- 1Department of Biological Sciences, College of Science, University of Hafr Al-Batin, Hafr Al- Batin, Saudi Arabia
- 2Department of Clinical Laboratory Sciences, Turbah University College, Taif University, Taif, Saudi Arabia
- 3Department of Biochemistry and Molecular Medicine, College of Medicine, Al-Faisal University, Riyadh, Saudi Arabia
- 4Research Center, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
Attention deficit hyperactivity disorder (ADHD) is a clinically and genetically heterogeneous neurodevelopmental syndrome characterized by behavioral appearances such as impulsivity, inattention, and hyperactivity. The prevalence of ADHD is high in childhood when compared to adults. ADHD has been significantly advanced by genetic research over the past 25 years. However, it is logically conceivable that both genetic and/or non-genetic factors, such as postnatal environmental and social influences, are associated with ADHD phenotype in Arab populations. While genetic influences are strongly linked with the etiology of ADHD, it remains obscure how consanguinity which is an underlying factor for many genetic diseases, contributes to ADHD subtypes. Arabian Gulf Nations have one the highest rates of consanguineous marriages, and consanguinity plays an important contributing factor in many genetic diseases that exist in higher percentages in Arabian Gulf Nations. Therefore, the current review aims to shed light on the genetic variants associated with ADHD subtypes in Arabian Gulf nations and Saudi Arabia in particular. It also focuses on the symptoms and the diagnosis of ADHD before turning to the neuropsychological pathways and subgroups of ADHD. The impact of a consanguinity-based understanding of the ADHD subtype will help to understand the genetic variability of the Arabian Gulf population in comparison with the other parts of the world and will provide novel information to develop new avenues for future research in ADHD.
1 Introduction
Attention-deficit hyperactivity disorder (ADHD) is a common clinically and genetically transmitted neurological condition that affects an individual’s behavior (American Academy of Pediatrics, 2001). Symptoms of ADHD such as mood and anxiety disorders, conduct disorder, and oppositional defiant disorder are usually noticed at an early age of childhood and are increasingly recognized in adulthood (Biederman et al., 2006). It has been reported that among 3%–10% of those diagnosed with ADHD disorder in their childhood, and 6% of the overall population continue to develop noticeable ADHD conditions (Wender et al., 2001). The concept of ADHD as a clinical condition has changed over the 20th century until the onset of the twin and family studies (Foreman, 2006). The historical descriptions associated with ADHD ranged from early brain damage “neurodevelopmental disorders” to the indirect contribution of genetic and environmental factors (Sonuga-Barke et al., 2023). Nevertheless, many descriptions are consistent with the modern diagnostic strategies for ADHD.
In 1798, a Scottish physician named Sir Alexander Crichton was the earliest to reveal that the incapacity of attending may be innate or due to unnatural nervous sensibility (Lange et al., 2010). Crichton saw numerous examples of insanity in his therapeutic practice and had a growing interest in mental illness. Fidgety Philip (Heinrich Hoffmann), a German physician, in 1840s was the first to write allegory stories for children with ADHD (American Academy of Pediatrics, 2001). In the 1990s century, the description of the characteristic features of an individual with ADHD became the focus of several scientists (Thome and Jacobs, 2004). Geroge Still, a British pediatrician, in 1902 described an abnormal defect of moral control in children and found that some could not control their behavior in a typical way, but were still intelligent (Lange et al., 2010). In 1919, ADHD was blamed on brain damage, and numerous words were associated with the disorder, such as impulsive, inattentive, hyperactive, and short attention span (Burd and Kerbeshian, 1988). Medications for symptoms were discovered during the 1930s, and research on ADHD in both children and adults continued throughout the 19th and 20th centuries as a worldwide interest (Abdelnour et al., 2022).
Attention deficit hyperactivity disorder accounts for over 5% of frequent disorders within child and adolescent psychiatry (Drechsler et al., 2020). Physicians still struggle with diagnosis and treatment despite the enormous volume of research, more than 20,000 publications have been mentioned in PubMed during the past 10 years. Due to its heterogeneous nature, ADHD can present in opposite forms with frequent and variable comorbidities (Wahlstedt et al., 2009). Additionally, there is an overlap between the symptoms of ADHD and other diseases, such as autism spectrum disorder (ASD), major depressive disorder, bipolar disorder, and schizophrenia, which may or may not show up on a clinical assessment (Morimoto et al., 2021). Biomarkers or other objective criteria that could result in an automatic algorithm for the reliable identification of ADHD in an individual within clinical practice are currently absent, despite the fact that the disorder’s neurological and genetic foundations are undeniable.
2 Symptoms of ADHD
The characteristic features of individuals with ADHD include hyperactivity, inattentiveness, and impulsiveness, which lead to impairments in daily living (Figure 1). Characterization of ADHD based on features is ensured by looking at a lack of moral control through inattentive and hyperactive-impulsive symptoms or a mix of both symptoms (Muller et al., 2011). Inattentive symptoms include trouble focusing, finishing tasks, following instructions, memories not always easy to recall, careless mistakes, and losing belongings (Muller et al., 2011). The hyperactive-impulsive symptoms include excessive talking and physical movement, interrupting conversations, answering before the question has been finished, dangerous behavior, choosing a smaller reward now rather than a larger reward later, finding boredom intolerable, and looking for stimulation. Some people have combined symptoms of both inattentive and hyperactive-impulsive symptoms (American Psychiatric Association, 1987).
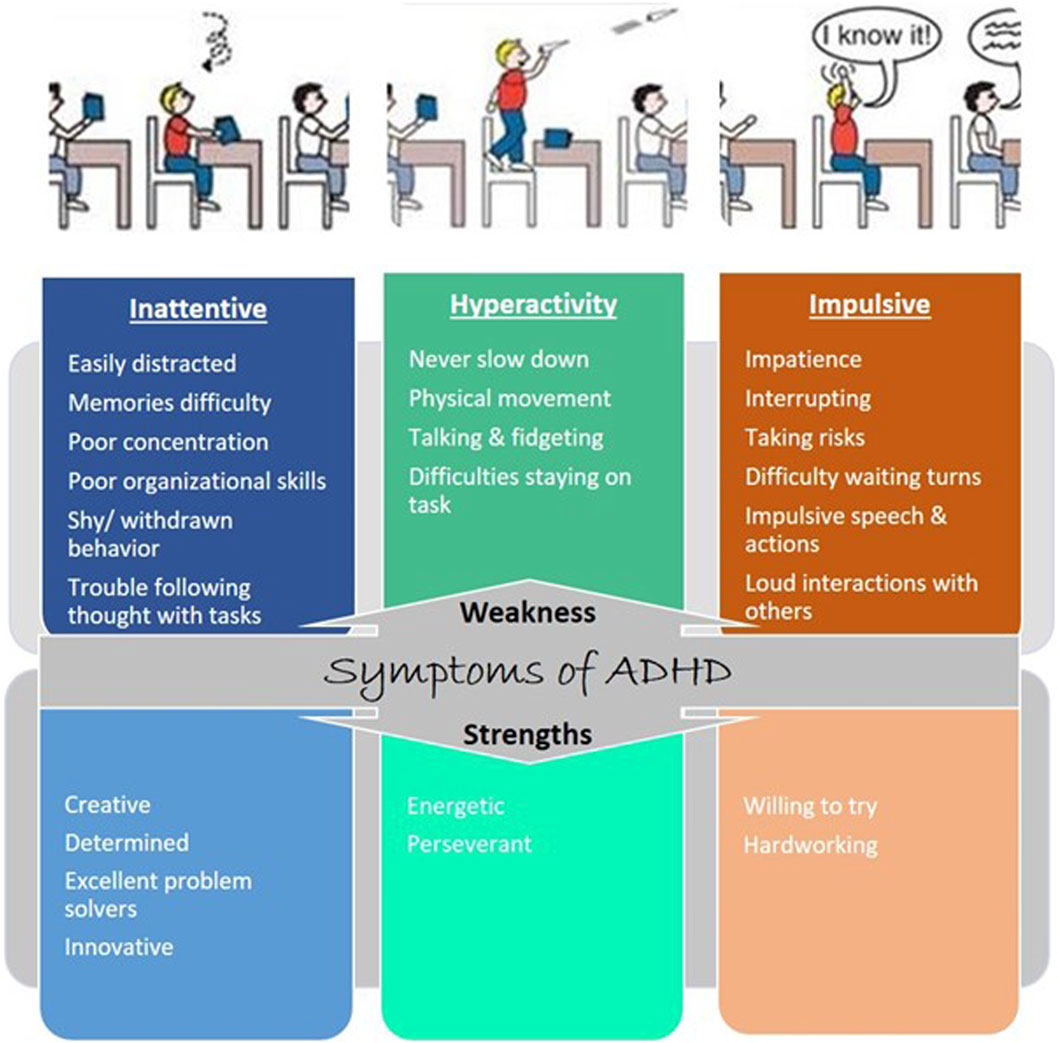
Figure 1. Symptoms of ADHD: weakness and strength. Each type of ADHD is tied to one or more characteristics: inattention and hyperactive-impulsive behavior.
Brain development and functions are achieved by expressing multiple genes distributed in four brain regions: frontal cortex, cerebellum, parietal lobe, and basal ganglion (Table 1). These genes are essential in regulating systemic sympathetic activity and contribute to the behaviors associated with dopamine levels. Normal brains and brains with ADHD develop in the same manner. However, the brain with ADHD develops more slowly overall, particularly in the frontal lobes that help with impulse control and concentration (Figure 2). As a result, neurodevelopmental disorders including ADHD may not mature to the same degree as a normal brain, though, depending on how severe the symptoms are (Gehricke et al., 2017). In fact, people with childhood ADHD diagnoses had a smaller overall brain capacity than adults without such a diagnosis, according to neuroscientists (Castellanos et al., 2002). In the areas of their brains impacted by ADHD, there was greater cortical thinning and less cortical thickness in the outer layer (Konrad et al., 2012). This essentially indicates that there were fewer brain cells in these regions in these people. Normal brains may produce brain cells more quickly than brains with ADHD, which could be one explanation for this. Therefore, before cortical thinning begins, those people have a higher initial level of grey matter. In addition, the brain with ADHD has a lower level of dopamine, which helps control the brain’s reward and regulates movement and emotional responses, in comparison with the normal brain (Figure 3). As a result, the ADHD brain cannot stay motivated and experiences functional changes and weak connectivity. In general, the structure, chemistry, and function of the ADHD brain differ from those of the non-ADHD brain.
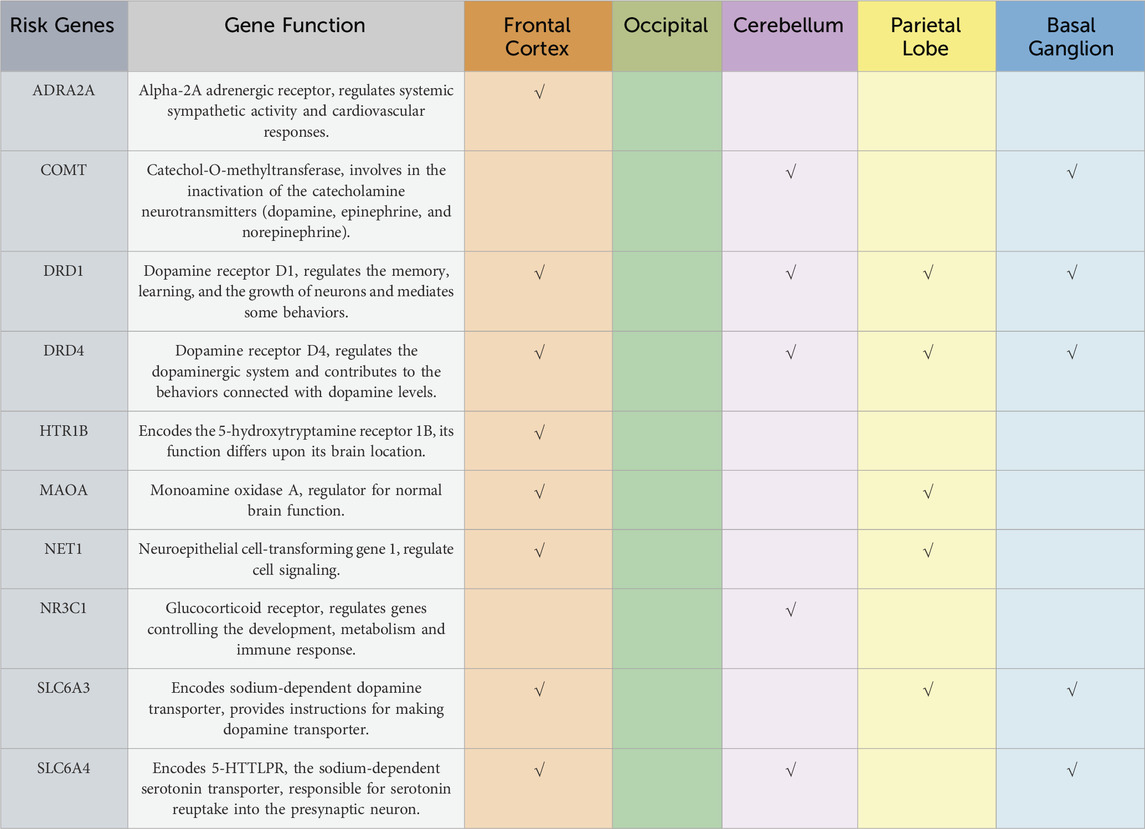
Table 1. A representation of a collective of genes that encode for primary altered structural and functional brain changes within the five brain regions that were found to be correlated with the mechanism of actions found in attention-deficit hyperactivity disorder (ADHD).
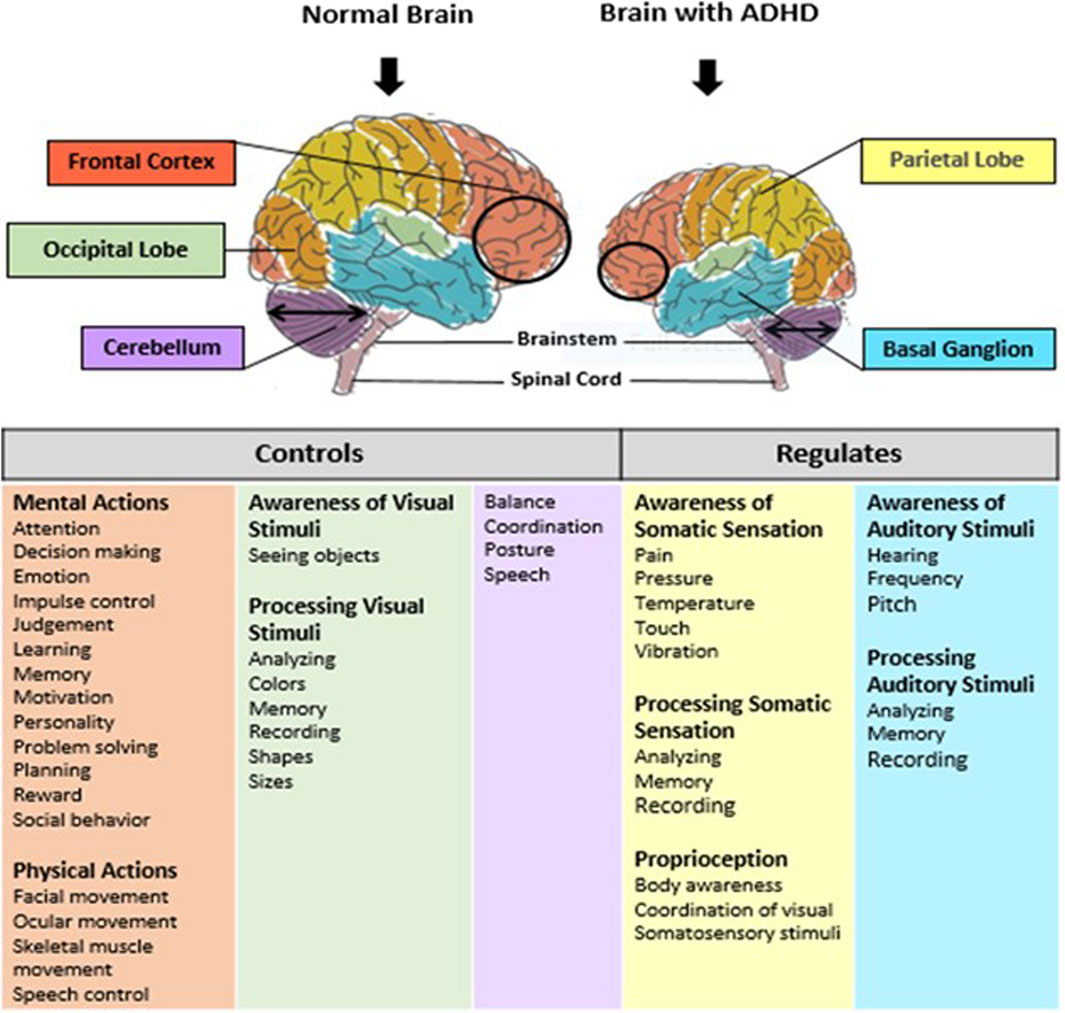
Figure 2. Schematic representation of normal brain and brain with ADHD. Brain consists of five main regions: prefrontal cortex, occipital lobe, cerebellum, the basal ganglia, and parietal lobe. These regions control and regulate several functions such as: inhibition, memory, planning and organization, motivation, processing speed, inattention and impulsivity. Brain development in individual with ADHD is smaller in the prefrontal cortex, and cerebellum regions as compare to a normal brain. Also, ADHD has been linked to deficits in the functioning of the prefrontal cortex, the basal ganglia, cerebellum and parietal lobe.
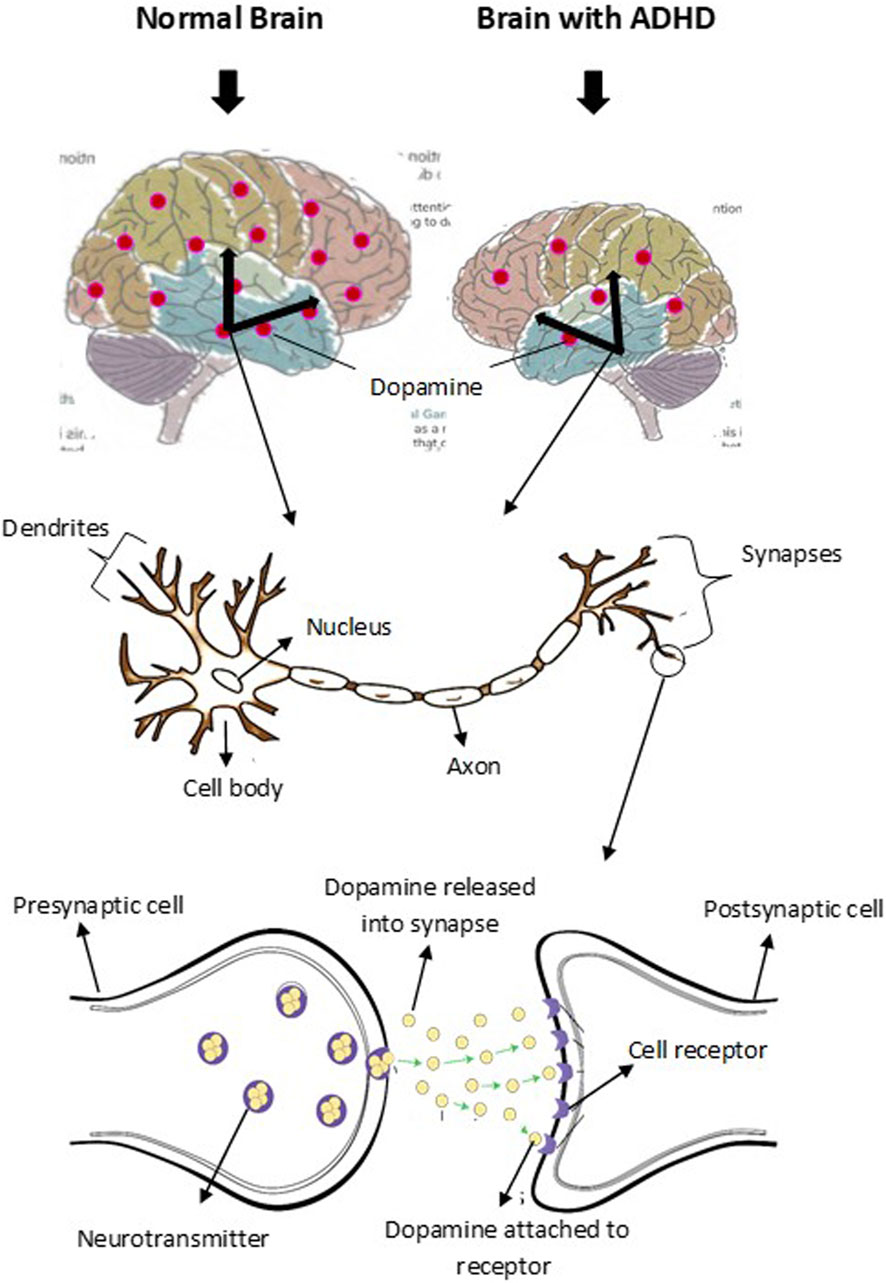
Figure 3. Schematic representation of dopamine level and transmission in normal brain and brain with ADHD. Dopaminergic neuron is located in the midbrain and control various functions. The level of dopamine in normal brain is higher than the level in brain with ADHD. Dopamine level contributes to the symptoms of inattention, hyperactivity, and impulsiveness in ADHD.
3 Diagnosis of ADHD
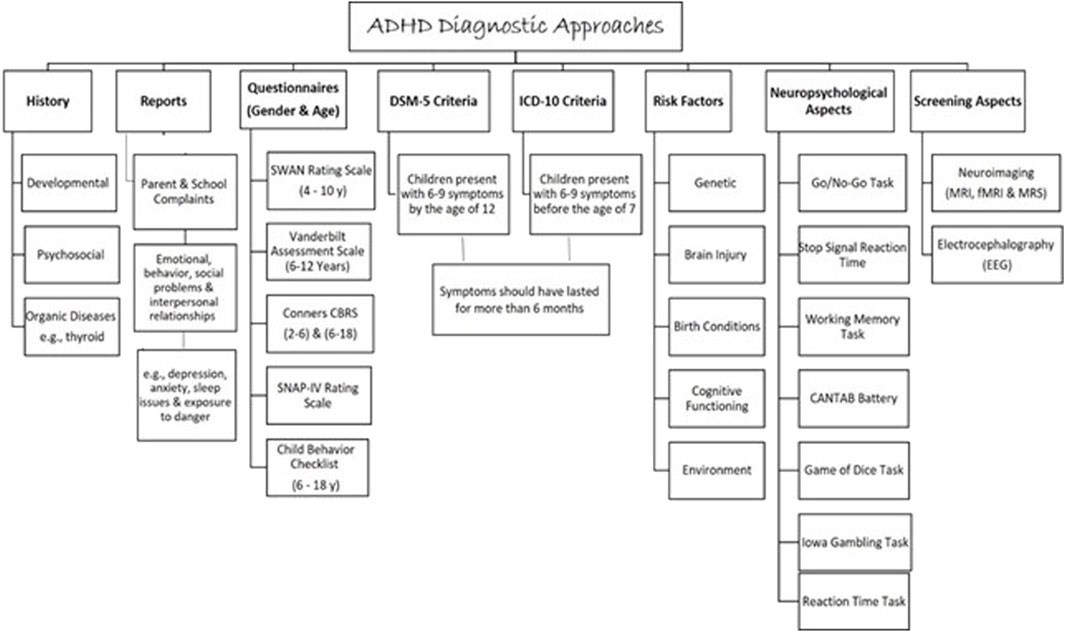
Attention deficit hyperactivity disorder is considered a major public health problem (Ferguson, 2000) as it causes serious impairment at school, home, and society (American Psychiatric Association, 1987). Therefore, to diagnose ADHD, impairment in school performance and daily life is assessed by using a symptoms inventory questionnaire for ADHD described in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) or the International Statistical Classification of Diseases and Related Health Problems (ICD-10) (Bell, 1994; Organization, 2004) (Figure 4). Children diagnosed with ADHD presented with 6–9 symptoms by the age of 12 for (DSM-5) or before the age of 7 for (ICD-10). Symptoms should have lasted for more than 6 months (Bell, 1994). The assessment must be carried out by a psychiatrist, pediatrician, or ADHD specialist. A full developmental and psychosocial history, and physical examination, alongside family and school reports, are essential to diagnose ADHD (Organization, 2004).
Current available ADHD objective assessment includes neuropsychological tests, neuroimaging, and electroencephalography (EEG). Research has shown that individuals with ADHD exhibit distinct genetic anomalies compared to those without the disorder. Unfortunately, there is insufficient evidence on the use of these tests to establish ADHD diagnosis (Kemper et al., 2018). Besides, there is no genetic testing in use to diagnose ADHD regardless of its high heritability (approximately 80%) (Thapar, 2018). However, numerous studies have utilized different types of genetic testing to understand and explore the genetic involvement in ADHD. Gene-wide association studies (GWAS) of single nucleotide polymorphisms (SNPs) and copy number variants (CNVs), next-generation sequencing (NGS), and single nucleotide variants (SNVs) for example, have illustrated the genetic contribution in the ADHD etiology (Hawi et al., 2015; Lima Lde et al., 2016; Grimm et al., 2018). The array of comparative genomic hybridization (aCGH) genetic testing has also helped identify the associated CNVs in many neuropsychiatric disorders including intellectual disability (ID), ASD, and ADHD (Baccarin et al., 2020). In this context, CNVs in multiple chromosomal regions have been linked with ADHD (5p13, 14q12, and 17p11) (Arcos-Burgos et al., 2004; Ogdie et al., 2006). In addition, Demontis et al. (2019) have shown many genetic variants correlated with ADHD on chromosomes 1–5, 7–8, 10, 12, and 15–16 (Demontis et al., 2019).
Given that, it is important to know that ADHD symptoms can overlap with other coexisting conditions, such as the development of substance use, motor control, conduct, language, learning, and mood disorders in children and bipolar, obsessive-compulsive, and personality disorders in adults (Bonati et al., 2018). While severe attention, hyperactivity, and impulsive impairments are hallmarks of ADHD, the disorder sometimes coexists with other neurological conditions, including ASD (Dougnon and Matsui, 2022). Along with repetitive and limited behavior and interests, ASD is linked to poor communication and social interaction abilities. Therefore, differentiation between these disorders during diagnosis is required.
Attention-deficit hyperactivity disorder is associated with a variety of neuropsychological (NP) characteristics and underlying neurobiological pathways (Curatolo et al., 2010). An early dual pathway model distinguished between an inhibitory/executive function pathway and a motivational/delay aversion pathway (also referred to as the “cool” and “hot” executive function pathways in later publications). These pathways are related to different neurobiological networks. Twenty-five years ago, ADHD was diagnosed as a disorder of inhibitory self-control (Castellanos and Tannock, 2002; Castellanos et al., 2006). Although other routes, such as time processing, have been introduced, it is challenging to pinpoint the exact number of pathways that could exist. For example, Coghill and colleagues (2014) distinguished six cognitive characteristics in children with ADHD, including working memory, inhibition, delay aversion, decision-making, timing, and response time variability, using data from seven subtests of the computerized Cambridge NP test battery (Coghill et al., 2014). Other empirical research have distinguished between three (Gomez et al., 2014) or four (Fair et al., 2012) NP profile groups, which all included children with ADHD as well as typically developing (TD) children, differing in severity but not in the type of profile. These studies used latent profile or cluster analysis of NP tasks in large ADHD samples. However, in the process of looking for subgroups, some additional empirical research found performance profiles that were specific to ADHD (poor cognitive control (van Hulst et al., 2015), with attentional lapses and fast processing speed (Bergwerff et al., 2019), among other profiles that were shared with TD controls). Divergent subgrouping outcomes can, of course, also be attributed to different compilations of domains that have been studied, which limits the comparability of these investigations.
3.1 Animal models of ADHD
Characteristics of ADHD are based upon the presence of three main criteria: attention, hyperactivity, and impulsiveness, which must be present before the age of 7. Identifying biological markers to describe the core disorder of ADHD may help to develop new diagnostic and treatment strategies. Several animal models of ADHD have been proposed to provide insight for this purpose although they cannot accurately reflect human psychiatric condition (Sontag et al., 2010; Russell, 2011).
Animal models should meet the necessary validation criteria: 1) face validity, which includes the three core symptoms of ADHD, etiology, treatment, and physiological basis of the modeled disease; 2) construct validity, which conforms to pathophysiological mechanisms; and 3) predictive validity, which is the ability to predict unknown aspects of the ADHD (Viggiano et al., 2004; Sontag et al., 2010; Russell, 2011). Various animal models with distinctly different neural defects (rats, mice, and monkeys) have been proposed to reflect the heterogeneous nature of ADHD, yet none can ensure which model best represents neither the disorder nor its subtypes. These models can be divided into: 1) genetic/trans-genetic, and 2) pharmacological animal models (Table 2) (Kim et al., 2024).
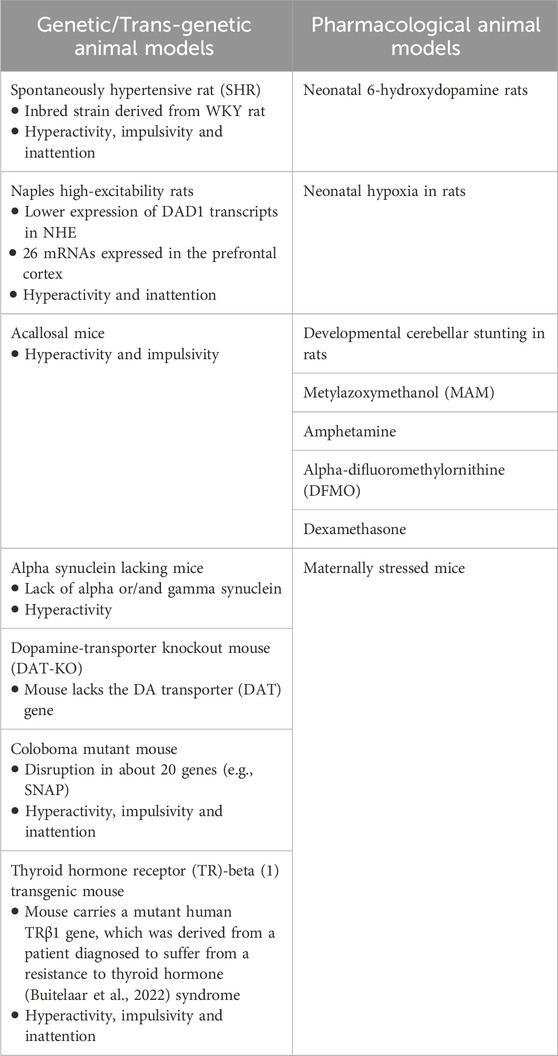
Table 2. Propose animal models in attention-deficit hyperactivity disorder (ADHD) research (Regan et al., 2022; Kim et al., 2024).
Of all the rodent models, research demonstrated that spontaneously hypertensive rat (SHR), a genetic model derived from Wistar-Kyoto (WKY) rats, fulfills many of the validation criteria for ADHD. Nevertheless, it shows several characteristic features of the disorder including inattention, impulsivity, and hyperactivity when compared to normotensive, WKY rats from various tests (Regan et al., 2022). Additionally, SHR shows neuropathological changes similar to those observed in patients with ADHD when compared with the WKY (e.g., smaller prefrontal cortex and hippocampus and fewer neurons). Several studies have reported developmental changes in the expression of dopamine transporter gene (DAT) in specific regions of the brain and dopamine uptake and utilization (Cook et al., 1995; Kirley et al., 2003; Bobb et al., 2005). Decreased expression of DAT4 in the prefrontal cortex and increased density of DAT1 and DAT5 in the neostriatum were observed in young male SHR (Li et al., 2007). However, transgenic animals such as Naples high excitability rat (Kim et al., 2020) performers promise to be useful models for investigating novel genes associated with ADHD in vivo. Other proposed animal models focus on the less important symptom of hyperactivity and may offer an opportunity to explore the role of dopamine in brain illness and assess new ADHD treatments.
4 Genetics and heritability of ADHD
Early molecular genetic research on ADHD aimed to link the disorder with genes that had prior plausibility in its development. Given that medications addressing ADHD primarily act on dopaminergic or noradrenergic transmission, many studies focused on investigating “candidate genes” within these pathways. Variants in genes related to neurotransmitter regulation, such as dopamine (Del Campo et al., 2011) and norepinephrine (Arnsten and Pliszka, 2011) pathways, appear to play a significant role. Furthermore, understanding the gene functions associated with altered brain function in neurodevelopmental disorders can unravel the biological pathways involved in ADHD.
The heritability of ADHD is between 75% and 90% based on twin and family studies. Recent studies have identified a strong hereditary component in ADHD, suggesting that genetics may account for up to 70%–80% of the variance in ADHD risk (Thapar, 2018). Multiple genes are implicated, each exerting a modest effect (Faraone and Larsson, 2019). Furthermore, it was discovered that the heritability of the inattentive and hyperactive-impulsive components of ADHD was comparable in males and females (Faraone and Larsson, 2019). Understanding the genetic underpinnings of ADHD is a complex and evolving field of research. This review delves into the intricate web of genetic factors that contribute to the development and manifestation of ADHD, shedding light on the interplay between genes and this neurodevelopmental disorder. It has recently been demonstrated that there are heritable components linked to ADHD in the structural and functional brain connections evaluated in families with children diagnosed with ADHD (Sudre et al., 2017). In a similar vein, it was discovered that there was a high heritability of the event-related potentials (ERPs) generated in a Go/No-Go test that measured reaction inhibition, which is known to be changed in ADHD (Anokhin et al., 2017).
Twin and family studies provide valuable insights into the genetic basis of traits and diseases. The key idea in twin studies is to compare the within-pair similarities of Monozygotic (MZ) twins to those of Dizygotic twins (DZ). If a trait or characteristic has a stronger correlation within MZ pairs compared to DZ pairs, it suggests a significant genetic influence on that trait. On the other hand, if the resemblance is similar between MZ and DZ pairs, it implies a greater role for environmental factors. Such studies also help researchers understand the mode of inheritance, estimate heritability, identify genetic markers, and unravel the interplay between genes and the environment. Furthermore, information gathered from family and twin studies is often used to design and interpret GWAS, which aims to identify specific genes associated with diseases.
A study with 894 individuals diagnosed with ADHD and 1,135 of their siblings, aged 5–17 years, uncovered a nine-fold increase in the likelihood of ADHD among the siblings of individuals with ADHD when compared to siblings in the control group (Chen et al., 2008). Another twin study conducted by Larsson et al. (2012) applied twin methods to investigate the extent to which ADHD is etiologically distinct from subthreshold variations in ADHD symptoms. Interestingly, when the extreme and subthreshold continuous ADHD characteristic symptoms were evaluated in the Swedish twins, a significant hereditary component was also discovered (Larsson et al., 2012). Family and twin studies have also contributed immensely in identifying and verifying genes that are associated with ADHD. A study by Lasky-Su et al. (2008) investigated 958 ADHD proband-parent trios by genotyping over 600,000 SNPs (Kim et al., 2020). Two novel variants were identified and are located in intronic regions of genes CDH13 and GFOD1. The CDH13 gene provides instructions for producing a calcium-dependent cell-cell adhesion protein crucial for influencing synaptic plasticity and neuronal development. Various genome-wide genetic methods, such as linkage analyses, association studies, and investigations into copy number variations, have identified connections between the CDH13 gene and diverse neurodevelopmental disorders. This suggests that alterations in CDH13 may contribute to the susceptibility or development of conditions impacting the nervous system during early development (Rivero et al., 2015). Another gene that was found to be significantly associated with the criteria is SLC9A9 (Lasky-Su et al., 2008). SLC9A9 is a gene of interest and has also been found to be implicated in other ADHD studies (Zhang-James et al., 2011; Zhang-James et al., 2012). The outcome of such studies underscores a significant genetic impact on the development of ADHD. In essence, the observed heightened risk in siblings of individuals with ADHD highlights the considerable influence of genetic factors in the onset of this neurodevelopmental disorder. This insight enhances our understanding of the complex interplay between genetics and environmental factors in the susceptibility to ADHD.
Genome-wide association studies have been instrumental in identifying specific genetic markers associated with ADHD (Franke et al., 2009). These markers are linked to the disorder and offer insights into related traits and comorbidities (Mehta et al., 2020). The intricate genetic landscape of ADHD involves a complex interplay of risk and protective factors, environmental influences, and gene-environment interactions (Kim et al., 2020). Moreover, epigenetic mechanisms contribute to the dynamic nature of ADHD genetics (Mill and Petronis, 2008). Environmental factors, such as prenatal exposure to certain substances or stressors, can modify gene expression, potentially influencing ADHD susceptibility (Mill and Petronis, 2008). Understanding these epigenetic processes is crucial for a more nuanced comprehension of the disorder’s etiology (Nigg, 2018).
In a genome-wide association (GWA) research meta-analysis of ADHD, Demontis et al. (2023) included 186,843 controls and 38,691 ADHD sufferers. About 27 genome-wide important loci were found, and 76 possible risk genes that are enriched in genes expressed specifically in the early stages of brain development were highlighted. In general, several brain-specific neuronal subtypes and midbrain dopaminergic neurons were linked to the hereditary risk of ADHD (Demontis et al., 2023). GWAS have led to the conclusion that there is an overlap in the genetics of ADHD and Bipolar disorder. For instance, DAT1, recently renamed as SLCA3 (Villani et al., 2018), is hypothesized to be associated with ADHD and has been confirmed to be linked to bipolar disorder. Interestingly, in psychiatry, genetic markers at the DAT1 locus appear to be able to predict clinical heterogeneity since the non-conduct disordered subtype of ADHD is associated with DAT1. In contrast, other subgroups do not appear to be connected. It is becoming increasingly apparent that the candidate genes for bipolar mania and candidate genes for an ADHD subtype are related (Polanczyk et al., 2014). In a study conducted in Oman, researchers investigated the frequency of VNTR alleles in DAT1 and their correlation with ADHD. They genotyped 92 Omani children with ADHD and 110 healthy controls, identifying two common alleles (DAT19 and DAT110). The DAT1*10 allele showed similar distribution between ADHD (64.6%) and control groups (60.9%), but was more prevalent in ADHD males (69.4%) compared to females (55%), a distinction absent in controls (Simsek et al., 2005). Given the high rate of consanguineous marriages in the GCC region, further twin studies and broader research are warranted to explore the genetic foundations of disorders like ADHD in the Arab world. The discovery of novel genetic variants associated with ADHD will foster the early detection of the disease.
Furthermore, epigenetic processes, which regulate gene expression, are proposed as a significant biological marker and potentially a molecular mediator of genetic and environmental influences on ADHD. A high level of DNA methylation has been associated with repression of gene expression, but recent data have shown that this association can vary depending on factors including the location of the methylation in the gene sequence. Several studies have concluded that investigating epigenetic effects on human behavior is challenging because epigenetic changes vary widely from one tissue to another. In the brain alone, DNA methylation can vary considerably from one region to another or from one system to another. However, there are also cross-tissue correlation patterns of individual-specific epigenetic marks, suggesting that DNA methylation studies of peripheral tissue DNA in psychiatry are generally considered informative (Wilmot et al., 2016; Mirkovic et al., 2020).
Vasoactive intestinal peptide receptor 2″encoded by VIPR2″ is present throughout the central nervous system and peripheral tissues. In a methylome-wide exploration of DNA methylation in peripheral tissue Wilmot et al. (2016) examined salivary DNA from children with ADHD compared to non-ADHD controls (Wilmot et al., 2016). They have found that probes in VIPR2 showed decreased CpG methylation in the saliva of children with ADHD. CpG methylation, which is essential for regulating gene expression, impacts a wide range of biological processes, from development to aging and inflammation. CpG islands, located in DNA methylation regions within promoters, are known to control gene expression by silencing transcription, playing a vital role in gene expression and tissue-specific functions (Wilmot et al., 2016).
Mutations in the ST3GAL3 gene, which encodes the Golgi enzyme β-galactoside-α2,3-sialyltransferase-III, are believed to play a role in the development of advanced cognitive abilities. This enzyme is crucial for the addition of sialic acid to glycoproteins and glycolipids, a process essential for proper brain function. Disruptions in this enzyme’s activity due to mutations in ST3GAL3 could potentially affect neural connectivity and signaling, thereby influencing higher-order cognitive functions such as learning, memory, and problem-solving. Research into these mutations helps to understand how specific genetic factors contribute to the complexities of cognitive development (Mijailovic et al., 2022). A genome-wide methylome analysis uncovered that ST3GAL3 undergoes differential methylation throughout the progression of ADHD. This suggests that gene-environment interactions might influence the expression of ST3GAL3, affecting the epigenetic regulation of brain development and maturation (Walton et al., 2017). Rivero et al. (2021) has established when investigating on mice models, that male St3gal3 heterozygous mice exhibit cognitive deficits, while female heterozygous mice show increased activity and improved cognitive control compared to the wildtype mice (Rivero et al., 2021). The research also identified subtle, sex-specific, and region-specific changes in the expression of various markers associated with oligodendrogenesis, myelin formation, protein sialylation, and cell adhesion/synaptic target glycoproteins of ST3GAL3. These findings suggest that ST3GAL3 haploinsufficiency leads to sex-dependent alterations in cognition, behavior, and brain plasticity markers. Additionally, St3gal3-deficient mice displayed behavioral changes similar to those observed in ADHD, such as differences in locomotor activity and cognitive performance, with male heterozygous mice specifically showing memory impairments in place learning tasks, a common issue in ADHD (Rivero et al., 2021).
Despite significant strides, challenges persist in unraveling the complete genetic architecture of ADHD. Heterogeneity within the disorder (Faraone, 2000), the influence of non-genetic factors (Mill and Petronis, 2008), and the intricate nature of gene interactions (Acosta et al., 2011) pose ongoing research challenges. In the realm of personalized medicine, genetic insights into ADHD hold promise for tailoring interventions (Buitelaar et al., 2022). Identifying genetic markers may pave the way for targeted therapies, allowing for more precise and effective treatment approaches (Mehta et al., 2020).
In the context of GWAS, there are only a few studies that directly identified variants with ADHD. The absence of positive findings suggests that the influence of individual common ADHD risk variants is likely to be minor, given the disorder’s high heritability. Alternatively, this prompts consideration that other types of variants, such as rare ones, might play a more significant role in contributing to the heritability of ADHD. These findings suggest that more GWAS should be performed from countries like Saudi Arabia with high consanguinity rates to aid researchers in identifying rare variants that are associated with ADHD.
Differences in linkage disequilibrium (LD) patterns among populations play a pivotal role in influencing the transferability of GWA findings. A variant strongly associated with a trait in one population may not exhibit a detectable association in another population due to differences in LD with the unknown causal variant. Given the pronounced disparities in LD observed among European, East Asian, and Arab populations, it becomes essential to account for these population-specific LD variations when evaluating the transferability of GWA-identified associations.
Research on ADHD in the Arab world, especially in the GCC region, has primarily concentrated on behavioral and epidemiological studies, with limited attention to the genetic and epigenetic factors underlying the disorder. The Arab population, characterized by large family sizes, high rates of consanguinity, and frequent first-cousin marriages, presents a unique genetic landscape that leads to increased homozygosity for both monogenic and polygenic diseases, and the accumulation of deleterious recessive alleles. This distinctive genetic profile makes the Arab population particularly valuable for genetic and epigenetic research, including twin and extended family studies, which could offer profound insights into the complex interplay between genetics and health. Despite these opportunities, the representation of the Arab population in global GWAS is inadequate, and the applicability of GWA findings to this group remains underexplored. Expanding genetic and epigenetic research on ADHD in the GCC is crucial to ensure that findings from global studies are relevant and beneficial to the Arab population, and to enhance our understanding of the disorder’s biological foundations in this unique context.
5 Epidemiology and prevalence of ADHD
Although prevalence rates and reported changes in the prevalence of ADHD vary greatly depending on country and region, technique, and sample, it appears to be a global phenomenon (Polanczyk et al., 2015). In 2014, Polanczyk et al. conducted a meta-analysis study that revealed a 5.8% global prevalence rate for children and adolescents (Polanczyk et al., 2014). Different criteria for identifying ADHD are causing slightly higher (e.g., 7.2%) prevalence percentages reported by other meta-analyses conducted by Thomas et al. (2015). Peak prevalence may be significantly greater in some age groups, such as 13% in 9-year-old boys although prevalence statistics in children and adolescents represent average values across the entire age range (Bachmann et al., 2017). The estimated prevalence of ADHD in adults worldwide is 2.8% with greater rates observed in high-income nations (3.6%) compared to low-income ones (1.4%) (Bachmann et al., 2017).
5.1 Current status of ADHD in Arabian Gulf Nations
The six Arab Gulf states that make up the Gulf Cooperation Council (GCC) consist of Bahrain, Kuwait, Oman, Qatar, the United Arab Emirates, and Saudi Arabia, which is the largest country, and are all disproportionately affected by the “youth bulge” with a combined population of approximately 54 million (Thygesen et al., 2021). However, the GCC is classified as in the “second phase of demography in transition” (Omran, 2005). As a result, this stage is frequently distinguished by high rates of conception and births and the prevalence of a population structure resembling a pyramid-like population structure. Systematic clinical data in this regard point to a significant number of children with disabilities in the GCC (Chan et al., 2021). This might be caused by the high rates of polygamous marriages and consanguinity among married couples, especially among older males, as well as high fertility and frequent childbirth. Moreover, the majority of GCC nations have not yet created policies to assist young people with special needs (Qoronfleh et al., 2019). Although it is still in its early stages, research on the prevalence of ADHD diseases in the GCC has emerged (Farah et al., 2009). To fill this gap in the literature, this study aimed to critically review the literature on the prevalence rates of ADHD disorders in the GCC (Table 3). A glimpse into the literature shows that ADHD disorder has received some attention in this region. In a research of PubMed, a total of 33 studies from the six countries were found (Chan et al., 2021). Focusing on the genetics of ADHD, only eight studies were found in the KSA, one in UAE, Kuwait, Qatar, and Oman.
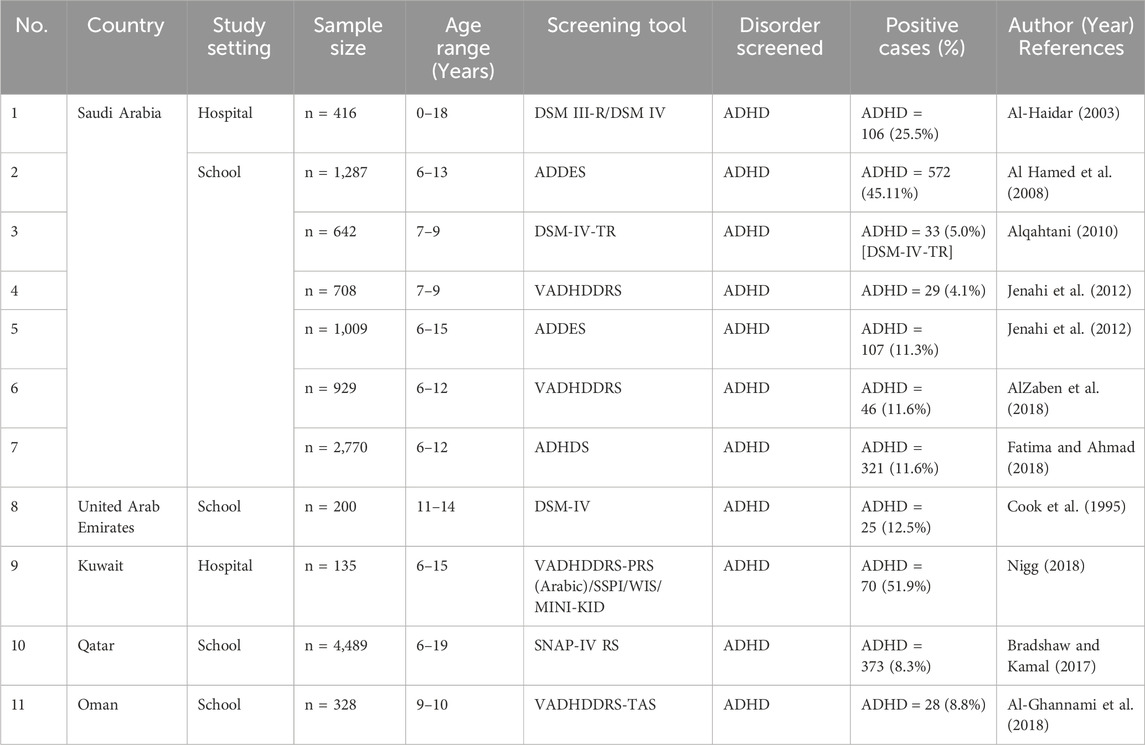
Table 3. Present studies conducted in the Gulf Cooperation Council (GCC) region. ADHD, attention-deficit hyperactivity disorder.
Research specifically focused on the genetics of ADHD within GCC populations is very limited. Most of the genetic studies on ADHD have been conducted in Western populations (Larsson et al., 2006; Thomas et al., 2015). However, genetic factors contributing to ADHD are expected to be similar across different populations due to the high heritability of the disorder (Faraone and Larsson, 2019). Generally, the GCC appears to be among the growing economies where the environmental aspect plays an important role in population health. In addition to genetic factors, cultural, social, and environmental factors in the GCC can influence the prevalence and presentation of ADHD (Zheng et al., 2020). For example, in 2022, a study by Setyanisa et al. presented those differences in educational systems, parenting styles, and societal attitudes toward mental health can impact the diagnosis and management of ADHD (Setyanisa et al., 2022). While the core genetic risk factors for ADHD are likely to be similar globally, there may be population-specific genetic variants in the GCC countries due to genetic drift, founder effects, and consanguinity, which is more common in the GCC than in many other regions specifically in Saudi Arabia (Bakry et al., 2023).
5.2 Consanguinity in Saudi Arabia
The Arabian Peninsula is known for its high rate of consanguinity. In Saudi Arabia, consanguineous marriage accounted for 52.0% of marriages, with an average inbreeding value of 0.0312 (Al-Abdulkareem and Ballal, 1998). Consanguineous marriage is one of the main risk factors that contribute to the spread of genetic syndrome. Therefore, there is a significant prevalence of genetic abnormalities in Saudi Arabia (Bakry et al., 2023). Several neurodevelopmental disorders were studied genetically and several variants were reported globally. However, Arab countries specifically Saudi Arabia need more attention to discover the genetic route of ADHD. In this land of the world, the genetic cause of ADHD is still a mystery due to the high rate of consanguinity.
In consanguineous populations such as Saudi Arabia, the probability of both parents carrying the same recessive gene mutation is significantly higher compared to low rates of consanguinity populations (Hamamy, 2012). Accordingly, the incidence of autosomal recessive disorders is more frequent in populations with a high rate of consanguinity (Khayat et al., 2024). High rates of consanguinity can increase the prevalence of certain genetic disorders, including those with complex inheritance patterns (Mahrous et al., 2023; Semlali et al., 2018a; Semlali et al., 2018b; Hawsawi et al., 2019), and ADHD. ADHD is a multifactorial disorder, meaning multiple genes and environmental factors influence it (Zayats and Neale, 2019). Certain genetic variants have been associated with an increased risk of ADHD, affecting neurotransmitter systems such as dopamine and serotonin (Yadav et al., 2021). Consanguineous marriages increase the probability of inheriting identical genetic variants from both parents. This can increase the risk of recessive genetic disorders and also contribute to the expression of complex genetic traits, including ADHD (Jackson et al., 2018). The increased genetic homogeneity in consanguineous populations can lead to a higher prevalence of certain alleles that may contribute to ADHD.
Studies in Saudi Arabia have identified a higher prevalence of certain genetic disorders due to high rates of consanguinity. Unfortunately, research specific to ADHD in consanguineous populations is limited but suggests a potential link between consanguinity and the genetic factors contributing to ADHD (Khayat et al., 2024). Therefore, the higher rates of ADHD in consanguineous populations could necessitate tailored public health strategies, including genetic counseling and targeted interventions. Understanding the genetic link can help in developing specific diagnostic tools and treatments for ADHD in populations with high consanguinity rates.
While the direct link between consanguinity and ADHD genetics in Saudi Arabia requires more research, the increased prevalence of genetic variants in consanguineous populations can contribute to a higher incidence of ADHD. This underscores the importance of genetic studies and targeted healthcare strategies in these populations (Alrefaei et al., 2022).
6 Current treatment and management of ADHD
Living with ADHD presents a unique set of challenges and opportunities. While the journey may be marked by moments of distraction and impulsivity (Wiklund et al., 2017), individuals with ADHD often possess remarkable creativity and an ability to think outside the box (White and Shah, 2011) (Figure 1). Attention deficit hyperactivity disorder is not a one-size-fits-all condition; it manifests differently in each person. Some may struggle with maintaining focus (Aliabadi et al., 2016), while others may grapple with impulsive decision-making (Hayashibara et al., 2023). However, it is crucial to recognize that ADHD is not a deficit of attention but a difference in attention (Baas et al., 2008). Embracing this perspective allows individuals to tap into their strengths and cultivate coping mechanisms (Fredrickson, 2000). One of the keys to navigating life with ADHD is developing personalized strategies (Lazar and Stein, 2017) (Figure 5). From utilizing visual aids and setting clear routines to breaking tasks into manageable chunks, individuals with ADHD can enhance their productivity and overall wellbeing (Bawden and Robinson, 2009; Spiel et al., 2022). By adopting a proactive mentality, people can transform possible obstacles into chances for development (Caniëls et al., 2018).
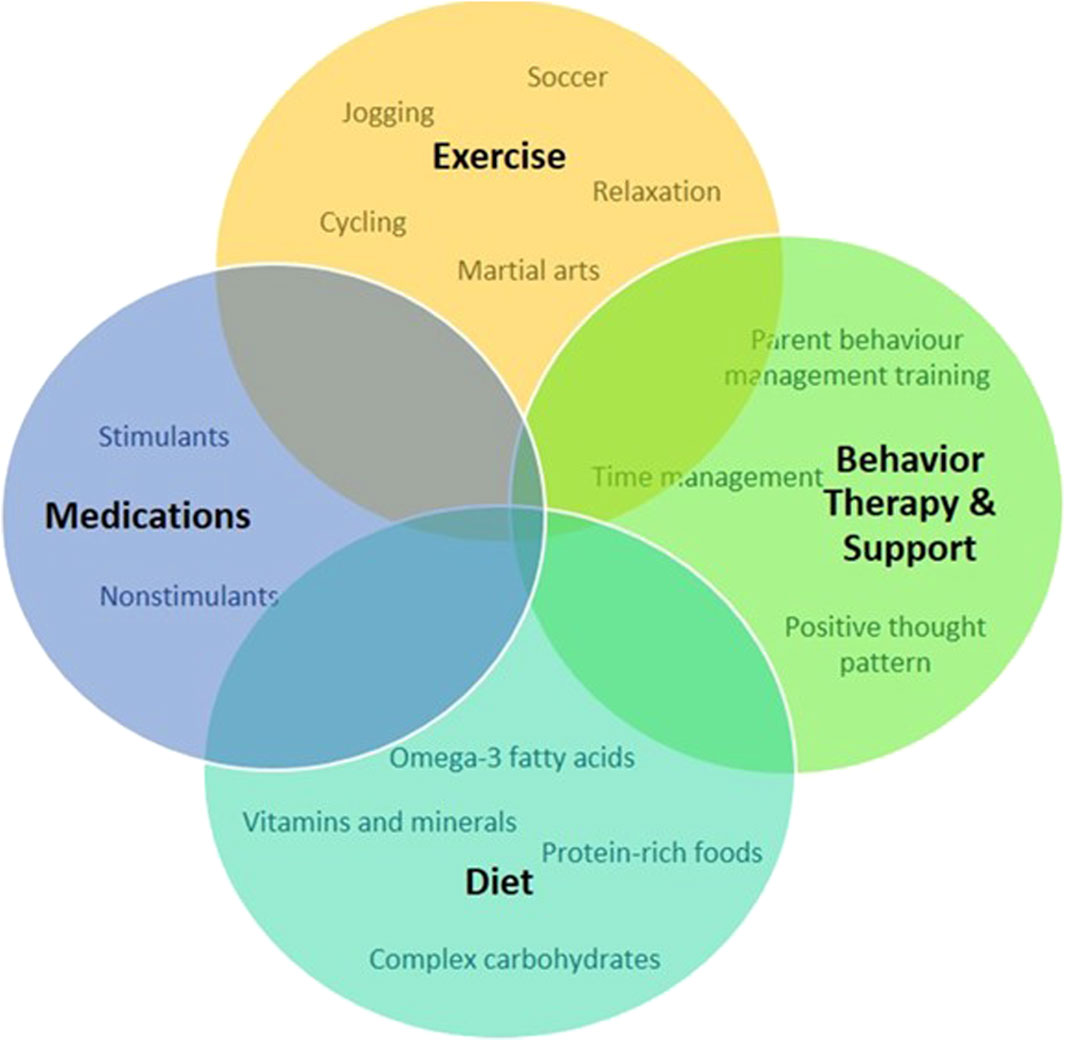
Figure 5. Schematic representation of approaches to treat and manage symptoms and promote positive behavior for individuals with ADHD.
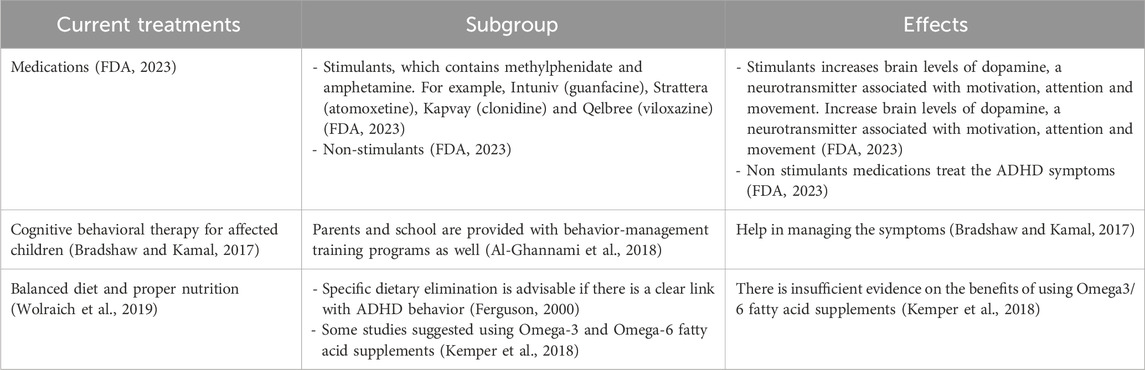
Support from friends, family, and the community plays a pivotal role in empowering individuals with ADHD (Craig et al., 2020; Claussen et al., 2022). Educating those around us about the nuances of ADHD fosters understanding (Claussen et al., 2022). Creating an environment that embraces neurodiversity not only reduces stigma but also encourages the celebration of unique perspectives and talents (Armstrong, 2012). In the workplace, accommodations that cater to the specific needs of individuals with ADHD can significantly enhance their performance (Sarkis, 2014). Flexible work arrangements, clear communication channels, and recognition of accomplishments contribute to a positive and inclusive professional environment (Kirby and Smith, 2021). While medication may be a part of the treatment plan for some (Dalton, 2013), it is important to emphasize that there’s no one-size-fits-all solution for managing ADHD (Coghill, 2017). Standard treatment and management for ADHD involve medication, behavioral intervention, nutrition, and supplements is presented in Table 4. Holistic approaches, including mindfulness, exercise, and proper nutrition, can complement traditional treatments and contribute to overall wellbeing (Kobe, 2023). In conclusion, living with ADHD is a journey filled with both challenges and opportunities. By fostering understanding, embracing uniqueness, and developing personalized strategies, individuals with ADHD can navigate life successfully while contributing their distinct talents to the world.
7 Conclusion
The characteristic features of individuals with ADHD include hyperactivity, inattentiveness, and impulsiveness, which lead to impairments in daily living. Research recognized that ADHD was not just a childhood disorder, that disappeared with age as was previously thought. Scientists have been trying to identify the causes of ADHD. It is a clinically and genetically heterogeneous syndrome that is comorbid with childhood conduct disorder, alcoholism, substance abuse, antisocial personality disorder, and affective disorders. The ADHD consistent overlap with autistic symptoms has also been established. Research points to a strong genetic link, yet it is not clear what role environmental factors play in determining who develops ADHD. Psychoeducation, counseling, supportive problem-directed therapy, behavioral intervention, coaching, cognitive remediation, and couples and family therapy are useful adjuncts to medication management.
In conclusion, this comprehensive review underscores the intricate genetic landscape of ADHD. While considerable progress has been made in identifying genetic markers and understanding heritability, continued research efforts are needed to unravel the full complexity of ADHD genetics in different populations, offering hope for more individualized and effective interventions in the future.
8 Future perspectives
In the realm of ADHD research in the Arab World, it is imperative to focus on several key aspects for future advancements. First and foremost, there is a critical need to enhance cultural awareness and education surrounding ADHD, dispelling misconceptions, and promoting understanding within the Arab community. Additionally, research efforts should span different demographic levels, exploring variations in ADHD prevalence across urban and rural settings, socioeconomic factors, and access to healthcare resources. Early intervention programs tailored to the unique needs of the Arab population should be established, with training initiatives for educators, parents, and healthcare professionals. Given the cultural landscape, investigating the correlation between consanguinity and ADHD prevalence is crucial, necessitating genetic research to identify specific risk factors. Moreover, GWA and genetic mapping studies should be actively pursued in the Arab World to provide a more holistic insight into the genetic underpinnings of ADHD. Furthermore, community engagement initiatives and support networks can contribute to a more inclusive and understanding environment for individuals affected by ADHD. Finally, improvements in healthcare infrastructure are essential to ensure widespread access to accurate diagnosis and effective treatment, addressing disparities and making ADHD interventions more accessible throughout the Arab World.
Author contributions
NM: Data curation, Validation, Visualization, Writing–original draft, Writing–review and editing, Formal Analysis, Software. AA: Formal Analysis, Visualization, Writing–original draft, Writing–review and editing, Investigation. RS: Investigation, Visualization, Writing–original draft, Writing–review and editing, Conceptualization, Project administration, Supervision, Validation. BK: Investigation, Visualization, Writing–original draft, Writing–review and editing, Data curation. MK: Data curation, Investigation, Visualization, Writing–original draft, Writing–review and editing, Formal Analysis, Funding acquisition, Project administration, Validation. YH: Data curation, Project administration, Validation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work has been funded by the King Salman Center for Disability Research for their financial support under grant no: KSRG-2023-145.
Acknowledgments
NNM would like to thank the University of Hafr Al-Batin and YH would like to thank King Faisal Specialist Hospital & Research Centre (Gen. Org) (KFSH and RC-Jed) for their support and approval (IRB approval # 2024-19).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelnour, E., Jansen, M. O., and Gold, J. A. (2022). ADHD diagnostic trends: increased recognition or overdiagnosis? Mo Med. 119 (5), 467–473.
Acosta, M., Velez, J., Bustamante, M., Balog, J., Arcos-Burgos, M., and Muenke, M. (2011). A two-locus genetic interaction between LPHN3 and 11q predicts ADHD severity and long-term outcome. Transl. Psychiatry 1 (7), e17. doi:10.1038/tp.2011.14
Al-Abdulkareem, A. A., and Ballal, S. G. (1998). Consanguineous marriage in an urban area of Saudi Arabia: rates and adverse health effects on the offspring. J. Community Health 23 (1), 75–83. doi:10.1023/a:1018727005707
Al-Ghannami, S. S., Al-Adawi, S., Ghebremeskel, K., Cramer, M. T., Hussein, I. S., Min, Y., et al. (2018). Attention deficit hyperactivity disorder and parental factors in school children aged nine to ten years in muscat, Oman. Oman Med. J. 33 (3), 193–199. doi:10.5001/omj.2018.37
Al-Haidar, F. A. (2003). Co-morbidity and treatment of attention deficit hyperactivity disorder in Saudi Arabia. East Mediterr. Health J. 9 (5-6), 988–995. doi:10.26719/2003.9.5-6.988
Al Hamed, J. H., Taha, A. Z., Sabra, A. A., and Bella, H. (2008). Attention deficit hyperactivity disorder (ADHD) among male primary school children in Dammam, Saudi Arabia: prevalence and associated factors. J. Egypt Public Health Assoc. 83 (3-4), 165–182.
Aliabadi, B., Davari-Ashtiani, R., Khademi, M., and Arabgol, F. (2016). Comparison of creativity between children with and without attention deficit hyperactivity disorder: a case-control study. Iran. J. psychiatry 11 (2), 99–103.
Alqahtani, M. M. (2010). Attention-deficit hyperactive disorder in school-aged children in Saudi Arabia. Eur. J. Pediatr. 169 (9), 1113–1117. doi:10.1007/s00431-010-1190-y
Alrefaei, A. F., Hawsawi, Y. M., Almaleki, D., Alafif, T., Alzahrani, F. A., and Bakhrebah, M. A. (2022). Genetic data sharing and artificial intelligence in the era of personalized medicine based on a cross-sectional analysis of the Saudi human genome program. Sci. Rep. 12 (1), 1405. doi:10.1038/s41598-022-05296-7
AlZaben, F. N., Sehlo, M. G., Alghamdi, W. A., Tayeb, H. O., Khalifa, D. A., Mira, A. T., et al. (2018). Prevalence of attention deficit hyperactivity disorder and comorbid psychiatric and behavioral problems among primary school students in western Saudi Arabia. Saudi Med. J. 39 (1), 52–58. doi:10.15537/smj.2018.1.21288
American Academy of Pediatrics. (2001). Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics 108 (4), 1033–1044. doi:10.1542/peds.108.4.1033
Anokhin, A. P., Golosheykin, S., Grant, J. D., and Heath, A. C. (2017). Heritability of brain activity related to response inhibition: a longitudinal genetic study in adolescent twins. Int. J. Psychophysiol. 115, 112–124. doi:10.1016/j.ijpsycho.2017.03.002
Arcos-Burgos, M., Castellanos, F. X., Pineda, D., Lopera, F., Palacio, J. D., Palacio, L. G., et al. (2004). Attention-deficit/hyperactivity disorder in a population isolate: linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11. Am. J. Hum. Genet. 75 (6), 998–1014. doi:10.1086/426154
Armstrong, T. (2012). Neurodiversity in the classroom: strength-based strategies to help students with special needs succeed in school and life. United States: ASCD.
Arnsten, A. F., and Pliszka, S. R. (2011). Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol. Biochem. Behav. 99 (2), 211–216. doi:10.1016/j.pbb.2011.01.020
American Psychiatric Association (1987). Diagnostic and statistical manual of mental health disorders (DSM-III-R). American Psychiatric Association.
Baas, M., De Dreu, C. K., and Nijstad, B. A. (2008). A meta-analysis of 25 years of mood-creativity research: hedonic tone, activation, or regulatory focus? Psychol. Bull. 134 (6), 779–806. doi:10.1037/a0012815
Baccarin, M., Picinelli, C., Tomaiuolo, P., Castronovo, P., Costa, A., Verdecchia, M., et al. (2020). Appropriateness of array-CGH in the ADHD clinics: a comparative study. Genes Brain Behav. 19 (6), e12651. doi:10.1111/gbb.12651
Bachmann, C. J., Philipsen, A., and Hoffmann, F. (2017). ADHD in Germany: trends in diagnosis and pharmacotherapy. Dtsch. Arztebl Int. 114 (9), 141–148. doi:10.3238/arztebl.2017.0141
Bakry, H., Alaiban, R. A., Alkhyyat, A. A., Alshamrani, B. H., Naitah, R. N., and Almoayad, F. (2023). Predictors of consanguinity marriage decision in Saudi Arabia: a pilot study. Healthc. (Basel) 11 (13), 1925. doi:10.3390/healthcare11131925
Bawden, D., and Robinson, L. (2009). The dark side of information: overload, anxiety and other paradoxes and pathologies. J. Inf. Sci. 35 (2), 180–191. doi:10.1177/0165551508095781
Bell, C. C. (1994). DSM-IV: diagnostic and statistical manual of mental disorders. Jama 272 (10), 828–829. doi:10.1001/jama.1994.03520100096046
Bergwerff, C. E., Luman, M., Weeda, W. D., and Oosterlaan, J. (2019). Neurocognitive profiles in children with ADHD and their predictive value for functional outcomes. J. Atten. Disord. 23 (13), 1567–1577. doi:10.1177/1087054716688533
Biederman, J., Monuteaux, M. C., Mick, E., Spencer, T., Wilens, T. E., Silva, J. M., et al. (2006). Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psychol. Med. 36 (2), 167–179. doi:10.1017/S0033291705006410
Bobb, A. J., Castellanos, F. X., Addington, A. M., and Rapoport, J. L. (2005). Molecular genetic studies of ADHD: 1991 to 2004. Am. J. Med. Genet. B Neuropsychiatr. Genet. 132B (1), 109–125.
Bonati, M., Reale, L., Zanetti, M., Cartabia, M., Fortinguerra, F., Capovilla, G., et al. (2018). A regional ADHD center-based network project for the diagnosis and treatment of children and adolescents with ADHD. J. Atten. Disord. 22 (12), 1173–1184. doi:10.1177/1087054715599573
Bradshaw, L. G., and Kamal, M. (2017). Prevalence of ADHD in Qatari school-age children. J. Atten. Disord. 21 (5), 442–449. doi:10.1177/1087054713517545
Buitelaar, J., Bölte, S., Brandeis, D., Caye, A., Christmann, N., Cortese, S., et al. (2022). Toward precision medicine in ADHD. Front. Behav. Neurosci. 16, 900981. doi:10.3389/fnbeh.2022.900981
Burd, L., and Kerbeshian, J. (1988). Historical roots of ADHD. J. Am. Acad. Child. Adolesc. Psychiatry 27 (2), 262. doi:10.1097/00004583-198803000-00029
Caniëls, M. C., Semeijn, J. H., and Renders, I. H. (2018). Mind the mindset! The interaction of proactive personality, transformational leadership and growth mindset for engagement at work. Career Dev. Int. 23 (1), 48–66. doi:10.1108/cdi-11-2016-0194
Castellanos, F. X., Lee, P. P., Sharp, W., Jeffries, N. O., Greenstein, D. K., Clasen, L. S., et al. (2002). Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 288 (14), 1740–1748. doi:10.1001/jama.288.14.1740
Castellanos, F. X., Sonuga-Barke, E. J., Milham, M. P., and Tannock, R. (2006). Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn. Sci. 10 (3), 117–123. doi:10.1016/j.tics.2006.01.011
Castellanos, F. X., and Tannock, R. (2002). Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat. Rev. Neurosci. 3 (8), 617–628. doi:10.1038/nrn896
Chan, M. F., Al Balushi, R., Al Falahi, M., Mahadevan, S., Al Saadoon, M., and Al-Adawi, S. (2021). Child and adolescent mental health disorders in the GCC: a systematic review and meta-analysis. Int. J. Pediatr. Adolesc. Med. 8 (3), 134–145. doi:10.1016/j.ijpam.2021.04.002
Chen, W., Zhou, K., Sham, P., Franke, B., Kuntsi, J., Campbell, D., et al. (2008). DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B (8), 1450–1460. doi:10.1002/ajmg.b.30672
Claussen, A. H., Holbrook, J. R., Hutchins, H. J., Robinson, L. R., Bloomfield, J., Meng, L., et al. (2022). All in the family? A systematic review and meta-analysis of parenting and family environment as risk factors for attention-deficit/hyperactivity disorder (ADHD) in children. Prev. Sci. 25, 249–271. doi:10.1007/s11121-022-01358-4
Coghill, D. (2017). Organisation of services for managing ADHD. Epidemiol. Psychiatric Sci. 26 (5), 453–458. doi:10.1017/S2045796016000937
Coghill, D. R., Seth, S., and Matthews, K. (2014). A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychol. Med. 44 (9), 1989–2001. doi:10.1017/S0033291713002547
Cook, E. H., Stein, M. A., Krasowski, M. D., Cox, N. J., Olkon, D. M., Kieffer, J. E., et al. (1995). Association of attention-deficit disorder and the dopamine transporter gene. Am. J. Hum. Genet. 56 (4), 993–998.
Craig, F., Savino, R., Fanizza, I., Lucarelli, E., Russo, L., and Trabacca, A. (2020). A systematic review of coping strategies in parents of children with attention deficit hyperactivity disorder (ADHD). Res. Dev. Disabil. 98, 103571. doi:10.1016/j.ridd.2020.103571
Curatolo, P., D’Agati, E., and Moavero, R. (2010). The neurobiological basis of ADHD. Ital. J. Pediatr. 36 (1), 79. doi:10.1186/1824-7288-36-79
Dalton, N. S. (2013). Neurodiversity and HCI. in CHI 13 Extended abstracts on human factors in computing systems, 2295–2304.
Del Campo, N., Chamberlain, S. R., Sahakian, B. J., and Robbins, T. W. (2011). The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol. Psychiatry 69 (12), e145–e157. doi:10.1016/j.biopsych.2011.02.036
Demontis, D., Walters, G. B., Athanasiadis, G., Walters, R., Therrien, K., Nielsen, T. T., et al. (2023). Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat. Genet. 55 (2), 198–208. doi:10.1038/s41588-022-01285-8
Demontis, D., Walters, R. K., Martin, J., Mattheisen, M., Als, T. D., Agerbo, E., et al. (2019). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 51 (1), 63–75. doi:10.1038/s41588-018-0269-7
Dougnon, G., and Matsui, H. (2022). Modelling autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) using mice and zebrafish. Int. J. Mol. Sci. 23 (14), 7550. doi:10.3390/ijms23147550
Drechsler, R., Brem, S., Brandeis, D., Grunblatt, E., Berger, G., and Walitza, S. (2020). ADHD: current concepts and treatments in children and adolescents. Neuropediatrics 51 (5), 315–335. doi:10.1055/s-0040-1701658
Fair, D. A., Bathula, D., Nikolas, M. A., and Nigg, J. T. (2012). Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc. Natl. Acad. Sci. U. S. A. 109 (17), 6769–6774. doi:10.1073/pnas.1115365109
Farah, L. G., Fayyad, J. A., Eapen, V., Cassir, Y., Salamoun, M. M., Tabet, C. C., et al. (2009). ADHD in the Arab world: a review of epidemiologic studies. J. Atten. Disord. 13 (3), 211–222. doi:10.1177/1087054708325976
Faraone, S. V. (2000). Genetics of childhood disorders: XX. ADHD, Part 4: is ADHD genetically heterogeneous? J. Am. Acad. Child Adolesc. Psychiatry 39 (11), 1455–1457. doi:10.1097/00004583-200011000-00022
Faraone, S. V., and Larsson, H. (2019). Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 24 (4), 562–575. doi:10.1038/s41380-018-0070-0
Fatima, W., and Ahmad, L. M. (2018). Prevalence of disordered eating attitudes among adolescent girls in Arar City, Kingdom of Saudi Arabia. Health Psychol. Res. 6 (1), 7444. doi:10.4081/hpr.2018.7444
FDA (2023). Treating and dealing with ADHD. Available at: https://www.fda.gov/consumers/consumer-updates/treating-and-dealing-adhd (Accessed October 29, 2023).
Ferguson, J. H. (2000). National institutes of health consensus development conference statement: diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD). J. Am. Acad. Child Adolesc. Psychiatry 39 (2), 182–193. doi:10.1097/00004583-200002000-00018
Foreman, D. M. (2006). Attention deficit hyperactivity disorder: legal and ethical aspects. Arch. Dis. Child. 91 (2), 192–194. doi:10.1136/adc.2004.064576
Franke, B., Neale, B. M., and Faraone, S. V. (2009). Genome-wide association studies in ADHD. Hum. Genet. 126, 13–50. doi:10.1007/s00439-009-0663-4
Fredrickson, B. L. (2000). Cultivating positive emotions to optimize health and well-being. Prev. Treat. 3 (1), 1a. doi:10.1037/1522-3736.3.1.31a
Gehricke, J. G., Kruggel, F., Thampipop, T., Alejo, S. D., Tatos, E., Fallon, J., et al. (2017). The brain anatomy of attention-deficit/hyperactivity disorder in young adults - a magnetic resonance imaging study. PLoS One 12 (4), e0175433. doi:10.1371/journal.pone.0175433
Gomez, R., Gomez, R. M., Winther, J., and Vance, A. (2014). Latent profile analysis of working memory performance in a sample of children with ADHD. J. Abnorm Child. Psychol. 42 (8), 1367–1379. doi:10.1007/s10802-014-9878-5
Grimm, O., Kittel-Schneider, S., and Reif, A. (2018). Recent developments in the genetics of attention-deficit hyperactivity disorder. Psychiatry Clin. Neurosci. 72 (9), 654–672. doi:10.1111/pcn.12673
Hamamy, H. (2012). Consanguineous marriages: preconception consultation in primary health care settings: preconception consultation in primary health care settings. J. Community Genet. 3 (3), 185–192. doi:10.1007/s12687-011-0072-y
Hawi, Z., Cummins, T. D., Tong, J., Johnson, B., Lau, R., Samarrai, W., et al. (2015). The molecular genetic architecture of attention deficit hyperactivity disorder. Mol. Psychiatry 20 (3), 289–297. doi:10.1038/mp.2014.183
Hawsawi, Y. M., Al-Numair, N. S., Sobahy, T. M., Al-Ajmi, A. M., Al-Harbi, R. M., Baghdadi, M. A., et al. (2019). The role of BRCA1/2 in hereditary and familial breast and ovarian cancers. Mol. Genet. Genomic Med. 7 (9), e879. doi:10.1002/mgg3.879
Hayashibara, E., Savickaite, S., and Simmons, D. (2023). Creativity and neurodiversity: towards an inclusive creativity measure for autism and ADHD.
Jackson, M., Marks, L., May, G. H. W., and Wilson, J. B. (2018). The genetic basis of disease. Essays Biochem. 62 (5), 643–723. doi:10.1042/EBC20170053
Jenahi, E., Khalil, M. S., and Bella, H. (2012). Prevalence of attention deficit hyperactivity symptoms in female schoolchildren in Saudi Arabia. Ann. Saudi Med. 32 (5), 462–468. doi:10.5144/0256-4947.2012.462
Kemper, A. R., Maslow, G. R., Hill, S., Namdari, B., NM, A. L., Goode, A. P., et al. (2018). Attention deficit hyperactivity disorder: diagnosis and treatment in children and adolescents.
Khayat, A. M., Alshareef, B. G., Alharbi, S. F., AlZahrani, M. M., Alshangity, B. A., and Tashkandi, N. F. (2024). Consanguineous marriage and its association with genetic disorders in Saudi Arabia: a review. Cureus 16 (2), e53888. doi:10.7759/cureus.53888
Kim, D., Yadav, D., and Song, M. (2024). An updated review on animal models to study attention-deficit hyperactivity disorder. Transl. Psychiatry 14 (1), 187. doi:10.1038/s41398-024-02893-0
Kim, J. H., Kim, J. Y., Lee, J., Jeong, G. H., Lee, E., Lee, S., et al. (2020). Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. Lancet Psychiatry 7 (11), 955–970. doi:10.1016/S2215-0366(20)30312-6
Kirby, A., and Smith, T. (2021). Neurodiversity at work: drive innovation, performance and productivity with a neurodiverse workforce. United Kingdom: Kogan Page Publishers.
Kirley, A., Lowe, N., Hawi, Z., Mullins, C., Daly, G., Waldman, I., et al. (2003). Association of the 480 bp DAT1 allele with methylphenidate response in a sample of Irish children with ADHD. Am. J. Med. Genet. B Neuropsychiatr. Genet. 121 (1), 50–54. doi:10.1002/ajmg.b.20071
Konrad, A., Dielentheis, T. F., El Masri, D., Dellani, P. R., Stoeter, P., Vucurevic, G., et al. (2012). White matter abnormalities and their impact on attentional performance in adult attention-deficit/hyperactivity disorder. Eur. Arch. Psychiatry Clin. Neurosci. 262 (4), 351–360. doi:10.1007/s00406-011-0251-1
Lange, K. W., Reichl, S., Lange, K. M., Tucha, L., and Tucha, O. (2010). The history of attention deficit hyperactivity disorder. Atten. Defic. Hyperact. Disord. 2 (4), 241–255. doi:10.1007/s12402-010-0045-8
Larsson, H., Anckarsater, H., Rastam, M., Chang, Z., and Lichtenstein, P. (2012). Childhood attention-deficit hyperactivity disorder as an extreme of a continuous trait: a quantitative genetic study of 8,500 twin pairs. J. Child. Psychol. Psychiatry 53 (1), 73–80. doi:10.1111/j.1469-7610.2011.02467.x
Larsson, H., Lichtenstein, P., and Larsson, J. O. (2006). Genetic contributions to the development of ADHD subtypes from childhood to adolescence. J. Am. Acad. Child. Adolesc. Psychiatry 45 (8), 973–981. doi:10.1097/01.chi.0000222787.57100.d8
Lasky-Su, J., Neale, B. M., Franke, B., Anney, R. J., Zhou, K., Maller, J. B., et al. (2008). Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B (8), 1345–1354. doi:10.1002/ajmg.b.30867
Lazar, J., and Stein, M. A. (2017). Disability, human rights, and information technology. University of Pennsylvania Press.
Li, Q., Lu, G., Antonio, G. E., Mak, Y. T., Rudd, J. A., Fan, M., et al. (2007). The usefulness of the spontaneously hypertensive rat to model attention-deficit/hyperactivity disorder (ADHD) may be explained by the differential expression of dopamine-related genes in the brain. Neurochem. Int. 50 (6), 848–857. doi:10.1016/j.neuint.2007.02.005
Lima Lde, A., Feio-dos-Santos, A. C., Belangero, S. I., Gadelha, A., Bressan, R. A., Salum, G. A., et al. (2016). An integrative approach to investigate the respective roles of single-nucleotide variants and copy-number variants in Attention-Deficit/Hyperactivity Disorder. Sci. Rep. 6, 22851. doi:10.1038/srep22851
Mahrous, N. N., Jamous, Y. F., Almatrafi, A. M., Fallatah, D. I., Theyab, A., Alanati, B. H., et al. (2023). A current landscape on Alport syndrome cases: characterization, therapy and management perspectives. Biomedicines 11 (10), 2762. doi:10.3390/biomedicines11102762
Mehta, T., Mannem, N., Yarasi, N. K., and Bollu, P. C. (2020). Biomarkers for ADHD: the present and future directions. Curr. Dev. Disord. Rep. 7, 85–92. doi:10.1007/s40474-020-00196-9
Mijailovic, N. R., Vesic, K., Arsenijevic, D., Milojevic-Rakic, M., and Borovcanin, M. M. (2022). Galectin-3 involvement in cognitive processes for new therapeutic considerations. Front. Cell Neurosci. 16, 923811. doi:10.3389/fncel.2022.923811
Mill, J., and Petronis, A. (2008). Pre-and peri-natal environmental risks for attention-deficit hyperactivity disorder (ADHD): the potential role of epigenetic processes in mediating susceptibility. J. child Psychol. Psychiatry 49 (10), 1020–1030. doi:10.1111/j.1469-7610.2008.01909.x
Mirkovic, B., Chagraoui, A., Gerardin, P., and Cohen, D. (2020). Epigenetics and attention-deficit/hyperactivity disorder: new perspectives? Front. Psychiatry 11, 579. doi:10.3389/fpsyt.2020.00579
Morimoto, Y., Yamamoto, N., Kanegae, S., Matsuzaka, R., Ozawa, H., and Imamura, A. (2021). “Genetic overlap among autism spectrum disorders and other neuropsychiatric disorders,” in Autism spectrum disorders. Editor A. M. Grabrucker (Brisbane, QLD: Exon Publications).
Muller, U. C., Asherson, P., Banaschewski, T., Buitelaar, J. K., Ebstein, R. P., Eisenberg, J., et al. (2011). The impact of study design and diagnostic approach in a large multi-centre ADHD study. Part 1: ADHD symptom patterns. BMC Psychiatry 11, 54. doi:10.1186/1471-244X-11-54
Nigg, J. T. (2018). “Future directions in ADHD etiology research,” in Future work in clinical child and adolescent psychology (Routledge), 290–299.
Ogdie, M. N., Bakker, S. C., Fisher, S. E., Francks, C., Yang, M. H., Cantor, R. M., et al. (2006). Pooled genome-wide linkage data on 424 ADHD ASPs suggests genetic heterogeneity and a common risk locus at 5p13. Mol. Psychiatry 11 (1), 5–8. doi:10.1038/sj.mp.4001760
Omran, A. R. (2005). The epidemiologic transition: a theory of the epidemiology of population change. 1971. Milbank Q. 83 (4), 731–757. doi:10.1111/j.1468-0009.2005.00398.x
Organization, W. H. (2004). International statistical classification of diseases and related health problems: alphabetical index. volume 3. Switzerland: World Health Organization.
Polanczyk, G. V., Salum, G. A., Sugaya, L. S., Caye, A., and Rohde, L. A. (2015). Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child. Psychol. Psychiatry 56 (3), 345–365. doi:10.1111/jcpp.12381
Polanczyk, G. V., Willcutt, E. G., Salum, G. A., Kieling, C., and Rohde, L. A. (2014). ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int. J. Epidemiol. 43 (2), 434–442. doi:10.1093/ije/dyt261
Qoronfleh, M. W., Essa, M. M., Alharahsheh, S. T., Al-Farsi, Y. M., and Al-Adawi, S. (2019). Autism in the Gulf States: a regional overview. Front. Biosci. (Landmark Ed.) 24 (2), 334–346. doi:10.2741/4721
Regan, S. L., Williams, M. T., and Vorhees, C. V. (2022). Review of rodent models of attention deficit hyperactivity disorder. Neurosci. Biobehav Rev. 132, 621–637. doi:10.1016/j.neubiorev.2021.11.041
Rivero, O., Alhama-Riba, J., Ku, H. P., Fischer, M., Ortega, G., Almos, P., et al. (2021). Haploinsufficiency of the attention-deficit/hyperactivity disorder risk gene St3gal3 in mice causes alterations in cognition and expression of genes involved in myelination and sialylation. Front. Genet. 12, 688488. doi:10.3389/fgene.2021.688488
Rivero, O., Selten, M. M., Sich, S., Popp, S., Bacmeister, L., Amendola, E., et al. (2015). Cadherin-13, a risk gene for ADHD and comorbid disorders, impacts GABAergic function in hippocampus and cognition. Transl. Psychiatry 5 (10), e655. doi:10.1038/tp.2015.152
Russell, V. A. (2011). Overview of animal models of attention deficit hyperactivity disorder (ADHD). Curr. Protoc. Neurosci. Chapter 9, Unit9 35. doi:10.1002/0471142301.ns0935s54
Sarkis, E. (2014). Addressing attention-deficit/hyperactivity disorder in the workplace. Postgrad. Med. 126 (5), 25–30. doi:10.3810/pgm.2014.09.2797
Semlali, A., Almutairi, M., Rouabhia, M., Reddy Parine, N., Al Amri, A., N, S. A.-N., et al. (2018b). Novel sequence variants in the TLR6 gene associated with advanced breast cancer risk in the Saudi Arabian population. PLoS One 13 (11), e0203376. doi:10.1371/journal.pone.0203376
Semlali, A., Parine, N. R., Al-Numair, N. S., Almutairi, M., Hawsawi, Y. M., Amri, A. A., et al. (2018a). Potential role of Toll-like receptor 2 expression and polymorphisms in colon cancer susceptibility in the Saudi Arabian population. Onco Targets Ther. 11, 8127–8141. doi:10.2147/OTT.S168478
Setyanisa, A. R., Setiawati, Y., Irwanto, I., Fithriyah, I., and Prabowo, S. A. (2022). Relationship between parenting style and risk of attention deficit hyperactivity disorder in elementary school children. Malays J. Med. Sci. 29 (4), 152–159. doi:10.21315/mjms2022.29.4.14
Simsek, M., Al-Sharbati, M., Al-Adawi, S., Ganguly, S. S., and Lawatia, K. (2005). Association of the risk allele of dopamine transporter gene (DAT1*10) in Omani male children with attention-deficit hyperactivity disorder. Clin. Biochem. 38 (8), 739–742. doi:10.1016/j.clinbiochem.2005.04.016
Sontag, T. A., Tucha, O., Walitza, S., and Lange, K. W. (2010). Animal models of attention deficit/hyperactivity disorder (ADHD): a critical review. Atten. Defic. Hyperact. Disord. 2 (1), 1–20. doi:10.1007/s12402-010-0019-x
Sonuga-Barke, E. J. S., Becker, S. P., Bolte, S., Castellanos, F. X., Franke, B., Newcorn, J. H., et al. (2023). Annual Research Review: perspectives on progress in ADHD science - from characterization to cause. J. Child. Psychol. Psychiatry 64 (4), 506–532. doi:10.1111/jcpp.13696
Spiel, K., Hornecker, E., Williams, R. M., and Good, J. (2022). “ADHD and technology research–investigated by neurodivergent readers,” in Proceedings of the 2022 CHI conference on human factors in computing systems. 1–21.
Sudre, G., Choudhuri, S., Szekely, E., Bonner, T., Goduni, E., Sharp, W., et al. (2017). Estimating the heritability of structural and functional brain connectivity in families affected by attention-deficit/hyperactivity disorder. JAMA Psychiatry 74 (1), 76–84. doi:10.1001/jamapsychiatry.2016.3072
Thapar, A. (2018). Discoveries on the genetics of ADHD in the 21st century: new findings and their implications. Am. J. Psychiatry 175 (10), 943–950. doi:10.1176/appi.ajp.2018.18040383
Thomas, R., Sanders, S., Doust, J., Beller, E., and Glasziou, P. (2015). Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 135 (4), e994–e1001. doi:10.1542/peds.2014-3482
Thome, J., and Jacobs, K. A. (2004). Attention deficit hyperactivity disorder (ADHD) in a 19th century children's book. Eur. Psychiatry 19 (5), 303–306. doi:10.1016/j.eurpsy.2004.05.004
Thygesen, J. H., Presman, A., Harju-Seppanen, J., Irizar, H., Jones, R., Kuchenbaecker, K., et al. (2021). Genetic copy number variants, cognition and psychosis: a meta-analysis and a family study. Mol. Psychiatry 26 (9), 5307–5319. doi:10.1038/s41380-020-0820-7
van Hulst, B. M., de Zeeuw, P., and Durston, S. (2015). Distinct neuropsychological profiles within ADHD: a latent class analysis of cognitive control, reward sensitivity and timing. Psychol. Med. 45 (4), 735–745. doi:10.1017/S0033291714001792
Viggiano, D., Vallone, D., and Sadile, A. (2004). Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling. Neural Plast. 11 (1-2), 97–114. doi:10.1155/NP.2004.97
Villani, V., Ludmer, J., Gonzalez, A., Levitan, R., Kennedy, J., Masellis, M., et al. (2018). Dopamine receptor D2 (DRD2), dopamine transporter solute carrier family C6, member 4 (SLC6A3), and catechol-O-methyltransferase (COMT) genes as moderators of the relation between maternal history of maltreatment and infant emotion regulation. Dev. Psychopathol. 30 (2), 581–592. doi:10.1017/S0954579417001122
Wahlstedt, C., Thorell, L. B., and Bohlin, G. (2009). Heterogeneity in ADHD: neuropsychological pathways, comorbidity and symptom domains. J. Abnorm Child. Psychol. 37 (4), 551–564. doi:10.1007/s10802-008-9286-9
Walton, E., Pingault, J. B., Cecil, C. A., Gaunt, T. R., Relton, C. L., Mill, J., et al. (2017). Epigenetic profiling of ADHD symptoms trajectories: a prospective, methylome-wide study. Mol. Psychiatry 22 (2), 250–256. doi:10.1038/mp.2016.85
Wender, P. H., Wolf, L. E., and Wasserstein, J. (2001). Adults with ADHD. An overview. Ann. N. Y. Acad. Sci. 931, 1–16. doi:10.1111/j.1749-6632.2001.tb05770.x
White, H. A., and Shah, P. (2011). Creative style and achievement in adults with attention-deficit/hyperactivity disorder. Personality Individ. Differ. 50 (5), 673–677. doi:10.1016/j.paid.2010.12.015
Wiklund, J., Yu, W., Tucker, R., and Marino, L. D. (2017). ADHD, impulsivity and entrepreneurship. J. Bus. Ventur. 32 (6), 627–656. doi:10.1016/j.jbusvent.2017.07.002
Wilmot, B., Fry, R., Smeester, L., Musser, E. D., Mill, J., and Nigg, J. T. (2016). Methylomic analysis of salivary DNA in childhood ADHD identifies altered DNA methylation in VIPR2. J. Child. Psychol. Psychiatry 57 (2), 152–160. doi:10.1111/jcpp.12457
Wolraich, M. L., Chan, E., Froehlich, T., Lynch, R. L., Bax, A., Redwine, S. T., et al. (2019). ADHD diagnosis and treatment guidelines: a historical perspective. Pediatrics 144 (4), e20191682. doi:10.1542/peds.2019-1682
Yadav, S. K., Bhat, A. A., Hashem, S., Nisar, S., Kamal, M., Syed, N., et al. (2021). Genetic variations influence brain changes in patients with attention-deficit hyperactivity disorder. Transl. Psychiatry 11 (1), 349. doi:10.1038/s41398-021-01473-w
Zayats, T., and Neale, B. M. (2019). Recent advances in understanding of attention deficit hyperactivity disorder (ADHD): how genetics are shaping our conceptualization of this disorder. F1000Res 8, F1000 Faculty Rev-2060. doi:10.12688/f1000research.18959.2
Zhang-James, Y., DasBanerjee, T., Sagvolden, T., Middleton, F. A., and Faraone, S. V. (2011). SLC9A9 mutations, gene expression, and protein-protein interactions in rat models of attention-deficit/hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 156B (7), 835–843. doi:10.1002/ajmg.b.31229
Zhang-James, Y., Middleton, F. A., Sagvolden, T., and Faraone, S. V. (2012). Differential expression of SLC9A9 and interacting molecules in the hippocampus of rat models for attention deficit/hyperactivity disorder. Dev. Neurosci. 34 (2-3), 218–227. doi:10.1159/000338813
Keywords: ADHD, ASD, consanguinity, genetic variants, neurodevelopmental disorders, learning disorder
Citation: Mahrous NN, Albaqami A, Saleem RA, Khoja B, Khan MI and Hawsawi YM (2024) The known and unknown about attention deficit hyperactivity disorder (ADHD) genetics: a special emphasis on Arab population. Front. Genet. 15:1405453. doi: 10.3389/fgene.2024.1405453
Received: 22 March 2024; Accepted: 15 July 2024;
Published: 06 August 2024.
Edited by:
J. Francis Borgio, Imam Abdulrahman Bin Faisal University, Saudi ArabiaCopyright © 2024 Mahrous, Albaqami, Saleem, Khoja, Khan and Hawsawi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yousef M. Hawsawi, aHlvdXNlZkBrZnNocmMuZWR1LnNh
 Nahed N. Mahrous
Nahed N. Mahrous Amirah Albaqami2
Amirah Albaqami2 Yousef M. Hawsawi
Yousef M. Hawsawi