
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 02 October 2023
Sec. Computational Genomics
Volume 14 - 2023 | https://doi.org/10.3389/fgene.2023.1230245
This article is part of the Research Topic The Connecting Role of Oral Microbiome and Inflammatory Mediators in Oral and Systemic Diseases View all 4 articles
 Jieqi Jin1†
Jieqi Jin1† Mengkai Guang2†
Mengkai Guang2† Simin Li3
Simin Li3 Yong Liu1
Yong Liu1 Liwei Zhang1
Liwei Zhang1 Bo Zhang1
Bo Zhang1 Menglin Cheng1
Menglin Cheng1 Gerhard Schmalz4*
Gerhard Schmalz4* Xiaofeng Huang1*
Xiaofeng Huang1*Background: Periodontits (PD) and Alzheimer’s disease (AD) are both associated with ageing and clinical studies increasingly evidence their association. However, specific mechanisms underlying this association remain undeciphered, and immune-related processes are purported to play a signifcant role. The accrual of publicly available transcriptomic datasets permits secondary analysis and the application of data-mining and bioinformatic tools for biological discovery.
Aim: The present study aimed to leverage publicly available transcriptomic datasets and databases, and apply a series of bioinformatic analysis to identify a robust signature of immune-related signature of PD and AD linkage.
Methods: We downloaded gene-expresssion data pertaining PD and AD and identified crosstalk genes. We constructed a protein-protein network analysis, applied immune cell enrichment analysis, and predicted crosstalk immune-related genes and infiltrating immune cells. Next, we applied consisent cluster analysis and performed immune cell bias analysis, followed by LASSO regression to select biomarker immune-related genes.
Results: The results showed a 3 gene set comprising of DUSP14, F13A1 and SELE as a robust immune-related signature. Macrophages M2 and NKT, B-cells, CD4+ memory T-cells and CD8+ naive T-cells emerged as key immune cells linking PD with AD.
Conclusion: Candidate immune-related biomarker genes and immune cells central to the assocation of PD with AD were identified, and merit investigation in experimental and clinical research.
With the rapid ageing of global populations, the burden of Alzheimer’s disease (AD) is rising. AD is a neurodegenerative disease marked by the formation of amyloid-β peptide (AβP) plaques aggregate in brain tissues. Inflammation and pathological aberrations in central and peripheral immune responses are implicated in AD (Campbell and Gear, 1995; Bettcher et al., 2021). While the relationship of systemic or peripheral inflammation with AD has been inconsistent (Eriksson et al., 2011), accruing research has highlighted the role of peripheral inflammation in AD pathogenesis. Gut microbiome dysbiosis is associated with neuroinflammation and synaptic dysfunction characteristic of AD (Bairamian et al., 2022). Research using a murine model of AD has demonstrated that low-grade peripheral inflammation is capable of aggravating brain pathology (Xie et al., 2021). ApoE4 allele of the Apolipoprotein E gene, a well-known genetic risk factor of AD, when coupled with chronic low-grade peripheral inflammation leads to earlier onset and greater morbidity from AD (Tao et al., 2018). Peripheral inflammation also alters the connectivity of large-scale cognitive networks in older individuals, particularly in ApoE4 carriers (Walker et al., 2020). Elevated levels of both peripheral and CSF inflammatory markers are associated with AD (Shen et al., 2019). Systemic infections are associated with enhanced immunosuppressive processes in the brains of patients with AD, with an increase in anti-inflammatory proteins including IL4R and CHI3L1 and a decrease in certain proinflammatory proteins, along with lowered T-cell recruitment (Rakic et al., 2018). Systemic inflammation can affect intra-brain drug distribution by altering ABCB1 and ABCG2 protein expression and can also perturb GluN1 protein expression in AD affected brains (Puris et al., 2021). Circulating IL-21, a key immunomodulatory cytokine is elevated in AD, possibly due to immune activation, resulting in neuroinflammation, microglial activation, and deposition of Aβ plaques (Agrawal et al., 2022).
Ageing is associated with higher levels of chronic inflammation and immune deregulation. Infections cause immune dysregulation, an increase circulating pro-inflammatory mediators such as TNFα and IL-6 along with the brain levels of IL-1β and IL-6 levels, aggravating neuroinflammation and accelerating cognitive decline in older adults (Holmes et al., 2011; Lopez-Rodriguez et al., 2021). Immune perturbation in AD is not restricted to the central nervous system (CNS), and peripheral immune dysregulation appears to affect homeostasis in AD-affected brains, where the barrier function is disrupted, allowing an ingress of T-cells (van Olst et al., 2022). Perturbed naive and memory CD4+ T cell subsets have been noted in the peripheral blood of patients with mild AD and dementia, with a lower proportion of naive cells and an increased proportion of effector memory and terminal differentiation effector memory (TEMRA) CD4+ cells (McManus et al., 2015). The deregulation of both the peripheral and central immune compartments marks AD. Peripheral immune activation is associated with neuroinflammation and AD pathogenesis. Sustained activation of the brain’s microglia and other immune cells is found to exacerbate both amyloid and tau pathology and may serve as the link between infections, chronic peripheral inflammation and AD (Kinney et al., 2018).
Periodontitis is a highly prevalent oral infectious disease that imposes both oral and systemic health burdens. It is an inflammatory disease caused by a complex interplay between dental plaque microbes and the host immune system (Hajishengallis, 2014a). The deposition of a microbial plaque biofilm initiates immune cell migration, and its dysbiosis sustains a local inflammatory response (Murakami et al., 2018). Key periodontal pathogens such as Porphyromonas gingivalis are immune evasive and can activate the complement system and pathogen recognition receptors such as TLRs, leading to chronic inflammation and periodontal tissue destruction (Xu et al., 2021). Virulence factors like Porphyromonas gingivalis fimbriae can activate TLR2 expressed by innate immune cells (Maekawa et al., 2014), leading to a cascade of cellular and humoral immune responses, and induction of adaptive immune responses (Hajishengallis, 2014a). Ageing is associated with a steep increase in the incidence and severity of periodontitis, attributed in part to increased susceptibility from age-dependent alterations in host innate immunity and inflammatory status (Hajishengallis, 2014b). Cellular senescence, stem cell failure, and immune senescence inherent to biological ageing impair periodontal tissue homeostasis and contribute to the pathophysiology of periodontitis (Baima et al., 2022).
Evidence showing the association of AD with periodontitis is rapidly accumulating (Dominy et al., 2019; Dioguardi et al., 2020; Hu et al., 2021). Porphyromonas gingivalis has been found to infiltrate the brains of tissue in AD and is proposed to be an important mechanistic link between periodontitis and AD (Ryder, 2020). Periodontitis also causes widespread systemic immune dysfunction, showing heightened pro-inflammatory responses to Porphyromonas gingivalis and attenuated T-cell responses (Gaudilliere et al., 2019). In the present study, we aimed to identify immunological perturbations and immune crosstalk potentially linking periodontitis with AD by leveraging gene expression data.
We downloaded gene expression profile datasets related to periodontitis (PD) and Alzheimer’s disease (AD) from the GEO (https://www.ncbi.nlm.nih.gov/geo/) database. For PD, we chose gingival tissue and for AD, we chose brain tissue data. The datasets are listed in Table1.
First, we converted the probe names into gene names based on the downloaded data. If the same gene had multiple expression values in the same sample, we obtained the mean of the expression values. As differences existed between the datasets, we first combined the datasets for AD and PD each based on common genes, and then applied the “ComBat” method in the R package “SVA” for batch correction. Among the datasets related to AD, since the series matrix of GSE33000 was a standardised dataset, the other four datasets were standardised separately. We then combined the 5 standardised datasets and applied the “ComBat” method to perform batch correction.
Differential expression gene analysis of the corrected datasets was performed using the R package “limma”. For the AD datasets, we used a threshold of p-value < 0.05, with | log2 (FC) | > 0 for upregulated genes and log2 (FC) < 0 for downregulated genes. For PD datasets we used p-value < 0.05, log2 (FC) ≥ 0.5 for upregulated genes and log2 (FC) < =0.5 for downregulated genes.
The differentially expressed genes of AD and PD were intersected and the shared genes were regarded as potential crosstalk genes. Functional enrichment analysis of the crosstalk genes was performed using “clusterProfiler” (GO Biological processes and KEGG pathways, at a threshold of p-value < 0.05.
We downloaded protein-protein related gene pairs from MINT (http://mint.bio.uniroma2.it/mint/Welcome.do), HPRD (http://www.hprd.org/index_html), BIOGRID (http://thebiogrid.org/), DIP (http://dip.doe-mbi.ucla.edu/dip/Main.cgi), mentha (http://mentha.uniroma2.it/index.php), PINA (http://cbg.garvan.unsw.edu.au/pina/), InnateDB (http://www.innatedb.com/), and Instruct (http://instruct.yulab.org/index.html) databases. Next, PPI relationship pairs for the crosstalk genes were extracted, and a PPI network was constructed using Cytoscape software, with the plug-in “NetworkAnalyzer” to analyse the network’s topological properties.
XCell (https://xcell.ucsf.edu/) was used for cell type enrichment of the crosstalk genes. XCell includes 64 cell types involving multiple adaptive and innate immune cells, hematopoietic progenitors, epithelial cells, and extracellular matrix cells, comprising 48 tumor microenvironment-related cells. We first extracted Case samples from AD and PD datasets and obtained the expression values of crosstalk genes. The gene number limit for the raw enrichment analysis method in the xCell package was reset and the scores for immune-infiltrating cells corresponding to the samples were calculated. Next, “transform scores” and “spill over” were used to obtain the final corrected immune infiltrating cell scores.
Immune-related genes were downloaded from an earlier publication (Charoentong et al., 2017) (782 genes, including 431 genes related to 15 adaptive immune cell types and 351 genes related to 13 innate immune cell types) and combined with immune-related genes from Innate DB (https://innatedb.com/annotatedGenes.do?type=innatedb) and ImmPort (https://www.immport.org/home) datasets. The expression values of these immune-related genes in the AD and PD case samples were extracted and the xCell algorithm was used to obtain the expression scores of infiltrating immune cells. The differences between the immune cell fractions in the two diseases was tested using the Wilcoxon’s test (p < 0.05).
We applied consensus clustering to the expression matrix profiles of immune-related genes in AD and PD each, using the “ConsensusClusterPlus” package. Average silhouette width, gap statistic, and the elbow method were used to determine the optimal number of clusters. Next, the samples were clustered using clustering consistency.
We combined the immune cell fractions and sample clusters of case samples from AD and PD. For each consensus cluster, statistics for cluster distribution of xCell scores for adaptive and innate immune-related genes were computed. The difference between scores of immune cells in different clusters was tested using the Kruskal Wallis test. We also noted the overall scores of immune cells in different clusters to determine immune cells that characterized a cluster.
We extracted the expression values of the crosstalk genes for the case and control groups and applied LASSO logistic regression. From the screened crosstalk genes, those common to AD and PD were considered biomarker crosstalk genes. Next, adaptive immune cell-related genes were identified based on the literature, and their expression profiles in AD and PD datasets were screened using LASSO logistic regression. The intersecting genes were recorded as biomarker adaptive immune genes. For Innate immune cell-related genes, we combined those obtained from literature with those obtained from the InnateDB dataset and obtained 1,335 innate immune-cell related genes. LASSO logistic regression was similarly applied, and biomarker innate immune genes were identified. In the next step, the intersections of biomarker crosstalk genes with biomarker adaptive immune cell-related genes and biomarker innate immune cell-related genes were determined. Receiver operating curve (ROC) analysis was performed using these genes’ expression values. Human KEGG pathways and related genes were obtained from the KEGG database (https://www.kegg.jp/) and pathways that correspond to these intersecting genes were identified and all genes in each such pathway were listed. Interactions between the KEGG pathways, biomarker crosstalk genes, biomarker adaptive immune-cell related genes, and biomarker innate immune cell-related genes were identified.
As evident in Figure 1, significant clustering by batch was noted for both AD and PD gene expression datasets before correction and was reduced post batch correction (Figure 1). Using the batch corrected data, we obtained 4,398 differentially expressed genes in AD and 1,041 differentially expressed genes in PD, respectively. A volcano map was used to display the distribution of the differentially expressed genes (Figure 2).
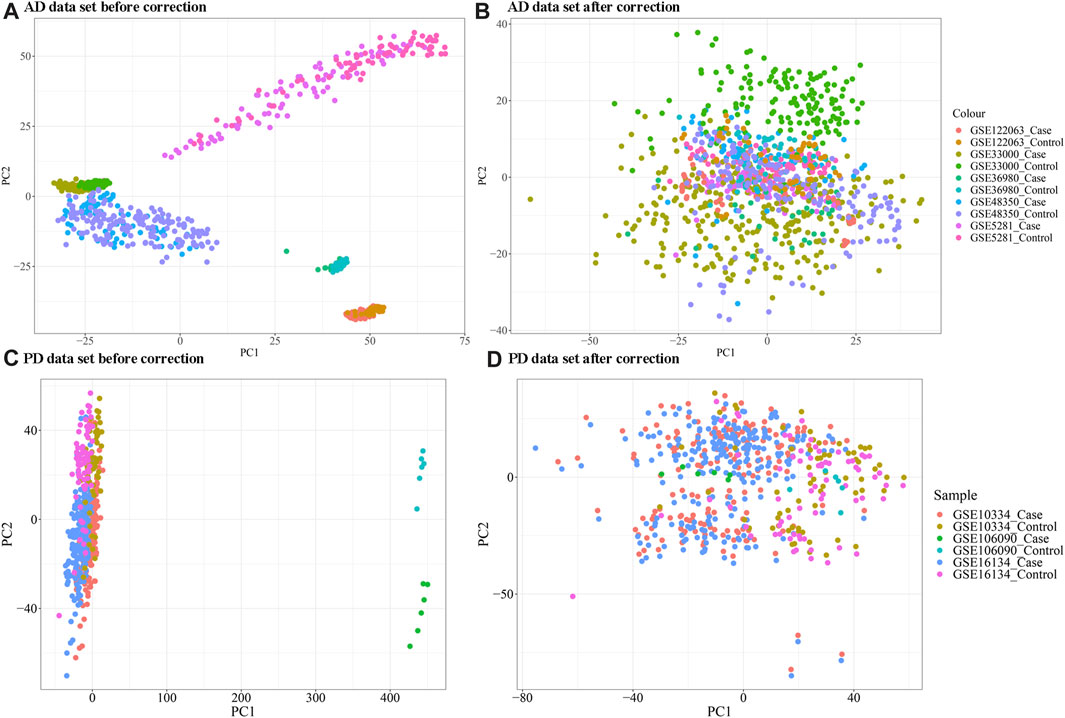
FIGURE 1. (A, B) Principal coordinate analysis (PCA) plot of AD samples clustering before and after batch correction; (C, D) PCA analysis plot of PD samples clustering before and after correction. The “ComBat” method in the R package “SVA” was used for batch correction.
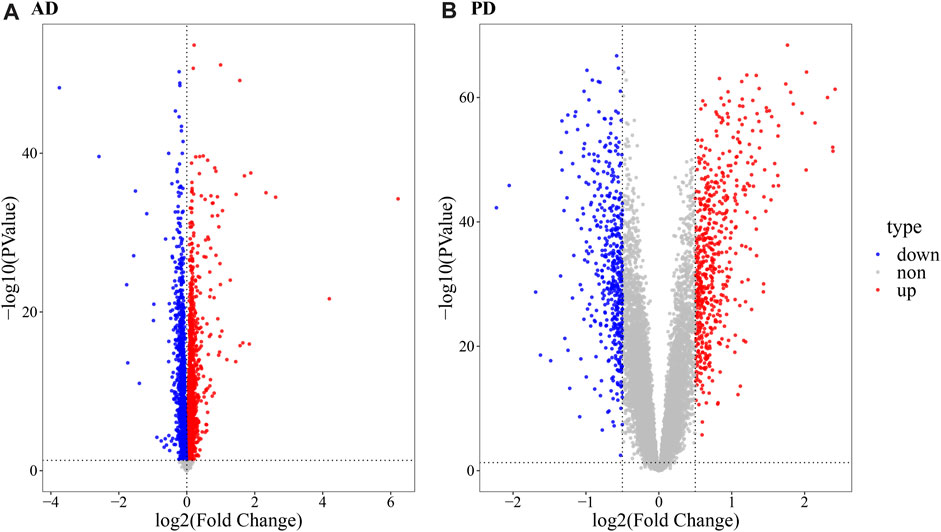
FIGURE 2. Volcano map depicting differentially expressed genes. (A) Volcano map ofdifferentially expressed genes in AD; (B) Volcano map of differentially expressed genes in PD. The R package “limma” was used for differential gene expression analysis. For the AD datasets, a threshold of p-value < 0.05,| log2 (FC) | > 0 for upregulated genes and log2 (FC) < 0 for downregulated genes was used. For PD datasets, a p-value < 0.05, log2 (FC) ≥ 0.5 for upregulated genes and log2 (FC) < = 0.5 for downregulated genes was used.
A total of 364 Crosstalk genes were obtained (Figure 3A) by intersection of the differentially expressed genes of PD and AD. To visualize changes in the expression values of crosstalk genes in different sample types, heat maps were plotted using the “pheatmap” R package, using top 50 Crosstalk genes as the input (Figures 3B, C). To further analyze the functions of the crosstalk genes functional enrichment analysis was performed and significantly enriched GO Biological process and KEGG Pathways were identified and the top 20 were visualized (Figures 3D–E).
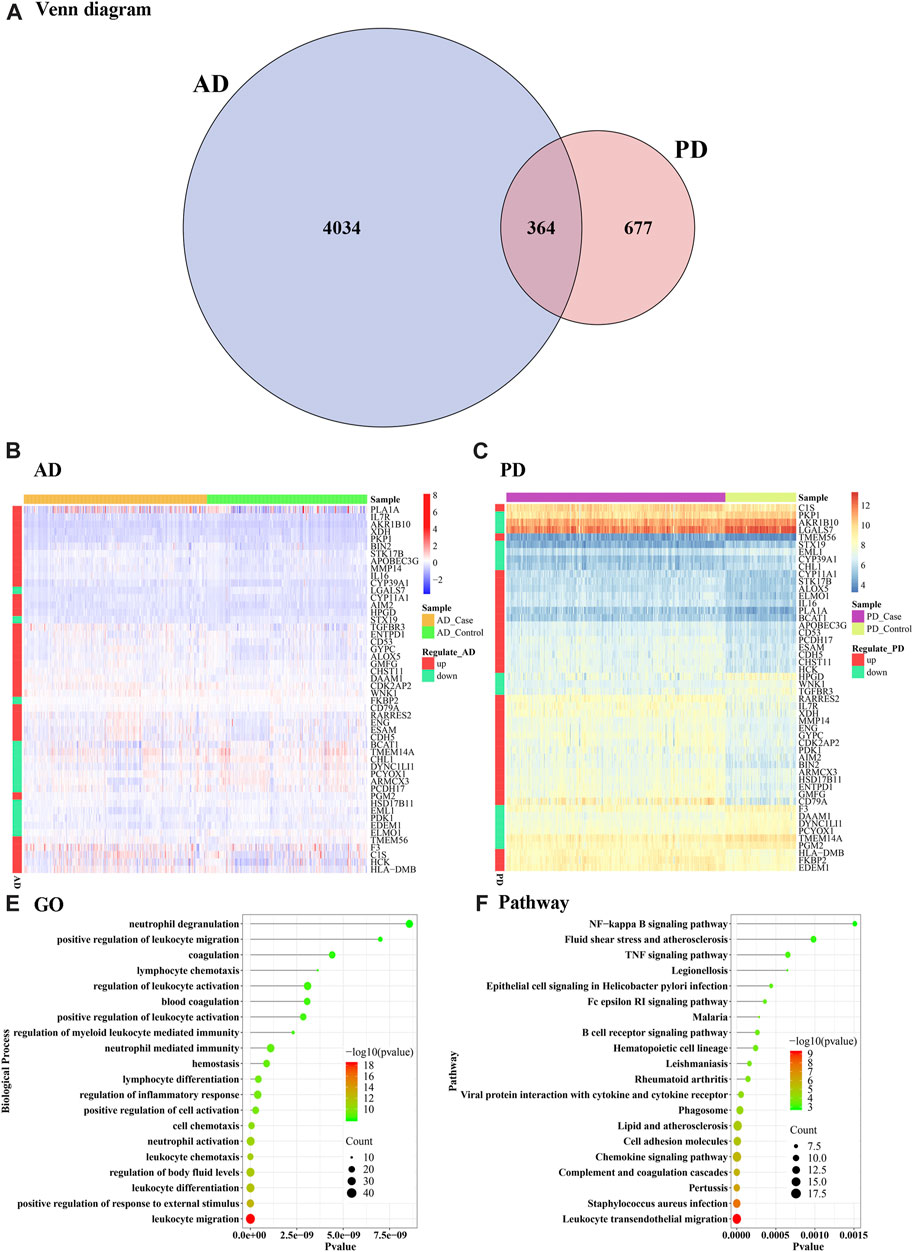
FIGURE 3. Crosstalk genes and functional enrichment analysis. (A) Venn diagram of differentially expressed genes obtained from AD and PD (B, C) Heat maps showing the expression levels of the cross talk genes in AD and PD (D) Top 20 enriched biological processes in the crosstalk genes; (E) KEGG pathways significantly enriched in the crosstalk genes. The top 50 crosstalk genes were used as input for the visualizations.
Gene ontology analysis showed that the crosstalk genes mainly regulated several leukocyte functions including chemotaxis, migration, differentiation, and myeloid leukocyte related immunity. In particular, neutrophil activation, degranulation, and associated immunity. Blood coagulation, hemostasis and body fluid balance regulation were also enriched among the crosstalk genes (Figure 3D). Among the enriched KEGG pathways, leukocyte transendothelial migration, S. auerus infection, and complement and coagulation cascades showed the top-most significance. Innate immune pathways including chemokine signaling, NF kappa beta signaling, and TNF signaling pathways were noted. Lipid and atherosclerosis pathway, epithelial cell signaling and cell-mediated immune pathway B cell receptor signaling were also notably enriched among others including rheumatoid arthritis, Fc epsilon R1 signaling and viral protein interaction with cytokine receptor (Figure 3E).
We extracted PPI relationship pairs for the 364 crosstalk genes and constructed a PPI network (Figure 4), which showed 4,870 nodes and 9,657 edges. The topological properties of the network were analyzed, and the top 30 hub node genes (Table 2) were identified based on the degree of gene connectivity and considered as the most important genes nodes in the protein interaction network relationship.
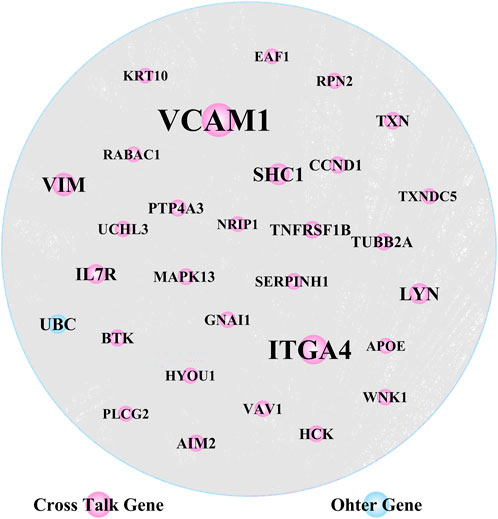
FIGURE 4. Crosstalk genes’ protein-protein interaction (PPI) network analysis (30 hub nodes obtained from the topological analysis are displayed).
VCAM1, ITGA4 and VIM, were noted as the top genes playing an important role in the network, and were upregulated in both AD and PD. Several of the gene nodes showed opposing patterns of regulation in the two diseases. KRT10, WNK1, MAPK13, TUBB2A and CCND1 were upregulated in AD but downregulated in PD. Conversely, RABAC1 and HYOU1 were downregulated in AD but upregulated in PD.
Using the xCell package, scores of immune-infiltrating cells corresponding to the 364 Crosstalk genes were calculated and “transform scores” and “spillOver” were applied to obtain the final corrected immune cell scores. Scores of 55 immune cell types in the AD and PD datasets were noted and a heatmap was used to display the scores of immune infiltrating cells in AD and PD datasets (Figure 5A).
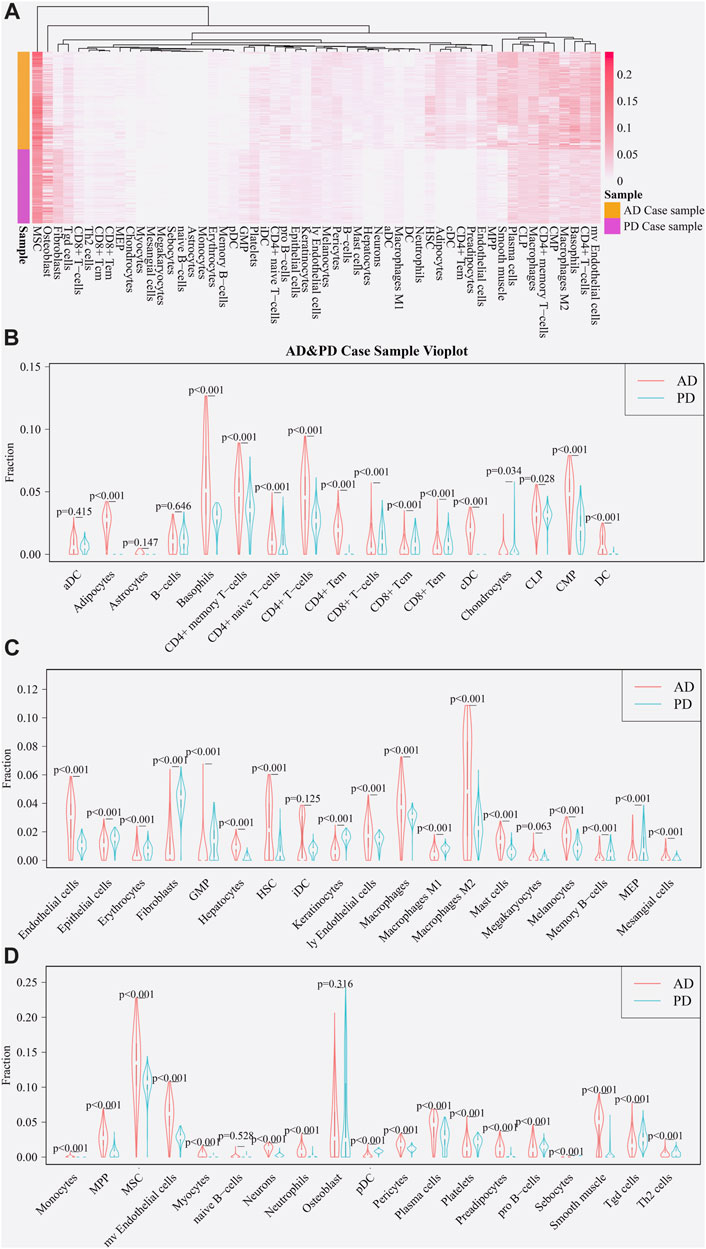
FIGURE 5. Expression of immune cells in AD and PD. (A) Thermographic representation of immune cell expression scores in AD and PD. (B–D) Violin diagram of immune cells expression scores in AD and PD. The “xCell” package was used to obtain scores of 55 immune infiltrating cells in both disease sample datasets and differences were tested using Wilcoxon’s analysis.
A violin diagram drawn using “vioplot” was used to depict the scores of each immune infiltrating cell in both diseases (Figures 5B–D). The difference in scores of immune infiltrating cells for the Case samples of AD and PD datasets was tested using Wilcoxson’s test. The cells were grouped in three categories and displayed (Figures 5B–D). We can see that several immune cell types are closely related in both diseases. AD samples showed highly significantly higher scores for adipocytes, CD4+ and CD8+ T-cell subsets, cDC and DC cell types. Endothelial cells, mast cells and macrophage cells, along with neutrophils, MSC, Th2, HSC, iDC, plasma cells and pro-B cells were comparatively overexpressed in AD, in particular, M2 macrophage cells. Chondrocytes, osteoblasts and fibroblast cells showed greater overexpression in PD.
To test the correlation between immune cells, a correlation analysis of xCell scores for Case samples for each immune cell type in the two data sets was applied (Figures 6A, B).
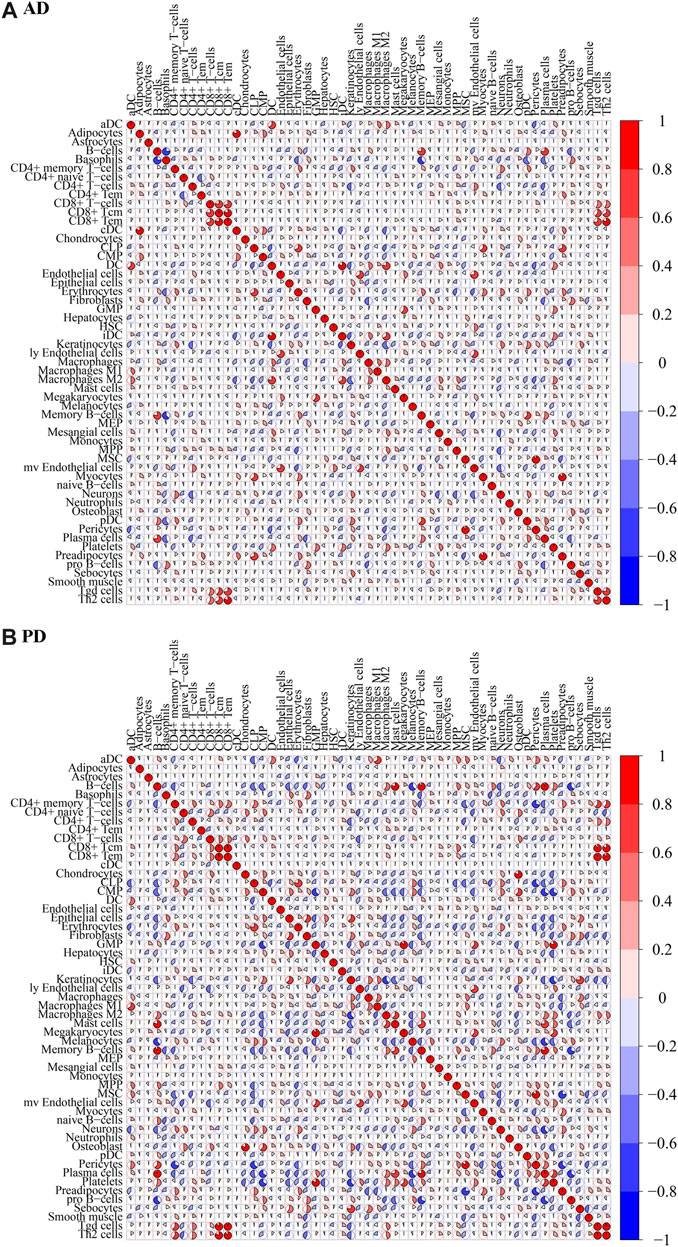
FIGURE 6. Correlation of immune cell scores in Case groups of (A) AD and (B) PD. xCell scores for each immune cell type in the case samples were subjected to Spearman’s correlation analysis.
For AD, immune cell CD8+ Tem and Th2 cells were highly positively correlated (COR = 0.8196), CD8+ Tcm and CD8+ Tem were highly positively correlated (COR = 0.8127), CD8+ T-cells and CD8+ Tcm were highly positively correlated (COR = 0.7822). B-cells were highly negatively correlated with Basophils (COR = −0.7763). For PD, CD8+ Tcm and CD8+ Tem were highly positively correlated (cor = 0.9695), Tgd cells and Th2 cells were highly positively correlated (cor = 0.9657), CD8+ Tem and Tgd cells were highly positively correlated (0.9500), CD8+ Tem and Th2 cells were highly positively correlated (cor = 0.8954). MSC and Preadipocytes were highly negatively correlated (cor = −0.7754), CD4+ memory T-cells and Pericytes were highly negatively correlated (cor = −0.7173), Memory B-cells and pro B-cells were highly negatively correlated (cor = −0.6979).
The immune-related genes downloaded from the literature included genes related to 15 Adaptive immune cell and 13 Innate immune cell types (Figure 7A). Further immune-related genes were obtained from InnateDB and ImmPort databases. We merged the immune-related genes acquired from literature, InnateDB, and ImmPort databases to obtain 3,046 immune genes as the final immune-related gene dataset. We extracted the expression values of these 3,046 immune genes in the Case samples of AD and PD and found that 1,142 immune genes were expressed in AD whereas 2,396 immune genes were expressed in PD. Using the xCell algorithm, we obtained the expression scores of immune cells in the Case samples of AD and PD. Since the names of 64 cell types included in xCell were different from the names of 28 cell types listed in literature, we identified and listed the cell types (Table 3).
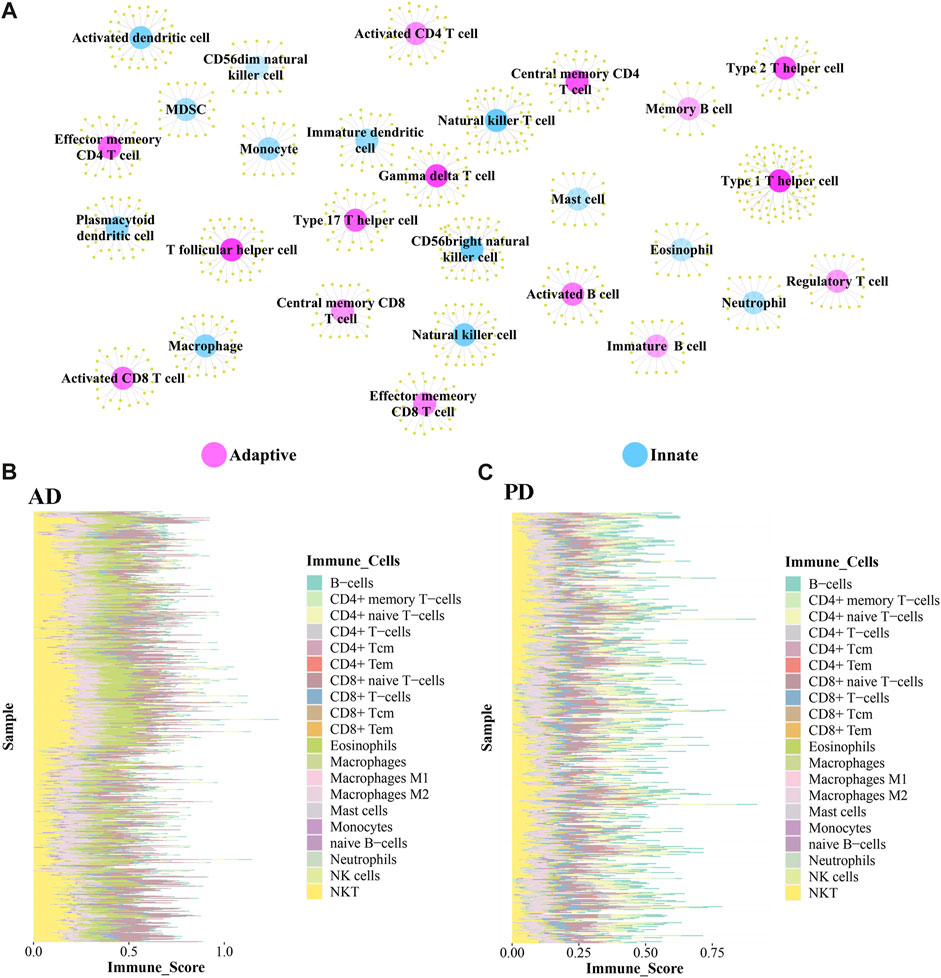
FIGURE 7. (A) Adaptive immune gene-associated immune cells and Innate gene-associated immune cells. (B, C) Expression levels of 20 Adaptive and Innate immune cells in AD and PD.
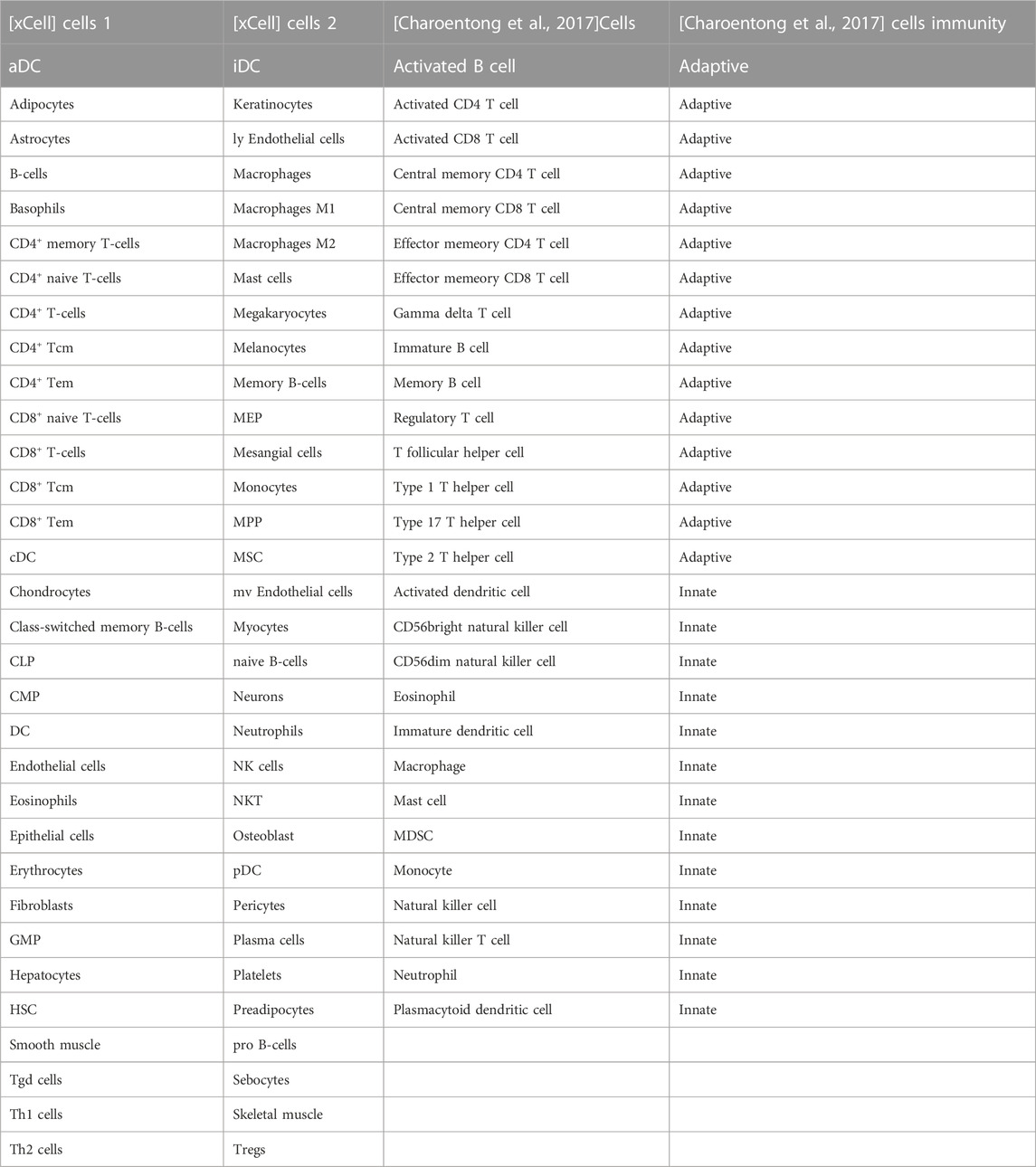
TABLE 3. Common immune cells xCell and literature (Charoentong et al., 2017).
10 adaptive and 10 innate immune cell types were noted in xCell and are marked with different colors. These 20 cell types were extracted for subsequent analysis and their scores were analysed. The fraction of these immune cells is depicted in Figure 7B.
Among the adaptive immune cells, most cells showed higher expression in AD, while CD4+ naive T−cells and B−cells were highly expressed in PD samples as compared with AD samples. The expression of Eosinophils was higher in AD disease samples than in PD disease samples. Macrophages M2, Natural killer T cell (NKT) and CD8+ naive T-cells were highly expressed in AD and PD samples. Macrophages M2 and Natural killer T cell (NKT) are innate immune cells, while CD8+ naive T-cells are adaptive.
A violin diagram was drawn to depict the scores of each immune infiltrating cell in both diseases (Figure 8A) and differences between AD and PD datasets were tested using Wilcoxon’s test (p < 0.05). Macrophages M2, Natural killer T cell (NKT) and CD8+ naive T-cells were found to be significantly different in AD and PD. Then, we examined the correlation between the 20 immune cells in AD and PD (Figures 8B, C). CD8+ T-cells and CD8+ naive T-cells, CD4+ memory T-cells and CD4+ T-cells were highly positively correlated in AD and PD (Table 4).
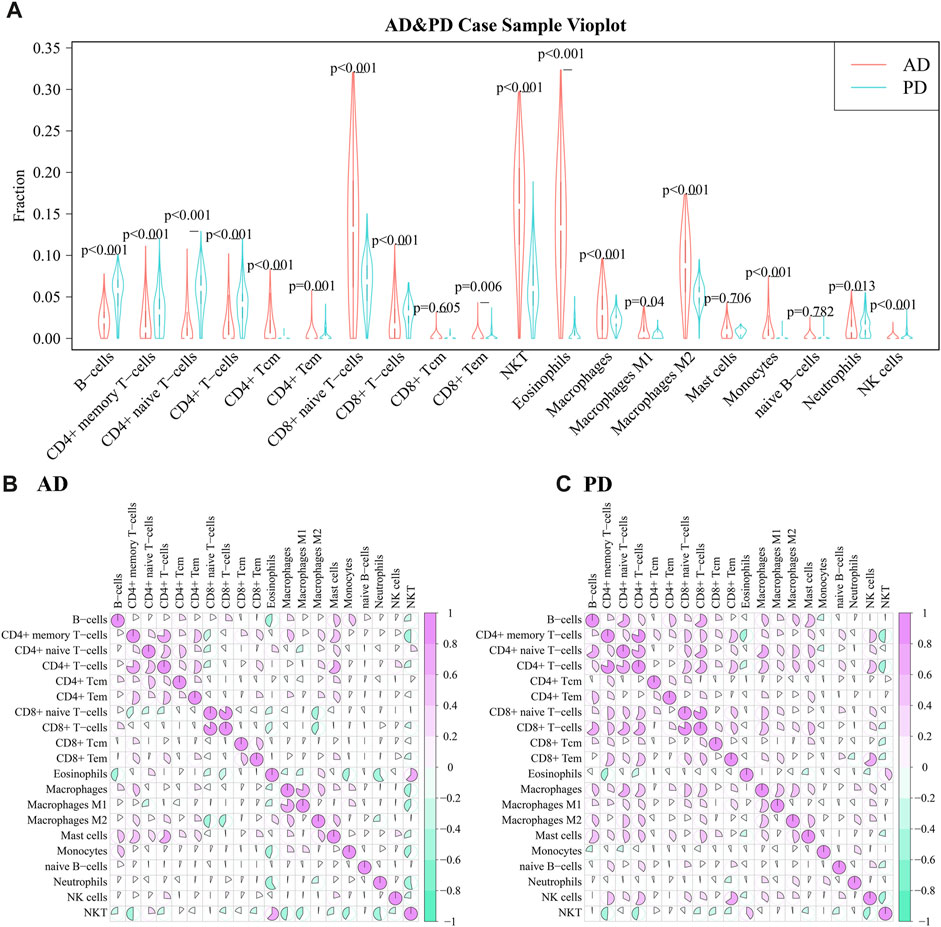
FIGURE 8. (A) Comparison of scores of 20 immune infiltrating cell scores in AD and PD; (B) Correlation between infiltrating immune cell scores in AD; Differences were tested using Wilcoxon’s analysis. (C) Correlation between infiltrating immune cell scores in PD. Spearman’s correlation analysis was performed.
1,142 immune genes found expressed in AD and 2,396 immune genes found expressed in PD were subjected to Consensus Clustering. The maxK values were determined using average silhouette width, gap statistic, and the elbow method to find the optimal number of clusters for the AD and PD expression matrices (Figures 9A–F).
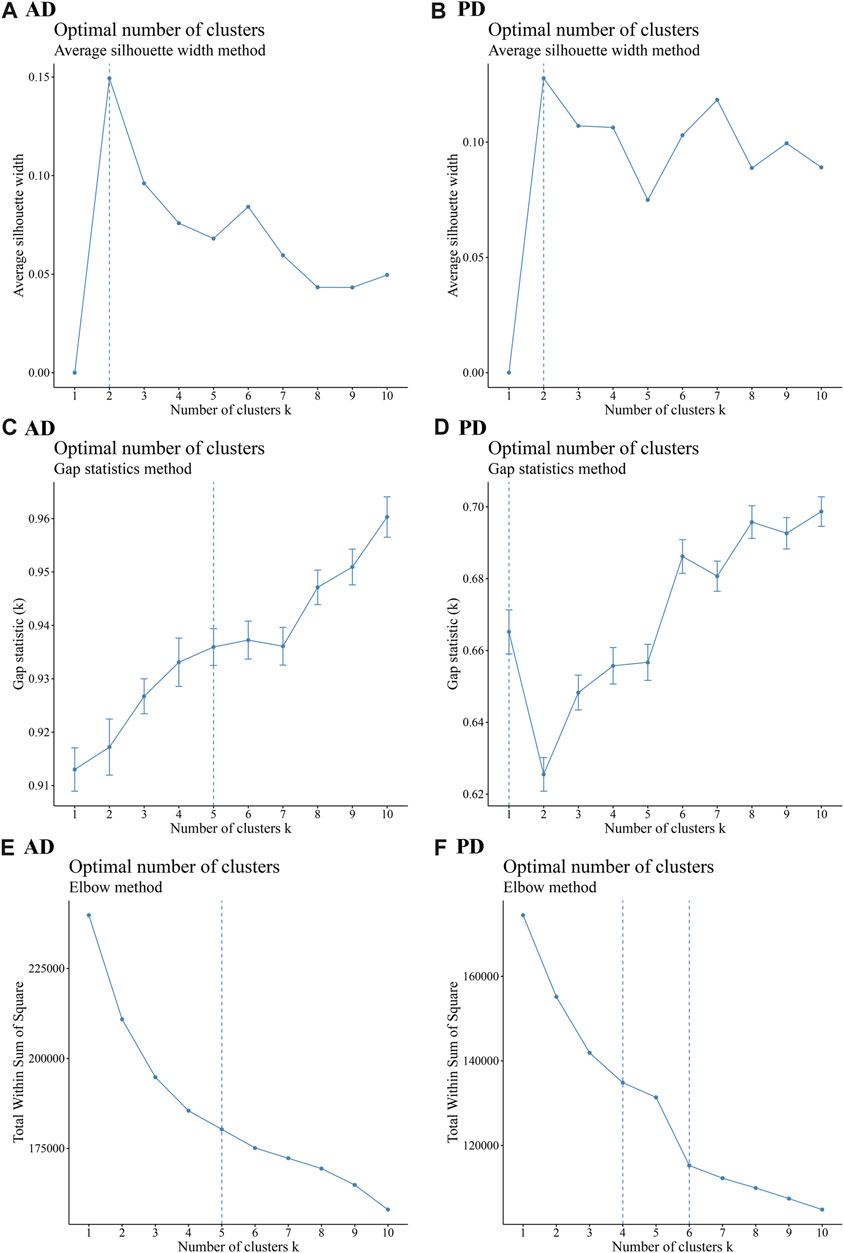
FIGURE 9. Cluster number analysis using different methods (A–C) Average silhouette width, Gap statistic, and Elbow method to analyse the number of AD clusters; (D–F) Average silhouette width, Gap statistic, and Elbow method to analyse the number of PD clusters. The “ConsensusClusterPlus” R package was applied for cluster analysis.
As seen in Figure 9, the number of optimal clusters denoted by the three methods were different, which may be related to the large number of gene features and the differences in the algorithms. In AD, the maximum number of clusters was 5 and the minimum number was 2. In PD, the maximum number was 6 and the minimum number was 1. Clustering consistency results for 2-5 clusters in AD, and 2-6 clusters in PD were analysed. Key clustering consistency results for AD and PD are depicted in Figures 10A–F.
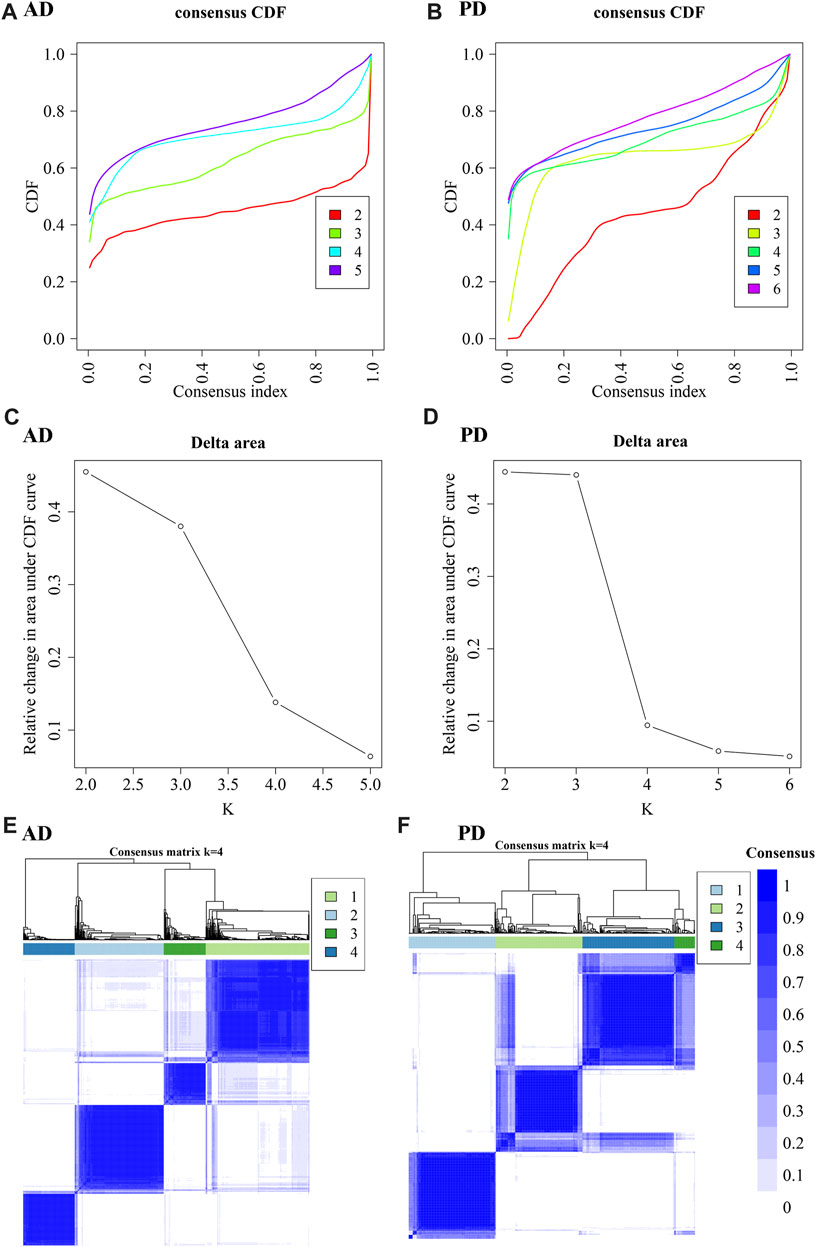
FIGURE 10. Cluster consistency analysis. (A, B) Consistent cumulative distribution function (CDF) plots for AD and PD. This figure shows the cumulative distribution function of scores with different values of K, which is used to determine the approximate maximum value of CDF for a selected k value, and the cluster analysis result that is the most reliable. That is, the k value with a small descending slope of CDF is considered. (C, D) Delta Area Plot of AD and PD: This figure shows the relative changes of areas under the CDF curve compared to k and k-1. When k = 2, since there is no k = 1, the first point represents the total area under the CDF curve at k = 2 (that is, the area of the center line in Figure AB), rather than the relative change in area. (E, F) Consistent clustering diagram of AD and PD.
The results show that to get the final k value, the descending slope of the Central Line and the relative change of the area under the CDF curve between K and K-1 should be as small as possible. We finally choose K = 4 for AD and PD both. Figure EF shows the correlation between AD and PD samples at the selected k values. The rows and columns of the matrix represent the samples. Consistency matrix values are shown in white to dark blue on a scale from 0 (impossible to cluster together) to 1 (always cluster together). The consistency matrix is arranged according to the consistency categories (tree at the top of the heatmap). The bar between the tree and the heat map is the category. The more scattered the dark blue squares, the weaker the clustering results. The cluster-consensus and item-consensus for AD and PD was analysed using the calcICL method in the ConsensusClusterPlus package (Figures 11A–D).
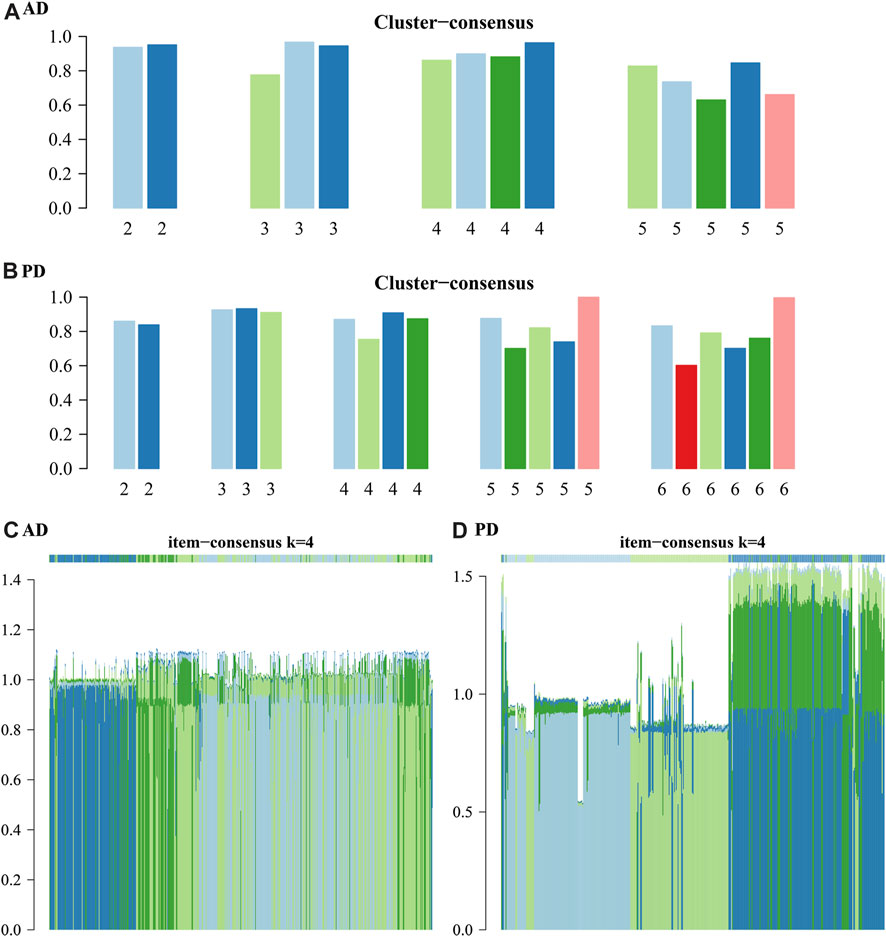
FIGURE 11. Cluster-consensus and item-consensus for AD and PD. (A–B) Cluster-Consensus Plot for AD and PD. These figures show the cluster-consensus value of each cluster under AD and PD (The mean value of pairwise consensus values of members in the cluster). The higher the value, the higher the stability. It can be used to assess the cluster-consensus values under the same and between different k values. We can see in (A), for AD, when k = 4, the mean values are high. In (B), for PD, when k = 4, the mean values are also very high. (C, D) Item-consensus Plot for AD and PD: This figure shows the score of each sample for AD and PD when k = 4.
From Figure 11 we can see whether the classification of each sample has sufficient fidelity, to help determine the k value. As shown in Figures 10, 11 we clustered the AD and PD disease samples in 4 clusters each. The sample clustering results and the 20 Adaptive and Innate immune cell scores for AD and PD across all samples are shown (Figure 7B). Next, we combined the immune cell fractions and sample cluster results for the Case samples from AD and PD for subsequent analysis.
For each consensus cluster, we calculated the cluster distribution of xCell scores of Adaptive and Innate immune genes in AD and PD and presented these in a box plot (Figures 12A–D). The Kruskal. Wallis test was performed to test differences in scores of immune cells in different clusters.
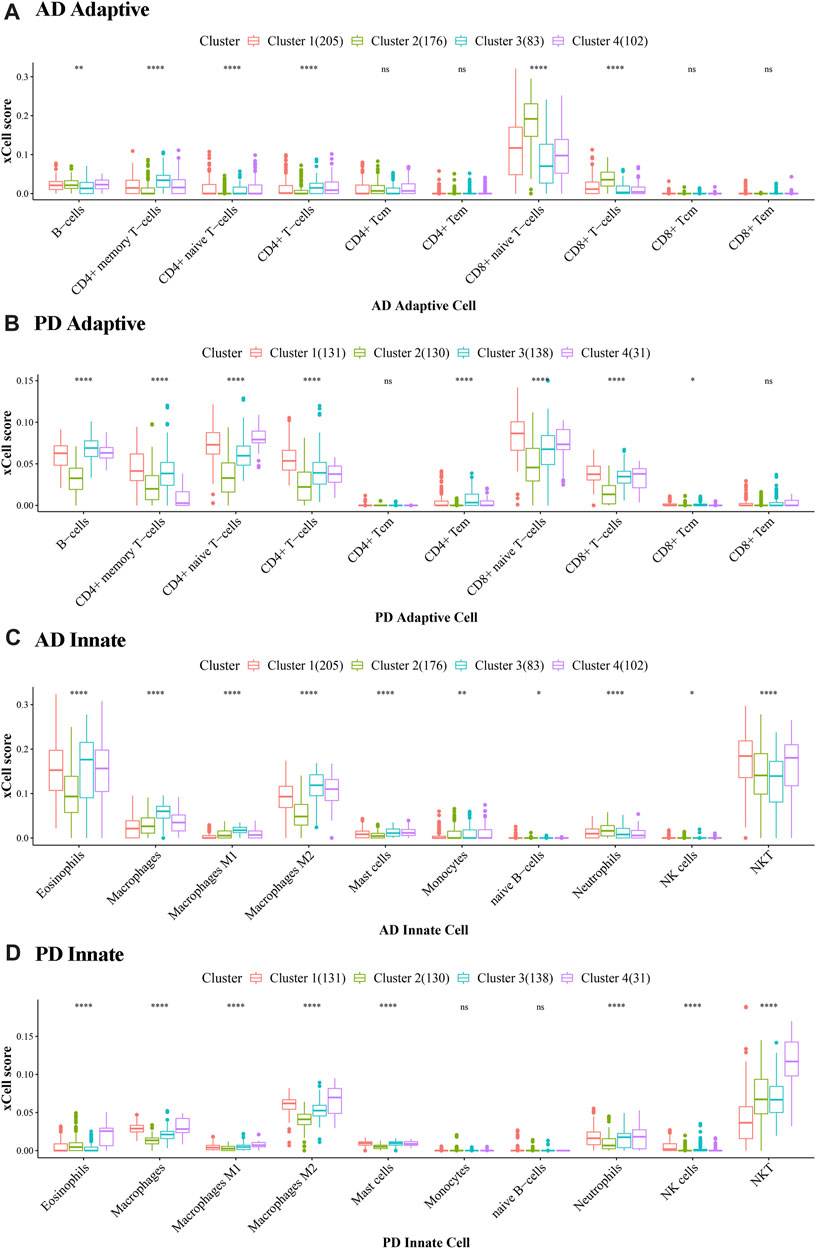
FIGURE 12. Cluster level expression of Adaptive and Innate immune genes in AD and PD. (A, B) Adaptive immune gene expression in AD and PD. (C, D) Innate immune gene expression in AD and PD. ns: p > 0.05; *: p ≤ 0.05; **: p ≤ 0.01; ***: p ≤ 0.001; ****: p ≤ 0.0001. The xCell scores of Adaptive and Innate immune genes in AD and PD were compared using Kruskal. Wallis tests to test differences in scores of immune cells in different clusters.
In Figure 12 we can see that there were significant differences between immune cells in different clusters, and the greater this difference, the more marked the difference between the clusters. We can also see the overall scores of immune cells in different clusters. The top 3 immune cells from the significant clusters in AD and PD were considered high expression immune cells that play an important role in disease pathology (Table 5).
The results showed that B-cells, CD4+ memory T-cells and CD8+ naive T-cells were adaptive immune cells that were highly expressed in all 4 clusters of both diseases, and innate immune cells Macrophages M2 and NKT, were similarly highly expressed in all clusters. Adaptive immune cells CD8+ naive T− Cells were significantly different between cluster 2 and cluster 3 in AD, and in PD, both.
We extracted the immune-related genes from the Crosstalk gene dataset and obtained 112 genes in total. Then we extracted the expression values of these 112 genes in the Case samples of AD and PD. Correlation analysis was conducted by combining these values with the xCell scores. These two datasets pertaining to each cluster were subjected to correlation analysis. Correlation results were obtained for each of the 8 clusters and are depicted (Figures 13A, B) allowing an estimation of immune cells bias in the different clusters, and also to allow for selection of specific cluster of subjects for longitudinal study.
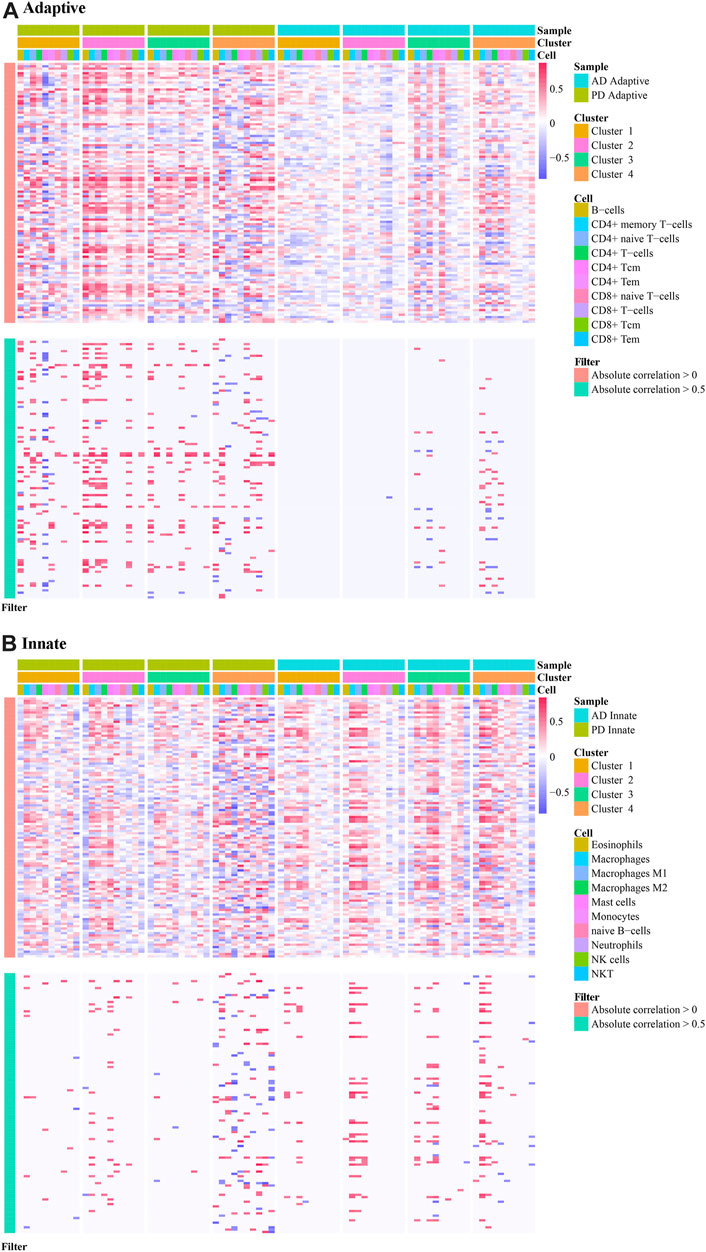
FIGURE 13. Correlation analysis applied to immune cell scores in different clusters. (A) The correlation of adaptive immune-related genes in different clusters in AD and PD; (B) The correlation of innate immune-related genes in different clusters in AD and PD. Spearman’s correlation analysis was performed.
The results show immune cell biases in different clusters. For adaptive immune-related genes, cluster1 and cluster4 in PD were highly correlated with a variety of immune cells. In cluster2 and cluster3, immune genes were positively correlated with immune cells. Adaptive immune related genes were highly correlated with a variety of immune cells in cluster 4 of AD, and participated in a variety of immune patterns (Figure 13A). For innate immune related genes, multiple immune cells in cluster4 of PD and cluster4 of AD were highly correlated, suggesting that innate genes were more active in cluster4 samples of PD and AD (Figure 13B).
Datasets of expression values for Case and Control samples of AD and PD were obtained. The expression values of the crosstalk genes were subjected to LASSO Logistic Regression to screen the crosstalk genes (Figures 14A, B). The intersecting genes were selected and 127 genes were recorded as the candidate biomarker crosstalk genes.
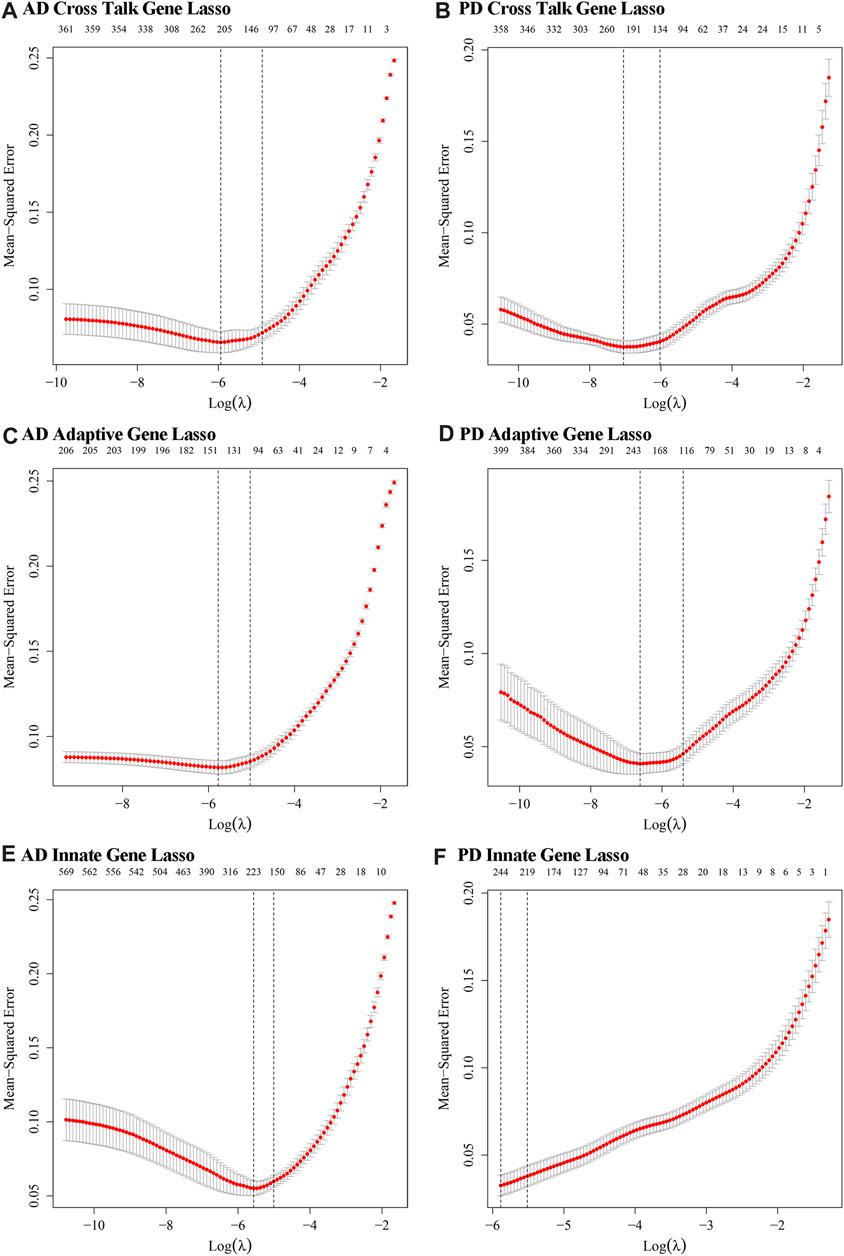
FIGURE 14. The optimal lambda values obtained from LASSO regression modelling of innate immune genes and adaptive immune genes. (A, B) Crosstalk genes: relationship between lambda value and mean square error in AD and PD Lasso regression analysis. The abscissa is log (lambda) and the ordinate is mean square error. There are two dashed lines in the figure, one is the value of λ with the minimum mean square error and the other is the value of λ with the standard error from the minimum mean square error. (C, D) Adaptive immune genes: lambda value and mean square error in AD and PD Lasso regression analysis. (E, F) Innate immune genes: lambda value and mean square error in AD and PD Lasso regression analysis.
For Adaptive immune-related genes, we extracted 431 genes’ expression profiles, which included 210 genes found in AD and 408 genes found in PD datasets and applied LASSO Logistic Regression (Figures 14C, D). The intersecting genes among AD and PD were selected and a total of 78 genes were recorded as the biomarker adaptive immune genes.
For Innate immune genes, we combined the innate immune genes obtained from literature with those obtained from InnateDB data to obtain 1,335 Innate immune genes. 571 such genes were found in AD and 1,183 in PD. LASSO Logistic Regression was applied (Figures 14E, F) and a total of 32 intersecting genes were recorded as biomarker Innate immune gene. A diagram displayed the variation of the remaining variables’ gene coefficients with different lambda values from the LASSO regression analysis.
We obtained 3 genes from the intersection of the biomarker crosstalk genes and the biomarker adaptive immune genes, and 1 gene (DEFB1) from the intersection of the biomarker crosstalk genes with the biomarker innate-immune genes. ROC analysis using the expression values of these 4 genes yielded 3 genes (DUSP14, F13A1, SELE) (Figures 15A, B).
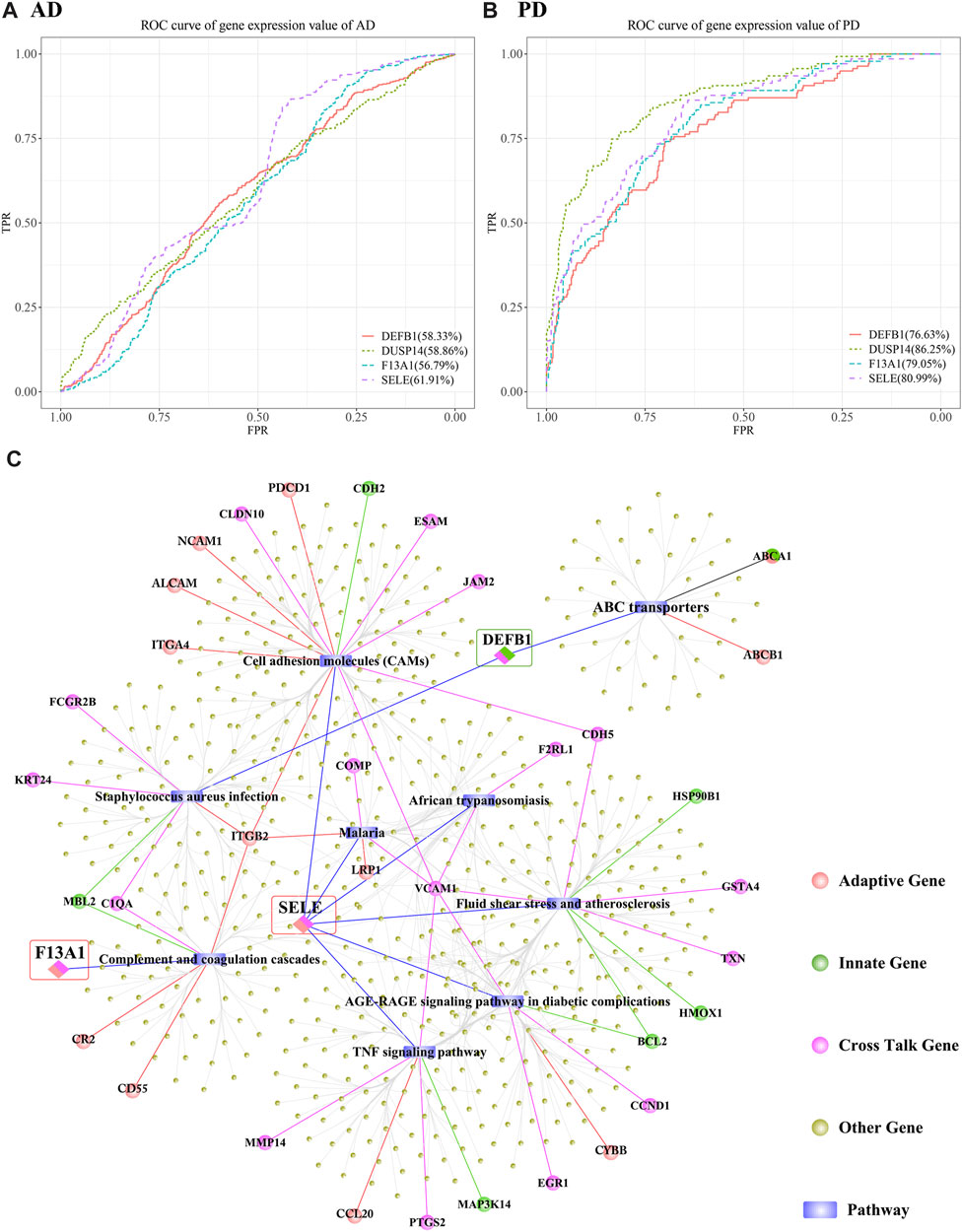
FIGURE 15. Predictive efficacy of DUSP14, F13A1, SELE and DEFB1 in AD and PD. (A, B) ROC results of DUSP14, F13A1, SELE and DEFB1 in AD and PD; (C) Functional correlation analysis results of DUSP14, F13A1, SELE and DEFB1.
AUC (AUC>70%) values obtained for DUSP14, F13A1, SELE and DEFB1 in discriminating PD were higher than those for AD prediction. SELE performed better than the other 3 genes in discriminating both AD and PD. To further analyse the functions of these genes, we obtained datasets of human KEGG pathways and related genes and mined the corresponding pathways, and then isolated all the genes in each pathway. We examined whether there is interaction between each pathway and the biomarker crosstalk genes, biomarker adaptive immune genes, and biomarker innate immune genes (Figure 15C). The results showed that SELE, an adaptive immune gene, mainly regulates the TNF signalling pathway, cell adhesion molecules (CAMs) and fluid shear stress and atherosclerosis. Within the TNF signalling pathway, VCAM1 represents a specific type of Cell adhesion Molecule (CAM). Within Cell adhesion Molecules (CAMs), the adaptive immune gene ITGB2 regulates both Staphylococcus aureus infection and complement and coagulation cascades. From Figure 15C, we can see that F13A1 is mainly involved in the regulation of complement and coagulation cascades pathway. DEFB1, an innate immune-related gene, is mainly involved in the regulation of S. aureus infection and ABC transporters. Within S. aureus infection, other crosstalk genes such as KRT24 and FCGR2B also participate in the regulation. In addition, ITGB2 and other genes are associated with other pathways to regulate the immune function in both AD and PD. It can be inferred from the above that immune-related crosstalk genes interact with other genes and jointly influence the two diseases.
The present bioinformatic study applied immunocorrelation analysis to identify immune-related genes, cells and pathways that might serve as key linkage mechanisms between AD and PD. We found that innate immune cells M2 macrophages and NKT are highly expressed in both AD and PD. M2 macrophages are primarily involved in the Th2 immune response. Th2 cells produce cytokines that promote the humoral immune response, including IL-4, IL-5, IL-6, IL-10 and IL-13 (Sun et al., 2021). NKT cells mediate proinflammatory and immunomodulatory effects, which range from B-cell regulation, production of specific antibodies, suppression of autoimmunity to cytokine production, dendritic cell crosstalk, and T/B cell interactions (Seidel et al., 2020a). Infiltration of the brain by peripheral NK cells with altered cytotoxic properties has been documented as a contributory mechanism to neuroinflammation in AD but the specific roles of infiltrating NKT cells, which share phenotypic and functional properties are less well understood in AD (Busse et al., 2021; Lu et al., 2021). In PD, NKT cells are known to be activated by several Gram-negative periodontal pathogens can play proinflammatory roles (Seidel et al., 2020b). Of note, gene expression based immune cell infiltration analysis may include the extrapolation of certain genes that may also be expressed in non-immune cell lineages under conditions such as stress or inflammation, and this could account for the prediction of adipocytes and hepatocyte expression, which is unsupported by experimental evidence. For instance, ICAM-1, expressed on immune cell lineages, is overexpressed on adipocytes and hepatocytes (Farhood et al., 1995; Singh et al., 2023).
Among the adaptive immune cells, B-cells, CD4+ memory T-cells and CD8+ naive T-cells were found highly expressed in all 4 clusters of AD and PD. B cells might exert protective functions in periodontitis. B-cell-deficient mice show alveolar bone loss without bacterial infection, while clinical evidence shows that B cells and plasma cells, along with osteoclastogenic factors, are involved in alveolar bone destruction in periodontitis (Zouali, 2017). AD is associated with B cell accumulation in brain tissue which can produce IgG to induce microglial activation (Park et al., 2022). Experimental evidence supports the notion of infectious disease driven microglial activation in AD (Hao et al., 2022) along with peripheral leukocyte infiltration of brain tissue secondary to persistent systemic inflammation (Lu et al., 2021), as seen in periodontitis, in particular NK cell infiltration (Le Page et al., 2018), and our findings were largely consistent. High levels of NKT cell-related immune genes were also implicated in AD in our results, and aberrant NKT cell homeostasis has been reported in AD (Sh et al., 2021). The CD8+ naïve T cell subset was also overrepresented in AD samples, consistent with experimental evidence demonstrating CD8+ T cell infiltration of AD-affected brain parenchyma which have been associated with upregulated IFN-β signalling and infection (Altendorfer et al., 2022). Eosiniophils were also markedly overrepresented, and eosinophilic inclusions are well documented in Alzheimer’s neurofibrillary tangles (Qian et al., 2022). Eosinophilic signatures have also been inversely correlated with AD stage. The M2 macrophage signature noted in AD samples is corroborated by peripheral macrophage infiltration in experimental models of AD (Rentsendorj et al., 2018) and the M2 phenotype has been correlated with AD but not experimentally validated (Lin C. et al., 2022). M1/M2 phenotype switch is also a key feature of microglial changes in AD, and while P. gingivalis infection is associated with M1 type microglial switch, the stage of AD is an important determinant of M1/M2 microglial balance (Lin J. et al., 2022). Ageing and senescence are associated with deregulation of immune responses, and higher risk of both AD and PD. Furthermore, gender-based differences in AD pathology are recognised. A limitation of this investigation is that the datasets were not matched for age, gender, and disease stage which may induce confounding and should be addressed in future clinical investigations. Cluster analysis revealed innate immunity associated genes were comparatively highly expressed in cluster4 samples of AD and PD, and whether these samples represent a distinct phenotype, a later stage of disease progression, or represent more advanced age, begets further questions which should be dissected in future longitudinal studies to fully understand the PD-AD link.
Using a data mining approach with a series of reductive analyses, we obtained a 3 gene set, DUSP14, F13A1 and SELE, as key crosstalk genes linking PD and AD, which was largely supported by experimental and clinical data. The mechanistic role of DUSP14 in mediating AD and PD is not investigated but several DUSP genes are shown to be deregulated during AD pathogenesis (An et al., 2021). Targeting DUSP 14 can counter NLRP2 inflammasome mediated immune-inflammatory pathways and has shown positive effects in ameliorating neuroinflammtion and cognitive dysfunction (Que et al., 2020). There are few reports regarding the roles of DUSP14 in the literature, mainly focusing on pathways related to T cells. DUSP14 can downregulate T-cell receptor signalling by inhibiting TGF-β-activated kinase 1-binding protein 1 (TAB1) activation (Yang et al., 2014). DUSP14 is a mitogen-activated protein kinase phosphatase that plays a critical role in the regulation of T cell activity. TRAF2 mediated Lys63-linked ubiquitination of DUSP14 leads to DUSP14 activation in T cells (Yang et al., 2016). DUSP14 directly interacts with TGF-beta-activated kinase 1 (TAK1)-binding protein 1 (TAB1) and dephosphorylated TAB1 at Ser(438), leading to TAB1-TAK1 complex inactivation in T cells and can downregulate T-cell receptor (TCR) signalling by inhibiting TAB1 activation (Yang et al., 2014). Activated DUSP14 also directly dephosphorylates extracellular signal-regulated kinases (ERK) and attenuates the ERK signalling pathway. TRAF2-mediated ubiquitination of Lys63-linked DUSP14 also enhances its phosphatase activity (Chen et al., 2019). Protein arginine methyltransferase (PRMT)5-mediated arginine methylation may sequentially stimulate TRAF2-mediated DUSP14 ubiquitination and phosphatase activity, leading to inhibition of TCR signalling (Yang et al., 2018). Therefore, enhancement/activation of DUSP14 or DUSP14 upstream molecules is a potential modality for the attenuation of autoimmune diseases such as systemic lupus erythematosus (SLE) (Chuang and Tan, 2019).
F13A1 is involved in clot stabilization and implicated in a number of immunoinflammatory diseases (Dull et al., 2021). The role of F13A1 has been investigated in AD. F13A1 subunit was detected by immunohistochemistry in a subset of AD reactive microglia, while F13A1 Val34Leu gene polymorphism is associated with sporadic AD where homozygous LL genotype shows about a fourfold higher risk of developing AD compared to the homozygous VV genotype (Gerardino et al., 2006). F13A1 may also influence the maintenance of neural connections (Festoff et al., 2001). The F13A1 204Phe allele is strongly associated with ischemic stroke in young women and the homozygous genotype (Phe/Phe) are associated with manyfold higher stroke risk than heterozygous (Tyr/Phe) genotype (Pruissen et al., 2008). Functionally, a pro-angiogenic function of F13A1 is affected by the interaction between vascular endothelial growth factor receptor 2 (VEGFR2) and integrin αvβ3 on the cell membrane, which facilitates important steps in granulation tissue formation at wound sites. F13A1 deficiency can thus present as intracranial haemorrhage, delayed bleeding or chronic wounding of the skin and impaired mucosal healing. F13A1 thus functions to link primary hemostasis, coagulation, and definitive tissue healing. Another important recently identified function of F13A1 is its ability to control cellular infiltration by binding to specific macromolecules, thereby limiting bacterial spread at the wound site and promoting host cell migration and survival (Gemmati et al., 2016). In the brain, F13A1 expression has been detected by immunohistochemistry in reactive microglia during glioma formation, which is a distinctive feature of AD pathogenesis (Gerardino et al., 2006). F13A1 levels are gradually elevated from controls to mild cognitive impairment (MCI) and AD. More importantly, F13A1 in the serum proteome can serve as a potential non-invasive early diagnostic marker of MCI and AD (Kang et al., 2016). Of note, PD pathogens can induce the upregulation of the coagulation cascade-related genes in endothelial cells (Salmina et al., 2010) and may contribute the endothelial dysfunction inherent to AD pathogenesis (Hossain et al., 2020).
SELE encodes for E-selectin and is involved in Leukocyte/endothelial cell adhesion, and its expression is reported to increase 4-fold in Treponema denticola oral infections (Chukkapalli et al., 2014), a subgingival oral spirochete species which is a key periodontal pathogen (Zeng et al., 2021). Its role in several age-associated conditions such as age-related macular degeneration (Mullins et al., 2011) and other conditions. SELE has been found to be related with peripheral arterial occlusive disease (Shaker et al., 2010). The serum level of SELE has been found significantly elevated in systemic sclerosis with early onset disease (Hasegawa et al., 2014). Regarding periodontitis, the Ser128Arg polymorphism is associated with periodontitis (Houshmand et al., 2009). SELE expression is also found positively correlated with the duration of Sjogren’s syndrome, characterised by dysregulation of circulating immune cells, T cells and antigen presenting cells and vascular endothelial extravasation (Turkcapar et al., 2005; Blochowiak et al., 2017). In an animal model of AD, SELE expression was found significantly elevated, indicating its role in AD development (Wang et al., 2020). The cell-surface glycoprotein E selectin plays an important role in immune adhesion (McEver, 2015). It is also associated with the accumulation of white blood cells at sites of inflammation by mediating cell adhesion to the intima of blood vessels. As a clinical diagnosis, AD shows variable pathology. Clinically, E-selectin has been found significantly raised in the cerebrospinal fluid (CSF) of AD patients without typical signature biomarker profiles, suggesting it may specifically mark the vascular mechanisms underlying AD pathology (Li et al., 2015). The SELE Ser128Arg gene polymorphism has also been associated with AD (Horstmann et al., 2010; Ribizzi et al., 2010; Flex et al., 2014) and SELE polymorphisms are also associated with Lewy body dementias (Rajkumar et al., 2020). The findings of the functional correlation analysis indicate an interaction between these candidate biomarker genes with key pathways intersecting adaptive immune responses, TNF alpha mediated inflammation, and endothelial dysfunction, supporting PD infection-mediated systemic immune dysregulation at the core of the AD-PD link.
Overall, the findings of this bioinformatic study were supported by exisiting experimental evidence addressing PD and AD but the roles of the discovered biomarkers DUSP14, F13A1 and SELE in mediating the link between the two diseases has not been addressed. A major limitation of the present study is the lack of validation experiments using cell, animal or clinical data. Therefore, the present data must be considered as a theoretical premise for further investigation that explores the validity of these biomarkers in large-scale clinical trials and their mechanistic roles in experimental or translational research focused on immune mechanisms implicated in AD and PD linkage.
Bioinformatic analysis integrating experimental transcriptomic data from Alzheimer’s disease and periodontitis revealed the most robust potentially shared molecular linkages. Three biomarker crosstalk genes; DUSP14, F13A1 and SELE were identified as the most robust signature. Macrophages M2 and NKT among innate immune cells, and B-cells, CD4+ memory T-cells and CD8+ naive T-cells among adaptive immune cells emerged as top immune cells linking PD and AD. These findings warrant future research in experimental and clinical studies.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Conceptualization, XH and GS; methodology, JJ; software, MG, and MC; validation, JJ, YL, and LZ; formal analysis, BZ; investigation, MC; resources, JJ and MG; data curation, JJ and MG; writing—original draft preparation, JJ and MG; writing—review and editing, XH and GS; visualization, MG; supervision, XH; project administration, XH. All authors contributed to the article and approved the submitted version.
We want to thank my colleagues for their collaboration and help during gathering data for my research project. We would also thank my parents and my lovely kid for their kindness to let me have time and energy to finish this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agrawal, S., Baulch, J. E., Madan, S., Salah, S., Cheeks, S. N., Krattli, R. P., et al. (2022). Impact of IL-21-associated peripheral and brain crosstalk on the Alzheimer's disease neuropathology. Cell Mol. Life Sci. 79 (6), 331. doi:10.1007/s00018-022-04347-6
Altendorfer, B., Unger, M. S., Poupardin, R., Hoog, A., Asslaber, D., Gratz, I. K., et al. (2022). Transcriptomic profiling identifies CD8+ T cells in the brain of aged and alzheimer’s disease transgenic mice as tissue-resident memory T cells. J. Immunol. 209 (7), 1272–1285. doi:10.4049/jimmunol.2100737
An, N., Bassil, K., Al Jowf, G. I., Steinbusch, H. W., Rothermel, M., de Nijs, L., et al. (2021). Dual-specificity phosphatases in mental and neurological disorders. Prog. Neurobiol. 198, 101906. doi:10.1016/j.pneurobio.2020.101906
Baima, G., Romandini, M., Citterio, F., Romano, F., and Aimetti, M. (2022). Periodontitis and accelerated biological aging: A geroscience approach. J. Dent. Res. 101 (2), 125–132. doi:10.1177/00220345211037977
Bairamian, D., Sha, S., Rolhion, N., Sokol, H., Dorothee, G., Lemere, C. A., et al. (2022). Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer's disease. Mol. Neurodegener. 17 (1), 19. doi:10.1186/s13024-022-00522-2
Bettcher, B. M., Tansey, M. G., Dorothee, G., and Heneka, M. T. (2021). Peripheral and central immune system crosstalk in Alzheimer disease - a research prospectus. Nat. Rev. Neurol. 17 (11), 689–701. doi:10.1038/s41582-021-00549-x
Blochowiak, K. J., Olewicz-Gawlik, A., Trzybulska, D., Nowak-Gabryel, M., Kociecki, J., Witmanowski, H., et al. (2017). Serum ICAM-1, VCAM-1 and E-selectin levels in patients with primary and secondary Sjogren's syndrome. Adv. Clin. Exp. Med. 26 (5), 835–842. doi:10.17219/acem/61434
Busse, S., Hoffmann, J., Michler, E., Hartig, R., Frodl, T., and Busse, M. (2021). Dementia-associated changes of immune cell composition within the cerebrospinal fluid. Brain, Behav. Immunity-Health 14, 100218. doi:10.1016/j.bbih.2021.100218
Campbell, S. L., and Gear, C. W. (1995). The index of general nonlinear DAEs. DAES. Numer. Math. 72 (2), 173–196. doi:10.1007/s002110050165
Charoentong, P., Finotello, F., Angelova, M., Mayer, C., Efremova, M., Rieder, D., et al. (2017). Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 18 (1), 248–262. doi:10.1016/j.celrep.2016.12.019
Chen, H. F., Chuang, H. C., and Tan, T. H. (2019). Regulation of dual-specificity phosphatase (DUSP) ubiquitination and protein stability. Int. J. Mol. Sci. 20 (11), 2668. doi:10.3390/ijms20112668
Chuang, H. C., and Tan, T. H. (2019). MAP4K family kinases and DUSP family phosphatases in T-cell signaling and systemic lupus erythematosus. Cells 8 (11), 1433. doi:10.3390/cells8111433
Chukkapalli, S. S., Rivera, M. F., Velsko, I. M., Lee, J. Y., Chen, H., Zheng, D., et al. (2014). Invasion of oral and aortic tissues by oral spirochete Treponema denticola in ApoE(-/-) mice causally links periodontal disease and atherosclerosis. Infect. Immun. 82 (5), 1959–1967. doi:10.1128/IAI.01511-14
Dioguardi, M., Crincoli, V., Laino, L., Alovisi, M., Sovereto, D., Mastrangelo, F., et al. (2020). The role of periodontitis and periodontal bacteria in the onset and progression of alzheimer’s disease: a systematic review. J. Clin. Med. 9 (2), 495. doi:10.3390/jcm9020495
Dominy, S. S., Lynch, C., Ermini, F., Benedyk, M., Marczyk, A., Konradi, A., et al. (2019). Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5 (1), eaau3333. doi:10.1126/sciadv.aau3333
Dull, K., Fazekas, F., and Törőcsik, D. (2021). Factor XIII-A in diseases: role beyond blood coagulation. Int. J. Mol. Sci. 22 (3), 1459. doi:10.3390/ijms22031459
Eriksson, U. K., Pedersen, N. L., Reynolds, C. A., Hong, M. G., Prince, J. A., Gatz, M., et al. (2011). Associations of gene sequence variation and serum levels of C-reactive protein and interleukin-6 with Alzheimer's disease and dementia. J. Alzheimers Dis. 23 (2), 361–369. doi:10.3233/JAD-2010-101671
Farhood, A., McGuire, G. M., Manning, A. M., Miyasaka, M., Smith, C. W., and Jaeschke, H. (1995). Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J. Leucocyte Biol. 57 (3), 368–374. doi:10.1002/jlb.57.3.368
Festoff, B. W., Suo, Z., and Citron, B. A. (2001). Plasticity and stabilization of neuromuscular and CNS synapses: interactions between thrombin protease signaling pathways and tissue transglutaminase. Int. Rev. Cytol. 211, 153–177. doi:10.1016/s0074-7696(01)11018-1
Flex, A., Giovannini, S., Biscetti, F., Liperoti, R., Spalletta, G., Straface, G., et al. (2014). Effect of proinflammatory gene polymorphisms on the risk of Alzheimer's disease. Neurodegener. Dis. 13 (4), 230–236. doi:10.1159/000353395
Gaudilliere, D. K., Culos, A., Djebali, K., Tsai, A. S., Ganio, E. A., Choi, W. M., et al. (2019). Systemic immunologic consequences of chronic periodontitis. J. Dent. Res. 98 (9), 985–993. doi:10.1177/0022034519857714
Gemmati, D., Vigliano, M., Burini, F., Mari, R., El Mohsein, H. H., Parmeggiani, F., et al. (2016). Coagulation factor XIIIA (F13A1): novel perspectives in treatment and pharmacogenetics. Curr. Pharm. Des. 22 (11), 1449–1459. doi:10.2174/1381612822666151210122954
Gerardino, L., Papaleo, P., Flex, A., Gaetani, E., Fioroni, G., Pola, P., et al. (2006). Coagulation factor XIII Val34Leu gene polymorphism and Alzheimer's disease. Neurol. Res. 28 (8), 807–809. doi:10.1179/016164106X110454
Hajishengallis, G. (2014b). Aging and its impact on innate immunity and inflammation: implications for periodontitis. J. Oral Biosci. 56 (1), 30–37. doi:10.1016/j.job.2013.09.001
Hajishengallis, G. (2014a). The inflammophilic character of the periodontitis-associated microbiota. Mol. Oral Microbiol. 29 (6), 248–257. doi:10.1111/omi.12065
Hao, X., Li, Z., Li, W., Katz, J., Michalek, S. M., Barnum, S. R., et al. (2022). Periodontal infection aggravates C1q-mediated microglial activation and synapse pruning in alzheimer’s mice. Front. Immunol. 13, 816640. doi:10.3389/fimmu.2022.816640
Hasegawa, M., Asano, Y., Endo, H., Fujimoto, M., Goto, D., Ihn, H., et al. (2014). Serum adhesion molecule levels as prognostic markers in patients with early systemic sclerosis: a multicentre, prospective, observational study. PLoS One 9 (2), e88150. doi:10.1371/journal.pone.0088150
Holmes, C., Cunningham, C., Zotova, E., Culliford, D., and Perry, V. H. (2011). Proinflammatory cytokines, sickness behavior, and Alzheimer disease. Neurology 77 (3), 212–218. doi:10.1212/WNL.0b013e318225ae07
Horstmann, S., Budig, L., Gardner, H., Koziol, J., Deuschle, M., Schilling, C., et al. (2010). Matrix metalloproteinases in peripheral blood and cerebrospinal fluid in patients with Alzheimer's disease. Int. Psychogeriatr. 22 (6), 966–972. doi:10.1017/S1041610210000827
Hossain, K. H., Okamoto, T., Usuda, H., Jahan, I., Niibayashi, T., and Wada, K. (2020). Differential expression of pro-inflammatory and pro-coagulant genes in endothelial cells induced by Porphyromonas gingivalis lipopolysaccharide, Escherichia coli lipopolysaccharide, and zymosan. Shimane J. Med. Sci. 37 (4), 123–132. doi:10.51010/sjms.37.4_123
Houshmand, B., Rafiei, A., Hajilooi, M., Mani-Kashani, K., and Gholami, L. (2009). E-selectin and L-selectin polymorphisms in patients with periodontitis. J. Periodontal Res. 44 (1), 88–93. doi:10.1111/j.1600-0765.2008.01092.x
Hu, X., Zhang, J., Qiu, Y., and Liu, Z. (2021). Periodontal disease and the risk of Alzheimer's disease and mild cognitive impairment: a systematic review and meta-analysis. Psychogeriatrics 21 (5), 813–825. doi:10.1111/psyg.12743
Kang, S., Jeong, H., Baek, J. H., Lee, S. J., Han, S. H., Cho, H. J., et al. (2016). PiB-PET imaging-based serum proteome profiles predict mild cognitive impairment and Alzheimer's disease. J. Alzheimers Dis. 53 (4), 1563–1576. doi:10.3233/JAD-160025
Kinney, J. W., Bemiller, S. M., Murtishaw, A. S., Leisgang, A. M., Salazar, A. M., and Lamb, B. T. (2018). Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement. (N Y) 4, 575–590. doi:10.1016/j.trci.2018.06.014
Le Page, A., Dupuis, G., Frost, E. H., Larbi, A., Pawelec, G., Witkowski, J. M., et al. (2018). Role of the peripheral innate immune system in the development of Alzheimer's disease. Exp. Gerontol. 107, 59–66. doi:10.1016/j.exger.2017.12.019
Li, G., Xiong, K., Korff, A., Pan, C., Quinn, J. F., Galasko, D. R., et al. (2015). Increased CSF E-selectin in clinical Alzheimer's disease without altered CSF Aβ42 and tau. J. Alzheimers Dis. 47 (4), 883–887. doi:10.3233/JAD-150420
Lin, C., Xu, C., Zhou, Y., Chen, A., and Jin, B. (2022a). Identification of biomarkers related to M2 macrophage infiltration in Alzheimer’s disease. Cells 11 (15), 2365. doi:10.3390/cells11152365
Lin, J., Huang, D., Xu, H., Zhan, F., and Tan, X. (2022b). Macrophages: A communication network linking Porphyromonas gingivalis infection and associated systemic diseases. Front. Immunol. 13, 952040. doi:10.3389/fimmu.2022.952040
Lopez-Rodriguez, A. B., Hennessy, E., Murray, C. L., Nazmi, A., Delaney, H. J., Healy, D., et al. (2021). Acute systemic inflammation exacerbates neuroinflammation in Alzheimer's disease: IL-1β drives amplified responses in primed astrocytes and neuronal network dysfunction. Alzheimers Dement. 17 (10), 1735–1755. doi:10.1002/alz.12341
Lu, Y., Li, K., Hu, Y., and Wang, X. (2021). Expression of immune related genes and possible regulatory mechanisms in Alzheimer’s disease. Front. Immunol. 12, 768966. doi:10.3389/fimmu.2021.768966
Maekawa, T., Krauss, J. L., Abe, T., Jotwani, R., Triantafilou, M., Triantafilou, K., et al. (2014). Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 15 (6), 768–778. doi:10.1016/j.chom.2014.05.012
McEver, R. P. (2015). Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 107 (3), 331–339. doi:10.1093/cvr/cvv154
McManus, R. M., Mills, K. H., and Lynch, M. A. (2015). T cells-protective or pathogenic in Alzheimer's disease? J. Neuroimmune Pharmacol. 10 (4), 547–560. doi:10.1007/s11481-015-9612-2
Mullins, R. F., Skeie, J. M., Folk, J. C., Solivan-Timpe, F. M., Oetting, T. A., Huang, J., et al. (2011). Evaluation of variants in the selectin genes in age-related macular degeneration. BMC Med. Genet. 12, 58. doi:10.1186/1471-2350-12-58
Murakami, S., Mealey, B. L., Mariotti, A., and Chapple, I. L. C. (2018). Dental plaque-induced gingival conditions. J. Periodontol. 89 (1), S17-S27–S27. doi:10.1002/JPER.17-0095
Park, J. C., Noh, J., Jang, S., Kim, K. H., Choi, H., Lee, D., et al. (2022). Association of B cell profile and receptor repertoire with the progression of Alzheimer’s disease. Cell Rep. 40 (12), 111391. doi:10.1016/j.celrep.2022.111391
Pruissen, D. M., Slooter, A. J., Rosendaal, F. R., van der Graaf, Y., and Algra, A. (2008). Coagulation factor XIII gene variation, oral contraceptives, and risk of ischemic stroke. Blood 111 (3), 1282–1286. doi:10.1182/blood-2007-08-110254
Puris, E., Auriola, S., Korhonen, P., Loppi, S., Kanninen, K. M., Malm, T., et al. (2021). Systemic inflammation induced changes in protein expression of ABC transporters and ionotropic glutamate receptor subunit 1 in the cerebral cortex of familial Alzheimer`s disease mouse model. J. Pharm. Sci. 110 (12), 3953–3962. doi:10.1016/j.xphs.2021.08.013
Qian, X. H., Liu, X. L., Chen, S. D., and Tang, H. D. (2022). Identification of immune hub genes associated with braak stages in Alzheimer’s Disease and their correlation of immune infiltration. Front. Aging Neurosci. 14, 887168. doi:10.3389/fnagi.2022.887168
Que, Y. Y., Zhu, T., Zhang, F. X., and Peng, J. (2020). Neuroprotective effect of DUSP14 overexpression against isoflurane-induced inflammatory response, pyroptosis and cognitive impairment in aged rats through inhibiting the NLRP3 inflammasome. Eur. Rev. Med. Pharmacol. Sci. 24 (12), 7101–7113. doi:10.26355/eurrev_202006_21704
Rajkumar, A. P., Bidkhori, G., Shoaie, S., Clarke, E., Morrin, H., Hye, A., et al. (2020). Postmortem cortical transcriptomics of Lewy body dementia reveal mitochondrial dysfunction and lack of neuroinflammation. Am. J. Geriatr. Psychiatry 28 (1), 75–86. doi:10.1016/j.jagp.2019.06.007
Rakic, S., Hung, Y. M. A., Smith, M., So, D., Tayler, H. M., Varney, W., et al. (2018). Systemic infection modifies the neuroinflammatory response in late stage Alzheimer's disease. Acta Neuropathol. Commun. 6 (1), 88. doi:10.1186/s40478-018-0592-3
Rentsendorj, A., Sheyn, J., Fuchs, D. T., Daley, D., Salumbides, B. C., Schubloom, H. E., et al. (2018). A novel role for osteopontin in macrophage-mediated amyloid-β clearance in Alzheimer’s models. Brain, Behav. Immun. 67, 163–180. doi:10.1016/j.bbi.2017.08.019
Ribizzi, G., Fiordoro, S., Barocci, S., Ferrari, E., and Megna, M. (2010). Cytokine polymorphisms and alzheimer disease: possible associations. Neurol. Sci. 31 (3), 321–325. doi:10.1007/s10072-010-0221-9
Ryder, M. I. (2020). Porphyromonas gingivalis and alzheimer disease: recent findings and potential therapies. J. Periodontology 91, S45-S49–S49. doi:10.1002/JPER.20-0104
Salmina, A. B., Inzhutova, A. I., Malinovskaya, N. A., and Petrova, M. M. (2010). Endothelial dysfunction and repair in alzheimer-type neurodegeneration: neuronal and glial control. J. Alzheimer's Dis. 22 (1), 17–36. doi:10.3233/JAD-2010-091690
Seidel, A., Seidel, C. L., Weider, M., Junker, R., Golz, L., and Schmetzer, H. (2020a). Influence of natural killer cells and natural killer T cells on periodontal disease: A systematic review of the current literature. Int. J. Mol. Sci. 21 (24), 9766. doi:10.3390/ijms21249766
Seidel, A., Seidel, C. L., Weider, M., Junker, R., Gölz, L., and Schmetzer, H. (2020b). Influence of natural killer cells and natural killer T cells on periodontal disease: A systematic review of the current literature. Int. J. Mol. Sci. 21 (24), 9766. doi:10.3390/ijms21249766
Sh, Y., Liu, B., Zhang, J., Zhou, Y., Hu, Z., and Zhang, X. (2021). Application of artificial intelligence modeling technology based on fluid biopsy to diagnose alzheimer’s disease. Front. Aging Neurosci. 13, 768229. doi:10.3389/fnagi.2021.768229
Shaker, O., Zahra, A., Sayed, A., Refaat, A., El-Khaiat, Z., Hegazy, G., et al. (2010). Role of ICAM-1 and E-selectin gene polymorphisms in pathogenesis of PAOD in Egyptian patients. Vasc. Health Risk Manag. 6, 9–15. doi:10.2147/vhrm.s8143
Shen, X. N., Niu, L. D., Wang, Y. J., Cao, X. P., Liu, Q., Tan, L., et al. (2019). Inflammatory markers in Alzheimer's disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry 90 (5), 590–598. doi:10.1136/jnnp-2018-319148
Singh, V., Kaur, R., Kumari, P., Pasricha, C., and Singh, R. (2023). ICAM-1 and VCAM-1: gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 548, 117487. doi:10.1016/j.cca.2023.117487
Sun, X., Gao, J., Meng, X., Lu, X., Zhang, L., and Chen, R. (2021). Polarized macrophages in periodontitis: characteristics, function, and molecular signaling. Front. Immunol. 12, 763334. doi:10.3389/fimmu.2021.763334
Tao, Q., Ang, T. F. A., DeCarli, C., Auerbach, S. H., Devine, S., Stein, T. D., et al. (2018). Association of chronic low-grade inflammation with risk of alzheimer disease in ApoE4 carriers. JAMA Netw. Open 1 (6), e183597. doi:10.1001/jamanetworkopen.2018.3597
Turkcapar, N., Sak, S. D., Saatci, M., Duman, M., and Olmez, U. (2005). Vasculitis and expression of vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E-selectin in salivary glands of patients with Sjogren's syndrome. J. Rheumatol. 32 (6), 1063–1070.
van Olst, L., Coenen, L., Nieuwland, J. M., Rodriguez-Mogeda, C., de Wit, N. M., Kamermans, A., et al. (2022). Crossing borders in Alzheimer's disease: A T cell's perspective. Adv. Drug Deliv. Rev. 188, 114398. doi:10.1016/j.addr.2022.114398
Walker, K. A., Gross, A. L., Moghekar, A. R., Soldan, A., Pettigrew, C., Hou, X., et al. (2020). Association of peripheral inflammatory markers with connectivity in large-scale functional brain networks of non-demented older adults. Brain Behav. Immun. 87, 388–396. doi:10.1016/j.bbi.2020.01.006
Wang, Y., Zhang, X., Song, Q., Hou, Y., Liu, J., Sun, Y., et al. (2020). Characterization of the chromatin accessibility in an Alzheimer's disease (AD) mouse model. Alzheimers Res. Ther. 12 (1), 29. doi:10.1186/s13195-020-00598-2
Xie, J., Gorle, N., Vandendriessche, C., Van Imschoot, G., Van Wonterghem, E., Van Cauwenberghe, C., et al. (2021). Low-grade peripheral inflammation affects brain pathology in the App(NL-G-F)mouse model of Alzheimer's disease. Acta Neuropathol. Commun. 9 (1), 163. doi:10.1186/s40478-021-01253-z
Xu, X. W., Liu, X., Shi, C., and Sun, H. C. (2021). Roles of immune cells and mechanisms of immune responses in periodontitis. Chin. J. Dent. Res. 24 (4), 219–230. doi:10.3290/j.cjdr.b2440547
Yang, C. Y., Chiu, L. L., Chang, C. C., Chuang, H. C., and Tan, T. H. (2018). Induction of DUSP14 ubiquitination by PRMT5-mediated arginine methylation. FASEB J. 32, fj201800244RR. doi:10.1096/fj.201800244RR
Yang, C. Y., Chiu, L. L., and Tan, T. H. (2016). TRAF2-mediated Lys63-linked ubiquitination of DUSP14/MKP6 is essential for its phosphatase activity. Cell Signal 28 (1), 145–151. doi:10.1016/j.cellsig.2015.10.017
Yang, C. Y., Li, J. P., Chiu, L. L., Lan, J. L., Chen, D. Y., Chuang, H. C., et al. (2014). Dual-specificity phosphatase 14 (DUSP14/MKP6) negatively regulates TCR signaling by inhibiting TAB1 activation. J. Immunol. 192 (4), 1547–1557. doi:10.4049/jimmunol.1300989
Zeng, H., Chan, Y., Gao, W., Leung, W. K., and Watt, R. M. (2021). Diversity of Treponema denticola and other oral treponeme lineages in subjects with periodontitis and gingivitis. Microbiol. Spectr. 9 (2), e0070121. doi:10.1128/Spectrum.00701-21
Keywords: Alzheimer’s disease, periodontitis, neuroinflammation, multimorbidity, immune pathways, gene expression signature
Citation: Jin J, Guang M, Li S, Liu Y, Zhang L, Zhang B, Cheng M, Schmalz G and Huang X (2023) Immune-related signature of periodontitis and Alzheimer’s disease linkage. Front. Genet. 14:1230245. doi: 10.3389/fgene.2023.1230245
Received: 27 June 2023; Accepted: 22 September 2023;
Published: 02 October 2023.
Edited by:
Jeffrey Miecznikowski, University at Buffalo, United StatesReviewed by:
Haoxiang Cheng, Icahn School of Medicine at Mount Sinai, United StatesCopyright © 2023 Jin, Guang, Li, Liu, Zhang, Zhang, Cheng, Schmalz and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng Huang, aHVhbmd4ZjE5OThAMTYzLmNvbQ==; Gerhard Schmalz, R2VyaGFyZC5TY2htYWx6QG1lZGl6aW4udW5pLWxlaXB6aWcuZGU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.