- 1Precision Medical Lab Center, People’s Hospital of Yangjiang, Yangjiang, Guangdong, China
- 2Precision Medical Lab Center, Chaozhou Central Hospital, Chaozhou, Guangdong, China
- 3Institute of Medicine and Nursing, Hubei University of Medicine, Shiyan, Hubei, China
- 4Department of Laboratory Medicine, People’s Hospital of Yangjiang, Yangjiang, Guangdong, China
- 5Department of Transfusion, People’s Hospital of Yangjiang, Yangjiang, Guangdong, China
Objectives: The prevalence of G6PD deficiency has not been reported in Yangjiang, a western city in Guangdong province. This study aims to investigate the molecular characteristics of G6PD deficiency in this region.
Methods: Blood samples were collected from adults at a local hospital to screen for G6PD deficiency. The deficient samples were subjected to further analysis using PCR and reverse dot blot to determine the specific G6PD variants.
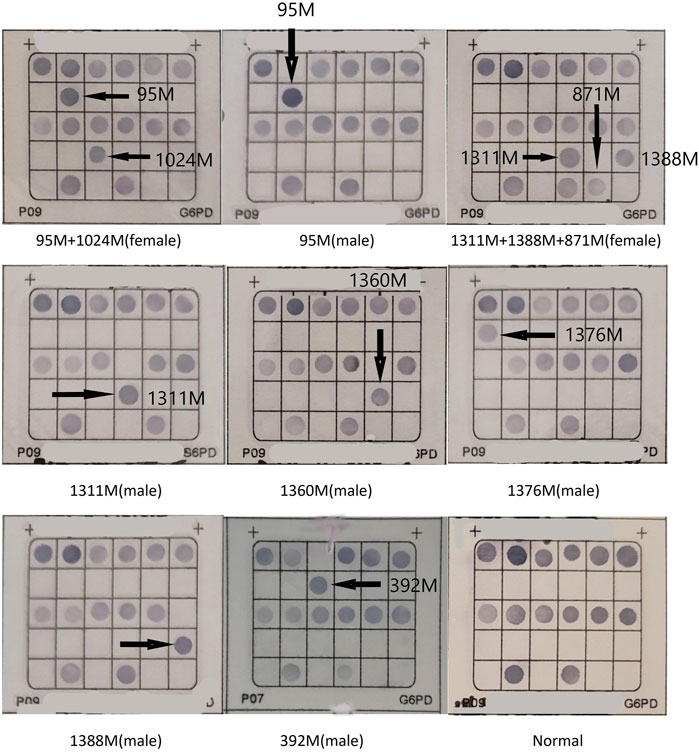
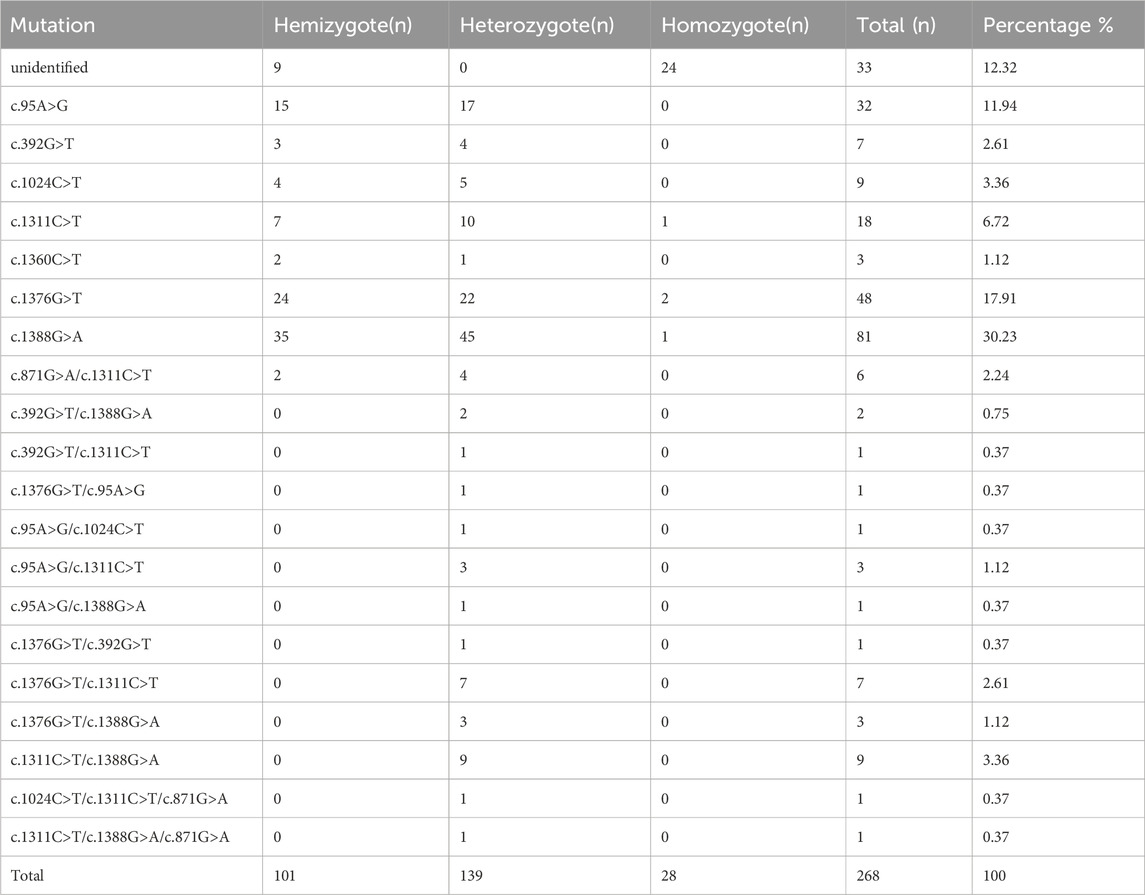
Results: Among the 3314 male subjects, 250 cases of G6PD deficiency were found using the G6PD enzyme quantitative assay, resulting in a prevalence of 7.54% (250/3314) in the Yangjiang region. The prevalence of G6PD deficiency in females was 3.42% (176/5145). Out of the 268 cases of G6PD deficiency tested for G6PD mutations, reverse dot blot identified 20 different G6PD variants. The most common G6PD variant was c.1388G>A (81/268), followed by c.1376G>T (48/268), c.95A>G (32/268), c.1024C>T (9/268), c.392G>T (7/268), and c.871G>A/c.1311C>T (6/268). It was observed that c.871G>A was always linked to the polymorphism of c.1311C>T in this population.
Conclusion: This investigation into G6PD deficiency in this area is expected to significantly improve our understanding of the prevalence and molecular characterization of this condition.
1 Introduction
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is a widely prevalent condition found worldwide. Individuals with G6PD deficiency have increased susceptibility to oxidative stress, such as from fava beans and infections, which can lead to acute hemolytic anemia (Lee et al., 2022). Moreover, newborns with G6PD deficiency face a higher risk of developing jaundice, which can rapidly progress to bilirubin encephalopathy and cause permanent and catastrophic neurological damage known as kernicterus (Xu et al., 2021).
The G6PD gene is located at the Xq28 band and encodes the G6PD enzyme. It consists of 13 exons, 12 introns, and spans approximately 18 kb. G6PD deficiency is predominantly caused by genetic mutations following an X-linked incomplete recessive inheritance pattern. These mutations often give rise to polymorphic variants that exhibit reduced enzyme activity and disrupt protein folding (Pfeffer et al., 2022). Currently, more than 230 clinically relevant genetic variants have been identified (Pfeffer et al., 2022). The frequency of G6PD deficiency varies among different regions and ethnic populations (Lee et al., 2022; Pfeffer et al., 2022). In southern China, G6PD deficiency is prevalent (Lin et al., 2018). However, while data on the general population’s prevalence of G6PD deficiency is available, limited information exists regarding the range of G6PD variants in specific regions and ethnic groups (He et al., 2020).
Previous surveys have shown a higher occurrence of G6PD deficiency in Guangdong province (Yang et al., 2015; Lin et al., 2018; He et al., 2020). Nonetheless, the specific genotypes of G6PD variants have not been identified, and no large-scale survey has been conducted to investigate the molecular characteristics of G6PD deficiency in Yangjiang, a city situated in the western region of Guangdong province. Hence, the current study aims to determine the prevalence and molecular characterization of G6PD deficiency in Yangjiang, with the objective of enhancing our understanding of this condition in the region.
2 Material and methods
2.1 Study population and sample collection
The data for G6PD screening was collected from August 2018 to November 2022. The study included two groups: one group consisted of individuals visiting the People’s Hospital of Yangjiang for routine check-ups, while the other group comprised pregnant women attending the obstetrics department as outpatients for regular prenatal examinations. All participants were unaware of their G6PD status and underwent G6PD quantitative assays along with routine blood tests. Following the routine medical blood tests, the remaining blood samples were used for G6PD gene variant analysis. The study population consisted of 3,314 healthy local males and 5,145 pregnant females, aged between 15 and 50 years, who underwent G6PD enzyme quantitative assays. The study was approved by the Ethics Committee of the People’s Hospital of Yangjiang (NO. 20220069).
2.2 G6PD enzyme assay
The G6PD enzyme assay involved detecting the production rate of NADPH, which served as a quantitative method for assessing G6PD activity. The assays were conducted following the manufacturers' protocols (Beijing Antu Bioengineering Co., Ltd., China). In adults, a threshold of <1300 U/L was used to define G6PD deficiency (Tang et al., 2018).
2.3 Molecular analysis of G6PD gene variants
In individuals diagnosed with G6PD deficiency, whole blood collected with EDTA anticoagulants was stored in a biobank at −40°C. 268 cases of G6PD deficiency received gene analysis. The whole blood DNA extraction kit (Chaozhou Hybribio Co., Ltd.) was used to extract DNA according to the instructions of the kit, and then the DNA quantity and purity were tested (NanoDrop One, Thermo Fisher Scientific Co., Ltd.). VeritiTM Dx 96-Well Thermal Cycler (Thermo Fisher Scientific) was used for PCR amplification, then, G6PD gene variant was detected by reverse dot blot method (Chaozhou Hybribio Co., Ltd.) for 13 common G6PD mutation types c.1376G>T (G6PD Canton), c.1388G>A (G6PD Kaiping), c.95A>G (G6PD Gaohe), c.871 G>A (G6PD Viangchan), c.392G>T (G6PD Qing Yan), c.487G>A (G6PD Mahidol), c.493A>G (G6PD Taipei), c.592G>T (G6PD Coimbra), c.1004C > T (G6PD Fushan), c.1024C>T (G6PD Chinese-5), c.1360C > T (G6PD Union), c.1387C>T (G6PD Keelung), c.1381G>A (G6PD Yunan) and one polymorphism c.1311C>T (Hu et al., 2015).
100 healthy cases (comprising both G6PD deficient and G6PD normal) were chosen randomly for G6PD gene analysis during routine check-ups, they were firstly tested for G6PD enzyme quantitative assay, and the gene sequencing method was described previously (Huang et al., 2023).
2.4 Statistical analysis
Statistical analysis in this study was carried out using SPSS 16.0 statistical software. Gene frequencies of the G6PD alleles were calculated using the Maximum Likelihood method. Hardy-Weinberg equilibrium was utilized to compare the observed and expected genotypes in the study. The distribution of various alleles responsible for G6PD deficiency in Yangjiang and other regions in China was compared using the chi-square test.
3 Results
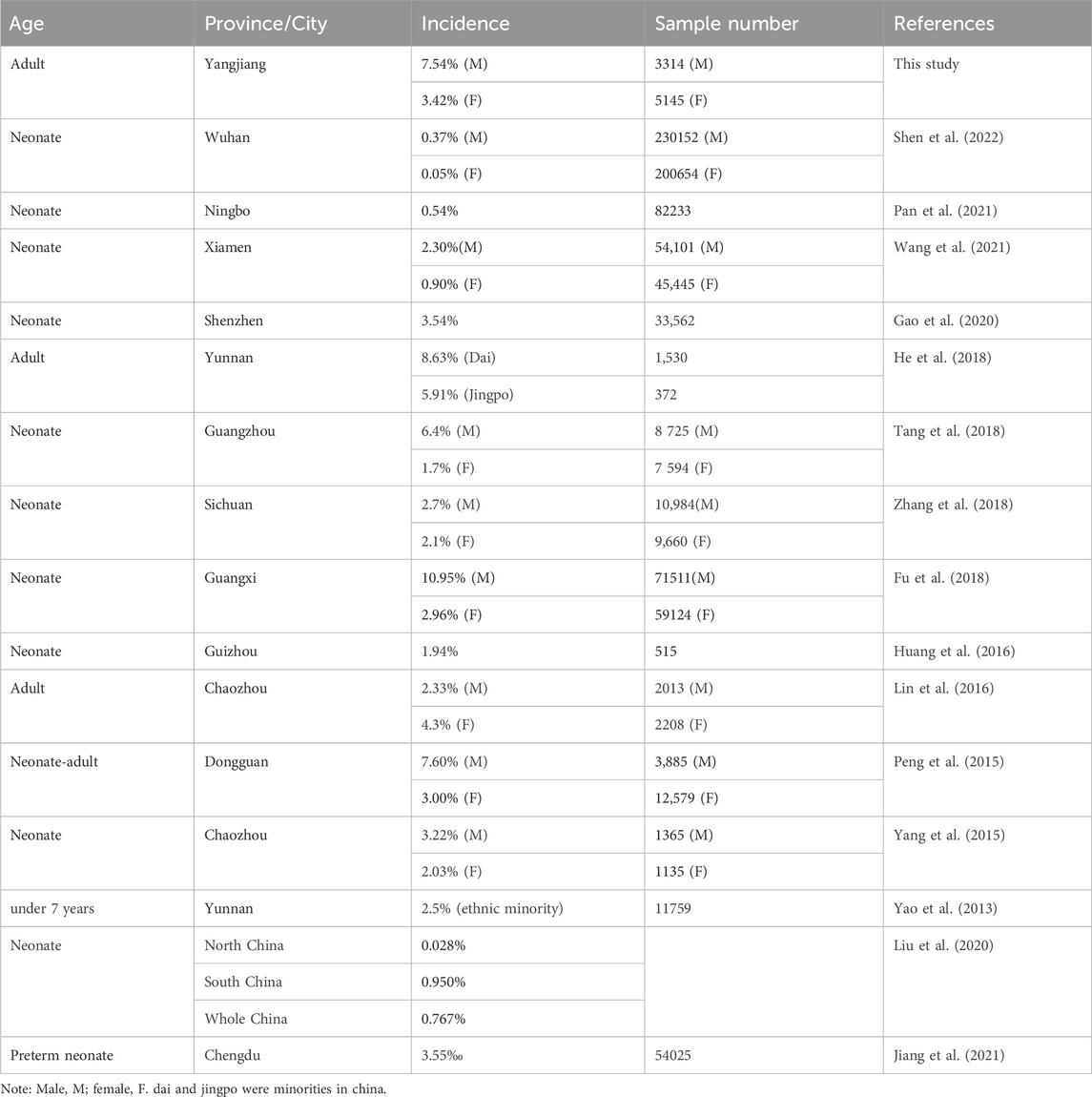
In this study, 426 cases G6PD deficiency were identified by G6PD enzyme quantitative assay in 8459 adults, the prevalence of G6PD deficiency was 5.04% in this population, 250 cases of G6PD deficiency were found in males, the prevalence of G6PD deficiency was 7.54% (250/3314) in Yangjiang region, while the prevalence of G6PD deficiency in females was 3.42% (176/5145), it was lower than that of males.
268 cases of G6PD deficiency were tested for G6PD mutations, 20 kinds of G6PD variants were identified by reverse dot blot. c.1388G>A (81/268) was the most common G6PD variant, followed by c.1376G>T (48/268), c.95A>G (32/268), c.1024C>T(9/268), c.392G>T(7/268), and c.871G>A/c.1311C>T (6/268). c.871G>A was always linked with polymorphism of c.1311C>T in this population (Figure 1; Table 1). 33 samples were not detected with G6PD mutations, the unidentified samples might be rare types not included in this detection kit (Table 1).
100 healthy adults (44 males and 56 females) for check-up were randomly selected for G6PD gene variants detection, they were firstly tested for G6PD enzyme quantitative assay, and 8 cases (8%) were G6PD deficient. Then, all 100 cases were genotyped for G6PD mutations (Huang et al., 2023), 4 female specimens showed gene mutation but without the enzyme activity decrease, while 3 specimens (2 males and one female) showed a decrease in enzyme activity but without gene mutations. 10 cases were detected with G6PD mutations, including c.1376G>T heterozygote (4 cases), c.1388G>A heterozygote (1 case), c.1388G>A hemizygote (1 case), c.95A>G heterozygote (3 cases), and c.95A>G hemizygote (1 case). The allele frequency of G6PD variants in this population and in females was 10% and 14.3% (8/56), respectively.
4 Discussion
This study is the first molecular investigation of G6PD deficiency in the Yangjiang area of western Guangdong province in southern China. A total of 8459 subjects underwent the G6PD enzyme assay, and the prevalence of G6PD deficiency was found to be 7.54% in males and 3.42% in females in our study cohort. Comparing the prevalence of G6PD deficiency in Yangjiang with other areas in China, the frequency of G6PD deficiency was higher than the average frequency of 13 provinces in the southern region of the Yangtze River and was also higher than the average frequency of 8 provinces in the northern region of the Yangtze River (Liu et al., 2020). In all, the frequency of G6PD deficiency in Yangjiang region of our study cohort was only secondary to Guangxi province (p < 0.001) and Yunnan (p < 0.001) for the whole China (Fu et al., 2018; He et al., 2018). Furthermore, the prevalence was similar to that report from the neighboring Guangzhou (p = 0.025) (Tang et al., 2018) and Dongguan (p = 0.964) (Peng et al., 2015). The prevalence of G6PD deficiency in China was shown in Table 2.
It has been reported that G6PD variants exhibit significant genetic polymorphisms in Chinese populations (Liu et al., 2017). The genotypes of G6PD variants show notable variations between the southern and northern regions of China, as well as among different ethnic groups (Yao et al., 2013; Han et al., 2016; Liu et al., 2020). Notably, c.1388G>A and c.1376G>T are prevalent throughout China, while c.487G>A, which was not found in our study cohort, is more common in northern China (Liu et al., 2017). In our study cohort, the three most common G6PD mutations were c.1388G > A, c.1376G > T, and c.95A > G, which accounted for 84% of the total disease alleles. Remarkably, the distribution of G6PD variants in Yangjiang was found to be similar to that in Guangzhou (Tang et al., 2018), Guangxi region (Fu et al., 2018), and Dongguan (Peng et al., 2015). The main genotypes observed in these regions were c.1388G>A, c.1376G>T, c.95A>G, and c.871G>A.
20 G6PD pathogenic variants were identified in our study, the polymorphism of c.1311C>T does not result in an amino acid change. In addition, c.1311 C>T/IVS-1193 T>C polymorphism had a relatively high frequency in the individuals with normal G6PD levels of Guangdong province (Lin et al., 2018). More interestingly, our study showed that c.871G>A was always linked with the c.1311C>T in this region. We hypothesized that the carriers with c.871G>A in Yangjiang might share the same ancestors. The polymorphism of 3′ UTR c.*+357A>G (rs1050757), which is often linked with c.1311C>T, may contribute to decreased G6PD (Amini and Ismail, 2013; Sirdah et al., 2017). However, we did not find this 3′ UTR polymorphism of G6PD gene in Guangdong province (Lin et al., 2016). 18 cases of c.1311C>T carriers with G6PD deficiency were identified in our study, and no other pathogenic variants was found, and there might be other mechanism to explain this conflict. The clinical significance of the c.1311C>T polymorphism remains unclear and requires further study. The exact reason why individuals with this polymorphism develop G6PD deficiency is yet to be determined.
G6PD deficiency presents a broad spectrum of clinical manifestations, ranging from asymptomatic cases to various hemolytic syndromes such as acute hemolytic anemia, favism, and neonatal hyperbilirubinemia. In severe cases, this deficiency can lead to kernicterus (Xu et al., 2021; Yang et al., 2023). Early identification of G6PD deficiency plays a critical role in effectively managing associated complications. The World Health Organization (WHO) recommends conducting G6PD screening for newborns if the male prevalence of deficiency is 3%–5% (WHO Working Group, 1989 19). However, in our study, the prevalence of G6PD deficiency in males in Yangjiang was found to be 7.54%, indicating the necessity of essential G6PD deficiency screening during the newborn period to alert parents about the potential risks of severe neonatal jaundice (Yang et al., 2023). Currently, G6PD deficiency is routinely included in newborn screening programs in southern China. Additionally, genetic counseling for couples may involve raising awareness and providing guidance on avoiding oxidative stressors to protect themselves from hemolytic triggers.
In our study cohort, the prevalence of hemizygous G6PD-deficient males in Yangjiang was found to be 7.54%. Theoretically, the corresponding prevalence in females can be predicted using the Hardy-Weinberg formula (Chan et al., 2008). Based on this formula, the prevalence of heterozygous G6PD-deficient females would be 14.51%, while 0.57% would represent homozygous females. However, our study only identified 3.42% of G6PD-deficient females. G6PD deficiency is classified as an X-linked recessive inborn error that primarily affects males, while heterozygous females may exhibit normal, intermediate, or deficient G6PD activity due to random X chromosome inactivation. Consequently, a significant proportion of heterozygous females with partial G6PD deficiency may have been overlooked in our study.
In our study, we randomly selected 100 healthy adults (44 males and 56 females) for check-up, and G6PD gene variant detection was performed. Among these individuals, 14.3% (8/56) of the females were identified as heterozygotes for G6PD variants, which is similar to the calculated prevalence of 14.51% in females.
5 Conclusion
In conclusion, this study provides valuable insights into the prevalence and molecular characterization of G6PD deficiency in the Yangjiang region, contributing to our understanding of this condition. Furthermore, offering genetic consultation to affected couples can effectively raise awareness about G6PD deficiency and neonatal jaundice. It also plays a crucial role in guiding individuals to avoid oxidative stressors and safeguarding their children from hemolytic triggers.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the People’s Hospital of Yangjiang. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
H-FL: Funding acquisition, Supervision, Writing–original draft. Y-BC: Investigation, Methodology, Writing–review and editing. FL: Software, Writing–review and editing. Y-KY: Investigation, Methodology, Writing–review and editing. Y-WL: Methodology, Software, Writing–review and editing. W-HO: Investigation, Methodology, Resources, Writing–review and editing. J-LC: Investigation, Methodology, Resources, Writing–review and editing. Y-QZ: Data curation, Methodology, Writing–review and editing. Y-CH: Data curation, Resources, Writing–review and editing. G-KZ: Data curation, Investigation, Methodology, Writing–review and editing. Z-XC: Data curation, Methodology, Resources, Writing–review and editing. J-WS: Data curation, Methodology, Resources, Writing–review and editing. J-XY: Methodology, Resources, Writing–review and editing. L-YY: Conceptualization, Funding acquisition, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the High Level Development Plan of People’s Hospital of Yangjiang (Nos G2020007 and 2021007) and the High Level and Key Health Research Plan of Yangjiang (No. 2023001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amini, F., and Ismail, E. (2013). 3'-UTR variations and G6PD deficiency. J. Hum. Genet. 58 (4), 189–194. doi:10.1038/jhg.2012.155
Chan, D. K. (2008). Glucose-6-phosphate dehydrogenase deficiency: correlation between the genotype, biochemistry and phenotype. Ann. Acad. Med. Singap. 37 (12 Suppl. l), 81–83.
Fu, C., Luo, S., Li, Q., Xie, B., Yang, Q., Geng, G., et al. (2018). Newborn screening of glucose-6-phosphate dehydrogenase deficiency in Guangxi, China: determination of optimal cutoff value to identify heterozygous female neonates. Sci. Rep. 8 (1), 833. doi:10.1038/s41598-017-17667-6
Gao, J., Lin, S., Chen, S., Wu, Q., Zheng, K., Su, J., et al. (2020). Molecular characterization of glucose-6-phosphate dehydrogenase deficiency in the shenzhen population. Hum. Hered. 85 (3-6), 110–116. doi:10.1159/000516808
Han, L., Su, H., Wu, H., Jiang, W., and Chen, S. (2016). Molecular epidemiological survey of glucose-6-phosphate dehydrogenase deficiency and thalassemia in uygur and kazak ethnic groups in xinjiang, northwest China. Hemoglobin 40 (3), 179–186. doi:10.3109/03630269.2016.1146618
He, M., Lin, K., Huang, Y., Zhou, L., Yang, Q., Li, S., et al. (2018). Prevalence and molecular study of G6PD deficiency in the dai and Jingpo ethnic groups in the dehong prefecture of the yunnan province. Hum. Hered. 83 (2), 55–64. doi:10.1159/000489009
He, Y., Zhang, Y., Chen, X., Wang, Q., Ling, L., and Xu, Y. (2020). Glucose-6-phosphate dehydrogenase deficiency in the Han Chinese population: molecular characterization and genotype-phenotype association throughout an activity distribution. Sci. Rep. 10 (1), 17106. doi:10.1038/s41598-020-74200-y
Hu, R., Lin, M., Ye, J., Zheng, B. P., Jiang, L. X., Zhu, J. J., et al. (2015). Molecular epidemiological investigation of G6PD deficiency by a gene chip among Chinese Hakka of southern Jiangxi province. Int. J. Clin. Exp. Pathol. 8 (11), 15013–15018.
Huang, Q., Liao, Y., Yu, T., Lei, W., Liang, H., Wen, J., et al. (2023). A retrospective analysis of preemptive pharmacogenomic testing in 22,918 individuals from China. J. Clin. Lab. Anal. 37 (5), e24855. doi:10.1002/jcla.24855
Huang, S., Xu, Y., Liu, X., Zhou, M., Wu, X., and Jia, Y. (2016). Molecular newborn screening of four genetic diseases in Guizhou Province of South China. Gene 591 (1), 119–122. doi:10.1016/j.gene.2016.07.019
Jiang, Z., Wang, M., Tang, L., Li, X. L., Li, C. R., and Cheng, X. R. (2021). Screening results and genetic features of glucose-6-phosphate dehydrogenase deficiency in 54 025 preterm infants in Chengdu, China. Zhongguo Dang Dai Er Ke Za Zhi 23 (5), 482–487. doi:10.7499/j.issn.1008-8830.2012012
Lee, H. Y., Ithnin, A., Azma, R. Z., Othman, A., Salvador, A., and Cheah, F. C. (2022). Glucose-6-Phosphate dehydrogenase deficiency and neonatal hyperbilirubinemia: insights on pathophysiology, diagnosis, and gene variants in disease heterogeneity. Front. Pediatr. 10, 875877. doi:10.3389/fped.2022.875877
Lin, F., Lou, Z. Y., Xing, S. Y., Zhang, L., and Yang, L. Y. (2018). The gene spectrum of glucose-6-phosphate dehydrogenase (G6PD) deficiency in Guangdong province, China. Gene 678, 312–317. doi:10.1016/j.gene.2018.07.068
Lin, F., Wu, J., Yang, H., Lin, M., and Yang, L. (2016). Molecular epidemiology of G6PD deficiency in Chaozhou area of eastern Guangdong Province. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 33 (1), 26–29. doi:10.3760/cma.j.issn.1003-9406.2016.01.007
Lin, F., Yang, H., Wu, J. R., and Yang, L. Y. (2016). c.1311C>T/IVS-1193T>C of G6PD gene and polymorphism of 3′-UTR. J. Mol. Diagnostics Ther. 8 (02), 78–81.
Liu, Z., Yu, C., Li, Q., Cai, R., Qu, Y., Wang, W., et al. (2020). Chinese newborn screening for the incidence of G6PD deficiency and variant of G6PD gene from 2013 to 2017. Hum. Mutat. 41 (1), 212–221. doi:10.1002/humu.23911
Pan, J., Zhuang, D., Yu, Q., Pan, X., Bao, Y., Pan, S., et al. (2021). Molecular genotyping of G6PD mutations for neonates in Ningbo area. J. Clin. Lab. Anal. 35 (12), e24104. doi:10.1002/jcla.24104
Peng, Q., Li, S., Ma, K., Li, W., Ma, Q., He, X., et al. (2015). Large cohort screening of G6PD deficiency and the mutational spectrum in the Dongguan District in Southern China. PLoS One 10 (3), e0120683. doi:10.1371/journal.pone.0120683
Pfeffer, D. A., Satyagraha, A. W., Sadhewa, A., Alam, M. S., Bancone, G., Boum, Y., et al. (2022). Genetic variants of glucose-6-phosphate dehydrogenase and their associated enzyme activity: a systematic review and meta-analysis. Pathogens 11 (9), 1045. doi:10.3390/pathogens11091045
Shen, S., Xiong, Q., Cai, W., Hu, R., Zhou, B., and Hu, X. (2022). Molecular heterogeneity of glucose-6-phosphate dehydrogenase deficiency in neonates in Wuhan: description of four novel variants. Front. Genet. 13, 994015. doi:10.3389/fgene.2022.994015
Sirdah, M. M., Shubair, M. E., Al-Kahlout, M. S., Al-Tayeb, J. M., Prchal, J. T., and Reading, N. S. (2017). Possible association of 3' UTR +357 A>G, IVS11-nt 93 T>C, c.1311 C>T polymorphism with G6PD deficiency. Hematology 22 (6), 370–374. doi:10.1080/10245332.2016.1276117
Tang, F., Huang, Y. L., Jiang, X., Jia, X. F., Li, B., Feng, Y., et al. (2018). Evaluations of newborn screening program performance and enzymatic diagnosis of glucose-6-phosphate dehydrogenase deficiency in Guangzhou. Zhonghua Er Ke Za Zhi 56 (5), 359–363. doi:10.3760/cma.j.issn.0578-1310.2018.05.010
Wang, X., Xia, Z., He, Y., Zhou, X., Zhang, H., Gao, C., et al. (2021). Newborn screening for G6PD deficiency in xiamen, China: prevalence, variant spectrum, and genotype-phenotype correlations. Front. Genet. 12, 718503. doi:10.3389/fgene.2021.718503
WHO Working Group (1989). Glucose-6-phosphate dehydrogenase deficiency. WHO working group. Bull. World Health Organ. 67, 601–611.
Xu, J. X., Lin, F., Chen, Z. K., Luo, Z. Y., Zhan, X. F., Wu, J. R., et al. (2021). Co-inheritance of G6PD deficiency and 211 G to a variation of UGT1A1 in neonates with hyperbilirubinemia in eastern Guangdong. Bmc. Pediatr. 21 (1), 564. doi:10.1186/s12887-021-03010-6
Yang, H., Wang, Q., Zheng, L., Zhan, X. F., Lin, M., Lin, F., et al. (2015). Incidence and molecular characterization of Glucose-6-Phosphate Dehydrogenase deficiency among neonates for newborn screening in Chaozhou, China. Int. J. Lab. Hematol. 37 (3), 410–419. doi:10.1111/ijlh.12303
Yang, Y. K., Lin, C. F., Lin, F., Chen, Z. K., Liao, Y. W., Huang, Y. C., et al. (2023). Etiology analysis and G6PD deficiency for term infants with jaundice in Yangjiang of western Guangdong. Front. Pediatr. 11, 1201940. doi:10.3389/fped.2023.1201940
Yao, L. Q., Zou, T. B., Wang, X. T., Quan, X., Chen, Q., Yang, F. B., et al. (2013). G6PD deficiency among children under 7 years old from Yunnan with unique ethnic minority origin. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 30 (2), 189–194. doi:10.3760/cma.j.issn.1003-9406.2013.04.015
Keywords: G6PD deficiency, G6PD mutation, DNA sequence, China, gene variant
Citation: Liang H-F, Cao Y-B, Lin F, Yang Y-K, Liao Y-W, Ou W-H, Chen J-L, Zeng Y-Q, Huang Y-C, Zeng G-K, Chen Z-X, Situ J-W, Yao J-X and Yang L-Y (2024) Molecular epidemiological investigation of G6PD deficiency in Yangjiang region, western Guangdong province. Front. Genet. 14:1345537. doi: 10.3389/fgene.2023.1345537
Received: 28 November 2023; Accepted: 28 December 2023;
Published: 09 January 2024.
Edited by:
Hui-Qi Qu, Children’s Hospital of Philadelphia, United StatesReviewed by:
Giovanni Mario Pes, University of Sassari, ItalyMin Lin, Hanshan Normal University, China
Ari Winasti Satyagraha, Eijkman Institute for Molecular Biology, Indonesia
Richard Oscar Francis, Columbia University, United States
Copyright © 2024 Liang, Cao, Lin, Yang, Liao, Ou, Chen, Zeng, Huang, Zeng, Chen, Situ, Yao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Ye Yang, eWFuZ2xlZXllZUBzaW5hLmNvbQ==
†ORCID: Li-Ye Yang, https://orcid.org/0000-0003-1581-9089
 Hong-Feng Liang1
Hong-Feng Liang1 Fen Lin
Fen Lin Li-Ye Yang
Li-Ye Yang