
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Genet. , 17 July 2023
Sec. Genetics of Common and Rare Diseases
Volume 14 - 2023 | https://doi.org/10.3389/fgene.2023.1198821
Parts of this article's content have been modified or rectified in:
Erratum: Case report: atypical Silver-Russell syndrome patient with hand dystonia: the valuable support of the consensus statement to the wide syndromic spectrum
 Alessandro Vimercati1
Alessandro Vimercati1 Pierpaola Tannorella1
Pierpaola Tannorella1 Eleonora Orlandini2
Eleonora Orlandini2 Luciano Calzari3
Luciano Calzari3 Mirella Moro4
Mirella Moro4 Sara Guzzetti1
Sara Guzzetti1 Angelo Selicorni5
Angelo Selicorni5 Milena Crippa1
Milena Crippa1 Lidia Larizza1
Lidia Larizza1 Maria Teresa Bonati6*
Maria Teresa Bonati6* Silvia Russo1*
Silvia Russo1*The amount of Insulin Growth Factor 2 (IGF2) controls the rate of embryonal and postnatal growth. The IGF2 and adjacent H19 are the imprinted genes of the telomeric cluster in the 11p15 chromosomal region regulated by differentially methylated regions (DMRs) or imprinting centers (ICs): H19/IGF2:IG-DMR (IC1). Dysregulation due to IC1 Loss-of-Methylation (LoM) or Gain-of-Methyaltion (GoM) causes Silver–Russell syndrome (SRS) or Beckwith–Wiedemann syndrome (BWS) disorders associated with growth retardation or overgrowth, respectively. Specific features define each of the two syndromes, but isolated asymmetry is a common cardinal feature, which is considered sufficient for a diagnosis in the BWS spectrum. Here, we report the case of a girl with right body asymmetry, which suggested BWS spectrum. Later, BWS/SRS molecular analysis identified IC1_LoM revealing the discrepant diagnosis of SRS. A clinical re-evaluation identified a relative macrocephaly and previously unidentified growth rate at lower limits of normal at birth, feeding difficulties, and asymmetry. Interestingly, and never previously described in IC1_LoM SRS patients, since the age of 16, she has developed hand-writer’s cramps, depression, and bipolar disorder. Trio-WES identified a VPS16 heterozygous variant [NM_022575.4:c.2185C>G:p.Leu729Val] inherited from her healthy mother. VPS16 is involved in the endolysosomal system, and its dysregulation is linked to autosomal dominant dystonia with incomplete penetrance and variable expressivity. IGF2 involvement in the lysosomal pathway led us to speculate that the neurological phenotype of the proband might be triggered by the concurrent IGF2 deficit and VPS16 alteration.
Silver–Russell syndrome (SRS, MIM #180860) is a rare imprinting disorder (1:30.000–1:100.000), characterized by severe prenatal and postnatal growth retardation, relative macrocephaly at birth, body asymmetry, prominent forehead, and feeding difficulties. Clinical diagnosis is based on the occurrence of at least four out of six clinical signs, in accordance with the Netchine–Harbison clinical scoring system (NH-CSS), but molecular testing is recommended in patients with ≥3/6 criteria. The most common underlying mechanisms are loss of methylation of the paternal allele at H19/IGF2:IG-DMR in the 11p15.5 region (IC1_LoM and 30%–60% of cases) and maternal uniparental disomy of chromosome 7 (UPD(7)mat and 5%–10% of cases) involving GRB10:alt-TSS-DMR, PEG10:TSS-DMR, and MEST:alt-TSS-DMR. Other rare abnormalities, such as UPD(14)mat, UPD(16)mat, UPD(20)mat, and pathogenic variants within the CDKN1C, IGF2, PLAG1, and HMGA2 genes have been described in a minor fraction of SRS patients. To date, the diagnosis remains clinically based in about 40% of SRS patients (Wakeling et al., 2017; Abi Habib et al., 2018).
Among the “non-cardinal” clinical features of SRS, Isolated LO Myoclonus-Dystonia (M-D) has been reported in six SRS patients, all carrying maternal uniparental disomy of chromosome 7 (UPD(7)mat) (Guettard et al., 2008; El-Shewy and Luttrell, 2009; Stark et al., 2010; Sheridan et al., 2013; Martins et al., 2020). M-D is a rare movement disorder with onset in the first 2 decades of life, characterized by truncal and upper-limb myoclonus associated with cervical dystonia and/or writer’s cramp. Depression, panic attacks, and obsessive–compulsive disorders are also included in the non-motor symptoms (Peall et al., 2011). Loss-of-function mutations in SGCE (7q21.3) have been identified in 20% of M-D patients (Peall et al., 2014), where the disorder shows autosomal dominant inheritance with reduced penetrance when transmitted by the mother, with the gene being maternally imprinted. This finding led to the hypothesis that the lack of gene expression in UPD(7)mat SRS patients exhibiting M-D might be the driver mechanism of the neurological manifestation. However, the prevalence of M-D in SRS cohorts with UPD(7)mat is low (Guettard et al., 2008; Sheridan et al., 2013; Lokulo-Sodipe et al., 2020; Martins et al., 2020). To our knowledge, unlike UPD(7)mat SRS patients, SRS patients exhibiting dystonia due to hypomethylation of the paternal allele at H19/IGF2:IG-DMR have not yet been described.
Here, we report the clinical history of a patient referred for apparently Isolated Lateralized Overgrowth (ILO) and molecularly diagnosed with SRS. Interestingly, since her 16th birthday, our SRS girl has displayed symptoms suggestive of hand dystonia.
In accordance with the manufacturer’s protocols, DNA was extracted from peripheral blood lymphocytes (automated extractor, Tecan Group Ltd.) of sibs and parents, from the proband’s epithelial buccal cells (Oragene tubes OG-575 and DNA kit, DNA Genotek Inc.), and from proband’s urine samples (Wizard Genomic DNA Purification Kit, Promega).
The MRC-Holland kit (MRC Holland, Amsterdam, Netherlands) ME-030 BWS/RSS C3-1,017 was used for the molecular diagnosis, in accordance with the kit instructions. Raw data were analyzed by using Coffalyser.Net software (version 140,701- MRC Holland).
Whole-exome sequencing (WES) was performed on genomic DNA from proband and parents (trio) at BIODIVERSA srl service (Milan, Italy) using the SureSelect Human All Exon V7 library and the Illumina HiSeq X platform. Bioinformatic analyses were carried out at the Bioinformatics Unit of the IRCCS Istituto Auxologico Italiano (Milano, Italy). FASTQ data were aligned to the reference genome assembly GRCh37/hg19 using the maximal exact matches algorithm in the Burrows–Wheeler Aligner (BWA) (v.0.7.10). PCR duplicate removal was performed by using Picard (v1.119) (picard.sourceforge.net/). UnifiedGenotyper (GATK v3.7) was used to locally realign insertion/deletions (InDels) and recalibrate base quality scores. Variants were visually inspected using the Integrative Genomics Viewer (IGV, Broad Institute) and further annotated using wANNOVAR (http://wannovar.wglab.org/). Variants with a coverage of >20x and a variant allele frequency of more than or equal to 0.35 were selected for quality filtering. Next, we designed the following two virtual panels of genes to scrutinize putative variants: one related to overgrowth syndromes and the other to dystonia (Supplementary Table S1). Variants were filtered by minor allele frequency (MAF) < 1% in the 1,000 Genomes, Genome Aggregation Databases (gnomAD), and Exome Aggregation Consortium (ExAC) databases. In silico prediction of missense variants’ pathogenicity was performed by combining the PolyPhen-2, SIFT, and CADD algorithms. The interpretation of the variants was based on the classification by the VarSome and InterVar databases (Li and Wang, 2017; Kopanos et al., 2019) in accordance with the ACMG–AMP (American College of Medical Genetics and Genomics/Association for Molecular Pathology) guidelines (Richards et al., 2015; Rehder et al., 2021). Selected variants were validated by Sanger sequencing.
Array CGH analysis was performed using the high-resolution Human Genome CGH Microarray Kits 2 × 400K (median resolution of 16 kb) (Agilent Technologies) in accordance with the manufacturer’s instructions. Data were analyzed using Agilent CytoGenomics 3.0. Detected copy number variations (CNVs) were interpreted in accordance with the work of Crippa et al. (2019).
The proband was second born to unrelated wealthy parents of Caucasian origin living in a small village in the province of Brescia, Northern Italy. Her father and elder brother suffer from depression. Figure 1 shows that her paternal and, especially, maternal family members exhibit tall stature. After 37 weeks of gestation, a cesarean section was performed because of intrauterine growth retardation (IUGR). Her birth weight was 2,310 g (−1.57 SDS), birth length was 46 cm (−1.11 SDS), and occipitofrontal circumference was (OFC) 53.5 cm (+0.08 SDS). Bilateral talipes equinovarus and left hip dysplasia were recorded. At the age of 2 months, she was referred to a clinical geneticist because of body asymmetry, with a predominance of the right side, involving the face, tongue, chest, and upper and lower limbs. Abdominal and renal ultrasounds were carried out, showing normal findings. The karyotype on peripheral blood lymphocytes was 46,XX. She was diagnosed with Isolated Lateralized Overgrowth (ILO) and started a follow-up composed of periodic abdomen ultrasound, orthopedic evaluation, and alpha-fetoprotein test. Developmental milestones were achieved at the expected age. During childhood, she developed kyphoscoliosis. At the age of 13, she underwent right tibial epiphysiodesis due to a worsening of lower limb asymmetry; 1 year later, the staples had to be removed because of rejection. Bone age has always been delayed by 1 year compared to chronological age. Growth parameters were apparently within the normal standard ranges.
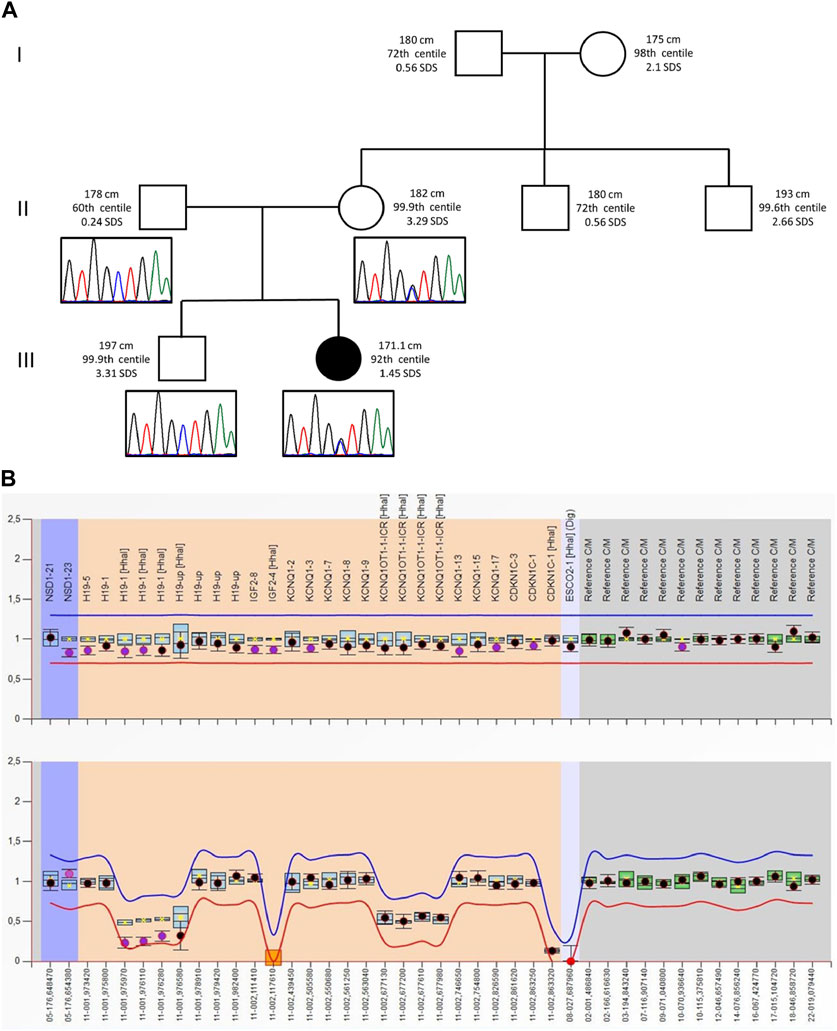
FIGURE 1. (A) Pedigree of the proband’s family: For each member, from the maternal side, the height (cm) and the respective centile and Z-score (SDS) are reported. Height 0–2 years: Who 0–5 years, 2006; height >2 years: Cacciari 2–20 years, Italy, 2006. Electropherograms of the VPS16 sequence are reported below each correspondent subject. (B) MS-MLPA plot of the SRS patient. Analysis of the BWS/RSS MS-MLPA (ME030) shows normal results for the copy numbers (top panel) and a hypomethylation methylation at the H19 locus (bottom panel).
At the age of 16, she has been suffering from episodes of sweating, tremors, and shortness of breath; CBC, thyroid function, blood glucose, and cortisol levels have been repeatedly assessed with normal findings. Furthermore, she started to complain of pain involving the ulnar side of the right hand and wrist, spreading to the forearm, arm, and shoulder, worsening during activity (writing and drawing), and leading to hand disuse, to the point that she was left-handed at the age of 19, at the clinical genetics re-evaluation. Indeed, the inability to use her right hand was a source of major stress for her, as she was studying to become a fashion designer. Several investigations were performed, including X-rays of the right arm and forearm, MRI of the cervical column, brachial plexus, right shoulder and arm, and ultrasound and EMG of the right median nerve. However, no cause has yet been found. The dystonia-specific writer’s type, also referred to as writer’s cramp, has been hypothesized (Sadnicka et al., 2016). Various therapies were employed (iontophoresis, oral intake of steroids Deltacortene 7.5 mg/day for 7 days, then 5 mg/day for 10 days, and 2.5 mg/day for 13 days and vitamin B12–Nicetile 1 tablet a day for 30 days, or antiepileptic pregabalin, and NSAIDs as needed) without any effect. In the last neurological examination, carried out at the age of 23, a slight weakness in the right hand finger V abductor and in the ulnar flexion of the right wrist was observed. The examination appeared otherwise normal, including the absence of a sensitivity deficit in the upper limbs and the absence of the appearance of dystonic aspects during graphic tests (writing and drawing). Based on the hypothesis of focal dystonia without motor symptoms (Peall et al., 2015; Sadnicka et al., 2016), a brain MRI with hemosiderin sequences, which the patient has not yet undergone, was recommended. Furthermore, at the age of 18, she was diagnosed with depressive disorder and treated successfully with benzodiazepines and selective serotonin reuptake inhibitors (SSRIs). At the age of 19, she has been suffering from bipolar mood disorder, which is well controlled with lithium salts. Current therapy involves the oral intake of a benzodiazepine as an anxiolytic drug (alprazolam, 15 drops three times a day), an SSRI antidepressant (escitalopram, 1 tablet in the morning), another antidepressant (trazodone, 1 tablet in the evening), and quetiapine (1 tablet of 100 mg in the evening), as well as lithium (1 tablet of 83 mg in the evening) as antipsychotic drugs.
Despite her behavioral hand dystonia and psychiatric diagnosis, she managed to continue her studies at the university. She particularly cares about her appearance.
Following the ILO diagnosis, the patient underwent a genetic test for the Beckwith–Wiedemann spectrum (BWSp) to confirm the clinical diagnosis. Methylation analysis at H19/IGF2:IG-DMR (IC1) and KCNQ1OT1:TSS-DMR (IC2) by MS-MLPA showed IC1_LoM in a mosaic pattern, which is consistent with SRS diagnosis. The same result was obtained by MS-MLPA on DNA from buccal swabs and urinary sediment samples. Furthermore, considering the atypical phenotype for SRS diagnosis and the co-occurrence of IC1_LoM with pathogenic CNVs and/or SNVs in genes associated with overgrowth syndromes, high-resolution array CGH and WES on DNA from peripheral blood were performed.
As the tests did not reveal any candidate CNVs or SNVs, the patient’s clinical history was revised in accordance with the SRS consensus statement (Wakeling et al., 2017). Growth parameters from clinical records are reported in Table 1. At the time of clinical re-evaluation, the patient was 19 years old with a height of 171.1 cm (+1.45 SDS), with a mid-parental height (MPH) of +1.91 SDS (Figure 1), a BMI of 17.3 kg/m2 (−1.80 SDS), and an OFC of 55 cm (+0.27 SDS). It is noteworthy that at the age of 2 years and 4 months, her growth was reported to be normal, although the body mass index (BMI) was −2.29 SDS and the height was −2 SDS below her target (Table 1); however, between the age of 2 years and 10 months and 17 years and 8 months, a catch-up occurred and her final height fits with the MPH. Furthermore, she displays asymmetry of the face, including ears and incisor teeth, causing left deviation of the nose, mouth, and tongue; her left ear is low-settled and posteriorly rotated. Asymmetry also involves the upper and lower limbs, with a leg length discrepancy of 0.5 cm after orthopedic surgery. She shows bilateral blepharoptosis and ligamentous laxity with a negative Beighton score. Table 2 shows that the patient exhibited three out of six NH-CSS diagnostic criteria.
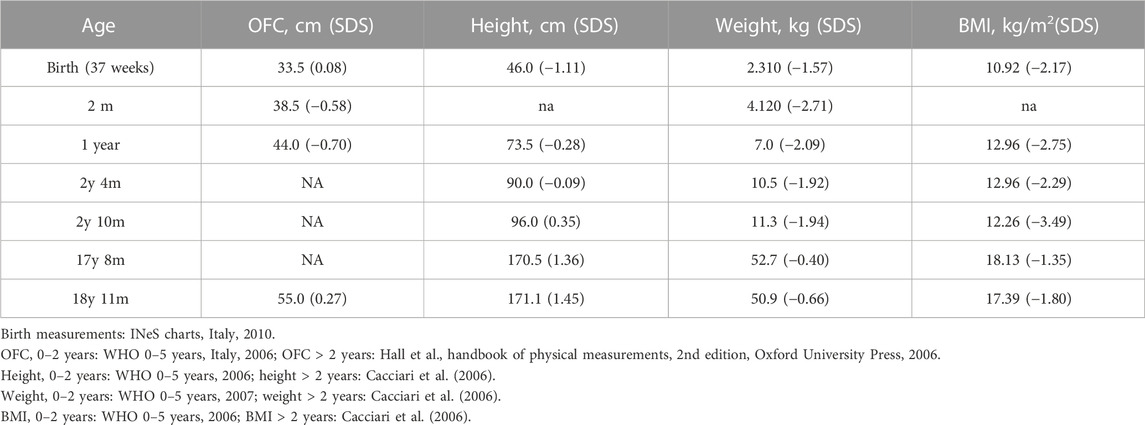
TABLE 1. Growth parameters of the proband at different ages. Female growth reference charts are provided in the footnote.
With the aim of revealing pathogenic variants explaining the neurological phenotype, we also interrogated WES for a wide panel of genes involved in the dystonia phenotype (Supplementary Table S1). The only interesting variant identified was the missense one (NM_022575.4:c.2185C>G:p.Leu729Val) in exon 22 of the VPS16 (vacuolar protein-sorting 16 homolog) gene. This variant has never been reported in 1,000 Genomes, ExAC, or gnomAD databases; it is in silico predicted to be damaging (CADD = 23.9; Polyphen-2 = 0.95; and SIFT = 0.005), and the aminoacid Leu729 is evolutionarily conserved. Using the ACMG criteria, we classified it as a variant of uncertain significance (PM1, PM2, PP3, and BP1). Variants in the VPS16 gene are associated with Dystonia 30 (DYT30, MIM#619291). The segregation analysis showed that the variant was inherited from the asymptomatic mother and it is absent in her brother.
Silver–Russell syndrome is a heterogeneous clinical and genetic condition, mainly characterized by lower growth parameters than the population. The growth rate and the final height in adulthood are highly heritable continuous quantitative traits. A number of genetic, hormonal, environmental, and nutritional factors can concur to influence the final phenotype. Given the complex etiology of this trait, suspicion of growth retardation is particularly difficult (Gudbjartsson et al., 2008). Here, we describe a patient initially diagnosed as BWSp by her ILO, in accordance with the diagnostic consensus of the syndrome (Brioude et al., 2018). Despite asymmetric body growth being one of the six specific clinical criteria listed in the NH-CSS for SRS (Wakeling et al., 2017), our patient’s growth parameters at birth and postnatally did not raise the suspicion of SRS (Table 1). However, her growth chart was below the familial target, since her mid-parental target height is higher than the reference one in the population. To our knowledge, few SRS patients have been reported with normal growth parameters (height > −2 SDS) (Bliek et al., 2006; Zeschnigk et al., 2008; Russo et al., 2016; Patti et al., 2018; Mackay et al., 2019; Lokulo-Sodipe et al., 2020), and only two patients have been described as having a final height of about 170 cm without receiving growth hormone treatment (Bliek et al., 2006; Lokulo-Sodipe et al., 2020). However, in both studies, parental height was not indicated. The patient reported by Bliek and others differed from our case for SGA (the birth length was unknown) and feeding difficulties, while that reported by Lokulo-Sodipe et al. (2020) shares two NH-CSS diagnostic criteria with our case. This suggests that, in evaluating patients with an ILO phenotype without marked growth delay, the presence of NH-CSS criteria (such as relative macrocephaly) and the mid-parental height should be considered, as the genetic background can influence the syndrome’s phenotypic expression.
Moreover, in the distinctive growth chart, our patient shows a neurological disorder, manifesting hand-writer’s cramp, and psychiatric disorders such as bipolar disorder and depression, which may be part of an M-D’s phenotype (Peall et al., 2011). To our knowledge, this patient is the first SRS exhibiting this disorder, associated with hypomethylation of the paternal allele at H19/IGF2:IG-DMR, and we hypothesized that an additional hit might be involved. WES analysis did not reveal pathogenic variants in candidate genes previously associated with M-D; out of the few identified VUSs (Supplementary Table S2), we focused on those with a putative interaction with the IGF2 pathway. Specifically, we focused on the VPS16 variant (p.Leu729Val). The VPS16 gene encodes a protein belonging to the HOPS (homotypic fusion and protein sorting) complex, a structural bridge composed of four Vps-C core proteins (VPS11, VPS16, VPS18, and VPS33A), and two additional subunits (VPS39 and VPS41), which are necessary for the fusion of late endosomes and autophagosomes with the lysosomes in the cytoplasm (Luzio et al., 2007; Ostrowicz et al., 2010; Monfrini et al., 2021). Figure 2A summarizes the connection between the degradation of IGF2 and the recovery of extracellular M6P-lysosomal enzymes through the plasma membrane cation-independent mannose-6-phosphate receptor (CI-M6PR/IGF2R) (Oka et al., 1985; Braulke et al., 1990; El-Shewy and Luttrell, 2009; Zavorka et al., 2016; Wang et al., 2017; 2020; Bajaj et al., 2019). Dysregulation of the endolysosomal and autophagic systems is linked to the pathogenesis of dystonia, which is a feature of the clinical presentation of many lysosomal storage disorders (Monfrini et al., 2021).
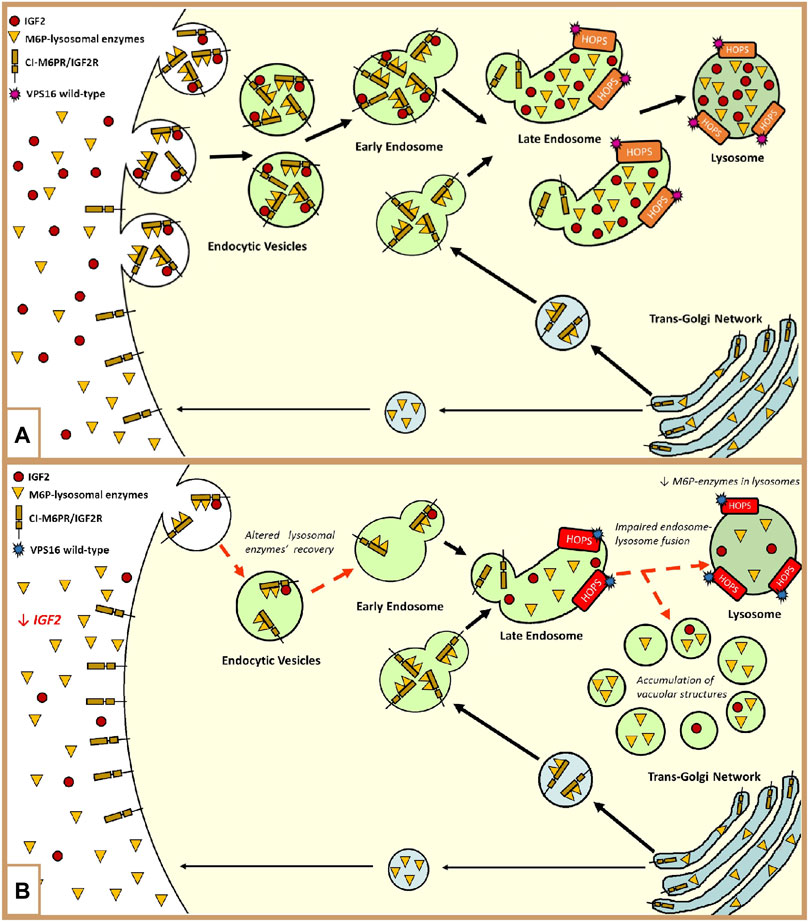
FIGURE 2. (A) Representation of the involvement of IGF2 and the HOPS complex in the endolysosomal pathway: The lysosomal enzymes are synthesized in the trans-Golgi network and delivered to the endolysosomal system through the Golgi cation-independent mannose-6-phosphate receptor (CI-M6PR/IGF2R). During trafficking, some proteins escape binding with the receptor, and they are exocytosed into the extracellular compartment (Bajaj et al., 2019). These enzymes can be retrieved and rerouted back to the endolysosomal system by the cell surface CI-M6PR/IGF2R. The recovery of these enzymes is modulated by the binding of extracellular IGF2 in an enzyme type-dependent manner. In both ways, lysosome enzymes reach their final destination by means of the fusion of late endosomes to lysosomes mediated by the HOPS complex, to which VPS16 belongs (El-Shewy and Luttrell, 2009; Wang et al., 2017; Monfrini et al., 2021). Furthermore, the endocytosis of extracellular IGF2 is important for its degradation and the regulation of its bioavailability (Oka et al., 1985; Olson et al., 2020; Braulke et al., 1990; El-Shewy and Luttrell, 2009; Zavorka et al., 2016; Wang et al., 2020). (B) Hypothetical dysregulation of the endolysosomal system in our patient: The loss of methylation at IC1 leads to low extracellular levels of IGF2. In this context, the recovery of the escaped M6P-lysosomal enzymes and IGF2 is impaired. The maturation of the endocytic vesicles, containing lysosomal enzymes, proceeds until the state of late endosome. At this level, the presence of the VPS16 variant slightly affects the activity of the HOPS complex in promoting the fusion between the late endosome and lysosome. This leads to an abnormal amount of M6P enzymes in the lysosomes and to the formation and accumulation of vacuolar structures, containing these enzymes, in the cytoplasm (Monfrini et al., 2021).
Several heterozygous VPS16 variants (17 loss-of-function and three missense variants, Supplementary Table S3) were found to co-segregate with dystonia in multigenerational families displaying a dominant pattern of inheritance with incomplete penetrance and wide clinical expressivity of the age of onset and dystonia severity (Steel et al., 2020; Ostrozovicova et al., 2021; Park et al., 2022. Patients with heterozygous VPS16 variants often display psychiatric features such as depression or bipolar disorder (Monfrini et al., 2021). Our patient fits the clinical phenotype of the reported VPS16-mutated patients and the segregation of their variants (overviewed in Supplementary Table S4). Clinical data were collected from all 32 heterozygous VPS16 patients described in the literature. Many patients displayed early-onset dystonia (0–20 years, 25/32), and the most frequent first symptom was isolated writer’s cramp (10/32) (Cai et al., 2016; Steel et al., 2020; Li L.-X. et al., 2021; Li X. et al., 2021; Ostrozovicova et al., 2021; Pott et al., 2021; Gu et al., 2022; Park et al., 2022; Petry-Schmelzer et al., 2022).
Despite few missense variants in the C-terminal domain of VPS16 being reported, in vitro mutagenesis experiments (Ala669Asp and Leu725Glu) support the relevant role of the HOPS domain by showing that variants in this domain may severely affect the VPS16-VPS33A binding and mutations proximal to the interaction surface could interfere in the binding (Graham et al., 2013). Furthermore, Park et al. identified 11 missense variants in a wide dystonic cohort. Although missense variants were not enriched in this cohort compared to non-dystonic patients, the authors did not exclude their roles in the disease, or other genetic, epigenetic, and environmental factors that may contribute to disease manifestation and severity (Park et al., 2022). Otherwise, since no other patients affected by dystonia have so far been reported with the VPS16 (NM_022575.4):c.2185C>G:p.Leu729Val, the proband’s hand dystonia might be one of the rare clinical phenotypes of the SRS spectrum, whose pathomechanism still needs to be elucidated. Based on this hypothesis, the patient’s psychiatric manifestations might be interpreted as a more severe manifestation of familial depressive disorder.
To sum up, we hypothesize that in our proband, the low extracellular levels of IGF2, due to the IC1_LoM, may worsen the effect of the co-present VPS16 variant and contribute to the expression of the neurological phenotype (Figure 2B).
Discrepant phenotypes have been reported for SRS and BWSp (Mackay et al., 2019), but confounding factors such as mid-parental height should be considered, as well as the presence of the diagnostic criteria of either syndrome. The path to the molecular diagnosis in our patient has been tortuous, and recognition of SRS came late. It is true to say that the result of the genetic test in childhood could have led to clinical diagnosis and the proper follow-up. Indeed, as an adult, the patient has expressed disappointment about the long-lasting and ineffective follow-up for the timely diagnosis of embryonic tumors. On the other hand, SRS patients are susceptible to developing metabolic syndrome in adulthood (Patti et al., 2018), whereas BWS/ILO patients can interrupt the follow-up. Interestingly, the deficit of IGF2 may correspond to the expression of a VPS16-related dystonia phenotype.
The description and discussion of this unique SRS patient, in addition to being useful in the clinical setting to reach the proper diagnosis and management, may enhance the understanding of the molecular basis of the broad SRS spectrum.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material. https://doi.org/10.7910/DVN/JBDF2Q.
This study was conducted according to the guidelines of the Declaration of Helsinki, and the studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Istituto Auxologico Italiano, IRCCS (protocol code CE: 2017_05_16_05 and date of approval 16/05/2017). The patients/participants provided their written informed consent to participate in this study.
AS made clinical suspicion at pediatric age; SG and SR detected the SRS molecular diagnosis; MTB, EO, and MM followed the patient in adult age and diagnosed the neurological phenotype; SR and MTB conceived and designed the study; MC performed array CGH; AV and PT performed WES molecular analyses; LC carried out bioinformatics data analysis; AV and PT filtered and validated the variants in probands and parents and disclosed the VPS16 variant; AV, MTB, and SR drafted the paper; LL supervised the manuscript; and all authors contributed to the article and approved the submitted version.
This work was supported by the Italian Ministry of Health, Ricerca Corrente grant 08C724.
The authors thank the patient and her family and AISRS Onlus.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2023.1198821/full#supplementary-material
Abi Habib, W., Brioude, F., Edouard, T., Bennett, J. T., Lienhardt-Roussie, A., Tixier, F., et al. (2018). Genetic disruption of the oncogenic HMGA2-PLAG1-IGF2 pathway causes fetal growth restriction. Genet. Med. 20, 250–258. doi:10.1038/gim.2017.105
Bajaj, L., Lotfi, P., Pal, R., Ronza, A., Sharma, J., and Sardiello, M. (2019). Lysosome biogenesis in health and disease. J. Neurochem. 148, 573–589. doi:10.1111/jnc.14564
Bliek, J., Terhal, P., van den Bogaard, M.-J., Maas, S., Hamel, B., Salieb-Beugelaar, G., et al. (2006). Hypomethylation of the H19 gene causes not only Silver-Russell syndrome (SRS) but also isolated asymmetry or an SRS-like phenotype. Am. J. Hum. Genet. 78, 604–614. doi:10.1086/502981
Braulke, T., Tippmer, S., Chao, H. J., and von Figura, K. (1990). Insulin-like growth factors I and II stimulate endocytosis but do not affect sorting of lysosomal enzymes in human fibroblasts. J. Biol. Chem. 265, 6650–6655. doi:10.1016/s0021-9258(19)39199-9
Brioude, F., Kalish, J. M., Mussa, A., Foster, A. C., Bliek, J., Ferrero, G. B., et al. (2018). Expert consensus document: Clinical and molecular diagnosis, screening and management of beckwith-wiedemann syndrome: An international consensus statement. Nat. Rev. Endocrinol. 14, 229–249. doi:10.1038/nrendo.2017.166
Cacciari, E., Milani, S., Balsamo, A., Spada, E., Bona, G., Cavallo, L., et al. (2006). Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Invest. 29, 581–593. doi:10.1007/BF03344156
Cai, X., Chen, X., Wu, S., Liu, W., Zhang, X., Zhang, D., et al. (2016). Homozygous mutation of VPS16 gene is responsible for an autosomal recessive adolescent-onset primary dystonia. Sci. Rep. 6, 25834. doi:10.1038/srep25834
Crippa, M., Bonati, M. T., Calzari, L., Picinelli, C., Gervasini, C., Sironi, A., et al. (2019). Molecular etiology disclosed by array CGH in patients with silver–russell syndrome or similar phenotypes. Front. Genet. 10, 955. doi:10.3389/fgene.2019.00955
El-Shewy, H. M., and Luttrell, L. M. (2009). Insulin-like growth factor-2/mannose-6 phosphate receptors. Vitam. Horm. 80, 667–697. doi:10.1016/S0083-6729(08)00624-9
Graham, S. C., Wartosch, L., Gray, S. R., Scourfield, E. J., Deane, J. E., Luzio, J. P., et al. (2013). Structural basis of Vps33A recruitment to the human HOPS complex by Vps16. Proc. Natl. Acad. Sci. U. S. A. 110, 13345–13350. doi:10.1073/pnas.1307074110
Gu, X., Lin, J., Hou, Y., Zhang, L., and Shang, H. (2022). De novo missense mutation of VPS16 in a Chinese patient with generalized dystonia with myoclonus. Mov. Disord. Clin. Pract. 9, 551–552. doi:10.1002/mdc3.13392
Gudbjartsson, D. F., Walters, G. B., Thorleifsson, G., Stefansson, H., Halldorsson, B. V., Zusmanovich, P., et al. (2008). Many sequence variants affecting diversity of adult human height. Nat. Genet. 40, 609–615. doi:10.1038/ng.122
Guettard, E., Portnoi, M.-F., Lohmann-Hedrich, K., Keren, B., Rossignol, S., Winkler, S., et al. (2008). Myoclonus-dystonia due to maternal uniparental disomy. Arch. Neurol. 65, 1380–1385. doi:10.1001/archneur.65.10.1380
Kopanos, C., Tsiolkas, V., Kouris, A., Chapple, C. E., Albarca Aguilera, M., Meyer, R., et al. (2019). VarSome: The human genomic variant search engine. Bioinformatics 35, 1978–1980. doi:10.1093/bioinformatics/bty897
Li, L.-X., Jiang, L.-T., Liu, Y., Zhang, X.-L., Pan, Y.-G., Pan, L.-Z., et al. (2021). Mutation screening of VPS16 gene in patients with isolated dystonia. Park. Relat. Disord. 83, 63–65. doi:10.1016/j.parkreldis.2020.12.014
Li, Q., and Wang, K. (2017). InterVar: Clinical interpretation of genetic variants by the 2015 ACMG-AMP guidelines. Am. J. Hum. Genet. 100, 267–280. doi:10.1016/j.ajhg.2017.01.004
Li, X. Y., Wang, L., Guo, Y., and Wan, X. (2021). Mutations in the VPS16 gene in 56 early-onset dystonia patients. Mov. Disord. 36, 780–781. doi:10.1002/mds.28540
Lokulo-Sodipe, O., Ballard, L., Child, J., Inskip, H. M., Byrne, C. D., Ishida, M., et al. (2020). Phenotype of genetically confirmed Silver-Russell syndrome beyond childhood. J. Med. Genet. 57, 683–691. doi:10.1136/jmedgenet-2019-106561
Luzio, J. P., Pryor, P. R., and Bright, N. A. (2007). Lysosomes: Fusion and function. Nat. Rev. Mol. Cell. Biol. 8, 622–632. doi:10.1038/nrm2217
Mackay, D. J. G., Bliek, J., Lombardi, M. P., Russo, S., Calzari, L., Guzzetti, S., et al. (2019). Discrepant molecular and clinical diagnoses in Beckwith-Wiedemann and Silver-Russell syndromes. Genet. Res. (Camb) 101, e3. doi:10.1017/S001667231900003X
Martins, J., Gabriel, D., Borges, T., Soares, G., and Temudo, T. (2020). Child neurology: Myoclonus-dystonia in russell-silver syndrome: Two syndromes caused by one genetic defect. Neurology 95, e936–e938. doi:10.1212/WNL.0000000000010076
Monfrini, E., Zech, M., Steel, D., Kurian, M. A., Winkelmann, J., and Di Fonzo, A. (2021). HOPS-Associated neurological disorders (HOPSANDs): Linking endolysosomal dysfunction to the pathogenesis of dystonia. Brain 144, 2610–2615. doi:10.1093/brain/awab161
Oka, Y., Rozek, L. M., and Czech, M. P. (1985). Direct demonstration of rapid insulin-like growth factor II Receptor internalization and recycling in rat adipocytes. Insulin stimulates 125I-insulin-like growth factor II degradation by modulating the IGF-II receptor recycling process. J. Biol. Chem. 260, 9435–9442. doi:10.1016/s0021-9258(17)39385-7
Olson, L. J., Misra, S. K., Ishihara, M., Battaile, K. P., Grant, O. C., Sood, A., et al. (2020). Allosteric regulation of lysosomal enzyme recognition by the cation-independent mannose 6-phosphate receptor. Communications biology 3 (1), 498. doi:10.1038/s42003-020-01211-w
Ostrowicz, C. W., Bröcker, C., Ahnert, F., Nordmann, M., Lachmann, J., Peplowska, K., et al. (2010). Defined subunit arrangement and rab interactions are required for functionality of the HOPS tethering complex. Traffic 11, 1334–1346. doi:10.1111/j.1600-0854.2010.01097.x
Ostrozovicova, M., Jech, R., Steel, D., Pavelekova, P., Han, V., Gdovinova, Z., et al. (2021). A recurrent VPS16 p.Arg187* nonsense variant in early-onset generalized dystonia. Mov. Disord. 36, 1984–1985. doi:10.1002/mds.28647
Park, J., Reilaender, A., Petry-Schmelzer, J. N., Stöbe, P., Cordts, I., Harmuth, F., et al. (2022). Transcript-specific loss-of-function variants in VPS16 are enriched in patients with dystonia. Neurol. Genet. 8, e644. doi:10.1212/NXG.0000000000000644
Patti, G., Giaccardi, M., Capra, V., Napoli, F., Cangemi, G., Notarnicola, S., et al. (2018). Clinical manifestations and metabolic outcomes of seven adults with silver-russell syndrome. J. Clin. Endocrinol. Metab. 103, 2225–2233. doi:10.1210/jc.2017-02589
Peall, K. J., Kuiper, A., de Koning, T. J., and Tijssen, M. J. (2015). Non-motor symptoms in genetically defined dystonia: Homogenous groups require systematic assessment. Park. Relat. Disord. 21, 1031–1040. doi:10.1016/j.parkreldis.2015.07.003
Peall, K. J., Kurian, M. A., Wardle, M., Waite, A. J., Hedderly, T., Lin, J.-P., et al. (2014). SGCE and myoclonus dystonia: Motor characteristics, diagnostic criteria and clinical predictors of genotype. J. Neurol. 261, 2296–2304. doi:10.1007/s00415-014-7488-3
Peall, K. J., Waite, A. J., Blake, D. J., Owen, M. J., and Morris, H. R. (2011). Psychiatric disorders, myoclonus dystonia, and the epsilon-sarcoglycan gene: A systematic review. Mov. Disord. 26, 1939–1942. doi:10.1002/mds.23791
Petry-Schmelzer, J. N., Park, J., Haack, T. B., Visser-Vandewalle, V., Barbe, M. T., and Wunderlich, G. (2022). Long-term benefit of pallidal deep brain stimulation in a patient with VPS16-associated dystonia. Neurol. Res. Pract. 4, 21. doi:10.1186/s42466-022-00185-w
Pott, H., Brüggemann, N., Reese, R., Zeuner, K. E., Gandor, F., Gruber, D., et al. (2021). Truncating VPS16 mutations are rare in early onset dystonia. Ann. Neurol. 89, 625–626. doi:10.1002/ana.25990
Rehder, C., Bean, L. J. H., Bick, D., Chao, E., Chung, W., Das, S., et al. (2021). Next-generation sequencing for constitutional variants in the clinical laboratory, 2021 revision: A technical standard of the American College of medical genetics and genomics (ACMG). Genet. Med. 23, 1399–1415. doi:10.1038/s41436-021-01139-4
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of medical genetics and genomics and the association for molecular Pathology. Genet. Med. 17, 405–424. doi:10.1038/gim.2015.30
Russo, S., Calzari, L., Mussa, A., Mainini, E., Cassina, M., Di Candia, S., et al. (2016). A multi-method approach to the molecular diagnosis of overt and borderline 11p15.5 defects underlying Silver–Russell and Beckwith–Wiedemann syndromes. Clin. Epigenetics 8, 23. doi:10.1186/s13148-016-0183-8
Sadnicka, A., Kassavetis, P., Pareés, I., Meppelink, A. M., Butler, K., and Edwards, M. (2016). Task-specific dystonia: Pathophysiology and management. J. Neurol. Neurosurg. Psychiatry 87, 968–974. doi:10.1136/jnnp-2015-311298
Sheridan, M. B., Bytyci Telegrafi, A., Stinnett, V., Umeh, C. C., Mari, Z., Dawson, T. M., et al. (2013). Myoclonus-dystonia and Silver-Russell syndrome resulting from maternal uniparental disomy of chromosome 7. Clin. Genet. 84, 368–372. doi:10.1111/cge.12075
Stark, Z., Ryan, M. M., Bruno, D. L., Burgess, T., and Savarirayan, R. (2010). Atypical Silver-Russell phenotype resulting from maternal uniparental disomy of chromosome 7. Am. J. Med. Genet. A 152A, 2342–2345. doi:10.1002/ajmg.a.33590
Steel, D., Zech, M., Zhao, C., Barwick, K. E. S., Burke, D., Demailly, D., et al. (2020). Loss-of-Function variants in HOPS complex genes VPS16 and VPS41 cause early onset dystonia associated with lysosomal abnormalities. Ann. Neurol. 88, 867–877. doi:10.1002/ana.25879
Wakeling, E. L., Amero, S. A., Alders, M., Bleik, J., Forsythe, E., Kumar, S., et al. (2010). Epigenotype-phenotype correlations in Silver-Russell syndrome. J. Med. Genet. 47, 760–768. doi:10.1136/jmg.2010.079111
Wakeling, E. L., Brioude, F., Lokulo-Sodipe, O., O’Connell, S. M., Salem, J., Bliek, J., et al. (2017). Diagnosis and management of silver-russell syndrome: First international consensus statement. Nat. Rev. Endocrinol. 13, 105–124. doi:10.1038/nrendo.2016.138
Wang, R., Qi, X., Schmiege, P., Coutavas, E., and Li, X. (2020). Marked structural rearrangement of mannose 6-phosphate/IGF2 receptor at different pH environments. Sci. Adv. 6, aaz1466. doi:10.1126/sciadv.aaz1466
Wang, Y., MacDonald, R. G., Thinakaran, G., and Kar, S. (2017). Insulin-like growth factor-II/cation-independent mannose 6-phosphate receptor in neurodegenerative diseases. Mol. Neurobiol. 54, 2636–2658. doi:10.1007/s12035-016-9849-7
Zavorka, M. E., Connelly, C. M., Grosely, R., and MacDonald, R. G. (2016). Inhibition of insulin-like growth factor II (IGF-II)-dependent cell growth by multidentate pentamannosyl 6-phosphate-based ligands targeting the mannose 6-phosphate/IGF-II receptor. Oncotarget 7, 62386–62410. doi:10.18632/oncotarget.11493
Keywords: Silver-Russell syndrome, unexpected molecular results, body asymmetry, IGF2, hand dystonia, VPS16, endolysosomal pathway
Citation: Vimercati A, Tannorella P, Orlandini E, Calzari L, Moro M, Guzzetti S, Selicorni A, Crippa M, Larizza L, Bonati MT and Russo S (2023) Case report: atypical Silver-Russell syndrome patient with hand dystonia: the valuable support of the consensus statement to the wide syndromic spectrum. Front. Genet. 14:1198821. doi: 10.3389/fgene.2023.1198821
Received: 03 April 2023; Accepted: 19 June 2023;
Published: 17 July 2023.
Edited by:
Pilar Giraldo, University of Zaragoza, SpainReviewed by:
Thomas Eggermann, RWTH Aachen University, GermanyCopyright © 2023 Vimercati, Tannorella, Orlandini, Calzari, Moro, Guzzetti, Selicorni, Crippa, Larizza, Bonati and Russo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Teresa Bonati, bWFyaWF0ZXJlc2EuYm9uYXRpQGJ1cmxvLnRyaWVzdGUuaXQ=; Silvia Russo, cy5ydXNzb0BhdXhvbG9naWNvLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.