- 1Department of Obstetrics and Gynecology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, China
- 3Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
Background: Research links arthropathies with adverse pregnancy outcomes. This study aims to explore its connection to postpartum hemorrhage (PPH) through Mendelian randomization (MR) analysis.
Methods: The study used GWAS data from the IEU OpenGWAS database for PPH and arthropathies. After selecting instrumental variables, bidirectional MR analysis was conducted using MR-Egger, Weighted median, Simple mode, Weighted mode, and IVW methods. Sensitivity analysis was then performed to assess MR results reliability. Finally, enrichment analysis of genes corresponding to arthropathies SNPs in forward MR was conducted to explore their biological function and signaling pathways.
Results: The forward MR results revealed that arthropathies was causally related to PPH, and arthropathies was a risk factor for PPH. Whereas, there was not a causal relationship between PPH and arthropathies by reverse MR analysis. It illustrated the reliability of the MR analysis results by the sensitivity analysis without heterogeneity, horizontal pleiotropy, and SNPs of severe bias by LOO analysis. Furthermore, a total of 33 genes corresponding to SNPs of arthropathies were obtained, which were mainly enriched in regulation of response to biotic stimulus, spliceosomal snRNP complex and ligase activity in GO terms, and natural killer cell-mediated cytotoxicity in KEGG pathways.
Conclusion: This study supported that arthropathies was a risk factor for PPH, and the pathways involved the genes corresponding to SNPs were analyzed, which could provide important reference and evidence for further exploring the molecular mechanism between arthropathies and PPH.
1 Introduction
Postpartum hemorrhage (PPH) remains a significant obstetric complication and a major risk during childbirth. Despite notable improvements in healthcare, which have reduced the maternal mortality rate in China to 3.0 per 1,00,000 live births by 2019 due to obstetric hemorrhage, PPH continues to be a leading cause of maternal death. Globally, it is responsible for about 27.1% of all maternal deaths, underscoring the need for ongoing focus on effective management and preventive strategies (Chen et al., 2021; Say et al., 2014). The challenges in managing PPH stem from various factors, including inadequate preventive strategies, limited early detection capabilities, and delayed intervention, underscoring the need for heightened preparedness against this life-threatening condition (Ende et al., 2021).
Arthropathies refer to a broad category of diseases that affect the joints. This term encompasses a wide range of joint disorders, from mild to severe, and includes conditions with different underlying causes, such as inflammatory, degenerative, infectious, and metabolic (Reinus et al., 2009). The common types of arthropathies include rheumatoid arthritis (RA), ankylosing spondylitis (AS), osteoarthritis (OA), gout, psoriatic arthritis (PsA) and arthritis of connective tissue diseases like systemic lupus erythematosus (SLE), scleroderma, and Sjögren’s syndrome. They have been linked to adverse pregnancy outcomes due to abnormal immune activation and subsequent alterations in platelet or coagulation function during pregnancy (Andreoli et al., 2023). This connection suggests a potential increase in PPH risk, further compounded by the effects of common treatments for joint diseases, such as non-steroidal anti-inflammatory drugs and immune modulators, on coagulation and platelet function (García-Fernández et al., 2021).
While there is a recognized association between joint diseases and adverse pregnancy outcomes, the specific relationship with PPH and the underlying mechanisms warrant further exploration. Traditional approaches to establishing causality, such as randomized controlled trials (RCTs), face limitations related to ethical concerns, methodological constraints, and resource demands (Smith and Ebrahim, 2002). Mendelian randomization (MR), leveraging genetic variations as instrumental variables from large-scale genome-wide association studies (GWAS), emerges as a robust alternative for causal inference, mitigating confounding biases inherent in observational studies (Yang et al., 2023; Zhong et al., 2023). Utilizing GWAS data from the IEU OpenGWAS database, this study employs bidirectional MR analysis to elucidate the genetic underpinnings of the association between arthropathies and PPH, offering novel insights into their interplay and informing future research directions (Ramessur et al., 2024).
2 Materials and methods
2.1 Data sources and summary
Genome-wide association studies (GWAS) summary data of PPH and arthropathies were downloaded from the Integrative Epidemiology Unit (IEU) OpenGWAS database. The dataset of PPH (finn-b-O15) was comprised of 3,670 cases, 98,626 controls and 16,379,289 single nucleotide polymorphisms (SNPs). The dataset finn-b-M13 for arthropathies contained 71,571 cases, 147,221 controls and 16,380,466 SNPs. Genes associated with arthropathies were selected using broad inclusion criteria to capture SNPs linked with a range of joint disorders, including autoimmune, inflammatory, and degenerative arthropathies.
2.2 Data pre-processing
The “extract_instruments” function in R package “TwoSampleMR” (Hemani et al., 2018) was employed to read exposure factors and screen instrumental variables (IVs), which were significantly correlated with exposure factors (Forward MR: P < 5 × 10−8; Reverse MR: P < 5 × 10−6). Whereafter, the IVs with linkage disequilibrium were discarded (Forward MR: clump = TRUE, r2 = 0.001, kb = 10,000; Reverse MR: clump = TRUE, r2 = 0.001, kb = 1,000). The “extract_outcome_data” function in R package “TwoSampleMR” (Hemani et al., 2018) was employed to obtain SNPs associated with exposure factors (proxies = TRUE, rsq = 0.8). Passingly, the exposure factor and outcome were arthropathies and PPH in forward MR analysis, respectively, which were swapped as outcome and exposure in reverse MR analysis.
2.3 Bidirectional Mendelian randomization (MR) analysis
The “harmonise_data” function in “TwoSampleMR” (Hemani et al., 2018) was utilized to harmonize the effect equipotential with effect size for follow-up analysis. The “mr” function was combined with five algorithms to execute bidirectional MR analysis, including MR Egger (Bowden et al., 2015), Weighted median (Bowden et al., 2016), Inverse variance weighted (IVW) (Burgess et al., 2015), Simple mode, and Weighted mode (Hartwig et al., 2017), while the result of IVW was decisive. For binary outcomes, the odds ratio (OR) and 95%CI was applied to estimate the degree of causality. In addition, in the MR analysis, we tested the effect of exposures on the outcome. Therefore, we used a p-value (P < 0.05) as the threshold for statistical significance. The results were exhibited by scatter plot, forest plot and funnel plot. Then we also performed the Heterogeneity, Horizontal pleiotropy and Leave-One-Out (LOO) analysis to determine the reliability of the MR results. Moreover, the “mr_pleiotropy_test” function was used for Horizontal pleiotropy to evaluate the presence of confounding factors in this study. For LOO analysis, the meta effect of the remaining SNPs was calculated through the “mr_leaveoneout” function after stepwise elimination of each SNP.
2.4 Functional enrichment of genes corresponding to SNPs
Based on SNPs acquired from forward MR analysis, the eQTLGen database was used to identify genes that influence gene expression via cis-eQTL. In order to explore the biological function and signal pathway of these genes corresponding to SNPs, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was carried out via R package “cluster Profiler” (P < 0.05) (Wu et al., 2021). The results of GO and KEGG enrichment were visualized by “ggpubr” and “enrichplot”, respectively.
3 Results
3.1 Forward MR analysis
3.1.1 Arthropathies was causally associated with an increased risk of PPH
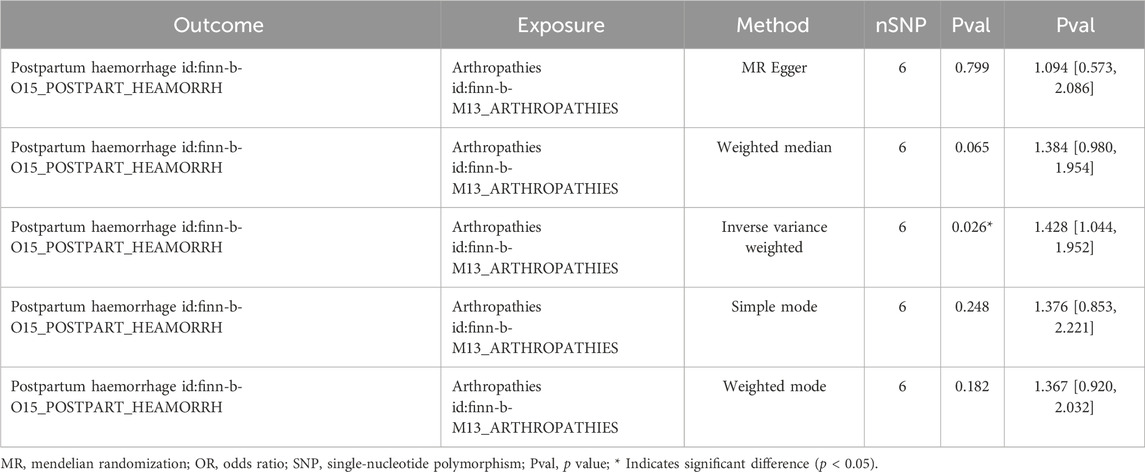
After filtrating, a total of six SNPs of arthropathies irrelevant to PPH were acquired as IVs for forward MR analysis (Supplementary Table S1). As presented in Table 1, a causal relationship between arthropathies and PPH was detected by IVW method (P = 0.026), and arthropathies was a risk factor for PPH (odds ratio (OR) = 1.428). The scatter plot also revealed that arthropathies was a risk factor for PPH (slope >0) in accordance with the previous MR results (Figure 1A). Obviously, the MR effect size in forest plot exceeded 0, further reflecting that the arthropathies was a risk factor for PPH (Figure 1B). The randomness judgement showed that the forward MR analysis was consistent with Mendel’s second law random grouping (Figure 1C).
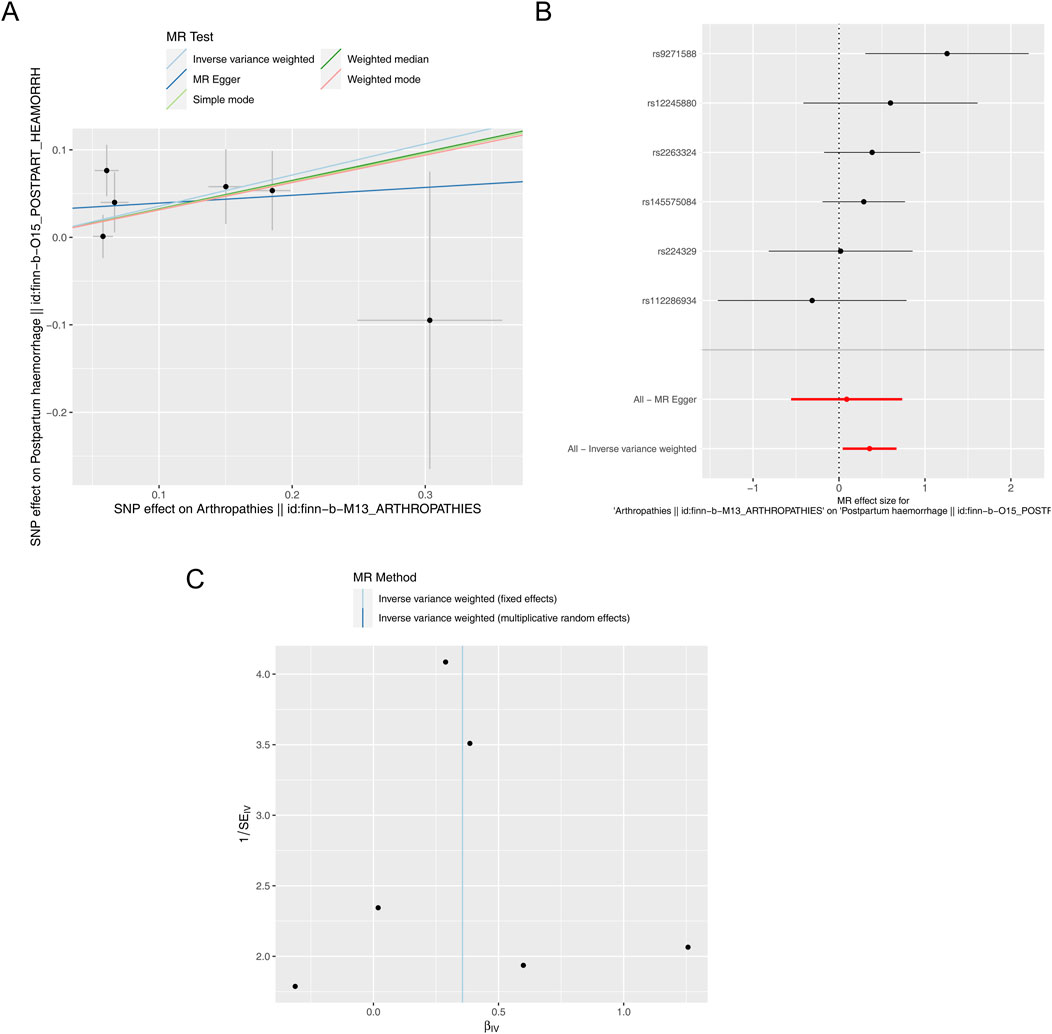
Figure 1. Forward MR analysis. (A) Scatter plot of causal relationship between arthropathies and PPH. A regular slope of a line indicates a risk factor, while a negative slope of a line indicates a safety factor. (B) Forest plot of causal relationship between arthropathies and PPH. (C) Funnel plot of causal relationship between arthropathies and PPH.
3.1.2 Reliability of the forward MR results was illustrated by sensitivity analysis
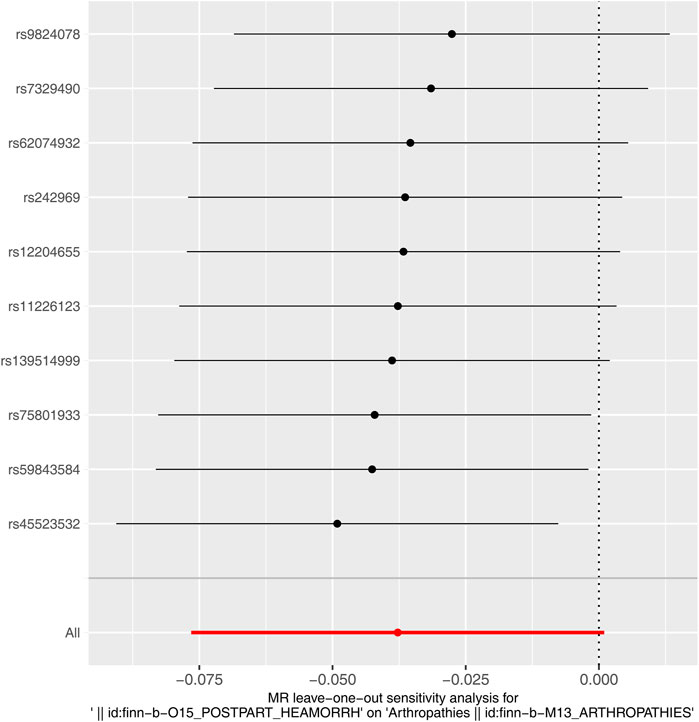
Immediately following the forward MR analysis, the sensitivity analysis was put into effect to evaluate the reliability of forward MR results. The Q_pval values of Heterogeneity test were greater than 0.05 based on MR Egger and IVW methods, suggesting that there was no heterogeneity (Supplementary Table S2). Meanwhile, there was no horizontal pleiotropy (P = 0.406), meaning there were no confounding factors (Supplementary Table S3). By eliminating a single SNP, there was no exaggerated influence on the model effect by LOO method (Figure 2). In conclusion, arthropathies was a risk factor for PPH occurrence with the proven reliability.
3.2 Reverse MR analysis
3.2.1 PPH was not causally related to arthropathies
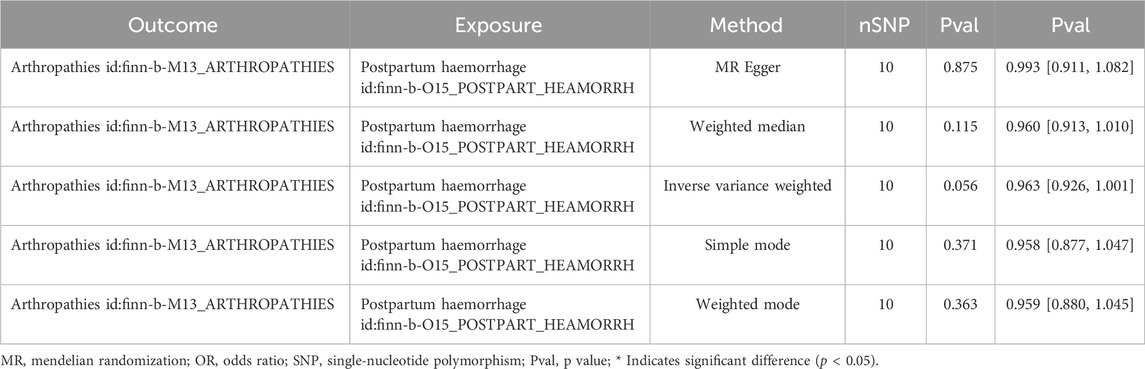
In addition, a total of 10 SNPs of PPH were acquired as IVs after filtrating for reverse MR analysis (Supplementary Table S4). The reverse MR results were presented in Table 2. The P values of five methods were greater than 0.05, meaning that PPH was not causally related to arthropathies. The scatter plot also supported this result (Figure 3A). The forest plot was created to evaluate the diagnostic efficiency of each SNP for outcome, suggesting that the overall effect of MR Egger and IVW models was not prominent (Figure 3B). The randomness judgement showed that the reverse MR analysis was consistent with Mendel’s second law random grouping (Figure 3C).
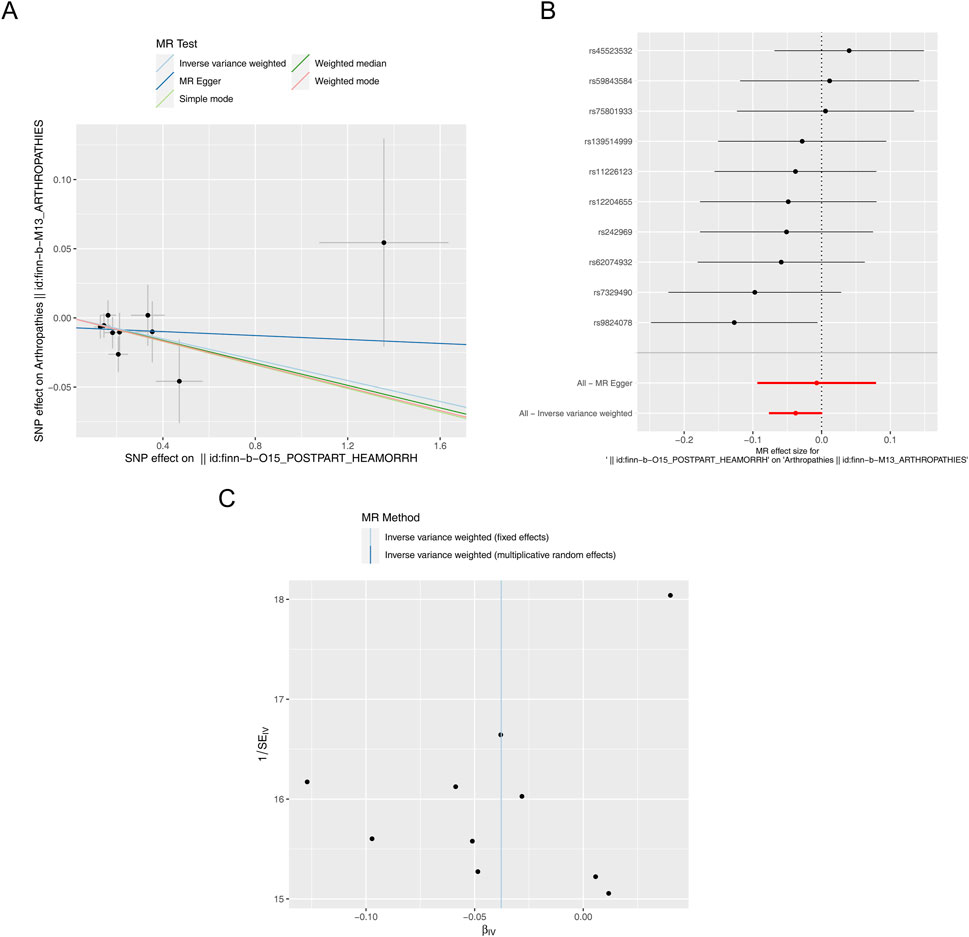
Figure 3. Reverse MR analysis. (A) Scatter plot of causal relationship between PPH and arthropathies. A regular slope of a line indicates a risk factor, while a negative slope of a line indicates a safety factor. (B) Forest plot of causal relationship between PPH and arthropathies. (C) Funnel plot of causal relationship between PPH and arthropathies.
3.2.2 Reliability of the reverse MR results was illustrated by sensitivity analysis
Similarly, immediately following the reverse MR analysis, the sensitivity analysis was put into effect to evaluate the reliability of reverse MR results. The Q_pval values of Heterogeneity test were greater than 0.05 based on MR Egger and IVW methods, suggesting that there was no heterogeneity (Supplementary Table S5). Furthermore, the Pleiotropy test suggested there was no horizontal pleiotropy (P = 0.459), meaning there were no confounding factors (Supplementary Table S6), and there were no points of severe bias by LOO method (Figure 4). In conclusion, PPH was not causally influenced on arthropathies.
3.3 Enrichment analysis of 33 genes corresponding to SNPs of arthropathies
In total, 33 genes corresponding to six SNPs of arthropathies were obtained for enrichment analysis (Supplementary Table S7). These genes were enriched in 190 GO terms, including 144 in biological process (BP), 25 cellular components (CC) and 21 molecular functions (MF), such as regulation of response to biotic stimulus and ribonucleoprotein (RNP) complex assembly in BP; spliceosomal snRNP complex and small nuclear ribonucleoprotein complex in CC; ligase activity, ribonucleoprotein complex binding, ribosomal large subunit binding and mannosyl-oligosaccharide mannosidase activity in MF (Figure 5A; Supplementary Table S8). Moreover, these 33 genes were markedly enriched in 3 KEGG pathways, including Natural Killer cell mediated cytotoxicity, Taurine and hypotaurine metabolism, and Kaposi sarcoma-associated herpesvirus infection (Figure 5B).
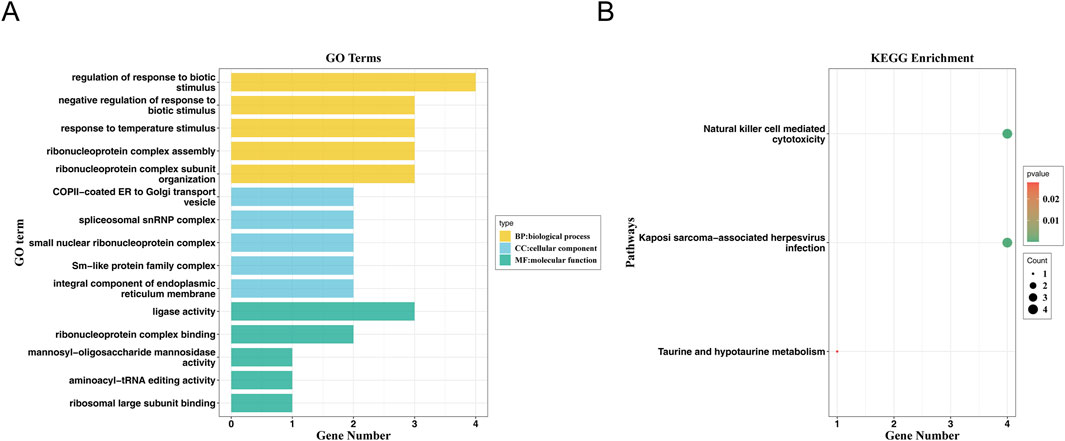
Figure 5. GO and KEGG enrichment analysis. (A) GO Function Analysis of Genes. The horizontal axis represents the number of genes enriched in the corresponding pathway, and the vertical axis represents the enriched GO function. (B) Bubble diagram of KEGG pathway enrichment of genes.
4 Discussion
Our investigation into the bidirectional associations between arthropathies and PPH, through a comprehensive Mendelian randomization (MR) analysis utilizing expansive GWAS datasets, predicts a significant genetic predisposition. This predisposition suggests that arthropathies increase the risk of PPH, while no reciprocal genetic evidence was found for PPH influencing the risk of arthropathies. This distinction underlines the critical impact of arthropathies on PPH risk, prompting a closer examination of the underlying biological mechanisms.
Generally, the causes of PPH can be summarized by the four “T’s”: tone (uterine atony), trauma (lacerations, expanding hematomas, or uterine rupture), tissue (retained placental tissue), and thrombin (defects of coagulation) (Bienstock et al., 2021; Committee on Practice Bulletins-Obstetrics, 2017). Abnormal uterine tone is estimated to cause approximately 70% of cases, followed by maternal trauma (approximately 20%), retained placental tissue (approximately 10%), and coagulation deficiencies (<1%) (Bienstock et al., 2021; Committee on Practice Bulletins-Obstetrics, 2017). It is essential to predict the risk and then initiate appropriate interventions (Bienstock et al., 2021; Committee on Practice Bulletins-Obstetrics, 2017).
Arthropathies, such as RA, JIA and SLE, pose unique challenges during pregnancy (Andreoli et al., 2023; Castro-Gutierrez et al., 2022). Women with these conditions are at higher risk for various pregnancy complications, including premature birth, small for gestational age (SGA) infants, disease flares, preeclampsia, miscarriage, and placental abruption et al. (Falcon et al., 2023; Fierro et al., 2023; Ehrmann Feldman et al., 2016; Keum et al., 2024; Zhang et al., 2022). Not all arthropathies behave similarly during pregnancy, disease like SLE, which could be complicated by HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count), coagulopathies and medications, could lead to PPH (Yan et al., 2024; Sun et al., 2021; Erazo-Martínez et al., 2021; Jiang et al., 2022). The management of JIA during pregnancy is also challenging (Gerosa et al., 2022). A significant concern for women with JIA during the postpartum period is hemorrhage (Chen et al., 2013). Ehrmann Feldman et al. (2017) conducted a population-based cohort study revealing that women with JIA are more likely to experience PPH compared to the controls. These complications highlight the need for effective management of arthropathies during pregnancy involves a multidisciplinary approach, including preconception counseling and close monitoring by rheumatologists and obstetricians (Andreoli et al., 2023). Treatment plans should balance disease control with minimizing fetal risks. Moreover, current evidence recommends the use of prophylactic low-dose aspirin in these patients for the prevention of preeclampsia, and it should be initiated between 12 weeks and 28 weeks of gestation (optimally before 16 weeks) and continued daily until delivery (ACOG Committee Opinion No, 2018).
Limited studies have suggest multiple potential mechanisms through which arthropathies may increase the risk of PPH, including systemic inflammation affecting platelets and vascular health, coagulation disturbance and autoimmune dysregulation, and medication effects. The chronic inflammation characteristic of arthropathies is known to disrupt both the function and number of platelets (Olumuyiwa-Akeredolu et al., 2019). This disruption can lead to a compromised coagulation cascade and hemostatic equilibrium, elevating the risk of bleeding during the postpartum period. In arthropathies, this balance is often skewed towards a pro-hemorrhagic state due to both intrinsic platelet defects and external influences on platelet dynamics (Becker et al., 2018; Tramś et al., 2022).
Beyond the platelet dysfunction, arthropathies are implicated in broader disturbances of the coagulation system, potentially predisposing individuals to either bleeding or thrombotic complications (Wang et al., 2024). The systemic inflammatory milieu can directly impact the endothelium, leading to a reduction in the expression and activity of key anticoagulant pathways and fibrinolytic systems (Vranic et al., 2019). The inflammatory cytokines prevalent in arthropathies, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), can disrupt the balance between procoagulant and anticoagulant factors (Kang and Kishimoto, 2021; Bouchnita et al., 2017). Commonly, autoimmune conditions can lead to a hypercoagulable state, paradoxically increasing the risk of bleeding by consuming clotting factors and platelets (Borensztajn et al., 2011). As the normal coagulation cascade essential for controlling bleeding during delivery may be impaired, it can significantly delay or prevent the formation of a stable clot, which is a crucial factor in the pathogenesis of PPH (Bank et al., 2023).
The role of inflammation in arthropathies could extend to the vascular endothelium, where chronic inflammatory mediators can degrade endothelial integrity and function (Ho-Tin-Noé et al., 2018). During childbirth, the integrity of the vascular system is paramount in managing the physiological challenges and preventing excessive bleeding. The pre-existing endothelial compromise in individuals with arthropathies may predispose them to hemorrhagic events (Deppermann, 2018). Moreover, autoantibody production and immune complex deposition are hallmark features of arthropathies, where the immune system erroneously targets self-antigens, leading to various clinical manifestations. In the context of pregnancy, such autoimmune activities can significantly impact vascular integrity, coagulation pathways and platelet function, increasing the risk of PPH (Sun et al., 2021).
On the other hand, medications such as low-dose aspirin is commonly used for the prevention of preeclampsia during pregnancy, but it may affect platelet function and increase bleeding risk (ACOG Committee Opinion No, 2018). Similarly, corticosteroids, can influence glucose regulation, wound healing, and blood pressure, which could indirectly increase the risk of PPH (Drechsel et al., 2020). Moreover, biological DMARDs (Disease-Modifying Anti-Rheumatic Drugs) targeting specific inflammatory pathways may also play a role, although their direct effects on pregnancy outcomes and PPH risk are less clear and require further investigation (Drechsel et al., 2020).
In this study, results indicated that arthropathies was genetic-causally related to PPH, and arthropathies was a risk factor for PPH. However, the current information in GWAS databases primarily focuses on the genetic variations related to the overall disease risk, like” arthropathies”, and does not directly provide a clear correspondence between each SNP and specific disease subtypes. To define the specific arthropathies corresponding to the SNPs, we reviewed several relevant studies. rs9271588, this SNP is located in the HLA-DRB1 gene region. Variations in HLA-DRB1 have been associated with several autoimmune conditions, including RA and AS (Tan et al., 2021; Ganjalikhani Hakemi and Nicknam, 2022). rs12245880, a SNP in the IL23R gene, has been linked to AS. Research has demonstrated that variations in the IL23R gene, can influence an individual’s susceptibility to AS (Dong et al., 2013). rs2263324, this SNP is located in the GDF5 gene, which plays a role in bone and cartilage development. Variations in GDF5 have been linked to OA and lumbar disc degeneration (Wang et al., 2023). rs145575084, this SNP has been found possibly related to PsA. It is linked to the IL12B gene, which influences the immune response, contributing to PsA (Filer et al., 2008). rs224329, also situated in the GDF5 gene, this SNP has been associated with chronic low back pain and lumbar disc degeneration (Qi et al., 2023). rs112286934, this SNP is linked to the STAT4 gene. Research indicates that variations in the STAT4 gene are associated with an increased risk of SLE (Liu et al., 2024). However, information on these specific SNPs’ association with arthropathies is limited. Further research is needed to elucidate the potential connections. It is important to note that while certain SNPs are associated with an increased risk of specific arthropathies, they are not definitive predictors. The development of these conditions is influenced by a combination of genetic, environmental, and lifestyle factors. Our study indicates a genetic association between arthropathies and increased PPH risk, seemingly with specific relevance noted for immune-related genes such as HLA and STAT4. However, some identified SNPs, including those associated with degenerative conditions, which seems not logical in any way to induce PPH, require further validation. While this broad selection strategy offered a broad genetic landscape, it also underscores the need for targeted validation studies to confirm the relevance of these findings to PPH risk.
While genetic factors may play a role in individual susceptibility to PPH (Westergaard et al., 2024), the found SNPs of arthropathies have not been identified as significant contributors in current medical literature. To explore the role of genes corresponding to the SNPs, GO functional enrichment and KEGG pathway analyses were performed. In GO analysis, for instance, the biological processes of response to temperature stimulus and RNP complex assembly are fundamental to cellular function. While their direct connections to arthropathies or PPH are not fully elucidated, current research might provide some insights. Temperature sensitivity, mediated by transient receptor potential (TRP) channels, plays a role in pain perception (Ji and Lee, 2021). Dysregulation of these channels may contribute to chronic pain conditions, including arthropathies (Ji and Lee, 2021). Besides, as temperature regulation is vital during labor and delivery, maintaining normothermia is essential for optimal uterine function and reducing PPH risk. Additionally, RNP complex assembly, is crucial for RNA processing and gene regulation (Staley and Woolford, 2009). Given the importance of RNPs in cellular stress responses and inflammation, further investigation into their potential role in arthropathies is warranted, and disruptions in their assembly could potentially impact uterine contractility and hemostasis, indirectly influencing PPH outcomes (McLintock, 2020). This area remains speculative and necessitates further research.
Although none of these genes was directly involved in the coagulation or platelet pathways, the KEGG findings suggest a novel area of exploration in the immunological dysregulation associated with arthropathies and pregnancy, including Natural killer cell mediated cytotoxicity, Taurine and hypotaurine metabolism, and Kaposi sarcoma-associated herpesvirus infection (KSHV). Natural Killer (NK) cells are a type of lymphocyte that plays a vital role in the immune system by targeting and destroying infected or malignant cells. Beyond these roles, NK cells significantly influence inflammatory processes and have been implicated in various autoimmune conditions. In RA, NK cells contribute to joint inflammation and destruction. They can produce pro-inflammatory cytokines like interferon-gamma (IFN-γ) and may exacerbate joint damage (Fathollahi et al., 2021). In systemic JIA, NK cells have been shown to play a role in the disease’s pathogenesis (Put et al., 2017). Recent research has begun to explore their potential involvement in reproductive processes. Studies have shown that uterine NK (uNK) cells can be categorized into different subsets with varying functions during the menstrual cycle and pregnancy (Whettlock et al., 2022). Increased NK cell cytotoxicity has been associated with recurrent spontaneous abortion, suggesting that dysregulation of NK activity can negatively affect placental and vascular integrity. Besides, during pregnancy, a specialized subset of NK cells, known as decidual NK (dNK) cells, accumulates in the uterine lining and is involved in placental development and fetal protection (Xu et al., 2022). While NK cells are primarily recognized for their immune functions, their role in PPH is not well-defined. Some studies suggest that dNK cells contribute to placental vasculature development, and abnormalities in their function could potentially affect uterine contractility and bleeding (Hidaka et al., 1991). However, direct evidence linking NK cell-mediated cytotoxicity to PPH is currently limited.
Disruptions in taurine and hypotaurine metabolism may play a role in the development or progression of arthropathies, possibly through mechanisms involving inflammation and oxidative stress (Yang et al., 2016; He et al., 2023). Moreover, it has been suggested in conditions like intrahepatic cholestasis of pregnancy (ICP), which can have obstetric implications (Tang et al., 2024). However, the direct impact on PPH has not been extensively studied, and current data do not establish a clear connection. Additionally, KSHV infection, while not a common cause of arthropathies, can lead to joint-related symptoms in certain cases, likely through its effects on host cell function and inflammatory pathways (Sychev et al., 2017). While direct evidence of KSHV’s impact on PPH is sparse, the virus’s capacity to induce severe hemorrhagic conditions in other contexts suggests a potential risk factor for similar outcomes in postpartum women, especially those with compromised immune systems (Matsushima et al., 1999; Kulkarni et al., 2023).
This bidirectional MR investigation provides novel genetic insights into the causal link between arthropathies and PPH, revealing potential mechanisms, yet acknowledging inherent limitations such as population homogeneity and the reliance on hypothesis-based instrumental variable validation methods. Although the findings of our study indicate that genetic predictors of arthropathies are notably associated with increased PPH risk, no reciprocal effect was observed, and none of the identified genes directly relate to PPH. This underscores the complexity of immune processes in arthropathies’ pathogenesis and their potential impact on pregnancy outcomes through indirect pathways.
The main concern is that the inclusion of SNPs across all forms of arthropathies presents a limitation. While this approach provided a comprehensive overview, it also encompassed genes related to degenerative conditions (e.g., OA, lumbar disc degeneration) that may lack direct relevance to PPH risk. This selection criterion was an initial exploratory approach, and we acknowledge its limitations in contributing to mechanistic understanding. Future studies should focus on validating genes with clearer immunological relevance, such as HLA and STAT4, and narrowing the inclusion criteria to specific types of arthropathies more plausibly related to PPH risk. This study’s exploratory nature aims to be a preliminary step, aimed at generating causal hypotheses that necessitate experimental follow-up, rather than providing conclusive mechanistic insights.
While experimental validation of this causal relationship is currently unavailable within the scope of this study, our results offer a robust foundation for future targeted in vitro and in vivo experimental investigations. Expanding the demographic and genetic diversity of study populations will also be crucial in validating and extending these findings to a broader spectrum of individuals affected by arthropathies. In light of these insights, further research could focus on evaluating the biological function of the identified SNPs and genes within relevant immune and coagulation pathways, thus providing more concrete evidence of the mechanisms suggested by our MR analysis. We believe that our reliance on extensive sensitivity analyses and gene expression pathway enrichment sets a strong base for future work, paving the way for experimental immunological research to confirm these associations.
5 Conclusion
This study’s Mendelian Randomization analysis elucidates a genetic causal relationship between arthropathies and PPH, with arthropathies identified as a genetic risk factor leading to an increased incidence of PPH. These findings not only contribute to our understanding of the genetic underpinnings linking arthropathies to PPH but also highlight the need for targeted clinical strategies to mitigate PPH risk in patients with arthropathies. Further research, especially in diverse populations and exploring the immunological mechanisms suggested by our findings, is essential for advancing our comprehension of these complex associations.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
ZW: Formal Analysis, Investigation, Methodology, Conceptualization, Supervision, Validation, Visualization, Writing–review and editing. CY: Formal Analysis, Investigation, Methodology, Data curation, Software, Writing–original draft. XP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Key Research and Development Program of China (2022YFC2704500, 2022YFC2704501), and grants from the Key Research Program of the Science & Technology Department of Sichuan Province, China (2023YFS0071).
Acknowledgments
We would like to express our sincere gratitude to all individuals and organizations who supported and assisted us throughout this research. We acknowledge the support by grants from the National Key Research and Development Program of China (2022YFC2704500, 2022YFC2704501), and grants from the Key Research Program of the Science & Technology Department of Sichuan Province, China (2023YFS0071).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1448754/full#supplementary-material
SUPPLEMENTARY TABLE S1 | SNPs information in forward MR analysis.
SUPPLEMENTARY TABLE S2 | Heterogeneity test.
SUPPLEMENTARY TABLE S3 | Horizontal pleiotropy test.
SUPPLEMENTARY TABLE S4 | SNPs information in reverse MR analysis.
SUPPLEMENTARY TABLE S5 | Heterogeneity test.
SUPPLEMENTARY TABLE S6 | Horizontal pleiotropy test.
SUPPLEMENTARY TABLE S7 | Information on 33 genes corresponding to 6 arthropathies of SNP in the enrichment analysis.
SUPPLEMENTARY TABLE S8 | GO enrichment information.
References
ACOG Committee Opinion No (2018). 743: low-dose aspirin use during pregnancy. Obstet. Gynecol. 132 (1), e44–e52. doi:10.1097/AOG.0000000000002708
Andreoli, L., Chighizola, C. B., Iaccarino, L., Botta, A., Gerosa, M., Ramoni, V., et al. (2023). Immunology of pregnancy and reproductive health in autoimmune rheumatic diseases. Update from the 11th international conference on reproduction, pregnancy and rheumatic diseases. Autoimmun. Rev. 22 (3), 103259. doi:10.1016/j.autrev.2022.103259
Bank, T. C., Ma'ayeh, M., and Rood, K. M. (2023). Maternal coagulation disorders and postpartum hemorrhage. Clin. Obstet. Gynecol. 66 (2), 384–398. doi:10.1097/GRF.0000000000000787
Becker, R. C., Sexton, T., and Smyth, S. S. (2018). Translational implications of platelets as vascular first responders. Circ. Res. 122 (3), 506–522. doi:10.1161/CIRCRESAHA.117.310939
Bienstock, J. L., Eke, A. C., and Hueppchen, N. A. (2021). Postpartum hemorrhage. N. Engl. J. Med. 384 (17), 1635–1645. doi:10.1056/NEJMra1513247
Borensztajn, K. S., von der Thüsen, J. H., and Spek, C. A. (2011). The role of coagulation in chronic inflammatory disorders: a jack of all trades. Curr. Pharm. Des. 17, 9–16. doi:10.2174/138161211795049813
Bouchnita, A., Miossec, P., Tosenberger, A., and Volpert, V. (2017). Modeling of the effects of IL-17 and TNF-α on endothelial cells and thrombus growth. C R. Biol. 340, 456–473. doi:10.1016/j.crvi.2017.10.002
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525. doi:10.1093/ije/dyv080
Bowden, J., Davey Smith, G., Haycock, P. C., and Burgess, S. (2016). Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. doi:10.1002/gepi.21965
Burgess, S., Scott, R. A., Timpson, N. J., Davey Smith, G., and Thompson, S. G.EPIC- InterAct Consortium (2015). Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 30, 543–552. doi:10.1007/s10654-015-0011-z
Castro-Gutierrez, A., Young, K., and Bermas, B. L. (2022). Pregnancy and management in women with rheumatoid arthritis, systemic lupus erythematosus, and obstetric antiphospholipid syndrome. Rheum. Dis. Clin. North Am. 48 (2), 523–535. doi:10.1016/j.rdc.2022.02.009
Chen, J. S., Ford, J. B., Roberts, C. L., Simpson, J. M., and March, L. M. (2013). Pregnancy outcomes in women with juvenile idiopathic arthritis: a population-based study. Rheumatol. Oxf. 52, 1119–1125. doi:10.1093/rheumatology/kes428
Chen, L., Feng, P., Shaver, L., and Wang, Z. (2021). Maternal mortality ratio in China from 1990 to 2019: trends, causes and correlations. BMC Public Health 21, 1536. doi:10.1186/s12889-021-11557-3
Committee on Practice Bulletins-Obstetrics (2017). Practice bulletin No. 183: postpartum hemorrhage. Obstet. Gynecol. 130 (4), e168–e186. doi:10.1097/AOG.0000000000002351
Deppermann, C. (2018). Platelets and vascular integrity. Platelets 29 (6), 549–555. doi:10.1080/09537104.2018.1428739
Dong, H., Li, Q., Zhang, Y., Tan, W., and Jiang, Z. (2013). IL23R gene confers susceptibility to ankylosing spondylitis concomitant with uveitis in a Han Chinese population. PLoS One 8 (6), e67505. doi:10.1371/journal.pone.0067505
Drechsel, P., Stüdemann, K., Niewerth, M., Horneff, G., Fischer-Betz, R., Seipelt, E., et al. (2020). Pregnancy outcomes in DMARD-exposed patients with juvenile idiopathic arthritis-results from a JIA biologic registry. Rheumatol. Oxf. 59, 603–612. doi:10.1093/rheumatology/kez309
Ehrmann Feldman, D., Vinet, É., Bernatsky, S., Duffy, C., Hazel, B., Meshefedjian, G., et al. (2016). Birth outcomes in women with a history of juvenile idiopathic arthritis. J. Rheumatol. 43 (4), 804–809. doi:10.3899/jrheum.150592
Ehrmann Feldman, D., Vinet, É., Sylvestre, M. P., Hazel, B., Duffy, C., Bérard, A., et al. (2017). Postpartum complications in new mothers with juvenile idiopathic arthritis: a population-based cohort study. Rheumatol. Oxf. 56, 1378–1385. doi:10.1093/rheumatology/kex168
Ende, H. B., Lozada, M. J., Chestnut, D. H., Osmundson, S. S., Walden, R. L., Shotwell, M. S., et al. (2021). Risk factors for atonic postpartum hemorrhage: a systematic review and meta-analysis. Obstet. Gynecol. 137, 305–323. doi:10.1097/AOG.0000000000004228
Erazo-Martínez, V., Nieto-Aristizábal, I., Ojeda, I., González, M., Aragon, C. C., Zambrano, M. A., et al. (2021). Systemic erythematosus lupus and pregnancy outcomes in a Colombian cohort. Lupus. 30 (14), 2310–2317. doi:10.1177/09612033211061478
Falcon, R. M. G., Alcazar, R. M. U., Mondragon, A. V., Penserga, E. G., and Tantengco, O. A. G. (2023). Rheumatoid arthritis and the risk of preterm birth. Am. J. Reprod. Immunol. 89 (3), e13661. doi:10.1111/aji.13661
Fathollahi, A., Samimi, L. N., Akhlaghi, M., Jamshidi, A., Mahmoudi, M., and Farhadi, E. (2021). The role of NK cells in rheumatoid arthritis. Inflamm. Res. 70 (10-12), 1063–1073. doi:10.1007/s00011-021-01504-8
Fierro, J. J., Prins, J. R., Verstappen, G. M., Bootsma, H., Westra, J., and de Leeuw, K. (2023). Preconception clinical factors related to adverse pregnancy outcomes in patients with systemic lupus erythematosus or primary Sjögren's syndrome: a retrospective cohort study. RMD Open 9 (3), e003439. doi:10.1136/rmdopen-2023-003439
Filer, C., Ho, P., Smith, R. L., Griffiths, C., Young, H. S., Worthington, J., et al. (2008). Investigation of association of the IL12B and IL23R genes with psoriatic arthritis [published correction appears in Arthritis Rheum. 2012 Apr;64(4):1302]. Arthritis Rheum. 58 (12), 3705–3709. doi:10.1002/art.24128
Ganjalikhani Hakemi, M. (2022). “Association of HLA and HLA-related genes with ankylosing spondylitis,” in Ankylosing spondylitis - axial spondyloarthritis. Editor M. H. Nicknam (Singapore: Springer).
García-Fernández, A., Gerardi, M. C., Crisafulli, F., Filippini, M., Fredi, M., Gorla, R., et al. (2021). Disease course and obstetric outcomes of pregnancies in juvenile idiopathic arthritis: are there any differences among disease subtypes? A single-centre retrospective study of prospectively followed pregnancies in a dedicated pregnancy clinic. Clin. Rheumatol. 40, 239–244. doi:10.1007/s10067-020-05404-w
Gerosa, M., Chighizola, C. B., Pregnolato, F., Pontikaki, I., Luppino, A. F., Argolini, L. M., et al. (2022). Pregnancy in juvenile idiopathic arthritis: maternal and foetal outcome, and impact on disease activity. Ther. Adv. Musculoskelet. Dis. 14, 1759720X221080375. doi:10.1177/1759720X221080375
Hartwig, F. P., Davey Smith, G., and Bowden, J. (2017). Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 46, 1985–1998. doi:10.1093/ije/dyx102
He, Y., Cheng, B., Guo, B. J., Huang, Z., Qin, J. H., Wang, Q. Y., et al. (2023). Metabonomics and 16S rRNA gene sequencing to study the therapeutic mechanism of Danggui Sini decoction on collagen-induced rheumatoid arthritis rats with Cold Bi syndrome. J. Pharm. Biomed. Anal. 222, 115109. doi:10.1016/j.jpba.2022.115109
Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., et al. (2018). The MR-Base platform supports systematic causal inference across the human phenome. Elife 7, e34408. doi:10.7554/eLife.34408
Hidaka, Y., Amino, N., Iwatani, Y., Kaneda, T., Mitsuda, N., Morimoto, Y., et al. (1991). Changes in natural killer cell activity in normal pregnant and postpartum women: increases in the first trimester and postpartum period and decrease in late pregnancy. J. Reprod. Immunol. 20, 73–83. doi:10.1016/0165-0378(91)90024-k
Ho-Tin-Noé, B., Boulaftali, Y., and Camerer, E. (2018). Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood 131 (3), 277–288. doi:10.1182/blood-2017-06-742676
Ji, R. R., and Lee, S. Y. (2021). Molecular sensors of temperature, pressure, and pain with special focus on TRPV1, TRPM8, and PIEZO2 ion channels. Neurosci. Bull. 37 (12), 1745–1749. doi:10.1007/s12264-021-00798-2
Jiang, Y., Cheng, Y., Ma, S., Li, T., Chen, Z., Zuo, X., et al. (2022). Systemic lupus erythematosus-complicating immune thrombocytopenia: from pathogenesis to treatment. J. Autoimmun. 132, 102887. doi:10.1016/j.jaut.2022.102887
Kang, S., and Kishimoto, T. (2021). Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 53, 1116–1123. doi:10.1038/s12276-021-00649-0
Keum, H., Bermas, B., Patel, S., Jacobe, H. T., and Chong, B. F. (2024). Patients with autoimmune skin diseases are at increased risk of adverse pregnancy outcomes. Am. J. Obstet. Gynecol. MFM 6 (1), 101226. doi:10.1016/j.ajogmf.2023.101226
Kulkarni, A., Bazou, D., and Santos-Martinez, M. J. (2023). Bleeding and thrombosis in multiple myeloma: platelets as key players during cell interactions and potential use as drug delivery systems. Int. J. Mol. Sci. 24 (21), 15855. doi:10.3390/ijms242115855
Liu, M., Wang, S., Liang, Y., Fan, Y., and Wang, W. (2024). Genetic polymorphisms in genes involved in the type I interferon system (STAT4 and IRF5): association with Asian SLE patients. Clin. Rheumatol. 43 (8), 2403–2416. doi:10.1007/s10067-024-07046-8
Matsushima, A. Y., Strauchen, J. A., Lee, G., Scigliano, E., Hale, E. E., Weisse, M. T., et al. (1999). Posttransplantation plasmacytic proliferations related to Kaposi's sarcoma-associated herpesvirus. Am. J. Surg. Pathol. 23 (11), 1393–1400. doi:10.1097/00000478-199911000-00010
McLintock, C. (2020). Prevention and treatment of postpartum hemorrhage: focus on hematological aspects of management. Hematol. Am. Soc. Hematol. Educ. Program 2020 (1), 542–546. doi:10.1182/hematology.2020000139
Olumuyiwa-Akeredolu, O. O., Page, M. J., Soma, P., and Pretorius, E. (2019). Platelets: emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat. Rev. Rheumatol. 15 (4), 237–248. doi:10.1038/s41584-019-0187-9
Put, K., Vandenhaute, J., Avau, A., van Nieuwenhuijze, A., Brisse, E., Dierckx, T., et al. (2017). Inflammatory gene expression profile and defective interferon-γ and granzyme K in natural killer cells from systemic juvenile idiopathic arthritis patients. Arthritis Rheumatol. 69 (1), 213–224. doi:10.1002/art.39933
Qi, L., Chen, L., Chang, Y., Zhu, Y., Yang, H., Zhou, D., et al. (2023). GDF5 rs143383 and the risk of lumbar disc degeneration: a meta-analysis and case-control study. Res. Square. doi:10.21203/rs.3.rs-2897119/v1
Ramessur, R., Saklatvala, J., Budu-Aggrey, A., Ostaszewski, M., Möbus, L., Greco, D., et al. (2024). Exploring the link between genetic predictors of cardiovascular disease and psoriasis. JAMA Cardiol. 9, 1009–1017. doi:10.1001/jamacardio.2024.2859
Reinus, W. R., Barbe, M. F., Berney, S., and Khurana, J. S. (2009). “Arthropathies,” in Bone pathology. Editor J. Khurana (Humana Totowa, NJ: Humana Press).
Say, L., Chou, D., Gemmill, A., Tunçalp, Ö., Moller, A. B., Daniels, J., et al. (2014). Global causes of maternal death: a WHO systematic analysis. Lancet Glob. Health 2, e323–e333. doi:10.1016/S2214-109X(14)70227-X
Smith, G. D., and Ebrahim, S. (2002). Data dredging, bias, or confounding. BMJ 325, 1437–1438. doi:10.1136/bmj.325.7378.1437
Staley, J. P., and Woolford, J. L. (2009). Assembly of ribosomes and spliceosomes: complex ribonucleoprotein machines. Curr. Opin. Cell Biol. 21 (1), 109–118. doi:10.1016/j.ceb.2009.01.003
Sun, S., Urbanus, R. T., Ten, C. H., de Groot, P. G., de Laat, B., Heemskerk, J. W. M., et al. (2021). Platelet activation mechanisms and consequences of immune thrombocytopenia. Cells 10 (12), 3386. doi:10.3390/cells10123386
Sychev, Z. E., Hu, A., DiMaio, T. A., Gitter, A., Camp, N. D., Noble, W. S., et al. (2017). Integrated systems biology analysis of KSHV latent infection reveals viral induction and reliance on peroxisome mediated lipid metabolism. PLoS Pathog. 13 (3), e1006256. doi:10.1371/journal.ppat.1006256
Tan, L. K., Too, C. L., Diaz-Gallo, L. M., Wahinuddin, S., Lau, I. S., Heselynn, H., et al. (2021). The spectrum of association in HLA region with rheumatoid arthritis in a diverse Asian population: evidence from the MyEIRA case-control study. Arthritis Res. Ther. 23 (1), 46. doi:10.1186/s13075-021-02431-z
Tang, M., Xiong, L., Cai, J., Fu, J., Liu, H., Ye, Y., et al. (2024). Intrahepatic cholestasis of pregnancy: insights into pathogenesis and advances in omics studies. Hepatol. Int. 18 (1), 50–62. doi:10.1007/s12072-023-10604-y
Tramś, E., Malesa, K., Pomianowski, S., and Kamiński, R. (2022). Role of platelets in osteoarthritis-updated systematic review and meta-analysis on the role of platelet-rich plasma in osteoarthritis. Cells 11 (7), 1080. doi:10.3390/cells11071080
Vranic, A., Pruner, I., Veselinovic, M., Soutari, N., Petkovic, A., Jakovljevic, V., et al. (2019). Assessment of hemostatic disturbances in women with established rheumatoid arthritis. Clin. Rheumatol. 38 (11), 3005–3014. doi:10.1007/s10067-019-04629-8
Wang, Q., Shao, G., Zhao, X., Wong, H. H., Chin, K., Zhao, M., et al. (2024). Dysregulated fibrinolysis and plasmin activation promote the pathogenesis of osteoarthritis. JCI Insight 9 (8), e173603. doi:10.1172/jci.insight.173603
Wang, Y. P., Di, W. J., Yang, S., Qin, S. L., Xu, Y. F., Han, P. F., et al. (2023). The association of growth differentiation factor 5 rs143383 gene polymorphism with osteoarthritis: a systematic review and meta-analysis. J. Orthop. Surg. Res. 18 (1), 763. doi:10.1186/s13018-023-04245-y
Westergaard, D., Steinthorsdottir, V., Stefansdottir, L., Rohde, P. D., Wu, X., Geller, F., et al. (2024). Genome-wide association meta-analysis identifies five loci associated with postpartum hemorrhage. Nat. Genet. 56 (8), 1597–1603. doi:10.1038/s41588-024-01839-y
Whettlock, E. M., Woon, E. V., Cuff, A. O., Browne, B., Johnson, M. R., and Male, V. (2022). Dynamic changes in uterine NK cell subset frequency and function over the menstrual cycle and pregnancy. Front. Immunol. 13, 880438. doi:10.3389/fimmu.2022.880438
Wu, T., Hu, E., Xu, S., Chen, M., Guo, P., Dai, Z., et al. (2021). clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innov. (Camb) 2, 100141. doi:10.1016/j.xinn.2021.100141
Xu, X., Zhou, Y., Fu, B., and Wei, H. (2022). Uterine NK cell functions at maternal-fetal interface. Biol. Reprod. 107 (1), 327–338. doi:10.1093/biolre/ioac094
Yan, P., Yao, J., Ke, B., and Fang, X. (2024). Mendelian randomization reveals systemic lupus erythematosus and rheumatoid arthritis and risk of adverse pregnancy outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 293, 78–83. doi:10.1016/j.ejogrb.2023.12.020
Yang, G., Zhang, H., Chen, T., Zhu, W., Ding, S., Xu, K., et al. (2016). Metabolic analysis of osteoarthritis subchondral bone based on UPLC/Q-TOF-MS. Anal. Bioanal. Chem. 408 (16), 4275–4286. doi:10.1007/s00216-016-9524-x
Yang, J., Wu, W., Amier, Y., Li, X., Wan, W., and Yu, X. (2023). Causal relationship of genetically predicted circulating micronutrients levels with the risk of kidney stone disease: a Mendelian randomization study. Front. Nutr. 10, 1132597. doi:10.3389/fnut.2023.1132597
Zhang, D., Hu, Y., Guo, W., Song, Y., Yang, L., Yang, S., et al. (2022). Mendelian randomization study reveals a causal relationship between rheumatoid arthritis and risk for pre-eclampsia. Front. Immunol. 13, 1080980. doi:10.3389/fimmu.2022.1080980
Keywords: Postpartum hemorrhage, arthropathies, Mendelian randomization, causality, enrichment analysis
Citation: Wu Z, Yuan C and Peng X (2024) Association between arthropathies and postpartum hemorrhage: a bidirectional Mendelian randomization study. Front. Genet. 15:1448754. doi: 10.3389/fgene.2024.1448754
Received: 13 June 2024; Accepted: 28 November 2024;
Published: 11 December 2024.
Edited by:
Angela Tincani, Rheumatology Unit ASST-Spedali Civili and University of Brescia, ItalyReviewed by:
Ruth D. Fritsch-Stork, Sigmund Freud University Vienna, AustriaAriela Hoxha, University Hospital of Padua, Italy
Copyright © 2024 Wu, Yuan and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue Peng, cGVuZ3h1ZXNub3dAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zhao Wu
Zhao Wu Chengyu Yuan1†
Chengyu Yuan1† Xue Peng
Xue Peng