
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet., 25 June 2024
Sec. Genetics of Aging
Volume 15 - 2024 | https://doi.org/10.3389/fgene.2024.1397352
This article is part of the Research TopicGenetic and Molecular Mechanisms of Healthy Aging and Age-related DiseasesView all 6 articles
 Hari Krishnan Krishnamurthy1*
Hari Krishnan Krishnamurthy1* Imbaasree Rajavelu2
Imbaasree Rajavelu2 Michelle Pereira2
Michelle Pereira2 Vasanth Jayaraman1
Vasanth Jayaraman1 Karthik Krishna1
Karthik Krishna1 Tianhao Wang1
Tianhao Wang1 Kang Bei1
Kang Bei1 John J. Rajasekaran1
John J. Rajasekaran1Genetics is a key factor that governs the susceptibility to oxidative stress. In the body, oxidative burden is regulated by the balance between the prooxidant genes that orchestrate processes that produce oxidant species, while the antioxidant genes aid those involved in scavenging these species. Together, the two components aid in maintaining the oxidative balance in the body. Genetic variations can influence the expression and activity of the encoded proteins which can then affect their efficiency in regulating redox processes, thereby increasing the risk of oxidative stress. This review studies single nucleotide polymorphisms (SNPs) that bear relevance to oxidative stress by exploring the variations in the prooxidant genes, such as XDH, CYBA, CYP1A1, PTGS2, NOS, and MAO and antioxidant genes including SOD, CAT, GPX, GSS, GLUL, GSR, GSTM1, GSTM5, GSTP1, TXN and HMOX1. Early identification of individuals at the increased risk of oxidative stress is possible from the assessment of sequence of these genes. Integrating genetic insights into oxidative stress management measures can pave the way for personalized medicine that tailors’ healthcare approaches to individual genetic profiles. Effective genetic assessment along with routine quantification of biological markers can improve and monitor treatment strategies, enhancing mitigation approaches that maintain cellular health and promote longevity.
Oxygen is a fundamental element for life and plays a crucial role in extracting energy through oxidation processes in the human body. This metabolic necessity, while essential, concurrently gives rise to transient entities, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), primarily originating from the mitochondria (Scandalios, 2005). Although pivotal for immune defense and cellular signaling, an excess of ROS and RNS can harm the body by modifying lipids, DNA, RNA, and proteins, instigating detrimental oxidative reactions (Scandalios, 2005). To counteract oxidative damage, the human body has evolved a sophisticated antioxidant defense mechanism comprising endogenous and exogenous antioxidants. Antioxidants play a crucial role in protecting against oxidative stress by preventing the formation of reactive species, scavenging, neutralizing, and removing reactive species, inhibiting oxidative chain reactions, and chelating reactive metals, therefore combatting oxidative stress. (Scandalios, 2005). Oxidative stress (OS) is defined as an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and molecular damage (Scandalios, 2005).
Oxidative stress has garnered attention due to its association with the onset and/or progression of several diseases, including cancer, diabetes, metabolic disorders, atherosclerosis, and cardiovascular diseases (CVD) (Bhattacharyya et al., 2014). Elevated levels of ROS and RNS attack cellular macromolecules which can trigger fundamental changes at the cellular level, leading to chronic inflammation, DNA damage, and disruptions in cell signaling pathways. Alterations in these critical pathways can be associated with the pathogenesis of various diseases (Ramírez-Expósito and Martínez-Martos, 2019). For instance, in cardiovascular diseases like atherosclerosis, oxidative stress can contribute to the development of arterial plaques (Scandalios, 2005). In diabetes, oxidative stress is implicated in insulin resistance and pancreatic beta-cell dysfunction (Scandalios, 2005). Persistent oxidative stress can also contribute to the development and promotion of cancer by causing genetic mutations, promoting angiogenesis, and facilitating metastasis (Andreadi et al., 2022). The global prevalence of these disorders underscores the significance of screening for oxidative stress.
Oxidative stress is known to be influenced by intrinsic elements such as genetic predispositions and epigenetic modifications (Barry, 2018). Prooxidant genes such as XDH, CYBA, CYP1A1, PTGS2, NOS, MAO encoding the enzymes, xanthine oxidase, NADPH oxidase, CYP1A1 enzyme, and cyclooxygenase-2, nitric oxide synthase, and monoamine oxidase, respectively, are involved in the generation of reactive species. Variations in these genes might pose the risk of higher oxidant production. Antioxidant genes, such as SOD, CAT, and GPX encode to the primary antioxidant enzymes, superoxide dismutase, catalase, and glutathione peroxidase, respectively. Various enzymes involved in the glutathione system encoded by genes, GSS, GLUL, GSTM1, GSTM5, GSTP1 as well as the thioredoxins (TXN) and heme oxygenase–1 (HMOX1) contribute to the body’s antioxidant system by maintaining redox homeostasis. Polymorphisms in these antioxidant genes can affect their activity and efficiency, thereby affecting the body’s antioxidant defences. For instance, variations in the SOD gene influence the enzyme’s function and reduce its activity, increasing the susceptibility to oxidative stress (Gusti et al., 2021).
Understanding the genetics of prooxidants and antioxidants can provide insights into the genetic factors influencing oxidative stress-related disease risk. Genetic assessment for these genes might enable the early identification of individuals at higher risk of oxidative stress. Moreover, the exploration into the genetic landscape opens a promising avenue for personalized medicine, where interventions can be tailored based on an individual’s unique genetic profile.
Prooxidant substances encompass both endogenous and exogenous compounds that instigate oxidative stress either by generating ROS or by impeding the function of antioxidant defense mechanisms (Di Meo and Venditti, 2020). Within the delicate equilibrium of cellular redox homeostasis, prooxidant genes play a significant role, often tipping the scale towards oxidative stress under certain conditions (Checa and Aran, 2020; Di Meo and Venditti, 2020). Among these genes, XDH, MAOB, NOS, and the enzymatic machinery involving NADPH and CYP1A1 stand out as key contributors to the generation and propagation of ROS (Checa and Aran, 2020).
To counteract oxidative stress, the body employs mechanisms involving enzymatic and non-enzymatic antioxidants. Antioxidants are encoded by genes that scavenge or eliminate a variety of free radicals including those generated during biological processes, thereby maintaining redox balance (Huchzermeyer et al., 2022). Among these defenders, SOD, CAT, and GPX genes serve as frontline protectors, neutralizing ROS and preventing their deleterious effects. Additionally, GSS, GLUL, GSR, and Glutathione S-transferases (GSTM1, GSTM5, GSTP1) collaborate to replenish and utilize glutathione, a vital antioxidant molecule, in the detoxification of ROS and xenobiotics. Furthermore, TXN and HMOX1 contribute to the antioxidant defense network by regulating redox-sensitive signaling pathways and degrading heme, respectively (Shaw and Chattopadhyay, 2020).
In response to oxidative stress, various signaling pathways orchestrate a complex cellular defense mechanism aimed at restoring the balance between oxidants and antioxidants. One prominent pathway is the Nrf2/ARE (nuclear factor erythroid 2-related factor 2/antioxidant response element) pathway (Liang et al., 2017; Liu et al., 2022). Under normal conditions, Nrf2 is sequestered in the cytoplasm by Keap1 (Kelch-like ECH associated protein 1). However, upon exposure to oxidative stress, Nrf2 dissociates from Keap1 and translocates into the nucleus, where it forms complexes with other transcription factors such as c-Jun and small Maf proteins. These complexes bind to the ARE in gene promoters, triggering the expression of over 200 genes involved in cellular defense against oxidative stress and inflammation. These genes include antioxidative enzymes like HO-1, which play a pivotal role in restoring the redox state balance by scavenging reactive oxygen species (ROS) (Liu et al., 2022).
Additionally, the NF-κB signaling pathway is activated in response to oxidative stress, regulating cellular proliferation and apoptosis in inflammatory states. NF-κB can induce the expression of specific genes that attenuate ROS production and promote cell survival. For instance (Liang et al., 2017; Liu et al., 2022), NF-κB activation may lead to the upregulation of genes encoding antioxidative enzymes such as SOD2 and GPX4 (Liu et al., 2022). Furthermore, the PI3K/AKT pathway modulates vascular tone by regulating the production of nitric oxide (NO) through the phosphorylation of endothelial nitric oxide synthase (eNOS). Dysregulation of this pathway under oxidative stress conditions can result in endothelial cell injury, highlighting the intricate interplay between oxidative stress and vascular function (Liu et al., 2022).
Another set of signaling pathways involved in oxidative stress response includes ferroptotic, apoptotic, FoxO, and ErbB pathways (Liang et al., 2017; Liu et al., 2022). These pathways collectively regulate cellular responses to oxidative stress, with key players such as FoxO3a and p53 orchestrating antioxidant responses by promoting the expression of antioxidative enzymes like SOD2 and GPX1 (Liu et al., 2022). Moreover, ErbB receptors, particularly EGFR and ErbB2, are implicated in the induction of oxidative stress and subsequent cellular survival mechanisms. Activation of these receptors can lead to the transcriptional activation of Nrf2, further enhancing the expression of antioxidant and detoxification proteins. Overall, these signaling pathways act in concert to mount a robust antioxidant response against oxidative stress, thereby safeguarding cellular integrity and function (Liang et al., 2017; Liu et al., 2022).
The generation of oxidant species involves both enzymatic and nonenzymatic reactions (Hajam et al., 2022). Enzymatic reactions in the respiratory chain, prostaglandin synthesis, phagocytosis, and the cytochrome P450 system significantly contribute to ROS production. Key enzymes, including NADPH oxidase, and xanthine oxidase play crucial roles in synthesizing superoxide radicals (O2⋅-), leading to the formation of hydrogen peroxide (H2O2), hydroxyl radicals (⋅OH), peroxynitrite (ONOO−), and hypochlorous acid (HOCl). H2O2 is a nonradical compound, generated by various oxidase enzymes, such as amino acid oxidase and xanthine oxidase. The highly reactive hydroxyl radical (⋅-OH) is formed through the interaction of O2⋅- with H2O2, catalysed by Fe2+ or Cu+ in the Fenton reaction. Additionally, the nitric oxide radical (NO⋅) is enzymatically synthesized from the oxidation of arginine to citrulline by nitric oxide synthase (NOS) (Hajam et al., 2022). Nonenzymatic reactions also contribute to free radical production, especially during mitochondrial respiration, where oxygen reacts with organic compounds. Exposure to toxins and ionizing radiation trigger nonenzymatic free radical formation (Hajam et al., 2022).
In this review, we delve into the molecular mechanisms underlying the involvement of XDH, MAOB, COX-2 NOS, NADPH, and CYP1A1 in oxidative stress, exploring their roles in cellular redox balance and their implications in various disease states.
Xanthine oxidase (XO) is a molybdoflavoprotein hydroxylase that can act both as an oxidase (XO) and reductase (called xanthine dehydrogenase). It is encoded by the XDH gene. Both forms of the enzyme aid in the final stage of purine catabolism. They catalyze the last two oxidative reactions that convert hypoxanthine to xanthine and xanthine to uric acid, a well-known antioxidant. However, this process results in the generation of O2⋅- and H2O2 (Kumar et al., 2018) (Figure 1). Additionally, XO is involved in the hydroxylation of various substrates and the production of NO⋅ under hypoxic conditions from nitrates and nitrites (Harrison, 2002). This increases the availability NO⋅ to react with O2⋅- to give ONOO− radicals (Harrison, 2002). This dual functionality of XO, in participating in the synthesis of uric acid and in being a source of ROS, underscores its significance in oxidative stress pathways.
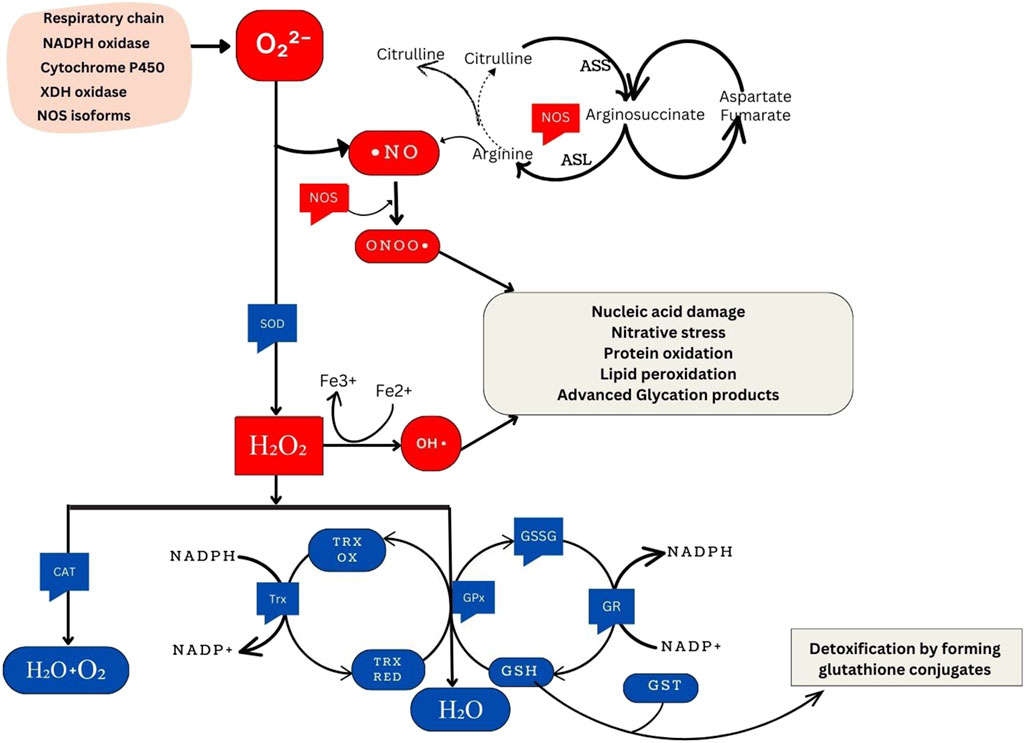
Figure 1. Overview of Cellular Oxidative Stress Pathways and Antioxidant Defense Mechanisms. NADPH oxidase, an essential component of the immune response, generates superoxide. Similarly, xanthine oxidase produces superoxide during purine metabolism. The respiratory chain, integral to cellular energy production, generates superoxide as a byproduct of electron transport. Cytochrome P450 enzymes, involved in metabolic processes, also contribute to superoxide production. Under certain conditions, nitric oxide synthase isoforms can produce superoxide, particularly when uncoupled. ⋅NO is synthesized from the citrulline cycle by NOS enzymes. These enzymes catalyze the conversion of L-arginine to L-citrulline and NO, a process involving the oxidation of L-arginine’s guanidino nitrogen. When NO reacts with O2⋅-, ONOO− forms, a highly reactive nitrogen species implicated in oxidative damage to biomolecules. SOD enzymes convert superoxide radicals into H2O2. These include cytosolic Cu/Zn-SOD (SOD1), mitochondrial Mn-SOD (SOD2), and extracellular SOD (SOD3). Hydrogen peroxide can produce ⋅OH through the Fenton reaction, a process catalyzed by transition metal ions such as Fe2+. This reaction initiates chain reactions leading to oxidative damage. Catalase (CAT), located in peroxisomes, breaks down hydrogen peroxide into water and oxygen. This catalysis facilitates the conversion of hydrogen peroxide molecules into water and oxygen molecules. Moreover, the glutathione and thioredoxin systems directly reduce H2O2 to water. In the glutathione system, glutathione peroxidase (GPx) reduces H2O2 using reduced glutathione (GSH) as a cofactor. Similarly, thioredoxin peroxidase (TPx) reduces hydrogen peroxide using thioredoxin as a cofactor. Both systems protect cells from oxidative damage by detoxifying hydrogen peroxide. GSH and glutathione S-transferase (GST) are crucial in detoxifying harmful compounds. Through conjugation, GSH binds to electrophilic centers on toxic molecules, enhancing their water solubility and facilitating excretion from cells. This detoxification process aids in protecting cells from the damaging effects of xenobiotics and reactive oxygen species (ROS).
Polymorphisms in the prooxidant, XDH gene associated with its increased activity results in higher ROS and RNS production leading to oxidative stress. Increased production of ROS by XDH has been described in experimental models of salt-sensitive, and glucocorticoid-induced hypertension (Laakso et al., 1998). Some studies have suggested that XDH activity is enhanced in patients with hypertension and a higher production of H2O2 mediated by XDH in hypertensives as compared with controls has been described (Suzuki et al., 1998). Among several XDH polymorphisms, variants at positions 565 + 64 CT and −337 GA are of particular interest. Individuals carrying specific genotypes, such as the CC genotype of the 565 + 64 CT polymorphism, have been found to exhibit higher levels of oxidative stress markers, including malondialdehyde (MDA) and eight-oxo-deoxyguanosine (8-oxo-dG) as compared to individuals with the CT and TT genotypes. This suggests that C allele enhances oxidase function and may predispose individuals to increased oxidative stress, potentially contributing to a range of oxidative stress-related conditions (Alderman et al., 1999). Similarly, the −337 GA polymorphism has shown associations with oxidative stress markers, primarily through elevated MDA levels seen among individuals with AA and AG genotypes in comparison with the GG genotypes. These studies strengthen the potential role of XDH variants in the risk of oxidative stress and related diseases such as hypertension (Chaves et al., 2007).
NADPH oxidase (NOX) is a transmembrane enzyme located in intracellular organelles. NOX is a transmembrane enzyme and is involved in the production of O2⋅- during cellular processes. However, exposure to diverse stimuli can amplify the production of ROS from these processes which leads to oxidative stress. The p22phox subunit, originating from the CYBA gene, plays a vital role in NOX function by stabilizing the catalytic subunit and providing a docking site for cytosolic factors, thereby facilitating NADPH oxidase activity (Aviello and Knaus, 2018). Upon translocation to the membrane and co-localization with p22phox and other NADPH subunits (p67phox, p47phox, and p40phox), NADPH oxidase stands out as the sole known enzyme family dedicated to producing ROS as its primary function. NOX orchestrates the transfer of electrons from cytosolic NADPH, traversing through FAD to penetrate the membrane via hemes, reaching oxygen and resulting in O2⋅- generation in the cytoplasm. Therefore, the CYBA gene through NADPH oxidase is involved in maintaining cellular processes by the generation of ROS (Kumar et al., 2015) (Figure 1).
Numerous genetic polymorphisms have been reported within the promoter and exonic regions of the CYBA gene. Some of these polymorphisms influence gene expression and subsequently, NADPH oxidase activity, leading to elevated free radical formation. Among several CYBA polymorphisms, rs4673 (C242T), rs9932581 (A-930G), and rs8854 variants have been extensively studied (Ortega-Loubon et al., 2021). In the promoter region, G alleles of rs9932581 and T alleles of rs8854 are associated with increased promoter activity, resulting in elevated oxidative stress (Tupurani et al., 2018). These polymorphisms are in the potential binding site of C/EBP (CCAAT/enhancer-binding protein) transcription factors, suggesting their role in modulating CYBA promoter activity and influencing CYBA transcription (San José et al., 2008). Studies have linked these genetic variations to susceptibility to oxidative stress-related diseases like hypertension, accompanied with increased oxidative stress markers such as eight-isoprostaglandin F2α (8-isoPGF2α) levels, along with reduced antioxidant CAT activity (Tupurani et al., 2018). For the rs4673 polymorphism, different rates of O2⋅- production have been demonstrated depending on the genotype. The T allele is associated with reduced NADPH oxidase activity, both at basal levels and when stimulated. This allele has been suggested to confer protection against oxidative stress pathologies (Najafi et al., 2012). Studies in patients with obstructive sleep apnea indicate that the CC genotype associates with higher oxidative marker levels, such as 8-Isoprostane levels, while the TT genotype associates with lower eight-isoPGF2α levels. This suggests that individuals with the CC genotype exhibit higher CYBA activity and experience increased oxidative stress compared to those with TT genotypes (Kheirandish-Gozal and Gozal, 2013). These genetic variations exert a significant influence on oxidative stress markers, antioxidant activity, and disease susceptibility.
Another single nucleotide polymorphism (SNP), rs3017887, located in the 5′untranslated region (UTR) of the Nox4 gene, has been associated with alanine aminotransferase (ALT) levels, a marker of inflammatory activity. Notably, the NOX4 CA and AA genotype of rs3017887 exhibited a significant association with ALT levels. Furthermore, a statistically significant difference in genotype frequencies was observed when the population was stratified by steatosis and nonalcoholic steatohepatitis (NASH) (Rabelo et al., 2018). The presence of the A allele of rs3017887 has been implicated in disease susceptibility, likely attributed to increased necrotic activity in the context of heightened oxidative stress. Elevated ALT levels are indicative of hepatocyte damage in nonalcoholic fatty liver disease (NAFLD). Importantly, oxidative stress, although more pronounced in cirrhotic patients, is not solely a late-stage phenomenon but is presumed to occur early, even when transaminase levels remain elevated. These findings suggest that functional polymorphisms influencing inflammation and/or oxidative stress may serve as heritable markers associated with other allelic variants predisposing individuals to oxidative stress and inflammation (Rabelo et al., 2018).
Cytochrome P450 (CYP) enzymes, specifically the CYP1A subfamily constitute a diverse group that plays pivotal roles in metabolizing both, internally generated (endobiotic) and foreign (xenobiotic) substances within the human body (Danielson, 2002). CYP1A1 belongs to this subfamily and it is mainly in extrahepatic tissues where it participates in the metabolism of a vast number of endobiotics and xenobiotic such as toxins and drugs. However, CYP1A1’s metabolic activity also results in the generation of ROS as byproducts, particularly when metabolizing certain procarcinogens like polycyclic aromatic hydrocarbons (PAHs) found in environmental pollutants and food contaminants. The overexpression of CYP1A1 usually caused due to exposure to PAHs results in the increase in ROS generation (Figure 1). CYP1A1 has been shown to regulate intracellular iron levels and contribute to the production of ROS through the Fenton reaction. As a result, variations in this gene might have implications in oxidative stress (Stading et al., 2020).
The CYP1A1 gene exhibits the polymorphism, rs4646903, located in the 3′-UTR. In rs4646903, the T>C alteration influences the enzyme’s activity which results in the increase in CYP1A1 activity (Kiyohara et al., 2012). As a result, individuals with variant genotypes (CC and TC) may experience higher ROS production during metabolic reactions as opposed to those with the wild-type genotype (TT) having optimum CYP1A1 activity (Kiyohara et al., 2012). It is fair to infer that the rs4646903 polymorphism predisposes the CC and TC genotypes to increased oxidative stress. This inference was supported by the observed increase in the levels of the oxidative marker, MDA and the decrease in the antioxidant, GPx (Peddireddy et al., 2016). The SNP can predispose these individuals to diseases associated with oxidative damage, such as chronic obstructive pulmonary disease (COPD) and coronary artery disease (Kim et al., 2018).
The PTGS2 or COX-2 gene which is responsible for encoding the cyclooxygenase-2 enzyme, plays a crucial role in susceptibility to oxidative stress. The enzyme contributes to the production of inflammatory molecules by catalysing the conversion of arachidonic acid into prostaglandins, specifically, prostaglandin G2 and prostaglandin H2. This process results in the generation of O2⋅- and subsequently other oxidant species. Moreover, COX-2 expression is upregulated during oxidative stress and inflammation. This creates a positive feedback loop where COX-2 activity is further boosted increasing the production of pro-inflammatory prostaglandins, which exacerbate oxidative stress and tissue damage (Simmons et al., 2004; Jaén et al., 2018). Variations in the COX-2 gene can be implicated in oxidative stress-related conditions such as cancer, cardiovascular diseases, and neurodegenerative disorders.
Polymorphisms within the COX-2 gene, such as rs20417 (−765G > C) significantly influence oxidative stress (Kim et al., 2016). The rs20417 polymorphism situated upstream from the transcription start site of the COX-2 gene introduces a critical alteration in a stimulatory protein binding site. This genetic variation leads to a consequential increase in transcription activity, resulting in elevated expression of the COX-2 enzyme (Benelli et al., 2018). The heightened expression of COX-2, in turn, is known to play a significant role in the intricate relationship between oxidative stress and cancer susceptibility (Benelli et al., 2018). By converting arachidonic acid into prostaglandins, COX-2 becomes a key player in oxidative stress-mediated inflammation and cytokine production. The CC genotype of rs20417 is associated with a higher incidence of oxidative stress (Roberts et al., 2009; Katkam et al., 2019). This polymorphism has been linked to a higher risk of colorectal and gastric diseases, potentially due to increase in oxidative stress levels (Rudnicka et al., 2019; Gholamalizadeh et al., 2022).
The family of nitric oxide synthase (NOS) proteins, which includes neuronal NOS (nNOS or NOS 1), inducible NOS (iNOS or NOS 2), and endothelial NOS (eNOS or NOS 3), plays a crucial role in catalyzing the oxidation of L-arginine, producing ⋅NO and L-citrulline (Supplementary Figure S1). These enzymes, encoded by separate genes, significantly contribute to cellular redox balance and various cellular functions (Cinel et al., 2002). ⋅NO, a multifaceted molecule, acts as a chain-breaking antioxidant in free radical-mediated lipid peroxidation. Optimal levels of ⋅NO are important for vasodilation, host defence, and other cellular signaling processes in the body. Generally, concentrations ranging from pico to nanomolar levels are considered the optimum range for ⋅NO, where it positively influences various physiological processes. It is crucial to know that the oxidative status of the underlying tissue can affect ⋅NO synthesis and bioavailability. When endogenous tissue oxidant levels are high (particularly, O2⋅-) they attack ⋅NO to form the cytotoxic, ONOO− (Supplementary Figure S1). This reduces ⋅NO levels aggravating NO-dependent oxidative stress. However, studies have proposed ⋅NO to represent a ‘double-edged sword’ with its overproduction leading to a multitude of ⋅NO by-products implicated in mutational events and carcinogenesis. It is hypothesized that metabolic oxygen and nitrogen species from ⋅NO may attack DNA bases, resulting in point mutations, strand breaks and interactions with sulfhydryl groups potentially leading to carcinogenesis (Chaaben et al., 2015). This indicates the need for maintaining ⋅NO at optimal levels. Therefore, understanding the role of NOS proteins is essential as their genetic variations can impact ⋅NO production.
Polymorphisms in the different NOS genes have been studied for their association with oxidative stress. Located in the intron region of the NOS1 gene, the C allele of the rs1879417 (g.117803515C > T) polymorphism was associated altered NOS1 function leading to increased oxidative stress. This allele correlated with an increased risk of oxidative stress-related conditions, such as stroke, when compared to individuals with T alleles (Synowiec et al., 2021). In the NOS2 gene, three SNPs in the promoter region, namely, −1659 C>T (rs8078340), −1026G>T (rs2779249), and −277A>G (rs2779248) contribute to increase ⋅NO production (Chaaben et al., 2015). Specifically, the T alleles of rs8078340 and rs2779249, along with G alleles of rs2779248, lead to higher ⋅NO production. These “high ⋅NO expressor” variants raise ⋅NO levels, potentially resulting in the generation of ROS and contributing to oxidative stress. Elevated concentrations of ⋅-NO under certain circumstances can generate ONOO− which is toxic and has carcinogenic potential (Chaaben et al., 2015). These polymorphisms are associated with conditions such as hypertension, diabetes mellitus, stroke, hypercholesterolemia, atherosclerosis, cardiovascular diseases, and kidney diseases. Polymorphisms in the NOS3 genes such as T-786C (Pacanowski et al., 2009), G894T (Glu298Asp) (Kondkar et al., 2020), and 27bp-VNTR (Nath et al., 2009) are linked to altered ⋅NO production leading to oxidative stress. For the SNPs, T-786C and G894T, the homozygous (NOS3−786 CC) and/or heterozygous (NOS3 894GT + TT) states are significantly associated with the with low ⋅NO and high oxidative stress (Chaaben et al., 2015). Similarly, 27bp-VNTR is seen to result in low ⋅NO bioavailability leading to disease progression (Chaaben et al., 2015). These polymorphisms, identified as low NO expressor alleles/genotypes, result in a global reduction in ⋅NO production due to a 50% reduction in promoter activity. This reduction in ⋅NO levels contribute to the observed heightened oxidative stress in individuals carrying these risk alleles/genotypes.
Monoamine oxidases (MAOs) are mitochondrial enzymes that oxidize monoamines, producing H2O2 and reactive aldehydes. There are two isoforms: MAO-A and MAO-B, with MAO-B playing a key role in regulating intracellular redox balance. Disruptions in monoamine metabolism and genetic variations in the MAO genes can cause oxidative stress, affecting cellular redox balance (Kaludercic et al., 2014; Ramsay, 2016). Among the MAO-B gene polymorphisms, rs1799836 is of great importance. This polymorphism is in intron 13 of the MAO-B gene and is thought to disrupt monoamine metabolism, leading to increased ROS production and oxidative stress within the central nervous system (Leko et al., 2021). In this polymorphism, the enzymatic activity of MAO-B is affected; the A allele is associated with elevated MAO-B activity, while the G allele is linked to lower MAO-B activity. Studies consistently show that individuals with the AA genotype exhibit higher MAO-B enzyme activity and protein levels, confirming the involvement of the A allele in heightened oxidative stress through increased MAO-B expression (Leko et al., 2021). The implications of rs1799836 extends to various neurodegenerative diseases such as Parkinson’s Disease (PD) and mental health conditions like bipolar disorder and panic disorder, mediated by the A allele’s effect in oxidative stress (Angelopoulou et al., 2022). Table 1 provides a gist of the mechanisms by which genetic polymorphisms influence prooxidant genes.
Antioxidants neutralize excess free radicals, protecting cells and contributing to disease prevention (Reuter et al., 2010). Endogenous antioxidants are classified as enzymatic antioxidants and non-enzymatic antioxidants. The primary antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) directly neutralize ROS and RNS. SOD catalyzes the dismutation of O2⋅- into H2O2, which is then transformed into water (H2O) and oxygen (O2) by CAT or GPx. Glutathione is an integral antioxidant in the body and it orchestrates its antioxidant functions with the help of various enzymes that together form the ‘glutathione system.’ Glutamate-cysteine ligase (GLUL) catalyzes the formation of the precursor to GSH while glutathione synthetase (GSS) is one of the enzymes participating GSH synthesis. GPx removes H2O2 by using it to oxidize reduced glutathione (GSH) into oxidized glutathione (GSSG) (Reuter et al., 2010) (Figure 1).
Glutathione reductase (GR) regenerates GSH from GSSG utilizing NADPH as a source of reducing power (Andreadi et al., 2022). The glutathione enzyme family, glutathione S-transferases (GSTs) also contribute to glutathione-mediated antioxidant actions. Additionally, the thioredoxin system comprising thioredoxin (Trx) and thioredoxin reductase (TR) mediate antioxidant functions by using NADPH. Heme oxygenase (HO) is another important enzyme that regulates oxidative stress by maintaining heme homeostasis (Figure 1). In addition to these internal enzymatic antioxidant defences, the body also has non-enzymatic antioxidants that are further divided into endogenous non-enzymatic antioxidants (e.g., glutathione, alpha-lipoic acid, coenzyme Q10, melatonin, uric acid, bilirubin) and exogenous non-enzymatic antioxidants (e.g., vitamin A, E, C, selenium, zinc, carotenoids, trace metals, flavonoids, omega-3 and omega-6 fatty acids) (Andreadi et al., 2022).
The enzymatic and non-enzymatic antioxidants together mount effective antioxidant defences against oxidant species in the body. The antioxidant process operates through chain-breaking or prevention mechanisms. In chain-breaking, antioxidants stabilize free radicals formed during reactions, preventing further damage, while in prevention, antioxidant enzymes reduce the rate of chain initiation, scavenging initiating free radicals or stabilizing transition metal radicals (Scandalios, 2005). This intricate process is critical for maintaining redox homeostasis and preventing oxidative damage (Scandalios, 2005).
This review explores the multifaceted roles of the enzymatic antioxidant genes in mitigating oxidative stress, unravelling their intricate mechanisms of action and their significance in maintaining cellular health and resilience.
Superoxide dismutase (SOD) is a group of enzymes found in oxygen-dependent organisms that convert the highly reactive, O2⋅- into less reactive, H2O2 and oxygen (O2) through redox reactions of metal ions within their active sites (Barry, 2018) (Figure 1). This is the integral mechanism by which SOD reduces oxidative stress in the body. Humans have three distinct SOD isoforms: copper-zinc superoxide dismutase (Cu/ZnSOD) or SOD1, manganese superoxide dismutase (MnSOD) or SOD2, and extracellular superoxide dismutase (ECSOD) or SOD3 (Ściskalska et al., 2020). Higher levels of SOD can enhance the antioxidant defense system, reducing oxidative damage to cells and potentially lowering the risk of various diseases, including cancer and neurodegenerative disorders such as Alzheimer’s disease (Aranda-Rivera et al., 2022; Saxena et al., 2022).
The SOD1 gene encodes for the enzyme, superoxide dismutase one present in cellular compartments (Eleutherio et al., 2021). Polymorphisms in the SOD1 gene have garnered attention due to their impact on oxidative stress regulation. One extensively studied polymorphism is rs2234694 (+35A/C), situated at the junction site between the intron and exon 3 (Ben Anes et al., 2019; Gallegos-Arreola et al., 2020). The AA genotype is associated with an increase in SOD1 enzyme activity, while the CC genotype correlates with a reduction in enzymatic activity. This reduction in enzyme activity can lead to a compromised ability to catalyze the conversion of O2⋅- into H2O2 and O2. As a result, the balance in the ROS levels is disrupted, contributing to an increased susceptibility to oxidative stress (Gallegos-Arreola et al., 2020). Another notable polymorphism, rs36232792 is the 50 bp Insertion/Deletion (Ins/Del) located 1,684 base pairs upstream of the ATG start codon in the SOD1 gene promoter region. The Del allele in this polymorphism is linked to a reduction in promoter activity which can result in decreased synthesis of the SOD1 enzyme, compromising the its ability to neutralize O2⋅- radicals (Mirsadraee and Saadat, 2019; Gallegos-Arreola et al., 2020; Sedaghat et al., 2024). This reduction in enzymatic activity and compromised ROS detoxification might contribute to an elevated oxidative stress environment within the cell (Mirsadraee and Saadat, 2019). The implications of these SOD1 gene polymorphisms extend to various diseases such as heart failure, cancer, diabetes, Down’s syndrome, and amyotrophic lateral sclerosis owing to their roles in altered redox signaling.
SOD2 encodes superoxide dismutase two that neutralizes O2⋅- generated during oxidative phosphorylation (Flynn and Melov, 2013). The SOD2 polymorphism, rs4880 located in exon 2, introduces a T to C substitution at position 2,734, resulting in the SOD2 Ala16Val genotype. The Val allele, a product of this SNP, significantly reduces SOD2 activity within the mitochondria via the accelerated degradation of SOD2 Val mRNA. As a result, individuals with the Val variant may experience higher oxidative stress. On the other hand, the Ala variants are associated with higher SOD2 Ala mRNA synthesis in cells, thereby having optimum antioxidant function. Additionally, the mitochondrial targeting sequence (MTS) of the SOD2 Ala precursor facilitates efficient mitochondrial import through an α-helix conformation while the MTS of the SOD2 Val precursor, adopting a β-sheet structure, results in a less efficient transport. Consequently, SOD2 activity is approximately 40% higher following the mitochondrial import in the SOD2 Ala precursor compared to its Val counterpart (Pourvali et al., 2016). The SOD2 rs4880 polymorphism is believed to be associated with the susceptibility to various diseases, including cancer, neurodegenerative disorders, chronic kidney disease (CKD), and cardiovascular diseases (Gao et al., 2008; Pourvali et al., 2016; Griess et al., 2017).
The SOD3 gene encodes for superoxide dismutase three playing a pivotal role as an extracellular antioxidant enzyme. Particularly abundant in the lungs, it contributes significantly to SOD activity in the airways and blood vessels protecting lung tissues from oxidative stress. (Gongora et al., 2008). The SOD3 gene, particularly in exon 3, is linked to a commonly studied SNP, specifically rs1799895 (R213G polymorphism). This SNP occurs in the heparin-binding domain of the SOD3 gene, leading to an arginine-to-glycine amino acid substitution at position 213 (R213G). The genetic variations among CC genotypes resulting from this polymorphism witness an impaired binding of ECSOD to the extracellular matrix, leading to lower tissue levels of the enzyme in comparison to individuals carrying CG and GG genotypes (Sørheim et al., 2010). This reduction in ECSOD levels results in decreased protection of lung matrix components against oxidative damage, indicating a potential involvement in the progression of chronic obstructive pulmonary disease (COPD) and a decline in lung function over time (Gongora et al., 2008; Sørheim et al., 2010).
The CAT gene encodes the catalase enzyme, primarily found in cell peroxisomes and the cytoplasm. It plays a crucial role in breaking down H2O2 produced during cellular respiration into oxygen and water (Figure 1). Catalase is consistently active in systems involved in electron transport with cytochromes, where H2O2 formation poses a threat to cellular integrity (Ighodaro and Akinloye, 2018). Genetic variations within the CAT gene, particularly in its promoter region and coding sequence, can affect catalase activity and may influence an individual’s susceptibility to oxidative stress-related diseases (Kodydková et al., 2014).
Variations in the CAT gene, including, −262C>T (rs1001179) (McCullough et al., 2012), −844C/T or −844G/A (rs769214) (Wu et al., 2023), and C111T (rs769217) (Hebert-Schuster et al., 2012) polymorphisms have been widely studied. These polymorphisms, located in the promoter region are associated with alterations in catalase expression levels and activity. Specifically, the rs1001179 polymorphism has been linked to variations in catalase levels and activity affecting the enzyme’s ability to neutralize intracellular H2O2. Carriers of the TT-genotype of the CAT gene rs1001179 polymorphism exhibited lower levels of catalase activity compared to carriers of CT- and CC-genotypes, suggesting a potential role in oxidative stress (McCullough et al., 2012). The other polymorphism, rs769214 has been associated with higher CAT activity in basal conditions, depending on the binding site of the transcriptional factor PAX6. The T allele of this polymorphism has been linked to increased CAT transcriptional activity (Wu et al., 2023). The rs769217 is responsible for alterations in CAT activity, with individuals carrying the TT genotype associated with lower CAT activity compared to those with the wild-type allele (Hebert-Schuster et al., 2012). While the variant allele in rs769214 is improving the enzyme’s activity, the variant allele of rs769217 is reducing CAT activity reading to oxidative stress.
Glutathione Peroxidase (GPx) catalyzes the reduction of H2O2 to water and oxygen (Figure 1). It also reduces peroxide radicals (ROO⋅) to alcohols and oxygen. Inactivity of GPx can result in oxidative damage and trigger inflammatory pathways associated with nuclear factor-κB (NF-κB) (Shaparov et al., 2021). GPx comprises at least eight different members in humans, labeled GPx1 to GPx8 (Flohé and Brigelius-Flohé, 2011). Most GPx enzymes use selenocysteine as a cofactor. While not all of them have selenocysteine, they all rely on GSH in their active sites. GPx5, GPx7, and GPx8 lack selenocysteine and instead use cysteine (CysGPxs). They are called thioredoxin-dependent peroxidases and use cysteine (Cys) in their redox-active sites (Flohé and Brigelius-Flohé, 2011). Due to their integral role in antioxidant activity, polymorphisms in GPx are implicated in various conditions, including cancer, hypertension, vitiligo, neurodegenerative diseases, and cardiovascular disease. (Toppo et al., 2008).
Glutathione Peroxidase 1 (GPx1), also known as cellular GPx, is encoded by the GPX1 gene and plays a crucial role in antioxidant defence mechanisms (Lubos et al., 2011; Mohammedi et al., 2016). A notable polymorphism in the GPX1 gene, rs1050450, is a leucine to proline change at codon position 198 (GPX1 Pro198Leu genotype). This SNP involves a C>T substitution at position 198, resulting in the replacement of proline (Pro) with leucine (Leu). The presence of the Leu allele in the GPX1 gene can affect the protein’s catalytic enzyme activity, substrate affinity, and structural stability. Specifically, the GPX1 Leu variant exhibits lower enzymatic activity compared to the GPX1 Pro enzymes which may weaken its ability to combat oxidative stress (Lubos et al., 2011; Mohammedi et al., 2016; Jerotić, 2021).
Glutathione Peroxidase 3 (GPx3) encoded by the GPX3 gene, is primarily released into the extracellular space. GPx3 serves as a crucial antioxidant enzyme in the vasculature. Its main function involves maintaining a delicate balance between various oxidant species and ⋅NO, a key vasorelaxant maintaining endothelial health (Chang et al., 2020). Therefore, GPx3’s role is essential in establishing an antithrombotic vascular environment, averting endothelial dysfunction, and reducing the likelihood of diseases associated with oxidative stress. Studies have identified the GPX3 gene to be associated with the risk of arterial ischemic stroke, cerebral venous thrombosis, and sudden sensorineural hearing loss (SSNHL), potentially due to its genetic influence on ROS (Voetsch et al., 2008; Chien et al., 2017). For the rs3805435 in the GPX3 gene, individuals with the AA genotypes exhibited a deficiency in the GPx3 enzyme, leading to heightened extracellular oxidant stress, platelet activation, poor antioxidant defenses, and potential oxidative modification of fibrinogen compared to the AG and GG genotypes (Voetsch et al., 2008; Chien et al., 2017). This sequence of events increases the risk of oxidative stress-related diseases, including acute ischemic stroke, hypertension, platelet-dependent thrombosis, coronary artery disease, and SSNHL (Voetsch et al., 2008; Chien et al., 2017).
Glutathione Peroxidase 4 (GPx4) encoded by the GPX4 gene, is a crucial antioxidant enzyme. It plays a key role in reducing H2O2 and lipid peroxides (LOOH) by utilizing GSH (Weaver and Skouta, 2022). The rs713041 SNP within the GPX4 gene introduces a C-T substitution, specifically located in the 3′untranslated region (3′UTR) of the mRNA. This region plays a crucial role in selenoprotein synthesis facilitating the incorporation of Secys. A genetic variation in this region has the potential to influence GPx4 activity, particularly under conditions of low selenium intake, rendering individuals more susceptible to oxidative stress-related diseases (Barbosa et al., 2022). The rs713041 polymorphism in GPX4 gene presents three distinct genotypes, CC (Homozygous wild), CT (Heterozygous), TT (Homozygous mutant). In this polymorphism, the C allele appears to confer a protective role against oxidative damage, particularly when selenium levels are sufficient. It also contributes to maintaining GPx4 concentrations in lymphocytes, particularly for individuals with the CC genotype, compared to those with the TT genotype in situations of inadequate selenium intake (Barbosa et al., 2022). The substitution of C allele with T allele has been linked to conditions such as obesity, endometriosis, thyroid diseases, Alzheimer’s disease, depression, multiple sclerosis, and various possibly owing to its implication in oxidative stress (Barbosa et al., 2022; Weaver and Skouta, 2022).
The glutathione system, anchored by glutathione (GSH), stands as a critical defense mechanism against oxidative stress. GSH, a tripeptide composed of L-glutamate, L-cysteine, and glycine, plays a pivotal role in maintaining cellular redox balance, essential for overall health (Deponte, 2022) (Supplementary Figure S2). Its synthesis is orchestrated by two key enzymes, γ-Glutamyl cysteine synthase and glutathione synthetase (GSS), fueled by ATP hydrolysis within the cytosol (Lu, 2013; Deponte, 2022). Additionally, glutamate-cysteine ligase (GLUL) catalyzes the formation of gamma-glutamylcysteine, a precursor to GSH, in the initial stage of GSH synthesis. GSH functions as a crucial substrate for enzymes like GPx, which scavenge peroxides to protect cells from oxidative damage. Glutathione reductase (GR) aids in GSH regeneration by converting oxidized glutathione (GSSG) back to its active form, thereby maintaining an optimal cellular pool of GSH for antioxidant defense and redox homeostasis (Lu, 2013) (Supplementary Figure S2). Furthermore, glutathione S-transferases (GSTs), including GSTM, GSTP, and GSTA, among others, contribute to detoxification processes within cells. These enzymes facilitate the conjugation of GSH with electrophilic compounds, enhancing their solubility and facilitating their removal from the cell. By neutralizing and eliminating harmful substances, GSTs play a crucial role in protecting cells from oxidative damage and maintaining overall cellular health (Nissar et al., 2017; Sarıkaya and Doğan, 2020) (Supplementary Figure S2). Together, these elements form the robust, glutathione defense network crucial for cellular health and resilience against oxidative insults. Polymorphisms in these enzymes have been associated with various diseases.
The GSS gene encodes the glutathione synthetase enzyme (GSS), a critical player in the synthesis of GSH (Kalamkar et al., 2023). GSS catalyzes the final step in GSH biosynthesis, using ATP to ligate γ-glutamylcysteine with glycine. This is the final step in the synthesis of GSH (Lu, 2013) Polymorphisms rs121909307 in the GSS gene can impact the activity of the GSS enzyme, influencing the production of GSH and, consequently, the cellular response to oxidative stress. Individuals with CC genotype exhibit optimum GSS activity, resulting in a lower risk of oxidative stress. In contrast, those with CT or TT genotypes experience reduced GSS activity, leading to decreased GSH production and a higher susceptibility to oxidative stress. The polymorphic variations in the GSS gene directly correlate with the enzyme’s function, influencing the cellular antioxidant capacity and the ability to combat oxidative stress (Kalamkar et al., 2023). Individuals carrying the CT or TT genotypes may face an increased risk of conditions where oxidative stress plays a pivotal role, such as neurodegenerative disorders, cardiovascular diseases, or certain types of cancers (Kalamkar et al., 2023).
The GLUL gene encodes the enzyme glutamate ammonia ligase, also known as glutamine synthetase. This enzyme is vital for maintaining cellular levels of glutamine, an amino acid with various functions, including antioxidant properties (Halama and Suhre, 2022). Glutamine is a precursor for GSH synthesis, crucial for controlling cellular redox status, highlighting the importance of the GLUL gene (Halama and Suhre, 2022). Polymorphisms in the GLUL gene, particularly, rs10911021 contribute to variations in oxidative stress susceptibility. Homozygous wild individuals (TT) have sufficient levels of glutamine synthetase and glutathione experience lower oxidative stress. Heterozygous individuals (TC) with decreased levels of glutamine synthetase enzyme and glutathione may face an increased risk of oxidative stress. Homozygous mutant individuals (CC) with reduced levels of glutamine synthetase enzyme and glutathione exhibit heightened susceptibility to oxidative stress (Tonin et al., 2024).
The GSR gene produces the glutathione-disulfide reductase protein, also known as the glutathione reductase (GR) enzyme. This enzyme plays a crucial role in maintaining the reduced form of GSH. This action mediated by GR is integral for replenishing the pool of GSH (Vyas et al., 2022). Mutations in the GSR gene can cause hereditary glutathione reductase deficiency, affecting cellular redox potential and increasing oxidative stress levels, especially in red blood cells. This deficiency is linked to conditions such as hereditary hemolytic anemia (Vyas et al., 2022). In the polymorphism, rs8190955 in the GSR gene, individuals with the C allele have optimum levels of GR while individuals with T allele are associated with a GR deficiency. As a result, homozygous wild individuals with the CC genotype have appropriate antioxidant function and lower levels of oxidative stress in red blood cells. On the other hand, heterozygous individuals and homozygous mutant individuals with the CT and TT genotypes, respectively, have impaired cellular redox potential and increased oxidative stress levels in red blood cells, owing to the GR deficiency. This deficiency is associated with hereditary hemolytic anemia (Vyas et al., 2022).
Glutathione transferases (GST) form a critical enzyme family in cellular detoxification and defense against oxidative stress. They facilitate the conjugation of GSH with electrophilic compounds, aiding in the elimination of harmful substances. These enzymes are categorized into cytosolic, mitochondrial, and microsomal members and are classified into multiple classes including Alpha (A), Mu (M), and Pi (P), each with distinct subtypes. GSTs are expressed predominantly in the liver and are involved in metabolizing various compounds. Their primary function lies in rendering substances more water-soluble for excretion (Hayes and McLellan, 1999). GSTs are crucial for maintaining cellular homeostasis and preventing the accumulation of toxic compounds, highlighting their role in cellular health maintenance. Genetic polymorphisms in GST genes can alter enzyme activity and may exhibit altered detoxification capacities and altered redox state, affecting susceptibility to diseases such as cancer, neurodegenerative disorders, and cardiovascular diseases (Grussy et al., 2023).
The GSTM1 gene produces an enzyme called glutathione S-transferase Mu 1 (GSMT1). The enzyme is involved in detoxifying toxic compounds by catalyzing the conjugation of GSH with a variety of electrophilic substrates, which makes the compounds more water-soluble and facilitating their elimination from the body. Found in cellular compartments such as mitochondria, lysosomes, and nuclei, this GSTM1 safeguards organelles, especially the mitochondria from oxidative stress. It achieves this by preventing cardiolipin peroxidation and cytochrome c release, making it a key regulator in fighting ROS (Mortazavi et al., 2020). Polymorphisms in the GSTM1 gene contribute to variations in oxidative stress susceptibility. The rs366631 polymorphism is characterized by the T>C change. Individuals with the TT genotype exhibit normal GSTM1 activity and have normal ROS scavenging abilities. On the other hand, individuals with the CT and CC genotypes display reduced GSTM1 activity, making them prone to oxidative stress due to the gene’s diminished ability to scavenge oxidant species (Mortazavi et al., 2020; Dai et al., 2021).
Similarly, the GSTM5 gene is part of the GST family and encodes the enzyme, glutathione S-transferase 5 (GSTM5) found in cellular compartments, such as the mitochondria. The enzyme is crucial for protecting cell organelles from oxidative stress. For rs3754446 polymorphism in GSTM5 individuals with TT genotypes exhibit normal GSTM5 activity, associated with normal mitochondrial function and a lower risk of oxidative stress. In contrast, individuals with GT and GG genotypes have altered GSTM5 activity and experience heightened oxidative stress due to ROS accumulation in the mitochondria (Butrym et al., 2021).
Glutathione S-transferase P1 (GSTP1) encoded by the GSTP1 gene, is a crucial enzyme found throughout various cellular compartments such as the cytoplasm, mitochondria, lysosomes, and nucleus. Its mitochondrial form plays a vital role in protecting organelles from oxidative stress by inhibiting cardiolipin peroxidation and preventing cytochrome c release (Santric et al., 2020). Polymorphisms within the GSTP1 gene, such as rs1138272 contribute to variations in oxidative stress susceptibility. Individuals with the AA genotype exhibit normal gene activity, leading to appropriate antioxidant activity and a lower risk of oxidative stress. In contrast, those with the AG genotype show partially abnormal gene activity, resulting in decreased antioxidant activity and an elevated risk of oxidative stress. Homozygous GG individuals experience decreased gene activity, reduced antioxidant capacity, and a higher risk of oxidative stress. These polymorphic variations directly impact the enzyme’s activity, influencing the cellular response to oxidative stress conditions (Santric et al., 2020).
The thioredoxin system is crucial for regulating redox processes. It consists of thioredoxin (Trx) and its partner, thioredoxin reductase (TR or TrxR), which uses NADPH to reduce Trx (Lee et al., 2013) (Supplementary Figure S3). Trx acts as an antioxidant by transferring electrons and protons, converting disulfides into dithiols (Lee et al., 2013). Trx maintains its active state mainly through the action of TR. It can also be reactivated by glutaredoxin (Grx) within the glutathione system. Trx serves as an antioxidant by directly quenching singlet oxygen (1O2) and hydroxyl radicals (⋅OH) or indirectly by reducing oxidized proteins. A significant target of Trx is peroxiredoxin (Prx), which directly reduces peroxides including H2O2 and various alkyl hydroperoxides. After Prx reduces its target, Trx recycles the oxidized form of Prx back to its reduced state (Hanschmann et al., 2013). Overall, the thioredoxin system collaborates with the glutathione system to maintain the organism’s redox balance and protect against oxidative stress.
The TXN2 gene encodes thioredoxin-2, that reduces Prx dimers that are formed upon reaction with H2O2, thereby keeping Prx in their reduced and active state (Supplementary Figure S3). TXN2 is essential in particular for the efficient cycling of PRDX3, which indicates its importance in the body’s antioxidant defences (Lee et al., 2013). Genetic variations within the TXN2 gene, particularly the rs35045487 polymorphism is known to be crucial in modulating of oxidative stress. This polymorphism, located in the proximal promoter region, involves an insertion/deletion impacting the transcriptional activity (Hanschmann et al., 2013). Alleles A2 (GA insertion), A4 (G insertion), and A5 (GGGA insertion) display decreased transcriptional activity, attributed to additional SP1 binding sites. This suggests a potential association with heightened oxidative stress, indicating that individuals carrying these alleles may be predisposed to an imbalance in redox homeostasis (Wen et al., 2009). Similarly, the rs4485648 polymorphism in intron one of the TXN2 gene is known to modulate oxidative stress-risk. The variant, ‘TT’ and ‘CT’ alleles of this polymorphism may have altered TXN2 expression which may compromise its functionality leading to oxidative stress. On the other hand, the ‘CC’ genotypes have appropriate gene expression associated with optimum antioxidant function. A study showed that the TT and CT genotypes were associated with the increased risk of diabetic retinopathy which could be mediated by elevated oxidative stress (Ramus et al., 2016).
Heme oxygenase (HO) plays a crucial role in regulating oxidative stress by maintaining heme homeostasis. There are three isoforms of heme oxygenase: HO-1, HO-2, and HO-3. Among these, HO-1 is upregulated in response to various stress stimuli, including oxidative stress. Its activation is a protective response against oxidative stress, as it helps to degrade heme, a pro-oxidant molecule, and generates products like biliverdin, which possess antioxidant properties HO-1 expression is regulated by the transcription factor, Nrf2, which activates antioxidant response elements (AREs) in the promoter region of the HMOX1 gene, encoding HO-1 (Chiang et al., 2021; Ryter, 2022). One notable polymorphism in the HMOX1 gene is rs2071746, where the A>T change is linked to various oxidative stress-related diseases like sickle cell anemia, ischemic heart disease, hypertension, and rheumatoid arthritis. Particularly in sickle cell anemia, the rs2071746TT genotype in the HMOX1 gene’s promoter is associated with elevated fetal hemoglobin (Hb F) levels. The T allele of rs2071746 is linked to reduced gene expression, potentially leading to higher free heme concentration and stress-induced erythropoiesis, consequently increasing Hb F levels. This association may contribute to the heightened oxidative stress observed in sickle cell anemia. (Pandey et al., 2023). Table 2 summarizes the mechanisms by which genetic polymorphisms influence antioxidant genes.
The review has explored the intricate relationship between genetic predispositions and oxidative stress which could be associated with the pathogenesis of various conditions. Through the assessment of single nucleotide polymorphisms (SNPs) relevant to oxidative stress, we have highlighted the significant impact of genetic variations in the prooxidant genes, XDH, CYBA, CYP1A1, PTGS2, NOS, MAO and the antioxidant genes, SOD, CAT, GPX, GSS, GLUL, GSR, GSTM1, GSTM5, GSTP1, TXN, and HMOX1 on oxidative stress susceptibility. These polymorphic variations can influence the expression and activity of the encoded proteins, thereby disrupting the delicate redox balance in the body.
Genetic assessment aids in understanding enzyme and pathway variations associated with oxidative stress, offering insights into individuals’ innate potential to produce and combat oxidant species. It allows for early detection of those at higher risk of oxidative stress-related conditions, enabling timely mitigation strategies. Integration of genetic insights into treatment facilitates personalized medicine tailored to one’s genetic profile. Additionally, assessment of biological markers represents actual oxidative stress levels in the body, crucial for managing oxidative stress effectively.
Furthermore, ongoing clinical trials explore various antioxidant approaches, including boosting glutathione levels using precursors, enhancing the synthesis of antioxidant enzymes, particularly via NRF2 pathway activation, NOX inhibition, and mimics such as SOD, GPX and catalase, to mitigate oxidative stress and associated pathologies. Further research is needed to optimize these interventions and understand their efficacy in managing oxidative stress-related conditions.
In conclusion, this review sheds light on the current understanding of genetic determinants of prooxidants and antioxidants offering a comprehensive perspective on how variations in these genes can modulate the risk of oxidative stress. Moving forward, further research is warranted to elucidate the precise molecular mechanisms underlying these genetic associations and to develop targeted interventions for mitigating the adverse effects of oxidative stress on health.
HK: Writing–review and editing, Writing–original draft, Conceptualization. IR: Writing–review and editing, Writing–original draft. MP: Writing–review and editing, Writing–original draft. VJ: Writing–review and editing, Writing–original draft, Conceptualization. KK: Writing–review and editing, Writing–original draft. TW: Writing–review and editing, Writing–original draft. KB: Writing–review and editing, Writing–original draft. JR: Writing–review and editing, Writing–original draft, Conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Vibrant America provided funding for this study in the form of salaries for authors (HK, IR, MP, VJ, KK, TW, KB, and JR). The specific roles of these authors are articulated in the “author contributions” section. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
We acknowledge Vibrant America LLC for supporting this research.
Authors HK, VJ, KK, TW, KB, and JR were employed by Vibrant Sciences LLC. Authors IR and MP were employed by Vibrant America LLC.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1397352/full#supplementary-material
CAT, Catalase; Fe2+, Ferrous iron; GPx, Glutathione peroxidase; GSH, Reduced glutathione; GST, Glutathione S-transferase; NO, Nitric oxide; NOS, Nitric oxide synthase; O22ˉ, Superoxide; ONOO−, Peroxynitrite; ROS, Reactive oxygen species; SOD, Superoxide dismutase; H2O2, Hydrogen peroxide; ⋅OH, Hydroxyl radicals; TPx, Thioredoxin peroxidase.
Alderman, M. H., Cohen, H., Madhavan, S., and Kivlighn, S. (1999). Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension 34 (1), 144–150. doi:10.1161/01.hyp.34.1.144
Andreadi, A., Bellia, A., Di Daniele, N., Meloni, M., Lauro, R., Della-Morte, D., et al. (2022). The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: a target for new therapies against cardiovascular diseases. Curr. Opin. Pharmacol. 62, 85–96. doi:10.1016/j.coph.2021.11.010
Angelopoulou, E., Bougea, A., Papageorgiou, S. G., and Villa, C. (2022). Psychosis in Parkinson’s disease: a lesson from genetics. Genes 13 (6), 1099. doi:10.3390/genes13061099
Aranda-Rivera, A. K., Cruz-Gregorio, A., Arancibia-Hernández, Y. L., Hernández-Cruz, E. Y., and Pedraza-Chaverri, J. (2022). RONS and oxidative stress: an overview of basic concepts. Oxygen 2 (4), 437–478. doi:10.3390/oxygen2040030
Aviello, G., and Knaus, U. G. (2018). NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol. 11 (4), 1011–1023. doi:10.1038/s41385-018-0021-8
Barbosa, P., Abo El-Magd, N. F., Hesketh, J., and Bermano, G. (2022). The role of rs713041 glutathione peroxidase 4 (GPX4) single nucleotide polymorphism on disease susceptibility in humans: a systematic review and meta-analysis. Int. J. Mol. Sci. 23 (24), 15762. doi:10.3390/ijms232415762
Barry, H. (2018). SUPEROXIDE DISMUTASE AND THE SUPEROXIDE THEORY OF OXYGEN TOXICITY. A CRITICAL APPRAISAL. Copp. Proteins Copp. Enzym. II, 2.
Ben Anes, A., Ben Nasr, H., Garrouche, A., Bchir, S., Dhaouefi, Z., Chabchoub, E., et al. (2019). The Cu/Zn superoxide dismutase+ 35A/C (rs2234694) variant correlates with altered levels of protein carbonyls and glutathione and associates with severity of COPD in a Tunisian population. Free Radic. Res. 53 (3), 293–303. doi:10.1080/10715762.2019.1572888
Benelli, R., Venè, R., and Ferrari, N. (2018). Prostaglandin-endoperoxide synthase 2 (cyclooxygenase-2), a complex target for colorectal cancer prevention and therapy. Transl. Res. 196, 42–61. doi:10.1016/j.trsl.2018.01.003
Bhattacharyya, A., Chattopadhyay, R., Mitra, S., and Crowe, S. E. (2014). Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94 (2), 329–354. doi:10.1152/physrev.00040.2012
Butrym, A., Łacina, P., Bogunia-Kubik, K., and Mazur, G. (2021). ABCC3 and GSTM5 gene polymorphisms affect overall survival in Polish acute myeloid leukaemia patients. Curr. Problems Cancer 45 (5), 100729. doi:10.1016/j.currproblcancer.2021.100729
Chaaben, A. B., Mariaselvam, C., Salah, S., Busson, M., Dulphy, N., Douik, H., et al. (2015). Polymorphisms in oxidative stress-related genes are associated with nasopharyngeal carcinoma susceptibility. Immunobiology 220 (1), 20–25. doi:10.1016/j.imbio.2014.09.021
Chang, C., Worley, B. L., Phaëton, R., and Hempel, N. (2020). Extracellular glutathione peroxidase GPx3 and its role in cancer. Cancers 12 (8), 2197. doi:10.3390/cancers12082197
Chaves, F. J., Corella, D., Blesa, S., Mansego, M. L., Marín, P., Portoles, O., et al. (2007). Xanthine oxidoreductase polymorphisms: influence in blood pressure and oxidative stress levels. Pharmacogenetics genomics 17 (8), 589–596. doi:10.1097/01.fpc.0000239970.23723.38
Checa, J., and Aran, J. M. (2020). Reactive oxygen species: drivers of physiological and pathological processes. J. Inflamm. Res. 13, 1057–1073. doi:10.2147/JIR.S275595
Chiang, S. K., Chen, S. E., and Chang, L. C. (2021). The role of HO-1 and its crosstalk with oxidative stress in cancer cell survival. Cells 10 (9), 2401. doi:10.3390/cells10092401
Chien, C. Y., Huang, T. Y., Tai, S. Y., Chang, N. C., Wang, H. M., Wang, L. F., et al. (2017). Glutathione peroxidase 3 gene polymorphisms and the risk of sudden sensorineural hearing loss. Kaohsiung J. Med. Sci. 33 (7), 359–364. doi:10.1016/j.kjms.2017.04.003
Cinel, L., Polat, A., Aydin, Ö., Düşmez, D., and Eğilmez, R. (2002). Bcl-2, iNOS, p53 and PCNA expression in normal, disordered proliferative, hyperplastic and malignant endometrium. Pathol. Int. 52 (5-6), 384–389. doi:10.1046/j.1440-1827.2002.01358.x
Dai, X., Bui, D. S., and Lodge, C. (2021). Glutathione S-transferase gene associations and gene-environment interactions for asthma. Curr. Allergy Asthma Rep. 21 (5), 31. doi:10.1007/s11882-021-01005-y
Danielson, P. Á. (2002). The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr. drug Metab. 3 (6), 561–597. doi:10.2174/1389200023337054
Deponte, M. (2022). “Glutathione and glutathione-dependent enzymes,” in Redox chemistry and biology of thiols (Academic Press), 241–275.
Di Meo, S., and Venditti, P. (2020). Evolution of the knowledge of free radicals and other oxidants. Oxidative Med. Cell. Longev. 2020, 9829176. doi:10.1155/2020/9829176
Eleutherio, E. C. A., Magalhães, R. S. S., de Araújo Brasil, A., Neto, J. R. M., and de Holanda Paranhos, L. (2021). SOD1, more than just an antioxidant. Archives Biochem. Biophysics 697, 108701. doi:10.1016/j.abb.2020.108701
Flohé, L., and Brigelius-Flohé, R. (2011). “Selenoproteins of the glutathione peroxidase family,” in Selenium: its molecular biology and role in human health (New York, NY: Springer New York), 167–180.
Flynn, J. M., and Melov, S. (2013). SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic. Biol. Med. 62, 4–12. doi:10.1016/j.freeradbiomed.2013.05.027
Gallegos-Arreola, M. P., Ramírez-Hernández, M. A., Figuera, L. E., Zúñiga-González, G. M., and Puebla-Pérez, A. M. (2020). The rs2234694 and 50 bp Insertion/Deletion polymorphisms of the SOD1 gene are associated with breast cancer risk in a Mexican population. Eur. Rev. Med. Pharmacol. Sci. 24 (15), 8017–8027. doi:10.26355/eurrev_202008_22485
Gao, F., Kinnula, V. L., Myllärniemi, M., and Oury, T. D. (2008). Extracellular superoxide dismutase in pulmonary fibrosis. Antioxidants redox Signal. 10 (2), 343–354. doi:10.1089/ars.2007.1908
Gholamalizadeh, M., Majidi, N., Tajaddod, S., Abdollahi, S., Poorhosseini, S. M., Ahmadzadeh, M., et al. (2022). Interactions of colorectal cancer, dietary fats, and polymorphisms of arachidonate lipoxygenase and cyclooxygenase genes: a literature review. Front. Oncol. 12, 865208. doi:10.3389/fonc.2022.865208
Gongora, M. C., Lob, H. E., Landmesser, U., Guzik, T. J., Martin, W. D., Ozumi, K., et al. (2008). Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome. Am. J. pathology 173 (4), 915–926. doi:10.2353/ajpath.2008.080119
Griess, B., Tom, E., Domann, F., and Teoh-Fitzgerald, M. (2017). Extracellular superoxide dismutase and its role in cancer. Free Radic. Biol. Med. 112, 464–479. doi:10.1016/j.freeradbiomed.2017.08.013
Grussy, K., Łaska, M., Moczurad, W., Król-Kulikowska, M., and Ściskalska, M. (2023). The importance of polymorphisms in the genes encoding glutathione S-transferase isoenzymes in development of selected cancers and cardiovascular diseases. Mol. Biol. Rep. 50 (11), 9649–9661. doi:10.1007/s11033-023-08894-4
Gusti, A. M., Qusti, S. Y., Alshammari, E. M., Toraih, E. A., and Fawzy, M. S. (2021). Antioxidants-Related Superoxide Dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione-S-transferase (GST), and nitric oxide synthase (NOS) gene variants analysis in an obese population: a preliminary case-control study. Antioxidants 10 (4), 595. doi:10.3390/antiox10040595
Hajam, Y. A., Rani, R., Ganie, S. Y., Sheikh, T. A., Javaid, D., Qadri, S. S., et al. (2022). Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells 11 (3), 552. doi:10.3390/cells11030552
Halama, A., and Suhre, K. (2022). Advancing cancer treatment by targeting glutamine metabolism—a roadmap. Cancers 14 (3), 553. doi:10.3390/cancers14030553
Hanschmann, E. M., Godoy, J. R., Berndt, C., Hudemann, C., and Lillig, C. H. (2013). Thioredoxins, glutaredoxins, and peroxiredoxins—molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxidants redox Signal. 19 (13), 1539–1605. doi:10.1089/ars.2012.4599
Harrison, R. (2002). Structure and function of xanthine oxidoreductase: where are we now? Free Radic. Biol. Med. 33 (6), 774–797. doi:10.1016/s0891-5849(02)00956-5
Hayes, J. D., and McLellan, L. I. (1999). Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 31 (4), 273–300. doi:10.1080/10715769900300851
Hebert-Schuster, M., Fabre, E. E., and Nivet-Antoine, V. (2012). Catalase polymorphisms and metabolic diseases. Curr. Opin. Clin. Nutr. Metabolic Care 15 (4), 397–402. doi:10.1097/MCO.0b013e328354a326
Huchzermeyer, B., Menghani, E., Khardia, P., and Shilu, A. (2022). Metabolic pathway of natural antioxidants, antioxidant enzymes and ROS providence. Antioxidants 11 (4), 761. doi:10.3390/antiox11040761
Ighodaro, O. M., and Akinloye, O. A. (2018). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 54 (4), 287–293. doi:10.1016/j.ajme.2017.09.001
Jaén, R. I., Prieto, P., Casado, M., Martín-Sanz, P., and Boscá, L. (2018). Post-translational modifications of prostaglandin-endoperoxide synthase 2 in colorectal cancer: an update. World J. gastroenterology 24 (48), 5454–5461. doi:10.3748/wjg.v24.i48.5454
Jerotić, Đ. (2021). Association of NRF2, SOD2 and GPX1 gene polymorphisms with markers of oxidative stress and prognosis in patients with end stage renal disease. Универзитет у Београду.
Kalamkar, S. D., Thorve, A. M., Gajjar, V., Divate, U., Karandikar-Iyer, S., Goel, P., et al. (2023). Single nucleotide polymorphism in glutathione synthetase gene in Indian population with type 2 diabetes. Chron. Diabetes Res. Pract. 2 (2), 67–72. doi:10.4103/cdrp.cdrp_6_23
Kaludercic, N., Mialet-Perez, J., Paolocci, N., Parini, A., and Di Lisa, F. (2014). Monoamine oxidases as sources of oxidants in the heart. J. Mol. Cell. Cardiol. 73, 34–42. doi:10.1016/j.yjmcc.2013.12.032
Katkam, S. K., Indumathi, B., Naushad, S. M., and Kutala, V. K. (2019). Impact of genetic and epigenetic factors on the oxidative stress in cardiovascular disease. Modul. Oxidative Stress Heart Dis., 107–128. doi:10.1007/978-981-13-8946-7_5
Kheirandish-Gozal, L., and Gozal, D. (2013). Genotype–phenotype interactions in pediatric obstructive sleep apnea. Respir. physiology Neurobiol. 189 (2), 338–343. doi:10.1016/j.resp.2013.03.016
Kim, H. J., Park, J. H., Seo, Y. S., Holsen, T. M., Hopke, P. K., Sung, J., et al. (2018). CYP1A1 gene polymorphisms modify the association between PM10 exposure and lung function. Chemosphere 203, 353–359. doi:10.1016/j.chemosphere.2018.03.196
Kim, J. H., Lee, M. R., and Hong, Y. C. (2016). Modification of the association of bisphenol A with abnormal liver function by polymorphisms of oxidative stress-related genes. Environ. Res. 147, 324–330. doi:10.1016/j.envres.2016.02.026
Kiyohara, C., Washio, M., Horiuchi, T., Asami, T., Ide, S., Atsumi, T., et al. (2012). Risk modification by CYP1A1 and GSTM1 polymorphisms in the association of cigarette smoking and systemic lupus erythematosus in a Japanese population. Scand. J. rheumatology 41 (2), 103–109. doi:10.3109/03009742.2011.608194
Kodydková, J., Vávrová, L., Kocík, M., and Zak, A. (2014). Human catalase, its polymorphisms, regulation and changes of its activity in different diseases. Folia Biol. 60 (4), 153–167.
Kondkar, A. A., Azad, T. A., Sultan, T., Osman, E. A., Almobarak, F. A., and Al-Obeidan, S. A. (2020). Association of endothelial nitric oxide synthase (NOS3) gene polymorphisms with primary open-angle glaucoma in a Saudi cohort. PLoS One 15 (1), e0227417. doi:10.1371/journal.pone.0227417
Kumar, R., Joshi, G., Kler, H., Kalra, S., Kaur, M., and Arya, R. (2018). Toward an understanding of structural insights of xanthine and aldehyde oxidases: an overview of their inhibitors and role in various diseases. Med. Res. Rev. 38 (4), 1073–1125. doi:10.1002/med.21457
Kumar, R., Kohli, S., Ali, Z., Duhan, K., Ram, R., Gupta, M., et al. (2015). CYBA (p22phox) variants associate with blood pressure and oxidative stress markers in hypertension: a replication study in populations of diverse altitudes. Hypertens. Res. 38 (7), 498–506. doi:10.1038/hr.2015.31
Laakso, J., Mervaala, E., Himberg, J. J., Teravainen, T. L., Karppanen, H., Vapaatalo, H., et al. (1998). Increased kidney xanthine oxidoreductase activity in salt-induced experimental hypertension. Hypertension 32, 902–906. doi:10.1161/01.hyp.32.5.902
Lee, S., Kim, S. M., and Lee, R. T. (2013). Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance. Antioxidants redox Signal. 18 (10), 1165–1207. doi:10.1089/ars.2011.4322
Leko, M. B., Perković, M. N., Erjavec, G. N., Klepac, N., Štrac, D. Š., Borovečki, F., et al. (2021). Association of the MAOB rs1799836 single nucleotide polymorphism and APOE ε4 allele in alzheimer’s disease. Curr. Alzheimer Res. 18 (7), 585–594. doi:10.2174/1567205018666210917162843
Liang, Y., Li, J., Lin, Q., Huang, P., Zhang, L., Wu, W., et al. (2017). Research progress on signaling pathway-associated oxidative stress in endothelial cells. Oxidative Med. Cell. Longev. 2017, 7156941. doi:10.1155/2017/7156941
Liu, R., Bian, Y., Liu, L., Liu, L., Liu, X., and Ma, S. (2022). Molecular pathways associated with oxidative stress and their potential applications in radiotherapy. Int. J. Mol. Med. 49 (5), 1–11.
Lu, S. C. (2013). Glutathione synthesis. Biochimica Biophysica Acta (BBA)-General Subj. 1830 (5), 3143–3153. doi:10.1016/j.bbagen.2012.09.008
Lubos, E., Loscalzo, J., and Handy, D. E. (2011). Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal 15 (7), 1957–1997. doi:10.1089/ars.2010.3586
McCullough, L. E., Santella, R. M., Cleveland, R. J., Bradshaw, P. T., Millikan, R. C., North, K. E., et al. (2012). Polymorphisms in oxidative stress genes, physical activity, and breast cancer risk. Cancer Causes Control 23, 1949–1958. doi:10.1007/s10552-012-0072-1
Mirsadraee, N., and Saadat, M. (2019). Association between a 50bp Ins/Del polymorphism at the promoter region of the superoxide dismutase-1 and age of onset of schizophrenia. EXCLI J. 18, 204–206. doi:10.17179/excli2019-1030
Mohammedi, K., Patente, T. A., Bellili-Muñoz, N., Driss, F., Le Nagard, H., Fumeron, F., et al. (2016). Glutathione peroxidase-1 gene (GPX1) variants, oxidative stress and risk of kidney complications in people with type 1 diabetes. Metabolism 65 (2), 12–19. doi:10.1016/j.metabol.2015.10.004
Mortazavi, Y., Rahimi, R., Azimi, F., Rostami, S., Moghimi, M., Faghihzadeh, S., et al. (2020). Role of Glutathione S-transferase (GSTM1, GSTT1) and CYP1A1 (cytochrome p450) Gene polymorphisms in susceptibility to acute myeloid leukemia. Middle East J. Cancer 11 (1), 12–20.
Najafi, M., Alipoor, B., Shabani, M., Amirfarhangi, A., and Ghasemi, H. (2012). Association between rs4673 (C/T) and rs13306294 (A/G) haplotypes of NAD (P) H oxidase p22phox gene and severity of stenosis in coronary arteries. Gene 499 (1), 213–217. doi:10.1016/j.gene.2012.02.032
Nath, S. D., He, X., Voruganti, V. S., Blangero, J., MacCluer, J. W., Comuzzie, A. G., et al. (2009). The 27-bp repeat polymorphism in intron 4 (27 bp-VNTR) of endothelial nitric oxide synthase (eNOS) gene is associated with albumin to creatinine ratio in Mexican Americans. Mol. Cell. Biochem. 331, 201–205. doi:10.1007/s11010-009-0159-5
Nissar, S., Sameer, A. S., Rasool, R., Chowdri, N. A., and Rashid, F. J. J. C. M. (2017). Glutathione S transferases: biochemistry, polymorphism and role in colorectal carcinogenesis. J. Carcinog. Mutagen 8 (2), 287. doi:10.4172/2157-2518.1000287
Ortega-Loubon, C., Martínez-Paz, P., García-Morán, E., Tamayo-Velasco, Á., López-Hernández, F. J., Jorge-Monjas, P., et al. (2021). Genetic susceptibility to acute kidney injury. J. Clin. Med. 10 (14), 3039. doi:10.3390/jcm10143039
Pacanowski, M. A., Zineh, I., Cooper-DeHoff, R. M., Pepine, C. J., and Johnson, J. A. (2009). Genetic and pharmacogenetic associations between NOS3 polymorphisms, blood pressure, and cardiovascular events in hypertension. Am. J. Hypertens. 22 (7), 748–753. doi:10.1038/ajh.2009.81
Pandey, H., Singh, K., Ranjan, R., Dass, J., Tyagi, S., Seth, T., et al. (2023). Prevalence and impact of HMOX1 polymorphism (rs2071746: A> T) in Indian sickle cell disease patients. J. Laboratory Physicians 15, 583–589. doi:10.1055/s-0043-1770068
Peddireddy, V., Badabagni, S. P., Gundimeda, S. D., Mamidipudi, V., Penagaluru, P. R., and Mundluru, H. P. (2016). Association of CYP1A1, GSTM1 and GSTT1 gene polymorphisms with risk of non-small cell lung cancer in Andhra Pradesh region of South India. Eur. J. Med. Res. 21, 17. doi:10.1186/s40001-016-0209-x
Pourvali, K., Abbasi, M., and Mottaghi, A. (2016). Role of superoxide dismutase 2 gene Ala16Val polymorphism and total antioxidant capacity in diabetes and its complications. Avicenna J. Med. Biotechnol. 8 (2), 48–56.
Rabelo, F., Stefano, J. T., Cavaleiro, A. M., Lima, R. V. C., de Campos Mazo, D. F., Carrilho, F. J., et al. (2018). Association between the CYBA and NOX4 genes of NADPH oxidase and its relationship with metabolic syndrome in non-alcoholic fatty liver disease in Brazilian population. Hepatobiliary Pancreat. Dis. Int. 17 (4), 330–335. doi:10.1016/j.hbpd.2018.06.005
Ramírez-Expósito, M. J., and Martínez-Martos, J. M. (2019). The delicate equilibrium between oxidants and antioxidants in brain glioma. Curr. Neuropharmacol. 17 (4), 342–351. doi:10.2174/1570159X16666180302120925
Ramsay, R. R. (2016). Molecular aspects of monoamine oxidase B. Prog. neuro-psychopharmacology Biol. Psychiatry 69, 81–89. doi:10.1016/j.pnpbp.2016.02.005
Ramus, S. M., Cilensek, I., Petrovic, M. G., Soucek, M., Kruzliak, P., and Petrovic, D. (2016). Single nucleotide polymorphisms in the Trx2/TXNIP and TrxR2 genes of the mitochondrial thioredoxin antioxidant system and the risk of diabetic retinopathy in patients with Type 2 diabetes mellitus. J. Diabetes its Complicat. 30 (2), 192–198. doi:10.1016/j.jdiacomp.2015.11.021
Reuter, S., Gupta, S. C., Chaturvedi, M. M., and Aggarwal, B. B. (2010). Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49 (11), 1603–1616. doi:10.1016/j.freeradbiomed.2010.09.006
Roberts, R. A., Laskin, D. L., Smith, C. V., Robertson, F. M., Allen, E. M., Doorn, J. A., et al. (2009). Nitrative and oxidative stress in toxicology and disease. Toxicol. Sci. 112 (1), 4–16. doi:10.1093/toxsci/kfp179
Rudnicka, K., Backert, S., and Chmiela, M. (2019). Genetic polymorphisms in inflammatory and other regulators in gastric cancer: risks and clinical consequences. Curr. Top. Microbiol. Immunol. 421, 53–76. doi:10.1007/978-3-030-15138-6_3
Ryter, S. W. (2022). Heme oxygenase-1: an anti-inflammatory effector in cardiovascular, lung, and related metabolic disorders. Antioxidants 11 (3), 555. doi:10.3390/antiox11030555
San José, G., Fortuno, A., Beloqui, O., Diez, J., and Zalba, G. (2008). NADPH oxidase CYBA polymorphisms, oxidative stress and cardiovascular diseases. Clin. Sci. 114 (3), 173–182. doi:10.1042/CS20070130
Santric, V., Djokic, M., Suvakov, S., Pljesa-Ercegovac, M., Nikitovic, M., Radic, T., et al. (2020). GSTP1 rs1138272 polymorphism affects prostate cancer risk. Medicina 56 (3), 128. doi:10.3390/medicina56030128
Sarıkaya, E., and Doğan, S. (2020). Glutathione peroxidase in health and diseases. Glutathione Syst. oxidative stress health Dis. 49. doi:10.5772/intechopen.91009
Saxena, P., Selvaraj, K., Khare, S. K., and Chaudhary, N. (2022). Superoxide dismutase as multipotent therapeutic antioxidant enzyme: role in human diseases. Biotechnol. Lett. 44, 1–22. doi:10.1007/s10529-021-03200-3
Scandalios, J. G. (2005). Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 38, 995–1014. doi:10.1590/s0100-879x2005000700003
Ściskalska, M., Ołdakowska, M., Marek, G., and Milnerowicz, H. (2020). Changes in the activity and concentration of superoxide dismutase isoenzymes (Cu/Zn SOD, MnSOD) in the blood of healthy subjects and patients with acute pancreatitis. Antioxidants 9 (10), 948. doi:10.3390/antiox9100948
Sedaghat, M. R., Shiri, H., Tavakkol-Afshari, J., Norouzmahani, M. E., Bahri, F., Fooladi, S., et al. (2024). Impact of a 50bp insertion/deletion polymorphism of the superoxide dismutase-1 on oxidative stress status and risk of keratoconus. Exp. Eye Res. 238, 109742. doi:10.1016/j.exer.2023.109742
Shaparov, M. G., Gudkov, S. V., Lankin, V. Z., and Novoselov, V. I. (2021). Role of glutathione peroxidases and peroxiredoxins in free radical-induced pathologies. Biochem. Mosc. 86, 1418–1433. doi:10.1134/S0006297921110067
Shaw, P., and Chattopadhyay, A. (2020). Nrf2–ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms. J. Cell. Physiology 235 (4), 3119–3130. doi:10.1002/jcp.29219
Simmons, D. L., Botting, R. M., and Hla, T. (2004). Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56 (3), 387–437. doi:10.1124/pr.56.3.3
Sørheim, I. C., DeMeo, D. L., Washko, G., Litonjua, A., Sparrow, D., Bowler, R., et al. (2010). Polymorphisms in the superoxide dismutase-3 gene are associated with emphysema in COPD. COPD J. Chronic Obstr. Pulm. Dis. 7 (4), 262–268. doi:10.3109/15412555.2010.496821
Stading, R., Chu, C., Couroucli, X., Lingappan, K., and Moorthy, B. (2020). Molecular role of cytochrome P4501A enzymes in oxidative stress. Curr. Opin. Toxicol. 20, 77–84. doi:10.1016/j.cotox.2020.07.001
Suzuki, H., DeLano, F. A., Parks, D. A., Jamshidi, N., Granger, D. N., Ishii, H., et al. (1998). Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc. Natl. Acad. Sci. U. S. A. 95, 4754–4759. doi:10.1073/pnas.95.8.4754
Synowiec, E., Wigner, P., Cichon, N., Watala, C., Czarny, P., Saluk-Bijak, J., et al. (2021). Single-nucleotide polymorphisms in oxidative stress-related genes and the risk of a stroke in a Polish population—a preliminary study. Brain Sci. 11 (3), 391. doi:10.3390/brainsci11030391
Tonin, G., Dolžan, V., and Klen, J. (2024). Genetic and transcriptomic background of oxidative stress and antioxidative therapies in late complications of type 2 diabetes mellitus: a systematic review. Antioxidants 13 (3), 277. doi:10.3390/antiox13030277
Toppo, S., Vanin, S., Bosello, V., and Tosatto, S. C. (2008). Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxidants redox Signal. 10 (9), 1501–1514. doi:10.1089/ars.2008.2057
Tupurani, M. A., Padala, C., Puranam, K., Galimudi, R. K., Kupsal, K., Shyamala, N., et al. (2018). Association of CYBA gene (-930 A/G and 242 C/T) polymorphisms with oxidative stress in breast cancer: a case-control study. PeerJ 6, e5509. doi:10.7717/peerj.5509
Voetsch, B., Jin, R. C., Bierl, C., Deus-Silva, L., Camargo, E. C., Annichino-Bizacchi, J. M., et al. (2008). Role of promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene as a risk factor for cerebral venous thrombosis. Stroke 39 (2), 303–307. doi:10.1161/STROKEAHA.107.490094
Vyas, B., Bhowmik, R., Akhter, M., and Ahmad, F. J. (2022). Identification, analysis of deleterious SNPs of the human GSR gene and their effects on the structure and functions of associated proteins and other diseases. Sci. Rep. 12 (1), 5474. doi:10.1038/s41598-022-09295-6
Weaver, K., and Skouta, R. (2022). The selenoprotein glutathione peroxidase 4: from molecular mechanisms to novel therapeutic opportunities. Biomedicines 10, 891. doi:10.3390/biomedicines10040891
Wen, S., Lu, W., Zhu, H., Yang, W., Shaw, G. M., Lammer, E. J., et al. (2009). Genetic polymorphisms in the thioredoxin 2 (TXN2) gene and risk for spina bifida. Am. J. Med. Genet. Part A 149 (2), 155–160. doi:10.1002/ajmg.a.32589
Keywords: oxidative stress, reactive oxygen species, genetic polymorphisms, prooxidants, antioxidants, repair genes
Citation: Krishnamurthy HK, Rajavelu I, Pereira M, Jayaraman V, Krishna K, Wang T, Bei K and Rajasekaran JJ (2024) Inside the genome: understanding genetic influences on oxidative stress. Front. Genet. 15:1397352. doi: 10.3389/fgene.2024.1397352
Received: 07 March 2024; Accepted: 03 June 2024;
Published: 25 June 2024.
Edited by:
Jiang-An Yin, University of Zurich, SwitzerlandReviewed by:
Mohammed S. Mustak, Mangalore University, IndiaCopyright © 2024 Krishnamurthy, Rajavelu, Pereira, Jayaraman, Krishna, Wang, Bei and Rajasekaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hari Krishnan Krishnamurthy, aGFyaUB2aWJyYW50c2NpLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.