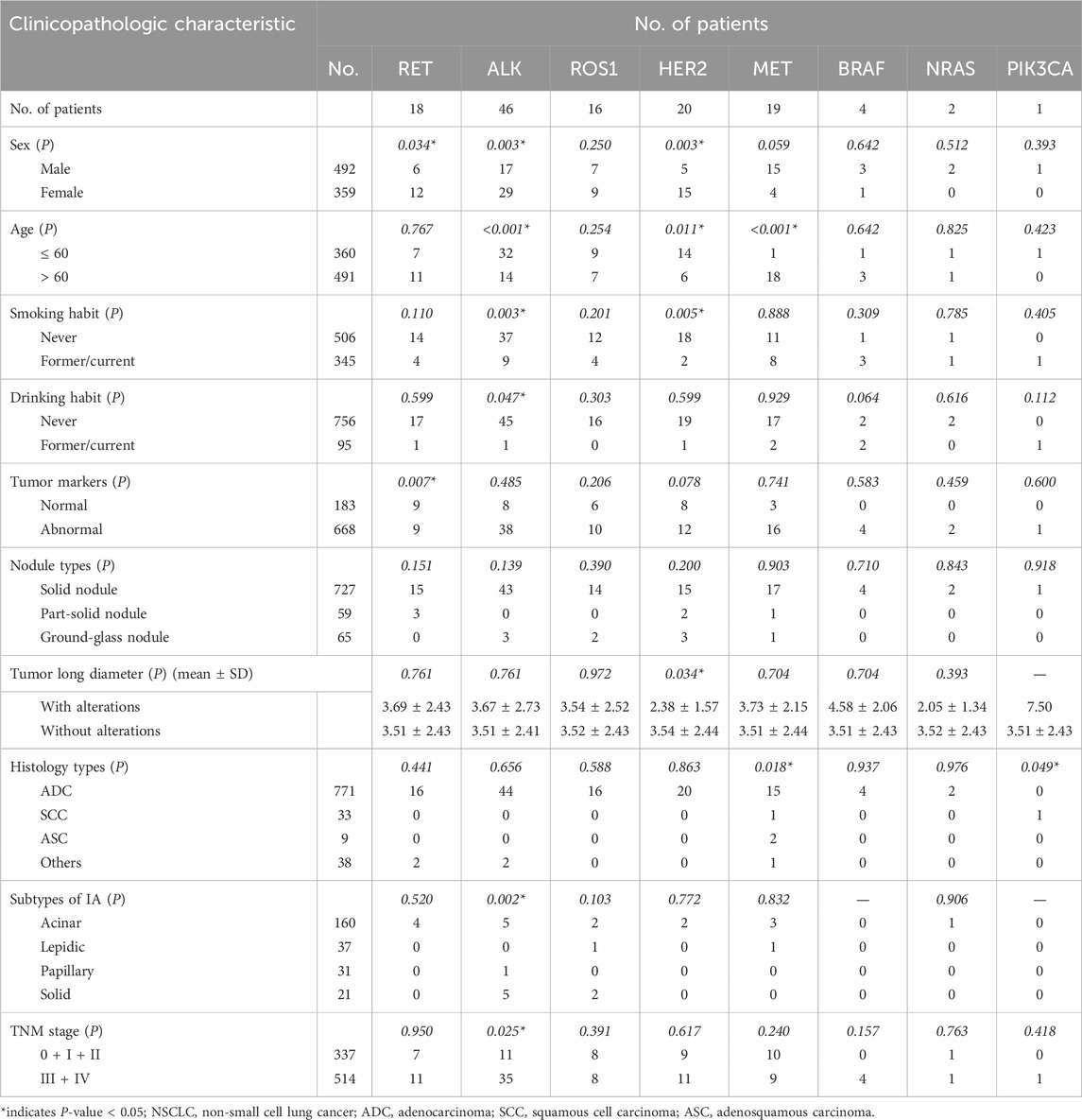
Table 8. Relationship between eight other targeted gene alterations and clinicopathologic characteristics in patients with NSCLC.
In this study, we assayed and investigated the status of EGFR, KRAS, MET, ALK, ROS1, HER2, RET, BRAF, NRAS, and PIK3CA somatic driver gene alterations derived from 851 Chinese NSCLC patients. The intent was to analyze the 10 targeted gene alterations status and their correlation with clinicopathological characteristics of NSCLC patients. We found that most NSCLC patients have at least one gene mutation, with the first two highest aberrant target oncogenes being EGFR (48.06%) and KRAS (9.51%). In patients with adenocarcinoma, the EGFR and KRAS mutation rates were 52.79% and 10.12%, respectively. Among women, 69.36% had EGFR mutations, and only 0.56% had KRAS mutations. Meanwhile, 32.52% of men had EGFR mutations, and 16.06% had KRAS mutations. EGFR and KRAS mutations were present in 61.26% and 4.55% of non-smokers, while in former/current smokers, the mutation rates were 28.70% and 16.81%, respectively. Furthermore, through three logistics regression models, we found that the probability of EGFR mutations was positively correlated with women, non-smokers, lung adenocarcinoma, and IA subtype of adenocarcinoma, while EGFR mutations were negatively correlated with solid nodule, the MIA subtype of adenocarcinoma, and the solid-predominant adenocarcinoma subtype of IA. KRAS mutations were positively associated with men and longer tumor long diameter and negatively associated with lung adenocarcinoma.
Some previous studies have analyzed the association of driver gene alterations with clinicopathologic features in patients with NSCLC (Chen et al., 2022). The most common mutation in NSCLC is an EGFR mutation, and there are significant regional differences in EGFR mutations in lung cancer patients. The prevalence of EGFR mutations in Asian and European NSCLC patients was 49.1% and 12.8%, respectively (Melosky et al., 2022). The most frequently aberrant target oncogene in our study was EGFR (48.06%), which was similar to that in East Asian patients (Liu et al., 2017; Melosky et al., 2022). However, it was lower than another study from China, which had an EGFR mutation rate of 57.7% (Li et al., 2020). This may be because the patients enrolled by Li et al. were mainly in the early stage, with TNM stage I and II patients accounting for 64.3%, while only 39.6% of the patients in our study were in the early stages of the disease. In this study, overall EGFR mutations and subtypes other than exon 18 G719 X and exon 21 L861Q mutations were more frequent in women, non-smokers, and patients with adenocarcinoma, which is consistent with previous findings (Melosky et al., 2022; Li et al., 2020; Gao et al., 2020). Some studies found no association between smoking status and EGFR mutations (Liu et al., 2017; Ess et al., 2017). This reminds us that the smoking index should be recorded clearly in future studies. Like most studies, we found that EGFR mutation and its subtypes were not associated with age, except for subtype exon 18 G719X (Liu and Xiong, 2021). This may be due to the absence of patients younger than 60 years in the eight patients with exon 18 G719X mutation. Similar to the results of Hong et al. (2016), we also found a lower mutation rate of EGFR in solid nodules than in part-solid nodules or ground-glass nodules, while the result of Zhao et al. was contrary (Zhao et al., 2018). This may be because Zhao classifies CT nodule types into two categories: ground-glass opacity (GGO)/ground-glass nodule (GGN) and no GGO/GGN, while we classified them into solid nodule, partial solid nodule, and ground-glass nodule.
Many studies have indicated that the EGFR mutation rate was higher in lung adenocarcinoma patients, but different studies had different methods for further pathologic classification of adenocarcinoma (Melosky et al., 2022). In this study, we used three logistic regression models to explore the association between histopathologic typing and NSCLC driver genes according to the latest WHO histologic classification (5th). In all three logistic regression models, we found EGFR was positively associated with ADC and subtype IA in ADC and negatively correlated with subtype MIA in ADC and subtype solid-predominant in IA. Among subtypes of IA, EGFR mutations were more common in the papillary, lepidic, and papillary-predominant subtypes, although no statistical association was found in the full adjusted logistic regression model.
The point mutations of EGFR exon 20 mainly include T790M and S768I, which are insensitive mutations (Romero, 2023; Wu et al., 2017). Previous studies have reported that most patients using EGFR-TKIs will develop resistance 9–14 months after treatment, with EGFR exon 20 T790M mutation being the most common (Rosell et al., 2012; Planchard et al., 2023). This also explains why the six patients with the T790M mutation in this study all had the EGFR-sensitive mutations, namely 19-Del (5/6) or L868R (1/6), and had a history of targeted therapy for EGFR-TKIs. The mutation rate of S768I accounts for about 1%–2% of EGFR mutations, including single mutations and combinations with other mutations (Kobayashi and Mitsudomi, 2016). In this study, nine of 851 patients developed the EGFR exon 20 S768I mutation (1.05%), all in combination with G719X, 19-Del, or L861R mutations. Studies have shown that when S768I was combined with the sensitive mutation 19-Del or L858R, the response effect of the sensitive mutation was not affected (Hao et al., 2023; Lin et al., 2023). Meanwhile, patients with S768I combined with the insensitive G719X mutation showed an enhanced response to EGFR-TKIs (Morimoto et al., 2023; Svaton et al., 2015). However, these are all small cohorts or even case reports, and studies with larger samples are needed to further explore the mechanism and clarify the therapeutic effect of S768I combined with other mutations.
Similar to the results of the current study, the KRAS mutation rate was higher in men, older patients, and current or former smokers by a chi-square test (Xu et al., 2011; Zhao et al., 2018). However, these studies did not conduct further univariate or multivariate regression analysis of the correlation. In this study, regression analysis showed that KRAS mutations had no statistical correlation with age and smoking status. Moreover, in the chi-square test and univariate regression analysis, there was no correlation between KRAS and histopathologic type. After preliminary and full adjustment, KRAS mutations were negatively correlated with the ADC subtype.
In this study, we found that ALK, RET, and ROS1 fusions accounted for 5.41%, 2.12%, and 1.88% of the total driver gene alterations, respectively. Their fusion rate in lung adenocarcinoma patients was 5.71%, 2.08%, and 2.59%, respectively, which was similar to previous studies (Gou and Wu, 2014; Li et al., 2021). ALK translocations were significantly more common in women, younger patients, non-smokers, non-drinkers, and patients in the advanced stages. Moreover, in the subtypes of invasive non-mucinous adenocarcinoma, the frequency of ALK translocations was significantly higher in patients with solid-predominant adenocarcinoma than in the other three subtypes of IA. RET fusions were significantly more frequent in women and patients with normal serum tumor markers. We found no significant relationship between clinicopathological features and ROS1 fusions. HER-2 mutations were more common in women, younger patients, non-smokers, and patients with a smaller tumor long diameter. MET exon 14 skipping mutations were more frequent in older patients and ASC.
There are some limitations to our study. First, this study is a single-center retrospective study, which can prove correlation but not causation. Future prospective cohort studies are needed to explore causal associations. Second, due to the brevity of the follow-up time, patient prognosis was not tracked in this study. Future studies are needed to analyze the relationship between gene alterations and patient clinical outcomes.
Overall, our work demonstrated that the probability of EGFR mutations was positively correlated with women, non-smokers, lung adenocarcinoma, and the IA subtype of adenocarcinoma, while EGFR mutations were negatively correlated with solid nodule, the MIA subtype of adenocarcinoma, and the solid-predominant adenocarcinoma subtype of IA. KRAS mutations were positively associated with men and longer tumor long diameter and negatively associated with lung adenocarcinoma. Specifically, our work may help thoracic surgeons, respirologists, and oncologists use the information obtained in this study to guide targeted treatments.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of the Sixth Affiliated Hospital of Guangzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin due to the retrospective nature of the study.
LD: data curation, formal analysis, funding acquisition, and writing–original draft. JL: methodology and writing–review and editing. ZQ: methodology and writing–review and editing. YW: conceptualization, investigation, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant from the Sixth Affiliated Hospital of Guangzhou Medical University, Qingyuan People’s Hospital (grant number 2022-11217).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1455502/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Distribution and proportion of gene alterations in 851 patients. (A) Distribution of gene alterations in 851 patients with NSCLC. (B) Proportions of 10 target gene alterations in 851 patients with NSCLC. NSCLC, non-small cell lung cancer.
Arbour, K. C., and Riely, G. J. (2019). Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. Jama 322 (8), 764–774. doi:10.1001/jama.2019.11058
Chen, P., Liu, Y., Wen, Y., and Zhou, C. (2022). Non-small cell lung cancer in China. Cancer Commun. (Lond) 42 (10), 937–970. doi:10.1002/cac2.12359
Di Maio, M. (2023). Osimertinib in resected EGFR-mutated NSCLC. N. Engl. J. Med. 389 (14), 1341–1342. doi:10.1056/NEJMc2309385
Eberhardt, W. E., De Ruysscher, D., Weder, W., Le Péchoux, C., De Leyn, P., Hoffmann, H., et al. (2015). 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann. Oncol. 26 (8), 1573–1588. doi:10.1093/annonc/mdv187
Ess, S. M., Herrmann, C., Frick, H., Krapf, M., Cerny, T., Jochum, W., et al. (2017). Epidermal growth factor receptor and anaplastic lymphoma kinase testing and mutation prevalence in patients with advanced non-small cell lung cancer in Switzerland: a comprehensive evaluation of real world practices. Eur. J. Cancer Care (Engl) 26 (6), e12721. doi:10.1111/ecc.12721
Ettinger, D. S., Wood, D. E., Aisner, D. L., Akerley, W., Bauman, J. R., Bharat, A., et al. (2022). Non-small cell lung cancer, version 3.2022, NCCN clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 20 (5), 497–530. doi:10.6004/jnccn.2022.0025
Gao, X. C., Wei, C. H., Zhang, R. G., Cai, Q., He, Y., Tong, F., et al. (2020). (18)F-FDG PET/CT SUV(max) and serum CEA levels as predictors for EGFR mutation state in Chinese patients with non-small cell lung cancer. Oncol. Lett. 20 (4), 61. doi:10.3892/ol.2020.11922
Gou, L. Y., and Wu, Y. L. (2014). Prevalence of driver mutations in non-small-cell lung cancers in the People's Republic of China. Lung Cancer (Auckl) 5, 1–9. doi:10.2147/lctt.S40817
Hao, Y., Xu, M., Jin, J., Si, J., Xu, C., and Song, Z. (2023). Comparison of efficacy and safety of second- and third-generation TKIs for non-small-cell lung cancer with uncommon EGFR mutations. Cancer Med. 12 (15), 15903–15911. doi:10.1002/cam4.6229
Hong, S. J., Kim, T. J., Choi, Y. W., Park, J. S., Chung, J. H., and Lee, K. W. (2016). Radiogenomic correlation in lung adenocarcinoma with epidermal growth factor receptor mutations: imaging features and histological subtypes. Eur. Radiol. 26 (10), 3660–3668. doi:10.1007/s00330-015-4196-z
Kobayashi, Y., and Mitsudomi, T. (2016). Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci. 107 (9), 1179–1186. doi:10.1111/cas.12996
Lancet, T. (2024). Lung cancer treatment: 20 years of progress. Lancet 403 (10445), 2663. doi:10.1016/s0140-6736(24)01299-6
Li, D., Ding, L., Ran, W., Huang, Y., Li, G., Wang, C., et al. (2020). Status of 10 targeted genes of non-small cell lung cancer in eastern China: a study of 884 patients based on NGS in a single institution. Thorac. Cancer 11 (9), 2580–2589. doi:10.1111/1759-7714.13577
Li, W., Guo, L., Liu, Y., Dong, L., Yang, L., Chen, L., et al. (2021). Potential unreliability of uncommon ALK, ROS1, and RET genomic breakpoints in predicting the efficacy of targeted therapy in NSCLC. J. Thorac. Oncol. 16 (3), 404–418. doi:10.1016/j.jtho.2020.10.156
Lin, Y., Chen, L., Li, R., Liu, X., Li, Q., Cai, J., et al. (2023). Survival analysis of patients with advanced non-small cell lung cancer receiving EGFR-TKI treatment of Yunnan in southwestern China: a real-world study. Front. Oncol. 13, 1156647. doi:10.3389/fonc.2023.1156647
Liu, L., Liu, J., Shao, D., Deng, Q., Tang, H., Liu, Z., et al. (2017). Comprehensive genomic profiling of lung cancer using a validated panel to explore therapeutic targets in East Asian patients. Cancer Sci. 108 (12), 2487–2494. doi:10.1111/cas.13410
Liu, L., and Xiong, X. (2021). Clinicopathologic features and molecular biomarkers as predictors of epidermal growth factor receptor gene mutation in non-small cell lung cancer patients. Curr. Oncol. 29 (1), 77–93. doi:10.3390/curroncol29010007
Melosky, B., Kambartel, K., Häntschel, M., Bennetts, M., Nickens, D. J., Brinkmann, J., et al. (2022). Worldwide prevalence of epidermal growth factor receptor mutations in non-small cell lung cancer: a meta-analysis. Mol. Diagn Ther. 26 (1), 7–18. doi:10.1007/s40291-021-00563-1
Morimoto, T., Yamasaki, K., Shingu, T., Higashi, Y., Maeda, Y., Uryu, T., et al. (2023). A rare case of double primary lung adenocarcinomas with uncommon complex EGFR G719X and S768I mutations and pleomorphic carcinoma. Thorac. Cancer 14 (29), 2981–2984. doi:10.1111/1759-7714.15085
Planchard, D., Jänne, P. A., Cheng, Y., Yang, J. C., Yanagitani, N., Kim, S. W., et al. (2023). Osimertinib with or without chemotherapy in EGFR-mutated advanced NSCLC. N. Engl. J. Med. 389 (21), 1935–1948. doi:10.1056/NEJMoa2306434
Ramalingam, S. S., Vansteenkiste, J., Planchard, D., Cho, B. C., Gray, J. E., Ohe, Y., et al. (2020). Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382 (1), 41–50. doi:10.1056/NEJMoa1913662
Rami-Porta, R., Bolejack, V., Crowley, J., Ball, D., Kim, J., Lyons, G., et al. (2015). The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J. Thorac. Oncol. 10 (7), 990–1003. doi:10.1097/jto.0000000000000559
Romero, D. (2023). Patients with uncommon EGFR mutations also benefit from first-line osimertinib. Nat. Rev. Clin. Oncol. 21, 84. doi:10.1038/s41571-023-00847-x
Rosell, R., Carcereny, E., Gervais, R., Vergnenegre, A., Massuti, B., Felip, E., et al. (2012). Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13 (3), 239–246. doi:10.1016/s1470-2045(11)70393-x
Svaton, M., Pesek, M., Chudacek, Z., and Vosmiková, H. (2015). Current two EGFR mutations in lung adenocarcinoma - case report. Klin. Onkol. 28 (2), 134–137. doi:10.14735/amko2015134
Tan, L., Brown, C., Mersiades, A., Lee, C. K., John, T., Kao, S., et al. (2024). A Phase II trial of alternating osimertinib and gefitinib therapy in advanced EGFR-T790M positive non-small cell lung cancer: OSCILLATE. Nat. Commun. 15 (1), 1823. doi:10.1038/s41467-024-46008-1
Tumours, T. (2021). WHO classification of Tumours editorial board. WHO classification of tumours. 5th edn. Lyon: IARC Press.
Vallee, A., Sagan, C., Le Loupp, A. G., Bach, K., Dejoie, T., and Denis, M. G. (2013). Detection of EGFR gene mutations in non-small cell lung cancer: lessons from a single-institution routine analysis of 1,403 tumor samples. Int. J. Oncol. 43 (4), 1045–1051. doi:10.3892/ijo.2013.2056
Wang, S., Ma, P., Ma, G., Lv, Z., Wu, F., Guo, M., et al. (2020). Value of serum tumor markers for predicting EGFR mutations and positive ALK expression in 1089 Chinese non-small-cell lung cancer patients: a retrospective analysis. Eur. J. Cancer 124, 1–14. doi:10.1016/j.ejca.2019.10.005
Wu, T. H., Hsiue, E. H., Lee, J. H., Lin, C. C., and Yang, J. C. (2017). New data on clinical decisions in NSCLC patients with uncommon EGFR mutations. Expert Rev. Respir. Med. 11 (1), 51–55. doi:10.1080/17476348.2017.1267569
Wu, Y. L., Dziadziuszko, R., Ahn, J. S., Barlesi, F., Nishio, M., Lee, D. H., et al. (2024). Alectinib in resected ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 390 (14), 1265–1276. doi:10.1056/NEJMoa2310532
Wu, Z., Dai, Y., and Chen, L. A. (2019). The prediction of epidermal growth factor receptor mutation and prognosis of EGFR tyrosine kinase inhibitor by serum ferritin in advanced NSCLC. Cancer Manag. Res. 11, 8835–8843. doi:10.2147/cmar.S216037
Xu, J., He, J., Yang, H., Luo, X., Liang, Z., Chen, J., et al. (2011). Somatic mutation analysis of EGFR, KRAS, BRAF and PIK3CA in 861 patients with non-small cell lung cancer. Cancer Biomark. 10 (2), 63–69. doi:10.3233/cbm-2012-0233
Yang, S. R., Schultheis, A. M., Yu, H., Mandelker, D., Ladanyi, M., and Büttner, R. (2022). Precision medicine in non-small cell lung cancer: current applications and future directions. Semin. Cancer Biol. 84, 184–198. doi:10.1016/j.semcancer.2020.07.009
Yang, Z. M., Ding, X. P., Pen, L., Mei, L., and Liu, T. (2014). Analysis of CEA expression and EGFR mutation status in non-small cell lung cancers. Asian Pac J. Cancer Prev. 15 (8), 3451–3455. doi:10.7314/apjcp.2014.15.8.3451
Keywords: EGFR, genomic alterations, non-small cell lung cancer, targeted therapy, KRAS
Citation: Deng L, Li J, Qiu Z and Wang Y (2024) Driver gene alterations in NSCLC patients in southern China and their correlation with clinicopathologic characteristics. Front. Genet. 15:1455502. doi: 10.3389/fgene.2024.1455502
Received: 27 June 2024; Accepted: 21 August 2024;
Published: 19 September 2024.
Edited by:
Triantafillos Liloglou, University of Liverpool, United KingdomReviewed by:
Carlos Gil Ferreira, Instituto Oncoclínicas, BrazilCopyright © 2024 Deng, Li, Qiu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanfen Wang, MjAyMjY5NTA0M0BnemhtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.