
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 31 August 2023
Sec. Cancer Genetics and Oncogenomics
Volume 14 - 2023 | https://doi.org/10.3389/fgene.2023.1245594
This article is part of the Research TopicInnovative Translational Research to Identify Colorectal Cancer Biomarkers for Personalized MedicineView all 7 articles
 Mladen Marinkovic1
Mladen Marinkovic1 Suzana Stojanovic-Rundic1,2
Suzana Stojanovic-Rundic1,2 Aleksandra Stanojevic3
Aleksandra Stanojevic3 Marija Ostojic3
Marija Ostojic3 Dusica Gavrilovic4
Dusica Gavrilovic4 Radmila Jankovic3
Radmila Jankovic3 Natasa Maksimovic5
Natasa Maksimovic5 Rafael Stroggilos6
Rafael Stroggilos6 Jerome Zoidakis6,7
Jerome Zoidakis6,7 Sergi Castellví-Bel8
Sergi Castellví-Bel8 Remond J. A. Fijneman9
Remond J. A. Fijneman9 Milena Cavic3*
Milena Cavic3*Introduction: The standard treatment for locally advanced rectal cancer (LARC) is neoadjuvant chemoradiotherapy (nCRT). To select patients who would benefit the most from nCRT, there is a need for predictive biomarkers. The aim of this study was to evaluate the role of clinical, pathological, radiological, inflammation-related genetic, and hematological parameters in the prediction of post-nCRT response.
Materials and methods: In silico analysis of published transcriptomics datasets was conducted to identify candidate genes, whose expression will be measured using quantitative Real Time PCR (qRT-PCR) in pretreatment formaline-fixed paraffin-embedded (FFPE) samples. In this study, 75 patients with LARC were prospectively included between June 2020—January 2022. Patients were assessed for tumor response in week 8 post-nCRT with pelvic MRI scan and rigid proctoscopy. For patients with a clinical complete response (cCR) and initially distant located tumor no immediate surgery was suggested (“watch and wait” approach). The response after surgery was assessed using histopathological tumor regression grading (TRG) categories from postoperative specimens by Mandard. Responders (R) were defined as patients with cCR without operative treatment, and those with TRG 1 and TRG 2 postoperative categories. Non-responders (NR) were patients classified as TRG 3-5.
Results: Responders group comprised 35 patients (46.6%) and NR group 53.4% of patients. Analysis of published transcriptomics data identified genes that could predict response to treatment and their significance was assessed in our cohort by qRT-PCR. When comparison was made in the subgroup of patients who were operated (TRG1 vs. TRG4), the expression of IDO1 was significantly deregulated (p < 0.05). Among hematological parameters between R and NR a significant difference in the response was detected for neutrophil-to-monocyte ratio (NMR), initial basophil, eosinophil and monocyte counts (p < 0.01). According to MRI findings, non-responders more often presented with extramural vascular invasion (p < 0.05).
Conclusion: Based on logistic regression model, factors associated with favorable response to nCRT were tumor morphology and hematological parameters which can be easily and routinely derived from initial laboratory results (NMR, eosinophil, basophil and monocyte counts) in a minimally invasive manner. Using various metrics, an aggregated score of the initial eosinophil, basophil, and monocyte counts demonstrated the best predictive performance.
In 2020, colorectal cancer (CRC) was the third most common malignant disease with 1.9 million new cases worldwide (Sung et al., 2021). With 0.9 million deaths, it held the second place of cancer-related mortality causes in 2020 (Sung et al., 2021). In Serbia, in 2020, there were 2,956 new cases and a total of 1,493 deaths related to rectal cancer, which placed Serbia in the group of countries with a high incidence and mortality rate for this disease (Sung et al., 2021). In the majority of cases, it is diagnosed in advanced stages, when treatment options are limited. In this regard, in the past we have profiled the diagnostic, prognostic and predictive factors for cancers of the digestive system, leading to improved research strategies for patient management and care (Brotto et al., 2012; Jakovljevic et al., 2012; Cavic et al., 2016; Nikolic et al., 2021; Stojanovic-Rundic et al., 2021; Stanojevic et al., 2023). However, there is a need for better primary prevention, more effective screening program, diagnosis at an earlier stage of the disease and improvement of existing treatment modalities in our country and on a global level.
The standard treatment for locally advanced rectal cancer (LARC) is neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision with or without adjuvant chemotherapy. The pathologic complete response (pCR) after nCRT is achieved in 10%–30% of cases (Zorcolo et al., 2012). It has been reported that pCR, independent of the initial clinical T and N stage of the disease, was associated with better local and distant disease control, as well as longer disease-free and overall survival (Maas et al., 2010). Other reports showed that radical surgical treatment was related to significant morbidity, including postoperative complications (Dossa et al., 2017). Further investigations were directed towards less invasive surgical treatment or avoiding surgery (“watch and wait” approach) in patients with favorable response to nCRT, in order to improve the quality of life. Since 2004, a group of researchers led by Angelita Habr-Gama have contributed greatly in this area by pointing out the effectiveness and safety of this approach (Habr-Gama et al., 2014). The current management of LARC uses the clinical complete response (cCR) as the point of reference for identifying patients for whom a non-operative approach may be a viable option (Ferrari and Fichera, 2015). However, the clinical response poorly correlates with the pathologic response (Maas et al., 2011).
Other research trends in this field were dedicated to prolonging the period between completion of neoadjuvant treatment and surgery, changing the type and regimen of chemotherapy, as well as increasing the radiotherapy doses. These approaches aimed to achieve a higher percentage of good response to the initial treatment. As not all patients will benefit from these treatment modifications, there is a need to categorize them initially before treatment. In order to select patients who would benefit the most from a neoadjuvant treatment, there is a strong demand to discover and characterize predictive biomarkers. Despite numerous studies in this field, until now no molecular marker has been implemented as a diagnostic or predictive parameter in routine clinical practice of LARC. This is stressed by the fact that there was not enough matching regarding results of published studies in this area and only two genes (MMP4 and FLNA) were shown to be significant in more than one study (Conde-Muíño et al., 2015). Limitations of previous studies included a small number of patients, the absence of reproducibility of measurements, the use of different methodologies, the retrospective nature of the studies, the heterogeneity of the studied groups and applied treatment modalities, as well as the lack of verification of the findings. Further research was aimed at examining the cumulative effect of molecular markers in combination with radiological and clinical data. An example of such successful research is the examination of the correlation between the expression of three protein molecular markers (c-MYC, PCNA and TIMP1) and magnetic resonance imaging (MRI) parameters (Li et al., 2016).
The association between inflammatory bowel disease and the higher risk of developing colorectal cancer is well known (Eaden et al., 2001; Bajpai et al., 2019). Also, there is evidence of the role of inflammation in sporadic colorectal cancer (Long et al., 2017; Schmitt and Greten, 2021). Chronic inflammation in the tumor microenvironment has also been shown to favorize tumor growth and invasiveness and stimulate synthesis of epithelial to mesenchymal transition promoting transcription factors (Vuletić et al., 2021). Yet, no inflammation-related genetic or circulating biomarkers have been investigated in detail or established as predictive parameters in the LARC setting so far.
The aim of this study was to evaluate the role of clinical, pathological, radiological, inflammation-related genetic and hematological parameters in prediction of response after nCRT in patients with LARC.
In silico analysis of published transcriptomics datasets was conducted to identify the best candidate genes, whose expression will be measured using quantitative Real Time PCR (qRT-PCR) in pretreatment formaline-fixed paraffin-embedded (FFPE) samples.
Gene expression patterns were analyzed using publicly available datasets. By searching the public database the National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO) using key words rectal cancer, chemoradiotherapy and response to treatment, five adequate sets of data that analyzed pretreatment samples were identified: GSE45404, GSE68204, GSE139255, GSE46862 and GSE3493 (Watanabe et al., 2006; Agostini et al., 2015; Gim et al., 2016; Millino et al., 2017; Park et al., 2020). Three datasets were selected where inflammatory response significantly correlated with treatment outcome to nCRT. Gene expression profiles of GSE46862, GSE139255, and GSE45404_570 were obtained from GEO database. The analysis of these three microarray datasets was conducted online using the GEO2R functionality of the GEO repository (https://www.ncbi.nlm.nih.gov/geo/geo2r). The volcano plots were automatically generated by the same application. The total number of patients of each dataset was 69, 156, and 42 respectively. In all selected studies, the response to treatment was classified according to pathohistological tumor regression grading (TRG) categories from the postoperative specimen using Mandard scoring system. Patients were subdivided into responders (TRG 1-2) and non-responders (TRG 3-5). Microarray data were processed and normalized with the Robust Multichip Average method (Irizarry et al., 2003). Analysis for statistically significant differences between the two groups was conducted using the standard moderated t-test from the limma package (Smyth, 2004; Ritchie et al., 2015). Gene set enrichment analysis (GSEA) was performed on selected datasets, and Hallmark, Kyoto encyclopedia of genes and genomes (KEGG), and Reactome gene sets were used to identify pathway alterations in patients who responded well to the therapy (TRG 1-2) versus those who did not (TRG 3-5) (Mootha et al., 2003; Subramanian et al., 2005).
Next, the top 100 genes from selected datasets, ranked by the default Signal2Noise metric used in previously described GSEA analysis, were extracted and overlapped using Venn diagram software. Cytoscape (version 3.10.0) was applied as bioinformatics software to evaluate the potential correlation between finally selected genes (Shannon et al., 2003; Smoot et al., 2011).
In this study 75 patients with LARC treated at the Institute for Oncology and Radiology of Serbia, between June 2020 and January 2022, were prospectively included. The inclusion criteria were histopathologically verified adenocarcinoma of the rectum, with a distant margin up to 12 cm from the anal verge by rigid proctoscopy. LARC was defined as T3-T4N0 or any T stage N positive. Pretreatment evaluation included an abdominal and pelvic MRI scan and a computed tomography (CT) scan or X ray of the chest. All patients were treated with long-course nCRT. Radiotherapy (RT) was delivered using volumetric modulated arc therapy-simultaneous integrated boost technique (VMAT-SIB). The dose to mesorectum and pelvic lymph nodes was 45 Gy (1.8 Gy/fraction). A SIB was delivered on macroscopic disease region expanded with 2 cm margin with a total dose of 54 Gy (2.16 Gy/fraction). Concomitant chemotherapy started on the first day of RT and was administered during the first and the fifth week of RT. The chemotherapy regimen included: 5-FU (350 mg/m2 on the first day of the first and fifth week of RT) and Leucovorine (25 mg/m2 daily, 5 days of the first and fifth week of RT).
Patients were assessed for tumor response in the eighth week after nCRT completion with pelvic MRI scan, rigid proctoscopy and digital rectal examination. For patients with cCR and initially distant located tumor no immediate radical surgery was suggested and they were enrolled in a strict follow-up program (“watch and wait” approach). Patients with cCR where sphincter preservation surgery treatment can be delivered, were referred to surgical resection between weeks 8 and 12 from nCRT completion. For patients with partial response (PR), surgery was delayed until week 12–15, approximately. The pathohistological response after surgery was assessed according to classification by Mandard. The response to treatment was classified according to pathohistological TRG categories from the postoperative specimen. Responders were defined as patients with cCR without operative treatment, and those with TRG 1 and TRG 2 postoperative categories. Non-responders were patients classified as TRG 3-5.
Formalin-fixed paraffin-embedded (FFPE) samples taken at the time of disease diagnosis were collected. The project was approved by the Ethics Committee of the Institute for Oncology and Radiology of Serbia (Approval No. 2211-01 from 11.06.2020.) and Ethics Committee of the Faculty of Medicine, University of Belgrade (Approval No. 1322/XII-17 from 03.12.2020.). All patients signed an informed consent.
Before initiation of treatment, ethylenediaminetetraacetic acid (EDTA) peripheral blood was drawn by venipuncture and hematological parameters were derived from the absolute differential counts of a complete blood count (CBC). The neutrophil-to-lymphocyte ratio (NLR) was calculated as a ratio of circulating neutrophil and lymphocyte counts, and the platelet-to-lymphocyte ratio (PLR) was defined as the absolute count of platelets divided by the absolute lymphocyte count. The derived neutrophil-to-lymphocyte ratio (dNLR) was calculated as absolute neutrophil count divided by absolute leukocyte minus absolute neutrophil count. The lymphocyte-to-monocyte ratio (LMR), platelet-to-monocyte ratio (PMR), and neutrophil-to-monocyte ratio (NMR) were also analyzed. Patients’ pre-treatment hemoglobin levels were obtained. The staging of the tumor was assessed according to the eighth edition of the Union for International Cancer Control (UICC) TNM staging system for rectal cancer (O’Sullivan et al., 2017). The general condition of the patients was classified using the Eastern cooperative oncology group (ECOG) Scale of Performance Status (Oken et al., 1982).
Total RNA was isolated from 2–5 10 μm thick FFPE tissue sections using RNeasy FFPE Kit (Qiagen, Manchester, United Kingdom). RNA quality and concentration were determined spectrophotometrically using BioSpec-nano (Shimadzu Scientific Instruments, Kyoto. Japan). The complementary DNA (cDNA) was accessed from 1 µg total RNA using random primers and MultiScribeTM Reverse Transcriptase (50 U/µL) from the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, United States). The reaction was performed in 20 μL, using the following program: 25°C for 10 min, 37°C for 120 min, and inactivation at 85°C for 5 min.
The mRNA levels of IL6 (RefSeq. NM_000600.5), CXCL9 (RefSeq. NM_002416.3), IDO1 (RefSeq. NM_002164.6) and CYBB (RefSeq. NM_000397.4) were detected by quantitative real-time PCR (qRT-PCR) using oligonucleotides primers (Integrated DNA Technology, Coralville, Iowa, United States) previously designed using NCBI Primer Blast and SybrGreen Gene Expression Master Mix (Applied Biosystems), on ABI Prism 7500 Sequence Detection System (Applied Biosystems). Oligonucleotides primer sequences used for determining expression levels of selected gene candidates are presented in Supplementary Table S1. The thermal cycling conditions consisted of an UDG activation at 50°C, initial denaturation step at 95°C for 2 min followed by 40 cycles of denaturation (15 s at 95°C) and annealing/extension (1 min at 60°C). All experiments were performed in duplicate, including non-template controls in each amplification. Gene expression data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH, RefSeq. NM_002046.5). Data was analyzed using the classical delta-delta-Ct method, and results expressed in relative units. qRT-PCR raw data are available in Supplementary Material S1.
For normal distribution data testing, the Kolmogorov-Smirnov and Shapiro-Wilk tests were used. Descriptive methods (frequencies, percentage, mean, median, standard deviation (SD) and range) were used to summarize the data. The statistical significance level was set at p < 0.05. For comparison of disease and treatment characteristics among different subgroups the Wilcoxon rank sum, Pearson chi-square and Fisher exact tests were used. Also, for evaluating potential predictors of the response, univariate and multivariate logistic regression was used (odds ratio with 95% CI for description, Likelihood Ratio and Wild test), and the responders versus non-responders was set as a dependent variable. We evaluated the sensitivity, specificity, positive predictive value, negative predictive value, and predictive accuracy for clinical assessment of disease presence in comparison with pathohistological response as a gold standard in group of patients where operative treatment was conducted (MedCalc Software Ltd, 2022). The Receiver Operating Characteristics curve (ROC) methods were applied to investigate the discriminative potential of NLR, PLR, dNLR, LMR, PMR, NMR, initial basophil, eosinophil and monocyte counts, for the good response to treatment (AUC ROC-Area Under the ROC curve according DeLong’s method; Likelihood ratio test for AUC ROC; the best cut-off value was set as value with maximum sensitivity and specificity). The statistical analysis was performed using the program R (version 3.3.2 (2016-10-31) --“Sincere Pumpkin Patch”; Copyright (C) 2016 The R Foundation for Statistical Computing; Platform: x86_64-w64-mingw32/× 64 (64-bit); downloaded: 21 January 2021). In the search for a measure that outperforms the individual variables, numerical variables that remained significant in the multivariate analysis were utilized to create the composite scores. The predictive power of these scores was then tested using the AUC, the Area Under Precision-Recall Curve (AUCRP), the Root Mean Square Error (RMSE) as a metric (using the ROCR package) and a random forest classifier (using the randomForest package, with the MeanDecreaseAccuracy metric) (Liaw and Wiener, 2001).
After extensive search of the GEO database according to the key words rectal cancer, chemoradiotherapy and response to treatment, three gene expression datasets were finally obtained. Volcano plots were employed to identify genes that exhibited statistically significant differential expression between responders and non-responders, as determined by the adjusted p-value, among selected datasets (Figure 1). The reduced number of genes plotted in Figure 1C (784) compared to 1A (54,620 genes) and 1B (32,327 genes) is derived from the fact that a custom array called nCounter human PanCancer Pathways Custom Codeset, designed by NanoString Technologies (GPL22330) was used to obtain this dataset. Significant alteration defined as those with adjusted p-value lower then 0.05 was obtained only for GSE 139255 dataset. The results of the differential expression analysis within GSE 139255 are reported in Supplementary Material S2. While KEGG and Reactome GSEA analysis yielded no overlap among the selected datasets, the Hallmark analysis exhibited consistent and significant parameters across two datasets. Results of GSEA Hallmark analysis presented in Table 1 showed parameters which reached significant levels within GSE46862 and GSE139255 datasets in relation to Hallmark inflammatory response pathway (NOM p-value <0.05, FDR q-value <0.25). Enrichment plots were used to present the expression of genes in selected datasets in Figure 2.
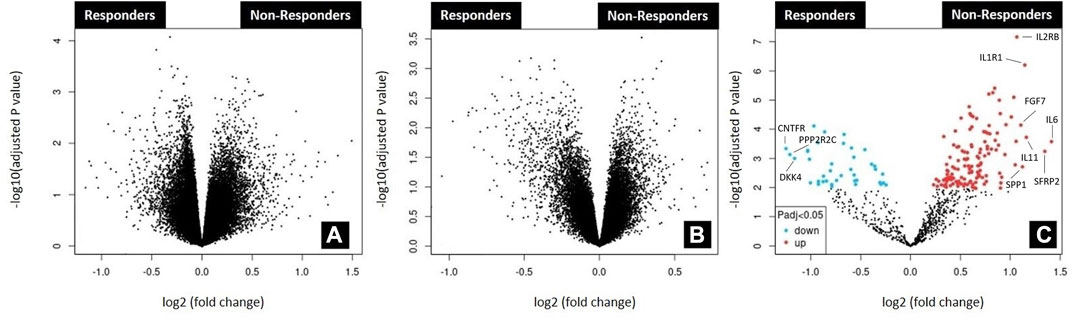
FIGURE 1. Analysis of microarray datasets GSE45404_570 (A); GSE46862 (B); GSE139255 (C). The analysis was performed using the GEO2R functionality available in the GEO repository (https://www.ncbi.nlm.nih.gov/geo/geo2r). Corresponding volcano plots were automatically generated by the same application. No differentially expressed genes (adjusted p-value<0.05) were found in datasets GSE45404_570 and GSE46862. The top ten differentially expressed genes found in dataset GSE139255 according to logFC value were labeled in 1C. Upregulated genes were labeled red (IL6, SFRP2, IL11, IL1R1, SPP1, FGF7, IL2RB) and downregulated blue (CNTFR, PPP2R2C, DKK4).
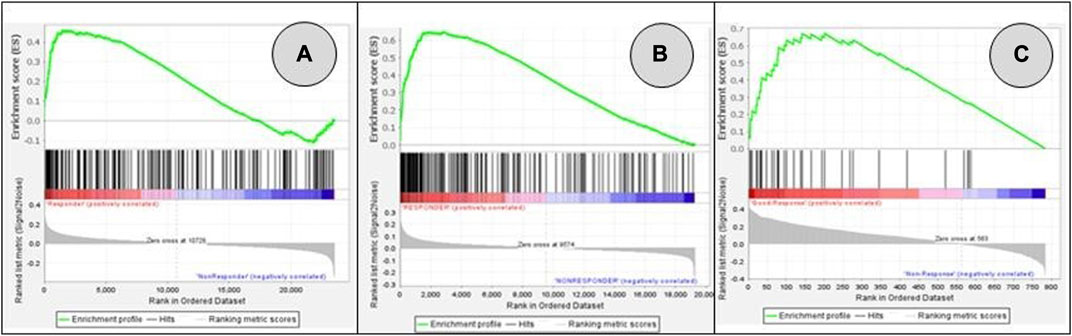
FIGURE 2. GSEA enrichment plots for genes included in Hallmark inflammatory response pathway: GSE45404_570 (A); GSE46862 (B); GSE139255 (C). False Discovery Rate q-value FDR q-values were 0.661 (A), 0.056 (B), 0.054 (C). Pathways associated datasets A and B showed significant enrichment (meeting the threshold of FDR <0.25).
The top 100 genes from each database (Supplementary Material S3) were chosen, and overlapped among these three datasets using Venn diagram. The results are presented in Figure 3. Our selection process was focused on identifying genes with potential predictive value for treatment outcomes in a comprehensive manner. We aimed to achieve a balance between genes implicated in established pathways as the Hallmark inflammatory response and genes with promising potential based on existing knowledge. As a result of overlapping three datasets, there were 11 genes present in two of them (PLAU, TGFB2, HGF, IL6, CXCL10, CXCL9, IDO1, INHBA, PDGFRB, CYBB, IL24). Statistical significance of these genes among responders and non-responders in all three datasets was examined and the results are presented in Table 2.
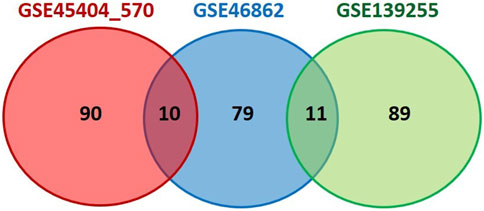
FIGURE 3. Venn diagram showing overlapping of inflammation-related genes between three analyzed datasets. Top 100 genes from selected datasets (ranked by the default Signal2Noise metric used in GSEA analysis), were extracted and overlapped using Venn diagram software. Cytoscape (version 3.10.0) was applied to evaluate the potential correlation between finally selected genes.
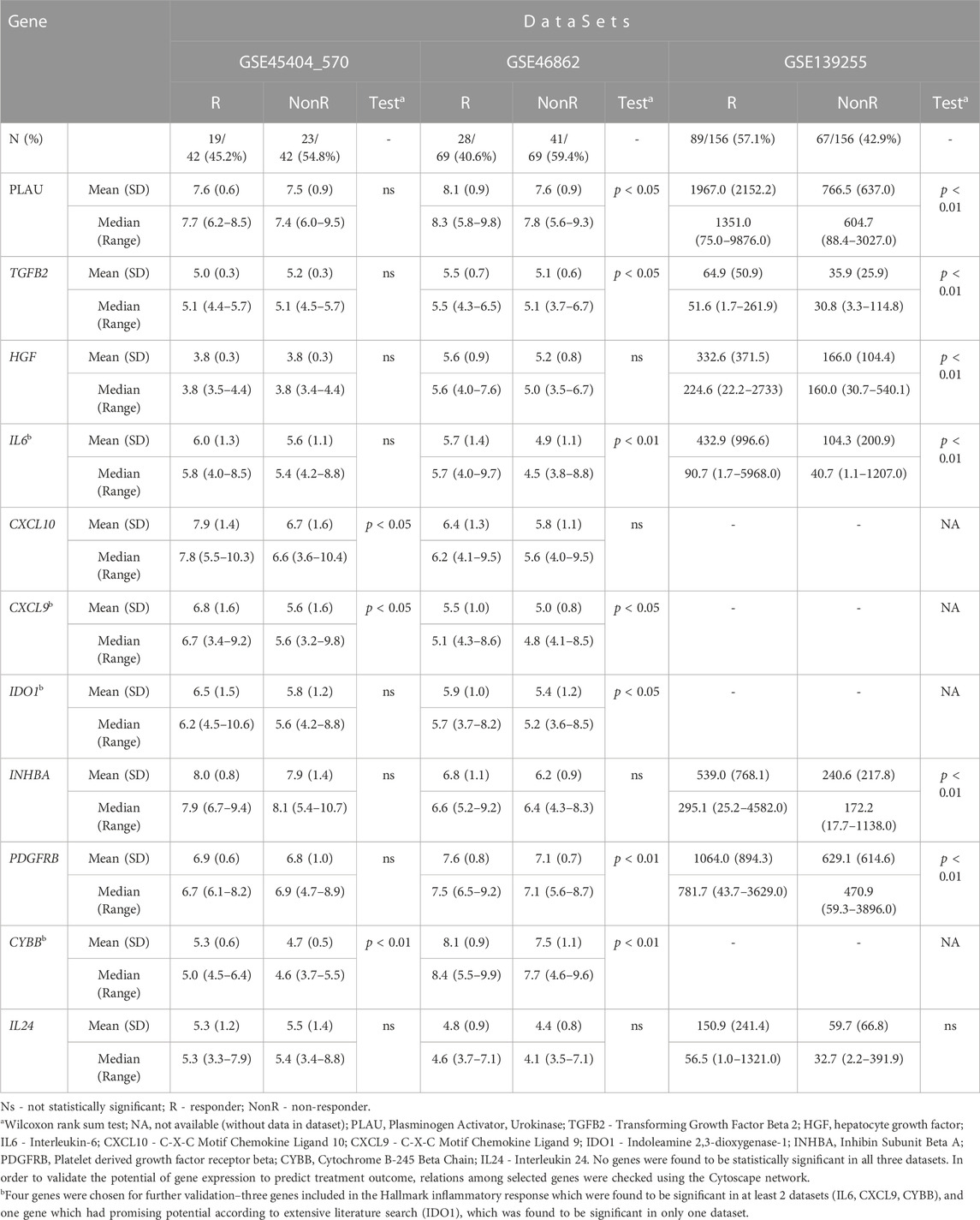
TABLE 2. Comparison between responders and non-responders within analyzed datasets in relation to expression of selected genes.
None of the selected genes were found to be statistically significant in all three datasets. In order to validate the potential of gene expression to predict treatment outcome, relations among selected genes were checked using the Cytoscape network. Three genes included in the Hallmark inflammatory response pathway related to avoidance of immune detection (IL6, CXCL9, CYBB) were chosen for further validation, along with a gene which had promising potential according to literature search (IDO1) (Chen et al., 2020; Takasu et al., 2022).
In order to explore the significance of in silico obtained results, the expression of candidate genes was analyzed in the cohort of LARC patients from the Institute for Oncology and Radiology of Serbia. Patients, disease, treatment and outcomes characteristics are presented in Table 3. The majority of patients had T3 stadium and N positive disease. One-third of patients were female. All patients completed the planned nCRT. Operative treatment was conducted in 63 patients, and the pathohistological complete response rate was 20.6% (Table 3). Twelve patients with distally located tumor and complete clinical response were involved in a “watch and wait” approach. One patient had to be excluded from the hematological ratios analysis, because of his chronic lymphocytic leukemia and its influence to the parameters of this analysis.
Correlation of clinical evaluation and pathological examination as a gold standard in a group of patients where operative treatment was conducted is presented in Table 4. Using disease prevalence of 79.4% the sensitivity, specificity, positive predictive value, negative predictive value and predictive accuracy were calculated (Table 5).
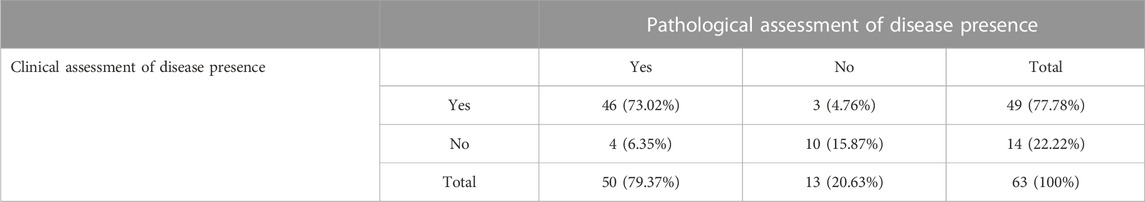
TABLE 4. Correlation of clinical and pathological CR within a group of patients where operative treatment was conducted.
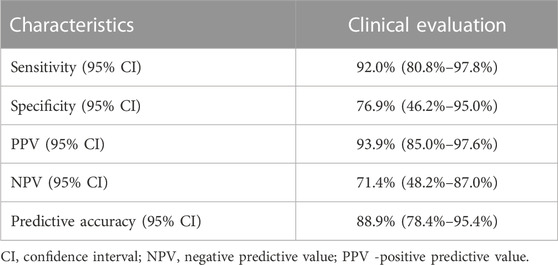
TABLE 5. Sensitivity, specificity, positive predictive value, negative predictive value and predictive accuracy of clinical evaluation for prediction of disease status using pathological examination as a gold standard.
Research interest was the comparisons between responders (comprised 35 patients) and non-responders (included 40 patients) (Table 6). Initial T and N stadium of disease were not significantly different between these two groups. Patients with poorly differentiated tumors and those with mucinous histological type responded to treatment significantly worse than patients with well or moderate tumor differentiation and those without mucinous type (p < 0.05 and p < 0.01, respectively). According to MRI findings, non-responders presented more often with extramural vascular invasion (EMVI) (p < 0.05). Among hematological parameters, significance was found for absolute basophil, eosinophil and monocyte counts, dNLR and NMR.
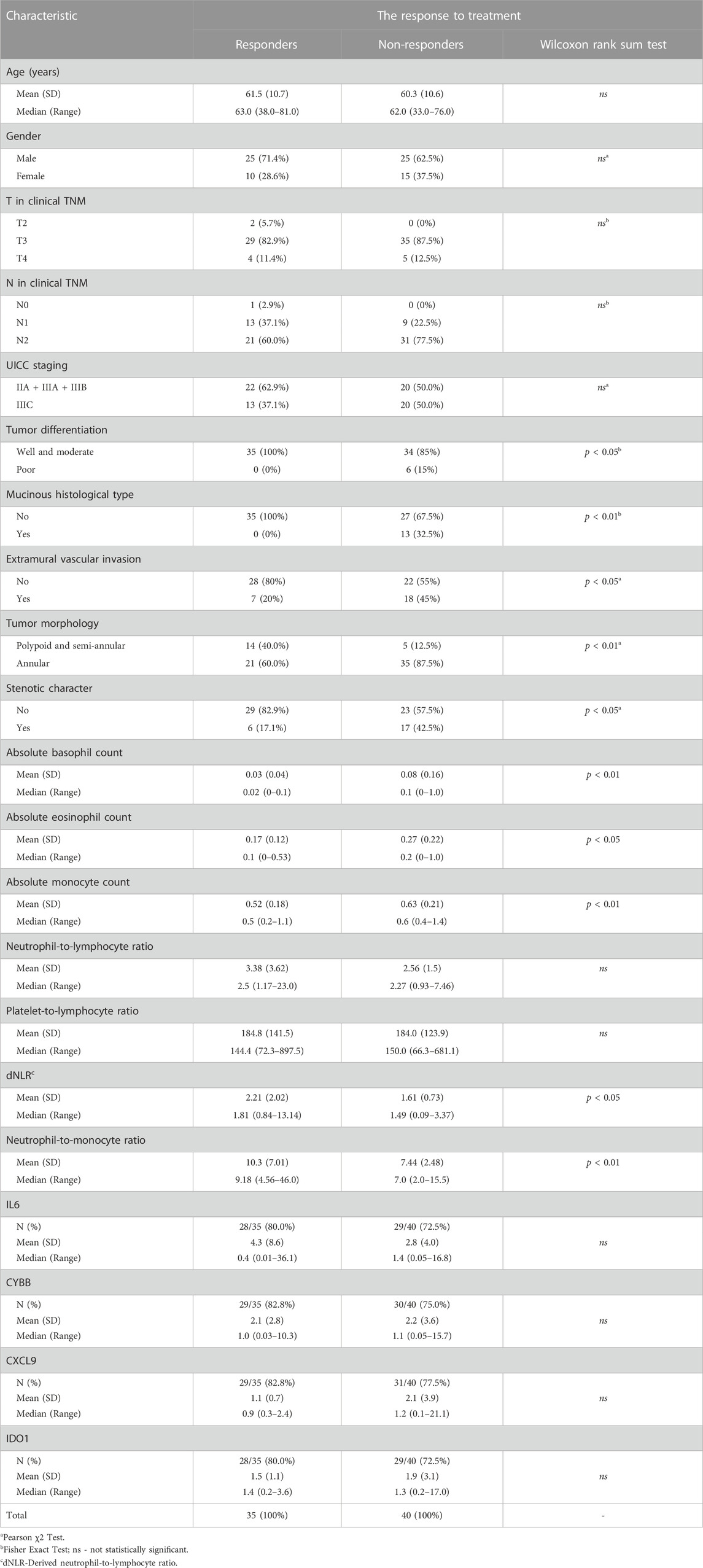
TABLE 6. Comparison of characteristics of responders and non-responders to neoadjuvant chemoradiotherapy.
In the whole patient group, there was no significant correlation between in silico selected genes (IL6, CYBB, CXCL9, IDO1) and response to treatment (Figure 4). On the other hand, when comparison between patients where pCR (TRG1) was detected and those who responded the worst (TRG4), statistical significance was found based on IDO1 expression (Wilcoxon rank sum test, p = 0.036) (Supplementary Table S2).

FIGURE 4. Gene expression levels of IL6, CXCL9, CYBB and IDO1 in responders (blue) and non-responders (green) normalized to GAPDH.
Next, ROC analysis was performed and it revealed the optimal cut-off values for absolute basophil, eosinophil and monocyte counts and NMR, above/below which the possibility of achieving favorable response after nCRT increased significantly (Table 7; Figure 5). The optimal cut-off value, which might distinguish patients with and without good response was not found only for dNLR.
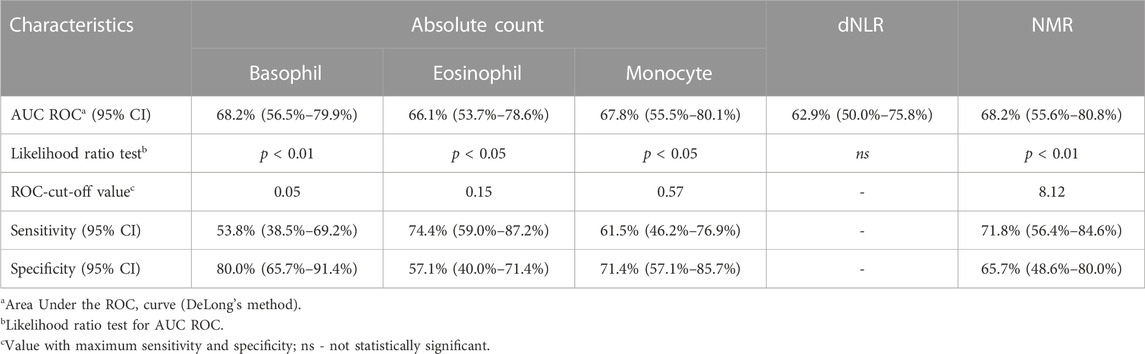
TABLE 7. Results of the ROC analysis for NMR, dNLR, absolute basophil, eosinophil and monocyte counts, and relevant events.
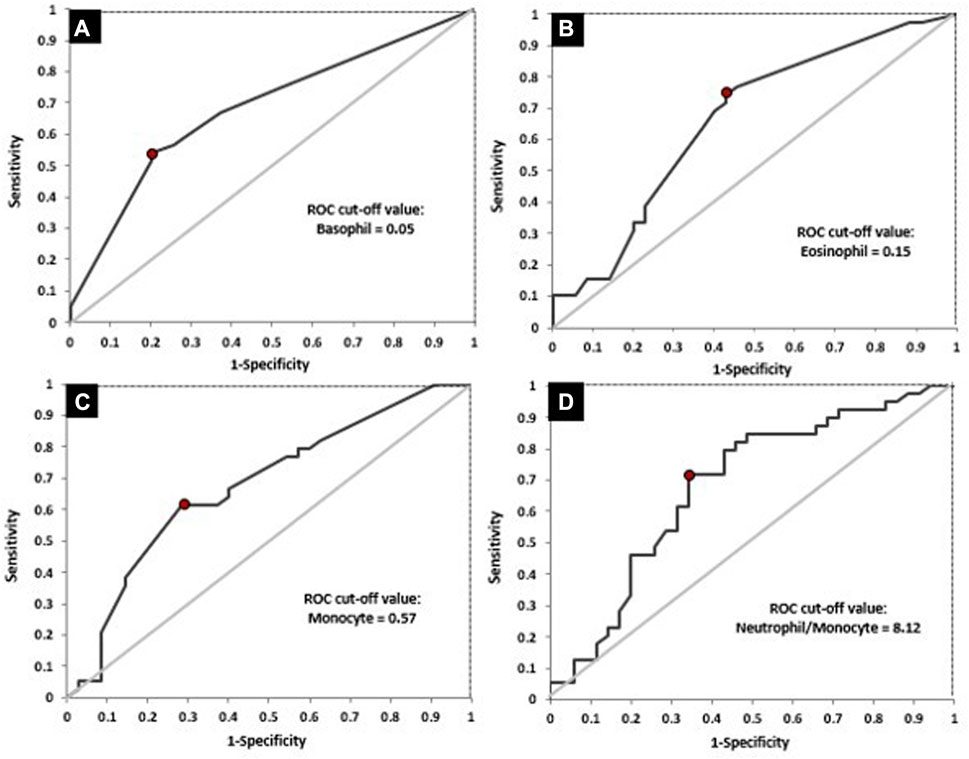
FIGURE 5. ROC curves for the absolute basophil count (A), absolute eosinophil count (B), absolute monocyte count (C) and NMR (D) in relation to response to treatment.
Afterwards, differences between responders and non-responders according to the cut-off values obtained by ROC analysis were examined (Table 8). According to the achieved cut-off values a statistically significant difference in the response was confirmed for the initial basophil, eosinophil and monocyte counts (p < 0.01 for all variables). Initial higher level of these parameters (greater than 0.05, 0.15, 0.57 respectively) were associated with unfavourable responses.
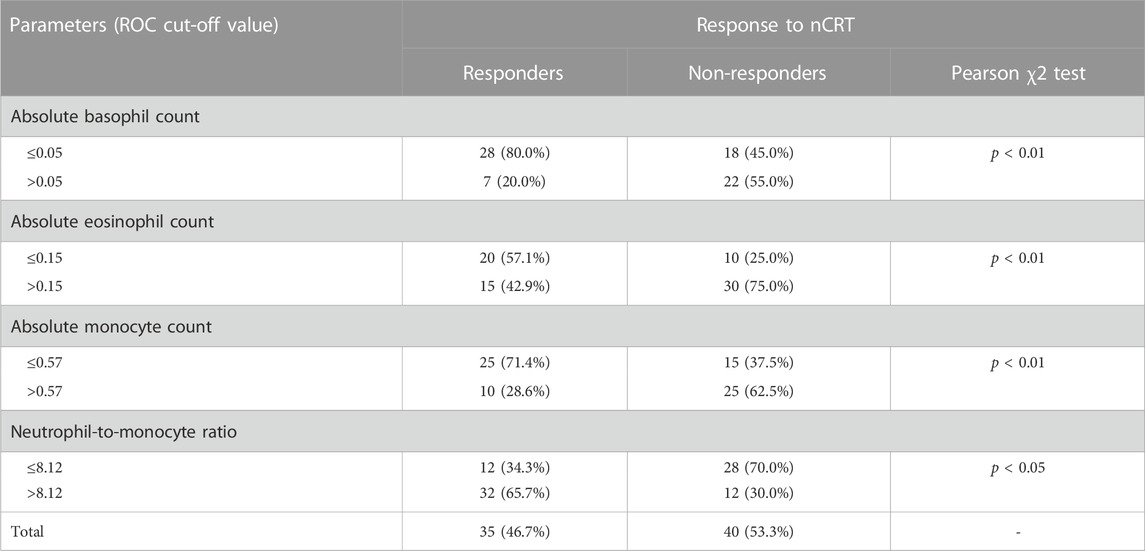
TABLE 8. The value of NMR, dNLR, absolute basophil, eosinophil and monocyte counts in prediction the response to nCRT.
Significant variables from the analyses were then used for the construction of a logistic regression model. The UICC staging was included as parameter which unit T and N stadium of disease and has high clinical importance. Finally, the model comprised ten variables: UICC staging, tumor differentiation, mucinous histological type, tumor morphology, stenotic character, extramural vascular invasion, as well as NMR, absolute basophil, eosinophil and monocyte counts (Table 9). After univariate anayses were conducted, the extremely high OR values were observed for tumor differentiation and mucinous histological type categories. These values were in correlation with the fact that all patients with mucinous histological type and/or poorly differentiated tumor had achieved bad response. Previously mentioned parameters as well as UICC staging were excluded after univariate analyses. The final model included tumor morphology, NMR, absolute basophil, eosinophil, and monocyte counts.
The numerical variables that remained significant in the multivariate analysis were utilized to create eleven different composite scores. These scores were calculated using various combinations of the significant variables (Supplementary Table S3). The best predictive power was observed when the initial eosinophil, basophil, and monocyte counts were combined (Figure 6). The changes in the false negative and true positive rates for the top three composite scores with respect to different cut-off values of these three scores are shown in Supplementary Figure S1.
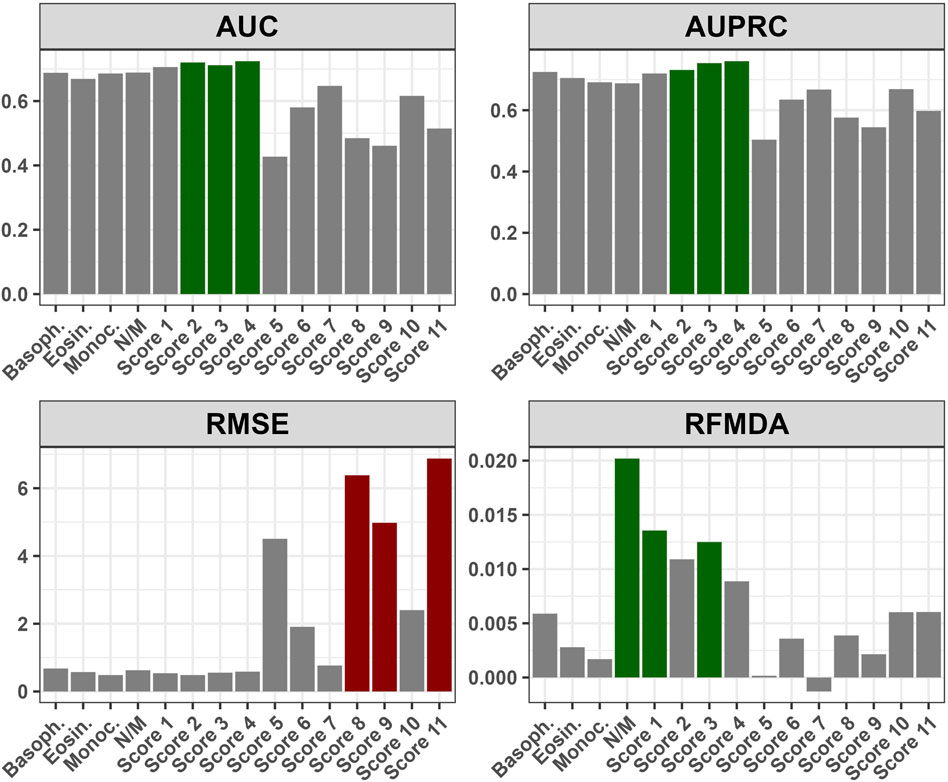
FIGURE 6. Performance of the composite scores with respect to various metrics: AUC - Area Under Curve; AUPRC - Area Under Precision-Recall Curve; RMSE - Root Mean Square Error; RFMDA - Random Forest Mean Decrease in Accuracy; Basoph. - Absolute basophil count; Eosin. - Absolute eosinophil count; Monoc. - Absolute monocyte count; N/M - Neutrophil-to-monocyte ratio; Score 1 - Absolute basophil + eosinophil count; Score 2 - Absolute basophil + monocyte count; Score 3 - Absolute eosinophil + monocyte count; Score 4 - Absolute basophil + eosinophil + monocyte count; Score 5 - Neutrophil-to-monocyte ratio + Absolute monocyte count; Score 6 - Neutrophil-to-monocyte ratio + Absolute eosinophil count; Score 7 - Neutrophil-to-monocyte ratio + Absolute basophil count; Score 8 - Neutrophil-to-monocyte ratio + Absolute monocyte + eosinophil count; Score 9 - Neutrophil-to-monocyte ratio + Absolute monocyte + Absolute basophil count; Score 10 - Neutrophil-to-monocyte ratio + Absolute eosinophil + basophil count; Score 11 - Neutrophil-to-monocyte ratio + Absolute monocyte + eosinophil count + basophil count; Supplementary Figure S1. Relationship between False Negative and True Positive Rates for Top Three Composite Scores at Different Cut-off Values: Score 2 - Absolute basophil + monocyte count; Score 3 - Absolute eosinophil + monocyte count; Score 4 - Absolute basophil + eosinophil + monocyte count.
The optimal time for assessment of tumor response after nCRT, time for surgery, and how to profile the best candidates for the „watch and wait” approach is still unknown. In this study, we aimed to select patients who would benefit the most from an increase of RT dose and waiting periods longer than 6 weeks after nCRT completion according to initial clinical, pathological, radiological, and hematological parameters, as well as inflammation-related genetic biomarkers chosen by in silico analysis. The identification of these predictive clinical and molecular markers would enable the intensification of treatment in selected groups of patients. Better selection of patients with a higher probability of a favorable response to neoadjuvant treatment would contribute to the reduction of morbidity, while improving survival and local control of the disease. On the other hand, patients where a good response to neoadjuvant treatment is not expected would be candidates for other treatment modalities in the initial approach, such as induction polychemotherapy, application of target therapy (e.g., epidermal growth factor receptor inhibitors) or surgery without delay after completion of neoadjuvant treatment.
In some cases, pelvic MRI scan performed at eighth week after nCRT completion cannot clearly distinguish residual tumor due to post treatment changes and still probably did not achieve the maximum response. The sensitivity of clinical evaluation, according to our results, was 92%. It refers to high probability that disease evaluation will indicate an incomplete response when viable tumor cells are present, which is confirmed with pathohystological examination as a gold standard. Therefore, the combination of MRI scan and proctoscopy examination is beneficial when it comes to a group of patients who still have residual disease after nCRT, at which point operative treatment is indicated. On the other hand, lower specificity and negative predictive value (NPV) (76.92%, 71.39%, respectively) suggest that this kind of evaluation is not selective enough for patients who are candidates for the “watch and wait” approach. This method is particularly important in the case of distally located rectal cancer when abdominoperineal resection is the only option. In our study, the majority of patients had distant located tumor (80%). The only way to confirm CR after nCRT is strict follow-up with reevaluation every 2–3 months in the first 2 years after treatment completion, followed by continuation of the protocols (Maas et al., 2015). Evidence suggests that in the case of local regrowth, salvage surgery can be done in 95% of patients, which indicate the safety of this approach (Dossa et al., 2017). However, when near CR is found at the first assessment, the protocols are not well established yet. It is well known that prolongation of period after nCRT completion is associated with higher pCR rate (Macchia et al., 2017). In the case when primary response 6–8 weeks after treatment completion is close to cCR, it is beneficial for patients who are not candidates for sphincter preservation surgery to delay surgery with one more clinical assessment after 8–12 weeks in order to achieve the maximum response. Simpson et al. reported local regrowth on repeated assessment for 37% of patients whose response was defined as near CR (Simpson et al., 2020). Another article which investigated the role of prolongation of period after nCRT in order to achieve the maximum response, found that 90% of patients with initial near CR at the first assessment were found to be cCR at the reassessment after 6–12 weeks (Hupkens et al., 2018). On the other hand, delaying surgery in order to achieve better response is associated with a higher probability of distant metastases (van der Valk et al., 2018). This fact can be related to local regrowth, but it has not been proved yet.
These circumstances stress the necessity of additional parameters which can guide the selection of patients who can be expected to achieve a complete response. Molecular markers in combination with good MRI and rigid proctoscopy examination may allow longer delays in surgery and one more pelvic MRI scan after 8–12 weeks.
In this study, we aimed to investigate some genetic factors that were found to be promising candidates using in silico methods of previously published datasets. As no genes were found to be statistically significant in all three datasets, four genes were chosen for further validation–three genes included in the Hallmark inflammatory response related to avoidance of immune detection that were found as significant for response in at least 2 analyzed datasets (IL6, CXCL9, CYBB), and one gene with prominent potential based on extensive literature search which was found to be significant in only one dataset (IDO1). Gene expression was evaluated by qPCR using GAPDH as a reference, which has been previously validated as one of optimal, most stably expressed housekeeping genes for gene expression-related experiments in rectal tumors (Vermani et al., 2020), which was confirmed in our cohort as well. However, statistical significance between responders and non-responders in relation to expression of selected genes (IL6, CYBB, CXCL9, IDO1) was not reached. Although there is significant potential of investigating genes associated with the inflammatory response in relation to therapy response in rectal cancer setting, the presence of population-specific parameters and limited number of samples might be potential reasons for the lack of validation in our study. When comparisons were made in the subgroup of patients who were operated, a significantly higher expression of IDO1 (p < 0.05) was found for TRG1 compared to TRG4. IDO1 is critical for tryptophan metabolism, and is regarded to have a significant effect on the modulation of T-cell behaviour and differentiation of regulatory T-cells. In a previous study which explored IDO1 expression using immunohistochemistry in postoperative specimens, the relation to pathological response was not found (p = 0.44). The same study showed that higher expression of IDO1 was associated with worse prognosis (Takasu et al., 2022). However, another study exploring nodal-positive LARC revealed that high IDO1 expression in specimens after nCRT completion was associated with improved overall survival (OS) (Schollbach et al., 2020). In our study, all but one patient was nodal-positive and our analyses were conducted on the initial specimens, which enabled us to analyze potential predictive biomarkers.
Concerning liquid biopsy parameters, periodic measurement of markers during patient follow up may also be crucial to prove the absence of the disease as well as for early detection of disease progression. This kind of approach has been investigated in metastatic colorectal cancer and it was shown that periodic sampling of liquid biopsy accompanied with ctDNA levels measurements can be valid for monitoring status of the disease and profile the response to treatment (van ’t Erve et al., 2022).
The importance of EMVI as a prognostic factor in LARC setting is well established. By comparison of disease-free survival (DFS) between II and III stage of disease, it was shown that independent from disease stadium, the presence of EMVI results in the worse prognosis (Chand et al., 2014). The predictive role of EMVI has not been defined yet. Sun et al. found that EMVI status was the only factor by multivariate analysis which influences the response to treatment. The focus of this research was the role of initial MRI characteristics on treatment outcome of T3 LARC patients. Patients with ypT0-2N0 postoperative category were previously defined as good responders (Sun et al., 2018). In our cohort, 33.3% of patients were EMVI positive, and it was shown that they were more likely to have poor response (p < 0.05). Worse response in EMVI positive group of patients can be connected with tumour hypoxia and consequent radioresistancy, due to the fact that primary mechanism of radiotherapy effectiveness is formation of reactive oxygen radicals. Hypoxia in solid tumors is a well-known problem because of insufficient vascularisation of rapid tumor growth. In order to resolve this in our study, we tried to increase the administrated dose per fraction on the gross disease region (2.16 Gy/fraction). By doing this, we attempt to cause cell death related to direct DNA damage caused by radiation, and to overcome lower level of oxygen in some parts of the tumor. By combining pCR rate in group of patients where operative treatment was conducted and patients who were enrolled in “watch and wait” program, we achieved 33.3% complete response rate. On the other hand, in EMVI positive group of patients, the complete response was achieved for only 16% of them. The option for this group of patients might be further dose escalation using adaptive MRI-guided radiotherapy which had shown potential for higher cCR rates and wider implementation of organ preservation approaches (Kensen et al., 2023).
The role of initial basophile count has been previously investigated as a prognostic factor in colorectal cancer. The association between lower basophile level and worse survival as well as aggressive tumor potential has been shown (Liu et al., 2020). To the best of our knowledge, this is the first study to find the predictive role of basophile counts in the rectal cancer settings. Patients with an initial basophile count lower than 0.05 are more likely to achieve good response (p < 0.01). Similar results were found in advanced gastric cancer, where worse response to programmed death 1 inhibitor (anti-PD-1 inhibitor) plus chemotherapy was in correlation with a higher level of peripheral basophils (Wu et al., 2022).
Comparing literature data on the predictive role of initial eosinophil counts, it has been proposed as a potential predictive marker for immunotherapy in lung cancer, with a higher level detected in patients with better treatment outcome (Caliman et al., 2022). It was also found that higher initial level of eosinophil is connected to more effective outcomes when immunotherapy is administrated together with chemotherapy in advanced melanoma (Ferrucci et al., 2017). In our study, a higher initial eosinophil level is associated with worse response, which might be explained by different treatment modalities and the addition of the radiotherapy component.
Analyzing initial monocyte counts, a predictive role was previously reported in the CRC settings, with higher levels detected in patients with poor outcome (Li et al., 2018). The same was found in our research where the absolute monocyte levels a higher than 0.57 were related to worse response. The NMR has been investigated in low-risk differentiated thyroid carcinoma as a prognostic factor, and it was found that lower initial level is related to a worse prognosis, which is in relation to our findings (Offi et al., 2021). Our group has previously shown that hematological parameters easily derived and routinely determined by low-cost and minimally invasive methods might be useful in predicting the response to chemoradiotherapy in patients with anal cancer (Stojanovic-Rundic et al., 2021). Also, we successfully evaluated the role of hematological parameters in predicting the survival and toxicity to specific treatment in the lung cancer setting (Jokic et al., 2021).
According to our results, mucinous tumor differentiation was significantly assocciated with poor response (p < 0.01). The study conducted by Simha et al. also found that presence of mucin is associated with larger residual disease and worse prognosis (Simha et al., 2014). Previously it has also been described that mucinous rectal carcinoma is associated with a unique genetic pattern, including more frequent presence of microsatellite instability (MSI), which is caused by a defect in DNA mismatch repair (Mekenkamp et al., 2012). The connection of MSI in rectal carcinoma and poorer prognosis has also been reported (Hasan et al., 2020). Bearing it in mind, recently presented preliminary results with focus on usefulness of introduction of the anti-PD-1 inhibitor dostarlimab in patients with mismatch repair–deficient (dMMR) LARC patients can be promising to individualise treatment in this group of patients (Cercek et al., 2022).
Some previous studies explored predictive biomarkers in similar patient cohorts (Krauthamer et al., 2013; Lu et al., 2022). Krauthamer et al. focused on predictors as serum albumin level, hemoglobin, and absolute blood cell counts in a retrospective, single-center approach. Our study adopted a prospective design, which allowed real-time data collection, reducing potential bias and providing more reliable results. Lu et al. explored the value of blood parameters after nCRT, 2 weeks before surgery, and considered fibrinogen-to-albumin ratio and sodium-to-globulin ratio as predictors. In our study, blood parameters were derived prior to nCRT, ensuring that they represent the initial state thus reducing the impact of treatment on their values. Our cohort of patients was also more homogenous, and rigorous control of factors like radiotherapy technique, doses, and chemotherapy regimens, allowing more confident assessment of the impact of specific predictors on treatment response. We also focused on parameters that are readily available in routine blood testing, which might ensure practicality and applicability of our prediction model in a clinical setting. The prediction strategy in our study generally offered a more comprehensive approach, as we incorporated a wide range of factors, including inflammatory hematological parameters, radiological features, pathohistological characteristics, and genetic markers. This comprehensive evaluation ensures a more holistic understanding of potential predictors for a favorable response to nCRT, providing valuable insights for personalized medicine approaches and meta-analyses taking into account potential population-specific differences.
This study has some limitations. The sample size is relatively low, but has met the criteria of a minimum number of LARC samples taking into account its incidence and population size in Serbia (95% confidence level) (Flikkema and Toledo-Pereyra, 2012). The evaluation of potential prognostic parameters has not been included, as the enrolled patients are currently under follow-up for long-term outcomes. The predictive model constructed in our study is currently being validated in an independent prospective cohort of patients with LARC treated with nCRT.
Based on the logistic regression model, important factors associated with favorable response to nCRT were tumor morphology and hematological parameters which can be easily and routinely derived from initial laboratory results (NMR, eosinophile, basophil and monocyte counts) in a minimally invasive manner. Here, we present evidence that a combined score derived by summing the initial absolute counts of basophils, eosinophils, and monocytes holds the highest predictive value and potential clinical utility.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by the Ethics Committee of the Institute for Oncology and Radiology of Serbia (Approval No. 2211-01 from 11.06.2020.) and Ethics Committee of the Faculty of Medicine, University of Belgrade (Approval No. 1322/XII-17 from 03.12.2020.). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Conceptualization (MM, MC, DG, SS-R, AS, and MO); Data curation (MM, MC, and SS-R); Formal analysis (MM, MC, SS-R, RS, DG, NM, AS, and MO); Funding acquisition (MC, RF, SC-B, and JZ); Investigation (MM, MC, SS-R, and AS); Methodology (MM, MC, AS, MO, SS-R, NM, and RF); Project administration (MM, MC, RJ, JZ, RF, and SC-B); Resources (MM, MC, SS-R, AS, DG, and SC-B); Software (DG, RS, MM, and MC); Supervision (MC, SS-R, NM, RJ, JZ, SC-B, and RF); Validation (MM, MC, and AS); Visualization (MC, MM, SS-R, RS, DG, and AS); Writing—original draft (MM, SS-R, AS, MO, DG, RS, and MC); Writing—review and editing (MM, SS-R, AS, MO, DG, RJ, NM, RS, JZ, SC-B, RF, and MC). All authors contributed to the article and approved the submitted version.
This study was funded by the Horizon Europe Twinning Project STEPUPIORS (Agreement No. 501 101079217) and the Ministry of Education and Science of the Republic of Serbia (Agreement No. 502 451-03-47/2023-01/200043).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2023.1245594/full#supplementary-material
SUPPLEMENTARY MATERIAL S1 | qRT-PCR raw data of expression levels of selected gene candidates.
SUPPLEMENTARY MATERIAL S2 | The results of the differential expression analysis within GSE 139255.
SUPPLEMENTARY MATERIAL S3 | The top 100 genes from each database.
SUPPLEMENTARY TABLE S1 | Oligonucleotides primer sequences for qRT-PCR designed using NCBI Primer Blast.
SUPPLEMENTARY TABLE S2 | Comparison of genes expression of patients with pCR and those with TRG4 postoperative category.
SUPPLEMENTARY TABLE S3 | Performance of the composite scores with respect to various metrics.
SUPPLEMENTARY FIGURE S1 | Relationship between False Negative and True Positive Rates for Top Three Composite Scores at Different Cut-off Values: Score 2 - Absolute basophil + monocyte count; Score 3 - Absolute eosinophil + monocyte count; Score 4 - Absolute basophil + eosinophil + monocyte count.
Agostini, M., Janssen, K.-P., Kim, I.-J., D’Angelo, E., Pizzini, S., Zangrando, A., et al. (2015). An integrative approach for the identification of prognostic and predictive biomarkers in rectal cancer. Oncotarget 6, 32561–32574. doi:10.18632/oncotarget.4935
Bajpai, M., Seril, D. N., Van Gurp, J., Geng, X., Alvarez, J., Minacapelli, C. D., et al. (2019). Effect of long-term mesalamine therapy on cancer-associated gene expression in colonic mucosa of patients with ulcerative colitis. Dig. Dis. Sci. 64, 740–750. doi:10.1007/s10620-018-5378-8
Brotto, K., Malisic, E., Cavic, M., Krivokuca, A., and Jankovic, R. (2012). The usability of allele-specific PCR and reverse-hybridization assays for KRAS genotyping in Serbian colorectal cancer patients. Dig. Dis. Sci. 58, 998–1003. doi:10.1007/s10620-012-2469-9
Caliman, E., Fancelli, S., Ottanelli, C., Mazzoni, F., Paglialunga, L., Lavacchi, D., et al. (2022). Absolute eosinophil count predicts clinical outcomes and toxicity in non-small cell lung cancer patients treated with immunotherapy. Cancer Treat. Res. Commun. 32, 100603. doi:10.1016/j.ctarc.2022.100603
Cavic, M., Krivokuca, A., Boljevic, I., Brotto, K., Jovanović, K., Tanic, M., et al. (2016). Pharmacogenetics in cancer therapy - 8 years of experience at the Institute for oncology and Radiology of Serbia. J. B.U.ON. 21, 1287–1295.
Cercek, A., Lumish, M., Sinopoli, J., Weiss, J., Shia, J., Lamendola-Essel, M., et al. (2022). PD-1 blockade in mismatch repair–deficient, locally advanced rectal cancer. N. Engl. J. Med. 386, 2363–2376. doi:10.1056/NEJMoa2201445
Chand, M., Bhangu, A., Wotherspoon, A., Stamp, G. W. H., Swift, R. I., Chau, I., et al. (2014). EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann. Oncol. 25, 858–863. doi:10.1093/annonc/mdu029
Chen, B., Alvarado, D. M., Iticovici, M., Kau, N. S., Park, H., Parikh, P. J., et al. (2020). Interferon-induced Ido1 mediates radiation resistance and is a therapeutic target in colorectal cancer. Cancer Immunol. Res. 8, 451–464. doi:10.1158/2326-6066.CIR-19-0282
Conde-Muíño, R., Cuadros, M., Zambudio, N., Segura-Jiménez, I., Cano, C., and Palma, P. (2015). Predictive biomarkers to chemoradiation in locally advanced rectal cancer. Biomed. Res. Int. 2015, 921435. doi:10.1155/2015/921435
Dossa, F., Chesney, T. R., Acuna, S. A., and Baxter, N. N. (2017). A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: A systematic review and meta-analysis. lancet. Gastroenterol. Hepatol. 2, 501–513. doi:10.1016/S2468-1253(17)30074-2
Eaden, J. A., Abrams, K. R., and Mayberry, J. F. (2001). The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 48, 526–535. doi:10.1136/gut.48.4.526
Ferrari, L., and Fichera, A. (2015). Neoadjuvant chemoradiation therapy and pathological complete response in rectal cancer. Gastroenterol. Rep. 3, 277–288. doi:10.1093/gastro/gov039
Ferrucci, P. F., Gandini, S., Cocorocchio, E., Pala, L., Baldini, F., Mosconi, M., et al. (2017). Baseline relative eosinophil count as a predictive biomarker for ipilimumab treatment in advanced melanoma. Oncotarget 8, 79809–79815. doi:10.18632/oncotarget.19748
Flikkema, R. M., and Toledo-Pereyra, L. H. (2012). Sample size determination in medical and surgical research. J. Investig. Surg. Off. J. Acad. Surg. Res. 25, 3–7. doi:10.3109/08941939.2011.648868
Gim, J., Cho, Y. B., Hong, H. K., Kim, H. C., Yun, S. H., Wu, H.-G., et al. (2016). Predicting multi-class responses to preoperative chemoradiotherapy in rectal cancer patients. Radiat. Oncol. 11, 50. doi:10.1186/s13014-016-0623-9
Habr-Gama, A., Gama-Rodrigues, J., São Julião, G. P., Proscurshim, I., Sabbagh, C., Lynn, P. B., et al. (2014). Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int. J. Radiat. Oncol. Biol. Phys. 88, 822–828. doi:10.1016/j.ijrobp.2013.12.012
Hasan, S., Renz, P., Wegner, R. E., Finley, G., Raj, M., Monga, D., et al. (2020). Microsatellite instability (MSI) as an independent predictor of pathologic complete response (PCR) in locally advanced rectal cancer: A national cancer database (ncdb) analysis. Ann. Surg. 271, 716–723. doi:10.1097/SLA.0000000000003051
Hupkens, B. J. P., Maas, M., Martens, M. H., van der Sande, M. E., Lambregts, D. M. J., Breukink, S. O., et al. (2018). Organ preservation in rectal cancer after chemoradiation: should we extend the observation period in patients with a clinical near-complete response? Ann. Surg. Oncol. 25, 197–203. doi:10.1245/s10434-017-6213-8
Irizarry, R. A., Bolstad, B. M., Collin, F., Cope, L. M., Hobbs, B., and Speed, T. P. (2003). Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15. doi:10.1093/nar/gng015
Jakovljevic, K., Malisic, E., Cavic, M., Krivokuca, A., Dobricic, J., and Jankovic, R. (2012). KRAS and BRAF mutations in Serbian patients with colorectal cancer. J. BUON. 17, 575–580. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23033302.
Jokic, V., Savic-Vujovic, K., Spasic, J., Stanic, N., Marinkovic, M., Radosavljevic, D., et al. (2021). Hematological parameters in EGFR-mutated advanced NSCLC patients treated with TKIs: predicting survival and toxicity. Expert Rev. Anticancer Ther. 21, 673–679. doi:10.1080/14737140.2021.1893694
Kensen, C. M., Betgen, A., Wiersema, L., Peters, F. P., Kayembe, M. T., Marijnen, C. A. M., et al. (2023). Online adaptive MRI-guided radiotherapy for primary tumor and lymph node boosting in rectal cancer. Cancers (Basel) 15, 1009. doi:10.3390/cancers15041009
Krauthamer, M., Rouvinov, K., Ariad, S., Man, S., Walfish, S., Pinsk, I., et al. (2013). A study of inflammation-based predictors of tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Oncology 85, 27–32. doi:10.1159/000348385
Li, X.-F., Jiang, Z., Gao, Y., Li, C.-X., and Shen, B.-Z. (2016). Combination of three-gene immunohistochemical panel and magnetic resonance imaging-detected extramural vascular invasion to assess prognosis in non-advanced rectal cancer patients. World J. Gastroenterol. 22, 8576–8583. doi:10.3748/wjg.v22.i38.8576
Li, Z., Xu, Z., Huang, Y., Zhao, R., Cui, Y., Zhou, Y., et al. (2018). The predictive value and the correlation of peripheral absolute monocyte count, tumor-associated macrophage and microvessel density in patients with colon cancer. Med. Baltim. 97, e10759. doi:10.1097/MD.0000000000010759
Liu, Q., Luo, D., Cai, S., Li, Q., and Li, X. (2020). Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer. Clin. Transl. Med. 9, 6. doi:10.1186/s40169-019-0255-4
Long, A. G., Lundsmith, E. T., and Hamilton, K. E. (2017). Inflammation and colorectal cancer. Curr. Colorectal Cancer Rep. 13, 341–351. doi:10.1007/s11888-017-0373-6
Lu, S., Liu, Z., Wang, Y., Meng, Y., Peng, R., Qu, R., et al. (2022). A novel prediction model for pathological complete response based on clinical and blood parameters in locally advanced rectal cancer. Front. Oncol. 12, 932853. doi:10.3389/fonc.2022.932853
Maas, M., Beets-Tan, R. G. H., Lambregts, D. M. J., Lammering, G., Nelemans, P. J., Engelen, S. M. E., et al. (2011). Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 29, 4633–4640. doi:10.1200/JCO.2011.37.7176
Maas, M., Lambregts, D. M. J., Nelemans, P. J., Heijnen, L. A., Martens, M. H., Leijtens, J. W. A., et al. (2015). Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Ann. Surg. Oncol. 22, 3873–3880. doi:10.1245/s10434-015-4687-9
Maas, M., Nelemans, P. J., Valentini, V., Das, P., Rödel, C., Kuo, L.-J., et al. (2010). Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet. Oncol. 11, 835–844. doi:10.1016/S1470-2045(10)70172-8
Macchia, G., Gambacorta, M. A., Masciocchi, C., Chiloiro, G., Mantello, G., di Benedetto, M., et al. (2017). Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: A population study on 2094 patients. Clin. Transl. Radiat. Oncol. 4, 8–14. doi:10.1016/j.ctro.2017.04.004
MedCalc Software Ltd (2022). Diagnostic test evaluation calculator. Available at: https://www.medcalc.org/calc/diagnostic_test.php.
Mekenkamp, L. J. M., Heesterbeek, K. J., Koopman, M., Tol, J., Teerenstra, S., Venderbosch, S., et al. (2012). Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur. J. Cancer 48, 501–509. doi:10.1016/j.ejca.2011.12.004
Millino, C., Maretto, I., Pacchioni, B., Digito, M., De Paoli, A., Canzonieri, V., et al. (2017). Gene and MicroRNA expression are predictive of tumor response in rectal adenocarcinoma patients treated with preoperative chemoradiotherapy. J. Cell. Physiol. 232, 426–435. doi:10.1002/jcp.25441
Mootha, V. K., Lindgren, C. M., Eriksson, K.-F., Subramanian, A., Sihag, S., Lehar, J., et al. (2003). PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273. doi:10.1038/ng1180
Nikolic, N., Radosavljevic, D., Gavrilovic, D., Nikolic, V., Stanic, N., Spasic, J., et al. (2021). Prognostic factors for post-recurrence survival in stage II and III colorectal carcinoma patients. Med. Kaunas. 57, 1108. doi:10.3390/medicina57101108
Offi, C., Romano, R. M., Cangiano, A., Candela, G., and Docimo, G. (2021). Clinical significance of neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, platelet-to-lymphocyte ratio and prognostic nutritional index in low-risk differentiated thyroid carcinoma. Acta Otorhinolaryngol. Ital. organo Uff. della Soc. Ital. Otorinolaringol. Chir. Cerv.-facc. 41, 31–38. doi:10.14639/0392-100X-N1089
Oken, M. M., Creech, R. H., Tormey, D. C., Horton, J., Davis, T. E., McFadden, E. T., et al. (1982). Toxicity and response criteria of the eastern cooperative oncology group. Am. J. Clin. Oncol. 5, 649–656. doi:10.1097/00000421-198212000-00014
O’Sullivan, B., Brierley, J., Byrd, D., Bosman, F., Kehoe, S., Kossary, C., et al. (2017). The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet. Oncol. 18, 849–851. doi:10.1016/S1470-2045(17)30438-2
Park, I. J., Yu, Y. S., Mustafa, B., Park, J. Y., Seo, Y. B., Kim, G.-D., et al. (2020). A nine-gene signature for predicting the response to preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Cancers (Basel) 12, 800. doi:10.3390/cancers12040800
Ritchie, M. E., Phipson, B., Wu, D., Hu, Y., Law, C. W., Shi, W., et al. (2015). Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47. doi:10.1093/nar/gkv007
Schmitt, M., and Greten, F. R. (2021). The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 21, 653–667. doi:10.1038/s41577-021-00534-x
Schollbach, J., Kircher, S., Wiegering, A., Anger, F., Rosenwald, A., Germer, C.-T., et al. (2020). The local immune phenotype influences prognosis in patients with nodal-positive rectal cancer after neoadjuvant chemoradiation. Int. J. Colorectal Dis. 35, 365–370. doi:10.1007/s00384-019-03466-0
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. doi:10.1101/gr.1239303
Simha, V., Kapoor, R., Gupta, R., Bahl, A., and Nada, R. (2014). Mucinous adenocarcinoma of the rectum: A poor candidate for neo-adjuvant chemoradiation? J. Gastrointest. Oncol. 5, 276–279. doi:10.3978/j.issn.2078-6891.2014.020
Simpson, G., Hopley, P., Wilson, J., Day, N., Haworth, A., Montazeri, A., et al. (2020). Long-term outcomes of real world “watch and wait” data for rectal cancer after neoadjuvant chemoradiotherapy. Color. Dis. Off. J. Assoc. Coloproctology Gt. Br. irel. 22, 1568–1576. doi:10.1111/codi.15177
Smoot, M. E., Ono, K., Ruscheinski, J., Wang, P.-L., and Ideker, T. (2011). Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27, 431–432. doi:10.1093/bioinformatics/btq675
Smyth, G. K. (2004). Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article3. doi:10.2202/1544-6115.1027
Stanojevic, A., Samiotaki, M., Lygirou, V., Marinkovic, M., Nikolic, V., Stojanovic-Rundic, S., et al. (2023). Data independent acquisition mass spectrometry (DIA-MS) analysis of FFPE rectal cancer samples offers in depth proteomics characterization of response to neoadjuvant chemoradiotherapy. doi:10.1101/2023.05.12.23289671
Stojanovic-Rundic, S., Marinkovic, M., Cavic, M., Karapandzic, V. P., Gavrilovic, D., Jankovic, R., et al. (2021). The role of haematological parameters in predicting the response to radical chemoradiotherapy in patients with anal squamous cell cancer. Radiol. Oncol. 55, 449–458. doi:10.2478/raon-2021-0039
Subramanian, A., Tamayo, P., Mootha, V. K., Mukherjee, S., Ebert, B. L., Gillette, M. A., et al. (2005). Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. 102, 15545–15550. doi:10.1073/pnas.0506580102
Sun, Y., Li, J., Shen, L., Wang, X., Tong, T., and Gu, Y. (2018). Predictive value of MRI-detected extramural vascular invasion in stage T3 rectal cancer patients before neoadjuvant chemoradiation. Diagn. Interv. Radiol. 24, 128–134. doi:10.5152/dir.2018.17286
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Takasu, C., Nishi, M., Yoshikawa, K., Tokunaga, T., Nakao, T., Kashihara, H., et al. (2022). Role of Ido expression in patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy. BMC Cancer 22, 1263. doi:10.1186/s12885-022-10357-1
van der Valk, M. J. M., Hilling, D. E., Bastiaannet, E., Meershoek-Klein Kranenbarg, E., Beets, G. L., Figueiredo, N. L., et al. (2018). Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the international watch and wait database (IWWD): an international multicentre registry study. Lancet (London, Engl. 391, 2537–2545. doi:10.1016/S0140-6736(18)31078-X
van ’t Erve, I., Medina, J. E., Leal, A., Papp, E., Phallen, J., Adleff, V., et al. (2022). Metastatic colorectal cancer treatment response evaluation by ultra-deep sequencing of cell-free DNA and matched white blood cells. Clin. Cancer Res. CCR- 29, 899–909. doi:10.1158/1078-0432.CCR-22-2538
Vermani, L., Kumar, R., and Kumar, N. S. (2020). GAPDH and PUM1: optimal housekeeping genes for quantitative polymerase Chain reaction-based analysis of cancer stem cells and epithelial-mesenchymal transition gene expression in rectal tumors. Cureus 12 (12), e12020. doi:10.7759/cureus.12020
Vuletić, A., Mirjačić Martinović, K., Tišma Miletić, N., Zoidakis, J., Castellvi-Bel, S., and Čavić, M. (2021). Cross-talk between tumor cells undergoing epithelial to mesenchymal transition and natural killer cells in tumor microenvironment in colorectal cancer. Front. Cell Dev. Biol. 9, 750022. doi:10.3389/fcell.2021.750022
Watanabe, T., Komuro, Y., Kiyomatsu, T., Kanazawa, T., Kazama, Y., Tanaka, J., et al. (2006). Prediction of sensitivity of rectal cancer cells in response to preoperative radiotherapy by DNA microarray analysis of gene expression profiles. Cancer Res. 66, 3370–3374. doi:10.1158/0008-5472.CAN-05-3834
Wu, C., Qiu, Y., Zhang, R., Li, X., Liang, H., Wang, M., et al. (2022). Association of peripheral basophils with tumor M2 macrophage infiltration and outcomes of the anti-PD-1 inhibitor plus chemotherapy combination in advanced gastric cancer. J. Transl. Med. 20, 386. doi:10.1186/s12967-022-03598-y
Zorcolo, L., Rosman, A. S., Restivo, A., Pisano, M., Nigri, G. R., Fancellu, A., et al. (2012). Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: A meta-analysis. Ann. Surg. Oncol. 19, 2822–2832. doi:10.1245/s10434-011-2209-y
Keywords: inflammation, locally advanced rectal cancer, neoadjuvant chemoradiotherapy, predictive biomarkers, hematological parameters
Citation: Marinkovic M, Stojanovic-Rundic S, Stanojevic A, Ostojic M, Gavrilovic D, Jankovic R, Maksimovic N, Stroggilos R, Zoidakis J, Castellví-Bel S, Fijneman RJA and Cavic M (2023) Exploring novel genetic and hematological predictors of response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Front. Genet. 14:1245594. doi: 10.3389/fgene.2023.1245594
Received: 23 June 2023; Accepted: 15 August 2023;
Published: 31 August 2023.
Edited by:
Qing Lin, Johns Hopkins University, United StatesReviewed by:
Yongkang Yang, Johns Hopkins University, United StatesCopyright © 2023 Marinkovic, Stojanovic-Rundic, Stanojevic, Ostojic, Gavrilovic, Jankovic, Maksimovic, Stroggilos, Zoidakis, Castellví-Bel, Fijneman and Cavic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milena Cavic, bWlsZW5hLmNhdmljQG5jcmMuYWMucnM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.