- Department of General Medicine, Xiangya Hospital, Central South University, Changsha, China
Introduction: Idiopathic systemic capillary leak syndrome (SCLS) is a rare disorder characterized by hemoconcentration, hypoproteinemia and edema. Chronic SCLS (cSCLS) presents as intractable edema, distinguishing it from the classic acute form, and only about 10 cases were reported worldwide. Nevertheless, the underlying pathogenesis of both types is obscure.
Case presentation: We report a case of a 58-year-old man with chronic edema persisting for 8 years, complicated by unique chylous polyserous effusions and hypotrichosis, which was successfully relieved by treatment with dexamethasone, intravenous immunoglobulin, and thalidomide. Furthermore, a variant c.5594A>G (p.K1865R) in the MYOF gene was identified as a potentially pathogenic mutation through whole-exome genetic sequencing. The proposed mechanism involves its impact on VEGF signaling, leading to increased capillary permeability.
Conclusion: Our case illustrates possible lymphatic capillaries involvement in SCLS, which may plays a potential role in immune disorder, and revealed a possible causative genetic mutation of SCLS.
Introduction
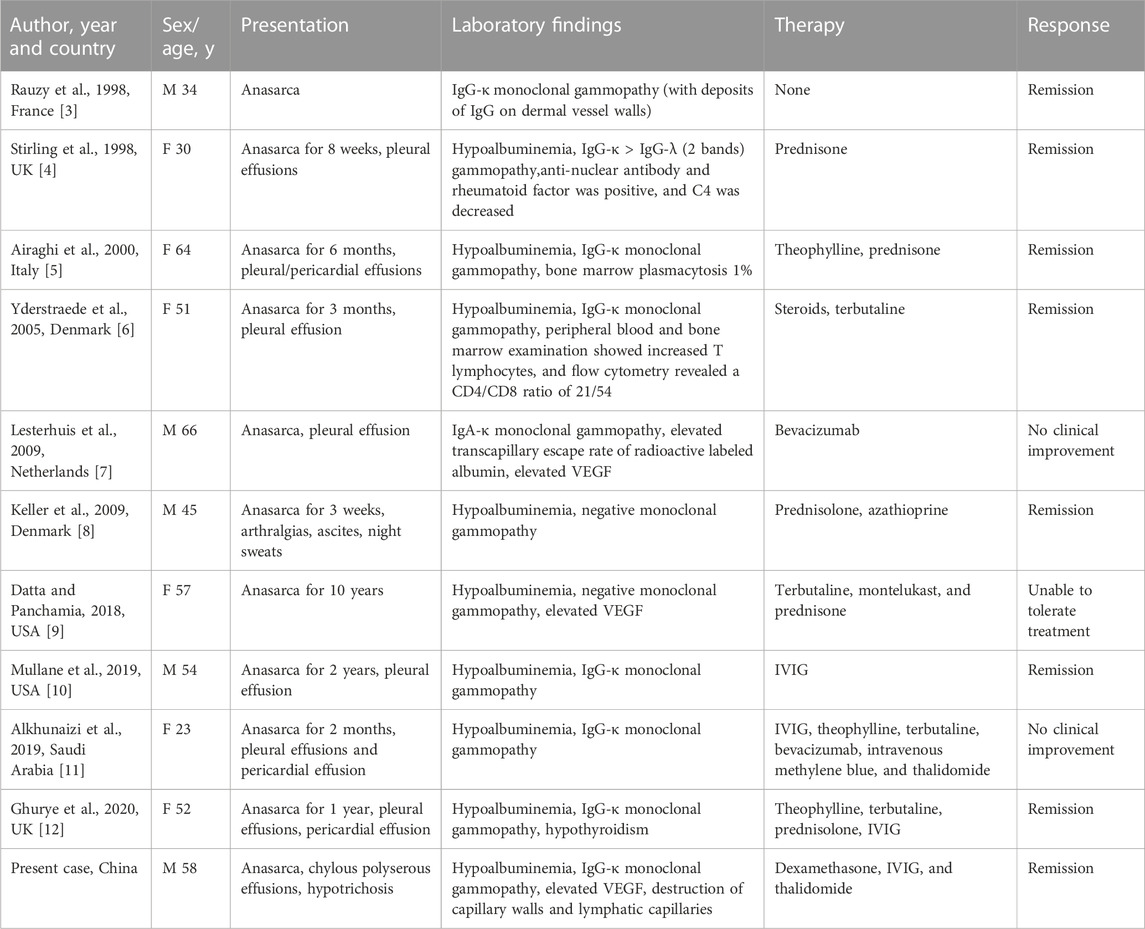
Idiopathic systemic capillary leak syndrome (SCLS), or Clarkson’s disease was first described by Clarkson in 1960 (Clarkson et al., 1960). It is an unusual and life-threatening disease caused by unexplained systemic capillary striking hyperpermeability, and the classic acute SCLS can be described as a triad of “3 Hs”, namely, hypotension, hypoalbuminemia and hemoconcentration (Dhir et al., 2007). However, chronic systemic capillary leak syndrome (cSCLS), a much rarer form of this syndrome, is characterized by persistent, intractable edema rather than fatal episodes of hypovolemic shock. Only 10 cases of cSCLS have been reported since its initial description in 1998 (Table 1) (Rauzy et al., 1998; Stirling et al., 1998; Airaghi et al., 2000; Yderstraede et al., 2005; Keller et al., 2009; Lesterhuis et al., 2009; Datta and Panchamia, 2018; Alkhunaizi et al., 2019; Mullane et al., 2019; Ghurye et al., 2020). SCLS typically affects individuals of Caucasian descent with a median age of onset around 49 years old (Druey and Greipp, 2010; Gousseff et al., 2011). The exact pathological mechanism of cSCLS remains unclear, but it has been observed that approximately 75.4% of SCLS patients have monoclonal immunoglobulinemia (Eo et al., 2018). In addition, several studies have elucidated vascular endothelial growth factor (VEGF) as a potential contributor to endothelial dysfunction in SCLS (Lesterhuis et al., 2009; Kinoshita et al., 2010; Xie et al., 2012; 2014). Besides, VEGF has been reported to play an important role in normal and pathological angiogenesis (Melincovici et al., 2018), and in increasing vascular permeability through the effects of Src family kinases (SFKs) on intercellular junctions and the regulation of focal adhesions (Wautier and Wautier, 2022). Cutaneous histopathology studies revealed inflammatory cell infiltration around vessels, and electron photomicrographs showed endothelial injury in previous literature (Johansson and Löfdahl, 1979; Assaly et al., 2001; Magro et al., 2022). Here, we report a Chinese patient diagnosed with cSCLS, presenting with an 8-year course of chronic edema, along with a rare manifestation of chylous polyserous effusions and hypotrichosis. Moreover, a variant of the MYOF gene, which encodes myoferlin, was found in this patient. This variant may be linked to cSCLS via a potential pathological pathway involving VEGF receptor disorder.
Case description
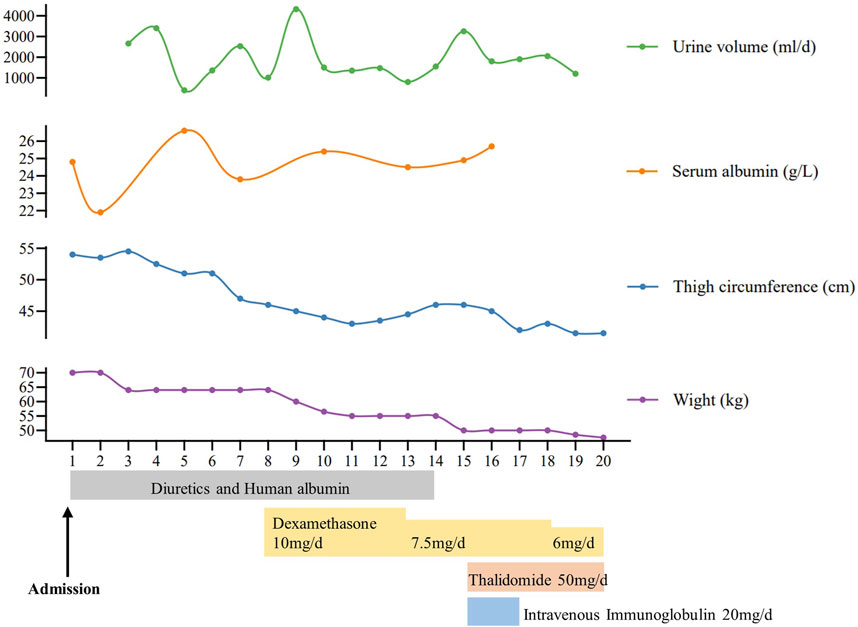
A 58-year-old man was referred to our hospital in February 2022 with an 8-year history of systemic edema and loss of eyebrow, axillary and pubic hair (Figures 1A–C). Previous history was unremarkable and no family history of alopecia. The patient’s edema initially presented as symmetrical edema of both lower limbs in 2014, then gradually extended to both upper limbs and the face. Despite receiving treatment with diuretics, the edema continued to progress, with occasional minor relief. Over time, fluid retention escalated, leading to the development of pleural and peritoneal effusions in April 2020. The pleural fluid was found to be chylous, and he had a thoracic duct ligation surgery at a local hospital in January 2021. Shortness of breath and pleural effusion were relieved after the operation, but relapsed 2 months later. No fever, rash or hemodynamic instability was exhibitedduring the course of the disease. After admission, routine laboratory tests were performed. Abnormal blood test results are showed in Supplementary Table S1. The serum albumin measured 24.8 g/L, and the blood IgGκ paraprotein tested weakly positive. We observed mild borderline elevations in white blood cell count, CRP, ESR, IL-1β, and IL-6, while PCT levels remained normal. The complement test revealed a slight decrease in C3 levels, but C4 and the C1 esterase inhibitor levels were within the normal range. Urinalysis, liver, renal and thyroid function tests, as well as cardiac enzymes, NT-proBNP assessments, did not indicate any common diseases associated with edema. Haemoglobin, haematocrit, blood glucose, autoimmune antibodies were all normal. Moderate amount of pericardial effusion and large amounts of bilateral pleural and abdominal fluid were found (Supplementary Figure S1). Thoracentesis and paracentesis were conducted. The hydrothorax and ascites were chyle like, and chylus qualitative test was positive, but no filarial worms were detected. Further, we found out that serum VEGF levels was elevated (264.29 pg/mL, normal values 0–142.2 pg/mL). A bone marrow biopsy revealed 0.1% clonal plasma cells. Additionally, histopathological examination of the skin on the right lower limb exhibited perivascular lymphocytic and plasma cell infiltration, along with suspicious mucinous deposits in the dermis (Supplementary Figure S2). Electron microscopy displayed breakdown of capillary walls (Figures 1E–G). Moreover, magnetic resonance lymphangiography (MRL) of the lower limbs indicated destruction of lymphatic capillaries, although the lymphatic trunk appeared normal (Figure 1D). Meanwhile, he and his son underwent whole-exome sequencing and Sanger sequencing, respectively, and the patient’s genomic DNA sample contained the potentially causal heterozygous mutation (c.5594A>G (p.K1865R)) in the MYOF gene, while his son’s did not (Figure 2A). In light of these findings, a multidisciplinary team diagnosed cSCLS as the primary condition. Subsequently, a treatment regimen was initiated, with the patient prescribed 10 mg of dexamethasone per day for 4 days, followed by a reduction to 5 mg for maintenance. Additionally, 20 mg of intravenous immunoglobulin (IVIG) was administered. Thalidomide was then introduced at a daily dose of 50mg, and human albumin infusion was conducted intermittently. Fortunately, he was discharged from the hospital after experiencing a remarkable 22.5 kg weight loss and significant relief from overall edema symptoms. The clinical course of the patient is shown in Figure 3. Presently, he has ceased all medications and maintains relatively good health, with only mild limb edema remaining.
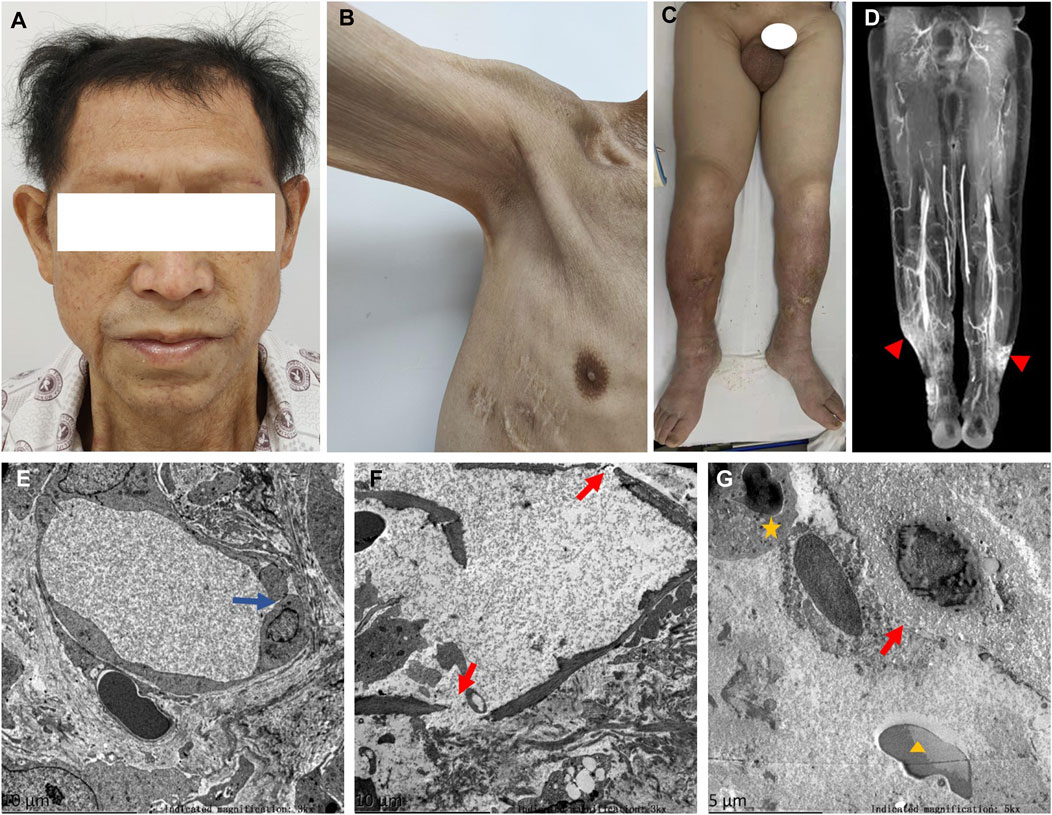
Figure 1. Symptoms, MRL image, and electron photomicrographs of skin biopsy. (A) Thinning hair and eyebrows hair. (B) Loss of axillary hair. (C) Loss of pubic hair and heavy edema of scrotum and both lower limbs. (D) Normal lymphatic trunks, but stagnant contrast media at ankle denoting disruption of lymphatic capillaries (▲). (E) Disruption of tight junctions between endothelial cells of capillaries (blue arrow). (F,G) Fractured capillary walls (red arrows), perivascular neutrophils (★) and erythrocytes (▲).
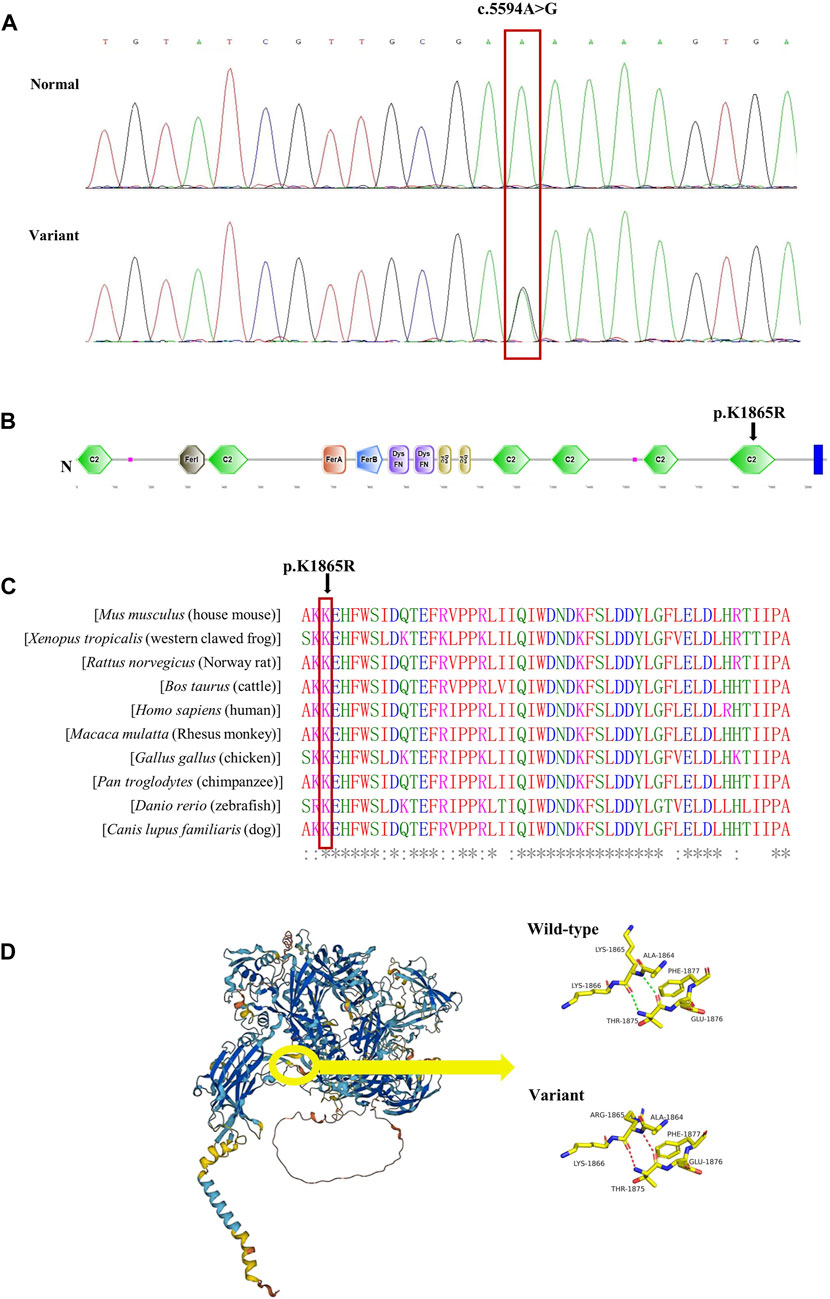
Figure 2. Validation of the missense variant of MYOF in the patient. (A) Sanger sequencing results from the patient and his son. The heterozygous variant in the MYOF gene was identified in the patient (variant), but not in his son (normal). (B) The location of the variant in the protein structure of MYOF. The arrow indicated the mutated site. (C) Amino acid alignment of the MYOF protein from several organisms. The position of Lys1865 residue (highlighted by a red box) was highly conserved among different species. (D) The 3D structure of MYOF protein, and schematic structures of amino acids showed a different steric hindrance of the residue between the WT and the variant.
We conducted bioinformatics analysis of the MYOF-1865R variant in our patient and suspect it was pathogenic. The MYOF-1865R variant was very rare, which was identified only 0.000472,233, 0.0008702 and 0.00006052 in Chinese, East Asian and global populations from the ChinaMap database and GnomAD. Furthermore, the variant was not observed in non-East Asian populations. The missense variation resulted in arginine replacing lysine in amino acid sequence 1865, which was in the conserved C2F domain of MYOF in humans and other species. (Figures 2B,C). Furthermore, we employed web-based software tools to assess the pathogenicity of the genetic variant, specifically, Mutation Taster (0.999, disease causing), CADD (22.3, potentially deleterious) and REVEL (0.338, neutral). The 3D structure of MYOF protein was created using the AlphaFold2. The mutant protein showed a different steric hindrance of the residue (the new residue has a smaller size), which may lead to protein misfolding, resulting in pathogenicity (Figure 2D).
Discussion
The patient experienced 8 years of persistent systemic edema and refractory hypoalbuminemia, along with unusual hair loss throughout the body and chylous polyserous effusions. Electron microscopy and MRL examination revealed the destruction of capillary walls and lymphatic capillaries (LC), respectively. After ruling out common diseases that could cause edema, the patient was ultimately diagnosed with cSCLS. Treatment with dexamethasone, IVIG, and thalidomide brought relief from the edema. Additionally, we identified a missense variant of the MYOF gene in this patient, suggesting a potential association with the development of cSCLS.
Differing from the traditional triad of SCLS, which typically includes rapid onset of hypotension (systolic blood pressure <90 mmHg), hemoconcentration (hematocrit >49%–50% in men and 43%–45% in women), and hypoalbuminemia (<3.0 g/dL) (Druey and Parikh, 2017), cSCLS is characterized by chronic systemic edema and hypoalbuminemia, and possibly serous cavity effusion, without episodes of hemodynamic instability (Kapoor et al., 2010). However, there is currently no consensus on diagnostic criteria or specific biomarkers for cSCLS, and other diseases need to be ruled out before a final diagnosis can be made. In our patient, the main clinical manifestations were chronic edema and hypoalbuminemia, and common disorders associated with edema, such as cardiac, renal, and hepatic insufficiency, as well as hypothyroidism, were ruled out through routine examination. But our patient exhibited unusual chylous effusion and hair loss, which had not been reported in previous cases of SCLS (Eo et al., 2018). The presence of chylous effusion suggested the possibility of a lymphatic duct leak, warranting consideration. Furthermore, the patient experienced body hair loss, which, from a dermatological perspective, could be linked to an immune disorder (Zhou et al., 2021). However, capillary walls disruption observed under electron microscopy suggested endothelial cell (ECs) dysfunction, which was in line with the characteristics of SCLS (Johansson and Löfdahl, 1979; Assaly et al., 2001; Druey and Greipp, 2010; Baloch et al., 2018). The skin biopsy seemed toreveal several diagnostic clues (Magro et al., 2022), showing perivascular lymphocytic and plasma cell infiltration, consistent with previous reports (Cicardi et al., 1997; Fardet et al., 2004; Magro et al., 2022). Besides, elevated levels of VEGF and the presence of monoclonal gammopathy of unknown significance (MGUS) served as supportive diagnostic evidences for SCLS (Druey and Parikh, 2017; E, M and J, 2017). The remarkable effectiveness of the treatment also in turn validated our diagnosis of cSCLS.
The immune-mediated hypothesis regarding the pathogenesis of SCLS has been extensively discussed in prior studies (Cicardi et al., 1997; Dowden et al., 2009; Baloch et al., 2018; Wu et al., 2021). Evidence of immune involvement includes perivascular lymphocytic infiltration in the skin (Cicardi et al., 1997; Fardet et al., 2004; Magro et al., 2022) and the observation of elevated peripheral blood T cells (Cicardi et al., 1990; Dowden et al., 2009). Notably, it has been reported that patients with higher levels of monoclonal components are more prone to severe recurrences (Pineton de Chambrun et al., 2017), underscoring the significance of immune dysregulation in the pathophysiology of SCLS. Furthermore, various drugs targeting immune system dysfunction have shown effectiveness in treating the disease, including IVIG, corticosteroids, infliximab, thalidomide, and others. Among these empirically employed treatments, prophylactic IVIG stands out as the most effective approach for reducing mortality, owing to its numerous immunomodulatory properties (Eo et al., 2018).
It has been widely reported that VEGF levels are elevated in many SCLS patients, coinciding with the course of the disease (Lesterhuis et al., 2009; Kinoshita et al., 2010; Xie et al., 2014). Bevacizumab has been suggested as a potential treatment for SCLS patients (Kouadri et al., 2021), and VEGF may be responsible for the immune systems disorder (Xie et al., 2012; Mo et al., 2020). VEGF/VEGFR-2 is an important angiogenic signaling pathway that also plays a potential role in lymphangiogenesis (Liu and Yu, 2022). The lymphatic system is crucial for immune regulation, and its role in the development of various diseases was gradually being revealed, especially lymphatic capillaries was highlighted by scientists for further study (Liu and Yu, 2022). Considering that high VEGF levels, lymphatic capillaries destruction, and chylous effusion were observed in our patient, we hypothesized that high VEGF levels in SCLS patients would lead to lymphatic capillaries damage and chylous polyserous effusions discovered in our patient, and possibly further connect with immune disorder in SCLS. In addition, we identified a variant of the MYOF gene, which encodes Myoferlin, a type II transmembrane protein highly expressed in ECs (Lek et al., 2012). It functions in VEGF signaling by regulating VEGFR-2 stability, thereby affecting VEGF-induced vascular permeability (Bernatchez et al., 2007). It is worth noting that the mutation of MYOF gene (c.651G>T, p. R217S) have also been reported in hereditary angioedema (HAE), a disease characterized by vascular leakage. And the MYOF-217S variant in HAE increased the VEGFR-2 localization on the cell membrane, which could raise VEGF-C levels (Ariano et al., 2020). Furthermore, some related genes like EDNRA, ARHGAP5, and MYOF were discussed in SCLS (Xie et al., 2013; Sek et al., 2015; Pierce et al., 2017). And the variant of MYOF in SCLS is one of the top-ranked single nucleotide polymorphisms (SNPs) detected by prior researchers (Xie et al., 2013). Therefore, it was assumed that MYOF-VEGF signal pathway disorder might induce the endothelial dysfunction in SCLS which needs further investigation.
Conclusion
We report a case of cSCLS characterized by persistent edema, with unusual chylous effusion and hypotrichosis. The treatment of dexamethasone, IVIG, and thalidomide was significantly effective in relieving edema. Additionally, a MYOF gene mutation (c.5594A>G, p. K1865R) was found in our patient, which might be a suspected pathological gene for SCLS.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Clinical Medical Ethics Committee of Xiangya Hospital, Central South University (No. 202310213). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DG: Writing–original draft, Visualization. WnZ: Writing–original draft. WiZ: Writing–review and editing. XW: Writing–review and editing. WL: Writing–original draft. JL: Writing–review and editing, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is supported by the Science and Health Collaboration Fund Project of Natural Science Foundation of Hunan province (Grant No. 2022JJ70159 of JL), the Natural Science Foundation of Hunan province (Grant Nos. 2022JJ40846 of WnZ, and 2023JJ30973 of WiZ), the Graduate Education and Teaching Reform Project of Central South University (Grant No. 2022JGB019 of WiZ). The Science and Health Collaboration Fund Project of Natural Science Foundation of Hunan province (Grant No. 2022JJ70159 of JL) and the Natural Science Foundation of Hunan province (2022JJ40846 of WnZ) has supported literature searches, article publication. The Natural Science Foundation of Hunan province (2023JJ30973 of WiZ) and the Graduate Education and Teaching Reform Project of Central South University (Grant No. 2022JGB019 of WiZ) has supported data collation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2023.1282711/full#supplementary-material
References
Airaghi, L., Montori, D., Santambrogio, L., Miadonna, A., and Tedeschi, A. (2000). Chronic systemic capillary leak syndrome. Report of a case and review of the literature. J. Intern. Med. 247 (6), 731–735. doi:10.1046/j.1365-2796.2000.00693.x
Alkhunaizi, A. M., Kabbani, A. H., and ElTigani, M. A. (2019). Chronic idiopathic systemic capillary leak syndrome: a case report. Allergy, Asthma, Clin. Immunol. Official J. Can. Soc. Allergy Clin. Immunol. 15, 34. doi:10.1186/s13223-019-0347-0
Ariano, A., D'Apolito, M., Bova, M., Bellanti, F., Loffredo, S., D'Andrea, G., et al. (2020). A myoferlin gain-of-function variant associates with a new type of hereditary angioedema. Allergy 75 (11), 2989–2992. doi:10.1111/all.14454
Assaly, R., Olson, D., Hammersley, J., Fan, P. S., Liu, J., Shapiro, J. I., et al. (2001). Initial evidence of endothelial cell apoptosis as a mechanism of systemic capillary leak syndrome. Chest 120 (4), 1301–1308. doi:10.1378/chest.120.4.1301
Baloch, N.U.-A., Bikak, M., Rehman, A., and Rahman, O. (2018). Recognition and management of idiopathic systemic capillary leak syndrome: an evidence-based review. Expert Rev. Cardiovasc. Ther. 16 (5), 331–340. doi:10.1080/14779072.2018.1456920
Bernatchez, P. N., Acevedo, L., Fernandez-Hernando, C., Murata, T., Chalouni, C., Kim, J., et al. (2007). Myoferlin regulates vascular endothelial growth factor receptor-2 stability and function. J. Biol. Chem. 282 (42), 30745–30753. doi:10.1074/jbc.M704798200
Cicardi, M., Gardinali, M., Bisiani, G., Rosti, A., Allavena, P., and Agostoni, A. (1990). The systemic capillary leak syndrome: appearance of interleukin-2-receptor-positive cells during attacks. Ann. Intern. Med. 113 (6), 475–477. doi:10.7326/0003-4819-113-6-475
Cicardi, M., Berti, E., Caputo, V., Radice, F., Gardinali, M., and Agostoni, A. (1997). Idiopathic capillary leak syndrome: evidence of CD8-positive lymphocytes surrounding damaged endothelial cells. J. Allergy Clin. Immunol. 99 (3), 417–419. doi:10.1016/s0091-6749(97)70061-7
Clarkson, B., Thompson, D., Horwith, M., and Luckey, E. H. (1960). Cyclical edema and shock due to increased capillary permeability. Am. J. Med. 29, 193–216. doi:10.1016/0002-9343(60)90018-8
Datta, R., and Panchamia, J. K. (2018). Anesthetic considerations for a patient with chronic systemic capillary leak syndrome: a case report. A&A Pract. 11 (10), 276–278. doi:10.1213/XAA.0000000000000808
Dhir, V., Arya, V., Malav, I. C., Suryanarayanan, B. S., Gupta, R., and Dey, A. B. (2007). Idiopathic systemic capillary leak syndrome (SCLS): case report and systematic review of cases reported in the last 16 years. Intern. Med. (Tokyo, Jpn. 46 (12), 899–904. doi:10.2169/internalmedicine.46.6129
Dowden, A. M., Rullo, O. J., Aziz, N., Fasano, M. B., Chatila, T., and Ballas, Z. K. (2009). Idiopathic systemic capillary leak syndrome: novel therapy for acute attacks. J. Allergy Clin. Immunol. 124 (5), 1111–1113. doi:10.1016/j.jaci.2009.06.043
Druey, K. M., and Greipp, P. R. (2010). Narrative review: the systemic capillary leak syndrome. Ann. Intern. Med. 153 (2), 90–98. doi:10.7326/0003-4819-153-2-201007200-00005
Druey, K. M., and Parikh, S. M. (2017). Idiopathic systemic capillary leak syndrome (Clarkson disease). J. Allergy Clin. Immunol. 140 (3), 663–670. doi:10.1016/j.jaci.2016.10.042
Eo, T. S., Chun, K. J., Hong, S. J., Kim, J. Y., Lee, I. R., Lee, K. H., et al. (2018). Clinical presentation, management, and prognostic factors of idiopathic systemic capillary leak syndrome: a systematic review. J. Allergy Clin. Immunol. Pract. 6 (2), 609–618. doi:10.1016/j.jaip.2017.07.021
Fardet, L., Kerob, D., Rybojad, M., Vignon-Pennamen, M. D., Schlemmer, B., Guermazi, A., et al. (2004). Idiopathic systemic capillary leak syndrome: cutaneous involvement can be misleading. Dermatol. (Basel, Switz. 209 (4), 291–295. doi:10.1159/000080851
Ghurye, R. R., Khan, A., Yung, T., Kiani-Alikhan, S., Pyne, D., and Grigoriadou, S. (2020). Successful treatment of a patient with chronic systemic capillary leak syndrome, neutropenia and thymoma. J. Clin. Immunol. 40 (1), 240–244. doi:10.1007/s10875-019-00722-4
Gousseff, M., Arnaud, L., Lambert, M., Hot, A., Hamidou, M., Duhaut, P., et al. (2011). The systemic capillary leak syndrome: a case series of 28 patients from a European registry. Ann. Intern. Med. 154 (7), 464–471. doi:10.7326/0003-4819-154-7-201104050-00004
Johansson, B. R., and Löfdahl, C. G. (1979). Ultrastructure of the microvessels in skeletal muscle in a case of systemic capillary leak syndrome. Acta Medica Scand. 206 (5), 413–416. doi:10.1111/j.0954-6820.1979.tb13537.x
Kapoor, P., Greipp, P. T., Schaefer, E. W., Mandrekar, S. J., Kamal, A. H., Gonzalez-Paz, N. C., et al. (2010). Idiopathic systemic capillary leak syndrome (Clarkson’s disease): the Mayo clinic experience. Mayo Clin. Proc. 85 (10), 905–912. doi:10.4065/mcp.2010.0159
Keller, K. K., Hauge, E. M., and Stengaard-Pedersen, K. (2009). Chronic systemic capillary leak syndrome: a case responding to prednisolone treatment. Scand. J. Rheumatology 38 (5), 400–401. doi:10.1080/03009740903161467
Kinoshita, Y., Kasaoka, S., Fujita, M., Oshima, C., Kawamura, Y., Tsuruta, R., et al. (2010). Synchronized changes in serum vascular endothelial growth factor during the clinical course of chronic systemic capillary leak syndrome. Intern. Med. (Tokyo, Jpn. 49 (8), 791–794. doi:10.2169/internalmedicine.49.2929
Kouadri, G., Perzo, N., Sauvetre, G., Lévesque, H., and Besnier, E. (2021). Refractory severe idiopathic systemic capillary leak syndrome successfully treated with bevacizumab: a case report. Angiogenesis 24 (3), 399–401. doi:10.1007/s10456-021-09769-7
Lek, A., Evesson, F. J., Sutton, R. B., North, K. N., and Cooper, S. T. (2012). Ferlins: regulators of vesicle fusion for auditory neurotransmission, receptor trafficking and membrane repair. Traffic (Copenhagen, Den. 13 (2), 185–194. doi:10.1111/j.1600-0854.2011.01267.x
Lesterhuis, W. J., Rennings, A. J., Leenders, W. P., Nooteboom, A., Punt, C. J., Sweep, F. C., et al. (2009). Vascular endothelial growth factor in systemic capillary leak syndrome. Am. J. Med. 122 (6), e5–e7. doi:10.1016/j.amjmed.2009.01.020
Liu, J., and Yu, C. (2022). Lymphangiogenesis and lymphatic barrier dysfunction in renal fibrosis. Int. J. Mol. Sci. 23 (13), 6970. doi:10.3390/ijms23136970
Magro, C. M., Mo, J. H., and Pecker, M. S. (2022). Idiopathic systemic capillary leak syndrome, a unique complement and interferon mediated endotheliopathy syndrome: the role of the normal skin biopsy in establishing the diagnosis and elucidating pathogenetic mechanisms. Ann. Diagnostic Pathology 61, 152028. doi:10.1016/j.anndiagpath.2022.152028
Melincovici, C. S., Boşca, A. B., Şuşman, S., Mărginean, M., Mihu, C., Istrate, M., et al. (2018). Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Romanian J. Morphol. Embryology 59 (2), 455–467.
Mo, L., Xu, G., Wu, C., Pan, K., Pan, P., Yu, L., et al. (2020). Key regulatory effect of activated HIF-1α/VEGFA signaling pathway in systemic capillary leak syndrome confirmed by bioinformatics analysis. J. Comput. Biol. A J. Comput. Mol. Cell Biol. 27 (6), 914–922. doi:10.1089/cmb.2019.0222
Mullane, R., Langewisch, E., Florescu, M., and Plumb, T. (2019). Chronic systemic capillary leak syndrome treatment with intravenous immune globulin: case report and review of the literature. Clin. Nephrol. 91 (1), 59–63. doi:10.5414/CN109467
Pierce, R. W., Merola, J., Lavik, J. P., Kluger, M. S., Huttner, A., Khokha, M. K., et al. (2017). A p190BRhoGAP mutation and prolonged RhoB activation in fatal systemic capillary leak syndrome. J. Exp. Med. 214 (12), 3497–3505. doi:10.1084/jem.20162143
Pineton de Chambrun, M., Gousseff, M., Mauhin, W., Lega, J. C., Lambert, M., Rivière, S., et al. (2017). Intravenous immunoglobulins improve survival in monoclonal gammopathy-associated systemic capillary-leak syndrome. Am. J. Med. 130 (10), 1219.e19–1219.e27. doi:10.1016/j.amjmed.2017.05.023
Rauzy, O., Adoue, D., and Arlet, P. (1998). Chronic systemic capillary leak syndrome not requiring treatment? Am. J. Med. 105 (4), 360.
Sek, A. C., Xie, Z., Terai, K., Long, L. M., Nelson, C., Dudek, A. Z., et al. (2015). Endothelial expression of endothelin receptor A in the systemic capillary leak syndrome. PloS One 10 (7), e0133266. doi:10.1371/journal.pone.0133266
Siddall, E., Khatri, M., and Radhakrishnan, J. (2017). Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int. 92, 37–46. doi:10.1016/j.kint.2016.11.029
Stirling, C. M., Boulton-Jones, J. M., and Simpson, K. (1998). Progressive oedema in a 30-year-old. Lancet (London, Engl. 352, 450. doi:10.1016/s0140-6736(98)05348-3
Wautier, J.-L., and Wautier, M.-P. (2022). Vascular permeability in diseases. Int. J. Mol. Sci. 23 (7), 3645. doi:10.3390/ijms23073645
Wu, M. A., Catena, E., Castelli, A., Rech, R., Borghi, B., Ottolina, D., et al. (2021). Autonomic biomarkers of shock in idiopathic systemic capillary leak syndrome. PloS One 16 (6), e0251775. doi:10.1371/journal.pone.0251775
Xie, Z., Ghosh, C. C., Patel, R., Iwaki, S., Gaskins, D., Nelson, C., et al. (2012). Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome). Blood 119 (18), 4321–4332. doi:10.1182/blood-2011-08-375816
Xie, Z., Nagarajan, V., Sturdevant, D. E., Iwaki, S., Chan, E., Wisch, L., et al. (2013). Genome-wide SNP analysis of the systemic capillary leak syndrome (Clarkson disease). Rare Dis. (Austin, Tex.) 1 (1), e27445. doi:10.4161/rdis.27445
Xie, Z., Ghosh, C. C., Parikh, S. M., and Druey, K. M. (2014). Mechanistic classification of the systemic capillary leak syndrome: Clarkson disease. Am. J. Respir. Crit. Care Med. 189 (9), 1145–1147. doi:10.1164/rccm.201310-1746LE
Yderstraede, K. B., Kassem, M. S., and Juhl, C. B. (2005). Systemic vascular leak syndrome. Ugeskrift Laeger 167 (15), 1648–1649.
Keywords: chronic systemic capillary leak syndrome, MYOF gene variant, lymphatic capillaries, VEGF, edema, chylous polyserous effusions, hypotrichosis
Citation: Gao D, Zhong W, Zhang W, Wang X, Li W and Liu J (2023) Chronic systemic capillary leak syndrome with lymphatic capillaries involvement and MYOF mutation: case report and literature review. Front. Genet. 14:1282711. doi: 10.3389/fgene.2023.1282711
Received: 26 August 2023; Accepted: 31 October 2023;
Published: 20 November 2023.
Edited by:
Raghu P. Kataru, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Chiara Suffritti, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyYashwant Kumar, Post Graduate Institute of Medical Education and Research (PGIMER), India
Copyright © 2023 Gao, Zhong, Zhang, Wang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Liu, ZG9jMi4wanVuQGhvdG1haWwuY29t
 Dehua Gao
Dehua Gao Wen Zhong
Wen Zhong Weiru Zhang
Weiru Zhang Xuan Wang
Xuan Wang Weiping Li
Weiping Li Jun Liu
Jun Liu