
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 13 March 2023
Sec. Molecular Cytogenetics
Volume 14 - 2023 | https://doi.org/10.3389/fgene.2023.1036467
This article is part of the Research Topic The Evolution of PGT-M in Diagnostics and Therapeutics View all 4 articles
 Bo-lan Sun1†
Bo-lan Sun1† Yong Wang1†
Yong Wang1† Sixi-Wen1
Sixi-Wen1 Liang Zhou1
Liang Zhou1 Chun-hui Zhang1
Chun-hui Zhang1 Ze-Xuan Wu1
Ze-Xuan Wu1 Jie Qiao2
Jie Qiao2 Qing-yuan Sun3
Qing-yuan Sun3 Ya-xin Yao4
Ya-xin Yao4 Jing Wang4
Jing Wang4 Zi-Yun Yi1*
Zi-Yun Yi1* Wei-Ping Qian1*
Wei-Ping Qian1*Purpose: To study the accuracy of non-invasive chromosomal screening (NICS) results, in normal chromosomes and chromosomal rearrangement groups and to investigate whether using trophoblast cell biopsy along with NICS, to choose embryos for transfer can improve the clinical outcomes of assisted pregnancy.
Methods: We retrospectively analyzed 101 couples who underwent preimplantation genetic testing at our center from January 2019 to June 2021 and collected 492 blastocysts for trophocyte (TE) biopsy. D3-5 blastocyst culture fluid and blastocyst cavity fluid were collected for the NICS. Amongst them, 278 blastocysts (58 couples) and 214 blastocysts (43 couples) were included in the normal chromosomes and chromosomal rearrangement groups, respectively. Couples undergoing embryo transfer were divided into group A, in which both the NICS and TE biopsy results were euploid (52 embryos), and group B, in which the TE biopsy results were euploid and the NICS results were aneuploid (33 embryos).
Results: In the normal karyotype group, concordance for embryo ploidy was 78.1%, sensitivity was 94.9%, specificity was 51.4%, the positive predictive value (PPV) was 75.7%, and the negative predictive value (NPV) was 86.4%. In the chromosomal rearrangement group, concordance for embryo ploidy was 73.1%, sensitivity was 93.3%, specificity was 53.3%, the PPV was 66.3%, and the NPV was 89%. In euploid TE/euploid NICS group, 52 embryos were transferred; the clinical pregnancy rate was 71.2%, miscarriage rate was 5.4%, and ongoing pregnancy rate was 67.3%. In euploid TE/aneuploid NICS group, 33 embryos were transferred; the clinic pregnancy rate was 54.5%, miscarriage rate was 5.6%, and ongoingpregnancy rate was 51.5%. The clinical pregnancy and ongoing pregnancy rates were higher in the TE and NICS euploid group.
Conclusion: NICS was similarly effective in assessing both normal and abnormal populations. Identification of euploidy and aneuploidy alone may lead to the wastage of embryos due to high false positives. More suitable reporting methods for NICS and countermeasures for a high number of false positives in NICS are needed. In summary, our results suggest that combining biopsy and NICS results could improve the outcomes of assisted pregnancy.
With the increase in work pressure and competition in modern society, the average childbearing age of couples is increasing; there is also a decline in fertility rates and an increase in infertility due to various reasons, such as chromosomal abnormalities of embryos which is mainly caused by age-dependent chromosome segregation errors during meiosis I (Xu et al., 2016). Therefore, an increasing number of couples are getting pregnant through assisted reproductive technology. However, chromosomal aneuploidy is an important factor that affects the success rate of assisted pregnancies. Chromosomal abnormalities can be prevented by performing embryo biopsies and preimplantation genetic testing (PGT). Multiple clinical trials have confirmed the clinical efficacy of PGT, including increased rates of implantation and clinical pregnancy, and decreased rates of miscarriages (Dreesen et al., 2014). However, biopsy of blastocyst trophoblast cells that are commonly used in clinics for PGT analysis is an invasive detection method and there are limitations to its clinical application: the biopsy is difficult to carry out and damage to embryos cannot completely be avoided (Zhang J. et al., 2019; Tiegs et al., 2019; Tocci, 2020; Makhijani et al., 2021). Furthermore, 30%–40% of embryos have chromosomal karyotype chimerism, and trophocyte (TE) biopsies cannot accurately represent the genome profile of the inner cell mass (ICM) and the remainder of the TE. Therefore, the accuracy of PGT detection is reduced by sample bias (Taylor et al., 2014; Munné et al., 2020).
Given the above limitations of TE biopsies, recent attention has been given to a non-invasive chromosome detection approach in embryos. Both genomic and mitochondrial DNA contents were found in blastocoel fluid (also called blastocyst cavity fluid; BF) and spent culture medium (Magli et al., 2016). In 2013, Stigliani et al. (Stigliani et al., 2013) first confirmed the existence of cell-free DNA in blastocyst culture media (SCM). Subsequently, DNA in the BF could be used for genetic analysis (Basile et al., 2015; Chen L. et al., 2021). Xu et al. reported a non-invasive chromosomal screening (NICS) method based on the sequencing of genomic DNA secreted into the culture medium from human blastocysts. This approach has the potential for a much wider chromosome screening applicability in clinical in vitro fertilization, due to its high accuracy and non-invasiveness (Gianaroli et al., 2014; Xu et al., 2016; Leaver and Wells, 2020; Rubio et al., 2021). Previous studies have shown that DNA testing using an embryo culture medium could on days 5 or 6 detect chromosome abnormalities with a reasonable positive predictive value (PPV) and high negative predictive value (NPV) (Rubio et al., 2021). Moreover, NICS, which is based on the sequencing of DNA from the SCM, may better represent the entire embryo compared to a TE biopsy alone (Poli et al., 2019). Moreover, in another study, concordance was higher in the SCM with the BF DNA analysis combination than in PGT with the TE biopsy alone (Huang et al., 2019). The one limitation to these methods is the low amount of DNA present in the BF and SCM; this can now be amplified for genetic analysis through whole-genome amplification (WGA) and detected through array comparative genomic hybridization and next-generation sequencing (NGS) (Shamonki et al., 2016; Jiao et al., 2019). In addition to this, several important issues need to be addressed before the routine clinical application of NICS. These include minimization of maternal DNA contamination (Kuznyetsov et al., 2018), determining factors that affect accuracy, and optimization of the WGA protocol for DNA in the SCM and BF.
For embryo screening, both the trophoblast cell biopsy and NICS methods have their advantages and disadvantages. We wanted to understand whether combining the two methods, to select embryos for transfer, improves the clinical outcome, as there are few such reports.
Therefore, in this study, we compared the consistency, sensitivity, specificity, PPV, and NPV of NICS in normal chromosome and chromosomal rearrangement groups. This is the first time that such a large sample size of different populations has been used to evaluate the performance of NICS. Furthermore, based on the results of TE biopsies and NICS, patients undergoing an embryo transfer were divided into two groups: euploid TE/euploid NICS group in which both the TE biopsy and NICS results suggested euploidy, and euploid TE/aneuploid NICS group in which the TE biopsy results suggested euploidy and the NICS results suggested aneuploidy. A series of clinical results from the two groups were compared in order to determine whether TE biopsies combined with NICS could improve clinical outcomes.
We performed a retrospective analysis of 101 couples who received PGT in our center from January 2019 to June 2021; 492 blastocysts were collected for TE biopsy. Meanwhile, D3-5 SCM and BF were collected for NICS. Among them, 278 blastocysts (58 couples) were included in the normal chromosome group and 214 blastocysts (43 couples) were included in the chromosomal rearrangement group. This study was approved by the Ethics Committee of the Peking University of Shenzhen Hospital ([2018] Issue no [014]) and was performed in accordance with the principles of the Declaration of Helsinki (1964) and its later amendments. Informed consent was obtained from all the patients included in this study.
In our study, we carefully removed and washed the cumulus-corona colliculus complex before intracytoplasmic sperm injection (ICSI). The embryos derived from ICSI were cultured to cleavage stage (D3). If there were any remaining granulosa cells, we removed the ramaining granulosa cells completely with a glass pipe. Then, each embryos was rinsed and transferred into an 20 µL droplet of blastocyst media, in the BD353001 petri dish (BD medical, Franklin Lakes, United States) to equilibration overnight. On the afternoon of D4, the embryos cultured in the collective culture were repeatedly blown, washed, and placed successively into the previously prepared blastocyst culture medium. Each embryo was placed into an individual drop of fresh blastocyst culture medium, which was then cultured in a three-gas incubator (37°C, 6% CO2, 5% O2). After the D5-D6 balstocyst was completely formed, artificial collapse of the blastocoel was induced by applying a laser pulse (300 μs), using the ZILOS-tkTM laser system (Hamilton Thorn Bioscience Inc., Beverly, MA, United States), at the junction of TE cells and the location of the juction was far from the inner cell mass. After 5 min of treatment, all the cluture media with the released blastocyst fluid were collected with a drawn glass straw and placed into a PCR tube containing 5 µL of cell lysis buffer (Yikon Genomics, Shanghai, China). The shriveled blastocyst was put into the biopsy operation dish and biopsied under an inverted microscope. The fixed needle was used to hold the cell mass inside the blastocyst at 9 o’clock, and a small hole was punctured, in the trophoblast cell junction, at three points opposite to the ICM. The biopsy needle entered the blastocyst at the perforated position and absorbed 3–6 trophectoderm ectoderm cells. The biopsied cells were completely separated from the blastocyst by a laser pulse (300 μs), using the ZILOS-tkTM laser system (Hamilton Thorn Bioscience Inc., Beverly, MA, United States), while being pulled. The biopsied blastocyst was removed under a stereomicroscope, and the biopsied trophoblast ectoderm cells were cleaned several times in phosphate-buffered saline. The egg stripping pipette (135 µm inner diameter) was used to move the biopsied trophoblast ectoderm cells into the PCR tube containing 5 µL of cell lysis buffer.
The SCM, BF and TE biopsy samples were subjected to cell lysis followed by WGA with the multiple annealing and looping-based amplification cycles (MALBAC) technique and library generation as described previously. The amplification products were sequenced on the Illumina HiSeq 2,500 platform (Illumina, San Diego, CA, United States) with approximately two million sequencing reads per sample. The read numbers were counted along the whole genome with a bin size of 1 Mb and normalized based on GC content and a reference dataset. A copy number gain from two to three copies results in a 50% increase in read counts, whereas a copy number loss from two copies to one copy results in a 50% decrease in read counts.
The standard for mosaicism in NICS (Jiao et al., 2019; Rubio et al., 2019; Yeung et al., 2019) and TE-PGT (Cram et al., 2019; Yeung et al., 2019; Lin et al., 2020) is not yet unified. We use the 30% as the threshold of TE-PGT. In the study reported by Yeung et al., the threshold of NICS and TE-PGT was also 30% (Yeung et al., 2019). For the detection results of TE cells and NICS, the mosaicism was reported as 30%. Embryos were classified as “mosaicism” if their mosaicism ranged from 30% to 70%. Embryos were classified as “euploid” if they were less than 30% mosaicism. Embryos were classified as “aneuploid” if they were more than 70% mosaicism. Due to the small fragment size and very minute DNA concentration, the window size for analysis was larger because sequencing date for SCM and BF were relatively noisier than for TE; and therefore, the detection limit was down to sub-chromosomal level (Yeung et al., 2019). Here, For the detection results of TE cells and SCM and BF results, the abnormal fragments were reported as ≥4 Mb and ≥10 Mb.
First, the NICS results were compared to the TE biopsy results in both groups. The consistency, sensitivity, specificity, PPV, and NPV were calculated. The parameters between the two groups were statistically analyzed (Figure 1). Thereafter, according to the TE biopsy and NICS results, couples undergoing embryo transfer were divided into two groups: euploid TE/euploid NICS group, wherein both the TE biopsy and NICS showed euploidy (52 embryos) and euploid TE/aneuploid NICS group, wherein the biopsy results showed euploidy and the NICS results showed aneuploidy (33 embryos). The clinical results from both groups (clinical pregnancy rate, miscarriage rate, ongoing pregnancy rate) were compared (Figure 2). In terms of outcomes, clinical pregnancy was diagnosed when a gestational sac was ascertained by transvaginal ultrasonography, ongoing pregnancy was defined as continued pregnancy at 8–12 weeks with positive fetal cardiac activity, while clinical miscarriage was defined as loss of pregnancy after some ultrasound findings were present (at least a gestational sac).
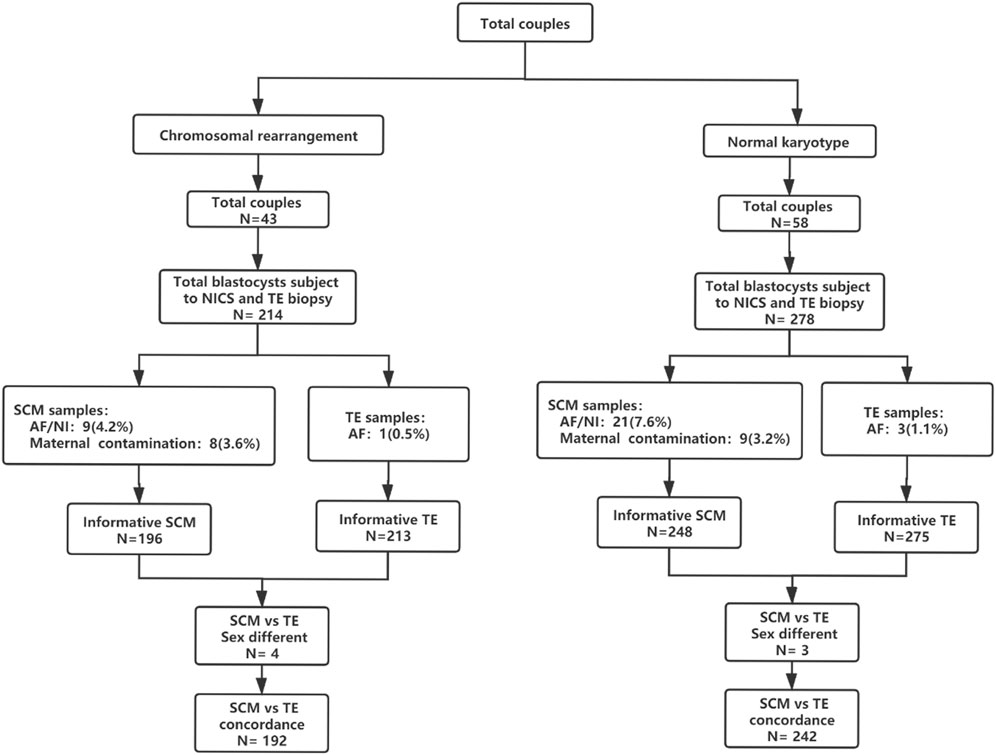
FIGURE 1. Flowchart of the study design. A total of 101 couples were included in this study; 278 blastocysts (58 couples) were included in the normal chromosome group and 214 blastocysts (43 couples) were included in the chromosomal rearrangement group. Abbreviations: AF, amplify failure; NI, non-information; NICS, non-invasive chromosomal screening; SCM, blastocyst culture fluid; TE, trophocyte.
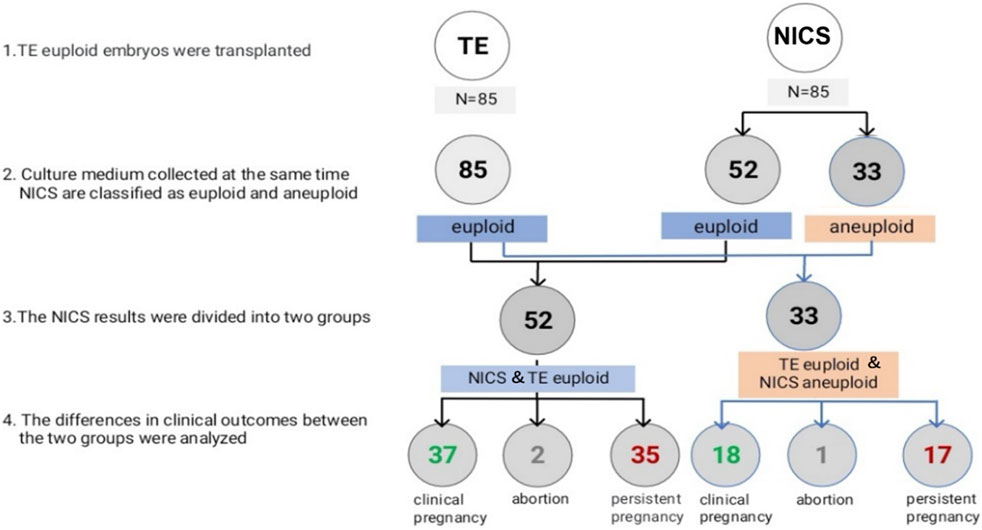
FIGURE 2. Euploidy and clinical outcomes of the transferred embryos. Abbreviations: NICS, non-invasive chromosomal screening; TE, trophocyte.
Descriptive data for the continuous variables are presented as the mean and standard deviation, and categorical data as numbers and percentages. Student’s t-tests and Mann–Whitney tests were used for parametric and non-parametric data, respectively. The chi-square or Fisher exact tests were used for the categorical variables of each group. All analyses were done using the SPSS version 25.0 (IBM, Armonk, NY, United States). Multiple logistic regression analyses were conducted to compare the outcomes of group A and group B after controlling for p < 0.10 and co-variables considered clinically influential, including female age, morphology (expansion, ICM, and trophectoderm), and cycle type of embryo transfer.
There were 278 blastocysts in the normal karyotype group; amongst these 9 blastocysts were contaminated with the parent source, 3 blastocysts were contaminated by exogenous sources (the extenrnal DNA may derived from plasticware, media, or manipulation during IVF), 21 blastocysts DNA samples from SCM and BF failed to amplify and could not be detected by NICS, and 3 blastocysts failed to amplify and could not be detected by TE biopsy. Finally, 242 blastocysts were used for consistent comparison (Figure 1). In the blastocyst culture medium of the 242 blastocysts included in the final analysis, the consistency rate was 73.1%, sensitivity was 93.3%, and specificity was 53.3%. There were 65 true negatives and 8 false negatives, with a false negative rate of 6.7% and an NPV of 89%. There were 112 true positives and 57 false positives, with a false positive rate of 46.7% and a PPV of 66.3% (Table 1).
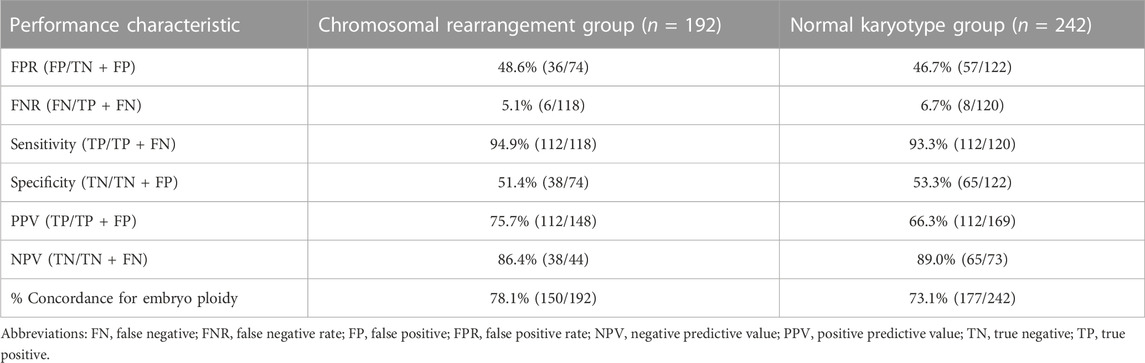
TABLE 1. Comparison of various parameters between the normal chromosome group and rearrangement group.
There were 214 blastocysts in the chromosomal rearrangement group; amongst these 8 blastocysts were contaminated with the parent source, 4 blastocysts were contaminated by exogenous sources, 9 blastocysts DNA samples from SCM and BF failed to amplify and could not be detected by NICS, and 1 blastocyst failed to amplify and could not be detected by TE biopsy. Finally, 192 were used for consistent comparison (Figure 1). In the blastocyst culture medium of the 192 blastocysts included in the final analysis, the consistency rate was 78.1%, sensitivity was 94.9%, and specificity was 51.4%. There were 38 true negatives and 6 false negatives, with a false negative rate of 5.1% and an NPV of 86.4%. There were 112 true positives and 36 false positives, with a false positive rate of 48.6% and a PPV of 75.7% (Table 1). There was no statistically significant difference (p > 0.05) in the performance characteristics between the chromosomal rearrangement and normal karyotype groups.
We further evaluated the embryos with different NICS CNV results. According the classification method of previous research (Xie et al., 2022), The CNV results from BCM can be classified into four different categories: 1) euploidy, 2) low-level mosaics (mosaic rates ≤50%) 3) high-level mosaics (mosaic rates >50%) 4) aneuploidy (Table 2). There was no statistically significant difference (p > 0.05) in the performance characteristics between the chromosomal rearrangement and normal karyotype groups.
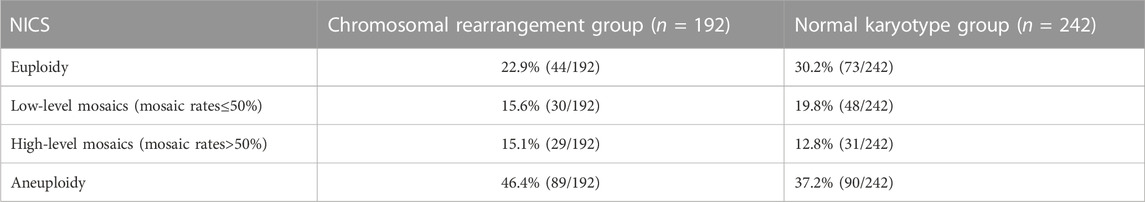
TABLE 2. Comparison of different NICS results between the normal chromosome group and rearrangement group.
In all, 85 TE euploid embryos were transferred, which were further divided into two groups: euploid TE/euploid NICS (52 embryos) and euploid TE/aneuploid NICS (33 embryos) groups (Figure 2). There was no statistical difference in any other basic conditions between the two groups except the endometrial preparation protocol (Table 3). The clinical pregnancy rates of the euploid TE/euploid NICS group and the euploid TE/aneuploid NICS group were 71.2% and 54.5%, ongoing pregnancy rates were 69.2% and 51.5%, and miscarriage rates were 5.4% and 5.6%, respectively. There were no statistical differences observed in terms of clinical pregnancy, miscarriage, and ongoing pregnancy rates between the two groups, however, the clinical pregnancy and ongoing pregnancy rates were higher in the euploid TE/euploid NICS group (Table 4).
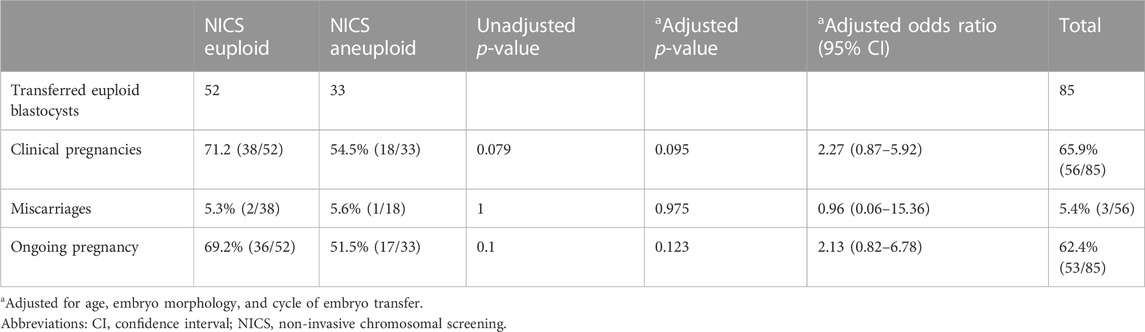
TABLE 4. Comparison of clinical outcomes between the euploid and aneuploid non-invasive chromosomal screening groups in trophocyte euploid embryo transplantation.
In recent years, more and more scholars have studied the effectiveness of NICS. Although NICS has the advantage of being a relatively simple and non-invasive sampling process compared to TE biopsy, contamination from the mother source and the low content of DNA in the culture medium can easily lead to the failure of amplification and affect the results. Wei Qiang Liu et al., observed that the DNA dectiction rate in SCM was slightly higher than that in BF samples (Liu et al., 2017), a finding that was also reported by Magil et al. (76.5%) and the Gianaroli group (82%) (Gianaroli et al., 2014) (Magli et al., 2016). Valeriy Kuznyetsov et al. (Kuznyetsov et al., 2018) and Li et al. (Li et al., 2018) found the higher DNA amplification rates were due to increased DNA availability caused by mixing BF and SBM. Subsequently, we collected samples after collapsingg the blastocyst by a laser without causing any other harm to the embryo. The BF DNA can be released into the embryo culture medium, which could notably increases the concerntration of the cf DNA. The rate of successful amplification across BCM samples was 93.9% (462 of 492), and the successful amplification occurered in 99.19% (488 of 492) of TE samples. Since the blastocyst was shriveled with a laser during collection, the protocal could be called less invasive and may be more appropriate than non-invasive. However, in the clinic, before blastocyst vitrification, people, release the blastocoel fluid through laser drilling so that to decrease the formation of ice crystals, which may affect the survival of the blastocyst (Zhang W. Y. et al., 2019). Mukaida et al., reported that the artificial shrinkage of blastocoels by microneedle or alaser pulse before vitrification improves the survival rate and clinical outcome of the embryo (Mukaida et al., 2006).
Maternal contamination is a common problem among discordant results. It is known that contamination by maternal DNA often leads to female bias in the sex ratio. Our research found that 17 samples had gender inconsistency, the TE sex chromosome was XY and the BCM was XX, which may be due to maternal contamination. Our maternal contamination rate (17 of 492) is lower than previously reported: 2 out of 27 (Zhang J. et al., 2019), and higher that of Jiao et al.’ research (0 of 41) (2019). Prevention is very critical. In our study, we carefully removed and washed the cumulus-corona colliculus complex before ICSI and again on the afternoon of day 4. And we wash and replace the culture medium on the afternoon of day 4. We have observed 7 male NICS results coming from a TE-diagnosed female embryo, indicating that external DNA contamination resulting from plasticware, media, or manipulation during IVF is crucial, and caution should be taken to prevent this. Human serum albumin in the embryo culture medium contains human DNA. The content of human DNA in different embryo culture medium batches was slightly different, and a high human DNA content may affect the sequencing result. Decreasing the volume of embryo culture medium or using serum free medium may be helpful for this issue (Zhang W. Y. et al., 2019). Here we use small volume of medium to culture the embryos (20 μL).
In previous studies, the consistency rate of NICS was low (Wong et al., 2014; Magli et al., 2016; Li et al., 2018; Vera-Rodriguez et al., 2018; Huang et al., 2019; Rubio et al., 2020; Chen Y. et al., 2021). Currently, there have been no studies done on the effectiveness of NICS, specifically for people with chromosomal abnormalities. We have compared the consistency, sensitivity, specificity, PPV, and NPV of NICS in the normal chromosome and chromosomal rearrangement groups. The consistency rates were 73.1% and 78.1% in the normal chromosome and chromosomal rearrangement groups, respectively, and did not show any statistical difference. There were also no significant differences in any other characteristics between the two groups. This suggested that NICS was equally effective in both the normal chromosome and chromosomal rearrangement groups. In our study, the sensitivity of the normal chromosome and chromosomal rearrangement groups was 93.3% and 94.9%, specificity was 53.3% and 51.4%, PPV was 66.3% and 75.7%, and NPV was 89% and 86.4%, respectively. Recently, Chen et al. used a whole embryo as the gold standard for conducting a large sample size study on the accuracy of NICS (Chen L. et al., 2021); the sensitivity (87.36%), PPV (73.08%), and NPV (91.2%) are consistent with our findings. A higher NPV indicates higher accuracy in determining NICS as euploidy, a poor PPV indicates poor accuracy in determining NICS as aneuploidy, and that there are high false positives. Mosaicism affects 30%–40% of human blastocyst (Huang et al., 2019). The TE test results may fail to reflect the genome profile of the inner cell mass (ICM), which ultimately forms the fetus (Huang et al., 2019). While the DNA in SCM likely originates form both of the ICM and TE cells, as they both undergo apoptosis during preimplantation development (Huang et al., 2019; Jiao et al., 2019). And, some embryos had different karyotypes in TE versus whole embryos and NICS, mostly because of mosaics (Jiao et al., 2019). What’s more, during embryonic development, some abnormal cells die and their DNA is released into the culture medium, which is the process of embryo self-repair (Vera-Rodriguez and Rubio, 2017; Huang et al., 2019). This self-repair process may lead to an increase in the false positive rates of the SCM. The above explanation might explain why some embryos were found to be aneuploid with the non-invasive method and euploid with the TE biopsy. Identification of euploidy and aneuploidy alone may lead to the wastage of embryos, due to a high number of false positives. Specificity was lower in our study than in Chen et al. (Chen Y. et al., 2021); this may be related to our use of TE biopsy results as the reference standard. Therefore, whole-embryo samples should be used as the gold standard in further studies.
Both TE biopsy and NICS have their limitations, that affect the accuracy of the two methods. These may affect the outcomes of assisted pregnancy, such as reduced pregnancy rate, increased abortion rate, etc., Rubio et al. reported that the ongoing pregnancy rates of euploidy embryos screened by TE biopsy and NICS were higher than that of NICS aneuploidy embryos after embryo transplantation, but there was no statistical difference between the two groups (Rubio et al., 2019). In our study, patients who underwent embryo transfer (TE euploid) were divided into two groups: euploid TE/euploid NICS group and euploid TE/aneuploid NICS group, the outcome of assisted pregnancy was retrospectively analyzed between the two groups. The clinical pregnancy rates were 71.2% and 54.5%, and the ongoing rates were 67.3% and 51.5% in the euploid TE/euploid NICS and euploid TE/aneuploid NICS groups, respectively. The clinical and ongoing pregnancy rates in the euploid TE/euploid NICS group were higher than in the euploid TE/aneuploid NICS group, but there was no statistical difference between the two groups. This was consistent with the findings of Rubio et al. The ongoing pregnancy rate in the present study was higher in both groups than that reported by Rubio et al. (52.9%, NCIS euploid; 16.7% NCIS aneuploid) (Rubio et al., 2019). Moreover, there was no significant difference in the abortion rates between the two groups. TE biopsies have the following problems. Embryo mosaicism gives rise to false positives and false negatives in PGT-A because the inner cell mass (ICM) cells, which forms the fetus are not tested by the TE biopsy, so false negatives of TE biopsy are of concern, because their transfer may lead to either no pregnancy or, worse yet, and abnormal fetus (Huang et al., 2019). While the DNA in SCM likely originates from both of the ICM and TE cell lineages (Huang et al., 2019). In our study, the high false positives may have led to insignificant differences in the clinical outcomes between the euploid TE/euploid NICS and euploid TE/aneuploid NICS groups. However, the population is relatively young, with good clinical conditions and a low probability of abortion, which may also have led to an insignificant difference. The sample sizes were small in our study as well as in the study of Rubio et al. (2019); in the latter, only 29 embryos were transferred. Therefore, whether the clinical outcomes can be improved, by selecting embryos that are euploid for both TE biopsies and NICS, needs to be further confirmed by expanding the sample size.
In summary, our results suggest that NICS was similarly effective in assessing both euploid and aneuploid chromosomes in embryos. Therefore, combining the TE biopsy and NICS results may improve the outcomes of assisted pregnancy; however, this needs to be studied further.
The authors acknowledge that the data presented in this study must be deposited and made publicly available in an acceptable repository, prior to publication. Frontiers cannot accept a manuscript that does not adhere to our open data policies.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Peking University of Shenzhen Hospital. The patients/participants provided their written informed consent to participate in this study.
B-lS and YW designed and conceived the experiments. B-lS, YW, and Z-YY wrote the manuscript and all the authors reviewed the manuscript. JQ, Q-yS, Z-YY, and W-PQ provided professional advice on experimental design and paper writing. C-hZ, S-W, LZ, Z-XW, Y-xY, and JW assisted with the experiments.
This study was supported by the Exploration of New Method of Non-invasive Fertility Evaluation and Establishment of National Standard (Grant No. (2018YFC1002104)); The National Natural Science Foundation of China (Grant No. (31530049)); The Research Team of Female Reproductive Health and Fertility Preservation (Grant No. (SZSM201612065)); The Natural Science Foundation of Guangdong Province (Grant No. (2018A030310673)); The Project for Medical Discipline Advancement of Health and Family Planning commission of Shenzhen Municipality (Grant No. (SZXJ2017003)); The National Natural Science Foundation of China (Grant No. (82001541)); and the National Natural Science Foundation of China (Grant No (8200062044)).
Authors Y-xY and JW were employed by the company Yikon Genomics Company Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Basile, N., Vime, P., Florensa, M., Aparicio Ruiz, B., Velasco, G. J. A., Remohí, J., et al. (2015). The use of morphokinetics as a predictor of implantation: A multicentric study to define and validate an algorithm for embryo selection. Hum. Reprod. 2015 (30), 276–283. doi:10.1093/humrep/deu331
Chen, L., Sun, Q., Xu, J., Fu, H., Liu, Y., Yao, Y., et al. (2021a). A non-invasive chromosome screening strategy for prioritizing in vitro fertilization embryos for implantation. Cell. Dev. Biol. 9, 708322. doi:10.3389/fcell.2021.708322
Chen, Y., Gao, Y., Jia, J., Chang, L., Liu, P., Qiao, J., et al. (2021b). DNA methylome reveals cellular origin of cell-free DNA in spent medium of human preimplantation embryos. J. Clin. Invest. 131, e146051. doi:10.1172/JCI146051
Cram, D. S., Leigh, D., Handyside, A., Rechitsky, L., Xu, K., Harton, G., et al. (2019). PGDIS position statement on the transfer of mosaic embryos 2019. Reprod. Biomed. Online 39 (1), e1–e4. doi:10.1016/j.rbmo.2019.06.012
Dreesen, J., Destouni, A., Kourlaba, G., Degn, B., Mette, W. C., Carvalho, F., et al. (2014). Evaluation of PCR-based preimplantation genetic diagnosis applied to monogenic diseases: A collaborative eshre pgd consortium study. Eur. J. Hum. Genet. 22, 1012–1018. doi:10.1038/ejhg.2013.277
Gianaroli, L., Magli, M. C., Pomante, A., Crivello, A. M., Cafueri, G., Valerio, M., et al. (2014). Blastocentesis: A source of DNA for preimplantation genetic testing. Results from a pilot study. Fertil. Steril. 102, 1692–1699. doi:10.1016/j.fertnstert.2014.08.021
Huang, L., Bogale, B., Tang, Y., Lu, S., Xie, X. S., and Racowsky, C. (2019). Noninvasive preimplantation genetic testing for aneuploidy in spent medium may be more reliable than trophectoderm biopsy. Proc. Natl. Acad. Sci. U. S. A. 116, 14105–14112. doi:10.1073/pnas.1907472116
Jiao, J., Shi, B., Sagnelli, M., Yang, D., Yao, Y., Li, W., et al. (2019). Minimally invasive preimplantation genetic testing using blastocyst culture medium. Hum. Reprod. 34, 1369–1379. doi:10.1093/humrep/dez075
Kuznyetsov, V., Madjunkova, S., Antes, R., Abramov, R., Motamedi, G., Ibarrientos, Z., et al. (2018). Evaluation of a novel non-invasive preimplantation genetic screening approach. PLOS ONE 13, e0197262. doi:10.1371/journal.pone.0197262
Leaver, M., and Wells, D. (2020). Non-invasive preimplantation genetic testing (niPGT): The next revolution in reproductive genetics? Hum. Reprod. Update 26, 16–42. doi:10.1093/humupd/dmz033
Li, P., Song, Z., Yao, Y., Huang, T., Mao, R., Huang, J., et al. (2018). Preimplantation genetic screening with spent culture medium/blastocoel fluid for in vitro fertilization. Sci. Rep. 8, 9275. doi:10.1038/s41598-018-27367-4
Lin, P. Y., Lee, C. I., Cheng, E. H., Huang, C. C., Lee, T. H., Shih, H. H., et al. (2020). Clinical outcomes of single mosaic embryo transfer: High-level or low-level mosaic embryo, does it matter? J. Clin. Med. 2 (6), 1695. doi:10.3390/jcm9061695
Liu, W., Liu, J., Du, H., Ling, J., Sun, X., and Chen, D. (2017). Non-invasive pre-implantation aneuploidy screening and diagnosis of beta thalassemia IVSII654 mutation using spent embryo culture medium. Ann. Of. Med. 49 (4), 319–328. doi:10.1080/07853890.2016.1254816
Magli, M. C., Pomante, A., Cafueri, G., Valerio, M., Crippa, A., Ferraretti, A. P., et al. (2016). Preimplantation genetic testing: Polar bodies, blastomeres, trophectoderm cells, or blastocoelic fluid? Fertil. Steril. 105, 676–683. doi:10.1016/j.fertnstert.2015.11.018
Makhijani, R., Bartels, C. B., Godiwala, P., Bartolucci, A., DiLuigi, A., Nulsen, J., et al. (2021). Impact of trophectoderm biopsy on obstetric and perinatal outcomes following frozen-–thawed embryo transfer cycles. Hum. Reprod. 36, 340–348. doi:10.1093/humrep/deaa316
Mukaida, T., Oka, C., Goto, T., and Takahashi, K. (2006). Artificial shrinkage of blastocoeles using either a micro-needle or a laser pulse prior to the cooling steps of vitrification improves survival rate and pregnancy outcome of vitrified human blastocysts. Oxf. Engl. 21 (12), 3246–3252. doi:10.1093/humrep/del285
Munné, S., Spinella, F., Grifo, J., Zhang, J., Beltran, M. P., Fragouli, E., et al. (2020). Clinical outcomes after the transfer of blastocysts characterized as mosaic by high resolution Next Generation Sequencing - further insights. Eur. J. Med. Genet. 63, 103741. doi:10.1016/j.ejmg.2019.103741
Poli, M., Girardi, L., Fabiani, M., Moretto, M., Romanelli, V., Patassini, C., et al. (2019). Past, present, and future strategies for enhanced assessment of embryo's genome and reproductive competence in women of advanced reproductive age. (Lausanne) 10, 154. doi:10.3389/fendo.2019.00154
Rubio, C., Navarro Sanchez, L., Pascual, G., Ocali, O., Cimadomo, D., Venier, W., et al. (2020). Multicenter prospective study of concordance between embryonic cell-free DNA and trophectoderm biopsies from 1301 human blastocysts. Am. J. Obstet. Gynecol. 223, 751.e1–751.e13. doi:10.1016/j.ajog.2020.04.035
Rubio, C., Racowsky, C., Barad, D. H., Scott, R. T., and Simon, C. (2021). Noninvasive preimplantation genetic testing for aneuploidy in spent culture medium as a substitute for trophectoderm biopsy. Fertil. Steril. 115, 841–849. doi:10.1016/j.fertnstert.2021.02.045
Rubio, C., Rienzi, L., Navarro-Sánchez, L., Cimadomo, D., García-Pascual, C. M., Albricci, L., et al. (2019). Embryonic cell-free DNA versus trophectoderm biopsy for aneuploidy testing: Concordance rate and clinical implications. Fertil. Steril. 112, 510–519. doi:10.1016/j.fertnstert.2019.04.038
Shamonki, M. I., Jin, H., Haimowitz, Z., and Liu, L. (2016). Proof of concept: Preimplantation genetic screening without embryo biopsy through analysis of cell-free DNA in spent embryo culture media. Fertil. Steril. 106, 1312–1318. doi:10.1016/j.fertnstert.2016.07.1112
Stigliani, S., Anserini, P., Venturini, P. L., and Scaruffi, P. (2013). Mitochondrial DNA content in embryo culture medium is significantly associated with human embryo fragmentation. Hum. Reprod. 28, 2652–2660. doi:10.1093/humrep/det314
Taylor, T. H., Gitlin, S. A., Patrick, J. L., Crain, J. L., Wilson, J. M., and Griffin, D. K. (2014). The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum. Reprod. Update 20, 571–581. doi:10.1093/humupd/dmu016
Tiegs, A. W., Tao, X., Whitehead, C. V., Neal, S. A., Osman, E. K., Kim, J. G., et al. (2019). Does preimplantation genetic testing for aneuploidy (PGT-A) harm embryos? No—a multi-center, prospective, blinded, non-selection study evaluating the predictive value of an aneuploid diagnosis and impact of biopsy. Fertil. Steril. 112, e31. doi:10.1016/j.fertnstert.2019.07.213
Tocci, A. (2020). The unknown human trophectoderm: Implication for biopsy at the blastocyst stage. J. Assist. Reprod. Genet. 37, 2699–2711. doi:10.1007/s10815-020-01925-0
Vera-Rodriguez, M., Diez-Juan, A., Jimenez-Almazan, J., Martinez, S., Navarro, R., Peinado, V., et al. (2018). Origin and composition of cell-free DNA in spent medium from human embryo culture during preimplantation development. Hum. Reprod. 33, 745–756. doi:10.1093/humrep/dey028
Vera-Rodriguez, M., and Rubio, C. (2017). Assessing the true incidence of mosaicism in preimplantation embryos. Fertil. Steril. 107, 1107–1112. doi:10.1016/j.fertnstert.2017.03.019
Wong, K. M., Mastenbroek, S., and Repping, S. (2014). Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil. Steril. 102, 19–26. doi:10.1016/j.fertnstert.2014.05.027.26
Xie, P., Zhang, S., Gu, Y., Jiang, B., Hu, L., Tan, Y. Q., et al. (2022). Non-invasive preimplantation genetic testing for conventional IVF blastocysts. J. Transl. Med. 20 (1), 396. doi:10.1186/s12967-022-03596-0
Xu, J., Fang, R., Chen, L., Chen, D., Xiao, J. P., Yang, W., et al. (2016). Noninvasive chromosome screening of human embryos by genome sequencing of embryo culture medium for in vitro fertilization. Proc. Natl. Acad. Sci. U. S. A. 113, 11907–11912. doi:10.1073/pnas.1613294113
Yeung, Q. S. Y., Zhang, Y. X., Chung, J. P. W., Lui, W. T., Kwok, Y. K. Y., Gui, B., et al. (2019). A prospective study of non-invasive preimplantation genetic testing for aneuploidies (NiPGT-A) using next-generation sequencing (NGS) on spent culture media (SCM). J. Assist. Reprod. Genet. 36 (8), 1609–1621. doi:10.1007/s10815-019-01517-7
Zhang, J., Xia, H., Chen, H., Yao, C., Feng, L., Song, X., et al. (2019a). Less-invasive chromosome screening of embryos and embryo assessment by genetic studies of DNA in embryo culture medium. J. Assist. Reprod. Gen. 36 (12), 2505–2513. doi:10.1007/s10815-019-01603-w
Keywords: non-invasive chromosomal screening, assisted reproductive technology, chromosomal ploidy, next-generation sequencing, blastocyst culture medium, clinical outcomes
Citation: Sun B-l, Wang Y, Sixi-Wen , Zhou L, Zhang C-h, Wu Z-X, Qiao J, Sun Q-y, Yao Y-x, Wang J, Yi Z-Y and Qian W-P (2023) Effectiveness of non-invasive chromosomal screening for normal karyotype and chromosomal rearrangements. Front. Genet. 14:1036467. doi: 10.3389/fgene.2023.1036467
Received: 04 September 2022; Accepted: 17 February 2023;
Published: 13 March 2023.
Edited by:
Firuza R. Parikh, Jaslok Hospital, IndiaReviewed by:
Byron Asimakopoulos, Democritus University of Thrace, GreeceCopyright © 2023 Sun, Wang, Sixi-Wen, Zhou, Zhang, Wu, Qiao, Sun, Yao, Wang, Yi and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zi-Yun Yi, c2Nzbnl6eUAxMjYuY29t; Wei-Ping Qian, cWlhbndlaXBpbmdzekAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.