- 1Prenatal Diagnosis Center, Boai Hospital of Zhongshan, Zhongshan, Guangdong, China
- 2Department of Urology, Zhongshan People’s Hospital, Zhongshan, Guangdong, China
- 3The First School of Clinical Medicine,Jinan University, Guangzhou, Guangdong, China
- 4The Second School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong, China
Complete trisomy 9 is a rare and lethal chromosomal anomaly characterized by multisystem dysmorphism and central nervous system (CNS) malformations. This study presents a case of complete trisomy 9 with an unusual phenotypic association and investigates the genetic pathways involved in this chromosomal abnormality. Trisomy 9 leads to a wide range of organ abnormalities, and this research contributes to a better understanding of the phenotype associated with this rare aneuploidy. The literature on the phenotypes of fetuses with various systems affected by complete trisomy 9 was reviewed and summarized. Correct diagnosis and appropriate counseling based on the characteristics of previous reports of fetuses with trisomy 9 is essential in maternity care and clinical management. To provide guidance and help for clinical diagnosis, this study aimed to explore the clinical and genetic characteristics of trisomy 9 syndrome to improve clinicians’ understanding of the disease.
Introduction
The prenatal diagnosis of complete trisomy 9 in fetuses presents several challenges. First, it is a rare condition during pregnancy, which may be unfamiliar to many doctors. Second, a significant number of affected fetuses do not survive in utero. Consequently, complete trisomy 9 is rarely observed and may not be initially considered or included in the list of potential diagnoses. Nonetheless, the accurate identification of fetuses with complete trisomy 9 remains crucial due to its poor prognosis. Chromosome 9 comprises approximately 141 million DNA base pairs, representing approximately 4.5% of the total DNA in cells. Chromosome 9 contains 2,466 genes, including 605 OMIM genes, and 162 of these 605 OMIM genes have been proven to be associated with diseases (data from https://www.gena.tech/; https://ghr.nlm.nih.gov/condition). The first report of trisomy 9 syndrome was published by Feingold and Atkins in 1973 (Feingold and Atkins, 1973). Complete trisomy 9 is characterized by the presence of an additional whole chromosome 9 in all cells, without evidence of mosaicism (Ferreres et al., 2008). Trisomy 9 is a fatal chromosomal disease, which mostly results in spontaneous abortion in early pregnancy. Trisomy 9 syndrome is a rare condition that affects multiple organ systems, including craniofacial dysmorphisms, cardiac abnormalities, genitourinary malformations, skeletal anomalies, and central nervous system abnormalities. Understanding the characteristics and manifestations of complete trisomy 9 can help doctors with early diagnosis. This knowledge can guide doctors in providing early interventions to minimize physical and psychological harm to pregnant women. For pregnant women, early detection and diagnosis of complete trisomy 9 can provide vital information to make early decisions, including further prenatal testing or the choice of pregnancy termination.
We present a case illustrating the complete form of this trisomy and performed a thorough review of the available literature to provide a comprehensive understanding of this syndrome. The aim was to help with the identification of clinical features and the performance of laboratory tests, prenatal genetic diagnosis, and genetic counseling for trisomy 9. We present a review of 59 cases of trisomy 9 to better define the phenotype and to determine its characteristics. Fetuses with complete trisomy 9 have multiple anomalies that can be readily detected prenatally by ultrasound. These anomalies primarily involve the craniofacial, cardiovascular, musculoskeletal, and genitourinary systems. However, some findings may be subtle and easily missed during routine ultrasound examinations (Sepulveda et al., 2003). Therefore, it is important to know the clinical characteristics of the various systems affected by trisomy 9 syndrome.
Case report
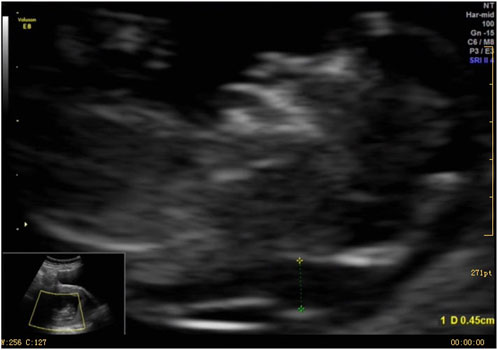
A 37-year-old female underwent routine fetal ultrasound examination at 12+2 weeks of pregnancy, which revealed thickened nuchal translucency. The examination was performed using a Voluson E8 ultrasound apparatus (GE Healthcare, Milwaukee, WI, United States) equipped with a multifrequency transabdominal RAB 4-8D probe. The procedures were performed according to the quality control standards of the British Fetal Medicine Foundation. As shown in Figure 1, thickened nuchal translucency (NT = 4.5 mm) was observed on fetal ultrasound. The woman had experienced a total of four pregnancies, including one ectopic pregnancy and one natural miscarriage at 8 weeks. She successfully delivered a healthy baby boy who is now 15 years old. The woman had no physical discomfort during the current pregnancy. Her partner was a 39-year-old healthy male. They had no history of medical conditions or medications, no abnormal family history, and no history of consanguineous marriages. At 19 weeks of gestation, fetal intrauterine growth restriction was noted on ultrasonography. The pregnant woman underwent amniocentesis at 19 weeks of pregnancy. Amniotic fluid specimens were collected by abdominal amniotic cavity puncture under the guidance of B-ultrasound. The results of G-banding karyotype analysis and CMA indicated trisomy 9 (Figures 2A, B). The pregnancy was terminated at 24 weeks of gestation, and a series of clinical examinations and genetic testing were conducted. The study protocols were approved by the Ethical Review Committee of the Boai Hospital of Zhongshan (KY-2023-004-47). A next-generation sequencing-based copy number variation (CNV-seq) assay was performed on the labor induction tissue. CNV-seq assay results of the placenta, fetal skin tissue, umbilical cord blood, kidney, and heart indicated trisomy 9 (Figure 2C). The examination revealed multiple anomalies (Supplementary Figure S1). The fetus’s face showed typical indications of trisomy 9. The face was dysmorphic, with a broad forehead, blepharophimosis, low-set malformed ears with small lobes, a prominent nose with a bulbous tip, and micrognathia. The fetus’s mouth was similar to a fish’s mouth, and the fetus had a broad neck, postural anomalies, broad thumbs, and clubfeet. Both renal malformations were connected and limited joint movement was observed.

FIGURE 2. (A) Results of karyotype analysis showed 47,XY,+9. (B) Chromosomal microarray results of the amniocyte indicated trisomy 9. (C) CNV-seq assay results of the placenta, fetal skin tissue, umbilical cord blood, kidney, and heart indicated trisomy 9.
Discussion
Complete trisomy 9 is typically associated with spontaneous abortion. Individuals with trisomy 9 seem either to die very early in embryonic life or survive to be born at term, many of the latter showing mosaicism (Saura et al., 1995; Saneto et al., 1998; Chen et al., 2023a). The prenatal diagnosis of trisomy 9 presents challenges in genetic counseling due to the need to differentiate between pseudo-mosaicism, fetal-placental discrepancy, and true trisomy 9. Studies have demonstrated varying levels of trisomy 9 mosaicism ranging from 99% to normal in different tissues (Tang et al., 2019; Ma et al., 2023). Trisomy 9 mosaicism tends to show different levels of mosaicism in various tissues. Low-level mosaic trisomy 9 at amniocentesis can be associated with a favorable fetal outcome (Chen et al., 2023b). When trisomy 9 mosaicism is suspected, genetic testing using uncultured cells is necessary to reflect the proportion of trisomy 9 mosaicism more accurately. Previous studies have suggested that the clinical phenotype of trisomy 9 mosaicism is similar to that of complete trisomy 9, while the clinical symptoms of trisomy 9 mosaicism are milder than those of complete trisomy 9 (Arnold et al., 1995; Saneto et al., 1998; Li et al., 2021). Individuals with low-level mosaic trisomy 9 can survive into young adulthood (Li et al., 2021).
We used CNV-seq to examine the placental tissue, skin tissue, umbilical cord blood, kidney, and heart of the fetus in this study. The results showed that the fetus had complete trisomy 9. The examination revealed multiple anomalies (Supplementary Figure S1). The fetus’s face showed typical indications of trisomy 9. The face was dysmorphic, with a broad forehead, blepharophimosis, low-set malformed ears with small lobes, a prominent nose with a bulbous tip, and micrognathia. The fetus’s mouth was similar to a fish’s mouth, and the fetus had a broad neck, postural anomalies, broad thumbs, and clubfeet. Both renal malformations were connected. Limited joint movement was observed.
The CNV-seq assay results of the placenta, fetal skin tissue, umbilical cord blood, kidney, and heart indicated trisomy 9. Therefore, the most likely cause of complete trisomy 9 was the non-disjunction of chromosome 9 during the diplotene phase of meiosis I. Complete trisomy 9 results from non-disjunction at meiosis and almost always occurs de novo. Almost all parents of fetuses with complete trisomy 9 have normal chromosomes. In addition, it cannot be ruled out that some phenotypically normal parents actually have mosaicism, with only a small percentage of abnormal cells, such as in some tissues or ovaries. Trisomy 9 cells in the ovary may lead to the birth of children with trisomy 9. Circumstantial evidence is scarce due to the normal phenotypes of the parents.
The prenatal diagnosis of trisomy 9 makes genetic counseling difficult since we must know the abnormal manifestations of various systems in fetuses with trisomy 9. To enhance our understanding, we conducted a comprehensive literature review on complete trisomy 9, including the addition of the case in our study. To date, a total of 59 cases of complete trisomy 9 have been reported. The first documented case of complete trisomy 9 was described in 1973 by Feingold and Atkins, wherein, remarkably, the male infant survived for 28 days despite presenting with multiple abnormalities (Feingold and Atkins, 1973). In 1978, one infant with trisomy 9 survived the longest (107 days) and had a karyotype of 47,XY,+9q- (Mace et al., 1978). Due to the presence of multisystem dysmorphism in their fetuses, 24 pregnant women chose to undergo induced abortion; 16 infants with complete trisomy 9 died shortly after birth, without passing through the neonatal period and 11 fetuses died in utero. Complete trisomy 9 has a lethal prognosis (Table 1).
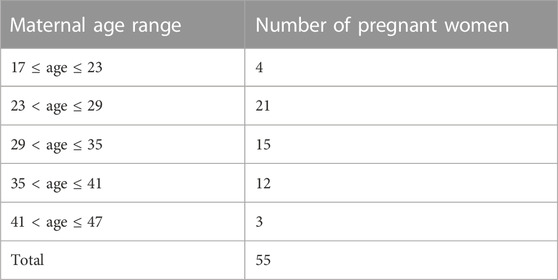
As shown in Table 2, the highest proportion of pregnant women were aged 23–29 years (21 cases). Only 16 of these pregnant women were aged ≥35 years old. Among the cases reported in the literature, it is worth noting that 39 instances of trisomy 9 were documented in mothers who were younger than 35 years old, rather than in those of advanced maternal age. This observation suggests that the occurrence of trisomy 9 does not appear to be significantly correlated with maternal age.
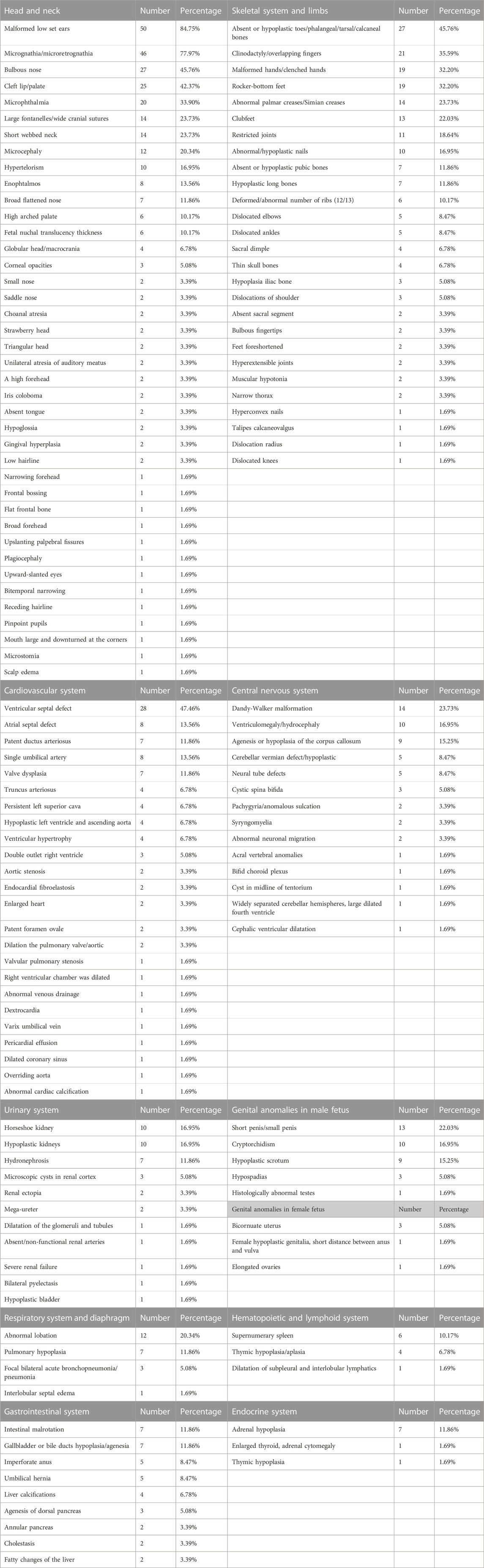
Fetuses with complete trisomy 9 exhibit anomalies in multiple systems, as summarized in Table 3. Notably, head and face abnormalities are the most prominent features. These included malformed low-set ears (50 cases, 84.75%), micrognathia/microretrognathia (45 cases, 77.97%), a bulbous nose (27 cases, 45.76%), cleft lip/palate (25 cases, 42.37%), and microphthalmia (20 cases, 33.90%). Among the cardiovascular system malformations, ventricular septal defects (28 cases, 47.46%) were the most frequently observed. In terms of the skeletal system and limbs, absent or hypoplastic toes/phalangeal/tarsal/calcaneal bones (27 cases, 45.76%), overlapping fingers (21 cases, 35.59%), malformed hands/clenched hands (19 cases, 32.20%), and rocker-bottom feet (19 cases, 32.20%) were the most common abnormalities. Dandy-Walker malformation (14 cases, 23.73%) was the most frequent malformation in the central nervous system, while horseshoe kidney (10 cases, 16.95%) and hypoplastic kidneys (10 cases, 16.95%) were the most common malformations in the urinary system. In male and female fetuses, the most frequent malformations were short penis/small penis (13 cases, 22.03%) and bicornuate uterus (3 cases, 5.08%), respectively. Abnormal lobation (12 cases, 20.34%) was the most common malformation in the respiratory system and diaphragm. A supernumerary spleen (6 cases, 10.17%) was the most frequently observed malformation in the hematopoietic and lymphoid system. For the gastrointestinal system, intestinal malrotation (7 cases, 11.86%) and gallbladder or bile duct hypoplasia/agenesia (7 cases, 11.86%) were the most frequent malformations. Lastly, adrenal hypoplasia (7 cases, 11.86%) was the most common malformation in the endocrine system. Fifty-nine cases with malformations in various systems had malformation rates of more than 30% for malformed low set ears, micrognathia/microretrognathia, a bulbous nose, cleft lip/palate, microphthalmia, absent or hypoplastic toes/phalangeal/tarsal/calcaneal bones, clinodactyly/overlapping finger, malformed hands/clenched hands, rocker-bottom feet, and ventricular septal defect.
Severe fetal malformations are typically identified during the second or third trimester of pregnancy or after birth. The head and neck regions are the most commonly affected areas by these anomalies (Table 3). Although these studies show the rate of malformation in each system, many of them did not perform further autopsy or did not observe the phenotypes in each system. It is possible that statistically significant abnormal phenotypes are easier to observe. There were six cases of thickened NT. Previous ultrasound technology was not advanced; therefore, in many cases, NT was not measured rather than NT not being thickened. In addition, many studies were not described in detail. For example, some studies mentioned cardiac abnormalities, among which only congenital heart disease was mentioned without being described in detail (Benacerraf et al., 1992; Seller et al., 1998; Zhang et al., 2021).
Based on the common malformations associated with trisomy 9 syndrome mentioned earlier, targeted sonographic examinations can be conducted to detect multiple abnormalities. These include microcephaly, Dandy-Walker malformation, abnormal facial features (such as malformed low-set ears, a hypoplastic nose, micrognathia, and cleft lip/palate), congenital heart defects (such as ventricular septal defect, atrial septal defect, cardiomegaly, double outlet right ventricle, and valvular pulmonary stenosis), abnormal limbs (such as malformed hands and clubfeet), and a single umbilical artery.
It is important to note that a morphological description alone cannot replace the diagnosis of complete trisomy 9 through karyotype analysis. However, if some of the characteristic features of trisomy 9 mentioned here are observed, this syndrome should be suspected. While 59 cases of complete trisomy 9 have been reported in the literature, it is encouraged to continue reporting new findings to further enhance our understanding of the morphological characteristics across various systems in fetuses with this syndrome.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Review Committee of the Boai Hospital of Zhongshan (KY-2023-004-47). The patients provided their written informed consent to participate in this study.
Author contributions
Conceptualization and methodology, CX; formal analysis and investigation, DW, GZ, and ML; resources, YZ; data curation, JP; writing—original draft preparation, CX; writing—review and editing, DW, ML, and HL; supervision, CX and DW. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FX declared a shared affiliation with the author DW to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2023.1241245/full#supplementary-material
References
AnneréN, G., and Sedin, G. (1981). Case report. Trisomy 9 syndrome. Acta Paediatr. Scand. 70, 125–128. doi:10.1111/j.1651-2227.1981.tb07185.x
Arnold, G. L., Kirby, R. S., Stern, T. P., and Sawyer, J. R. (1995). Trisomy 9: review and report of two new cases. Am. J. Med. Genet. 56, 252–257. doi:10.1002/ajmg.1320560303
Ben Slama, S., Ouertani, I., Dimassi, K., Bacha, D., Lahmar, A., and Mzabi, S. (2016). Complete Trisomy 9 with unusual phenotypic associations. Tunis. Med. 94, 895.
Benacerraf, B. R., Pauker, S., Quade, B. J., and Bieber, F. R. (1992). Prenatal sonography in trisomy 9. Prenat. Diagn 12, 175–181. doi:10.1002/pd.1970120306
Carpenter, B. F., and Tomkins, D. J. (1982). The trisomy 9-syndrome. Perspect. Pediatr. Pathol. 7, 109–120.
Chen, C. P., Chern, S. R., Cheng, S. J., Chang, T. Y., Yeh, L. F., Lee, C. C., et al. (2004). Second-trimester diagnosis of complete trisomy 9 associated with abnormal maternal serum screen results, open sacral spina bifida and congenital diaphragmatic hernia, and review of the literature. Prenat. Diagn 24, 455–462. doi:10.1002/pd.900
Chen, C. P., Ko, T. M., Chen, S. W., Chern, S. R., Wu, F. T., Pan, Y. T., et al. (2023a). Low-level mosaic trisomy 9 at amniocentesis associated with a positive non-invasive prenatal testing for trisomy 9, maternal uniparental disomy 9, intrauterine growth restriction and a favorable fetal outcome in a pregnancy. Taiwan J. Obstet. Gynecol. 62, 457–460. doi:10.1016/j.tjog.2023.03.008
Chen, C. P., Lai, T. H., Chen, S. W., Chern, S. R., Wu, F. T., Wu, P. S., et al. (2023b). Low-level mosaic trisomy 9 at amniocentesis in a pregnancy associated with a favorable fetal outcome, intrauterine growth restriction, cytogenetic discrepancy between cultured amniocytes and uncultured amniocytes and perinatal progressive decrease of the aneuploid cell line. Taiwan J. Obstet. Gynecol. 62, 461–465. doi:10.1016/j.tjog.2023.03.009
Chitayat, D., Hodgkinson, K., Luke, A., Winsor, E., Rose, T., and Kalousek, D. (1995). Prenatal diagnosis and fetopathological findings in five fetuses with trisomy 9. Am. J. Med. Genet. 56, 247–251. doi:10.1002/ajmg.1320560302
Delicado, A., IñIGUEZ, L., Lopez Pajares, I., and Omeñaca, F. (1985). Complete trisomy 9. Two additional cases. Ann. Genet. 28, 63–66.
Feingold, M., and Atkins, L. (1973). A case of trisomy 9. J. Med. Genet. 10, 184–187. doi:10.1136/jmg.10.2.184
Ferreres, J. C., Planas, S., MartíNEZ-SáEZ, E. A., Vendrell, T., Peg, V., Salcedo, M. T., et al. (2008). Pathological findings in the complete trisomy 9 syndrome: three case reports and review of the literature. Pediatr. Dev. Pathol. 11, 23–29. doi:10.2350/06-08-0143.1
Francke, U., Benirschke, K., and Jones, O. W. (1975). Prenatal diagnosis of trisomy 9. Humangenetik 29, 243–250. doi:10.1007/BF00297630
Frohlich, G. S. (1982). Delineation of trisomy 9. J. Med. Genet. 19, 316–317. doi:10.1136/jmg.19.4.316-a
Fuma, K., Kotani, T., Nakamura, N., Ushida, T., and Kajiyama, H. (2022). Severe congenital diaphragmatic hernia with trisomy 9: a case report and review of the literature. Cureus 14, e28395. doi:10.7759/cureus.28395
Golden, J. A., and Schoene, W. C. (1993). Central nervous system malformations in trisomy 9. J. Neuropathol. Exp. Neurol. 52, 71–77. doi:10.1097/00005072-199301000-00009
Kannan, T. P., Hemlatha, S., Ankathil, R., and Zilfalil, B. A. (2009). Clinical manifestations in trisomy 9. Indian J. Pediatr. 76, 745–746. doi:10.1007/s12098-009-0158-2
Khan, A. H., Khilji, Z., Azim, M., and Khurshid, M. (2001). Trisomy 9: a case report. J. Pak Med. Assoc. 51, 128–130.
Khoury-Collado, F., Anderson, V. M., Haas, B. R., Fisher, A. J., Bombard, A. T., and Weiner, Z. (2004). Trisomy 9 screened positive for trisomy 18 by maternal serum screening. Prenat. Diagn 24, 836–838. doi:10.1002/pd.857
Kor-Anantakul, O., Suwanrath, C., Kanngurn, S., Rujirabanjerd, S., Suntharasaj, T., and Pinjaroen, S. (2006). Prenatal diagnosis of complete trisomy 9: a case report and review of the literature. Am. J. Perinatol. 23, 131–135. doi:10.1055/s-2006-931804
Kurnick, J., Atkins, L., Feingold, M., Hills, J., and Dvorak, A. (1974). Trisomy 9: predominance of cardiovascular, liver, brain, and skeletal anomalies in the first diagnosed case. Hum. Pathol. J. 5, 223–232. doi:10.1016/s0046-8177(74)80068-7
Lam, Y. H., Lee, C. P., and Tang, M. H. (1998). Low second-trimester maternal serum human chorionic gonadotrophin in a trisomy 9 pregnancy. Prenat. Diagn 18, 1212. doi:10.1002/(sici)1097-0223(199811)18:11<1212:aid-pd430>3.0.co;2-0
Li, M., Glass, J., Du, X., Dubbs, H., Harr, M. H., Falk, M., et al. (2021). Trisomy 9 mosaic syndrome: sixteen additional patients with new and/or less commonly reported features, literature review, and suggested clinical guidelines. Am. J. Med. Genet. A 185, 2374–2383. doi:10.1002/ajmg.a.62251
Li, H., Lu, L., Yao, Y., Gao, T., Jiang, Y., Zhang, C., et al. (2022). Perinatal outcomes of prenatal cases testing positive for trisomy 9 by noninvasive prenatal testing. Taiwan J. Obstet. Gynecol. 61, 965–970. doi:10.1016/j.tjog.2022.07.006
Lim, J. E., Jeong, Y. A., Cho, G. J., Shin, J. H., Oh, M. J., and Kim, H. J. (2006). A complete trisomy 9 associated with abnormal triple screening result. J. Med. Screen 13, 108. doi:10.1258/096914106777589597
Ma, N., Zhu, Z., Hu, J., Pang, J., Yang, S., Liu, J., et al. (2023). Case report: detection of fetal trisomy 9 mosaicism by multiple genetic testing methods: report of two cases. Front. Genet. 14, 1121121. doi:10.3389/fgene.2023.1121121
Mace, S. E., Macintyre, M. N., Turk, K. B., and Johnson, W. E. (1978). The trisomy 9 syndrome: multiple congenital anomalies and unusual pathological findings. J. Pediatr. 92, 446–448. doi:10.1016/s0022-3476(78)80444-2
Mantagos, S., Mcreynolds, J. W., Seashore, M. R., and Breg, W. R. (1981). Complete trisomy 9 in two liveborn infants. J. Med. Genet. 18, 377–382. doi:10.1136/jmg.18.5.377
Marino, B., Digilio, M. C., Giannotti, A., and Dallapiccola, B. (1989). Atrioventricular canal associated with trisomy 9. Chest 96, 1420–1421. doi:10.1378/chest.96.6.1420
Mcduffie, R. S. (1994). Complete trisomy 9: case report with ultrasound findings. Am. J. Perinatol. 11, 80–84. doi:10.1055/s-2007-994561
Murta, C., Moron, A., Avila, M., França, L., and Vargas, P. (2000). Reverse flow in the umbilical vein in a case of trisomy 9. Ultrasound Obstet. Gynecol. 16, 575–577. doi:10.1046/j.1469-0705.2000.00280.x
Nakagawa, M., Hashimoto, K., Ohira, H., Hamanaka, T., Ozaki, M., and Suehara, N. (2006). Prenatal diagnosis of trisomy 9. Fetal Diagn Ther. 21, 68–71. doi:10.1159/000089051
Perez, M. J., Schneider, A., Chaze, A. M., Bigi, N., Lefort, G., Rouleau, C., et al. (2009). Epiphyseal punctate calcifications (stippling) in complete trisomy 9. Prenat. Diagn 29, 1085–1088. doi:10.1002/pd.2350
Pinette, M. G., Pan, Y., Chard, R., Pinette, S. G., and Blackstone, J. (1998). Prenatal diagnosis of nonmosaic trisomy 9 and related ultrasound findings at 11.7 weeks. J. Matern. Fetal Med. 7, 48–50. doi:10.1002/(SICI)1520-6661(199801/02)7:1<48:AID-MFM11>3.0.CO;2-H
Pruksanusak, N., Rujirabanjerd, S., Kanjanapradit, K., Kor-anantakul, O., Suntharasaj, T., Suwanrath, C., et al. (2014). Prenatal diagnosis of complete trisomy 9 with a novel sonographic finding of heart calcification. J. Ultrasound Med. 33, 1871–1873. doi:10.7863/ultra.33.10.1871
Quigg, M. H., Diment, S., and Roberson, J. (2005). Second-trimester diagnosis of trisomy 9 associated with abnormal maternal serum screening results. Prenat. Diagn 25, 966–967. doi:10.1002/pd.1278
Raffi, F., Geneix, A., Satge, D., Fallouh, B., Brunerie, A. M., Lemery, D., et al. (1992). [Complete and homogenous trisomy 9 detected in utero]. J. Gynecol. Obstet. Biol. Reprod. Paris. 21, 251–254.
Romain, D. R., and Sullivan, J. (1983). Delineation of trisomy 9 syndrome. J. Med. Genet. 20, 156–157. doi:10.1136/jmg.20.2.156
Roshanfekr, D., Dahl-Lyons, C., Pressman, E., Ural, S., and Blakemore, K. (1998). Complete trisomy 9 in a term fetus: a case report. J. Matern. Fetal Med. 7, 247–249. doi:10.1002/(SICI)1520-6661(199809/10)7:5<247:AID-MFM8>3.0.CO;2-1
Sandoval, R., Sepulveda, W., Gutierrez, J., Be, C., and Altieri, E. (1999). Prenatal diagnosis of nonmosaic trisomy 9 in a fetus with severe renal disease. Gynecol. Obstet. Invest. 48, 69–72. doi:10.1159/000010138
Saneto, R. P., Applegate, K. E., and Frankel, D. G. (1998). Atypical manifestations of two cases of trisomy 9 syndrome: rethinking development delay. Am. J. Med. Genet. 80, 42–45. doi:10.1002/(sici)1096-8628(19981102)80:1<42:aid-ajmg7>3.0.co;2-s
Satge, D., Gasser, B., Geneix, A., Malet, P., and Stoll, C. (1994). Hepatic calcifications in a fetus with trisomy 9 that underwent cordocentesis. Prenat. Diagn 14, 303–306. doi:10.1002/pd.1970140411
Saura, R., Traore, W., Taine, L., Wen, Z. Q., Roux, D., Maugey-Laulom, B., et al. (1995). Prenatal diagnosis of trisomy 9. Six cases and a review of the literature. Prenat. Diagn 15, 609–614. doi:10.1002/pd.1970150704
Seabright, M., Gregson, N., and Mould, S. (1976). Trisomy 9 associated with an enlarged 9qh segment in a liveborn. Hum. Genet. 34, 323–325. doi:10.1007/BF00295299
Seller, M. J., Bergbaum, A., and Daker, M. G. (1998). Trisomy 9 in an embryo with spina bifida. Clin. Dysmorphol. 7, 217–219. doi:10.1097/00019605-199807000-00012
Sepulveda, W., Wimalasundera, R. C., Taylor, M. J., Blunt, S., Be, C., and De La Fuente, S. (2003). Prenatal ultrasound findings in complete trisomy 9. Ultrasound Obstet. Gynecol. 22, 479–483. doi:10.1002/uog.233
Stevenson, D. A., Low, J., King, J., Opitz, J. M., and Miller, M. E. (2004). Pseudoaminopterin syndrome and trisomy 9. Am. J. Med. Genet. A 128a, 217–218. doi:10.1002/ajmg.a.30044
Sutherland, G. R., Carter, R. F., and Morris, L. L. (1976). Partial and complete trisomy 9: delineation of a trisomy 9 syndrome. Hum. Genet. 32, 133–140. doi:10.1007/BF00291495
Suzumori, N., Sato, T., Okada, J., Nakanishi, T., Shirai, K., Tanemura, M., et al. (2003). Prenatal findings for complete trisomy 9. Prenat. Diagn 23, 866–868. doi:10.1002/pd.704
Tang, H. S., Wang, D. G., Huang, L. Y., and Li, D. Z. (2019). Chromosomal microarray analysis detects trisomy 9 mosaicism in a prenatal case not revealed by conventional cytogenetic analysis of cord blood. J. Obstet. Gynaecol. 39, 123–125. doi:10.1080/01443615.2018.1439905
Tonni, G., and Grisolia, G. (2013). Ultrasound diagnosis of central nervous system anomalies (bifid choroid plexus, ventriculomegaly, dandy-walker malformation) associated with multicystic dysplastic kidney disease in a trisomy 9 fetus: case report with literature review. J. Clin. Ultrasound 41, 441–447. doi:10.1002/jcu.21999
Tonni, G., Lituania, M., Chitayat, D., Bonasoni, M. P., Keating, S., Thompson, M., et al. (2014). Complete trisomy 9 with unusual phenotypic associations: dandy-walker malformation, cleft lip and cleft palate, cardiovascular abnormalities. Taiwan J. Obstet. Gynecol. 53, 592–597. doi:10.1016/j.tjog.2014.01.005
Williams, T., Zardawi, I., Quaife, R., and Young, I. D. (1985). Complex cardiac malformation in a case of trisomy 9. J. Med. Genet. 22, 230–233. doi:10.1136/jmg.22.3.230
Yeo, L., Waldron, R., Lashley, S., Day-Salvatore, D., and Vintzileos, A. M. (2003). Prenatal sonographic findings associated with nonmosaic trisomy 9 and literature review. J. Ultrasound Med. 22, 425–430. doi:10.7863/jum.2003.22.4.425
Zhang, L., Hu, T., Zhu, H., Huang, M., Bao, Y., Wang, L., et al. (2021). The genome of Nautilus pompilius illuminates eye evolution and biomineralization. Chin. J. Med. Genet. 38, 927–938. doi:10.1038/s41559-021-01448-6
Keywords: aneuploid, complete trisomy 9, chromosomal disorder, prenatal diagnosis, genetic counseling
Citation: Xu C, Li M, Peng J, Zhang Y, Li H, Zheng G and Wang D (2023) Case report: A case report and literature review of complete trisomy 9. Front. Genet. 14:1241245. doi: 10.3389/fgene.2023.1241245
Received: 16 June 2023; Accepted: 08 August 2023;
Published: 31 August 2023.
Edited by:
Yongchu Pan, Nanjing Medical University, ChinaReviewed by:
Nadja Kokalj Vokač, UKC MB, SloveniaPei-Song Chen, Sun Yat-sen University, China
Fu Xiong, Southern Medical University, China
Copyright © 2023 Xu, Li, Peng, Zhang, Li, Zheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Degang Wang, d2RnMDMyMUAxNjMuY29t
†These authors have contributed equally to this work
 Chenxia Xu
Chenxia Xu Miaoyuan Li2,3†
Miaoyuan Li2,3† Haijun Li
Haijun Li Degang Wang
Degang Wang