- 1Department of Gynecology, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Department of Gynecology, The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Luzhou, China
Background: With the increasing number of new cancer cases and mortality rates, cancer has become a serious global health problem, but there are no ideal cancer biomarkers for effective diagnosis. Currently, mounting evidence demonstrates that lncRNAs play a fundamental role in cancer progression. BBOX1 anti-sense RNA 1 (BBOX1-AS1) is a recently clarified lncRNA and has been identified as dysregulated in various carcinomas, and it contributes to poor survival in cancer patients.
Methods: We thoroughly searched six databases for eligible articles published as of 27, April 2023. The association of BBOX1-AS1 expression levels with prognostic and clinicopathological parameters was assessed by odds ratios (OR) and hazard ratios with 95% CIs. Additionally, we further validated our results utilizing the GEPIA online database.
Results: Eight studies comprising 602 patients were included in this analysis. High BBOX1-AS1 expression indicated poor overall survival (OS) (hazard ratios = 2.30, 95% Cl [1.99, 2.67], p < 0.00001) when compared with low BBOX1-AS1 expression. Furthermore, BBOX1-AS1 expression was positively correlated with lymph node metastasis (OR = 3.00, 95% CI [1.71–5.28], p = 0.0001) and advanced tumor stage (OR = 3.74, 95% CI [2.63–5.32], p < 0.00001) for cancer patients. Moreover, BBOX1-AS1 was remarkably upregulated in 12 malignancies, and the elevated BBOX1-AS1 expression predicted poorer OS and worse disease-free survival (DFS) confirmed through the GEPIA online gene analysis tool.
Conclusion: The findings highlight that BBOX1-AS1 was significantly associated with detrimental overall survival, disease-free survival, lymph node metastasis and tumor stage; thus, it could act as a novel promising biomarker to predict the clinicopathological characteristics and prognosis for various cancers.
Introduction
With rising morbidity and mortality, cancer has become a serious global health concern, and an additional economic burden is attributable to improving cancer survival worldwide (Ward et al., 2021). The GLOBOCAN 2020 estimated there were nearly 19.3 million new malignant tumors patients and 10.0 million deaths in 2020. Besides, approximately 28.4 million cases will be diagnosed in 2040 (Sung et al., 2021). Currently, cancer patients with lymph node metastasis or advanced tumor stage exhibit a poor prognosis. However, no ideal cancer biomarker can diagnose cancer effectively (Jayanthi et al., 2017). Therefore, a novel biological target capable of early detection and prediction prognosis is urgently required.
Long non-coding RNAs (lncRNAs) are defined as RNAs with longer than 200 nucleotides that lack protein coding potential (Engreitz et al., 2016). Additionally, with the application of nascent transcriptomics technology, abundant molecular mechanisms of lncRNAs have been identified (Nojima and Proudfoot, 2022). Mounting evidence demonstrates that lncRNAs play an indispensable role in cancer progression, tumorigenesis, and immune responses (Bhan et al., 2017; Ebrahimi et al., 2022; Park et al., 2022). For instance, increased lncRNA H19 expression is notably associated with elevated metastasis rates, decreased therapeutic sensitivity and enhanced cancer progression via modulation of numerous targets and molecular pathways, such as RUNX1, STAT3, β-catenin, and FOXM1 in various cancers (Hashemi et al., 2022). Furthermore, high lncRNA KCNQ1OT1 expression correlates with worse overall survival (OS) and affects the CD8+ T-cell responses in colorectal tumors (Lin et al., 2021). Therefore, lncRNAs could be developed as novel targets for various cancer diagnostics and drug discovery.
BBOX1 anti-sense RNA 1 (BBOX1-AS1) is a recently clarified lncRNA and has been identified to be dysregulated in several carcinomas, which include lung cancer (Shi et al., 2021; Zhang et al., 2022), cervical cancer (Xu et al., 2020), oral squamous cell carcinoma (OSCC) (Zhao et al., 2022), ovarian cancer (Yao et al., 2021), colorectal cancer (Liu et al., 2022; Shi et al., 2022), nasopharyngeal carcinoma (Jiang et al., 2021), hepatocellular carcinoma (Tao et al., 2021), pituitary adenoma (Wu et al., 2022), and esophageal squamous cell carcinoma (ESCC) (Sheng et al., 2022). Clinically, high BBOX1-AS1 expression is closely associated with poor OS and clinicopathological characteristics, such as tumor stage, lymph node metastasis, tumor size, and differentiation in various malignancies (Lian et al., 2021; Tao et al., 2021; Sheng et al., 2022; Shi et al., 2022). Moreover, BBOX1-AS1 contributes to tumor cell metastasis and proliferation, along with reduces cell apoptosis and ferroptosis via various mechanisms (Pan et al., 2022; Zhang et al., 2022). However, owing to the limited clinical patient sample size, the effect of this relevance is poorly characterized. Thus, we conducted this meta-analysis to evaluate the application of BBOX1-AS1 in the prognosis of various malignancies.
Materials and methods
Search strategy
We thoroughly searched six databases, which included SinoMed, Web of Science, EBSCO, Cochrane Library, PubMed, and Ovid for eligible articles published up as of 27 April, 2023. The following search keywords were adopted to identify relevant publications: “long non-coding RNA BBOX1-AS1” OR “BBOX1 anti-sense RNA 1” OR “BBOX1-AS1” OR “lncRNA BBOX1-AS1.” Furthermore, the references in these retrieved articles were also manually searched to examine potentially eligible studies. Only manuscripts written in English or Chinese were included.
Inclusion and exclusion criteria
Inclusion criteria: 1) BBOX1-AS1 expression was identified in cancerous tissues and corresponding non-cancerous tissues; 2) all patients did not receive preoperative anticancer treatment; 3) the approach of detecting BBOX1-AS1 expression was qRT-PCR; 4) studies reported clinicopathological characteristics or prognostic outcomes such as OS, disease-free survival (DFS), and Kaplan–Meier (K-M) curves to calculate the hazard ratios (HR) (95% CI) indirectly; and 5) patients were divided into high- and low-expression groups, according to BBOX1-AS1 expression levels.
Exclusion criteria: 1) studies absent of clinicopathological characteristics or prognostic outcomes; 2) duplicated articles; 3) case reports, review articles, meta-analysis, editorials, non-human studies and conference reports; and 4) studies based on the public database.
Data extraction and quality assessment
After reviewing all the eligible articles, two investigators (GYL and YZW) independently extracted the requisite data, and all disagreements were resolved by discussing with two additional reviewers (LD and TY). The necessary information was carefully extracted from each study: author’s last name, publication year, cancer types, total number of patients, the number of patients in the high- and low-BBOX1-AS1 expression groups, detection methods, outcomes, the HR and corresponding 95% CIs for OS, follow-up time, and extraction methods for OS data. The HR (95% CI) for OS was calculated using the Engauge Digitizer 4.1 software indirectly through K-M curves if the eligible articles failed to offer the HR (95% CI) directly (Tierney et al., 2007). If both univariate and multivariate methods were performed in analyzing the data, the latter was preferred.
Validation of bioinformatics database
We compared the differences between BBOX1-AS1 expression in various human cancerous and non-cancerous tissues. Furthermore, we validated its prognostic value utilizing the GEPIA online database based on the TCGA and GTEx data (http://gepia.cancer-pku.cn/) (Tang et al., 2017; Li et al., 2021). Survival plots of the association with BBOX1-AS1 expression and DFS or OS were displayed as K-M curves derived from different cancer data sets. The median was established for the cut-off value. All p-value <0.05 was defined as statistically significant.
Statistical analysis
Data management was conducted with the EndNote 20.2 software. All analysis were carried out with the Review Manager 5.3 software. The effect of BBOX1-AS1 levels on OS, tumor differentiation, lymph node metastasis, tumor stage, tumor size, patient age, and gender were assessed by odds ratio (OR) and HRs with 95% CIs. Cochrane Higgins I2 statistics was applied to calculate heterogeneity among all included studies. The fixed-effects model was used for data analysis if heterogeneity was absent (I2< 50%). Otherwise, the more appropriate random-effects model was adopted. A p-value <0.05 was considered to be statistically significant.
Results
Included articles
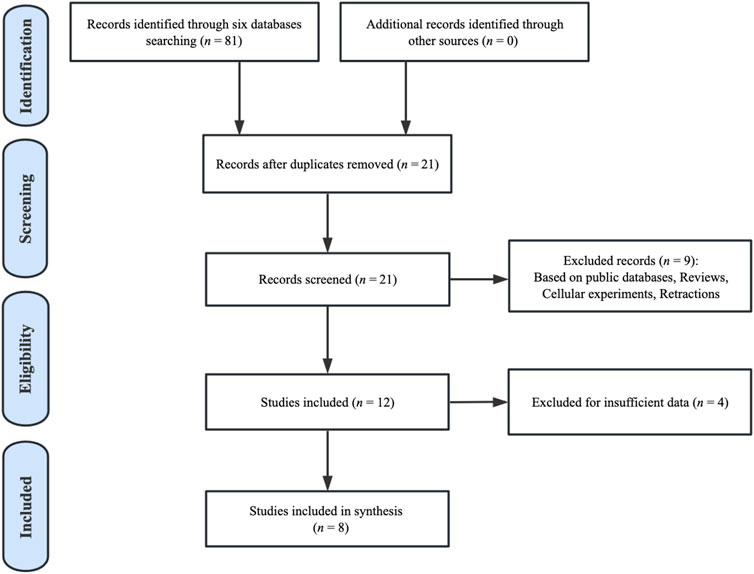
By searching six databases (up to 27 April 2023), a total of 81 publications concerning the prognosis of BBOX1-AS1 and cancer patients were retrieved preliminarily. Among the 81 pieces of literature, 60 duplicate studies were excluded and 21 studies were selected for abstract screening. Nine publications were excluded since they were retraction articles, cell-based experiments, reviews, or research studies based on public databases. Then, we carefully removed another four studies because they lacked sufficient data regarding clinicopathologic characteristics or prognosis for analysis. Finally, eight studies from 2020 to 2023 were considered eligible for the meta-analysis. The selection flowchart for the included publications is depicted in Figure 1.
Study characteristics
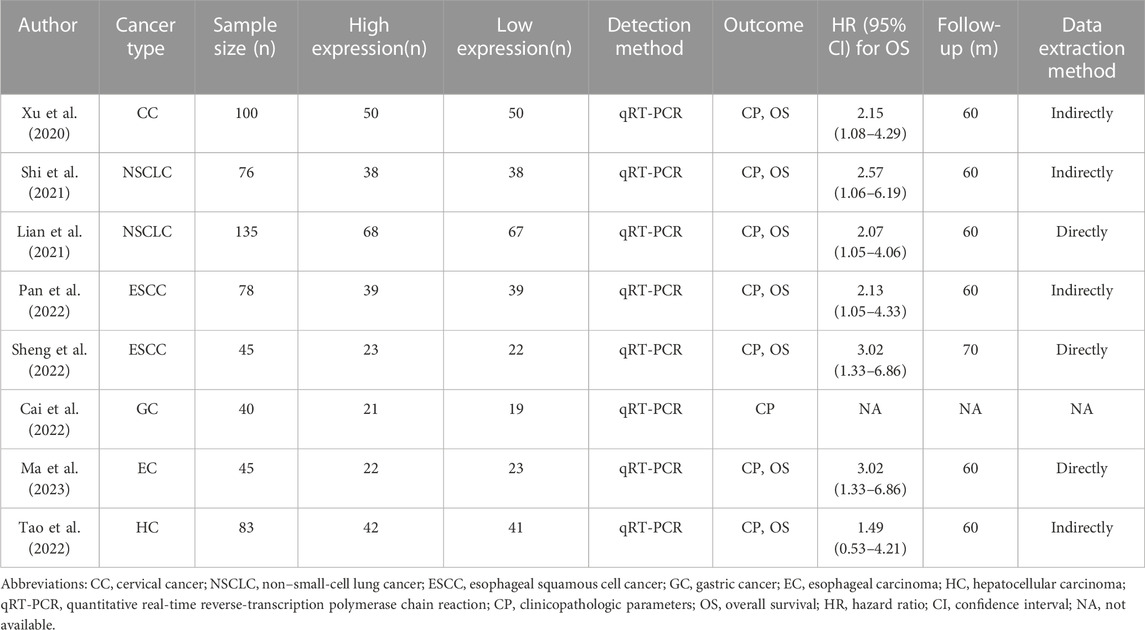
These studies were published between 2020 and 2023, and the sample sizes ranged from 40 to 135. A total of 602 patients were divided into the low- and high-BBOX1-AS1 expression groups, with 299 and 303 cases in each group, respectively. The studies were performed in different regions of China and six types of malignancies were examined: lung cancer, cervical cancer, ESCC, gastric cancer, esophageal carcinoma, and hepatocellular carcinoma. Among these included studies, seven estimated the correlation between BBOX1-AS1 and OS of patients. Table 1 summarizes the detailed characteristics of these included studies.
Association between BBOX1-AS1 expression and clinical covariates
This meta-analysis examined the association between BBOX1-AS1 expression and clinical covariates. The results revealed that BBOX1-AS1 overexpression was remarkably correlated with lymph node metastasis (I2 = 53%, p = 0.0001) (Figure 2A) and more advanced tumor stage (I2 = 2%, p < 0.00001) (Figure 2B). However, BBOX1-AS1 expression did not associate with tumor differentiation (I2 = 58%, p = 0.26) (Figure 2C) and patient gender (I2 = 0%, p = 0.77) (Figure 2D). Subsequently, as for patient age and tumor size, the meta-analysis was performed stratified by a cut-off values such as age ≥ 60 years (elder) versus <60 years (young) and tumor size ≥ 5 cm (large size) versus <5 cm (small size). The pooled results demonstrated high BBOX1-AS1 expression was not significantly associated with patient age (I2 = 0%, p = 0.99) (Figure 2E) and tumor size (I2 = 40%, p = 0.07) (Figure 2F).
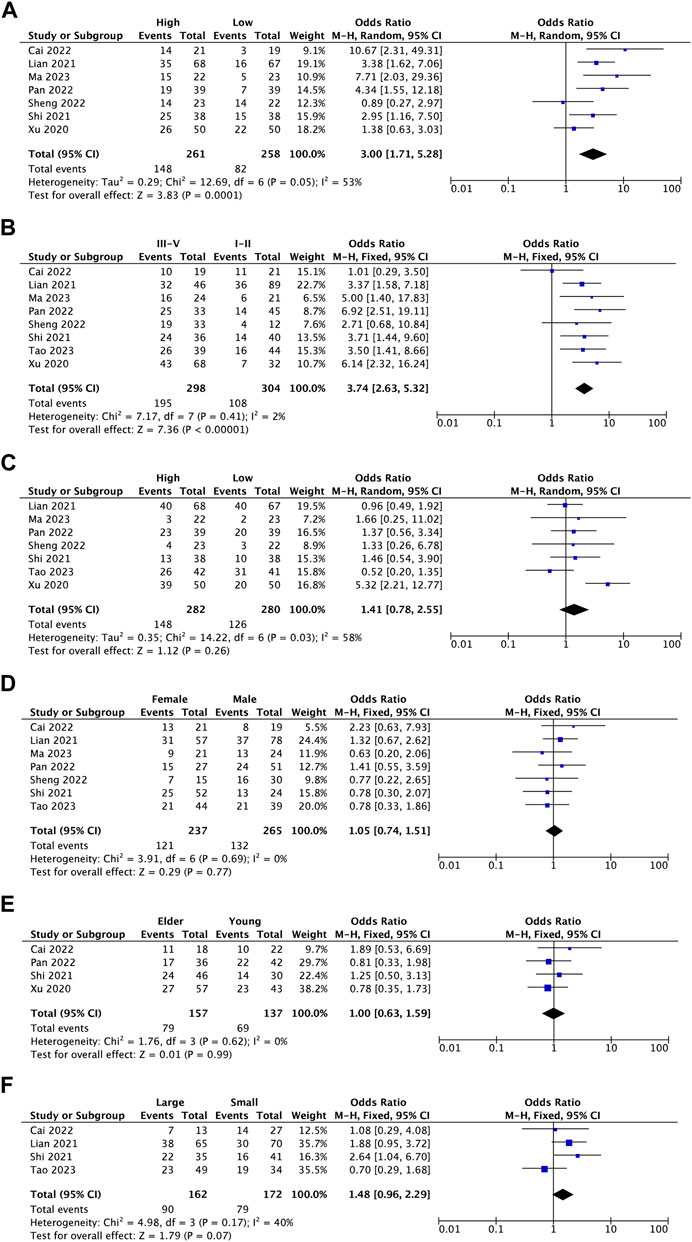
FIGURE 2. Forest plots assessing the association between BBOX1-AS1 expression and clinicopathological parameters [(A) lymph node metastasis, (B) stage, (C) differentiation, (D) gender, (E) age, and (F) tumor size].
Association between BBOX1-AS1 expression and overall survival
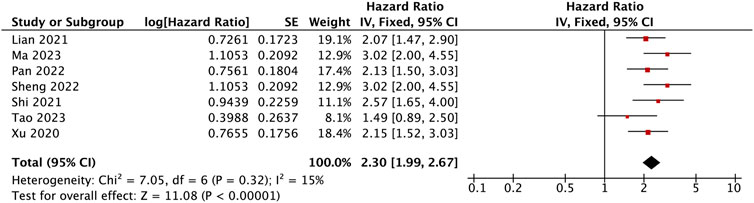
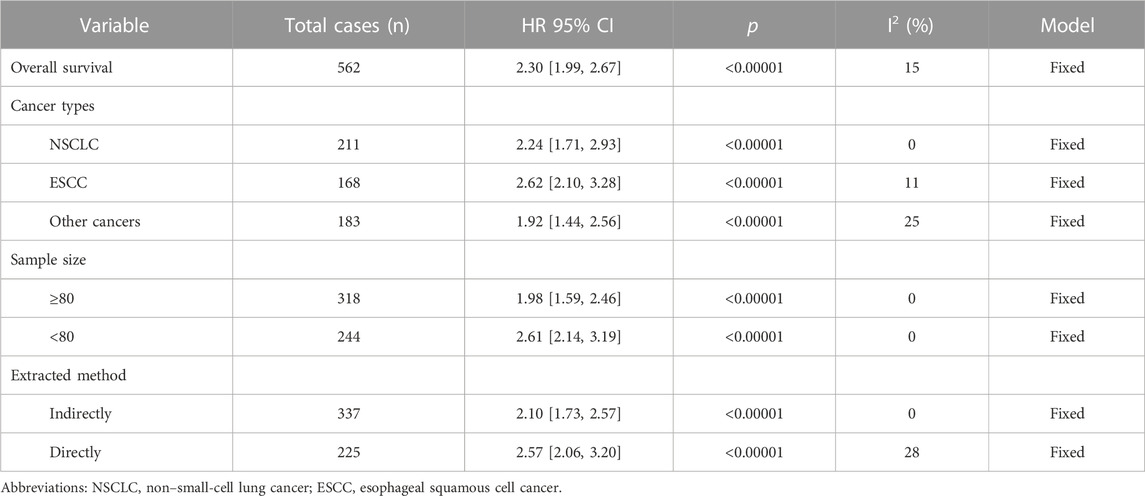
Seven studies with data from 562 patients reported the association between BBOX1-AS1 as a prognostic biomarker of malignancies and OS. Pooled HR was 2.30 (95% Cl: 1.99, 2.67, p < 0.00001). A fixed-effects model was applied to evaluate the HR of these studies because of low heterogeneity (I2 = 15%). The result suggested that the upregulation of BBOX1-AS1 predicted poor OS among various malignancies (Figure 3). Furthermore, the subgroup analysis was carried out, based on cancer types, sample sizes, and extracted methods. As presented in Table 2, when compared with low BBOX1-AS1 expression in these subgroup analysis, high BBOX1-AS1 expression revealed poor OS in cancer patients, regardless of the cancer types, sample sizes, and extracted methods (p < 0.00001). A fixed-effects model was utilized since all these stratified analysis manifested low or no heterogeneity.
Validation of results based on TCGA
To further validate the significance of BBOX1-AS1 in diverse cancers, the GEPIA online gene analysis tool was utilized. Figure 4 shows that the overexpression of BBOX1-AS1 was identified in 12 malignancies. In addition, the connection between BBOX1-AS1 expression and prognosis with various malignancies was verified with K-M curves. Similar to our meta-analysis, the upregulated BBOX1-AS1 expression was dramatically correlated to a poorer OS in three malignancies, which included brain lower grade glioma (LGG), mesothelioma (MESO), and breast invasive carcinoma (BRCA) (p < 0.05) (Figures 5A–C). Besides, the overexpression of BBOX1-AS1 was negatively related to a poorer DFS in LGG, MESO, and sarcoma (p < 0.05) (Figures 5D–F).
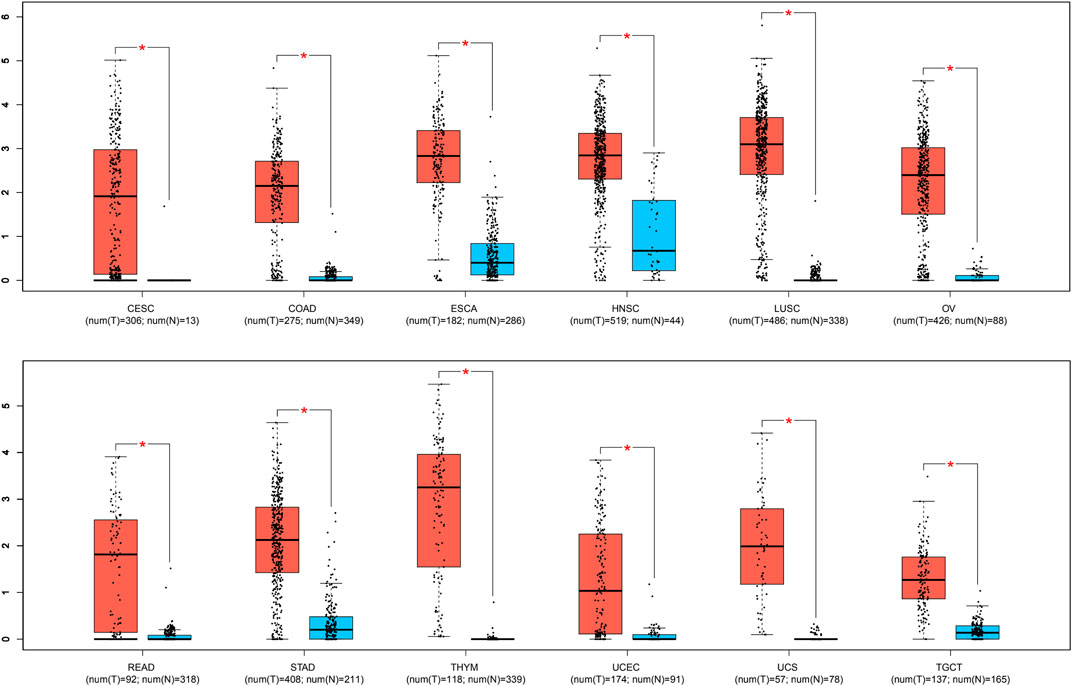
FIGURE 4. BBOX1-AS1 expression in 12 types of tumor tissues (red) vs. normal tissues (blue). “*” p < 0.01. Abbreviations: T, tumor tissues; N, normal tissues; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, colon adenocarcinoma; ESCA, esophageal carcinoma; HNSC, head and neck squamous cell carcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; READ, rectum adenocarcinoma; STAD, stomach adenocarcinoma; THYM, thymoma, UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; TGCT, testicular germ cell tumors.
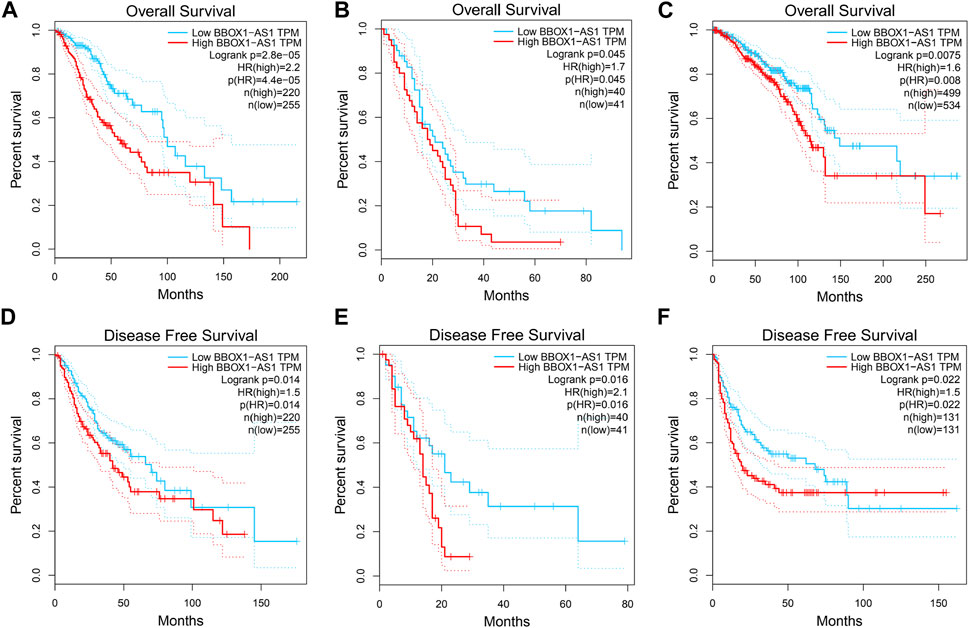
FIGURE 5. Verification of the prognostic value of BBOX1-AS1 in the TCGA database. (A) OS plots of BBOX1-AS1 in LGG, (B) OS plots of BBOX1-AS1 in MESO, (C) OS plots of BBOX1-AS1 in BRCA, (D) DFS plots of BBOX1-AS1 in LGG, (E) DFS plots of BBOX1-AS1 in MESO, and (F) DFS plots of BBOX1-AS1 in SARC. Abbreviations: TCGA, The Cancer Genome Atlas; LGG, brain lower grade glioma; MESO, mesothelioma; BRCA, breast invasive carcinoma; SARC, sarcoma.
Discussion
Recently, a burgeoning number of studies discovered that lncRNAs were dysregulated in diverse human malignancies. Many may act as novel potential markers for early diagnosis, therapy, and prediction of prognosis in cancers. Hence, in recent years, numerous reviews, clinical trials, basic experiments, and systematic review publications, such as our meta-analysis, have been performed to gradually demonstrate the correlation between lncRNAs and diverse tumor prognoses (Shen et al., 2018; Tan et al., 2021; Li et al., 2022). Furthermore, numerous previous studies investigated that BBOX1-AS1 may serve as a novel lncRNA to advance the development of cancers through various biological mechanisms including cell migration, invasion, proliferation, and apoptosis inhibition (Liao et al., 2020; Xu et al., 2020; Shi et al., 2021). Moreover, elevated BBOX1-AS1 may negatively interact with the miRNAs expression, such as miR-27a-5p (Shi et al., 2021), miR-361-3p (Xu et al., 2020; Lian et al., 2021; Yao et al., 2021; Wu et al., 2022), miR-3940-3p (Jiang et al., 2021; Liu et al., 2022; Zhao et al., 2022), miR-513a-3p (Pan et al., 2022), and miR-125b(a)-5p (Wang et al., 2021) in multiple cancers. For example, Sheng et al. (2022) proved that the upregulation of BBOX1-AS1 could accelerate ESCC progression by activating the Wnt/β-catenin pathway via promoting HOXB7 expression. In the respiratory system, Shi et al. (2021) reported that BBOX1-AS1 played tumor-promoting roles in non–small-cell lung cancer by activating the MELK/FAK signaling axis. In addition, BBOX1-AS1 could boost cell migration, proliferation, and the malignant phenotype by upregulating LAMC2 expression in OSCC (Zhao et al., 2022). Collectively, these studies demonstrated that BBOX1-AS1 acted a vital role in human malignancies' development and progression. Nevertheless, the prognostic effect of BBOX1-AS1 in clinical application was limited by the small sample capacity. To the best of our knowledge, no meta-analysis has been performed to investigate the effects of BBOX1-AS1 expression on the prognosis of patients with malignancy.
In this meta-analysis, we identified the relevance between BBOX1-AS1 expression and human malignancies’ prognostic parameters. The results illustrated that an increase in BBOX1-AS1 was dramatically related to unfavorable OS (HR = 2.30, 95% CI: 1.99–2.67, p < 0.00001). Furthermore, subgroup analysis demonstrated the same result despite cancer types, sample sizes, and extracted methods. Additionally, high BBOX1-AS1 expression significantly was connected with lymph node metastasis (OR = 3.00, 95% CI: 1.71–5.28, p = 0.0001) and more advanced tumor stage (OR = 3.74, 95% CI: 2.63–5.32, p < 0.00001) in cancer patients. Clinically, a study involving 10,126 cases demonstrated that lymph node metastasis was tightly correlated with a high incidence of regional recurrence and distant metastasis, which directly lowered the survival rate of cancer patients (Zhang et al., 2019). Besides, mounting evidence have suggested that advanced tumor stage tended to have worse prognosis, which includes shorter progression-free survival (PFS), OS, and post-progression survival (PPS) (Fallowfield and Fleissig, 2011; Ogasawara et al., 2016). However, no remarkable association was detected between high BBOX1-AS1 expression and tumor differentiation (OR = 1.41, 95% CI: 0.78–2.55, p = 0.26), patient age (OR = 1.00, 95% CI: 0.63–1.59, p = 0.99), patient gender (OR = 1.05, 95% CI: 0.74–1.51, p = 0.77), and tumor size (OR = 1.48, 95% CI: 0.96–2.29, p = 0.07). Meanwhile, the GEPIA database was applied to confirm our results as extensively as possible. Elevated BBOX1-AS1 expression levels were also established in 12 different types of malignancies. Moreover, increased BBOX1-AS1 expression was associated with poor OS in LGG, MESO, and BRCA and with worse DFS in LGG, MESO, and sarcoma. Taken together, this consequence demonstrated that BBOX1-AS1 could be applied as a novel biomarker for prognosis or detection of various malignancies.
Nevertheless, several limitations in this study should be noted as well. First, as a novel biomarker, the earliest study demonstrating the association between BBOX1-AS1 and cancers was published in 2020. Although we thoroughly searched six databases, only eight studies were finally enrolled for this analysis after careful screening. This may hence be one of the explanations for the relatively small amount of literature included in our study. However, the GEPIA database and subgroup analysis were adopted to strengthen our results, and they demonstrated that our results were reliable. Second, therapeutic regimens may also be considered to play a vital role in cancer patient survival. Thus, different treatments, to some extent, may impact the calculation of HR or OR values. Third, there was moderate heterogeneity in some analyses such as lymph node metastasis (I2 = 53%) and tumor differentiation (I2 = 58%). Since all included studies were from different hospitals and examination approaches used by individual studies might not be uniform, these may likely contributed to heterogeneity. Consequently, an appropriate random-effects model had to be utilized, which might somewhat degrade the accuracy of results. Fourth, studies published only in English or Chinese were included, whereas studies exploring the relationship between BBOX 1-AS 1 and survival in cancer patients in other languages were omitted. Fifth, some HRs were extracted indirectly through rebuilding the K-M curves, which might inevitably cause possible deviations. Notwithstanding the intrinsic deficiencies, our results render robust evidence that overexpressed BBOX1-AS1 indicates poor prognosis in cancer patients.
Conclusion
In conclusion, our meta-analysis demonstrated that overexpressed BBOX1-AS1 is exceedingly correlated with worse OS, DFS, lymph node metastasis, and advanced tumor stage in carcinomas. Specifically, BBOX1-AS1 can be served as a novel promising biomarker for predicting the clinicopathological characteristics and prognosis for various cancers. Furthermore, in vitro/in vivo validation of the promotion of BBOX1-AS1 in different malignancies and its effects on tumor pathology could be investigated in future studies.
Data availability statement
The original contributions presented in the study are included in the article further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: GYL and YZW; methodology: GYL, YZW, LD and TY; formal analysis and investigation: TY and GYL; manuscript writing—original draft preparation: GYL and LD; manuscript writing—review and editing: YZW and GYL; funding acquisition: LD; resources: GYL; and supervision: TY. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, editors, and reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bhan, A., Soleimani, M., and Mandal, S. S. (2017). Long noncoding RNA and cancer: A new paradigm. Cancer Res. 77 (15), 3965–3981. doi:10.1158/0008-5472.CAN-16-2634
Cai, T., Peng, B., Hu, J., and He, Y. (2022). Long noncoding RNA BBOX1-AS1 promotes the progression of gastric cancer by regulating the miR-361-3p/Mucin 13 signaling axis. Bioengineered 13 (5), 13407–13421. doi:10.1080/21655979.2022.2072629
Ebrahimi, N., Parkhideh, S., Samizade, S., Esfahani, A. N., Samsami, S., Yazdani, E., et al. (2022). Crosstalk between lncRNAs in the apoptotic pathway and therapeutic targets in cancer. Cytokine Growth Factor Rev. 65, 61–74. doi:10.1016/j.cytogfr.2022.04.003
Engreitz, J. M., Ollikainen, N., and Guttman, M. (2016). Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nat. Rev. Mol. Cell Biol. 17 (12), 756–770. doi:10.1038/nrm.2016.126
Fallowfield, L. J., and Fleissig, A. (2011). The value of progression-free survival to patients with advanced-stage cancer. Nat. Rev. Clin. Oncol. 9 (1), 41–47. doi:10.1038/nrclinonc.2011.156
Hashemi, M., Moosavi, M. S., Abed, H. M., Dehghani, M., Aalipour, M., Heydari, E. A., et al. (2022). Long non-coding RNA (lncRNA) H19 in human cancer: from proliferation and metastasis to therapy. Pharmacol. Res. 184, 106418. doi:10.1016/j.phrs.2022.106418
Jayanthi, V. S. P. K. S. A., Das, A. B., and Saxena, U. (2017). Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 91, 15–23. doi:10.1016/j.bios.2016.12.014
Jiang, H., He, Q., and Liu, T. (2021). BBOX1-AS1 accelerates nasopharyngeal carcinoma progression by sponging miR-3940-3p and enhancing KPNA2 upregulation. Cancer Manag. Res. 13, 9049–9062. doi:10.2147/CMAR.S327211
Li, C., Tang, Z., Zhang, W., Ye, Z., and Liu, F. (2021). GEPIA2021: integrating multiple deconvolution-based analysis into GEPIA. Nucleic Acids Res. 49 (1), W242–W246. doi:10.1093/nar/gkab418
Li, Y., Shen, Y., Xie, M., Wang, B., Wang, T., Zeng, J., et al. (2022). LncRNAs LCETRL3 and LCETRL4 at chromosome 4q12 diminish EGFR-TKIs efficiency in NSCLC through stabilizing TDP43 and EIF2S1. Signal Transduct. Target Ther. 7 (1), 30. doi:10.1038/s41392-021-00847-2
Lian, Y. F., Jiang, X. M., and Shi, X. J. (2021). Targeted regulation of BBOX1-AS1 on miR-361-3p and its effect on the biological function of non-small cell lung cancer cell. J. Biol. Regul. Homeost. Agents 35 (4). doi:10.23812/21-143-L
Liao, C., Zhang, Y., Fan, C., Herring, L. E., Liu, J., Locasale, J. W., et al. (2020). Identification of BBOX1 as a therapeutic target in triple-negative breast cancer. Cancer Discov. 10 (11), 1706–1721. doi:10.1158/2159-8290.CD-20-0288
Lin, Z.-B., Long, P., Zhao, Z., Chu, X. D., and Zhao, X. X. (2021). Long noncoding RNA KCNQ1OT1 is a prognostic biomarker and mediates CD8+ T cell exhaustion by regulating CD155 expression in colorectal cancer. Int. J. Biol. Sci. 17 (7), 1757–1768. doi:10.7150/ijbs.59001
Liu, J., Zhu, J., Xiao, Z., Wang, X., and Luo, J. (2022). BBOX1-AS1 contributes to colorectal cancer progression by sponging hsa-miR-361-3p and targeting SH2B1. FEBS Open Bio 12 (5), 983–992. doi:10.1002/2211-5463.12802
Ma, R., Lu, Y., He, X., and Zeng, X. (2023). LncRNA BBOX1-AS1 targets miR-361-3p/COL1A1 axis to drive the progression of oesophageal carcinoma. Eur. J. Clin. Invest. 53 (4), e13929. doi:10.1111/eci.13929
Nojima, T., and Proudfoot, N. J. (2022). Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell Biol. 23 (6), 389–406. doi:10.1038/s41580-021-00447-6
Ogasawara, S., Chiba, T., Ooka, Y., Suzuki, E., Kanogawa, N., Saito, T., et al. (2016). Post-progression survival in patients with advanced hepatocellular carcinoma resistant to sorafenib. Invest. New Drugs 34 (2), 255–260. doi:10.1007/s10637-016-0323-1
Pan, C., Chen, G., Zhao, X., Xu, X., and Liu, J. (2022). lncRNA BBOX1-AS1 silencing inhibits esophageal squamous cell cancer progression by promoting ferroptosis via miR-513a-3p/SLC7A11 axis. Eur. J. Pharmacol. 175317, 175317. doi:10.1016/j.ejphar.2022.175317
Park, E.-G., Pyo, S.-J., Cui, Y., Yoon, S. H., and Nam, J. W. (2022). Tumor immune microenvironment lncRNAs. Brief. Bioinform 23 (1). doi:10.1093/bib/bbab504
Shen, X., Piao, L., Zhang, S., Cui, Y., Cui, Y., Quan, X., et al. (2018). Long non-coding RNA activated by TGF-β expression in cancer prognosis: A meta-analysis. Int. J. Surg. 58, 37–45. doi:10.1016/j.ijsu.2018.08.004
Sheng, J., Zhou, M., Wang, C., Jia, J., Chu, J., Ju, C., et al. (2022). Long non-coding RNA BBOX1-AS1 exacerbates esophageal squamous cell carcinoma development by regulating HOXB7/β-catenin axis. Exp. Cell Res. 415 (1), 113117. doi:10.1016/j.yexcr.2022.113117
Shi, J., Yang, C., An, J., Hao, D., Liu, C., Liu, J., et al. (2021). KLF5-induced BBOX1-AS1 contributes to cell malignant phenotypes in non-small cell lung cancer via sponging miR-27a-5p to up-regulate MELK and activate FAK signaling pathway. J. Exp. Clin. Cancer Res. 40 (1), 148. doi:10.1186/s13046-021-01943-5
Shi, Z.-L., Zhou, G.-Q., Guo, J., Yang, X. L., Yu, C., Shen, C. L., et al. (2022). Identification of a prognostic colorectal cancer model including LncRNA FOXP4-AS1 and LncRNA BBOX1-AS1 based on bioinformatics analysis. Cancer Biother Radiopharm. 37 (10), 893–906. doi:10.1089/cbr.2020.4242
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tan, Y.-T., Lin, J.-F., Li, T., Xu, R. H., and Ju, H. Q. (2021). LncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun. (Lond) 41 (2), 109–120. doi:10.1002/cac2.12108
Tang, Z., Li, C., Kang, B., Gao, G., and Zhang, Z. (2017). Gepia: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45 (1), W98–W102. doi:10.1093/nar/gkx247
Tao, H., Zhang, Y., Li, J., Liu, J., Yuan, T., Wang, W., et al. (2022). Oncogenic lncRNA BBOX1-AS1 promotes PHF8-mediated autophagy and elicits sorafenib resistance in hepatocellular carcinoma. Mol. Ther. Oncolytics 28, 88–103. doi:10.1016/j.omto.2022.12.005
Tao, H., Li, J., Liu, J., Yuan, T., Zhang, E., Liang, H., et al. (2021). Construction of a ceRNA network and a prognostic lncRNA signature associated with vascular invasion in hepatocellular carcinoma based on weighted gene Co-expression network analysis. J. Cancer 12 (13), 3754–3768. doi:10.7150/jca.57260
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S., and Sydes, M. R. (2007). Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16. doi:10.1186/1745-6215-8-16
Wang, T., Zhang, X.-D., and Hua, K.-Q. (2021). A ceRNA network of BBOX1-AS1-hsa-miR-125b-5p/hsa-miR-125a-5p-CDKN2A shows prognostic value in cervical cancer. Taiwan J. Obstet. Gynecol. 60 (2), 253–261. doi:10.1016/j.tjog.2020.12.006
Ward, Z. J., Scott, A. M., Hricak, H., and Atun, R. (2021). Global costs, health benefits, and economic benefits of scaling up treatment and imaging modalities for survival of 11 cancers: A simulation-based analysis. Lancet Oncol. 22 (3), 341–350. doi:10.1016/S1470-2045(20)30750-6
Wu, H., Zhou, S., Zheng, Y., Pan, Z., Chen, Y., and Wang, X. (2022). LncRNA BBOX1-AS1 promotes pituitary adenoma progression via sponging miR-361-3p/E2F1 axis. Anticancer Drugs 33 (7), 652–662. doi:10.1097/CAD.0000000000001309
Xu, J., Yang, B., Wang, L., Zhu, Y., Zhu, X., Xia, Z., et al. (2020). LncRNA BBOX1-AS1 upregulates HOXC6 expression through miR-361-3p and HuR to drive cervical cancer progression. Cell Prolif. 53 (7), e12823. doi:10.1111/cpr.12823
Yao, H., Chen, R., Yang, Y., and Jiang, J. (2021). LncRNA BBOX1-AS1 aggravates the development of ovarian cancer by sequestering miR-361-3p to augment PODXL expression. Reprod. Sci. 28 (3), 736–744. doi:10.1007/s43032-020-00366-5
Zhang, Y., Zhang, Z. C., Li, W. F., Liu, X., Liu, Q., and Ma, J. (2019). Prognosis and staging of parotid lymph node metastasis in nasopharyngeal carcinoma: an analysis in 10,126 patients. Oral Oncol. 95, 150–156. doi:10.1016/j.oraloncology.2019.06.013
Zhang, Y., Wang, X., Cheng, X.-K., Zong, Y. Y., He, R. Q., et al. (2022). Clinical significance and effect of lncRNA BBOX1-AS1 on the proliferation and migration of lung squamous cell carcinoma. Oncol. Lett. 23 (1), 17. doi:10.3892/ol.2021.13135
Keywords: lncRNA BBOX1-AS, human malignancies, meta-analysis, prognosis, review
Citation: Lin G, Wang Y, Deng L and Ye T (2023) Prognostic effect of lncRNA BBOX1-AS1 in malignancies: a meta-analysis. Front. Genet. 14:1234040. doi: 10.3389/fgene.2023.1234040
Received: 03 June 2023; Accepted: 21 July 2023;
Published: 11 August 2023.
Edited by:
Harikrishnan Radhakrishnan, SRI International, United StatesReviewed by:
Kartik Soni, University of Southern California, United StatesVikash Singh, Penn State Milton S. Hershey Medical Center, United States
Osheen Sahay, National Centre for Cell Science, India
Ganesh Kumar Barik, National Centre for Cell Science Pune, India in collaboration with reviewer OS
Copyright © 2023 Lin, Wang, Deng and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Ye, bGVyZWwtc3RAaG90bWFpbC5jb20=
 Guangyao Lin
Guangyao Lin Yongzhou Wang2
Yongzhou Wang2