
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 24 May 2024
Sec. Applied Genetic Epidemiology
Volume 15 - 2024 | https://doi.org/10.3389/fgene.2024.1392745
Background: Numerous epidemiological studies have elucidated the intricate connection between inflammation and cancer, highlighting how sustained inflammatory responses can fuel carcinogenesis by fostering proliferation, angiogenesis, and metastasis, while dampening immune responses and sensitivity to chemotherapy. Previous clinical investigations have underscored the potential of anti-inflammatory medications in either preventing or mitigating tumor formation. Here, the causal relationship between anti-inflammatory drugs and cancer was further explored through Mendelian randomization studies.
Methods: Employing Mendelian randomization, we scrutinized the causal links between three anti-inflammatory drugs—NSAIDs, Aspirin, and Anilide—and 37 types of cancer. We primarily utilized inverse variance weighting (IVW) as the primary analytical approach to delineate the causal association between these drugs and cancer types. Concurrently, sensitivity analyses were conducted to ascertain the absence of horizontal pleiotropy and heterogeneity.
Results: Our investigation revealed a discernible causal relationship between certain anti-inflammatory drugs and a subset of cancers, albeit without a pervasive impact across all cancer types. Specifically, NSAIDs exhibited a risk-reducing effect on non-small cell lung cancer (OR: 0.76, 95% CI: 0.59–0.97, p-value: 0.03) and gastric cancer (OR: 0.57, 95% CI: 0.34–0.98, p-value: 0.04). Conversely, aspirin was associated with an increased risk of oral malignant tumors (OR: 2.18, 95% CI: 1.13–4.21, p-value: 0.02). Notably, no statistically significant findings were observed for anilide drugs (p < 0.05).
Conclusion: We identified several cancers with potential causal links to NSAIDs, including non-small cell lung cancer and gastric cancer. Despite our extensive analysis, we did not identify a substantial causal relationship between the use of anti-inflammatory drugs and the development of various cancers.
Inflammation plays a pivotal role in cancer development, a concept first proposed by Rudolph Virchow in 1863 (Balkwill and Mantovani, 2001). Since then, the relationship between inflammation and carcinogenesis has gained widespread acceptance (Coussens and Werb, 2002; Perwez Hussain and Harris, 2007). Numerous studies have demonstrated a direct association between inflammation and the onset of various cancers (Wang et al., 2014; Hasche et al., 2017).
Given the strong correlation between inflammation and cancer, numerous studies have demonstrated the potential of common anti-inflammatory drugs, such as NSAIDs, in cancer prevention and risk reduction. Long-term NSAID use has been associated with decreased incidence of colorectal cancer, esophageal cancer, breast cancer, and lung cancer (Chan et al., 2005; Pereg and Lishner, 2005; Khandia and Munjal, 2020). Additionally, NSAIDs can enhance sensitivity to conventional therapies while reducing cancer invasion and metastasis (Zappavigna et al., 2020).
Indeed, various anti-inflammatory drugs exert their effects through different mechanisms. NSAIDs, for instance, primarily reduce inflammation by inhibiting cyclooxygenase (COX), which consequently impedes the conversion of arachidonic acid into thromboxane and prostaglandins (Vane, 1971; Bindu et al., 2020).
The efficacy of anti-inflammatory drugs in preventing and reducing cancer incidence remains an area of ongoing investigation, with varied conclusions across clinical studies. Inconsistent findings can often be attributed to inspection bias, unavoidable biases, and confounding factors, all of which contribute to the complexity of determining the relationship between anti-inflammatory drugs and cancer (Mahmud et al., 2004; Menter and Bresalier, 2023).
Navigating the relationship between anti-inflammatory drugs and cancer in real-world settings poses inherent challenges due to confounding factors and experimental design limitations. Mendelian randomization (MR) studies, employing genetic variation as instrumental variables (IVs), offer a promising avenue to mitigate biases arising from confounding factors and reverse causality (Katan, 2004; Lawlor et al., 2008). Thus, In this study we leveraged Mendelian randomization to investigate the causal relationship between anti-inflammatory drugs and cancer, with the aim of shedding new light on the potential role of anti-inflammatory drugs in cancer prevention and treatment.
In this study, the anti-inflammatory drugs under investigation—NSAIDs, aniline drugs, and aspirin—were sourced from Wu et al. (2019)’s study, which aggregated GWAS data from the UK Biobank. This cohort comprised 502,616 participants, with approximately 54% being female and a mean age of 56.53 years (SD 8.09) (Wu et al., 2019). The mean body mass index (BMI) was 27.43 (SD 4.80). Study cohort characteristics were presented in Supplementary Table S4. Medications and diagnoses were obtained through nurse-led interviews. Only regular medications and supplements taken weekly, monthly, or three monthly were recorded. UK Biobank did not collect specific dosage and duration of the medications (Wu et al., 2019). Medications were classified using the Anatomical Therapeutic Chemical (ATC) classification system (Santos et al., 2017). Categories named by active ingredients were mapped directly to ATC codes. Categories named by brand name were first mapped to their active ingredients and then further mapped to ATC codes based on dosage and route of administration (if available). To ensure minimal sample overlap and facilitate cross-sectional comparability across studies, our outcome variables were derived from the Finnish project (Kurki et al., 2023). This encompassed data on 37 cancer types, including subtypes, and cancer summary GWAS data, accessible at https://www.finngen.fi/en.
In our Mendelian randomization study, we employed rigorous methods to explore the potential causal relationship between anti-inflammatory drugs and cancer. We adhered to three fundamental assumptions when selecting instrumental variables (Davey Smith and Hemani, 2014): 1. Utilizing SNPs strongly correlated with exposure factors as instrumental variables; 2. Ensuring instrumental variables are independent of potential confounding factors that could influence exposure and outcomes; 3. Verifying that the selected SNPs do not influence results through pathways other than the exposure under investigation.
We applied three primary analysis methods: Inverse variance weighting (IVW), MR-Egger, and weighted median methods. Initially, we confirmed consistency in the direction of B values across IVW, MR-Egger, and weighted median results, prioritizing IVW results for the main analysis (Bowden et al., 2017). Furthermore, sensitivity analysis was conducted to concurrently assess the presence of horizontal pleiotropy and heterogeneity. A p-value exceeding 0.05 for MR-Egger indicated the absence of significant horizontal pleiotropy. Heterogeneity was evaluated using Conchrane’s Q test, with a p-value exceeding 0.05 suggesting the absence of significant heterogeneity. Additionally, leave-one-out sensitivity analyses were conducted to evaluate result robustness. The MR pleiotropy residual sum and outliers (MR-PRESSO) test was used to further confirm the existence of horizontal pleiotropy, and MR-PRESSO has higher accuracy and can be used to identify horizontal pleiotropy and outliers (p > 0.05 indicates no horizontal pleiotropy) (Figure 1).
All analyses were performed using the TwoSampleMR R package, with statistical significance set at p < 0.05.
To ensure robustness in our selection of instrumental variables, we adopted stringent criteria. We selected single nucleotide polymorphisms (SNPs) with a p-value below the locus-wide significance threshold of 1 × 10−6 as instrumental variables. Additionally, we set the SNP linkage disequilibrium parameter (R2) to 0.001 to ensure independence among selected SNPs. To further guarantee the independence of instrumental variables, we maintained a genetic distance of 10,000 kb between them. Moreover, we verified that the p-values of both instrumental variables and outcomes exceeded 5 × 10−5, ensuring that only SNPs strongly associated with exposure factors and outcomes were included in our analysis. To guarantee the strength of the selected IVs, we used the variance (R2) and F statistics. R2 was calculated as follows: 2× (1−MAF) ×MAF × β2 (MAF, minor allele frequency; β, effect size of exposure). The F statistic is calculated using formula F = R2(N−2)/(1−R2). Among them, R2 represents the proportion of exposure variance explained by the independent variable, and N represents the sample size. An F statistic >10 indicates a strong correlation between IV and exposure. The instrumental variables (SNPs) related to exposures included in this study were in Supplementary Table S5.
In our analysis of the causal relationship between NSAIDs and various cancers, we observed statistically significant risk reductions for non-small cell lung cancer (OR: 0.76, 95% CI: 0.59–0.97, p-value: 0.03) and gastric cancer (OR: 0.57, 95% CI: 0.34–0.98, p-value: 0.04) (Figure 2). We also performed horizontal pleiotropy and heterogeneity analyzes to assess potential bias in their results. The analysis showed that the selected SNPs did not exhibit horizontal pleiotropy or heterogeneity, as evidenced by a p-value greater than 0.05 (Supplementary Table S1). Overall, NSAIDs exhibited a risk-reducing effect (OR<1) in 22 cancers and cancer pools, including ER-breast (OR: 0.98, 95% CI: 0.71–1.34, p-value: 0.89), colorectal cancer (OR: 0.89, 95% CI: 0.70–1.13, p-value: 0.32), colon cancer (OR: 0.82, 95% CI: 0.62–1.09, p-value: 0.18), and cervical cancer (OR: 0.86, 95% CI: 0.28–2.63, p-value: 0.80), among others. However, NSAIDs demonstrated an increased risk effect for prostate cancer (OR: 1.22, 95% CI: 1.00–1.50, p-value: 0.05) (Figure 3; Supplementary Table S1).

Figure 2. Forest plot of statistically significant summary results in Mendelian randomization analysis of anti-inflammatory drugs and cancer (p < 0.05).
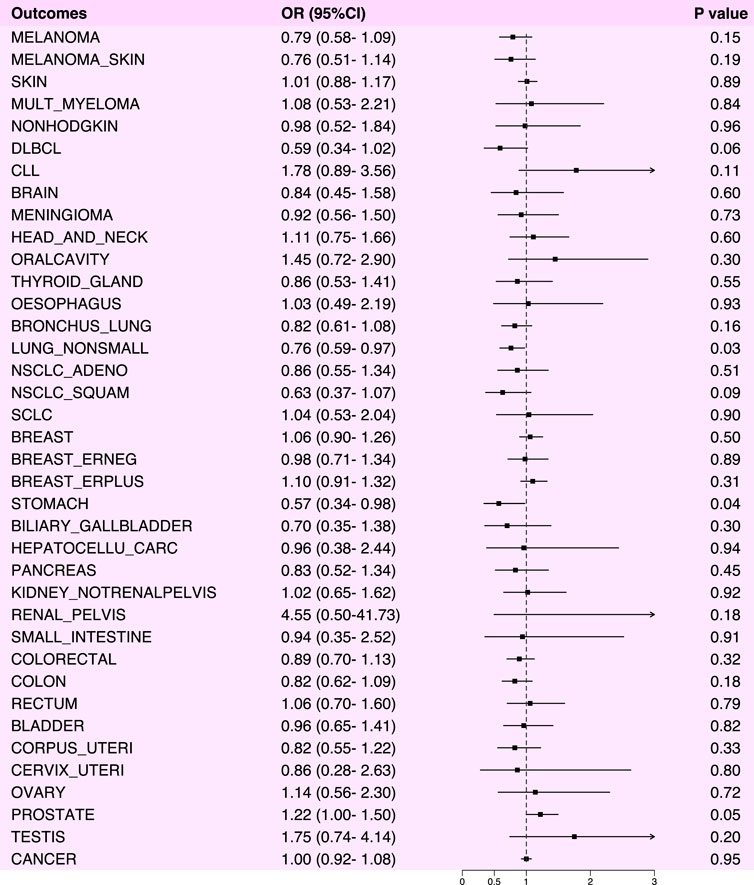
Figure 3. Forest plot of Mendelian randomization analysis results between NSAIDs and various cancers.
Aspirin was demonstrated a protective effect against chronic lymphocytic leukemia (OR: 0.45, 95% CI: 0.21–0.93, p-value: 0.03). However, it exhibited an increased risk effect against oral malignancies (OR: 2.18, 95% CI: 1.13–4.21, p-value: 0.02) (Figures 2, 4; Supplementary Table S2). Notably, in the analysis results with chronic lymphocytic leukemia, the B value of MR-Egger was >0, suggesting that the result lacked robustness. Likewise, we also performed horizontal pleiotropy and heterogeneity analyzes to assess potential biases in this part. The analysis showed that the selected SNPs did not exhibit horizontal pleiotropy or heterogeneity, as evidenced by a p-value greater than 0.05.
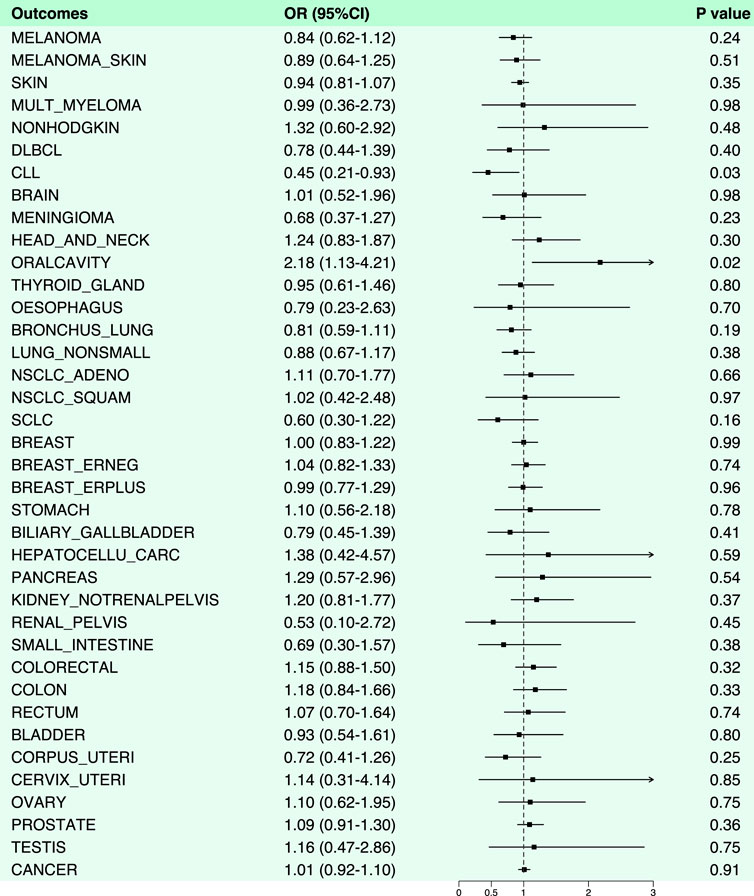
Figure 4. Forest plot of results from Mendelian randomization analysis between aspirin and various cancers.
In the Mendelian randomization analysis of anilide and cancer, no statistically significant results were observed. Overall, anilide drugs exhibited a risk-reducing effect (OR < 1) for 18 cancers and cancer pools, including breast (OR: 0.91, 95% CI: 0.77–1.07, p-value: 0.25), colon cancer (OR: 0.86, 95% CI: 0.61–1.23, p-value: 0.42), among others. Conversely, they showed an increased risk effect (OR > 1) for 19 types of cancer, including prostate cancer (OR: 1.01, 95% CI: 0.81–1.27, p-value: 0.91) (Figure 5; Supplementary Table S3).
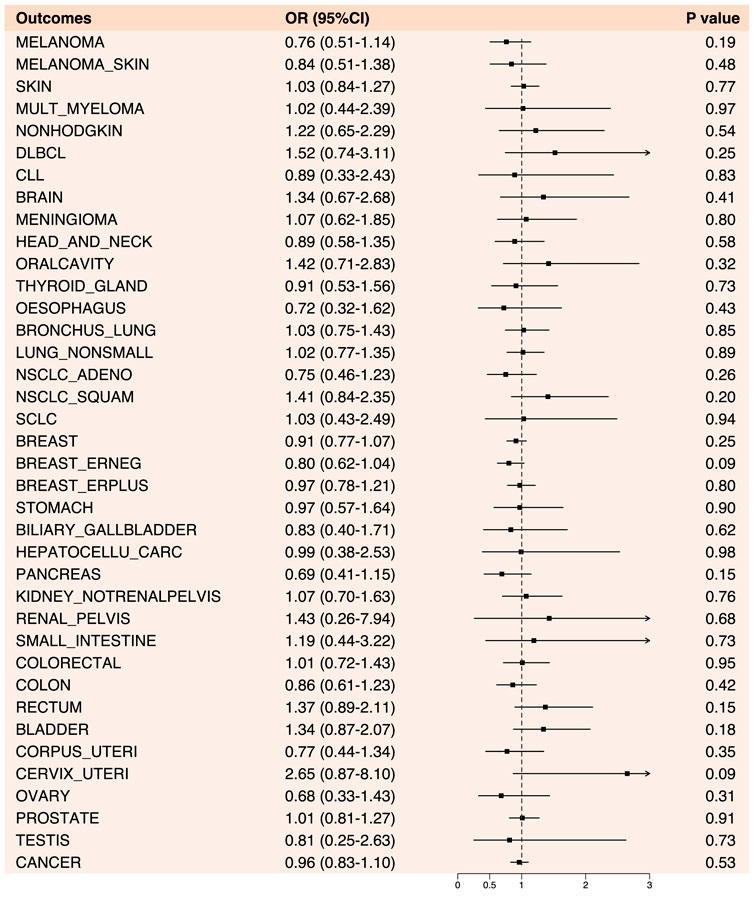
Figure 5. Forest plot of results from Mendelian randomization analysis between anilide drugs and various cancers.
The connection between chronic inflammation and cancer is widely acknowledged. Numerous epidemiological studies have provided compelling evidence of a robust correlation between inflammation and cancer (Hoepelman, 2001). This association stems from the ability of inflammation to foster a microenvironment conducive to cancer cell proliferation, migration, and metastasis, thus facilitating the development of cancer (Khandia and Munjal, 2020). Chronic inflammation, characterized by heightened cell proliferation and impaired DNA repair mechanisms, is particularly implicated in elevating cancer risk (Guo et al., 2017).
The profound connection between inflammation and cancer underscores the rationale for utilizing anti-inflammatory drugs as a therapeutic strategy. Aspirin and NSAIDs, long established in clinical practice, offer multifaceted benefits beyond their anti-inflammatory, antipyretic, and analgesic properties, including cardioprotective effects. Emerging evidence further suggests their potential in cancer prevention and risk reduction (Cuzick et al., 2009; Kolawole and Kashfi, 2022).
Indeed, NSAIDs exert their anti-inflammatory effects primarily by inhibiting cyclooxygenase, which plays a pivotal role in the synthesis of prostaglandins and thromboxane from arachidonic acid (Simmons et al., 2004; Panchal and Prince Sabina, 2023). However, their impact extends beyond mere inflammation, as NSAIDs have been implicated in anticancer activities through diverse mechanisms (Kopp and Ghosh, 1994; Arico et al., 2002; Shureiqi et al., 2003; Gurpinar et al., 2014; Kolawole and Kashfi, 2022).
Our study delves into the potential causal association between anti-inflammatory drugs and cancer through Mendelian randomization analysis, aiming to illuminate novel insights into their interplay. In our examination of NSAIDs and cancer, we observed protective effects against non-small cell lung cancer (OR: 0.76, 95% CI: 0.59–0.97, p-value: 0.03) and gastric cancer (OR: 0.57, 95% CI: 0.34–0.98, p-value: 0.04), suggesting a potential avenue for their therapeutic utility (p < 0.05). Notably, COX-2, typically minimally expressed in normal lung tissue, was found to be overexpressed in specimens from non-small cell lung cancer (NSCLC) patients, correlating with poorer prognosis (Khuri et al., 2001; Petkova et al., 2004; Sandler and Dubinett, 2004). Clinical evidence further supported the notion that NSAIDs may exert protective effects against lung cancer through pathways beyond mere anti-inflammatory actions. Although research on NSAIDs and gastric cancer remains limited, a meta-analysis has demonstrated aspirin’s efficacy in mitigating the risk of non-cardia gastric cancer and Helicobacter pylori infection (Yang et al., 2010). Elevated COX-2 expression in gastric cancer underscores its potential etiological role, with studies indicating a reduction in COX-2 expression following H. pylori eradication (Ji et al., 2021).
In our Mendelian randomization study concerning aspirin and cancer, we uncovered a risk-increasing effect on oral malignancies (OR: 2.18, 95% CI: 1.13–4.21, p-value: 0.02), a finding that currently lacks robust research support. Regarding the extensively studied impact of NSAIDs on colorectal cancer prevention, our analysis revealed no statistically significant protective effect for either NSAIDs (OR: 0.89, 95% CI: 0.70–1.13, p-value: 0.32) or aspirin alone (OR: 1.15, 95% CI: 0.88–1.50, p-value: 0.32). The onset of colorectal cancer is intricately linked to various factors, encompassing COX activity, platelet-mediated effects, inflammation, intestinal microorganisms, and more. The multifaceted effects of NSAIDs hint at complex underlying mechanisms yet to be fully elucidated. Similarly, the nuanced biochemistry of aspirin may yield divergent outcomes. Notably, the US Preventive Services Task Force’s recent revisions suggested insufficient evidence to support the notion that low-dose aspirin reduces mortality from colorectal cancer (Dehmer et al., 2022; US Preventive Services Task Force et al., 2022).
While in our Mendelian randomization analysis examining the association between aniline anti-inflammatory drugs and cancer, we did not observe any statistically significant results. Aniline anti-inflammatory drugs, characterized as relatively mild analgesics and antipyretics, are exemplified by acetaminophen, which has been a mainstay in clinical use for over a century. Despite its long history, the exact mechanism of action remains elusive. Traditionally, it was believed to share a mechanism akin to classic non-steroidal anti-inflammatory drugs (NSAIDs) by inhibiting COX enzymes (Graham et al., 2013). However, emerging evidence suggested distinctions from traditional NSAIDs as it largely lacks peripheral anti-inflammatory effects. Notably, when levels of arachidonic acid and peroxide are elevated, its activity is minimal, resulting in weak anti-inflammatory effects (Ghanem et al., 2016). Consequently, the limited breadth of COX enzyme inhibitory activity may undermine the efficacy of aniline anti-inflammatory drugs against inflammation and cancer.
The pharmacological differences among NSAIDs, Aspirin, and Anilide may be closely associated with their anticancer effects. Acetaminophen, the most common Anilide, is widely utilized for its antipyretic and analgesic properties. Its pharmacokinetic characteristics support its centrally mediated mechanism of action (Ayoub, 2021). Acetaminophen is essentially nonionizable within the physiological pH range, and its lipid solubility facilitates rapid penetration of cell membranes and easy passage across the blood-brain barrier (Clissold, 1986). Consequently, acetaminophen primarily exerts its antipyretic effect by inhibiting the synthesis and release of prostaglandins in the hypothalamus, resulting in weaker peripheral effects (Seppälä et al., 1983; Muth-Selbach et al., 1999).
Studies have demonstrated that anti-inflammatory drugs, such as NSAIDs, can modulate the pharmacokinetics of other medications (Zappavigna et al., 2020). By altering the metabolism of these drugs, they can enhance efficacy and reduce toxicity. For instance, diclofenac inhibits the glucuronidation of DMXAA, thereby impeding its metabolism and elevating its plasma concentration (Wang et al., 2009).
In addition to their ability to reduce the toxicity of conventional drugs by altering their metabolism, the effective anticancer effects of NSAIDs when combined with chemotherapy drugs may also be attributed to their chemosensitizing effects (Thiruchenthooran et al., 2023). Several preclinical studies have demonstrated that the combination of celecoxib with etoposide, doxorubicin, vincristine, or irinotecan can result in additive or synergistic effects in vitro (Ponthan et al., 2007; Arunasree et al., 2008). Moreover, celecoxib enhances the response of prostate cancer cells to docetaxel both in vitro and in vivo (Nakata et al., 2004). Currently, combining chemotherapy drugs with NSAIDs provides a promising approach to enhancing drug activity and increasing the lipophilicity of chemotherapy agents (Ravera et al., 2019).
In our study, we delved into the potential causal relationship between NSAIDs anti-inflammatory drugs and cancer using Mendelian randomization analysis. Our findings unveiled a risk-reducing effect of NSAIDs on non-small cell lung cancer and gastric cancer, while conversely, aspirin exhibited an increased risk effect on oral cancer. Notably, anilide did not yield statistically significant results. The comprehensive analysis did not reveal a widespread risk-reducing impact of anti-inflammatory drugs on cancer. The application of Mendelian randomization analysis enabled effective control over the influence of confounding factors. Given the multifaceted mechanisms of action of NSAIDs drugs, our research contributes valuable insights to existing clinical studies and charts new directions for future research endeavors.
While our study provides valuable insights, it’s important to acknowledge its limitations. Firstly, the data primarily focused on European populations, potentially limiting the generalizability of our findings to other ethnic groups. Additionally, human factors during data acquisition, processing, and analysis may have influenced the accuracy of our results. These limitations highlight the need for further research involving diverse populations and meticulous data handling to ensure robust and comprehensive findings.
In our Mendelian randomization study, we delved into the causal association between NSAIDs anti-inflammatory drugs and cancer. Despite our extensive analysis, we found that only a select few cancers exhibited a risk-reducing effect, leading us to refrain from concluding that NSAIDs anti-inflammatory drugs possess a broad protective effect against cancer.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
SG: Data curation, Investigation, Software, Writing–original draft. GW: Conceptualization, Data curation, Formal Analysis, Methodology, Writing–original draft. QM: Investigation, Methodology, Validation, Writing–original draft. XW: Investigation, Methodology, Writing–original draft. SW: Data curation, Software, Writing–original draft. YN: Conceptualization, Supervision, Visualization, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank the participants and researchers of the FinnGen study. Additionally, we thank Wu et al., 2019 for their study and their contribution to the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1392745/full#supplementary-material
Arico, S., Pattingre, S., Bauvy, C., Gane, P., Barbat, A., Codogno, P., et al. (2002). Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J. Biol. Chem. 277, 27613–27621. doi:10.1074/jbc.M201119200
Arunasree, K. M., Roy, K. R., Anilkumar, K., Aparna, A., Reddy, G. V., and Reddanna, P. (2008). Imatinib-resistant K562 cells are more sensitive to celecoxib, a selective COX-2 inhibitor: role of COX-2 and MDR-1. Leuk. Res. 32, 855–864. doi:10.1016/j.leukres.2007.11.007
Ayoub, S. S. (2021). Paracetamol (acetaminophen): a familiar drug with an unexplained mechanism of action. Temp. (Austin) 8, 351–371. doi:10.1080/23328940.2021.1886392
Balkwill, F., and Mantovani, A. (2001). Inflammation and cancer: back to Virchow? Lancet 357, 539–545. doi:10.1016/S0140-6736(00)04046-0
Bindu, S., Mazumder, S., and Bandyopadhyay, U. (2020). Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem. Pharmacol. 180, 114147. doi:10.1016/j.bcp.2020.114147
Bowden, J., Del Greco M, F., Minelli, C., Davey Smith, G., Sheehan, N., and Thompson, J. A. (2017). A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Statistics Med. 36, 1783–1802. doi:10.1002/sim.7221
Chan, A. T., Giovannucci, E. L., Meyerhardt, J. A., Schernhammer, E. S., Curhan, G. C., and Fuchs, C. S. (2005). Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 294, 914–923. doi:10.1001/jama.294.8.914
Clissold, S. P. (1986). Paracetamol and phenacetin. Drugs 32 (Suppl. 4), 46–59. doi:10.2165/00003495-198600324-00005
Coussens, L. M., and Werb, Z. (2002). Inflammation and cancer. Nature 420, 860–867. doi:10.1038/nature01322
Cuzick, J., Otto, F., Baron, J. A., Brown, P. H., Burn, J., Greenwald, P., et al. (2009). Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 10, 501–507. doi:10.1016/S1470-2045(09)70035-X
Davey Smith, G., and Hemani, G. (2014). Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23, R89–R98. doi:10.1093/hmg/ddu328
Dehmer, S. P., O’Keefe, L. R., Grossman, E. S., and Maciosek, M. V. (2022) Aspirin use to prevent cardiovascular disease and colorectal cancer: an updated decision analysis for the U.S. Preventive Services Task Force; U.S. Preventive Services Task Force evidence syntheses, formerly systematic evidence reviews. Rockville (MD): Agency for Healthcare Research and Quality US.
Ghanem, C. I., Pérez, M. J., Manautou, J. E., and Mottino, A. D. (2016). Acetaminophen from liver to brain: new insights into drug pharmacological action and toxicity. Pharmacol. Res. 109, 119–131. doi:10.1016/j.phrs.2016.02.020
Graham, G. G., Davies, M. J., Day, R. O., Mohamudally, A., and Scott, K. F. (2013). The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacol 21, 201–232. doi:10.1007/s10787-013-0172-x
Guo, Y., Nie, Q., MacLean, A. L., Li, Y., Lei, J., and Li, S. (2017). Multiscale modeling of inflammation-induced tumorigenesis reveals competing oncogenic and oncoprotective roles for inflammation. Cancer Res. 77, 6429–6441. doi:10.1158/0008-5472.CAN-17-1662
Gurpinar, E., Grizzle, W. E., and Piazza, G. A. (2014). NSAIDs inhibit tumorigenesis, but how? Clin. Cancer Res. 20, 1104–1113. doi:10.1158/1078-0432.CCR-13-1573
Hasche, D., Stephan, S., Braspenning-Wesch, I., Mikulec, J., Niebler, M., Gröne, H.-J., et al. (2017). The interplay of UV and cutaneous papillomavirus infection in skin cancer development. PLoS Pathog. 13, e1006723. doi:10.1371/journal.ppat.1006723
Hoepelman, A. (2001). Title “inflammation. Basic principles and clinical correlates” 3rd edition, 1999, ed. By J.I. Gallin and R. Snyderman, lippincott williams and wilkins publishers, ISBN: 0 39 751 759 9, price $245.00, dfl. 620.00, pp. 1335, large format, hard cover, including 21 full colour pages. Neth. J. Med. 58, 41. doi:10.1016/S0300-2977(00)00085-1
Ji, X.-K., Madhurapantula, S. V., He, G., Wang, K.-Y., Song, C.-H., Zhang, J.-Y., et al. (2021). Genetic variant of cyclooxygenase-2 in gastric cancer: more inflammation and susceptibility. WJG 27, 4653–4666. doi:10.3748/wjg.v27.i28.4653
Katan, M. B. (2004). Commentary: mendelian randomization, 18 Years on. Int. J. Epidemiol. 33, 10–11. doi:10.1093/ije/dyh023
Khandia, R., and Munjal, A. (2020). Interplay between inflammation and cancer. Adv. Protein Chem. Struct. Biol. 119, 199–245. ISBN 978-0-12-816844-8. doi:10.1016/bs.apcsb.2019.09.004
Khuri, F. R., Wu, H., Lee, J. J., Kemp, B. L., Lotan, R., Lippman, S. M., et al. (2001). Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin. Cancer Res. 7, 861–867.
Kolawole, O. R., and Kashfi, K. (2022). NSAIDs and cancer resolution: new paradigms beyond cyclooxygenase. IJMS 23, 1432. doi:10.3390/ijms23031432
Kopp, E., and Ghosh, S. (1994). Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 265, 956–959. doi:10.1126/science.8052854
Kurki, M. I., Karjalainen, J., Palta, P., Sipilä, T. P., Kristiansson, K., Donner, K. M., et al. (2023). FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518. doi:10.1038/s41586-022-05473-8
Lawlor, D. A., Harbord, R. M., Sterne, J. A. C., Timpson, N., and Davey Smith, G. (2008). Mendelian randomization: using genes as instruments for making causal inferences in Epidemiology. Statistics Med. 27, 1133–1163. doi:10.1002/sim.3034
Mahmud, S., Franco, E., and Aprikian, A. (2004). Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br. J. Cancer 90, 93–99. doi:10.1038/sj.bjc.6601416
Menter, D. G., and Bresalier, R. S. (2023). An aspirin a day: new pharmacological developments and cancer chemoprevention. Annu. Rev. Pharmacol. Toxicol. 63, 165–186. doi:10.1146/annurev-pharmtox-052020-023107
Muth-Selbach, U. S., Tegeder, I., Brune, K., and Geisslinger, G. (1999). Acetaminophen inhibits spinal prostaglandin E2 release after peripheral noxious stimulation. Anesthesiology 91, 231–239. doi:10.1097/00000542-199907000-00032
Nakata, E., Mason, K. A., Hunter, N., Husain, A., Raju, U., Liao, Z., et al. (2004). Potentiation of tumor response to radiation or chemoradiation by selective cyclooxygenase-2 enzyme inhibitors. Int. J. Radiat. Oncology*Biology*Physics 58, 369–375. doi:10.1016/j.ijrobp.2003.09.061
Panchal, N. K., and Prince Sabina, E. (2023). Non-steroidal anti-inflammatory drugs (NSAIDs): a current insight into its molecular mechanism eliciting organ toxicities. Food Chem. Toxicol. 172, 113598. doi:10.1016/j.fct.2022.113598
Pereg, D., and Lishner, M. (2005). Non-steroidal anti-inflammatory drugs for the prevention and treatment of cancer. J. Intern. Med. 258, 115–123. doi:10.1111/j.1365-2796.2005.01519.x
Perwez Hussain, S., and Harris, C. C. (2007). Inflammation and cancer: an ancient link with novel potentials. Intl J. Cancer 121, 2373–2380. doi:10.1002/ijc.23173
Petkova, D. K., Clelland, C., Ronan, J., Pang, L., Coulson, J. M., Lewis, S., et al. (2004). Overexpression of cyclooxygenase-2 in non-small cell lung cancer. Respir. Med. 98, 164–172. doi:10.1016/j.rmed.2003.09.006
Ponthan, F., Wickström, M., Gleissman, H., Fuskevåg, O. M., Segerström, L., Sveinbjörnsson, B., et al. (2007). Celecoxib prevents neuroblastoma tumor development and potentiates the effect of chemotherapeutic drugs in vitro and in vivo. Clin. Cancer Res. 13, 1036–1044. doi:10.1158/1078-0432.CCR-06-1908
Ravera, M., Zanellato, I., Gabano, E., Perin, E., Rangone, B., Coppola, M., et al. (2019). Antiproliferative activity of Pt(IV) conjugates containing the non-steroidal anti-inflammatory drugs (NSAIDs) ketoprofen and naproxen. IJMS 20, 3074. doi:10.3390/ijms20123074
Sandler, A. B., and Dubinett, S. M. (2004). COX-2 inhibition and lung cancer. Seminars Oncol. 31, 45–52. doi:10.1053/j.seminoncol.2004.03.045
Santos, R., Ursu, O., Gaulton, A., Bento, A. P., Donadi, R. S., Bologa, C. G., et al. (2017). A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 16, 19–34. doi:10.1038/nrd.2016.230
Seppälä, E., Laitinen, O., and Vapaatalo, H. (1983). Comparative study on the effects of acetylsalicylic acid, indomethacin and paracetamol on metabolites of arachidonic acid in plasma, serum and urine in man. Int. J. Clin. Pharmacol. Res. 3, 265–269.
Shureiqi, I., Jiang, W., Zuo, X., Wu, Y., Stimmel, J. B., Leesnitzer, L. M., et al. (2003). The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc. Natl. Acad. Sci. U. S. A. 100, 9968–9973. doi:10.1073/pnas.1631086100
Simmons, D. L., Botting, R. M., and Hla, T. (2004). Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56, 387–437. doi:10.1124/pr.56.3.3
Thiruchenthooran, V., Sánchez-López, E., and Gliszczyńska, A. (2023). Perspectives of the application of non-steroidal anti-inflammatory drugs in cancer therapy: attempts to overcome their unfavorable side effects. Cancers (Basel) 15, 475. doi:10.3390/cancers15020475
US Preventive Services Task Force Davidson, K. W., Barry, M. J., Mangione, C. M., Cabana, M., Chelmow, D., Coker, T. R., et al. (2022). Aspirin use to prevent cardiovascular disease: US preventive Services Task Force recommendation statement. JAMA 327, 1577–1584. doi:10.1001/jama.2022.4983
Vane, J. R. (1971). Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 231, 232–235. doi:10.1038/newbio231232a0
Wang, F., Meng, W., Wang, B., and Qiao, L. (2014). Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 345, 196–202. doi:10.1016/j.canlet.2013.08.016
Wang, L.-C. S., Ching, L.-M., Paxton, J. W., Kestell, P., Sutherland, R., Zhuang, L., et al. (2009). Enhancement of the action of the antivascular drug 5,6-dimethylxanthenone-4-acetic acid (DMXAA; ASA404) by non-steroidal anti-inflammatory drugs. Invest. New Drugs 27, 280–284. doi:10.1007/s10637-008-9167-7
Wu, Y., Byrne, E. M., Zheng, Z., Kemper, K. E., Yengo, L., Mallett, A. J., et al. (2019). Genome-wide association study of medication-use and associated disease in the UK Biobank. Nat. Commun. 10, 1891. doi:10.1038/s41467-019-09572-5
Yang, P., Zhou, Y., Chen, B., Wan, H.-W., Jia, G.-Q., Bai, H.-L., et al. (2010). Aspirin use and the risk of gastric cancer: a meta-analysis. Dig. Dis. Sci. 55, 1533–1539. doi:10.1007/s10620-009-0915-0
Zappavigna, S., Cossu, A. M., Grimaldi, A., Bocchetti, M., Ferraro, G. A., Nicoletti, G. F., et al. (2020). Anti-inflammatory drugs as anticancer agents. IJMS 21, 2605. doi:10.3390/ijms21072605
Keywords: Mendelian randomization analysis, NSAIDs, aspirin, anilide, pancancer, anti-inflammatory medications
Citation: Gao S, Wei G, Ma Q, Wang X, Wang S and Niu Y (2024) Causal relationship between anti-inflammatory drugs and cancer: a pan-cancer study with Mendelian randomization. Front. Genet. 15:1392745. doi: 10.3389/fgene.2024.1392745
Received: 28 February 2024; Accepted: 08 May 2024;
Published: 24 May 2024.
Edited by:
Min Qi, Central South University, ChinaReviewed by:
Sandra Donnini, University of Siena, ItalyCopyright © 2024 Gao, Wei, Ma, Wang, Wang and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanjie Niu, bml1eXVhbmppZTk2OEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.