- 1Division of Genetics, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 2ICAR- National Institute for Plant Biotechnology, New Delhi, India
- 3Division of Plant Physiology, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 4Division of Environment Science, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 5Centre for Quantitative Genetics and Genomics (QGG), Aarhus University, Aarhus, Denmark
A Genome-wide association (GWAS) study was conducted for phosphorous (P)-use responsive physiological traits in bread wheat at the seedling stage under contrasting P regimes. A panel of 158 diverse advanced breeding lines and released varieties, and a set of 10,800 filtered single nucleotide polymorphism (SNP) markers were used to study marker-trait associations over the eight shoot traits. Principle component analysis separated the two environments (P regimes) because of the differential response of the traits indicating the essentiality of the separate breeding programmes for each environment. Significant variations for genotypic, environmental, and genotype × environment (GEI) effects were observed for all the traits in the combined analysis of variance with moderately high broad sense heritability traits (0.50–0.73). With the different algorithms of association mapping viz., BLINK, FarmCPU, and MLM, 38 unique QTLs under non-limiting P (NLP) and 45 QTLs for limiting P (LP) conditions for various shoot traits were identified. Some of these QTLs were captured by all three algorithms. Interestingly, a Q.iari.dt.sdw.1 on chromosome 1D was found to explain the significant variations in three important physiological traits under non-limiting phosphorus (NLP) conditions. We identified the putative candidate genes for QTLs namely Q.iari.dt.chl.1, Q.iari.dt.sdw.16, Q.iari.dt.sdw.9 and Q.iari.dt.tpc.1 which are potentially involved in the mechanism regulating phosphorus use efficiency through improved P absorption due to improved root architectural traits and better mobilization such as sulfotransferase involved in postembryonic root development, WALLS ARE THIN1 (WAT1), a plant-specific protein that facilitates auxin export; lectin receptor-like kinase essentially involved in plant development, stress response during germination and lateral root development and F-box component of the SKP-Cullin-F box E3 ubiquitin ligase complex and strigolactone signal perception. Expression profiling of putative genes located in identified genomic regions against the wheat expression atlas revealed their significance based on the expression of these genes for stress response and growth development processes in wheat. Our results thus provide an important insight into understanding the genetic basis for improving PUE under phosphorus stress conditions and can shape the future breeding programme by developing and integrating molecular markers for these difficult-to-score important traits.
Introduction
Wheat (Triticum aestivum L.), a major cereal crop, meets one-fourth of the protein and one-fifth of the calorie requirement of the total human diet, worldwide. Considering the continuously increasing demand for wheat, its production needs to be increased by at least 50 percent by 2050. Additionally, wheat scientists have to look for avenues to increase production under the constraints of declining natural resources and changing climatic conditions (Yadav et al., 2011; Gupta et al., 2016). Globally, the sharp gain in wheat yield was realized with the development and cultivation of lodging tolerant and fertilizer-responsive semi-dwarf wheat varieties during the green revolution era. Since then, yield gain rates are declining in many parts of the world (Yadav et al., 2010). In one of our recent assessments of varieties released over a century-long period (since 1905) for India’s north-western plain zone, the wheat yield has grown at the rate of 0.544% (Yadav et al., 2021).
Globally phosphorus (P), being the essential macronutrient, is among the various major yield-deciding factor for increased crop productivity. P, besides being an essential constituent of genetic material, plays a vital role in photosynthesis, energy transfer, improved root development, starch and sugar transformation, and nutrient flow within the plant system (Pasek, 2008). P-limiting conditions have been reported to be causing stunted growth, reduced effective tillering, thin stems, enhanced root:shoot ratio, and substantial yield reduction in rice and many other cereals (Dobermann and Fairhurst, 2000). At early stages, P deficiency affects the emergence and growth of seedlings (Singh et al., 2013) which in turn leads to decreased root volume, total leaf area, and plant dry weight; however, there is also a significant increase in the density of root hairs and root to shoot ratio (Ahmed et al., 2018). Hence, P feeding during the early stages of wheat growth is crucial for better establishment and any supplementation at later stages would not be compensated in plant growth, which would cause a significant decrease in tiller development and head formation (Grant et al., 2001). Early seedling variations correlate well with the high P uptake. A deeper understanding of the biomass and optimized root traits like root length, root width, root tips number, root diameter, root biomass, and shoot biomass can throw some light on dissecting mechanisms underlying PUE. Hence, exploring the variations for seedlings and their initial biomass accumulation traits under limiting and non-limiting P conditions would provide a better scope for improving elite lines for PUE (YANG et al., 2021). In wheat, the realized yield is also significantly compromised under P deficit conditions worldwide (Zhang et al., 2005). Due to its essential role in physiological activity the high energy requirement of modern high-yielding varieties and the limitation of naturally available P in soil, inorganic P fertilizers are much needed in great quantities in contemporary agriculture. It is anticipated that 52.9 MT of P fertilizers will be used in agriculture by 2030 (Brears, 2015). Nevertheless, large-scale injudicious fertilization raises environmental concerns with unused chemical fertilizers seeping into water systems resulting in eutrophication and affecting marine life adversely (Cassman et al., 2003; Chiou and Lin, 2011). Phosphate rock is a limited and non-renewable global resource with a very short expected exhaustion period (50–200 years) at the current pace of P utilization (Herrera-Estrella and López-Arredondo, 2016), it is high time for its judicious application in agriculture.
Among the various management practices for the rational use of P, the development of P use efficient crop cultivars which come with no additional costs (Heuer et al., 2017) is the most economical. Moreover, for wheat, with the largest area and comparatively lower P utilization rate (10.7%) than rice (13.1%) and maize (11%) (Ma et al., 2011), focuses on the fast-track approaches to identify P efficient cultivars is very essential (Dharmateja et al., 2021). Productivity gains with low P-application by growing P-efficient cultivars would pave the way to meet future global food requirements in an eco-friendly, economically feasible, and socially sustainable manner. Moreover, compared to modern wheat varieties which are bred under optimal P conditions, landraces and traditional local germplasm have greater PUE under unfertilized and P-fixed soil due to their natural selection in the evolutionary process and adaptation to P-deficient conditions (Wissuwa and Ae, 2001). The use of such genetic variability would help to improve modern cultivars for these traits.
To develop P-efficient wheat varieties, knowledge about fundamental traits and their associated genomic regions controlling P uptake and utilisation is of utmost importance. The advances in genomics in wheat over the last decade enabled breeders to identify the genetic markers liked to traits of interest and to facilitate integrating them into the breeding lines for various difficult-to-measure target traits. High throughput genotyping arrays have revolutionized marker-trait-associations studies and have helped to fine-map the target genomic regions. PUE-related traits are largely governed by several minor genes with small cumulative effects. Previously, QTLs for P-deficiency-tolerance have been identified through linkage mapping in biparental wheat populations (Yuan et al., 2017). But, considering large minor genes affecting PUE and its complexity association mapping by exploring more than two possible alleles at various genomic regions in a genetic panel consisting of lines with a diverse pedigree will have more probability of capturing factors explaining the phenotypic variation for P use efficiency than the bi-parental mapping population. Accordingly, Genome-wide association studies (GWAS) have effectively explored allelic variations for P-deficiency tolerance in Aegilops tauschii (Liu et al., 2015), Arabidopsis (Bouain, Doumas, and Rouached, 2016) and soybean (Ning et al., 2016).
When measuring a trait response of P among genotypes, precise phenotyping becomes the major limiting factor. Many researchers have struggled to precisely quantify the impact of P on plant growth modifications. Testing genotypes against a particular concentration of measured P is not a feasible task under field conditions. This approach has been limited due to the uncontrollable natural contribution of P through inorganic supply from manures and microbial interaction, and moreover, it is difficult to maintain a uniform supply of P throughout the growing period in the soil. To overcome this limitation and to measure genotypic responses to sole P concentration variations, hydroponic systems are being used in many crops to accurately quantify the P and its uptake by plants. Due to their subterranean nature, these systems have been considered to be the best way to study the root system modifications which would also permit for precise estimation of root and shoot parameters. The simplified hydroponics systems based on aerated nutrient solutions with various levels of P with an option for complete replacement of nutrient solutions at fixed intervals were reported (Byrne et al., 2011; Gong et al., 2011) and successfully used in PUE studies. Hydroponic systems can also prove to be an efficient approach for screening large populations under different nutrient conditions and less amount of space.
In recent decades, several quantitative trait loci (QTLs) for PUE and related traits have been identified and mapped on all 21 chromosomes in wheat under hydroponic culture trials (Guo et al., 2012; Zhang and Wang, 2015). In addition, the phosphorus uptake (PupE), the utilization efficiency (PutE), and the response of morphological traits under different P levels were investigated in hydroponic culture (Yuan et al., 2017b). Categorization of wheat germplasm for PUE was done based on P use efficiency parameters, recorded on genotypes grown in hydroponics with two P regimes (Bilal et al., 2018). PUE-enhanced crop cultivars can have more growth and biomass for the same quantity of P taken up at a given time (Rengel and Marschner, 2005). However, having low heritability for this trait, breeding for PUE is complex and influenced by multiple physiological processes, besides enormous environmental impacts (Heuer et al., 2017). Therefore, dissecting its inheritance patterns into underlying genetic factors at the genomic level can circumvent these environmental influences. With this background, in our current study, we performed a GWAS on a set of high-yielding genotypes with diverse pedigree/parentage to identify marker-trait associations (MTAs) for P use efficiency with a 35 K breeders Affymetrix SNPs array for genotyping. More interestingly, this study has been planned on a panel of indigenously bred, high-yielding advanced breeding lines so that the subsequent gain in PUE can be built upon among these elite lines without compromising yield gain. In addition, the insight gained in understanding P use efficiency among selected genotypes and identified QTNs (Quantitative traits nucleotides) governing PUE will pave the way to develop better genotypes for this challenging-to-score and complex-to-study trait in wheat.
Materials and methods
Plant materials and hydroponic culture
A set of 158 advanced bread wheat lines of spring type with great diversity in their pedigree and lineage were grown under non-limited and limited phosphorus conditions in hydroponics. This set also included released varieties with their wider adaptation to different agro-climatic conditions (Supplementary Table S1). The phenotypic assay under hydroponics was conducted at National Phytotron Facility at the ICAR-Indian Agricultural Research Institute, New Delhi, India. Individual genotypes were grown under a controlled environment with typical growing parameters of 12°C–22°C day/night temperature, 10 h day length of photoperiod, and 70% relative humidity were maintained throughout the growing period. The seeds of individual genotypes were germinated in a separate petri dish with blotting paper containing sufficient moisture for 5–6 days. The uniform-sized five-day-old seedlings were transferred to the hydroponics tank system consisting of a tray of 18-litre capacity covered with a black ceramic lid. The basal nutrient solution used in the hydroponics experiment consisted of (NH4)2SO4·H2O (1 mmol/L), Ca(NO3)2·4H2O (1 mmol/L), KCl (1.8 mmol/L), MgSO4·7H2O (0.5 mmol/L), CaCl2 (1.5 mmol/L), H3BO3 (1 μmol/L), CuSO4·5H2O (0.5 μmol/L), ZnSO4·7H2O (1 μmol/L), MnSO4·H2O (1 μmol/L), FeEDTA (100 μmol/L), and (NH4)6Mo7O24·4H2O (0.1 μmol/L), and two levels of P were maintained using KH2PO4 as Non limiting P (0.2 mmol/L) and limiting P (0.02 mmol/L) (Yuan et al., 2017; Bilal et al., 2018). The nutrient solution was continuously aerated, and the pH was maintained between 6 and 6.5 using 1 M KOH and 1 M HCL. The consistent nutrient provision is supported by basal solution supplanted with fresh solution every 4 days.
Advanced phenotyping for phosphorous-responsive traits
The experimental material was tested in the completely randomized design (CRD) design with three replications, and five plants of thirty-day-old seedlings (Zadok’s scale: growth stage 29) (Zadoks, Chang, and Konzak, 1974) from each replication under NLP and LP hydroponic conditions were taken out for recording the observations on traits under study. The root and shoots of the individual plant were separated carefully using scissors. LI-COR 3000 (Lincoln, NE, United States) leaf area meter was used to measure the total leaf area (TLA, m2/plant). The Chl content (µmmol/m2) was measured with SPAD (Minolta Camera Co., Osaka, Japan) (Minolta 1989). The SPAD readings are measured based on the transmission of red light (650 nm) and infrared light (940 nm), in which red light is absorbed by chlorophyll (Xiong et al., 2015). The Chl data with SPAD was recorded on a fully-opened, well-developed topmost leaf of 30 days old seedlings in each replication on five plants with an average of three readings between 11 a.m. and 12:30 p.m. The variability in SPAD reading is influenced by environmental factors, diurnal variation, and genotypic differences in Chl content under treatment. Therefore, we tried to keep the SPAD reading variability to the minimum level by taking the observations under controlled conditions and with a minimum time difference in data recording. The separated plant parts, i.e., shoot and roots, were dried in a hot air oven at 60°C until stable dry biomass was obtained, then measured for shoot dry weight (SDW, g/plant) and root dry weight (RDW, g/plant). The RSR was calculated as shoot-dry weight (SDW) to root-dry weight (RDW) ratio. The combined shoot and root-dry weight of samples were considered as total dry weight (TDW, g/plant). To estimate the P content, the fine-grind and dried sample of each plant was digested in a diacid mixture (HNO3:HCLO4) until a clear solution was obtained. The total P (mg) was analyzed using the vanadium molybdate yellow colourimetric method (Ma et al., 2021a). The tissue phosphorus content multiplied by total dry weight is used to calculate the total P uptake by the plant (TPU, mg) (Wang et al., 2017). The P utilization efficiency (PUtE, dry weight (g)/P (mg)) was calculated using the following formula under both NLP and LP conditions (Wang et al., 2017).
PUtE [dry weight (g)/P (mg)] = Total dry weight/total P uptake by plant.
Phenotypic data analysis
The presence of outliers in the data was confirmed by boxplot analysis and with the Z score test. The phenotypic data generated through trials in a completely randomised design were subjected to the analysis of variance (ANOVA) with STAR version 2.1.0 (Statistical Tool for Agricultural Research) an R-based software (Gulles et al., 2014). The variability analysis and adjusted means were calculated as best linear unbiased predictions (BLUPs) considering the replication and genotypes as random effects separately for each P regimes using META-R version 6.0 (Alvarado et al., 2020). The adjusted means of replicates in the trial were obtained by fitting mixed linear models (MLM) using the equation.
where
Where
Where t is the cumulative “Student’s t-test” distribution; df error is the degrees of freedom for the variance of error, and ASED is the average standard error of the differences between pairs of means. And the coefficient of variation (%) is calculated using the formula:
The phosphorus deficiency tolerance coefficient (PDTC) was calculated as the ratio of LP over NLP treatment (Li et al., 2015). The correlations were calculated as simple pairwise Pearson’s correlations among traits. Further, principal component analysis (PCA) was performed to identify the number of principal components required to explain the variation across the environments and to study the relationship between traits and the effect of treatments using R package version 4.0.1 (R core Team, 2022).
Genome-wide studies to establish marker traits associations
Genomic DNA was extracted by following the CTAB method (Doyle and Doyle, 1987) and the quantity and quality of DNA were estimated by using an agarose gel electrophoresis approach and UV spectrophotometer. The 35 K Axiom® Wheat Breeder’s Array (Affymetrix UK Ltd., United Kingdom) was used for genome-wide association analysis. Monomorphic markers, markers with >30% missing data, 5% minor allele frequency, and greater than 20% heterozygosity were removed. A filtered set of 10,800 highly informative SNP markers was used for GWAS. Population structure among the 158 genotypes was determined by using STRUCTURE v. 2.3.4 (Pritchard, Stephens, and Donnelly, 2000). Population structure was generated the over 10,000 length of burnin period and 100,000 MCMC reps with three iterations. The optimum population number (K) value was determined by the ad-hoc, delta K method (Evanno et al., 2005). Constellation plot plotted by using the Ward method in JMP v.14 (Lehman et al., 2005). Linkage disequilibrium (LD) analysis was performed on a sub-population basis, using the LD function in TASSEL (Bradbury et al., 2007) and draw the LD plot with R software version 4.2.1 (R core Team 2022). Associations between genotypic and phenotypic data were evaluated in GAPIT version 3.0 (Wang et al., 2021) using MLM, BLINK and FarmCPU algorithms. The Mixed linear model (MLM) is carried out by taking into account both the Q-matrix and the K-matrix (the kinship matrix expressing family relatedness among the genotypes) (Yu and Buckler, 2006; Tadesse et al., 2015). The FarmCPU makes use of stepwise regression [fixed-effect model (FEM)] and the mixed linear model (MLM) (X. Liu et al., 2016). The FEM is used to evaluate genetic markers, while the REM is used to control false positives by including associations or pseudo-quantitative trait nucleotides as covariates in the model. The Bayesian-information and Linkage-disequilibrium Iteratively Nested Keyway (BLINK) is very computationally efficient and reliable, and algorithum is based on the fixed effect model involving the Bayesian information criteria and linkage disequilibrium information (Huang et al., 2019).
The kinship matrix was calculated from the 10,800 markers, and QQ and Manhattan plots were generated to evaluate the results. The adjusted p-value threshold of significance was corrected for multiple comparisons according to the false-discovery rate (FDR) with cut-off ≤ 0.05 with Benjamini and Hochberg method (https://tools.carbocation.com/FDR) (Benjamini and Hochberg, 1995). After the identification of MTAs, an in silico search of the putative candidate genes with their annotated functions was conducted in the Ensembl Plants database (http://plants.ensembl.org/index.html) of the bread wheat genome (Triticum aestivum L.). The Chinese spring wheat cultivar was utilized in making of genome assembly IWGSC-refseq version 1.0. The data from CerealDB was utilized to locate the genes in the wheat version hosted on Ensembl. The gene expression atlas was used to analyse the expression of detected putative candidate genes (http://www.wheat-expression.com/) (Borrill et al., 2016).
Results
Phenotypic variation and heritability for traits under non-limiting phosphorus and limiting phosphorus conditions
Phenotypic data on P-responsive traits among the 158 wheat genotypes were recorded under NLP and LP conditions. The combined analysis of variance indicated significant variations due to the genotype (G), different P-treatment (T) regimes (NLP and LP), and genotype-P treatment interaction effect (G × T) for all the traits. The broad-sense heritability expressed as the proportion of total phenotypic variance for each trait under this study was moderate, ranging from 0.50 (PUtE) to 0.73 (TDW), indicating the involvement of both genetic and environmental variations in governing these traits related to P-response (Table 1). The boxplots of various traits under study have been presented in (Figure 1), with mean values as “*”. The traits namely TLA, SDW, TDW, TPU, and TPC were higher in NLP than LP, while RSR, Chl content, and PUtE were higher in LP. The spread of variability for all traits under study was higher in NLP except for PUtE, which was limited. The overall coefficient of variation was ranging from 3.10% to 13.70% across the traits tested. Limiting the phosphorous supply reduced TLA, SDW, TDW, TPC, and TPU. TLA ranged from 18.94 to 97.55 m2/plant in NLP and 13.32–55.65 m2/plant in LP while SDW ranged from 0.114 to 0.451 g/plant for NLP and 0.067–0.214 g/plant in LP. TDW ranged from 0.139 to 0.545 g/plant under NLP condition against 0.111–0.362 g/plant in LP. A higher value was observed for TPC and TPU in a P-rich environment with a range of 3.07–9.33 against 0.499–2.54 for LP. TPU varied from 0.541 to 4.075 under NLP and 0.092–0.647 in LP. In contrast, under LP, increased values were observed for Chl, RSR, and PUtE. Chl varied from 25.14 to 40.66 μm mol/m2 for NLP and 23.42–48.06 μm mol/m2 for LP, with a mean value higher in LP than NLP (Table 2). Similarly, a higher ratio was observed for RSR in LP across all genotypes tested, with a range of 0.287–1.03 in LP. The PUtE was found higher under limiting phosphorus with a range of 0.396–2.02 against 0.107–0.326 under NLP. The phosphorus deficiency tolerance coefficient (PDTC) was calculated as the ratio of different trait mean values in LP over NLP for a better and quicker understanding of trait response under two conditions. The traits like Chl (1.15), RSR (2.33), and PUtE (4.73) with >1 PDTC value indicated their better expression under LP. The remaining traits TLA (0.556), SDW (0.550), TDW (0.677), TPC (0.231), TPU (0.154) expressed better with availability of sufficient phosphorus as their PDTC value was < 1.
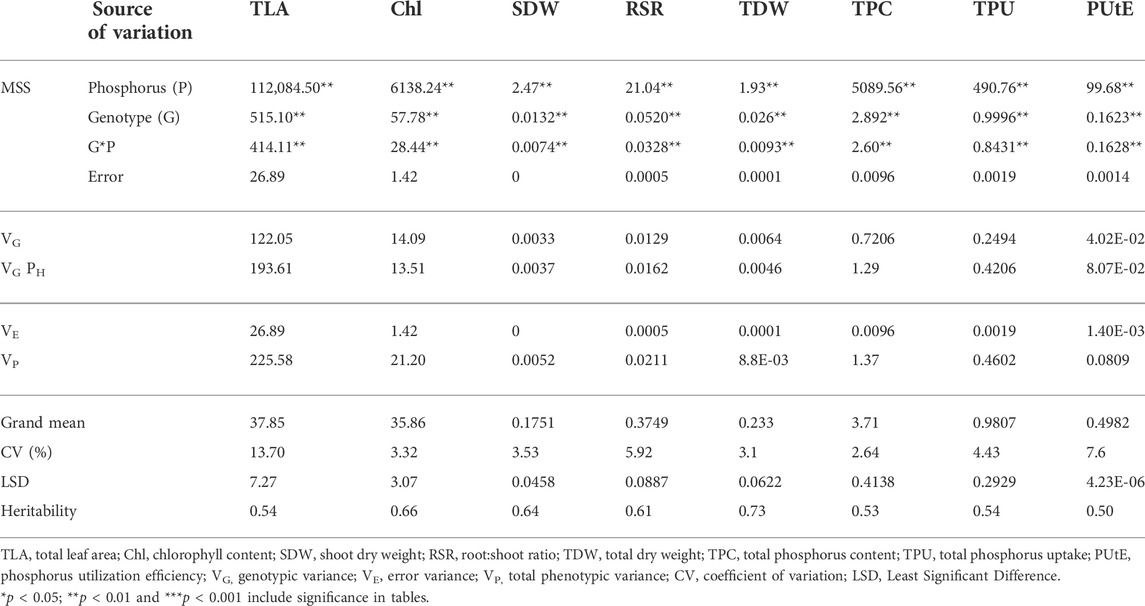
TABLE 1. Analysis of variance and heritability for the traits under non-limiting and limiting phosphorus.
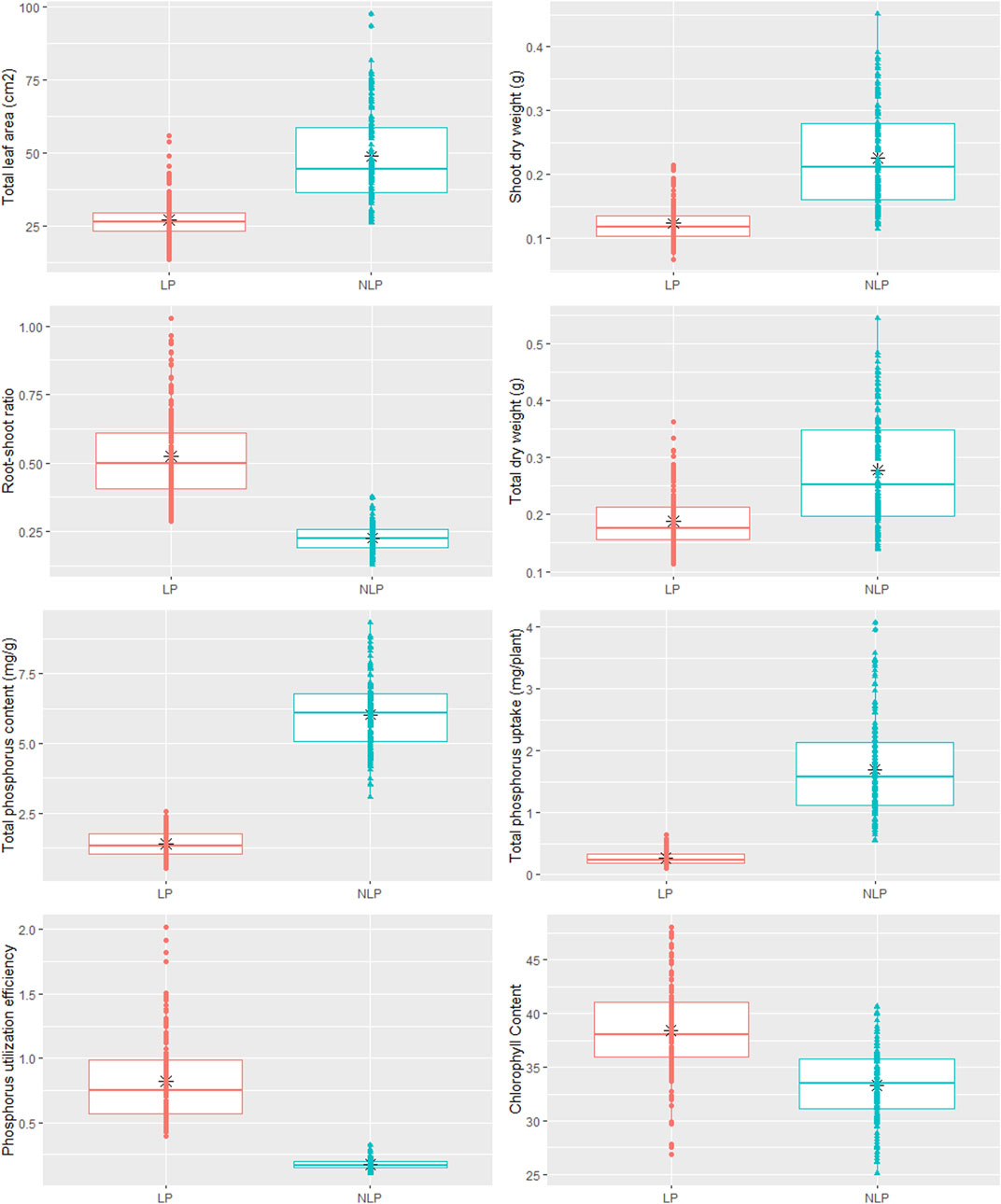
FIGURE 1. Box plot showing phenotypic variation in wheat genotypes in non-limiting and limiting phosphorus conditions.
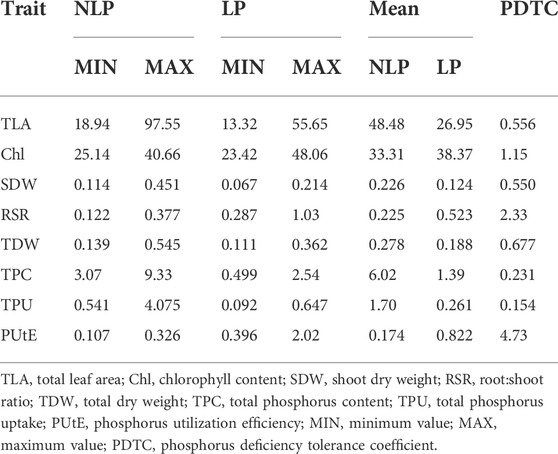
TABLE 2. Measures of variability and phosphorus deficiency tolerance coefficient (PDTC) for the traits under study.
Trait correlations and principal component analysis
Pearson’s pairwise correlation coefficient was calculated among various traits under both environments. Chl was not associated with any other traits, neither in NLP nor in LP. The most significant (p < 0.001) correlations were observed between TLA and SDW under both conditions (Figures 2A,B). Interestingly, RSR was positively correlated with TLA under NLP and negatively under LP conditions. Very strong positive correlations were observed for TLA with SDW, TDW and TPU; SDW with TDW and TPU and TPU with TDW and TPC under both NLP and LP conditions indicating their strong influence on each other. The TDW showed a significant positive correlation with TPC in the NLP condition, and a non-significant correlation was observed in the LP condition. TPC had a significant positive correlation with TPU, and it was interesting to note that both of these showed a strong negative association with PUtE under both environments. In addition, the PUtE was also negatively associated with most of the traits in NLP and LP conditions except for a significant positive association with RSR in LP conditions. PCA-based grouping of traits over LP and NLP conditions indicated that the first two principal components (PC1 and PC2) had explained 84.5% (68.9% and 15.6%) of the total variation (Figure 3). The existence of a high G x T effect indicates that these treatments significantly affected the traits studied. PCA analysis showed that TLA was highly dependent on SDW, TDW, TPC, and TPU and least dependent on Chl, RSR, and PUtE. Similarly, Chl was dependent on RSR and, PUtE, with the least dependence on SDW, TDW, TPC, and TLA. The results clearly depicted in the figure that the trait expression varies with the availability of P. The genotypes under NLP condition exhibit better expression for TLA, SDW, TDW, TPC, and TPU, and in the LP condition exhibit higher expression for Chl, RSR, and PUtE. Along with the treatment, genotypes are separated and all eight traits fall into two distinct groups. The correlation between these traits is represented by the angle between their vectors. The traits TLA, SDW, TDW, TPC, TPU and Chl, RSR, and PUtE are highly correlated because the angle between them is very small (acute angle i.e., < 90°). But there is a significant crossover between the traits of these distinct groups because the presence of a wider angle (i.e., > 90°).
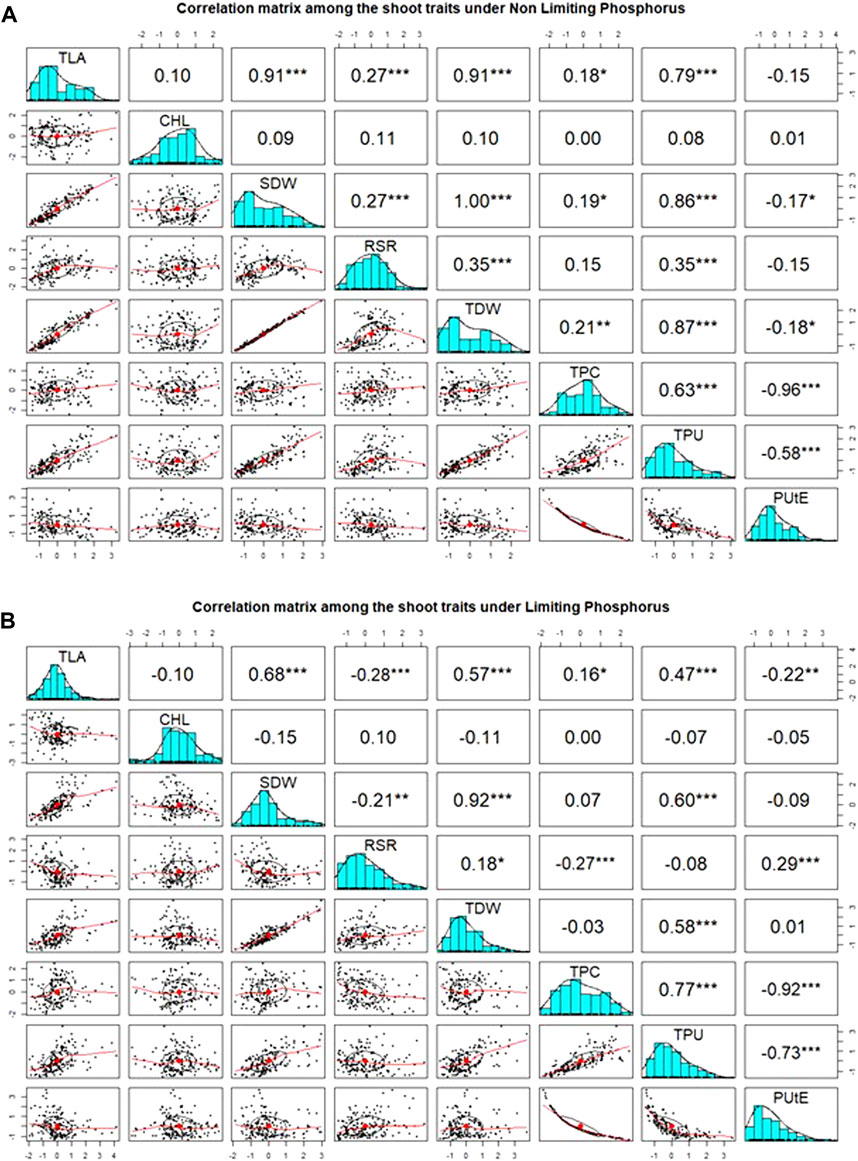
FIGURE 2. Association between measured traits and distribution among wheat genotypes under non-limiting (NLP) (A) and limiting (LP) phosphorus (B) conditions. TLA, total leaf area; Chl, chlorophyll content; SDW, shoot dry weight; RSR, root:shoot ratio; TDW, total dry weight; TPC, total phosphorus content; TPU, total phosphorus uptake; PUtE, phosphorus utilization efficiency.
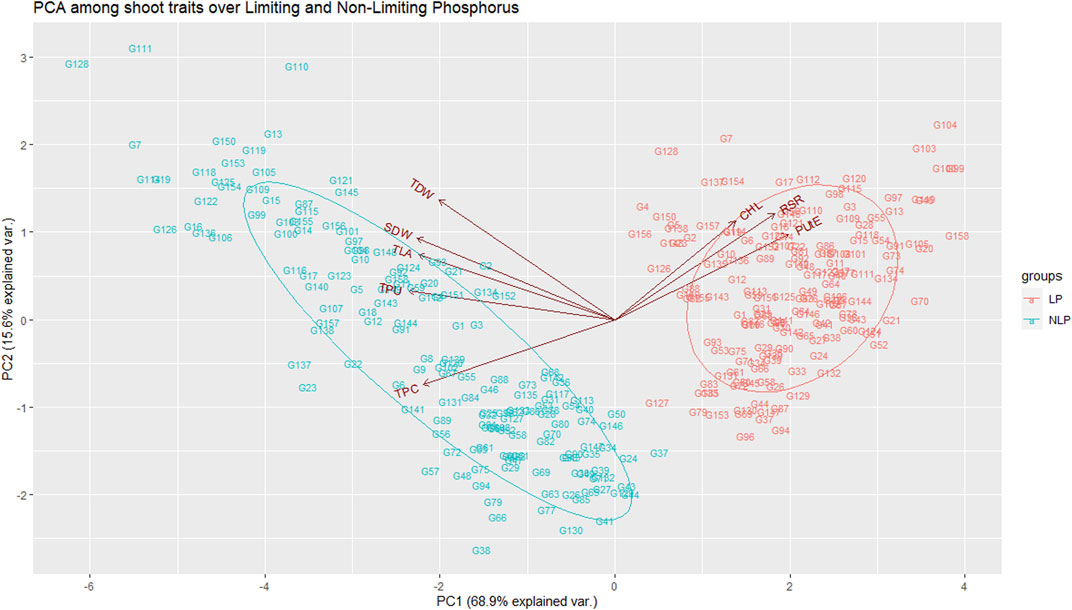
FIGURE 3. Principal component analysis among the traits over non-limiting and limiting conditions. TLA, total leaf area; Chl, chlorophyll content; SDW, shoot dry weight; RSR, root:shoot ratio; TDW, total dry weight; TPC, total phosphorus content; TPU, total phosphorus uptake; PUtE, phosphorus utilization efficiency.
Genome-wide association mapping of phosphorous-responsive physiological traits
The association analysis was performed using three algorithms (Blink, FarmCPU, and MLM) for the physiological traits studied under NLP and LP conditions. A total of 10,800 SNP’s derived from the SNP chip array for 158 genotypes were used for mapping and their distribution across the chromosomes was depicted (Supplementary Table S2). Among association mapping panel population structure was calculated by using STRUCTUREv2.3.4 software (Figure 4A). The population structure showed a sharp peak at K = 2 when the clusters were plotted against ΔΚ indicating two subgroups in the population and the dendrogram, constellation plot revealed two major groups, thus results were further confirmed (Figures 4B,C). A set of 10,800 high-quality SNP markers were distributed across the genome with the B genome (4041) having the highest number of markers, followed by the A genome (3409) and the D genome (3350) respectively. According to chromosome-wise distribution, chromosome 2B (749) had the most markers mapped, followed by chromosome 2D (707). The least number of markers were found on chromosomes 4D (180) and 6B (277). The LD was estimated by calculating the squared correlation coefficient (r2) for all the 10,800 markers. The genome-wide LD decay with physical distance, the LD decay to its half at 9.04 Mb for the whole genome (Figure 5). The −log p-value = 3.5 is considered as threshold to call the MTAs as significant associations and corrected according to the false-discovery rate (FDR cut-off ≤ 0.05). A total of 83 QTLs were detected, among them 45 in NLP and 38 in LP conditions identified for the eight traits under study. With different algorithms under NLP conditions, 19 (BLINK), 32 (Farm CPU), 9 (MLM) QTLs were identified, and similarly, under LP conditions, 26 (BLINK), 34 (FARM CPU), and 21 (MLM) QTLs were detected (Supplementary Table S3) (Figures 6A,B; Supplementary Figures S1A–D). Fourteen QTLs (4 in NLP and 10 in LP) were detected in all the algorithms (Supplementary Table S4). Four QTLs were detected for TLA, located one each on chromosome 7B, 1D, 6A, and 1B across both the conditions. In NLP condition Q.iari.dt.tla.1 and Q.iari.dt.tla.2 were detected on chromosome 7B and 1D with their associated SNPs AX94470386 and AX94765690 with −log10 p-value with 3.70 and 3.5 respectively. Two QTLs namely Q.iari.dt.tla.3 and Q.iari.dt.tla.4 with their associated SNPs as AX94770913 and AX95190390 with −log10 p-value ranging from 4.2 to 4.6 and 4.0 to 4.3, respectively, were detected in LP conditions on chromosome 6A and 1B. For Chl content, three QTLs, namely Q.iari.dt.chl.1, Q.iari.dt.chl.2, and Q.iari.dt.chl.3 associated with SNPs AX94832883, AX94676652, and AX95105278 were detected in NLP conditions on chromosomes 7D, 2A, and 2B. Under LP condition, Q.iari.dt.chl.4, Q.iari.dt.chl.5, Q.iari.dt.chl.6, Q.iari.dt.chl.7, and Q.iari.dt.chl.8 were detected on chromosomes 6B, 6A, 6D, 2D, and 6D for Chl content with their associated SNPs AX94597699, AX95241386, AX94702861, AX94622481, and AX95230097 with −log10 p-value ranging from 3.5 to 4.8. Seven QTLs under NLP and thirteen QTLs under LP were detected for SDW with a range of −log10 p-value from 3.74 to 8.13. For RSR, thirteen QTLs in NLP and seven QTLs in LP conditions were detected. SDW is very strongly associated with TDW, seven QTLs in the NLP condition and twelve QTLs in the LP condition were detected to explain the variation for TDW. For TPC five QTLs, two each on chromosomes, 3A and 3 D and one on 7A were identified under both the treatments. Under NLP conditions, three QTLs and in LP four QTLs were detected for the trait TPU. However, only two QTLs were found to explain the variation for PUtE in both NLP and LP conditions.
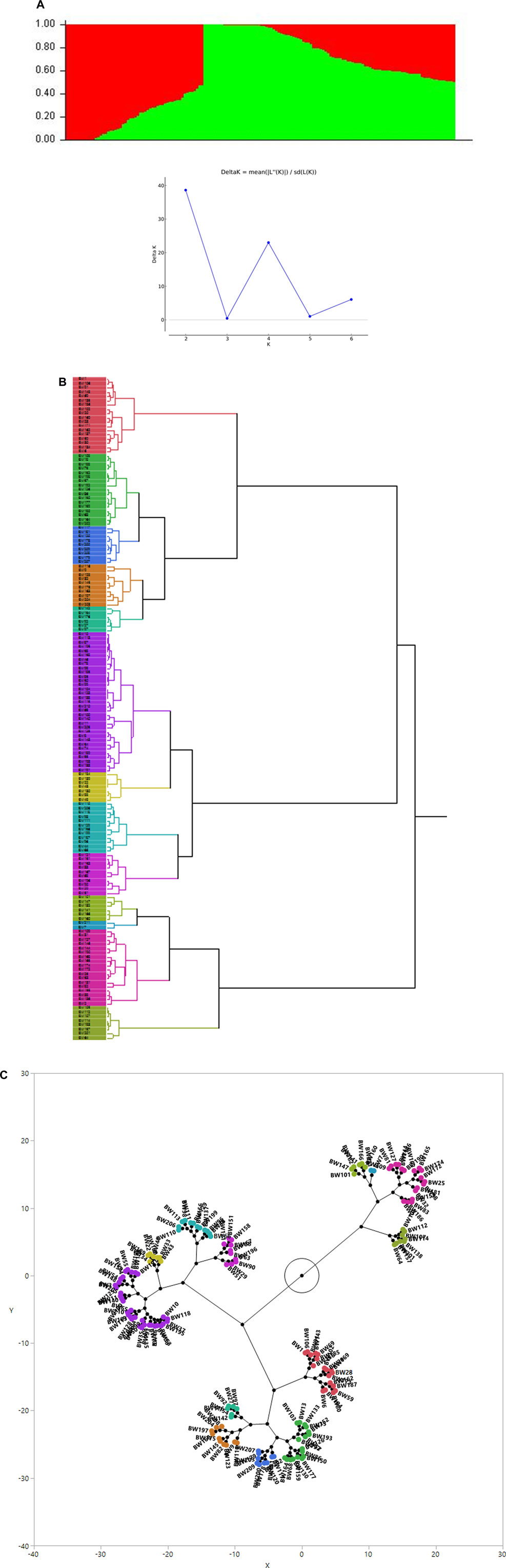
FIGURE 4. (A) Population genetic structure plot in association panel of 158 wheat genotypes (optimal population number K = 2 with two different colours) and Delta K plot depicting peak at K = 2. (B) Dendrogram (C) Constellation plot (showing three groups) using the Ward method in JMP v.14.Figure.
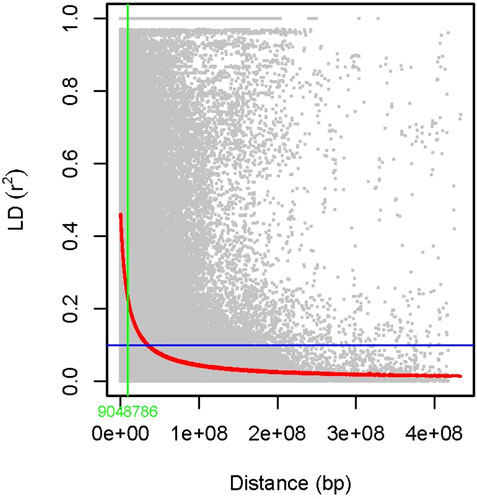
FIGURE 5. Scatter plot showing linkage disequilibrium (LD) decay estimated by plotting (r2) against genetic distance (bp).
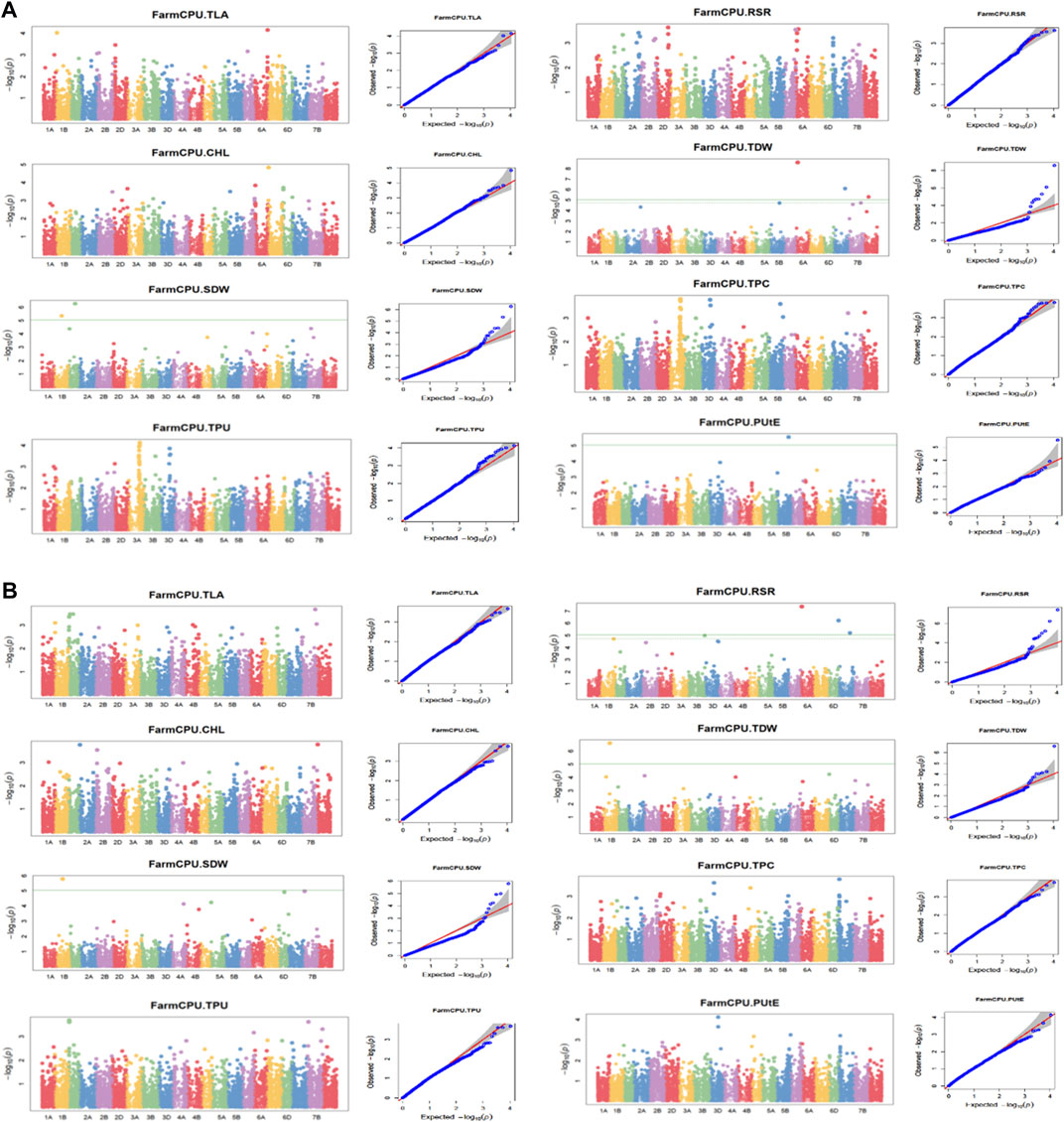
FIGURE 6. (A) (NLP) and (B) (LP): Manhattan plots and Q-Q plots (using FARM CPU) for Phosphorus use efficiency traits. TLA, total leaf area; Chl, chlorophyll content; SDW, shoot dry weight; RSR, root: shoot ratio; TDW, total dry weight; TPC, total phosphorus content; TPU, total phosphorus uptake; PUtE, phosphorus utilization efficiency.
Interestingly, a QTL Q.iari.dt.sdw.1 associated with SNP AX94514240 located on chromosome 1D was found to explain the significant variation for all three traits, namely SDW, TDW, and TPU under NLP conditions (Table 3). Though SDW is part of TDW, only four common QTLs viz. Q.iari.dt.sdw.2 associated with SNP AX94646448 on 1B; Q.iari.dt.sdw.8 and Q.iari.dt.sdw.14 on 7B associated with SNPs AX94638774 and AX94626370, under NLP condition, and a QTL Q.iari.dt.sdw.20 associated with SNP AX94456805 detected on chromosome 2D under LP condition explained the variation for both traits. The traits like SDW and TPU shared a common QTL named Q.iari.dt.sdw.3 present on chromosome 7B and associated with SNP AX94396598 was detected to explain the variation for traits SDW and TPU, simultaneously under NLP condition only. Another QTL named Q.iari.dt.rsr.1 on chromosome 6A and associated with SNP AX94475513 was associated with two traits, RSR and TDW under both conditions. In the NLP condition, TPC shared common QTLs named Q.iari.dt.tpc.2 and Q.iari.dt.tpc.3 which were associated with SNPs AX94584110 and AX94397869 on the chromosome 3A and 3D with the other traits PUtE and TPU, respectively.
The annotation of putative candidate genes functions
Based on the physical locations of associated SNPs and their linked QTLs, an attempt was made to identify the candidate genes harbouring the associated SNPs (Table 4). The SNP AX94470386 linked to QTL Q.iari.dt.tla.1 of TLA was found in the gene TraesCS7B02G382300 coding for ATPase-associated with cation transmembrane transporter activity. Similarly, the SNP AX94765690 is associated with QTL Q.iari.dt.tla.2 of TLA was found within the gene TraesCS1D02G075500 coding for basic region/leucine zipper protein; a positive regulator of transcription. The SNP AX94770913 linked to QTL Q.iari.dt.tla.3 of TLA was also found in TraesCS6A02G406500 gene transcribing ribosomal protein L9 in bacteria/chloroplast. The SNP AX94832883 is linked to QTL Q.iari.dt.chl.1 and was found in gene TraesCS7D02G040300 coding for the multi-protein family of sulfotransferase involved in postembryonic root development. In the same way, the QTLs namely. Q.iari.dt.chl.4, Q.iari.dt.chl.5, Q.iari.dt.chl.6, and Q.iari.dt.chl.8 linked to SNPs AX94597699, AX95241386, AX94702861, and AX95230097 were harbouring the genes TraesCS6B02G056100, TraesCS6A02G037800, TraesCS6D02G046800, and TraesCS6D02G047400 coding for RNA-binding domain S1, ribosomal small subunit biogenesis (cleavage involved in rRNA processing), F-box-like domain superfamily and chloroplast rRNA processing, respectively.
The SNP AX94514240 linked to the QTL Q.iari.dt.sdw.1 was found in gene TraesCS1D02G029900 coding for leucine-rich repeat domain superfamily, NB ARC, P-loop containing nucleoside triphosphate hydrolase and in our study this gene has been found to play role in the expression in several traits like SDW, TDW, and TPU. The QTLs namely Q.iari.dt.sdw.4, Q.iari.dt.sdw.5, Q.iari.dt.sdw.6, and Q.iari.dt.sdw.9 linked to SNPs AX94503640, AX94812403, AX94635019, and AX95081347 were having the genes TraesCS6D02G193000, TraesCS5A02G023600, TraesCS4A02G307900 and TraesCS2D02G126300 coding for elongation factor Tu and translational elongation, glycosyltransferase activity, polysaccharide catabolic process and bulb-type lectin domain superfamily (LecRLKs play important roles in plant development and stress responses, respectively). The protein coded by these genes plays an important role in seed germination and lateral root development. Other SNPs AX94544797, AX94939596, and AX95113278 and their associated QTLs Q.iari.dt.sdw.12, Q.iari.dt.sdw.15, and Q.iari.dt.sdw.16 are localised in genes TraesCS1D02G218300, TraesCS1D02G058900, and TraesCS5D02G496600 coding for Phospho-2-dihydro-3-dioxyheptonate aldolase, protein phosphorylation and WAT1-related protein (facilitates auxin export), respectively.
Incidentally, the QTLs namely Q.iari.dt.sdw.14 and Q.iari.dt.sdw.20 linked to SNPs AX94626370 and AX94456805 and associated with SDW and TDW carried the genes TraesCS7B02G149200 and TraesCS2D02G584900 coding for Heat shock protein 90, cellular response to heat, protein stabilization, and Tubby-like F-box protein. The SNP AX94475513 linked with Q.iari.dt.rsr.1 and associated with RSR and TDW has localized in gene TraesCS6A02G095100 coding for F-box-like domain superfamily, leucine-rich repeat domain superfamily, and leucine-rich repeat 2. The four QTLs associated with RSR viz., Q.iari.dt.rsr.2, Q.iari.dt.rsr.6, Q.iari.dt.rsr.12, and Q.iari.dt.rsr.13 are located in genes coding for protein dephosphorylation, acetylglucosaminyltransferase activity, aldehyde dehydrogenase and calcium-dependent protein binding. Similarly, six QTLs Q.iari.dt.tdw.4, Q.iari.dt.tdw.6, Q.iari.dt.tdw.7, Q.iari.dt.tdw.9, Q.iari.dt.tdw.10, and Q.iari.dt.tdw.14 are localized in genes TraesCS4B02G000900, TraesCS7A02G383000, TraesCS7D02G243800, TraesCS5B02G271700, TraesCS2A02G556400, and TraesCS7B02G149200 coding for ATG8-interacting protein, protein phosphorylation, negative regulation of mRNA polyadenylation, protein Iojap, chloroplastic, methyltransferase activity, and heat shock protein 90, respectively.
The QTLs namely Q.iari.dt.tpc.1 and Q.iari.dt.tpc.4 linked to SNPs AX94905933 and AX94978370 were harboring the genes TraesCS7A02G110500 and TraesCS3A02G298600 coding for the F-box component of the skp-cullin-f box (SCF) E3 ubiquitin ligase complex (Strigolactone (SL) signal perception, the unidirectional movement of auxin in the stem from tip to base basipetal) and integral component of membrane. Q.iari.dt.tpc.2 QTL associated with traits TPC and PUtE was having the gene TraesCS3D02G267000 coding for glycerol lipid biosynthetic process, diacylglycerol O-acyltransferase activity. The Q.iari.dt.tpu.3 was having the gene TraesCS3A02G288900 coding for Zinc ion binding.
Putative candidate genes against wheat gene expression atlas
Using the publicly available global gene expression atlas of wheat, the identified putative genes for P-responsive traits in our study were analysed against the gene expression atlas of wheat targeting the leaf and root traits under normal and phosphate deprivation conditions, to know the reliable expression of these genes (Borrill et al., 2016; Ramírez-González et al., 2018). Most of the identified genes were having the moderate to high consecutive expression in leaves and roots over normal and phosphate-deprivation conditions (Figure 7). These genes are TraesCS7B02G382300 and TraesCS1D02G075500 for TLA-NLP; TraesCS6A02G037800 for Chl-LP; TraesCS1D02G058900 and TraesCS2D02G584900 for SDW/TDW-LP; TraesCS2D02G190700, TraesCS7A02G374700, TraesCS4D02G094500 and TraesCS2D02G435000 for RSR-LP; TraesCS4B02G000900; TraesCS7A02G383000; TraesCS7D02G243800 and TraesCS7B02G149200 for TDW-LP; TraesCS7A02G110500 for TPC-NLP; TraesCS3D02G267000 for TPC/PUtE-NLP and TraesCS3A02G288900 for TPU-LP, which are consecutively expressed genes, except the non-expressed genes like TraesCS7D02G040300, TraesCS7A02G383000, TraesCS2A02G556400, and TraesCS3A02G298600. The other genes like TraesCS6A02G406500 for TLA-LP; TraesCS6B02G056100 and TraesCS6D02G047400 for Chl-LP, TraesCS6D02G193000 for SDW-LP; TraesCS1D02G218300 for SDW-LP and TraesCS5B02G271700 for TDW-LP were expressed in leaves and shoot of the wheat plants under normal and phosphorus deprivation conditions which coincides with our finding. The genes, TraesCS5A02G023600 for SDW-NLP; TraesCS2D02G126300 for SDW-LP; and TraesCS5D02G496600 for SDW/TDW-LP were expressed in root tissues under normal and phosphorus deprivation conditions.
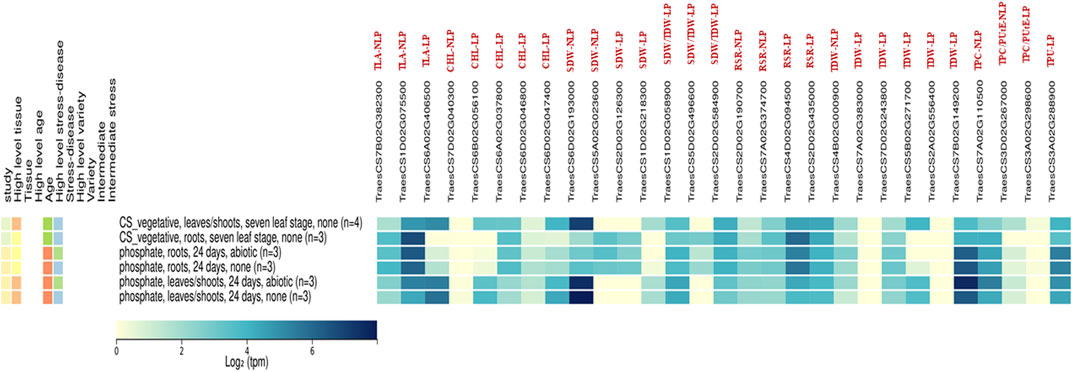
FIGURE 7. The expression analysis for identified Putative Candidate genes in Non-limiting and Limiting conditions by using the gene expression atlas.
Discussion
To sustain wheat production globally through breeding intervention is the target of most breeding programs, primarily because wheat is the most important source of food and energy. The challenges to meeting this target are compounded by quickly depleting natural resources due to unsustainable management practices, declining soil health, and changing climatic conditions (Yadav et al., 2010). Intensive cropping with no concern for resource use efficiency—such as is presently the case with phosphorus fertilizers—cannot be sustained for a very long period (Syers, Johnston, and Curtin, 2008; Lott et al., 2011). Under intensive cropping systems, increasing phosphorus efficiency has long been a target to sustain food production (Shenoy and Kalagudi, 2005; Schröder et al., 2011). The residual soil phosphorus from past intense fertilization though contributes considerably to future crop output but with a large lag period as most of the applied phosphorus stays in the soil in the absence of larger uptake and efficient utilization (Sattari et al., 2012). As a result, improved plant capacity to utilize phosphorus effectively will be highly beneficial to crop output. The declining availability of rock phosphate as a source of phosphorus fertilizer and growing awareness about the negative consequences on the environment has piqued interest in improving plant phosphorus uptake and use efficiency (van de Wiel et al., 2016).
The results showed an increase in Chl, RSR, and PUtE and a decrease in TLA, SDW, TDW, TPC, and TPU under P-limited conditions. Improved phosphorus scavenging and uptake (phosphorus acquisition efficiency, PAE) achieved through better RSR with more economical and better utilization in the plant (phosphorus utilization efficiency, PUtE) as indicated in the present study can both improve phosphorus use efficiency (Wang, Shen, and Liao, 2010; Rose and Wissuwa, 2012; Veneklaas et al., 2012; van de Wiel et al., 2016). Correlation studies help to detect the relation between the traits, with respect to a specific treatment. Leaf area plays the most important role in carbon assimilation and therefore, was positively correlated with SDW, TDW, TPC, and TPU. The substantial contribution of TDW, phosphorus concentration, and total phosphorus uptake towards PUE was reported in rice (Wissuwa and Ae, 2001) and wheat (Valizadeh, Rengel, and Rate, 2002). However, better cell expansion to achieve optimum leaf area requires sufficient P in various plant parts, assimilation of which in fact depends upon better root proliferation and higher RSR. RSR exhibited considerably better expression in LP condition. In crop plants, non-availability of P predominantly stimulates root growth as opposed to shoot growth (Lambers et al., 2011). Chl was observed to have weak-to-no association with most of the other traits, but its expression manifests in LP conditions. Plants turn dark green in color when P deprivation is more severe (Hoppo, Elliott, and Reuter, 1999). Respiration and photosynthesis can be slowed down under phosphorus-deprived conditions (Glass, Beaton, and Bomke, 1980), but if respiration is slowed down more than photosynthesis, carbohydrates will be deposited, resulting in dark green leaves.
Principle component analysis clearly shows the importance of chlorophyll content, RSR, and PUtE under P-limited conditions whereas TPU, TPC, TLA, and TDW under nonlimiting P achieve higher yield. Plants have evolved highly specialized adaptive mechanisms through morphological, physiological, and molecular modifications such as an increased root/shoot ratio, an increase in the number of root hairs, association with arbuscular mycorrhizal fungi (AMF), synthesis and release of phosphatases and organic acids, and enhanced expression of phosphatases, to optimize access to soil phosphorus under limited phosphorus (Péret et al., 2011). The length of the vector (distance from the origin) explains the variation contributed by each trait. Along with mean values and correlation, PCA explains the traits TPU, TPC, TLA, SDW, and TDW are highly correlated and explains the large amount of variation contributed by these traits. The significant crossover interaction between traits across environments (i.e > 90°) helps to identify the traits to be useful for the selection/improvement of genotypes for a specific environment (Yan and Tinker, 2006). In the present study HDCSW18, a wheat variety of very high yield potential and specifically bred for conservation agriculture conditions, exhibits very high phosphorus limitation tolerance largely because of its strong root traits. This genotype because of its strong RSA traits (Dharmateja et al., 2021) has the inherent ability to explore even the deeper layers of soil. Our study clearly shows that better P uptake though, largely depends upon root traits, and is essential for better ground coverage and C assimilation but can improve PUE up to a limited extent because of the limitation imposed by PUtE. It also vouches for a separate breeding programme for both sets of conditions.
For mapping, a panel of 158 wheat genotypes was used, including advanced breeding lines, obsolete varieties, and recently released varieties. The population structure revealed by STRUCTURE analysis infers two major sub-populations (Figure 4A). The presence of two major sub-populations, was further confirmed with the dendrogram and constellation plot. The genotypes in a group shared alleles descended from common parents leading to genetic relatedness among the genotypes. The genotypes are mostly grouped based on pedigree lineage, and evolutionary and geographical origin (Gorafi et al., 2018; Tomar et al., 2021). In view of genetic relatedness, we have adopted the FarmCPU, BLINK, and MLM with population structure and kinship relatedness matrix in association analysis to avoid spurious false positives. The genomic regions responsible for better trait expression both under abundant and deficit P conditions were identified through GWAS. In total 38 QTLs under NPL and 45 QTLs under LP were associated with various traits in the present study. Four QTLs for TLA, eight for Chl, twenty for SDW, seventeen for RSR, eighteen for TDW, five for TPC, seven for TPU, and four QTLs for PUtE were found to be associated in both conditions. In developing countries like India, the major focus of the breeders throughout the 20th century was to achieve higher yields with almost nil or negligible effort on the development of nutrient-efficient genotypes. However, with the faster depletion of natural resources for P and stronger dependence on imports, it becomes inevitable to focus on the development of phosphorus efficient genotypes in wheat. In contrast, breeding phosphorus-efficient wheat genotypes has received a lot of attention (Davies et al., 2002; Wang et al., 2005) in the developed world where genotypic differences in phosphorus acquisition efficiency and phosphorus utilization efficiency for wheat have been frequently reported (Batten, 1993; Manske and Vlek, 2002; Wang et al., 2005; Gunes et al., 2006). The shoot traits are mostly associated and their biological process are also presumed to coordinate with their expression. Strong pleiotropic gene action or tight linkage between the genes results in strong correlation between the traits. Five QTLs in NLP, three QTLs in LP, and one in both treatments were detected for multiple traits. The loci affecting multiple traits should be a potential marker for marker-assisted selection for varietal improvement (Chebib and Guillaume, 2021).
Putative candidate gene functions of identified QTLs
Studying the annotated genomic region in wheat enabled us to identify the genes within the associated SNPs/identified QTLs. The identified putative candidate gene for Q.iari.dt.tla.1 on 7B for TLA is reported to be responsible for ATPase-coupled cation transmembrane transporter activity. H + -ATPase had a role in nutrient uptake in the root and translocation of these nutrients to the shoots (Sondergaard, Schulz, and Palmgren, 2004). Similarly, Q.iari.dt.tla.2 for TLA was linked with the gene responsible for basic leucine zipper protein, which is reported to have a role in the positive regulation of transcription. Plants regulate various physiological processes through a regulatory network of transcription factors. Under nutrient starvation, the conserved sucrose-non-fermenting-1-related protein kinase-1 (SnRK1) mediates the phosphorylation of S1-bZIPs (basic region/leucine zipper) to regulate plant growth and development (Lastdrager et al., 2014). SnRK1 also play a major role in low energy syndrome response under stress condition (Zhang et al., 2020). Mitochondrial ribosomal protein L9, crucial for cells’ survival and kernel development regulation (Qi et al., 2019), was linked with Q.iari.dt.tla.3.
Q.iari.dt.chl.1 on chromosome 7D is linked to the gene responsible for sulfotransferase. In Arabidopsis thaliana, tyrosyl protein sulfotransferase (TPST) was found to act in the auxin/plethora pathway to maintain the stem cell niche of the roots (Zhou et al., 2010). Q.iari.dt.chl.4 is associated with the RNA-binding domain, S1. As distinct RNA-binding domains (RBDs) are very limited in number, they often combine with multiple RNA-binding motifs for higher affinity and target selectivity. S1 domains binding RNA specifically and non-specifically with high affinity indicate its importance in Rrp5 (ribosomal assembly factor), pre-rRNA complex (Young and Karbstein, 2011). Q.iari.dt.chl.6 was an ensemble with a gene F-box-like domain superfamily. F-box containing highly conserved motif and their association with cellular degradation with other interacting domains have a robust adaptive role under biotic and abiotic stress conditions, including low-P stress in crops (Pérez-Torres et al., 2008). Q.iari.dt.chl.8 on chromosome 6D is associated with the gene responsible for chloroplast rRNA processing. The rRNA processing is critical for chloroplast biogenesis and photosynthetic activity resulting in the normal growth of Arabidopsis (Han et al., 2015).
Q.iari.dt.sdw.1 is present on chromosome 1D and associated with nucleotide-binding sites -leucine-rich repeat domain responsible for plant proteins’ key role in host-pathogen interaction (Flor, 1956; Hammond-Kosack and Jones, 1997). Similarly, Q.iari.dt.sdw.5 associated with gene glycosyltransferase OsUGT90A1 on chromosome 5A helps in protecting the plasma membrane during stress (Shi et al., 2020). Q.iari.dt.sdw.9 present on chromosome 2D is linked to the bulb-type lectin domain superfamily. Lectin receptor-like kinase (LecRLKs) is reported to play essential roles in plant development and stress responses (Vaid, Macovei, and Tuteja, 2013) besides their involvement in germination processes (Cheng et al., 2013) and lateral root development (Deb et al., 2014). Q.iari.dt.sdw.14 is associated with heat shock protein 90 which plays an important role in plant adaptation under different environmental conditions (Wang et al., 2011; Kumar et al., 2020). Q.iari.dt.sdw.15 is associated with gene-responsible protein phosphorylation, which is an integral part of abiotic stress-responsive pathways including phytohormones and ion homeostasis. Auxin hormone plays a very important role in plant adaptation response and the QTL Q.iari.dt.sdw.16 is associated with a candidate gene responsible for WALLS ARE THIN1 (WAT1), a plant-specific protein that facilitates auxin export from vacuoles (Ranocha et al., 2013). Tubby-like proteins (TLPs) are present in all eukaryotic species (Liu, 2008), including wheat (Hong, Kim, and Seo, 2016). Q.iari.dt.sdw.20 on chromosome 2D was found associated with a Tubby-like F-box protein-producing gene reported to have a role in adaptation response though with some indistinct mechanism.
QTL Q.iari.dt.rsr.1 was found to be putatively associated with the gene F-box-like domain superfamily. F-box genes in plants control many important processes including embryogenesis, hormonal responses, seedling development, floral organogenesis, senescence, and pathogen resistance (Lechner et al., 2006). Q.iari.dt.rsr.2 on chr2D was found putatively associated with gene coding protein dephosphorylation. Plants regulate protein through phosphorylation and dephosphorylation during their response to biotic and abiotic stresses (Ma et al., 2021b). Gene regulating acetylglucosaminyltransferase was found putatively associated with Q.iari.dt.rsr.6, the role in various developmental processes under stress conditions has been established in Arabidopsis (Yoo et al., 2021). Q.iari.dt.rsr.12 was associated with gene-regulating Aldehyde dehydrogenase production, which oxidizes excessive endogenous and exogenous aliphatic and aromatic aldehyde molecules into corresponding carboxylic acids (Tola et al., 2020). Q.iari.dt.rsr.13 on chromosome 2D is putatively associated with the calcium-dependent protein binding gene. Calcium ions are a messenger for physiological responses to various developmental signals (Reddy and Reddy, 2004).
During the energy deprivation-induced by limiting nutrient supply or other biotic and abiotic stresses, including toxicity, plants have evolved the autophagy process to counter the negative outcome. Q.iari.dt.tdw.4 links were found putatively associated with ATG8-interacting protein. Similarly, phosphorylation is an important means through which plants regulate post-translational gene expression. Q.iari.dt.tdw.6 was found to be associated with genes responsible for phosphorylation. Q.iari.dt.tdw.7 was found to be associated with gene-regulating negative mRNA polyadenylation. Plants also regulate gene expression quantitatively and qualitatively through mRNA polyadenylation (Hunt, 2012). Q.iari.dt.tdw.9 is an ensemble with a gene responsible for IOJAP protein localized in the chloroplast in Arabidopsis and found to have a role in adaptation to cold stress. Q.iari.dt.tdw.10 is associated with gene methyltransferase conferring tolerance to salinity stress in Arabidopsis (Mishra et al., 2019). Q.iari.dt.tpc.1 is related to the gene F-box component of the SKP-Cullin-F box (SCF) E3 ubiquitin ligase complex. The complex is involved in various growth, flower development, and other physiological processes in wheat (Liu et al., 2021).
As earlier discussed, some of the QTLs were associated with more than one trait and which harbors the putative candidate genes. The Q.iari.dt.sdw.1 associated with SDW, TDW, and TPU under NLP, and harboring the TraesCS1D02G029900 which code for Leucine-rich repeat domain superfamily, NB ARC, P-loop containing nucleoside triphosphate hydrolase responsible for plant proteins’ key role in host-pathogen interaction (Flor, 1956; Hammond-Kosack and Jones, 1997) (Table 3). Another two more QTLs viz. Q.iari.dt.sdw.14 and Q.iari.dt.sdw.20 were associated with SDW and TDW, and harbouring the TraesCS7B02G149200 and TraesCS2D02G584900, respectively. The putative candidate genes, TraesCS7B02G149200 codes for heat shock protein 90 which plays an important role in plant adaptation under different environmental conditions (Wang et al., 2011; Kumar et al., 2020), while, TraesCS2D02G584900 codes for Tubby-like F-box protein involved in various physiological activities (Hong, Kim, and Seo, 2016). Likewise, QTL named Q.iari.dt.rsr.1 was associated with RSR and TDW under both conditions, and harbouring the TraesCS6A02G095100 responsible for F-box-like domain super family, FBD domain, Leucine-rich repeat domain superfamily influences the embryogenesis, hormonal responses, seedling development, floral organogenesis, senescence, and pathogen resistance (Lechner et al., 2006). The QTL, Q.iari.dt.tpc.2 associated with PUtE and TPU, was harbouring the TraesCS3D02G267000 which codes for Glycerolipid biosynthetic process, diacylglycerol O-acyltransferase activity responsible for lipid metabolism (Sanjaya et al., 2013).
In the Wheat Gene Expression Atlas data, several transcripts of different wheat tissues with various ID contents exhibit differential gene expression (Borrill, Ramirez-Gonzalez, and Uauy, 2016). The several genes expressed in leaves, roots, and shoots of the wheat plants under normal and phosphorus-deprived conditions which indicate the potential tissue-specific roles that these genes play in phosphorus stress. Many QTLs explaining the significant variation for many root traits relevant for PUE in the present study are co-localized in the chromosome regions harbouring essential pertinent genes for stress response and growth development processes in crop plants. Isolation, cloning, and verifying their role in PUE may pave the way for developing stable molecular markers in the crop improvement programme. The expression pattern of identified putative genes against the gene expression atlas indicates that the identified genes in this study have relevance, which can be converted into PCR-based primers for marker-assisted selection.
Conclusion
Identifying regions on the chromosome in the form of QTLs to explain the phenotypic variation for the various breeding traits is an important tool to improve breeding programme efficiency. The identified genomic loci (83 loci across the models and treatments) in the present study explain the significant variation in phosphorus uptake and utilization or its associated root and shoot traits. Their connotation with putative functions or proteins can lead to the validation of gene(s) underlying these loci. The discovered common QTLs controlling several phenotypes may serve as candidate markers for marker-assisted breeding. However, the functional markers need to be validated in a separate independent panel with different genetic backgrounds of wheat genotypes. The proteins encoded by the identified genes are involved in many developmental processes, particularly stress responses. Therefore, there is a need to investigate the functions, activation, deactivation, or changes in the expression rate of these genes in the different developmental stages, to know how these genes enhance the efficiency of a few genotypes under P deficient or P surplus conditions.
Further research into the genic regions of associated SNPs at the transcriptional level is needed to determine the trustworthy source of efficiency imparted in the genotypes studied, which will aid in the identification of distinct developmental pathways. Given the rising cost and relevance of phosphorus as an agricultural input, Crop improvement, PUE is an intrinsically worthwhile objective. However, a PUE-focused breeding programme will compete with other breeding goals like disease resistance and climate change adaptability. The discovery of QTL allows for the creation of trait-relevant markers for marker-assisted or genomic selection methods. Overall, the vast diversity of the genetic resources used in this study will help develop new cultivars of wheat with higher PUE by genomic-assisted breeding.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://doi.org/10.6084/m9.figshare.20298006.v1.
Author contributions
RY and PD conceptualized the idea and arranged the resources. All the authors contributed to conducting the experiments and article edits. All the authors contributed to the article and approved the submitted version.
Funding
This research was partly funded by ICAR-World bank-funded NAHEP-CAAST project and the Division of Genetics, ICAR-Indian Agricultural Research.
Acknowledgments
PD acknowledges the National Fellowship for Other Backward Classes (NFOBC) and ICAR-Indian Agricultural Research Institute (IARI), New Delhi for scholarships to complete this work as part of a Ph.D. thesis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.984720/full#supplementary-material
References
Ahmed, M., Khan, S., Irfan, M., Aslam, M. A., Shabbir, G., and Ahmad, S. (2018). Effect of phosphorus on root signaling of wheat under different water regimes. Global Wheat Production.
Alvarado, G., Rodriguez, F. M., Pacheco, A., Burgueno, J., Crossa, J., Vargas, M., et al. (2020). META-R: A software to analyze data from multi-environment plant breeding trials. Crop J. 8 (5), 745–756. doi:10.1016/j.cj.2020.03.010
Batten, G. D. (1993). ‘A review of phosphorus efficiency in wheat’, Genetic Aspects of Plant Mineral Nutrition, 215–220.
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57 (1), 289–300. doi:10.1111/j.2517-6161.1995.tb02031.x
Bilal, H. M., Aziz, T., Maqsood, M. A., Farooq, M., and Yan, G. (2018). Categorization of wheat genotypes for phosphorus efficiency. PLoS ONE 13 (10), e02054711. doi:10.1371/journal.pone.0205471
Borrill, P., Ramirez-Gonzalez, R., and Uauy, C. (2016). expVIP: a customizable RNA-seq data analysis and visualization platform. Plant Physiol. 170 (4), 2172–2186. doi:10.1104/pp.15.01667
Bouain, N., Doumas, P., and Rouached, H. (2016). Recent advances in understanding the molecular mechanisms regulating the root system response to phosphate deficiency in Arabidopsis. Curr. Genomics 17 (4), 308–304. doi:10.2174/1389202917666160331201812
Brears, R. C. (2015). The circular economy and the water-food nexus. Futur. Food J. Food, Agric. Soc. 3, 53–59.
Byrne, S. L., Foito, A., Hedley, P. E., Morris, J. A., Stewart, D., and Barth, S. (2011). Early response mechanisms of perennial ryegrass (Lolium perenne) to phosphorus deficiency. Ann. Bot. 107 (2), 243–254. doi:10.1093/aob/mcq234
Cabot, C., Martos, S., Llugany, M., Gallego, B., Tolra, R., and Poschenrieder, C. (2019). A role for zinc in plant defense against pathogens and herbivores. Front. Plant Sci. 10, 1171. doi:10.3389/fpls.2019.01171
Carey, A. L. (2016). Chloroplastic protein IOJAP is important for cold-acclimation in Arabidopsis thaliana. Knoxville: University of Tennessee.
Cassman, K. G., Dobermann, A., Walters, D. T., and Yang, H. (2003). Meeting cereal demand while protecting natural resources and improving environmental quality. Annu. Rev. Environ. Resour. 28 (1), 315–358. doi:10.1146/annurev.energy.28.040202.122858
Chebib, J., and Guillaume, F. (2021). Pleiotropy or linkage? Their relative contributions to the genetic correlation of quantitative traits and detection by multitrait GWA studies. Genetics 219 (4), iyab159. doi:10.1093/genetics/iyab159
Cheng, X., Wu, Y., Guo, J., Du, B., Chen, R., Zhu, L., et al. (2013). A rice lectin receptor‐like kinase that is involved in innate immune responses also contributes to seed germination. Plant J. 76 (4), 687–698. doi:10.1111/tpj.12328
Chiou, T.-J., and Lin, S.-I. (2011). Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant Biol. 62, 185–206. doi:10.1146/annurev-arplant-042110-103849
Cvrcková, F. (2000). Are plant formins integral membrane proteins? Genome Biol. 1 (1), RESEARCH001–7. doi:10.1186/gb-2000-1-1-research001
Davies, T. G. E., Ying, J., Xu, Q., Li, Z. S., Li, J., and Gordon-Weeks, R. (2002). Expression analysis of putative high‐affinity phosphate transporters in Chinese winter wheats. Plant, Cell & Environ. 25 (10), 1325–1339. doi:10.1046/j.1365-3040.2002.00913.x
Deb, S., Sankaranarayanan, S., Wewala, G., Widdup, E., and Samuel, M. A. (2014). The S-domain receptor kinase arabidopsis receptor kinase2 and the U box/armadillo repeat-containing E3 ubiquitin ligase9 module mediates lateral root development under phosphate starvation in Arabidopsis. Plant Physiol. 165 (4), 1647–1656. doi:10.1104/pp.114.244376
Dharmateja, P., Kumar, M., Pandey, R., Mandal, P. K., Babu, P., Bainsla, N. K., et al. (2021). Deciphering the change in root system architectural traits under limiting and non-limiting phosphorus in Indian bread wheat germplasm. PloS one 16 (10), e0255840. doi:10.1371/journal.pone.0255840
Dobermann, A., and Fairhurst, T. H. (2000). Nutrient disorders and nutrient management. Singapore: Potash and Phosphate Institute, Potash and Phosphate Institute of Canada and International Rice Research Institute.
Doyle, J. J., and Doyle, J. L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bulletin 19 (1), 11–15.
Entus, R., Poling, M., and Herrmann, K. M. (2002). Redox regulation of Arabidopsis 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase. Plant Physiol. 129 (4), 1866–1871. doi:10.1104/pp.002626
Evanno, G., Regnaut, S., and Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 14 (8), 2611–2620. doi:10.1111/j.1365-294X.2005.02553.x
Flor, H. H. (1956). The complementary genic systems in flax and flax rust. Adv. Genet. 8, 29–54. doi:10.1016/s0065-2660(08)60498-8
Glass, A. D. M., Beaton, J. D., and Bomke, A. (1980). “Role of P in plant nutrition,” in Proceedings of the western Canada phosphate symposium, 357–368.
Gong, Y., Guo, Z., He, L., and Li, J. (2011). Identification of maize genotypes with high tolerance or sensitivity to phosphorus deficiency. J. plant Nutr. 34 (9), 1290–1302. doi:10.1080/01904167.2011.580816
Gorafi, Y. S. A., Ishii, T., Kim, J. S., Elbashir, A. A. E., and Tsujimoto, H. (2018). Genetic variation and association mapping of grain iron and zinc contents in synthetic hexaploid wheat germplasm. Plant Genet. Resour. 16 (1), 9–17. doi:10.1017/s1479262116000265
Grant, C. A., Flaten, D. N., Tomasiewicz, D. J., and Sheppard, S. C. (2001). The importance of early season phosphorus nutrition. Can. J. Plant Sci. 81 (2), 211–224. doi:10.4141/P00-093
Gulles, A. A., Bartolome, V., Morantte, R., Nora, L. A., Relente, C., Talay, D., et al. (2014). Randomization and analysis of data using STAR [statistical tool for agricultural research]. Philippines: Philippine Journal of Crop Science.
Gunes, A., Inal, A., Alpaslan, M., and Cakmak, I. (2006). Genotypic variation in phosphorus efficiency between wheat cultivars grown under greenhouse and field conditions. Soil Sci. Plant Nutr. 52 (4), 470–478. doi:10.1111/j.1747-0765.2006.00068.x
Guo, Y., Kong, F. m., Xu, Y. f., Zhao, Y., Liang, X., Wang, Y. y., et al. (2012). QTL mapping for seedling traits in wheat grown under varying concentrations of N, P and K nutrients. Theor. Appl. Genet. 124 (5), 851–865. doi:10.1007/s00122-011-1749-7
Gupta, S., Yadav, R., Gaikwad, K., Kushwah, A., Singh, A. M., and Bainsla, N. K. (2016). Genetic improvement trend analysis for end-use quality characteristics among wheat cultivars of North-Western India. Indian J. Genet. Plant Breed. 76, 137–143. doi:10.5958/0975-6906.2016.00030.4
Hammond-Kosack, K. E., and Jones, J. D. G. (1997). Plant disease resistance genes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 (1), 575–607. doi:10.1146/annurev.arplant.48.1.575
Han, J. H., Lee, K., Lee, K. H., Jung, S., Jeon, Y., Pai, H. S., et al. (2015). A nuclear‐encoded chloroplast‐targeted S1 RNA‐binding domain protein affects chloroplast rRNA processing and is crucial for the normal growth of Arabidopsis thaliana. Plant J. 83 (2), 277–289. doi:10.1111/tpj.12889
Harvey, K. L., Jarocki, V. M., Charles, I. G., and Djordjevic, S. P. (2019). The diverse functional roles of elongation factor Tu (EF-Tu) in microbial pathogenesis. Front. Microbiol. 10, 2351. doi:10.3389/fmicb.2019.02351
Herrera-Estrella, L., and López-Arredondo, D. (2016). Phosphorus: The underrated element for feeding the world. Trends Plant Sci. 21 (6), 461–463. doi:10.1016/j.tplants.2016.04.010
Heuer, S., Gaxiola, R., Schilling, R., Herrera-Estrella, L., Lopez-Arredondo, D., Wissuwa, M., et al. (2017). Improving phosphorus use efficiency: A complex trait with emerging opportunities. Plant J. 90 (5), 868–885. doi:10.1111/tpj.13423
Hong, M. J., Kim, D. Y., and Seo, Y. W. (2016). Interactions between wheat tubby-like and SKP1-like proteins Genes Genet. Syst. 90 (5), 293–304. doi:10.1266/ggs.14-00084
Hoppo, S. D., Elliott, D. E., and Reuter, D. J. (1999). Plant tests for diagnosing phosphorus deficiency in barley (Hordeum vulgare L.) Aust. J. Exp. Agric. 39 (7), 857–872. doi:10.1071/ea99029
Huang, M., Liu, X., Zhou, Y., Summers, R. M., and Zhang, Z. (2019). Blink: A package for the next level of genome-wide association studies with both individuals and markers in the millions. Gigascience 8 (2), giy154. doi:10.1093/gigascience/giy154
Hunt, A. G. (2012). RNA regulatory elements and polyadenylation in plants. Front. Plant Sci. 2, 109. doi:10.3389/fpls.2011.00109
Kumar, Ashish, Sharma, S., Chunduri, V., Kaur, A., Kaur, S., Malhotra, N., et al. (2020). Genome-wide identification and characterization of Heat Shock Protein Family reveals role in development and stress conditions in Triticum aestivum L. Sci. Rep. 10, 7858. doi:10.1038/s41598-020-64746-2
Lambers, H., Finnegan, P. M., Laliberte, E., Pearse, S. J., Ryan, M. H., Shane, M. W., et al. (2011). Phosphorus nutrition of proteaceae in severely phosphorus-impoverished soils: Are there lessons to be learned for future crops? Plant Physiol. 156 (3), 1058–1066. doi:10.1104/pp.111.174318
Lastdrager, J., Hanson, J., and Smeekens, S. (2014). Sugar signals and the control of plant growth and development. J. Exp. Bot. 65 (3), 799–807. doi:10.1093/jxb/ert474
Lechner, E., Achard, P., Vansiri, A., Potuschak, T., and Genschik, P. (2006). F-box proteins everywhere. Curr. Opin. Plant Biol. 9 (6), 631–638. doi:10.1016/j.pbi.2006.09.003
Lehman, A., Creighton, L., Sall, J., Vang, E., Blackwelder, M., Zangi, A. D., et al. (2005). JMP IN 5 software. Nc: SAS Inc., Ltd.,JMP.
Li, R., Zeng, Y., Xu, J., Wang, Q., Wu, F., Cao, M., et al. (2015). Genetic variation for maize root architecture in response to drought stress at the seedling stage. Breed. Sci. 65 (4), 298–307. doi:10.1270/jsbbs.65.298
Li, X., Sanagi, M., Lu, Y., Nomura, Y., Stolze, S. C., Yasuda, S., et al. (2020). Protein phosphorylation dynamics under carbon/nitrogen-nutrient stress and identification of a cell death-related receptor-like kinase in Arabidopsis. Front. Plant Sci. 11, 377. doi:10.3389/fpls.2020.00377
Liu, Q. (2008). Identification of rice TUBBY‐like genes and their evolution. FEBS J. 275 (1), 163–171. doi:10.1111/j.1742-4658.2007.06186.x
Liu, R., Xia, R., Xie, Q., and Wu, Y. (2021). Endoplasmic reticulum-related E3 ubiquitin ligases: Key regulators of plant growth and stress responses. Plant Commun. 2 (3), 100186. doi:10.1016/j.xplc.2021.100186
Liu, Y., Wang, L., Deng, M., Li, Z., Lu, Y., Wang, J., et al. (2015). Genome-wide association studies of phosphorus-deficiency-tolerance traits in Aegilops tauschii. Theor. Appl. Genet. 128 (11), 2203–2212. doi:10.1007/s00122-015-2578-x
Lott, J. N. A., Kolasa, J., Batten, G. D., and Campbell, L. C. (2011). The critical role of phosphorus in world production of cereal grains and legume seeds. Food Secur. 3 (4), 451–462. doi:10.1007/s12571-011-0144-1
Ma, C., Xie, P., Zhang, K., Yang, J., Li, X., Liu, F., et al. (2021b). Contribution of the flag leaf to lead absorption in wheat grain at the grain-filling stage. Ecotoxicol. Environ. Saf. 225, 112722. doi:10.1016/j.ecoenv.2021.112722
Ma, W., Ma, L., Li, J., Wang, F., Sisak, I., and Zhang, F. (2011). Phosphorus flows and use efficiencies in production and consumption of wheat, rice, and maize in China. Chemosphere 84 (6), 814–821. doi:10.1016/j.chemosphere.2011.04.055
Ma, Z., Wang, J., Li, C., Ren, P., Yao, L., Li, B., et al. (2021a). Global profiling of phosphorylation reveals the barley roots response to phosphorus starvation and resupply. Front. Plant Sci. 12, 676432. doi:10.3389/fpls.2021.676432
Manske, G. G. B., and Vlek, P. L. G. (2002). Root architecture–wheat as a model plant. Plant roots hidden half 3, 249–259.
Michaeli, S., Honig, A., Levanony, H., Peled-Zehavi, H., and Galili, G. (2014). Arabidopsis ATG8-INTERACTING PROTEIN1 is involved in autophagy-dependent vesicular trafficking of plastid proteins to the vacuole. Plant Cell 26 (10), 4084–4101. doi:10.1105/tpc.114.129999
Mishra, P., Jain, A., Takabe, T., Tanaka, Y., Negi, M., Singh, N., et al. (2019). Heterologous expression of serine hydroxymethyltransferase-3 from rice confers tolerance to salinity stress in E. coli and Arabidopsis. Front. Plant Sci. 10, 217. doi:10.3389/fpls.2019.00217
Ning, L., Kan, G., Du, W., Guo, S., Wang, Q., Zhang, G., et al. (2016). Association analysis for detecting significant single nucleotide polymorphisms for phosphorus-deficiency tolerance at the seedling stage in soybean [Glycine max (L) Merr.]. Breed. Sci. 66 (2), 191–203. doi:10.1270/jsbbs.66.191
Pasek, M. A. (2008). Rethinking early Earth phosphorus geochemistry. Proc. Natl. Acad. Sci. U. S. A. 105 (3), 853–858. doi:10.1073/pnas.0708205105
Péret, B., Clement, M., Nussaume, L., and Desnos, T. (2011). Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 16 (8), 442–450. doi:10.1016/j.tplants.2011.05.006
Pérez-Torres, C. A., Lopez-Bucio, J., Cruz-Ramirez, A., Ibarra-Laclette, E., Dharmasiri, S., Estelle, M., et al. (2008). Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20 (12), 3258–3272. doi:10.1105/tpc.108.058719
Pritchard, J. K., Stephens, M., and Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics 155 (2), 945–959. doi:10.1093/genetics/155.2.945
Qi, W., Lu, L., Huang, S., and Song, R. (2019). Maize Dek44 encodes mitochondrial ribosomal protein L9 and is required for seed development. Plant Physiol. 180 (4), 2106–2119. doi:10.1104/pp.19.00546
Ramírez-González, R. H., Borrill, P., Lang, D., Harrington, S. A., Brinton, J., Venturini, L., et al. (2018). The transcriptional landscape of polyploid wheat. Science 361 (6403), eaar6089. doi:10.1126/science.aar6089
Ranocha, P., Dima, O., Nagy, R., Felten, J., Corratge-Faillie, C., Novak, O., et al. (2013). Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nat. Commun. 4 (1), 2625–2629. doi:10.1038/ncomms3625
Reddy, V. S., and Reddy, A. S. N. (2004). Proteomics of calcium-signaling components in plants. Phytochemistry 65 (12), 1745–1776. doi:10.1016/j.phytochem.2004.04.033
Rengel, Z., and Marschner, P. (2005). Nutrient availability and management in the rhizosphere: Exploiting genotypic differences. New Phytol. 168 (2), 305–312. doi:10.1111/j.1469-8137.2005.01558.x
Rose, T. J., and Wissuwa, M. (2012). “Rethinking internal phosphorus utilization efficiency: A new approach is needed to improve PUE in grain crops,” in Advances in agronomy (Elsevier), 185–217.
Sanjaya, , Miller, R., Durrett, T. P., Kosma, D. K., Lydic, T. A., and Muthan, B. (2013). Altered lipid composition and enhanced nutritional value of Arabidopsis leaves following introduction of an algal diacylglycerol acyltransferase 2. Plant Cell 25 (2), 677–693. doi:10.1105/tpc.112.104752
Sattari, S. Z., Bouwman, A. F., Giller, K. E., and van Ittersum, M. K. (2012). Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. U. S. A. 109 (16), 6348–6353. doi:10.1073/pnas.1113675109
Schröder, J. J., Smit, A. L., CorDell, D., and RosemArin, A. (2011). Improved phosphorus use efficiency in agriculture: A key requirement for its sustainable use. Chemosphere 84 (6), 822–831. doi:10.1016/j.chemosphere.2011.01.065
Shenoy, V. V., and Kalagudi, G. M. (2005). Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol. Adv. 23 (7–8), 501–513. doi:10.1016/j.biotechadv.2005.01.004
Shi, Y., Phan, H., Liu, Y., Cao, S., Zhang, Z., Chu, C., et al. (2020). Glycosyltransferase OsUGT90A1 helps protect the plasma membrane during chilling stress in rice. J. Exp. Bot. 71 (9), 2723–2739. doi:10.1093/jxb/eraa025
Singh, S. K., Badgujar, G. B., Reddy, V. R., Fleisher, D. H., and Timlin, D. J. (2013). Effect of phosphorus nutrition on growth and physiology of cotton under ambient and elevated carbon dioxide. J. Agron. Crop Sci. 199 (6), 436–448. doi:10.1111/jac.12033
Sondergaard, T. E., Schulz, A., and Palmgren, M. G. (2004). Energization of transport processes in plants. Roles of the plasma membrane H+-ATPase. Plant Physiol. 136 (1), 2475–2482. doi:10.1104/pp.104.048231
Syers, J. K., Johnston, A. E., and Curtin, D. (2008). Efficiency of soil and fertilizer phosphorus use. FAO Fertilizer plant Nutr. Bull. 18 (108).
Tadesse, W., Ogbonnaya, F. C., Jighly, A., Sanchez-Garcia, M., Sohail, Q., Rajaram, S., et al. (2015). Genome-wide association mapping of yield and grain quality traits in winter wheat genotypes. PloS one 10 (10), e0141339. doi:10.1371/journal.pone.0141339
Team, R. C. (2013). R: A language and environment for statistical computing. Vienna, Austria’: R Foundation for Statistical Computing.
Tola, A. J., Jaballi, A., Germain, H., and Missihoun, T. D. (2020). Recent development on plant aldehyde dehydrogenase enzymes and their functions in plant development and stress signaling. Genes 12 (1), 51. doi:10.3390/genes12010051
Tomar, V., Singh, D., Dhillon, G. S., Singh, R. P., Poland, J., Joshi, A. K., et al. (2021). New QTLs for spot blotch disease resistance in wheat (Triticum aestivum L.) using genome-wide association mapping. Front. Genet. 11, 613217. doi:10.3389/fgene.2020.613217
Vaid, N., Macovei, A., and Tuteja, N. (2013). Knights in action: Lectin receptor-like kinases in plant development and stress responses. Mol. Plant 6 (5), 1405–1418. doi:10.1093/mp/sst033
Valizadeh, G. R., Rengel, Z., and Rate, A. W. (2002). Wheat genotypes differ in growth and phosphorus uptake when supplied with different sources and rates of phosphorus banded or mixed in soil in pots. Aust. J. Exp. Agric. 42 (8), 1103–1111. doi:10.1071/ea01087
van de Wiel, C. C. M., van der Linden, C. G., and Scholten, O. E. (2016). ‘Improving phosphorus use efficiency in agriculture: Opportunities for breeding’, euphytica. Netherlands: Springer, 1–22. doi:10.1007/s10681-015-1572-3
Veneklaas, E. J., Lambers, H., Bragg, J., Finnegan, P. M., Lovelock, C. E., Plaxton, W. C., et al. (2012). Opportunities for improving phosphorus‐use efficiency in crop plants. New Phytol. 195 (2), 306–320. doi:10.1111/j.1469-8137.2012.04190.x
Wang, G., Wei, X., Fan, R., Zhou, H., Wang, X., Yu, C., et al. (2011). Molecular analysis of common wheat genes encoding three types of cytosolic heat shock protein 90 (Hsp90): Functional involvement of cytosolic Hsp90s in the control of wheat seedling growth and disease resistance. New Phytol. 191 (2), 418–431. doi:10.1111/j.1469-8137.2011.03715.x
Wang, Q., Li, J., Li, Z., and Christie, P. (2005). Screening Chinese wheat germplasm for phosphorus efficiency in calcareous soils. J. Plant Nutr. 28 (3), 489–505. doi:10.1081/pln-200049186
Wang, X., Chen, Y., Thomas, C. L., Ding, G., Xu, P., Shi, D., et al. (2017). Genetic variants associated with the root system architecture of oilseed rape (Brassica napus L.) under contrasting phosphate supply. DNA Res. 24 (4), 407–417. doi:10.1093/dnares/dsx013
Wang, X. X., Zhang, J., Wang, H., Rengel, Z., and Li, H. (2021). Plasticity and co‐variation of root traits govern differential phosphorus acquisition among 20 wheat genotypes. Oikos.
Wang, X., Shen, J., and Liao, H. (2010). Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops? Plant Sci. 179 (4), 302–306. doi:10.1016/j.plantsci.2010.06.007
Wissuwa, M., and Ae, N. (2001). Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed. 120 (1), 43–48. doi:10.1046/j.1439-0523.2001.00561.x
Yadav, R., Kumar, L., Jat, M. L., and Gupta, R. (2011). “Tailoring wheat genotypes for conservation agriculture in different cropping systems: An innovative and much needed breeding paradigm,” in 5 th world congress on conservation agriculture.
Yadav, R., Gupta, S., Gaikwad, K. B., Bainsla, N. K., Kumar, M., Babu, P., et al. (2021). Genetic gain in yield and associated changes in agronomic traits in wheat cultivars developed between 1900 and 2016 for irrigated ecosystems of northwestern plain zone of India. Front. Plant Sci. 12, 719394. doi:10.3389/fpls.2021.719394
Yadav, R., Singh, S. S., Jain, N., Singh, G. P., and Prabhu, K. (2010). Wheat production in India: Technologies to face future challenges. J. Agric. Sci. (Tor). 2 (2). doi:10.5539/jas.v2n2p164
Yan, W., and Tinker, N. A. (2006). Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 86 (3), 623–645. doi:10.4141/P05-169
Yang, M., Wang, C. r., Hassan, M. A., Wu, Y. y., Xia, X. c., Shi, S. b., et al. (2021). QTL mapping of seedling biomass and root traits under different nitrogen conditions in bread wheat (Triticum aestivum L.) J. Integr. Agric. 20 (5), 1180–1192. doi:10.1016/s2095-3119(20)63192-6
Yoo, J. Y., Ko, K. S., Vu, B. N., Lee, Y. E., Yoon, S. H., Pham, T. T., et al. (2021). N-Acetylglucosaminyltransferase II is involved in plant growth and development under stress conditions. Front. Plant Sci. 12, 761064. doi:10.3389/fpls.2021.761064
Young, C. L., and Karbstein, K. (2011). The roles of S1 RNA-binding domains in Rrp5’s interactions with pre-rRNA. Rna 17 (3), 512–521. doi:10.1261/rna.2458811
Yu, J., and Buckler, E. S. (2006). Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 17 (2), 155–160. doi:10.1016/j.copbio.2006.02.003
Yuan, Y., Gao, M., Zhang, M., Zheng, H., Zhou, X., Guo, Y., et al. (2017). QTL mapping for phosphorus efficiency and morphological traits at seedling and maturity stages in wheat. Front. Plant Sci. 8, 614. doi:10.3389/fpls.2017.00614
Yuan, Y., Zhang, J., Kallman, J., Liu, X., Meng, M., and Lin, J. (2019). Polysaccharide biosynthetic pathway profiling and putative gene mining of Dendrobium moniliforme using RNA-Seq in different tissues. BMC Plant Biol. 19 (1), 521. doi:10.1186/s12870-019-2138-7
Zadoks, J. C., Chang, T. T., and Konzak, C. F. (1974). A decimal code for the growth stages of cereals. Weed Res. 14 (6), 415–421. doi:10.1111/j.1365-3180.1974.tb01084.x
Zhang, Huanchao, Cao, F., Fang, S., Wang, G., and Cao, Z. (2005). Effects of agricultural production on phosphorus losses from paddy soils: A case study in the taihu lake region of China. Wetl. Ecol. Manag. 13 (1), 25–33. doi:10.1007/s11273-003-5033-8
Zhang, H., and Wang, H. (2015). QTL mapping for traits related to P-deficient tolerance using three related RIL populations in wheat. Euphytica 203 (3), 505–520. doi:10.1007/s10681-014-1248-4
Zhang, L., Xie, J., Wang, L., Si, L., Zheng, S., Yang, Y., et al. (2020). Wheat TabZIP8, 9, 13 participate in ABA biosynthesis in NaCl-stressed roots regulated by TaCDPK9-1. Plant Physiol. biochem. 151, 650–658. doi:10.1016/j.plaphy.2020.03.039
Keywords: wheat, GWAS, single nucleotide polymorphism, PUE, non-limiting phosphorus and limiting phosphorus
Citation: Dharmateja P, Yadav R, Kumar M, Babu P, Jain N, Mandal PK, Pandey R, Shrivastava M, Gaikwad KB, Bainsla NK, Tomar V, Sugumar S, Saifi N and Ranjan R (2022) Genome-wide association studies reveal putative QTLs for physiological traits under contrasting phosphorous conditions in wheat (Triticum aestivum L.). Front. Genet. 13:984720. doi: 10.3389/fgene.2022.984720
Received: 02 July 2022; Accepted: 17 October 2022;
Published: 11 November 2022.
Edited by:
Vijay Gahlaut, Institute of Himalayan Bioresource Technology (CSIR), IndiaReviewed by:
Amit Kumar Singh, National Bureau of Plant Genetic Resources (ICAR), IndiaRahul Kumar, Chaudhary Charan Singh University, India
Copyright © 2022 Dharmateja, Yadav, Kumar, Babu, Jain, Mandal, Pandey, Shrivastava, Gaikwad, Bainsla, Tomar, Sugumar, Saifi and Ranjan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Palaparthi Dharmateja, ZGhhcm1hdGVqYS5wYWxhcGFydGhpQGdtYWlsLmNvbQ==; Rajbir Yadav, cmFqYmlyeWFkYXZAeWFob28uY29t
†These authors have contributed equally to this work
 Palaparthi Dharmateja
Palaparthi Dharmateja Rajbir Yadav
Rajbir Yadav Manjeet Kumar
Manjeet Kumar Prashanth Babu
Prashanth Babu Neelu Jain1
Neelu Jain1 Pranab Kumar Mandal
Pranab Kumar Mandal Manoj Shrivastava
Manoj Shrivastava Kiran B. Gaikwad
Kiran B. Gaikwad Naresh Kumar Bainsla
Naresh Kumar Bainsla Vipin Tomar
Vipin Tomar Nasreen Saifi
Nasreen Saifi Rumesh Ranjan
Rumesh Ranjan