- 1Faculty of Electronic Information and Electrical Engineering, Dalian University of Technology, Dalian, Liaoning, China
- 2Department of Breast Surgery, The Second Hospital of Dalian Medical University, Dalian, Liaoning, China
Gene clustering is one of the important techniques to identify co-expressed gene groups from gene expression data, which provides a powerful tool for investigating functional relationships of genes in biological process. Self-training is a kind of important semi-supervised learning method and has exhibited good performance on gene clustering problem. However, the self-training process inevitably suffers from mislabeling, the accumulation of which will lead to the degradation of semi-supervised learning performance of gene expression data. To solve the problem, this paper proposes a self-training subspace clustering algorithm based on adaptive confidence for gene expression data (SSCAC), which combines the low-rank representation of gene expression data and adaptive adjustment of label confidence to better guide the partition of unlabeled data. The superiority of the proposed SSCAC algorithm is mainly reflected in the following aspects. 1) In order to improve the discriminative property of gene expression data, the low-rank representation with distance penalty is used to mine the potential subspace structure of data. 2) Considering the problem of mislabeling in self-training, a semi-supervised clustering objective function with label confidence is proposed, and a self-training subspace clustering framework is constructed on this basis. 3) In order to mitigate the negative impact of mislabeled data, an adaptive adjustment strategy based on gravitational search algorithm is proposed for label confidence. Compared with a variety of state-of-the-art unsupervised and semi-supervised learning algorithms, the SSCAC algorithm has demonstrated its superiority through extensive experiments on two benchmark gene expression datasets.
1 Introduction
The recent development of biological experiments has generated vast amounts of gene expression data. Thus, comprehending and interpreting the enormous number of genes has become a significant challenge (Diniz et al., 2019; Maâtouk et al., 2019; Li and Yang, 2020; Summers et al., 2020; Nisar et al., 2021; Dang et al., 2022). Semi-supervised learning (Chapelle et al., 2006) is a focused issue in the analysis of gene expression data, the research branches mainly include semi-supervised gene clustering (Yu et al., 2014; Yu et al., 2016; Xia et al., 2018; Liu et al., 2021), semi-supervised gene classification (Huang and Feng, 2012; Zhang et al., 2021), semi-supervised gene selection (Mahendran et al., 2020), and semi-supervised gene dimensionality reduction (Feng et al., 2021). In this paper, we focus on the semi-supervised gene clustering problem for identify co-expressed gene groups, which can provide a useful basis for the further investigation of gene function and gene regulation in the field of functional genomics (Maâtouk et al., 2019). When clustering gene expression data, practical dataset usually exists in the form of a large amount of unlabeled data and a small amount of labeled data. However, unsupervised clustering algorithms inherently lack the ability to utilize the label information in exploring the pattern of gene expression data, and the clustering results are often unsatisfactory. Comparatively speaking, semi-supervised clustering can make full use of prior knowledge, such as pairwise information or class labels, to guide the partition of unlabeled data, thus can improve the clustering quality of gene expression data.
Most of the existing semi-supervised learning methods use raw data directly for analysis (Gan et al., 2013; Wu et al., 2018; Li et al., 2019). In recent years, many scholars have found in their research that the intrinsic structure of data is often smaller than its actual dimensionality, and it may be easier to mine the cluster structure of data in subspaces (Basri and Jacobs, 2003). Subspace-based low-dimensional feature representation of data has been successfully applied to various applications, such as image segmentation (Liu et al., 2013; Fei et al., 2017; Xu et al., 2023) and biological data analysis (Shi et al., 2019; Wang et al., 2019; Zheng et al., 2019; Lu et al., 2020; Sun et al., 2021; Huang and Wu, 2022). One of the representative algorithm is low-rank representation (LRR) (Liu et al., 2013), which assumes that the dataset is sampled from multiple mutually orthogonal subspaces in the data space, and uses rank to measure the sparsity of matrix. LRR only focuses on the global structure of data, and ignores the local structure hidden in data. To overcome this drawback, Wang et al. (2019) introduced mixed-norm and Laplacian regularization into LRR to identify differentially expressed genes for tumor clustering. Lu et al. (2020) incorporated the constraints of the non-negative symmetric low-rank matrix and graph regularization for cancer clustering. To preserve the neighbor relationship among data, Fei et al. (2017) proposed a low-rank representation algorithm with distance penalty (LRRADP), which adds a distance penalty term on the basis of LRR to ensure that the representation vectors of the neighboring data in the original data space are still close in the representation space, thereby enhancing the locality of the model and data discriminability. Aiming at guaranteeing block diagonal property of LRR, Xu et al. (2023) presented a projective block diagonal representation approach, which rapidly pursues a representation matrix with block diagonal structure. By assuming that cells with the same type are in the same subspace, Zheng et al. (2019) proposed a self-expression clustering method with non-negative and low-rank constraints for cell type detection. Besides, to effectively integrate multiple omics data, various multi-view subspace clustering algorithms based on LRR were developed for cancer subtyping (Shi et al., 2019; Sun et al., 2021; Huang and Wu, 2022).
As an essential semi-supervised learning method, self-training (Nie et al., 2012; Gan et al., 2013; Wu et al., 2018; Xia et al., 2018; Li et al., 2019) has been successfully applied to various applications including the analysis of gene expression data. Self-training can be regarded as a kind of self-learning method, which consists of two main steps (Li et al., 2019): semi-supervised learning using labeled data to update the predicted labels of unlabeled data; expansion of labeled dataset by selecting unlabeled data as newly labeled data based on some rules. These two steps are repeated until some stopping criteria are reached. For the task of self-training classification, Gan et al. (2013) suggested utilizing unlabeled and labeled data to reveal the true data space structure by cluster analysis, along with a semi-supervised fuzzy c-means technique, to improve self-training. However, the algorithm is not appropriate for non-spherically distributed data (Wu et al., 2018; Li et al., 2019). To overcome this weakness, Wu et al. (2018) proposed a method of self-training based on density peak of data (STDP), which uses clustering by fast search and find of density peaks (DPC) (Rodriguez and Laio, 2014) to build the density-pointing relationship between data, and newly labeled data are selected to iteratively strengthen the classification performance of SVM, KNN, and CART on this basis. Although STDP achieves good classification results for non-spherically distributed data, the problem of mislabeling in the self-training process is not considered. In fact, mislabeling of newly labeled data in a self-training approach is an unavoidable and very intractable problem (Li et al., 2019). Iterative self-training based on these mislabeled data will further reinforce the misinformation and generate more mislabels, leading to mistaken reinforcement (Xia et al., 2018; Li and Zhu, 2020). To solve this problem, researchers have proposed various self-training methods based on partial noise filters in recent years, including multi-label self-training with editing (Wei et al., 2013), dynamic safety assessment self-training based on semi-supervised learning and data editing (Liu et al., 2019), etc. To further exploit unlabeled data in the filter and overcome the parameter dependence problem, Li et al. (2019) proposed a self-training method based on density peaks and an extended parameter-free local noise filter (STDPNF), which can filter out part of mislabeled newly labeled data. However, as with other self-training algorithms using local noise filters, STDPNF still needs to entirely solve the problem of mislabeling.
On the other hand, for the self-training clustering task, Nie et al. (2012) proposed an active self-training clustering (ASTC), which utilizes Gaussian fields and harmonic functions (GFHF) (Zhu et al., 2003) to achieve label propagation. ASTC considers the probability of data being partitioned into various clusters as Bayesian posterior probability, and iteratively selects unlabeled data with large probability values as newly labeled data to optimize the label fitness process of GFHF and improve the label prediction accuracy. To address the problem of partitioning cancer gene expression data, Xia et al. (2018) proposed a self-training subspace clustering algorithm under low-rank representation (SSC-LRR), which introduces LRR to extract subspace structures from cancer gene expression data, iteratively clusters low-rank representation matrix and noise matrix using the K-means algorithm, and selects unlabeled data with the same clustering labels on the two matrices as newly labeled data for self-training learning. SSC-LRR achieves encouraging cancer classification on several benchmark gene expression datasets, and the advantage of low-rank representation in extracting discriminative features from data is analyzed through experimental results.
Despite the success of the above self-training methods, mislabeling a considerable amount of newly labeled data is inevitable (Xia et al., 2018; Li et al., 2019), and its accumulation will lead to the problem of mistaken reinforcement and seriously affect the performance of the self-training methods. In fact, in the self-training clustering problem on gene expression data, different newly labeled data should have different label confidences. The higher the semi-supervised learning value of a newly labeled datum, the more likely this datum has a correctly predicted label, so it should be assigned a higher label confidence. Based on the above analysis, for gene expression data with partial labels, a self-training subspace clustering algorithm based on adaptive confidence (SSCAC) is proposed in this paper, with the following main contributions. Firstly, a self-training subspace clustering framework based on GFHF is designed in this paper, which reveals the subspace structure of gene expression data through low-rank representation, and achieves iterative semi-supervised learning of unlabeled data using the label propagation capability of GFHF on the basis of the constructed similarity matrix. Secondly, to tackle the problem of mislabeling, an improved GFHF objective function with label confidence and the corresponding adaptive adjustment strategy of label confidence based on the gravitational search algorithm (Rashedi et al., 2009) are proposed. The negative impact of mislabeled data can be mitigated by reducing the label confidences of low-value newly labeled data, and the clustering accuracy on gene expression data can thus be improved.
2 Proposed algorithm
Although existing self-training methods have improved the partition accuracy of unlabeled data to some extent, the mislabeling problem of newly labeled data is still one of the important challenges in self-training methods (Xia et al., 2018; Li et al., 2019), which makes it difficult to accurately identify co-expressed gene groups on gene expression data with partial labels. During the iterative self-training, the falsely predicted labels will be accumulated gradually and lead to the problem of mistaken reinforcement. One major reason is that once the newly labeled data are selected, self-training methods always fully trust their predicted labels in the semi-supervised classification or clustering process, i.e., it is implicitly assumed that all newly labeled data have the same label confidence. This will obviously make both correctly and incorrectly labeled data act on the semi-supervised learning task with equal strength, and ignore the difference in value of different newly labeled data for semi-supervised learning. In view of this, a self-training subspace clustering algorithm based on adaptive confidence for gene expression data (SSCAC) is proposed in this paper. The proposed algorithm uses density relationships to select newly labeled data, and constructs a self-training subspace clustering framework based on GFHF and the low-rank representation with distance penalty. SSCAC differs from the existing self-training methods in that the semi-supervised clustering objective function with label confidence and the adaptive adjustment strategy of label confidences. The proposed algorithm aims to weaken the supervisory guidance of low-value newly labeled data by reducing their label confidences, thus alleviating the problem of mislabeling in the self-training process and improving the generalization ability of the algorithm.
2.1 SSCAC objective function
Currently, low-rank representation has achieved good clustering results as a typical representation model for learning the subspace structure of gene expression data (Xia et al., 2018; Shi et al., 2019; Wang et al., 2019; Zheng et al., 2019; Lu et al., 2020; Sun et al., 2021; Huang and Wu, 2022). In this paper, the proposed SSCAC algorithm constructs a self-training subspace clustering framework based on the low-rank representation with distance penalty (LRRADP) (Fei et al., 2017) by using the high coordination between Gaussian fields and harmonic functions (GFHF) (Zhu et al., 2003) and low-rank representation.
In a semi-supervised learning framework, the dataset is usually formulated as X = [x1, x2, …, xl, xl+1, …, xn] = [XL, XU] ∈ Rm×n, where
where
In LRRADP, Z ∈ Rn×n is the low-rank representation matrix and Zi is the vector of coefficients of datum xi represented by other data; E ∈ Rm×n is the noise matrix. The iterative update equations are as follows (Fei et al., 2017):
where ‖Z‖* = ∑iσi(Z) is the nuclear norm of Z, which is used as a convex approximation of matrix rank, σi(Z) denotes the i-th singular value of Z; ‖.‖1 and ‖.‖2 are the l1-norm and l2-norm, respectively; auxiliary variable H, Lagrange multipliers Λ1, Λ2 and penalty parameter β are determined by the following equations:
In the update equations, λ1 > 0 and λ2 > 0 are balance parameters to trade off among the low-rank representation, noise and adaptive distance penalty.
In the SSCAC objective function defined by Eq. 1, the first term is the same as that of the original GFHF, which ensures the smoothness of data labels on the LRRADP graph. The second term is the label fitness term, which incorporates the label confidence μi and applies it to the label indication vector Yi of the labeled datum xi. Actually, the objective function of GFHF is a special case of that of SSCAC with μi = 1 for each labeled datum xi(i = 1, 2, …, l). That is, the SSCAC objective function is the extension of that of GHFH, which further considers the label confidences of the labeled data and can be applied to self-training clustering. Minimizing Eq. 1 can achieve both the manifold smoothness of the partition results in subspaces and the maximum matching between the predicted label and the label of labeled data under the effect of label confidence.
In the self-training process of SSCAC, newly labeled data are selected based on density-pointing relationships between data (Wu et al., 2018; Li et al., 2019) and added to the labeled dataset XL to guide the next iteration of self-training learning. The newly labeled data selection strategy will be detailed in the next Subsection. The rules for setting the label confidence μi in Eq. 1 are as follows: 1) if xi is an initially labeled datum, set the label confidence μi = 1 with complete confidence in its label accuracy; 2) if xi is a newly labeled datum of the current iteration of self-training, μi is initialized to a random number within (0,1], and then adaptively adjusted based on the semi-supervised learning value of xi. The specific strategy is detailed in Section 2.3; 3) only the label confidences of the newly labeled data selected in the current iteration are adjusted, the adjusted confidences remain unchanged in the subsequent iterations of self-training.
The advantage of adding the label confidence in Eq. 1 is that the value can effectively regulate the supervision strength of newly labeled data on semi-supervised gene clustering, which improves the clustering accuracy on gene expression data. The analysis is as follows: 1) if the newly labeled datum xi is mislabeled, i.e., the position of 1 in the label indication vector Yi does not match that of the actual label, the label prediction vector Fi will be predicted in the wrong direction under the effect of the second term of Eq. 1, and the larger the label confidence μi, the larger the prediction bias. In the first term of Eq. 1, the elements corresponding to data in the same subspace in the LRRADP similarity matrix W are relatively large and those corresponding to data in different subspaces are small, so that labels are mainly propagated among data in the same subspace, then the mislabeled datum xi will lead to the label prediction bias of unlabeled data in the same gene clustering. Therefore, reducing the label confidence of mislabeled datum xi can effectively mitigate its negative impact on semi-supervised gene clustering; 2) if the newly labeled datum xi has correct label, the second term of Eq. 1 can guide Fi to obtain correct prediction, and then realize correct label propagation for unlabeled data in the same subspace under the effect of the first term of Eq. 1. Obviously, increasing the label confidence of correctly labeled datum is beneficial to improve the partition accuracy of unlabeled data.
The matrix form of the SSCAC objective function is:
where L ∈ Rn×n is the graph Laplacian matrix, L = D − W, D is a diagonal matrix, Dii = ∑jWi,j;U ∈ Rn×n is also a diagonal matrix, the first l and the remaining n − l diagonal elements are λ∞ and 0, respectively; ⊗ denotes the Hadamard product; μ ∈ Rn×c, if the label of xi(i = 1, 2, …, l) is k(k = 1, 2, …, c), then the k-th element in the i-th row vector is the label confidence of xi, and all the other elements in the row vector are 0. For each unlabeled datum, all elements in the corresponding row vector are set to 0. By setting the derivative of Eq. 9 with respect to F to zero, the following equation can be easily obtained:
Then the predicted label of the unlabeled datum xi can be assigned by:
2.2 Newly labeled data selection strategy based on density relationships
In the self-training process, how to select newly labeled data from the unlabeled dataset XU and iteratively expand the labeled dataset XL is an important issue. Most self-training learning methods (Nie et al., 2012; Xia et al., 2018) rely entirely on the performance of learning models and ignore the potential density information in datasets. Relatively speaking, the strategy based on the data density relationships is not restricted by the distribution of initially labeled data and entire data space (Wu et al., 2018), and is more suitable for self-training learning on non-spherically distributed data.
In the self-training process of SSCAC, newly labeled data are selected based on density-pointing relationships between data (Wu et al., 2018; Li et al., 2019). The strategy utilizes clustering by fast search and find of density peaks (DPC) (Rodriguez and Laio, 2014), and for each datum xi, its local density ρi can be defined as:
where dij is the Euclidean distance between xi and xj, and dc is the cut-off distance. It can be seen that the value of local density ρi is the number of data whose distance from xi is less than dc. In addition, DPC defines the minimum distance between xi and other data with higher local densities as follows:
The newly labeled data selection strategy calculates ρi and δi for each datum xi and make xi point to its nearest datum xj with a higher local density, then xj is called the “next” datum of xi and xi is the “previous” datum of xj. Then, the strategy constructs the density-pointing relationships of low-density data to high-density data by selecting the “next” and “previous” unlabeled data of labeled data in batches and set their selection orders. Specifically, all the “next” data of data in the original labeled dataset XL are firstly selected from the unlabeled dataset XU, and their selection orders are set to 1. That is, these data are viewed as the ones that should be labeled in the first iteration of self-training and used as the newly labeled data to expand the labeled dataset. In the next iteration, all the “next” data of the newly labeled data of the previous iteration are selected from XU, and their selection orders increase by 1. This step repeats until there exists no “next” data of the newly labeled data of the previous iteration in XU. If there are still unselected data in XU, the selection orders of these remaining data can be set according to the “previous” relationships using the similar process. It can be seen that the unlabeled data with the same selection orders form the newly labeled dataset of the same iteration of self-training, on which basis the proposed SSCAC algorithm can expand the labeled dataset XL iteratively and realize self-training clustering.
2.3 Adaptive adjustment of label confidence based on gravitational search algorithm
According to the analysis of the SSCAC objective function in the previous subsection, it is obvious that the value of different newly labeled data should vary for semi-supervised learning. If the newly labeled datum xi is mislabeled, its incorrect label will propagate to the unlabeled data in the same subspace, making these data together with xi have significant differences in the label prediction vectors from those of the correctly labeled data in that subspace. In this case, Eq. 1 will inevitably result in a large function value, and xi can be regarded as a low-value newly labeled datum. Conversely, the newly labeled datum xi with correct label can propagate its correct label in the subspace it belongs to, so that the unlabeled data in this subspace will obtain similar label prediction vectors to those of the correctly labeled data. In this case, the objective function value of Eq. 1 will be relatively small, and xi can be regarded as a high-value newly labeled datum. Therefore, the SSCAC algorithm proposed in this paper measures the semi-supervised learning value of newly labeled data by the objective function value of Eq. 1, and on this basis, achieves the adaptive adjustment of label confidence.
Gravitational search algorithm (GSA) (Rashedi et al., 2009) is an optimization method based on the law of gravity, which is easy to implement and requires fewer parameters. It has been proven in the literature that GSA outperforms heuristic search algorithms such as PSO and GA (Mirjalili et al., 2012; Kumar et al., 2013). The search particles in GSA are a set of individuals that attract each other and generate motion in the solution space, the position of the individual is the solution of the optimization problem. Under the influence of gravity, the individuals move toward the individuals with heavier masses, which correspond to better solutions. To distinguish from the iterations of self-training learning, the iteration index of GSA is referred to as time in this paper. In the r-th iteration of self-training, let I be the number of newly labeled data,
where N is the population size, μi,a(t) is the label confidence of the i-th newly labeled datum
Based on the SSCAC objective function given in Eq. 9, the GSA fitness function of particle a at time t is defined as:
For the i-th newly labeled datum
where G(t) is gravitational constant at time t, Ma(t) and Mb(t) are the inertial masses of particle a and particle b, Rab(t) is the Euclidean distance between particle a and particle b, and ɛ is a tiny constant to avoid zero denominator. The following equation can be used to determine the gravitational constant G(t):
where G0 is the initial value of the gravitational coefficient, α is the decay coefficient, G0 and α are usually taken as 100 and 20 (Rashedi et al., 2009), and T is the maximum time.
During the motion of a particle, the inertial mass Ma(t) of particle a can be updated according to the adapted value:
where ma(t) is the intermediate variable, best(t) and worst(t) are the best and worst fitness values among all particles at time t, respectively. In this paper, the particle position that makes the fitness value Eq. 15 obtain the minimum value is selected as the label confidence of the newly labeled data. Here, best(t) and worst(t) are respectively given by:
According to Newtonian gravity and the laws of motion, the gravitational force on particle a in the i-th dimension at time t is the sum of the gravitational forces from all other particles.
where randb is a random number within [0,1]. According to Newton’s second law, the acceleration of particle a in the i-th dimension is:
Therefore, the velocity and position of particle a in the i-th dimension at the next time are updated by:
where randa is a random number within [0,1], the initial velocity vi,a(0) is 0.
When time t reaches T, the position of the particle that obtains the minimum fitness value is used as the label confidence vector μr for the newly labeled data Xr at the r-th iteration of self-training. Then, we can update μ, U and Y in Eq. 9 based on the obtained label confidence vector μr, the newly labeled data Xr, their predicted labels respectively, and guide the subsequent iterations of self-training. It can be seen that the proposed strategy can adaptively adjust the label confidence based on the semi-supervised learning value of the newly labeled data. By reducing the label confidences of low-value newly labeled data, we can effectively reduce their effect on semi-supervised learning and thus alleviate the problem of mistaken reinforcement in the self-training gene clustering.
2.4 The procedure of the proposed SSCAC algorithm
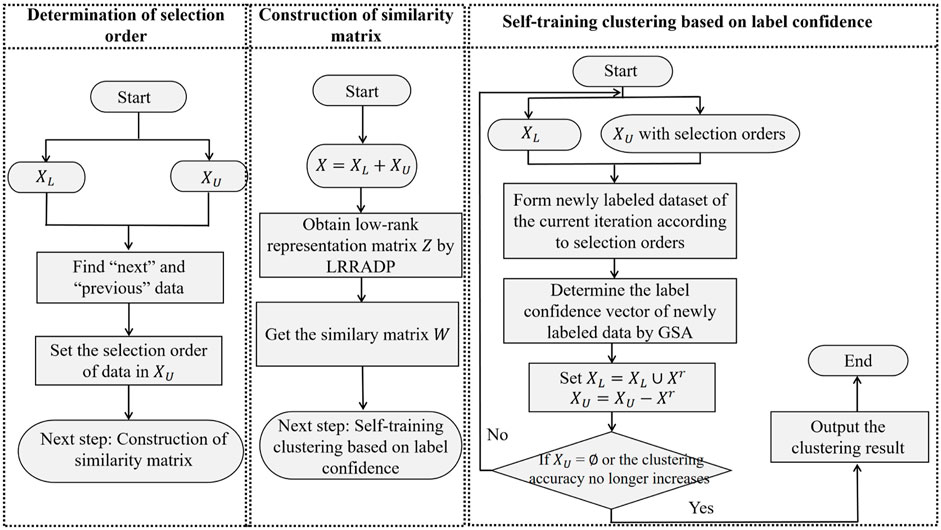
For a set of gene expression data X = [x1, x2, …, xl, xl+1, …, xn] = [XL, XU] ∈ Rm×n, the detailed procedure of SSCAC is given in Algorithm 1, and the framework of SSCAC is shown in Figure 1. In SSCAC, the stopping condition is set to XU = ∅ or the clustering accuracy no longer increases as suggested in the literature (Qu et al., 2019).
Algorithm 1. Note that when the stopping condition is that the clustering accuracy no longer increases, the labels of the remaining data in XU are obtained based on F.
Step 1: Set the parameters, including maximum value of penalty parameter βmax, iteration stop parameter ξ, constant ρ, balance parameters λ1 and λ2 of the LRRADP algorithm, and population size N, maximum time T, constant ɛ of the GSA algorithm.
Step 2: For each datum xi in X, initialize its selection order O(i) = 0, calculate ρi, δi according to Eqs 12, 13, and find the “next” and “previous” data of xi based on ρi, δi. Set the iteration index of the unlabeled data selection r = 1, then set the selection order of unlabeled data by the following steps.
1) For each datum xi in XU, if xi is the “next” datum of a datum in XL, set its selection order O(i) = r.
2) Set r = r + 1. For each unselected datum xi in XU, if xi is the “next” datum of a datum whose selection order is r − 1, set O(i) = r.
3) If there still exists “next” data of data whose selection orders are r in XU, then return to 2); otherwise, set r = r + 1 and go to 4).
4) For each unselected datum xi in XU, if xi is the “previous” datum of the selected data, then set O(i) = r.
5) Set r = r + 1. For each unselected datum xi in XU, if xi is the “previous” datum of a datum whose selection order is r − 1, set O(i) = r.
6) If there still exists “previous” data of data whose selection orders are r in XU, then return to 5); otherwise, get the vector O of selection order for unlabeled data and go to Step3.
Step 3: Initialize Z = H = E = Λ1 = Λ2 = 0, β0 = 1. Set the iteration index of the LRRADP algotirhm p = 0, calculate Eqs 3–8 iteratively until
Step 4: For the r-th iteration of self-training, initialize the newly labeled dataset Xr = ∅. For each datum xi whose O(i) = r, label xi according to its predicted label
Step 5: Determine the label confidence vector μr for the newly labeled data Xr by the following steps.
1) For each particle a(1 ≤ a ≤ N), randomly generate each element of its initial position
2) For each particle a, calculate its fitness value at time t according to Eq. 15, update its position
3) If t < T, then set t = t + 1 and return to 2); otherwise, the position of the particle with minimum fitness value is used as the label confidence vector μr and go to Step 6.
Step 6: Set XL = XL ∪ Xr, XU = XU − Xr, update μ, U and Y. Update the label prediction matrix F and predicted labels of the data according to Eqs 10, 11. If XU = ∅ or the clustering accuracy no longer increases compared with the previous iteration, stop and output the clustering result; otherwise, set r = r + 1 and return to Step 4.
3 Experimental results and analysis
3.1 Experimental setup
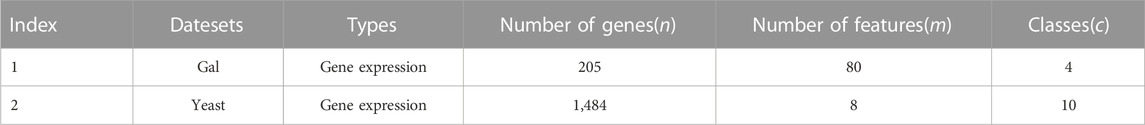
In this paper, comparative experiments are conducted in two benchmark gene expression datasets, as shown in Table 1. The Gal dataset (Ideker et al., 2001) is composed of gene expression measurements for 205 genes involved in galactose use in Saccharomyces cerevisiae. The gene expression profiles were measured with four replicate assays across 20 time points and the expression patterns reflect four functional categories. Yeast is a UCI dataset, which aims to predict the localization sites of proteins in cells and contains 1,484 yeast genes with eight methods of predicting protein localization sites in dimensions. Besides, we also demonstrate the applications of the proposed algorithm in other datasets, details of the datasets are tabulated in Supplementary Table S1, and the clustering results can be seen in Supplementary Tables S2, S3.
To verify the effectiveness of the SSCAC algorithm proposed in this paper for gene expression data, SSCAC is compared with three unsupervised clustering algorithms and four semi-supervised learning algorithms, including the K-means clustering based on the original gene expression data X, the K-means clustering based on the low-rank representation matrix Z (LRR + Kmeans) (Xia et al., 2018), the NCut clustering based on the LRR similarity matrix W (LRR + NCut) (Liu et al., 2013), SSC-LRR (Xia et al., 2018), STDP (Wu et al., 2018), STDPNF (Li et al., 2019), and LRRADP + GFHF (Fei et al., 2017) algorithms, where SSC-LRR, STDP, and STDPNF are self-training methods. To illustrate the effectiveness of the filter in the STDPNF algorithm, both STDP and STDPNF use KNN as the base classifier. Based on the suggestion of the literature (Xia et al., 2018), the balance parameter λ in LRR and SSC-LRR algorithms is tuned within [2−3, 24], and the parameter value corresponding to the optimal clustering result is selected, so we set λ = 0.1 for all the datasets. In LRRADP + GFHF and SSCAC, we set the balance parameters λ1 = 100, λ2 = 1 and λ∞ = 1 × 105, and the maximum value of penalty parameter βmax = 104, iteration stop parameter ξ = 10−5, constant ρ = 1.01. And we set the maximum time of the adaptive adjustment of label confidence T = 100, population size N = 50, and constant ɛ = 2.2204e − 16 in SSCAC, the cut-off distance dc is the corresponding value of data distance sorted in ascending order of 2%, and the other parameters in comparison methods are set as suggested in the original studies. Similar to literature (Nie et al., 2012; Fei et al., 2017), the experiments in this paper form the initial labeled dataset XL by randomly selecting 10% of the data in each dataset, and the rest of the data form the unlabeled dataset XU. All algorithms are run 10 times with randomly selected initial labeled data, and the algorithm performance is evaluated using the mean value of the results.
3.2 Evaluation metrics
To assess the partition performance, we use two popular metrics, accuracy (ACC) and Normalized mutual information (NMI).
(1) ACC is calculated by
where yi and
(2) NMI is calculated by
where A and B denote the vectors consisting of the true and predicted labels corresponding to the partition results, respectively. I(A, B) denotes the mutual information measure, H(A) and H(B) denote the entropy of A and B, respectively. The value of NMI is between 0 and 1, and a larger value of NMI indicates a better partition performance.
3.3 Comparative results and analysis
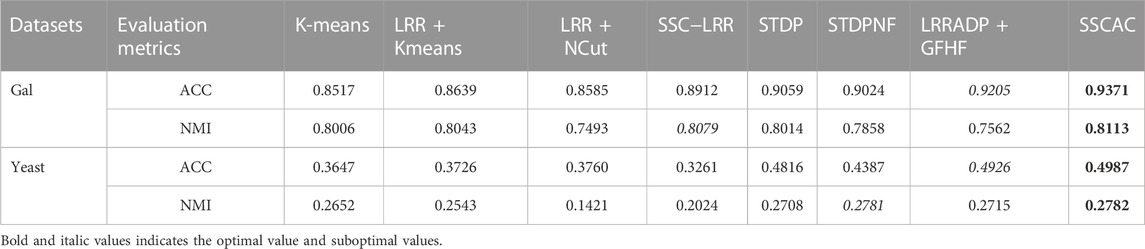
Table 2 shows the ACC and NMI results of eight algorithms on two benchmark gene expression datasets. The optimal and suboptimal results are marked with bold and italics, respectively.
From the results in Table 2, it can be seen that.
(1) For the two benchmark gene expression datasets, the clustering results of the SSCAC algorithm proposed in this paper are significantly better than those of the comparison algorithms, indicating the effectiveness of the proposed self-training subspace clustering framework and the adaptive adjustment strategy of label confidence. In addition, the performance of the semi-supervised learning methods outperforms that of the unsupervised clustering algorithms in general, reflecting the advantages of the semi-supervised learning methods.
(2) Among the unsupervised clustering algorithms, LRR + Kmeans and LRR + NCut perform better overall than the K-means algorithm based on the original gene expression data X. Compared with K-means, LRR + Kmeans and LRR + NCut improve ACC by an average of 1.80% and 1.95% for two benchmark gene expression datasets. This is because the low-rank representation matrix Z and the similarity matrix W can better reflect the properties of the gene expression data in the low-dimensional subspace, thus more discriminative features can be extracted from the data (Xia et al., 2018). Compared with LRR, the LRRADP used in the proposed SSCAC algorithm further enhances the locality of the model and can better capture the subspace structure of gene expression data. This advantage of SSCAC will be further demonstrated and analyzed in Section 3.7.
(3) Compared with the self-training algorithms SSC-LRR, STDP, and STDPNF, the SSCAC algorithm proposed in this paper has significant advantages. One of the main reasons is that the compared self-training methods implicitly assume that all newly labeled data have the same label confidence. As pointed out in the literature (Mellor et al., 2015; Xia et al., 2018; Li et al., 2019), the problem of mislabeling is inevitable, so setting the same label confidence for both mislabeled and correctly labeled data will lead to continuous reinforcement of incorrect labels during label propagation. Besides, the proposed SSCAC algorithm also outperforms the semi-supervised LRRADP + GFHF, the analysis and comparison will be detailed in the following ablation study.
In order to verify the convergence of the proposed updating strategy of confidence vector in SSCAC, convergence analysis experiments regarding the number of iterations versus fitness value are done for two benchmark gene expression datasets, Gal and Yeast. As shown in Figure 2, the fitness values flatten out with increasing iteration number and finally converge in approximately 100 iterations. Then, the position of the particle that obtains the minimum fitness value is used as the label confidence vector for the newly labeled data, on which basis SSCAC yields superior clustering results.
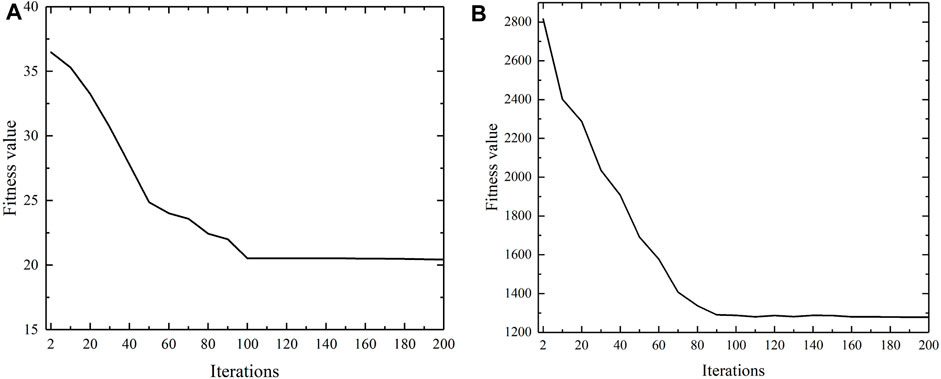
FIGURE 2. Convergence analysis of proposed updating strategy of confidence vector in SSCAC. (A) Gal; (B) Yeast.
3.4 Ablation study
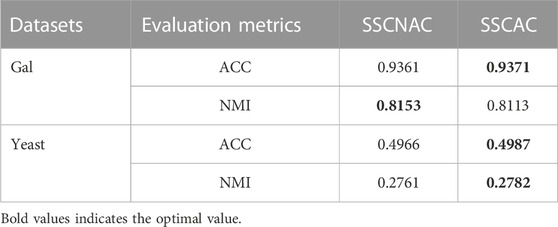
In order to validate the effectiveness of label confidence, we also conduct an ablation study. The ablation algorithm is referred to as SSCNAC, i.e., SSCAC without label confidence. In SSCNAC, the same label confidence μi = 1 is implicitly set for each newly labeled datum xi in the self-training process, thus SSCNAC is a self-training subspace clustering algorithm based on original GFHF. The parameter setting of SSCNAC is the same as that of SSCAC, and the performance of SSCNAC and SSCAC in terms of ACC and NMI is reported in Table 3. The optimal values of Table 3 are shown in bold. From Table 3, it can be seen that the proposed SSCAC algorithm achieves better clustering performance over SSCNAC. As with other self-training algorithms, SSCNAC performs self-training with complete confidence in the label accuracy of newly labeled data, and therefore suffers from the problem of mislabeling. Comparatively speaking, the proposed SSCAC algorithm introduces label confidences into the semi-supervised clustering objective function and adaptively adjusts them based on semi-supervised learning values, thus can effectively mitigate the negative impact of mislabeled data on self-training learning. This advantage of SSCAC will be further demonstrated in Table 4.
Moreover, from Tables 2, 3, we can also observe that the clustering results of SSCNAC outperform those of LRRADP + GFHF, with an average improvement of 1.25% and 4.75% in ACC and NMI, respectively. In essence, the SSCNAC algorithm with fixed-label confidence is a direct extension of LRRADP + GFHF on self-training, which gives SSCNAC the ability to learn from unlabeled data in self-training framework and therefore has better generalization performance. The above results demonstrate the positive role of unlabeled data in self-training learning and the effectiveness of the proposed self-training subspace clustering framework based on GFHF for gene expression data.
3.5 Analysis of hyper-parameters
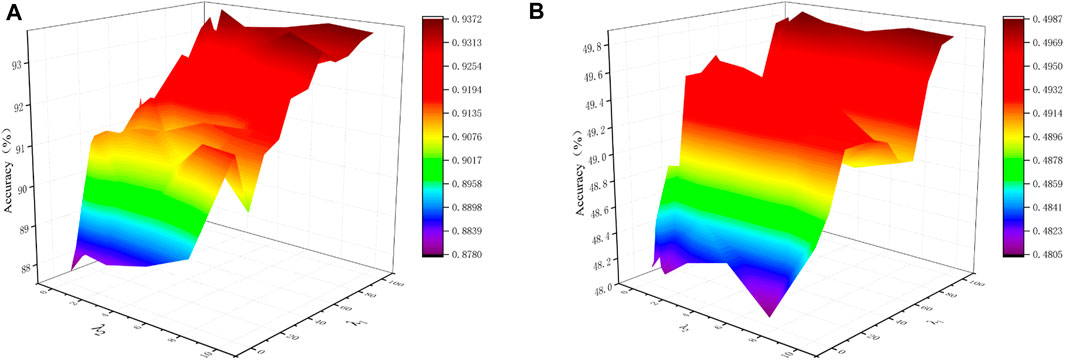
In the proposed SSCAC algorithm, λ1 and λ2 are balance parameters to trade off among the low-rank representation, noise and adaptive distance penalty. Figure 3 shows the impact of the two hyper-parameters on the performace of SSCAC. As can be observed, the proposed SSCAC algorithm is comparatively unaffected by hyper-parameters that are close to the ideal. To be more precise, we advise setting λ1 = 100 and λ2 = 1.
3.6 Analysis of the impact of initially labeled data ratio
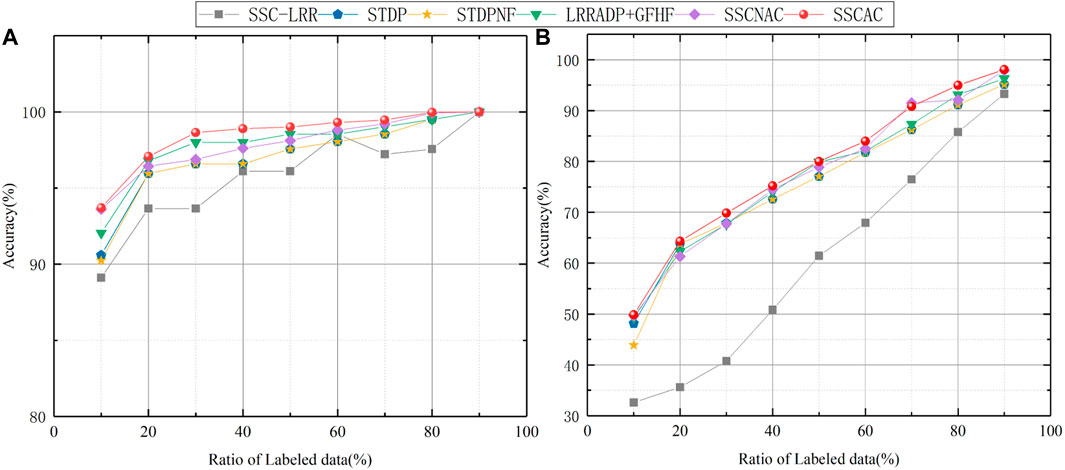
In order to analyze the impact of initially labeled data size on algorithm performance, we increase the initially labeled data ratio from 10% to 90% and conducted experiments, all algorithms are run 10 times. The average ACC curves of semi-supervised SSC-LRR, STDP, STDPNF, LRRADP + GFHF, SSCNAC and SSCAC algorithms are given in Figure 4.
It can be seen from Figure 4 that, in general, the partition accuracy of each algorithm increases along with the size of initially labeled data, the reason is that the increase in available label information helps to obtain models that fit the data more closely. It can also be seen from Figure 4 that in all cases, the ACC values of the proposed SSCAC algorithm are higher than those of the comparison algorithms, and this advantage of SSCAC is more evident in the cases when the initially labeled data ratios are relatively low. This is because that in such cases, the newly labeled data occupies a larger proportion of the labeled dataset and therefore plays a dominant role in the self-training process. Thus, the adaptive adjustment strategy of label confidence of SSCAC can reduce the influence of mislabeled data to a greater extent. As the proportion of initially labeled data increases, the dominant role of the newly labeled data in the self-training process decreases, and the semi-supervised learning performance of each algorithm tends to be similar. The above results and analysis indicate that the SSCAC algorithm proposed in this paper is more suitable for solving the semi-supervised clustering problem with less initially labeled data.
3.7 Analysis of the contribution of each part of the proposed SSCAC model
In this section, we discuss the contribution of each part of the proposed model. The SSCAC model described by Eq. 9 consists of two parts:

FIGURE 5. Visualization of original gene expression data and low-rank representation matrices on Gal. (A) Image of original data matrix X; (B) Image of LRR low-rank representation matrix Z; (C) Image of LRRADP low-rank representation matrix Z.
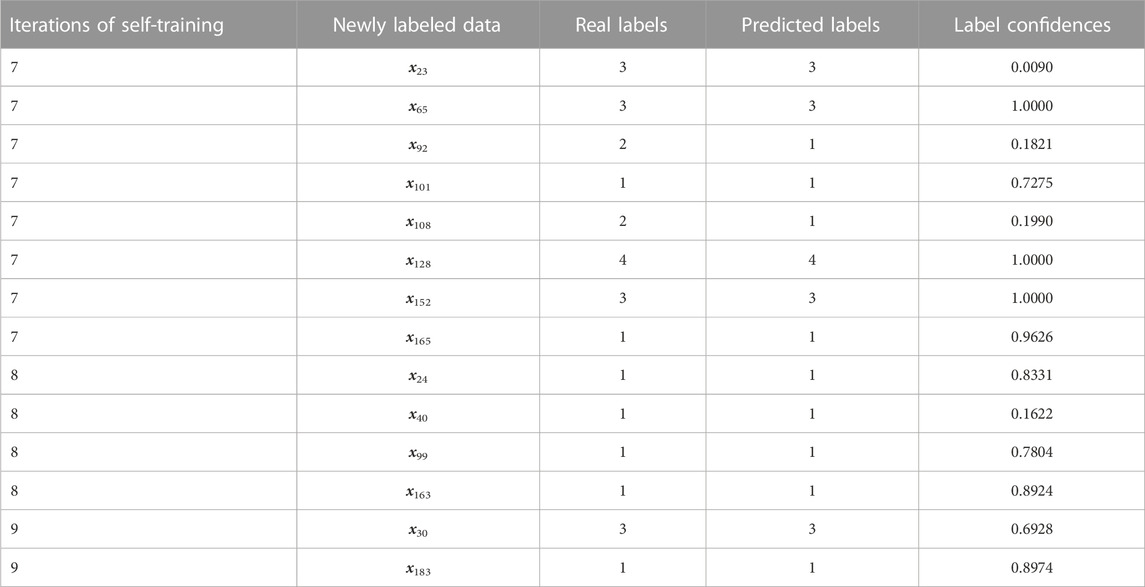
On the other hand, the second term of the SSCAC model, tr(F − μY)TU(F − μY), incorporates the label confidence μ with the aim to reduce the label confidences of mislabeled data through the proposed adaptive adjustment strategy of label confidence, and mitigate their negative impact during the self-training iterations. In order to verify the effectiveness of the adaptive adjustment strategy of label confidence in the SSCAC model, we focus on the newly labeled data, as well as their real labels, predicted labels, and label confidences during the self-training process. In our experiments, all algorithms are run 10 times with randomly selected initial labeled data. Thus, the newly labeled data selected during the iteration of SSCAC are different for different initial labeled data. Here, we take one case of random selection of initial labeled data on GAL as an example, where SSCAC achieves convergence in nine iterations. The newly labeled data selected in the last three iterations and their label confidences are reported in Table 4, and similar results can be obtained for other iterations.
As seen from Table 4, the adaptive adjustment strategy proposed in this paper can effectively reduce the label confidences of the mislabeled data, such as x92 and x108 in the 7-th iteration, and assign large confidences to the correctly labeled data. From Table 4, we can also observe that the label confidence of the correctly labeled datum x23 is rather small. As pointed out in the literature (Chen et al., 2011), even though some datum has correct label, it may have less impact on supervised learning due to its low partition uncertainty. Therefore, it is reasonable to assign a lower confidence to such correctly labeled datum. Compared with the existing self-training methods that do not consider the label confidence of newly labeled data, SSCAC can adaptively adjust the strength of supervisory guidance for different newly labeled data in the self-training process and effectively mitigate the negative impact of mislabeled data, which helps to significantly improve the clustering accuracy on gene expression data.
4 Conclusion
To deal with the widely existing problem of mislabeling in self-training learning tasks, a novel self-training subspace clustering algorithm for gene clustering is proposed in this paper. In particular, label confidences are integrated into the self-training clustering model, and the corresponding determination strategy of label confidences is proposed to adaptively adjust the supervision strength of newly labeled data according to their semi-supervised learning values. Moreover, the low-rank representation with distance penalty is adopted to improve discriminative property of gene expression data. Compared with other state-of-the-art unsupervised and semi-supervised learning algorithms, the proposed SSCAC algorithm can effectively mitigate the negative impact of mislabeling and improve the stability and accuracy of gene clustering. In our future work, we will consider biological knowledge such as Gene Ontology annotation information, and extend the proposed model to multi-view clustering framework to further improve clustering performance on gene expression data.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://archive.ics.uci.edu/ml/datasets/Yeast, http://genomebiology.com/2003/4/5/R34.
Author contributions
DL: conceptualization, methodology, software. HL: data curation, writing- original draft preparation. PQ: visualization, investigation. JW: writing- reviewing and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2023.1132370/full#supplementary-material
References
Basri, R., and Jacobs, D. (2003). Lambertian reflectance and linear subspaces. IEEE Trans. Pattern Analysis Mach. Intell. 25, 218–233. doi:10.1109/TPAMI.2003.1177153
Chapelle, O., Scholkopf, B., and Zien, A. (2006). Semi-supervised learning. Cambridge, Massachusettes: The MIT Press View Article.
Chen, R., Cao, Y., and Sun, H. (2011). Multi-class image classification based on active learning and semi-supervised learning. Acta Autom. Sin. 37, 954–962.
Dang, R., Qu, B., Guo, K., Zhou, S., Sun, H., Wang, W., et al. (2022). Weighted co-expression network analysis identifies rnf181 as a causal gene of coronary artery disease. Front. Genet. 12, 818813. doi:10.3389/fgene.2021.818813
Diniz, W. J. S., Mazzoni, G., Coutinho, L. L., Banerjee, P., Geistlinger, L., Cesar, A. S. M., et al. (2019). Detection of co-expressed pathway modules associated with mineral concentration and meat quality in nelore cattle. Front. Genet. 10, 210. doi:10.3389/fgene.2019.00210
Fei, L., Xu, Y., Fang, X., and Yang, J. (2017). Low rank representation with adaptive distance penalty for semi-supervised subspace classification. Pattern Recognit. 67, 252–262. doi:10.1016/j.patcog.2017.02.017
Feng, T., Davila, J. I., Liu, Y., Lin, S., Huang, S., and Wang, C. (2021). Semi-supervised topological analysis for elucidating hidden structures in high-dimensional transcriptome datasets. IEEE/ACM Trans. Comput. Biol. Bioinforma. 18, 1620–1631. doi:10.1109/TCBB.2019.2950657
Gan, H., Sang, N., Huang, R., Tong, X., and Dan, Z. (2013). Using clustering analysis to improve semi-supervised classification. Neurocomputing 101, 290–298. doi:10.1016/j.neucom.2012.08.020
Huang, H., and Feng, H. (2012). Gene classification using parameter-free semi-supervised manifold learning. IEEE/ACM Trans. Comput. Biol. Bioinforma. 9, 818–827. doi:10.1109/TCBB.2011.152
Huang, Z., and Wu, J. (2022). A multiview clustering method with low-rank and sparsity constraints for cancer subtyping. IEEE/ACM Trans. Comput. Biol. Bioinforma. 19, 1–3223. doi:10.1109/tcbb.2021.3122917
Ideker, T., Thorsson, V., Ranish, J. A., Christmas, R., Buhler, J., Eng, J. K., et al. (2001). Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science 292, 929–934. doi:10.1126/science.292.5518.929
Kumar, J., Kumar, D., and Edukondalu, K. (2013). Strategic bidding using fuzzy adaptive gravitational search algorithm in a pool based electricity market. Appl. Soft Comput. 13, 2445–2455. doi:10.1016/j.asoc.2012.12.003
Li, J., and Zhu, Q. (2020). A boosting self-training framework based on instance generation with natural neighbors for k nearest neighbor. Appl. Intell. 50, 3535–3553. doi:10.1007/s10489-020-01732-1
Li, J., Zhu, Q., and Wu, Q. (2019). A self-training method based on density peaks and an extended parameter-free local noise filter for k nearest neighbor. Knowledge-Based Syst. 184, 104895. doi:10.1016/j.knosys.2019.104895
Li, Z., and Yang, L. (2020). Underlying mechanisms and candidate drugs for Covid-19 based on the connectivity map database. Front. Genet. 11, 558557. doi:10.3389/fgene.2020.558557
Liu, G., Lin, Z., Yan, S., Sun, J., Yu, Y., and Ma, Y. (2013). Robust recovery of subspace structures by low-rank representation. IEEE Trans. Pattern Analysis Mach. Intell. 35, 171–184. doi:10.1109/TPAMI.2012.88
Liu, G., Liu, B., Li, A., Wang, X., Yu, J., and Zhou, X. (2021). Identifying protein complexes with clear module structure using pairwise constraints in protein interaction networks. Front. Genet. 12, 664786. doi:10.3389/fgene.2021.664786
Liu, R., Verbič, G., and Ma, J. (2019). A new dynamic security assessment framework based on semi-supervised learning and data editing. Electr. Power Syst. Res. 172, 221–229. doi:10.1016/j.epsr.2019.03.009
Lu, C., Wang, J., Liu, J., Zheng, C., Kong, X., and Zhang, X. (2020). Non-negative symmetric low-rank representation graph regularized method for cancer clustering based on score function. Front. Genet. 10, 1353. doi:10.3389/fgene.2019.01353
Maâtouk, O., Ayadi, W., Bouziri, H., and Duval, B. (2019). Evolutionary biclustering algorithms: An experimental study on microarray data. Soft Comput. 23, 7671–7697. doi:10.1007/s00500-018-3394-4
Mahendran, N., Durai Raj Vincent, P. M., Srinivasan, K., and Chang, C.-Y. (2020). Machine learning based computational gene selection models: A survey, performance evaluation, open issues, and future research directions. Front. Genet. 11, 603808. doi:10.3389/fgene.2020.603808
Mellor, A., Boukir, S., Haywood, A., and Jones, S. (2015). Exploring issues of training data imbalance and mislabelling on random forest performance for large area land cover classification using the ensemble margin. ISPRS J. Photogrammetry Remote Sens. 105, 155–168. doi:10.1016/j.isprsjprs.2015.03.014
Mirjalili, S., Hashim, S., and Sardroudi, H. (2012). Training feedforward neural networks using hybrid particle swarm optimization and gravitational search algorithm. Appl. Math. Comput. 218, 11125–11137. doi:10.1016/j.amc.2012.04.069
Nie, F., Xu, D., and Li, X. (2012). Initialization independent clustering with actively self-training method. IEEE Trans. Syst. Man, Cybern. Part B 42, 17–27. doi:10.1109/TSMCB.2011.2161607
Nisar, M., Paracha, R. Z., Arshad, I., Adil, S., Zeb, S., Hanif, R., et al. (2021). Integrated analysis of microarray and rna-seq data for the identification of hub genes and networks involved in the pancreatic cancer. Front. Genet. 12, 663787. doi:10.3389/fgene.2021.663787
Qu, Z., Wu, C., and Wang, X. (2019). Semi-supervised self-training for aspect extraction. CAAI Trans. Intelligent Syst. 14, 635–641.
Rashedi, E., Nezamabadi-pour, H., and Saryazdi, S. (2009). Gsa: A gravitational search algorithm. Inf. Sci. 179, 2232–2248. doi:10.1016/j.ins.2009.03.004
Rodriguez, A., and Laio, A. (2014). Machine learning. Clustering by fast search and find of density peaks. Science 344, 1492–1496. doi:10.1126/science.1242072
Shi, Q., Hu, B., Zeng, T., and Zhang, C. (2019). Multi-view subspace clustering analysis for aggregating multiple heterogeneous omics data. Front. Genet. 10, 744. doi:10.3389/fgene.2019.00744
Summers, K. M., Bush, S. J., Wu, C., Su, A. I., Muriuki, C., Clark, E. L., et al. (2020). Functional annotation of the transcriptome of the pig, sus scrofa, based upon network analysis of an rnaseq transcriptional atlas. Front. Genet. 10, 1355. doi:10.3389/fgene.2019.01355
Sun, Y., Ou-Yang, L., and Dai, D.-Q. (2021). Wmlrr: A weighted multi-view low rank representation to identify cancer subtypes from multiple types of omics data. IEEE/ACM Trans. Comput. Biol. Bioinforma. 18, 2891–2897. doi:10.1109/TCBB.2021.3063284
Wang, J., Liu, J., Kong, X., Yuan, S., and Dai, L. (2019). Laplacian regularized low-rank representation for cancer samples clustering. Comput. Biol. Chem. 78, 504–509. doi:10.1016/j.compbiolchem.2018.11.003
Wei, Z., Wang, H., and Zhao, R. (2013). Semi-supervised multi-label image classification based on nearest neighbor editing. Neurocomputing 119, 462–468. doi:10.1016/j.neucom.2013.03.011
Wu, D., Shang, M., Luo, X., Xu, J., Yan, H., Deng, W., et al. (2018). Self-training semi-supervised classification based on density peaks of data. Neurocomputing 275, 180–191. doi:10.1016/j.neucom.2017.05.072
Xia, C., Han, K., Qi, Y., Zhang, Y., and Yu, D. (2018). A self-training subspace clustering algorithm under low-rank representation for cancer classification on gene expression data. IEEE/ACM Trans. Comput. Biol. Bioinforma. 15, 1315–1324. doi:10.1109/TCBB.2017.2712607
Xu, Y., Chen, S., Li, J., Xu, C., and Yang, J. (2023). Fast subspace clustering by learning projective block diagonal representation. Pattern Recognit. 135, 109152. doi:10.1016/j.patcog.2022.109152
Yu, Z., Chen, H., You, J., Wong, H.-S., Liu, J., Li, L., et al. (2014). Double selection based semi-supervised clustering ensemble for tumor clustering from gene expression profiles. IEEE/ACM Trans. Comput. Biol. Bioinforma. 11, 727–740. doi:10.1109/TCBB.2014.2315996
Yu, Z., Luo, P., You, J., Wong, H.-S., Leung, H., Wu, S., et al. (2016). Incremental semi-supervised clustering ensemble for high dimensional data clustering. IEEE Trans. Knowl. Data Eng. 28, 701–714. doi:10.1109/TKDE.2015.2499200
Zhang, X., Liang, L., Liu, L., and Tang, M. (2021). Graph neural networks and their current applications in bioinformatics. Front. Genet. 12, 690049. doi:10.3389/fgene.2021.690049
Zheng, R., Li, M., Liang, Z., Wu, F.-X., Pan, Y., and Wang, J. (2019). Sinnlrr: A robust subspace clustering method for cell type detection by non-negative and low-rank representation. Bioinformatics 35, 3642–3650. doi:10.1093/bioinformatics/btz139
Keywords: self-training, subspace clustering, label confidence, adaptive adjustment, gravitational search algorithm, gene expression data
Citation: Li D, Liang H, Qin P and Wang J (2023) A self-training subspace clustering algorithm based on adaptive confidence for gene expression data. Front. Genet. 14:1132370. doi: 10.3389/fgene.2023.1132370
Received: 27 December 2022; Accepted: 07 March 2023;
Published: 21 March 2023.
Edited by:
Cong Liu, Columbia University, United StatesReviewed by:
Hong Peng, Chinese Academy of Sciences (CAS), ChinaJiaogen Zhou, Huaiyin Normal University, China
Copyright © 2023 Li, Liang, Qin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pan Qin, cXAxMTJjbkBkbHV0LmVkdS5jbg==; Jia Wang, d2FuZ2ppYTc3QGhvdG1haWwuY29t
 Dan Li
Dan Li Hongnan Liang
Hongnan Liang Pan Qin
Pan Qin Jia Wang
Jia Wang