- Reproductive Medical Center, Guangdong Women and Children Hospital, Guangzhou, Guangdong, China
Objective: Complex chromosome rearrangements (CCR) are rare structural abnormalities involving at least three breakpoints, categorized into three types based on their structure: type A (three-way rearrangements), type B (double two-way translocations), and type C (exceptional CCR). However, thus far, limited data exists on preimplantation genetic testing for chromosomal structural rearrangements (PGT-SR) in CCR carriers. This study aims to evaluate the clinical outcomes and influencing factors of PGT-SR in couples with CCR.
Methods: Fifteen couples with unique CCR recruited from 793 couples following PGT-SR between January 2017 and May 2023. In addition, a total of 54 CCR cases, 39 previously reported as well as 15 newly added, were included in the analysis of factors associate with normal/balanced embryos.
Results: A total of 100 blastocysts were biopsied and analyzed in 15 CCR couples after 17 PGT-SR cycles, with 16.0% being euploid, 78.0% aneuploid and 6.0% mosaic. 11 normal/balanced embryos and one mosaic embryo were transferred, resulting in eight live births. Furthermore, based on the combined data from 54 CCR carriers, the proportion of normal/balanced embryos was 10.8%, with a significant decrease observed among female carriers compared to male heterozygotes (6.5% vs. 15.5%, p = 0.002). Type B exhibited the lowest rate of euploid embryos at only 6.7%, followed by type A at 11.6% and type C at 14.0%, although the differences were not significant (p = 0.182). After completing the multivariate generalized estimating equation (GEE) analysis, type B (p = 0.014) and female carrier (p = 0.002) were identified as independent risk factors for fewer euploid embryos.
Conclusion: The occurrence of balanced CCR in patients with reproductive abnormalities may be more frequent than we expected. Despite the proportion of normal/balanced embryos being significantly low, which can be influenced by CCR type and carrier’s sex, PGT-SR may improve the reproductive outcomes among CCR cases. These findings can optimize the clinical management and genetic counseling of CCR carriers seeking assisted reproductive technology (ART).
Introduction
Complex chromosomal rearrangements (CCR) are structural abnormalities characterized by two or more chromosomes with at least three breakpoints, resulting in the exchange of genetic material between non-homologous chromosomes (Pellestor et al., 2011a). CCR can be classified into three categories according to their structure (Kausch et al., 1988; Pellestor et al., 2011a): type A (three-way rearrangements), type B (double two-way translocations) and type C (exceptional CCR) respectively; the first two types involve only translocations, while exceptional CCR often combine translocations with other structural aberrations such as inversions and insertions. CCR occurring in phenotypically normal persons are extremely rare, either familial or de novo, with an estimated incidence of 0.003% in newborns (Giardino et al., 2009). Nevertheless, they are more prevalent in individuals with reproductive abnormalities such as infertility, recurrent pregnancy loss and/or offspring abnormality, ranging from 0.1% to 0.2% frequency (Mau- and Holzmann, 2005; Liao et al., 2017). For carriers of balanced CCR, it is likely that a significant number of unbalanced gametes will be generated due to abnormal segregation patterns during meiosis (Loup et al., 2010; Pellestor et al., 2011b; Godo et al., 2013; Rossi et al., 2023).
Preimplantation genetic testing for chromosomal structural rearrangements (PGT-SR) has been widely applied for carriers of common chromosomal rearrangements, including reciprocal translocations, Robertsonian translocations and inversions (Coonen et al., 2020; Spinella et al., 2023). However, the available data on preimplantation genetic testing for structural rearrangements (PGT-SR) in carriers with CCR remains insufficient. To date, only a few case reports or small series have been published regarding carriers with balanced CCR undergoing PGT-SR (Escudero et al., 2008; Lim et al., 2008; Vanneste et al., 2011; Scriven et al., 2014; Frumkin et al., 2017; Brunet et al., 2018; Hu et al., 2018; Mas et al., 2018; Li et al., 2020; Ou et al., 2020; Dufton and Bouzayen, 2021; Özer et al., 2022; Ren et al., 2023). Here, we assessed the clinical outcomes of 15 couples with novel CCR undergoing PGT-SR using next-generation sequencing (NGS). To our knowledge, this is the most extensive series of CCR carriers undergoing PGT-SR ever reported. Moreover, we conducted a systematic analysis to identify factors influencing the outcomes of PGT-SR in individuals with CCR by integrating our data with previously reported cases. These data would provide valuable insights for the clinical management and genetic counseling of CCR carriers seeking assisted reproductive technology (ART).
Materials and methods
Study patients
Fifteen couples were retrospectively selected from a large cohort of 793 couples who underwent PGT-SR at the Reproductive Medicine Center of Guangdong Women and Children Hospital between January 2017 and May 2023. One partner in each couple was a CCR carrier, including five female carriers and ten male heterozygotes. Among the remaining 778 couples, one partner carried a common chromosomal rearrangement, such as reciprocal translocation, Robertsonian translocation or inversion. Classical pericentric inversion “inv (9)(p11q12)” was excluded. The present study was reviewed and approved by the Institutional Review Board (IRB) of Guangdong Women and Children Hospital, ensuring compliance with ethical guidelines. Written consent was obtained from all participating patients prior to their inclusion in the study.
For ease of reference, we assigned numbers to the 15 couples as cases 1 to 15 (Table 1). More than half of the female participants (cases 3, 6, 7, 9, 10, 11, 12, 14 and 15) had experienced abnormal pregnancy outcomes, including spontaneous or induced abortions. Oligoasthenoteratozoospermia (OAT) was diagnosed in three of the male carriers (cases 4, 5 and 11), while the rest exhibited normal sperm parameters. None of these couples had achieved a healthy live birth before undergoing PGT-SR.
Considering the limited prevalence of CCR carriers, we expanded our sample size by collecting data from PGT-SR studies involving individuals with CCR that have been reported in PubMed up until now, with the aim of exploring factors influencing euploidy of embryos in CCR carriers undergoing PGT-SR.
Cytogenetic analysis
Using the standard G-banding technique, cytogenetic analysis was performed on cultured peripheral blood lymphocytes from the 15 couples.
Controlled ovarian stimulation and PGT-SR procedure
The procedures reported previously were followed (Liu et al., 2021; Dong et al., 2023). Briefly, controlled ovarian stimulation was induced using a gonadotropin-releasing hormone (GnRH) agonist, recombinant follicular stimulating hormone (FSH) and human chorionic gonadotropin (HCG). Standard techniques were employed in IVF treatment process at the Reproductive Medical Centre of Guangdong Women and Children Hospital, including fertilization, embryo culture, blastocyst biopsy, and blastocyst transfer. Blastocysts on day 5/6 with a grading score ≥3BC were selected for biopsy.
Trophectoderm (TE) cells obtained from day 5/6 biopsies for PGT-SR were subjected to whole-genome amplification (WGA) using the PicoPLEX single-cell WGA kit (Rubicon Genomics, Ann Arbor, United States). Subsequently, sequencing libraries were prepared using the WGA products of the embryos and then analyzed for the detection of copy number variation (CNV) in all 24 chromosomes using next-generation sequencing (NGS) following standard protocols. Euploid or mosaic embryos (Leigh et al., 2022), accompanied by genetic counseling, could be transferred into the uterine cavity.
Statistical methods
The demographic characteristics and clinical outcomes were typically presented as mean values with standard deviations (SD) for continuous variables, and as frequency with proportion for categorical variables. The differences between groups were assessed using the ANOVA test for continuous variables and the Pearson’s chi-square test for categorical variables.
As multiple embryos from the same woman were included in the cohort, a multivariate generalized estimating equation (GEE) with an exchangeable working correlation matrix was utilized to examine the associations between patient demographics and embryonic euploidy. The following potential influencing factors were considered for inclusion in the GEE: female age (<35 years or ≥35 years), time of biopsy (day 3 or day 5/6), type of CCR (type A, B or C) and carrier’s sex (female or male). All statistical analyses were performed using R Version 4.3.1. All p values were two-sided, and less than 0.05 was considered statistically significant.
Results
Karyotyping
Fifteen couples, in which one partner was identified as a CCR carrier through G-banding analysis of peripheral blood (Supplementary Figure S1), were categorized into three groups: two couples belonged to type A, five to type B, and eight to type C (Table 1).
Clinical characteristics and PGT-SR outcomes of CCR carriers
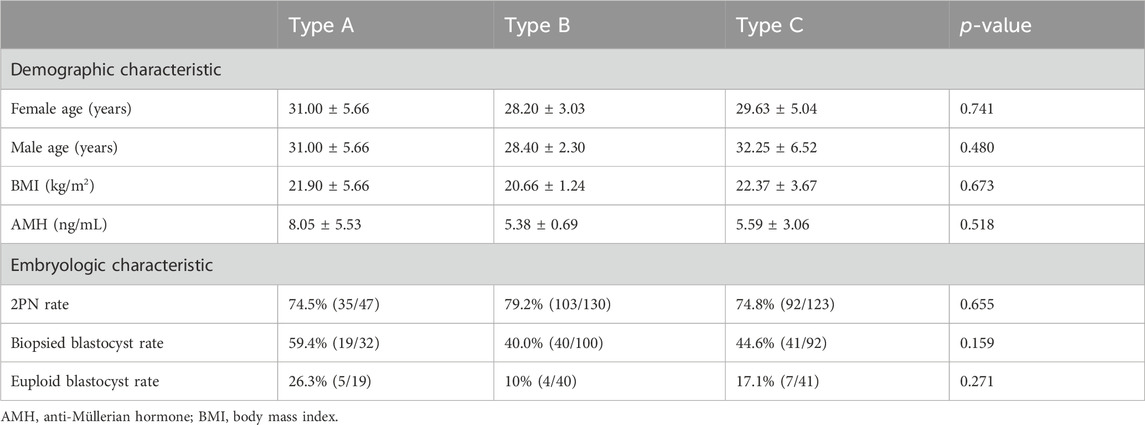
No statistically significant differences were observed among the three groups in terms of baseline information (Table 2).
As presented in Tables 1, 2, 17 PGT-SR cycles were performed in 15 cases, resulting in the retrieval of 337 oocytes, with 300 (89.0%) available for fertilization. Subsequently, 230 (76.7%) oocytes developed into two-pronuclear embryos (2PN), and out of these, 224 (97.4%) embryos reached day 3 of development. Finally, a total of 100 (44.6%) blastocysts were eligible for biopsy and detection on day 5/6. The rates of 2PN in types A, B and C were found to be 74.5% (35/47), 79.2% (103/130) and 74.8% (92/123), respectively; however, no significant differences were observed among the three types (χ2 = 0.85, p = 0.655) (Table 2). Similarly, there were no significant differences in the rates of blastocyst formation on day 5/6 for biopsy between the three groups: 59.4% (19/32, type A), 40.0% (40/100, type B), and 44.6% (41/92, type C) respectively (χ2 = 3.68, p = 0.159) (Table 2). However, two patients (case 14 and 15) had no eligible blastocysts for biopsy within one PGT-SR cycle.
The NGS-based PGT-SR results are presented in Supplementary Table S1. All of the 100 biopsied blastocysts were diagnosed successfully, with 16.0% (16/100) embryos identified as balanced or normal, 79.0% (79/100) as aneuploid, and 5.0% (5/100) as mosaic (Table 1). In addition, 26 out of 100 embryos involved de novo abnormal chromosomes that were not present in the carriers. The rate of euploid blastocysts was found to be the highest in type A CCR at 26.3% (5/19), followed by type C with a rate of 17.1% (7/41). In contrast, type B CCR had the lowest rate at 10.0% (4/40). However, no statistically significant differences were observed among the three types (χ2 = 2.61, p = 0.271) (Table 2).
In the 17 PGT cycles, there were eight cycles (8/17, 47.1%) in which no euploid embryo could be transplanted. A total of 11 normal/balanced embryos (46, XN) (XN means XX or XY) and one mosaic embryo [46, XN, + (mosaic)(16)(q22.2-q24.3)(17.51 Mb)(40%)] (case 10, Supplementary Table S1) were transplanted with frozen-thawed embryo transfer, resulting in eight live births for 7 couples (cases 2, 5, 7, 9, 10, 12 and 13) (Table 1).
Factors influencing the proportion of normal/balanced embryos
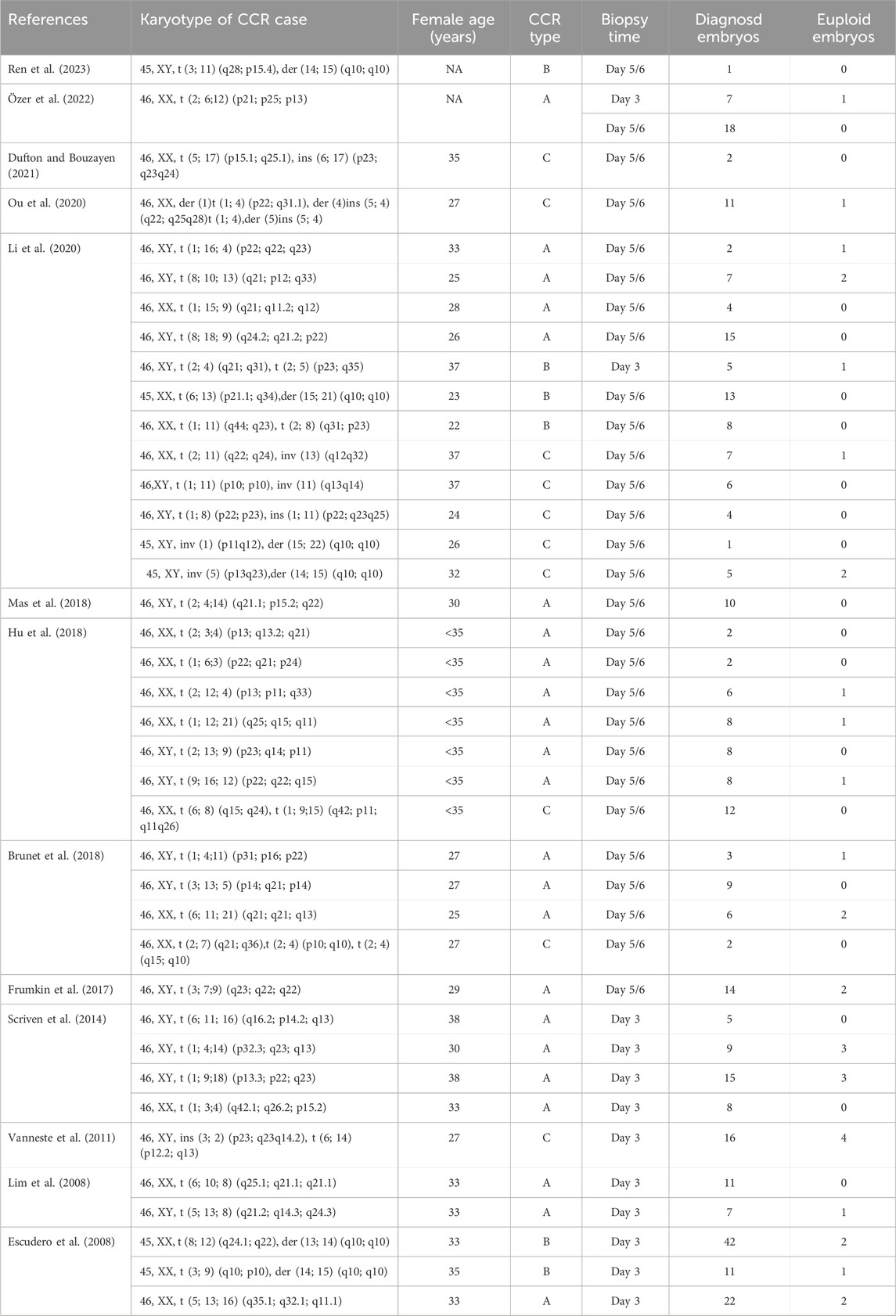
To date, an extensive literature review has identified a total of 39 individuals with balanced CCR who have undergone PGT-SR (Table 3). A total of 352 embryos were successfully tested on either day 3 or day 5/6. Consequently, 54 CCR carriers (24 females and 30 males), comprising 25 type A, 11 type B, and 18 type C cases, were included in the analysis. In summary, as presented in Figure 1A, the overall proportion of normal/balanced embryos among CCR carriers was 10.8% (49/452). The rates of euploid embryos for female aged <35 and ≥35 were 10.7% (39/363) and 14.3% (9/63), respectively (χ2 = 0.67, p = 0.412). Similarly, the euploid embryos rates for day 3 and day 5/6 biopsies were found to be 11.4% (18/158) and 10.5% (31/294), respectively (χ2 = 0.08, p = 0.782). Additionally, type B CCR exhibited the lowest euploid embryos rate at 6.7% (8/120), whereas type A and C displayed the rates of 11.6% (26/225) and 14.0% (15/107), respectively; however, the observed differences did not reach statistical significance (χ2 = 3.40, p = 0.183). Notably, the proportion of normal/balanced embryos was significantly lower in female carriers (6.5%, 15/232) compared to male heterozygotes (15.5%, 34/220) (χ2 = 9.44, p = 0.002).
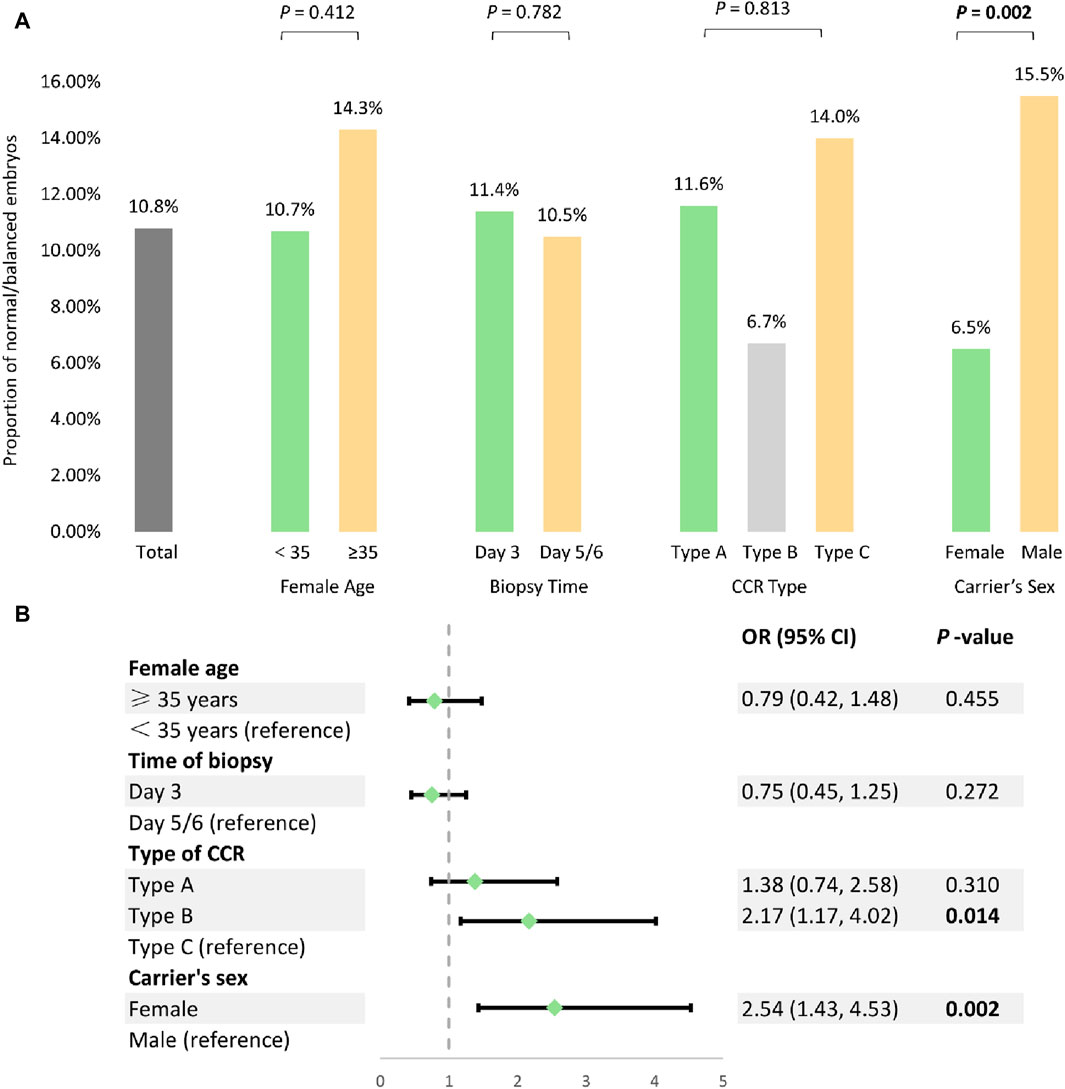
Figure 1. Analysis of the potential factors influencing PGT-SR outcomes among 54 CCR carriers. (A) Univariate analysis of the potential factors affecting normal/balanced embryo proportions. (B) The multivariable generalized estimating equations analysis to model predictors for normal/balanced embryos.
These potential factors were further analyzed using multivariate GEE to determine their impact on the proportion of balanced embryos following PGT-SR. The result indicated that type B CCR (OR = 2.17, 95% CI 1.17−4.02, p = 0.014) and female carrier (OR = 2.54, 95% CI 1.43−4.53, p = 0.002) were independent risk factors associated with a decrease in the proportion of euploid embryos, while female age (OR = 0.79, 95% CI 0.42−1.48, p = 0.455) and time of biopsy (OR = 0.75, 95% CI 0.45−1.25, p = 0.272) had no significant effect (Figure 1B).
Discussion
Although CCR are uncommon events in humans, the frequency of CCR carriers in the PGT-SR population is described here for the first time. In our reproductive center, 1.9% (15/793) of couples undergoing PGT-SR cycles were identified as CCR carriers, who have normal physical health but may be at risk for spontaneous abortion or chromosomally abnormal offspring. The proportion of carriers with balanced CCR in the PGT-SR population has not been reported previously, and this proportion may exceed expectations.
We assessed the PGT-SR outcomes in 15 couples carrying three different types of CCR. Our data showed that the fertilization rates (76.7%), the embryo formation rates on day 3 (97.4%), and the blastocyst formation rates for biopsy (44.6%) were within the normal range, with no significant differences observed among the three groups (Table 2). Two studies have suggested some CCR may lead to poor early embryonic development (Hu et al., 2018; Li et al., 2020), which is consistent with our observed cases 3, 14 and 15. However, the phenomenon should be further investigated with larger samples to derive a more precise conclusion. Among the 100 blastocysts biopsied from 15 CCR couples after 17 PGT-SR cycles, only 16.0% were diagnosed as normal/balanced. The live birth rate after transfer of euploid embryos was 63.6% (7/11), and it was 100% (1/1) for mosaic embryo. Therefore, considering the relatively low incidence of normal/balanced embryos for CCR patients after PGT-SR cycles, transferring an embryo with a trophectoderm mosaic-range result could be considered as a viable clinical strategy (ASRM, 2023).
Several studies attribute a portion of the de novo aneuploidies to the inter chromosomal effect (ICE) (Lejeune, 1963; Wang et al., 2019), but the existence of ICE in CCR carriers remains controversial (Pellestor et al., 2011b; Wang et al., 2015; Ogur et al., 2023). In the present study, 26 out of 100 embryos involved unrelated chromosomal imbalances, providing limited evidence for the occurrence of ICE in CCR carriers. Comprehensive analysis (such as NGS) of all chromosomes in parallel with rearrangement-related chromosome testing is nonetheless essential.
In addition, for the first time, we conducted a systematic analysis using a relatively large sample size from our research and the published literature (Table 3). The results revealed that the odds of obtaining a euploid embryo was 10.8%, which was significantly lower compared to the genetically transferable embryos (26.8%, 3991/14883) in individuals with common chromosomal rearrangements (Coonen et al., 2020). After the GEE analysis of 452 embryos from 52 CCR individuals undergoing PGT-SR, we found that CCR type (OR = 2.17, 95% CI 1.17−4.02, p = 0.014) and carrier’s sex (OR = 2.54, 95% CI 1.43−4.53, p = 0.002) were independent risk factors that may be associated with the proportion of normal/balanced embryos (Figure 1B). Type B (double two-way translocations) CCR reduced the percentage of normal/balanced embryos, whereas a previous small-sample study demonstrated that different types of CCR had little effect on the embryonic molecular karyotype (Li et al., 2020). Also, the likelihood of obtaining at least one embryo for transfer following PGT-SR may be substantially less for female carriers, suggesting the different mechanisms and checkpoints involved in male and female meiosis (Zhang et al., 2019; Lin et al., 2021). Interestingly, the majority of familial CCR are transmitted through female carriers (70% maternal versus 30% paternal) (Pellestor et al., 2011a). This observation is mainly due to spermatogenesis failure in half of males, frequently linked with CCR and leading to sterility or subfertility (Liang et al., 2022). Additionally, some studies demonstrated that female age (Dang et al., 2023) and biopsy time (Beyer and Willats, 2017) might impact the proportion of genetically normal/balanced embryos for translocation carriers. Nevertheless, no significant influence of female age or biopsy time was observed on the normal/balanced embryos in our study. The possible reasons for this discrepancy could be the small sample size or the heterogeneity of CCR. However, larger cohort studies will be required to accurately assess the clinical outcomes, influencing factors, and efficacy of PGT-SR in carriers with CCR.
In conclusion, we evaluated the clinical outcomes of NGS-based PGT-SR in 15 carriers with three different types of CCR. This is the most extensive series of CCR carriers undergoing PGT-SR ever reported. PGT-SR may improve the reproductive outcomes in individuals with CCR, even though the proportion of normal/balanced embryos is relatively low. Moreover, type B CCR and female carrier are independent risk factors that may reduce the proportion of normal/balanced embryos. These findings may help optimize the genetic counseling and clinical management of these complex cases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Review Board (IRB) of Guangdong Women and Children Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DL: Data curation, Investigation, Methodology, Supervision, Writing–original draft, Writing–review and editing. CC: Data curation, Investigation, Writing–original draft, Writing–review and editing. QH: Data curation, Investigation, Writing–original draft, Writing–review and editing. YD: Data curation, Investigation, Methodology, Supervision, Writing–original draft, Writing–review and editing. LX: Data curation, Investigation, Writing–review and editing. MD: Data curation, Investigation, Writing–review and editing. ZZ: Formal Analysis, Writing–review and editing. LH: Formal Analysis, Writing–review and editing. FW: Formal Analysis, Writing–review and editing. LZ: Data curation, Investigation, Writing–review and editing. XZ: Data curation, Formal Analysis, Investigation, Writing–original draft, Writing–review and editing. FL: Data curation, Formal Analysis, Investigation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Guangzhou Science and Technology Plan Project (grant 202102021170, 202102020449 and 202201011058).
Acknowledgments
The authors would like to thank all the families enrolled in our study. Also thank all staff of laboratory for their works.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1401549/full#supplementary-material
References
ASRM (2023). Clinical management of mosaic results from preimplantation genetic testing for aneuploidy of blastocysts: a committee opinion. Fertil. Steril. 120 (5), 973–982. doi:10.1016/j.fertnstert.2023.08.969
Beyer, C. E., and Willats, E. (2017). Natural selection between day 3 and day 5/6 PGD embryos in couples with reciprocal or Robertsonian translocations. J. Assist. Reprod. Genet. 34 (11), 1483–1492. doi:10.1007/s10815-017-1009-0
Brunet, B., Shen, J., Cai, L., Xie, J., Cui, Y., Liu, J., et al. (2018). Preimplantation genetic testing for complex chromosomal rearrangement carriers by next-generation sequencing. Reprod. Biomed. Online 37 (3), 375–382. doi:10.1016/j.rbmo.2018.07.001
Coonen, E., van Montfoort, A., Carvalho, F., Kokkali, G., Moutou, C., Rubio, C., et al. (2020). ESHRE PGT consortium data collection XVI-XVIII: cycles from 2013 to 2015. Hum. Reprod. Open 2020 (4), hoaa043. doi:10.1093/hropen/hoaa043
Dang, T., Xie, P., Zhang, Z., Hu, L., Tang, Y., Tan, Y., et al. (2023). The effect of carrier characteristics and female age on preimplantation genetic testing results of blastocysts from Robertsonian translocation carriers. J. Assist. Reprod. Genet. 40 (8), 1995–2002. doi:10.1007/s10815-023-02853-5
Dong, Y., Liu, D., Zou, Y., Wan, C., Chen, C., Dong, M., et al. (2023). Preimplantation genetic testing for human blastocysts with potential parental contamination using a quantitative parental contamination test (qPCT): an evidence-based study. Reprod. Biomed. Online 46 (1), 69–79. doi:10.1016/j.rbmo.2022.08.103
Dufton, M., and Bouzayen, R. (2021). Complex reciprocal translocations, more complex than initially thought: a case report. F. S Rep. 2 (4), 487–492. doi:10.1016/j.xfre.2021.08.003
Escudero, T., Estop, A., Fischer, J., and Munne, S. (2008). Preimplantation genetic diagnosis for complex chromosome rearrangements. Am. J. Med. Genet. A 146a (13), 1662–1669. doi:10.1002/ajmg.a.32286
Frumkin, T., Peleg, S., Gold, V., Reches, A., Asaf, S., Azem, F., et al. (2017). Complex chromosomal rearrangement-a lesson learned from PGS. J. Assist. Reprod. Genet. 34 (8), 1095–1100. doi:10.1007/s10815-017-0954-y
Giardino, D., Corti, C., Ballarati, L., Colombo, D., Sala, E., Villa, N., et al. (2009). De novo balanced chromosome rearrangements in prenatal diagnosis. Prenat. Diagn 29 (3), 257–265. doi:10.1002/pd.2215
Godo, A., Blanco, J., Vidal, F., Parriego, M., Boada, M., and Anton, E. (2013). Sequential FISH allows the determination of the segregation outcome and the presence of numerical anomalies in spermatozoa from a t(1;8;2)(q42;p21;p15) carrier. J. Assist. Reprod. Genet. 30 (9), 1115–1123. doi:10.1007/s10815-013-0063-5
Hu, L., Wei, Y., Luo, K., Xie, P., Gong, F., Xiong, B., et al. (2018). Clinical outcomes in carriers of complex chromosomal rearrangements: a retrospective analysis of comprehensive chromosome screening results in seven cases. Fertil. Steril. 109 (3), 486–492. doi:10.1016/j.fertnstert.2017.11.021
Kausch, K., Haaf, T., Köhler, J., and Schmid, M. (1988). Complex chromosomal rearrangement in a woman with multiple miscarriages. Am. J. Med. Genet. 31 (2), 415–420. doi:10.1002/ajmg.1320310221
Leigh, D., Cram, D. S., Rechitsky, S., Handyside, A., Wells, D., Munne, S., et al. (2022). PGDIS position statement on the transfer of mosaic embryos 2021. Reprod. Biomed. Online 45 (1), 19–25. doi:10.1016/j.rbmo.2022.03.013
Li, G., Shi, W., Niu, W., Xu, J., Guo, Y., Su, Y., et al. (2020). The influence of balanced complex chromosomal rearrangements on preimplantation embryonic development potential and molecular karyotype. BMC Genomics 21 (1), 326. doi:10.1186/s12864-020-6731-9
Liang, Y., Xie, Y., Kong, S., Pan, Q., Qiu, W., Wang, D., et al. (2022). Complex chromosomal rearrangement causes male azoospermia: a case report and literature review. Front. Genet. 13, 792539. doi:10.3389/fgene.2022.792539
Liao, Y. P., Wang, C. J., Liang, M., Hu, X. M., and Wu, Q. (2017). Analysis of genetic characteristics and reproductive risks of balanced complex chromosome rearrangement carriers in China. Yi Chuan 39 (5), 396–412. doi:10.16288/j.yczz.16-322
Lim, C. K., Cho, J. W., Kim, J. Y., Kang, I. S., Shim, S. H., and Jun, J. H. (2008). A healthy live birth after successful preimplantation genetic diagnosis for carriers of complex chromosome rearrangements. Fertil. Steril. 90 (5), 1680–1684. doi:10.1016/j.fertnstert.2007.08.016
Lin, L., Chen, X., Wang, J., Li, R., Ding, C., Cai, B., et al. (2021). Effect of carriers’ sex on meiotic segregation patterns and chromosome stability of reciprocal translocations. Reprod. Biomed. Online 43 (6), 1011–1018. doi:10.1016/j.rbmo.2021.08.017
Liu, D., Chen, C., Zhang, X., Dong, M., He, T., Dong, Y., et al. (2021). Successful birth after preimplantation genetic testing for a couple with two different reciprocal translocations and review of the literature. Reprod. Biol. Endocrinol. 19 (1), 58. doi:10.1186/s12958-021-00731-2
Loup, V., Bernicot, I., Janssens, P., Hedon, B., Hamamah, S., Pellestor, F., et al. (2010). Combined FISH and PRINS sperm analysis of complex chromosome rearrangement t(1;19;13): an approach facilitating PGD. Mol. Hum. Reprod. 16 (2), 111–116. doi:10.1093/molehr/gap105
Mas, J., Sabouni, R., and Bocca, S. (2018). A novel male 2;4;14 complex chromosomal translocation with normal semen parameters but 100% embryonic aneuploidy. J. Assist. Reprod. Genet. 35 (5), 907–912. doi:10.1007/s10815-018-1126-4
Mau-Holzmann, U. A. (2005). Somatic chromosomal abnormalities in infertile men and women. Cytogenet Genome Res. 111 (3-4), 317–336. doi:10.1159/000086906
Ogur, C., Kahraman, S., Griffin, D. K., Cinar Yapan, C., Tufekci, M. A., Cetinkaya, M., et al. (2023). PGT for structural chromosomal rearrangements in 300 couples reveals specific risk factors but an interchromosomal effect is unlikely. Reprod. Biomed. Online 46 (4), 713–727. doi:10.1016/j.rbmo.2022.07.016
Ou, J., Yang, C., Cui, X., Chen, C., Ye, S., Zhang, C., et al. (2020). Successful pregnancy after prenatal diagnosis by NGS for a carrier of complex chromosome rearrangements. Reprod. Biol. Endocrinol. 18 (1), 15. doi:10.1186/s12958-020-00572-5
Özer, L., Aktuna, S., Unsal, E., Baltaci, A., and Baltaci, V. (2022). An incidental detection of a cryptic complex chromosome rearrangement found during NGS based PGT-SR: a case report. J. Reprod. Infertil. 23 (4), 303–309. doi:10.18502/jri.v23i4.10817
Pellestor, F., Anahory, T., Lefort, G., Puechberty, J., Liehr, T., Hédon, B., et al. (2011a). Complex chromosomal rearrangements: origin and meiotic behavior. Hum. Reprod. Update 17 (4), 476–494. doi:10.1093/humupd/dmr010
Pellestor, F., Puechberty, J., Weise, A., Lefort, G., Anahory, T., Liehr, T., et al. (2011b). Meiotic segregation of complex reciprocal translocations: direct analysis of the spermatozoa of a t(5;13;14) carrier. Fertil. Steril. 95 (7), 2433.e17–e22. doi:10.1016/j.fertnstert.2011.01.159
Ren, J., Keqie, Y., Li, Y., Li, L., Luo, M., Gao, M., et al. (2023). Case report: optical genome mapping revealed double rearrangements in a male undergoing preimplantation genetic testing. Front. Genet. 14, 1132404. doi:10.3389/fgene.2023.1132404
Rossi, C., Siffroi, J. P., Ruosso, L., Rogers, E., Becker, M., Cassuto, N. G., et al. (2023). Chromosomal segregation analysis and HOST-based sperm selection in a complex reciprocal translocation carrier. J. Assist. Reprod. Genet. 40 (1), 33–40. doi:10.1007/s10815-022-02665-z
Scriven, P. N., Bint, S. M., Davies, A. F., and Ogilvie, C. M. (2014). Meiotic outcomes of three-way translocations ascertained in cleavage-stage embryos: refinement of reproductive risks and implications for PGD. Eur. J. Hum. Genet. 22 (6), 748–753. doi:10.1038/ejhg.2013.237
Spinella, F., Bronet, F., Carvalho, F., Coonen, E., De Rycke, M., Rubio, C., et al. (2023). ESHRE PGT Consortium data collection XXI: PGT analyses in 2018. Hum. Reprod. Open 2023 (2), hoad010. doi:10.1093/hropen/hoad010
Vanneste, E., Melotte, C., Voet, T., Robberecht, C., Debrock, S., Pexsters, A., et al. (2011). PGD for a complex chromosomal rearrangement by array comparative genomic hybridization. Hum. Reprod. 26 (4), 941–949. doi:10.1093/humrep/der004
Wang, J., Li, D., Xu, Z., Diao, Z., Zhou, J., Lin, F., et al. (2019). Analysis of meiotic segregation modes in biopsied blastocysts from preimplantation genetic testing cycles of reciprocal translocations. Mol. Cytogenet 12, 11. doi:10.1186/s13039-019-0423-7
Wang, L., Iqbal, F., Li, G., Jiang, X., Bukhari, I., Jiang, H., et al. (2015). Abnormal meiotic recombination with complex chromosomal rearrangement in an azoospermic man. Reprod. Biomed. Online 30 (6), 651–658. doi:10.1016/j.rbmo.2015.02.015
Zhang, L., Wei, D., Zhu, Y., Jiang, W., Xia, M., Li, J., et al. (2019). Interaction of acrocentric chromosome involved in translocation and sex of the carrier influences the proportion of alternate segregation in autosomal reciprocal translocations. Hum. Reprod. 34 (2), 380–387. doi:10.1093/humrep/dey367
Keywords: complex chromosomal rearrangements (CCR), PGT, NGS, carrier’s sex, genetic counseling
Citation: Liu D, Chen C, Huang Q, Dong Y, Xu L, Dong M, Zhu Z, Huang L, Wang F, Zhang L, Zhang X and Liu F (2024) Preimplantation genetic testing for complex chromosomal rearrangements: clinical outcomes and potential risk factors. Front. Genet. 15:1401549. doi: 10.3389/fgene.2024.1401549
Received: 15 March 2024; Accepted: 09 July 2024;
Published: 29 July 2024.
Edited by:
Roberto Valli, University of Insubria, ItalyReviewed by:
Antonio Musio, National Research Council (CNR), ItalyFrancesco Pasquali, University of Insubria, Italy
Copyright © 2024 Liu, Chen, Huang, Dong, Xu, Dong, Zhu, Huang, Wang, Zhang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiqian Zhang, emhhbmd4aXFpYW4yMDE1QDE2My5jb20=; Fenghua Liu, bGl1c2hpbmUyMDA2QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Dun Liu
Dun Liu Chuangqi Chen†
Chuangqi Chen† Qianwen Huang
Qianwen Huang Zhenghong Zhu
Zhenghong Zhu Li Huang
Li Huang Fenghua Liu
Fenghua Liu